Introduction
Intervertebral disc (IVD) degeneration is the
leading cause of chronic back pain, which impairs daily quality of
life (1). Until recent
developments, conservative treatment or surgical procedures were
the only strategies put in place to ameliorate the clinical
symptoms, due to poor understanding of the pathophysiology of IVD
degeneration (2). The major goals
of numerous studies include the development of etiological
treatments and the reversal of the degeneration process (3).
An increasing number of recent studies have shown
that growth factors play important roles in the occurrence and
development of IVD degeneration (4-6).
Growth factors constitute a group of low molecular weight proteins
involved in mitosis and the stimulation of the synthesis of
extracellular matrix (1,7). Various growth factors, such as
insulin-like growth factor (IGF), TGF-β and bone morphogenetic
proteins, have been identified as anabolic regulators that alter
homeostasis by shifting the cellular metabolism to the anabolic
state in IVD (8-10).
Members of the fibroblast growth factor (FGF) family
have been shown to contribute to the regulation of articular and
IVD homeostasis (11,12). FGF-18 has been identified as an
anabolic growth factor involved in cartilage homeostasis (13). A single intravenous injection of
pharmacologic doses of FGF-18 can stimulate the expansion of
various cartilage depots in rats, including the rib-sternum
junction, trachea, spine and articular cartilage, within 2 weeks
(14). In a rat osteoarthritis
model, a series of FGF-18 intra-articular injections was able to
increase cartilage formation and reduce cartilage degeneration in
the tibial plateau (15). However,
the role of FGF-18 in IVD degeneration has not been
investigated.
IVD degeneration is a chronic process; hence, a
single injection may not achieve satisfactory therapeutic effect
because the half-life of FGF-18 in the nucleus pulposus is unknown
(16). Prolonged exposure to growth
factors may be needed to stimulate biological repair. Gene therapy
techniques have been used to explore the effects of growth factors
on IVD (17,18). In the present study, a
lentivirus-mediated gene transfer approach was used to investigate
the effect of FGF-18 treatment on IVD degeneration in a rabbit
model. In addition, tert-butyl hydroperoxide (TBHP) was used to
induce the apoptosis of nucleus pulposus cells (NPs), and the
protective effects of FGF-18 overexpression were evaluated in
vitro.
The purpose of the present study was to perform a
preliminarily assessment of the application of FGF-18 for the
treatment of IVD degeneration. The effects of the
lentivirus-mediated delivery of FGF-18 on IVD degeneration were
evaluated in a puncture-induced IVD degeneration in vivo
model in rabbits and in TBHP-treated NP cells in vitro. The
results suggested that FGF-18 can delay IVD degeneration by
inhibiting the apoptosis of NPs and the expression of
matrix-degrading enzymes.
Materials and methods
Animals
Adult New Zealand 45 female white rabbits (age,
21-23 weeks; weight, 3.0-3.5 kg) were purchased from Experimental
Animal Center of Wenzhou Medical University (license no.
SYXK(Zhe)2010-0150). All procedures and experimental operations
were approved by the Animal Care and Use Committee of Wenzhou
Medical University and conformed to the Guide for the Care and Use
of Laboratory Animals of the National Institutes of Health
(19).
Reagents and antibodies
TBHP and type II collagenase were obtained from
Sigma-Aldrich; Merck KGaA. Rabbit anti-FGF-18 antibody was
purchased from LifeSpan Biosciences. Mouse monoclonal anti-Bcl-2
(cat. no. ab692) and anti-Bax (cat. no. ab3191) antibodies were
purchased from Abcam. Mouse monoclonal anti-GAPDH antibody (cat.
no. #51332), rabbit monoclonal anti-cleaved caspase-3 antibody
(cat. no. #9664), goat anti-rabbit IgG-HRP (cat. no. #7047) and
goat anti-mouse IgG-HRP (cat. no. #7076) were purchased from Cell
Signaling Technology, Inc. Mouse monoclonal anti-Flag antibody
(cat. no. F3165) was purchased from Sigma-Aldrich; Merck KGaA. An
in situ cell death detection kit, POD (cat. no. 11684817910)
was purchased from Roche Diagnostics.
Lentiviral vector construction and
lentivirus production
The rabbit FGF-18 sequence was obtained from the
NCBI database (https://www.ncbi.nlm.nih.gov/nuccore/XM_002710377.3).
A Ubi-MCS-3FLAG-SV40-EGFP vector (Gv287 backbone; Shanghai GeneChem
Co., Ltd.) was linearized using the AgeI restriction enzyme
(New England BioLabs, Inc.). To create the recombinant Ubi-LV
vector, a fragment containing FGF-18 was constructed into the GV287
vector. Then, the recombinant Ubi-LV vector was transformed into
293T cells [(cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.), containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin, and placed at 37˚C
in a humidified incubator containing 5% CO2)]. After
identification by PCR and sequencing (data not shown), the 20 µg
Ubi-LV vector in combination with the lentiviral packaging vectors
(15 µg pHelper1.0 and 20 µg pHelper2.0) (Shanghai GeneChem Co.,
Ltd.) were transfected into 293T cells, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) to generate the virus. The virus titer was
determined using the gradient dilution method (20).
NP culture and viability assay
Rabbits were euthanized with an overdose of 10%
sodium pentobarbital (100 mg/kg) via intravenous injection. Death
was verified by cessation of the heartbeat and lack of movement.
The gel-like nucleus pulposus tissue was separated from the anulus
fibrosus under a dissection microscope, and the tissue was treated
with 0.1% collagenase and 2 U/ml hyaluronidase for 4 h at 37˚C.
Subsequently, the digested tissue was transferred as explants
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% heat-inactivated FBS (Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (100 U/ml penicillin and 100
µg/ml streptomycin) in a humidified atmosphere containing 5%
CO2 at 37˚C. The NPs separated from the explant culture
after 1 week. Confluent cells were harvested by using 0.25%
trypsin-EDTA solution and sub-cultured in 10-cm dishes or 6-well
plates. When reaching 20-30% confluence, corresponding to the stage
with optimal infection efficiency, the cells at first passage were
infected with various concentrations of lentivirus (with MOI
ranging from 1-100). Fluorescent protein expression in the cells
was observed using an inverted fluorescence microscope (Nikon
Corporation) at 24, 48 and 72 h post-infection to assess the
infection efficiency. After a second passage, the cells were
treated with TBHP at various concentration (0, 50, 100, 200, 300,
500 µM) for 4 h at 37˚C. Then, cell viability was determined by
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.)
at 37˚C for 2 h, according to the manufacturer's instructions.
Animal model and lentivirus-mediated
gene transfer
A rabbit annular puncture model (5 rabbits/group)
was generated to induce IVD degeneration at L3/4, L4/5 and
L5/6(21). All animals were
anesthetized intraperitoneally with 2% (w/v) pentobarbital (40
mg/kg). After puncture, ~109 PFU of the FGF-18
overexpression lentivirus were injected into the center of the
rabbit nucleus pulposus using a 100-µl micro-syringe (Hamilton
Company) along the initial foramina. Negative control lentivirus
with no gene overexpression was injected in the control group. In
the sham group, IVDs were only exposed and were not punctured.
Histologic and immunohistochemical
analysis
At 8 weeks after surgery, the rabbits were
sacrificed via an intravenous injection of 10% sodium pentobarbital
(100 mg/kg). The IVDs were separated, fixed in 10% neutral buffered
formalin at 4˚C for 3 days and decalcified in the presence of 10%
EDTA for 2-6 weeks. The samples were incubated in a mixture of
xylene and paraffin at 25˚C for 15 min, and paraffin I and paraffin
II were then added for 50-60 min each. The tissue was subsequently
sliced into 5-µm-thick sections. For H&E staining, the sections
were washed three times with PBS for 5 min each. and incubated with
hematoxylin for 5 min and eosin for another 5 min at room
temperature. After three more 5-min washes with PBS, the sections
were dehydrated in 95% alcohol, permeabilized with xylene and
mounted with neutral resin. A widely used grading scale was
subsequently used to assess the anulus fibrosus, the border between
the anulus fibrosus and nucleus pulposus, the cellularity of the
nucleus purpose and the matrix of the nucleus pulposus (14). Grades ranged from 4 to 12, where
‘normal’ is 1 point for each of the four categories listed above,
for a total of four points (grade 4). Since there is a maximum of
three points for each parameter, a total of 12 points (grade 12) is
representative of severe degeneration (20). A grading system was used to evaluate
semi-quantitatively disc degeneration as previously described
(22).
Immunohistochemical staining for cleaved caspase-3
was performed using the sections obtained 8 weeks after the
operation. After incubation with 3% H2O2 at
room temperature for 10 min, the tissue was washed with PBS, boiled
in 0.1% trisodium citrate at 90˚C for 15 min for antigen retrieval
and blocked with BSA (cat. no. 4240GR100; NeoFroxx GmbH) at room
temperature for 1 h. Subsequently, the sections were incubated with
a rabbit anti-cleaved caspase-3 monoclonal antibody (1:200) diluted
in PBS overnight at 4˚C, followed by an incubation with
HRP-conjugated secondary antibodies (1:1,000) for 1 h at room
temperature. The staining was visualized with 3,3'-diaminobenzidine
(OriGene Technologies, Inc.), and the sections were counterstained
with hematoxylin at room temperature for 1 min, dehydrated with
graded ethanol, clarified with dimethylbenzene and sealed with
neutral resin. Images (magnification, x200) were acquired using a
light imaging microscope (BX53; Olympus Corporation), and the
percentage of cleaved caspase-3-positive cells in five randomly
selected fields per sample was determined, and the images were
analyzed by ImageJ v1.44 software (National Institutes of
Health).
TUNEL assay
TUNEL assay was performed to detect DNA
fragmentation induced by apoptotic signaling cascades. Transverse
sections prepared as aforementioned were washed with distilled
water and incubated with protein digestion enzyme K for 20 min at
37˚C. Then, the in situ cell death detection kit, POD (Roche
Diagnostics) was used for the TUNEL assay according to the
manufacturer's instructions. Sections were stained with DAPI
(1:1,000; MilliporeSigma) at room temperature for 10 min to
visualize the nucleus. Images were evaluated using a fluorescence
microscope (DP70; Olympus Corporation) at x200 magnification, the
percentage of positive cells was quantified in five randomly
selected fields per sample, and the images were analyzed using
ImageJ v1.44 software (National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from the nucleus pulposus
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and then RNA was reversely transcribed into cDNA
with a SuperScript™ One-Step Reverse Transcription kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. The mRNA expression of matrix
metalloproteinase-3 (MMP-3) and A disintegrin and metalloproteinase
with thrombospondin motifs 5 (ADAMTS-5) were quantified using the
iTaq™ Universal SYBR-Green Supermix (Bio-Rad
Laboratories, Inc.) on the Real-Time PCR detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions used were as follows: 95˚C for 10 min, followed by 40
cycles of 95˚C for 15 sec, 60˚C for 30 sec and 72˚C for 30 sec.
Samples were amplified independently at least three times. Relative
gene expression was converted using the 2-ΔΔCq method
against GAPDH (23). All primer
sequences were designed and synthesized by Sangon Biotech Co. Ltd.
and are presented in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer | Sequence | Size, bp |
|---|
| MMP3 | Forward |
5'-CCCAAAGTGGACAAAAACTCA-3' | 118 |
| | Reverse |
5'-AGTCACCTCCTCCCAGACCT-3' | |
| ADAMTS-5 | Forward |
5'-GTGGAGTATGCGGAGGAGAC-3' | 139 |
| | Reverse |
5'-TCTTTGGCTTTGAACTGTCG -3' | |
| GAPDH | Forward |
5'-TGAACGGGAAACTCACTGG-3' | 117 |
| | Reverse |
5'-TCACCACCTTCTTGATGTCG-3' | |
Western blot analysis
The nucleus pulposus tissues or cultured cells were
lysed in ice-cold RIPA buffer (cat. no. P0013B; Beyotime Institute
of Biotechnology), 100 mM Na3VO4, 100 mM NaF,
100 mM PMSF (cat. no. ST506; Beyotime Institute of Biotechnology)
at 4˚C for 30 min, then centrifuged at 12,000 x g for 30 min at
4˚C, and protein concentration was determined using the BCA method
(Pierce BCA Protein Assay kit; Thermo Fisher Scientific, Inc.).
Finally, lysates were mixed with 5X loading buffer (cat. no. P1040;
Beijing Solarbio Science & Technology Co., Ltd.) and boiled at
100˚C for 10 min. The equivalent of 20 µg of protein was separated
via 12% SDS-PAGE and then transferred onto a PVDF membrane
(MilliporeSigma). The membranes were blocked with 5% non-fat milk
at room temperature for 1 h and then incubated overnight at 4˚C
with anti-Flag (1:800), anti-Bcl-2 (1:1,000), anti-Bax (1:1,000)
and anti-GAPDH (1:1,000) antibodies. After the membranes were
washed with TBS-Tween-20 (0.1%), HRP-conjugated antibodies
(1:5,000) was added to develop the signal at room temperature for
1.5 h. Immunoreactive proteins were visualized using an ECL
detection kit (Bio-Rad Laboratories, Inc.) and blots were analyzed
using Quantity One software v4.6.2 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
The results are presented as the mean ± SD unless
specified otherwise. All experiments were repeated at least three
times. Statistical significance was evaluated by using Student's
t-test, or comparison between multiple groups was assessed using
one-way ANOVA followed by Tukey's post hoc test. For histological
grades, data are expressed as a median (interquartile range) and
the Kruskal-Wallis test with Dunn's post hoc test was applied to
compare multiple groups. SPSS 22.0 (IBM Corp.) and GraphPad Prism
7.0 software (GraphPad Software, Inc.) were used to perform
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Overexpression of FGF-18 inhibits
apoptosis in NPs in vitro
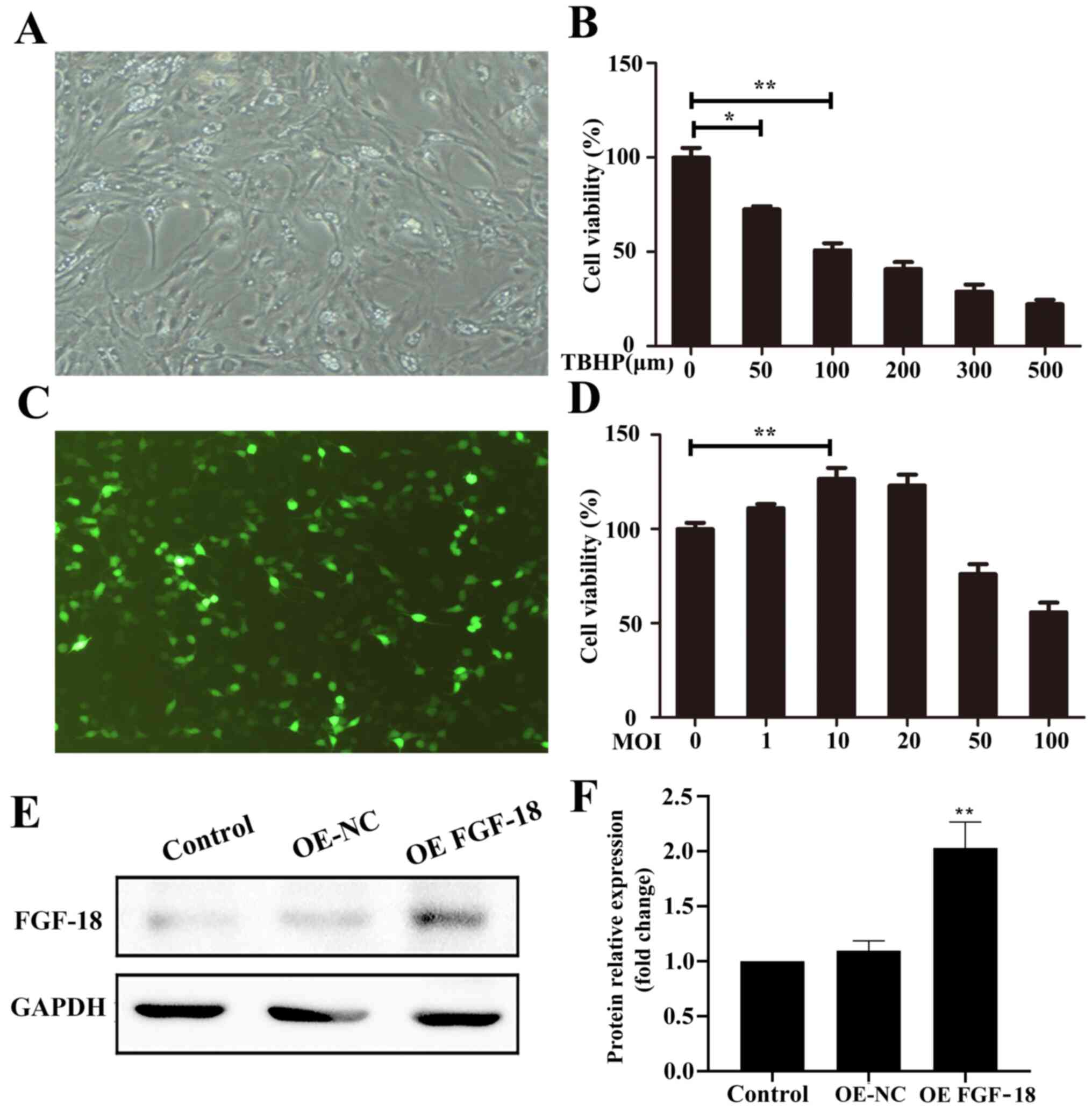
Pre-confluent NPs (Fig.
1A) were transfected with various concentrations of lentivirus
(with MOI ranging from 1-100). Cells in green indicated successful
transfection (Fig. 1C). CCK-8 assay
results indicated that lentiviral transfection at concentrations
≤20 MOI improved cell viability after 72 h (Fig. 1D). However, cell viability was
decreased by TBHP treatment in a dose-dependent manner (Fig. 1B). The transfection efficiency in
vitro was confirmed with quantification of FGF-18 expression in
NPs via western blotting (Fig. 1E
and F).
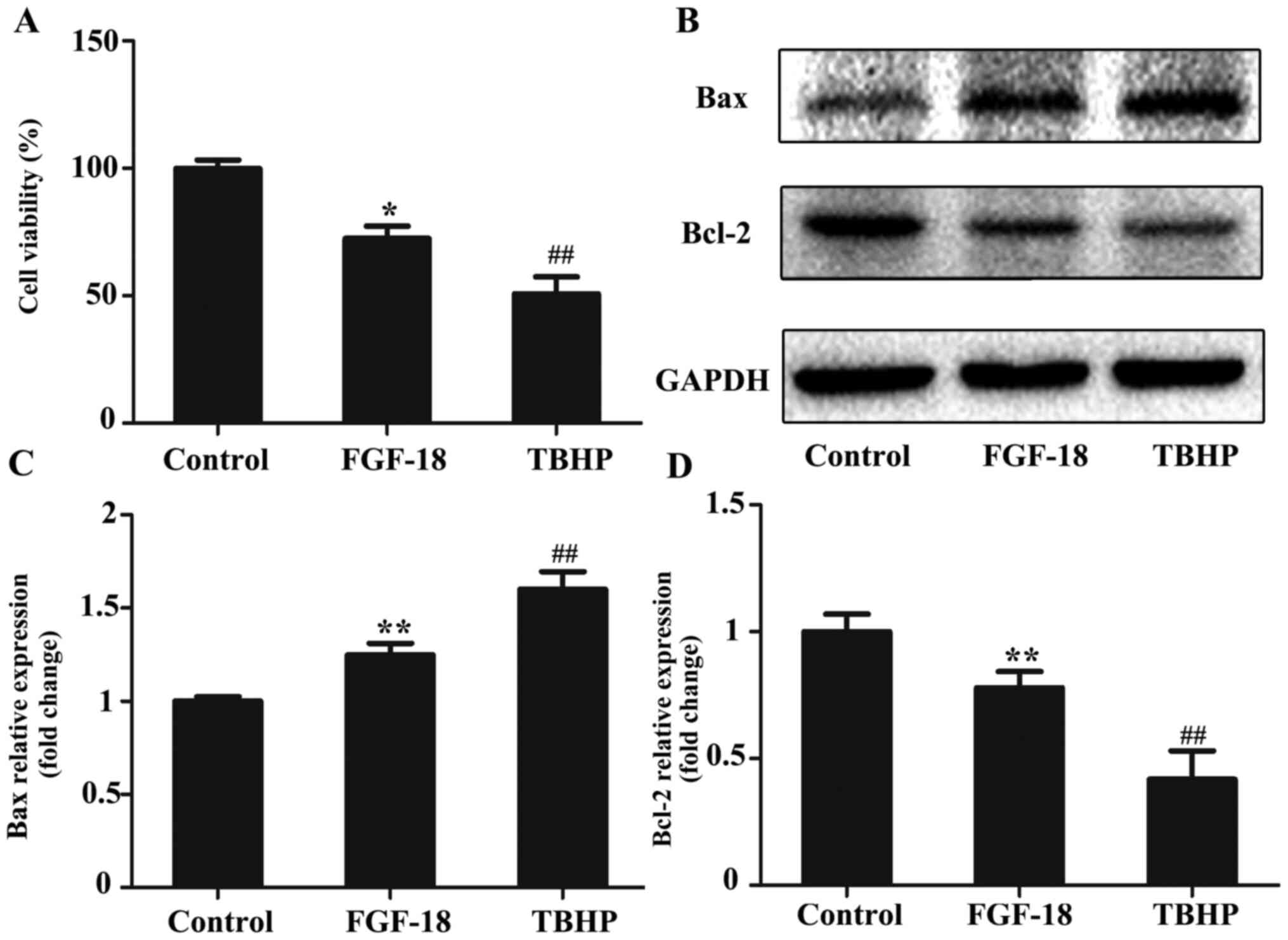
FGF-18 overexpression significantly protected NPs
against TBHP-induced cell death (Fig.
2A). The expression levels of Bax and Bcl-2 were detected using
western blot analyses (Fig. 2B).
The results indicated that 100 µM TBHP markedly increased the
expression of an apoptosis-related protein (Bax) and decreased the
expression of an anti-apoptotic protein (Bcl-2), whereas
overexpression of FGF-18 inhibited TBHP-induced apoptosis (Fig. 2C and D).
Anti-catabolic and anti-apoptotic
effects of FGF-18 on NPs in vivo
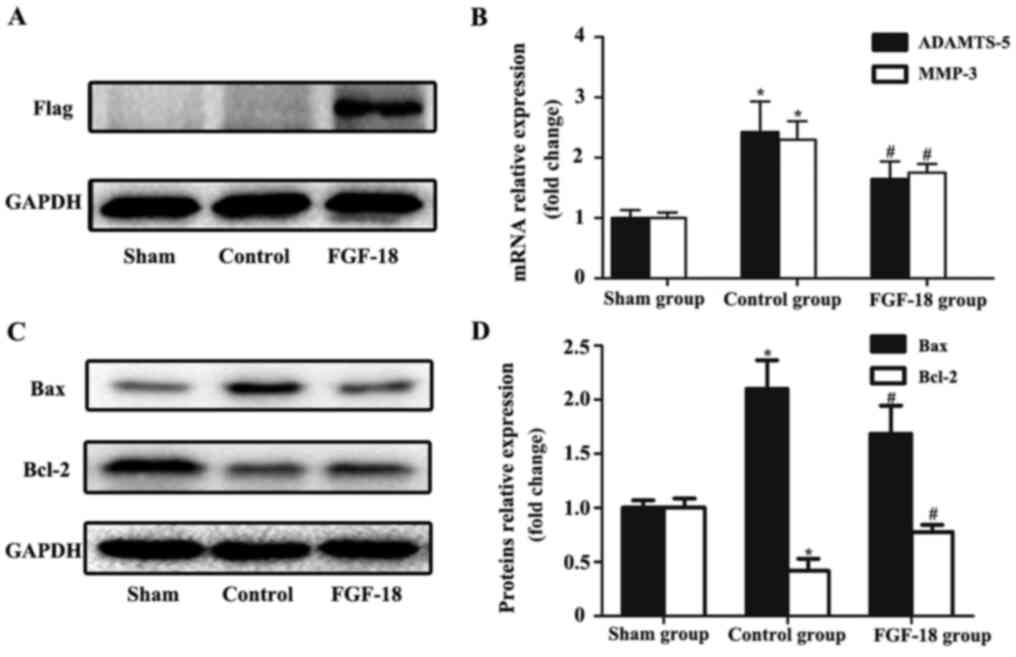
To evaluate the transfection efficiency of FGF-18
overexpression lentivirus in NPs, western blot analysis of
exogenous Flag protein was performed. The Flag protein was designed
to be co-expressed with FGF-18 and was not expressed in
non-infected NPs. As presented in Fig.
3A, the protein level of Flag in rabbit NPs was increased 8
weeks after they were transfected with FGF-18 overexpression
lentivirus, whereas it was not detected in the sham or control
group.
RT-qPCR results demonstrated that the mRNA levels of
the pro-catabolic indicators MMP-3 and ADAMTS-5 in the control
group were markedly increased compared with the sham group
(Fig. 3B). FGF-18 treatment
decreased the expression levels of both MMP-3 and ADAMTS-5 at the
mRNA level compared with the control group (Fig. 3B). As indicated by western blot
analysis, the expression levels of Bax in the FGF-18 group were
significantly reduced compared with those in the control group, and
the expression level of Bcl-2 was increased in the FGF-18 group
compared with the control group (Fig.
3C and D).
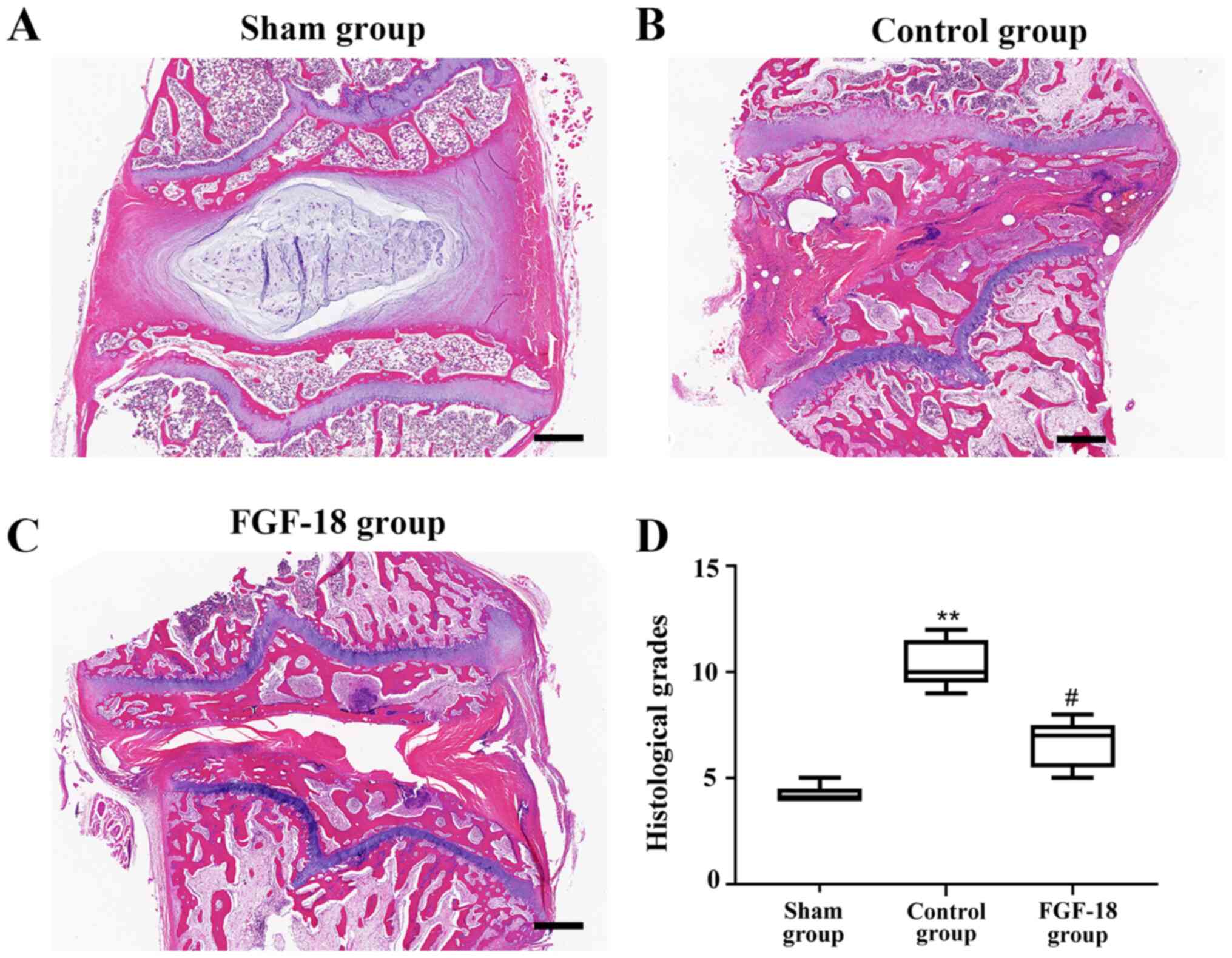
Histologic examination of the rabbit
discs after transfer of the FGF-18 overexpression lentivirus
H&E-stained IVDs were observed 8 weeks after the
operation (Fig. 4). Abnormalities,
such as inflammation or cancerous growth, were not detected in any
of the groups. However, after 8 weeks, NPs of the control group had
collapsed. As illustrated in Fig.
4B, the destruction structure was accompanied by the absence of
vacuoles and fewer NPs, and the space was filled with a
hypocellular fibrocartilaginous matrix. By contrast, the rabbits
treated with the FGF-18 overexpression lentivirus exhibited
numerous large vacuolated cells and smaller chondrocyte-like cells,
and the structure was more complete as observed in Fig. 4C. The histological grades in the
control group were significantly higher compared with in the sham
group (Fig. 4D), and they were
significantly lower in the FGF-18 group compared with in the
control group (Fig. 4D).
Immunohistochemical analysis of
cleaved caspase-3 in rabbit NPs
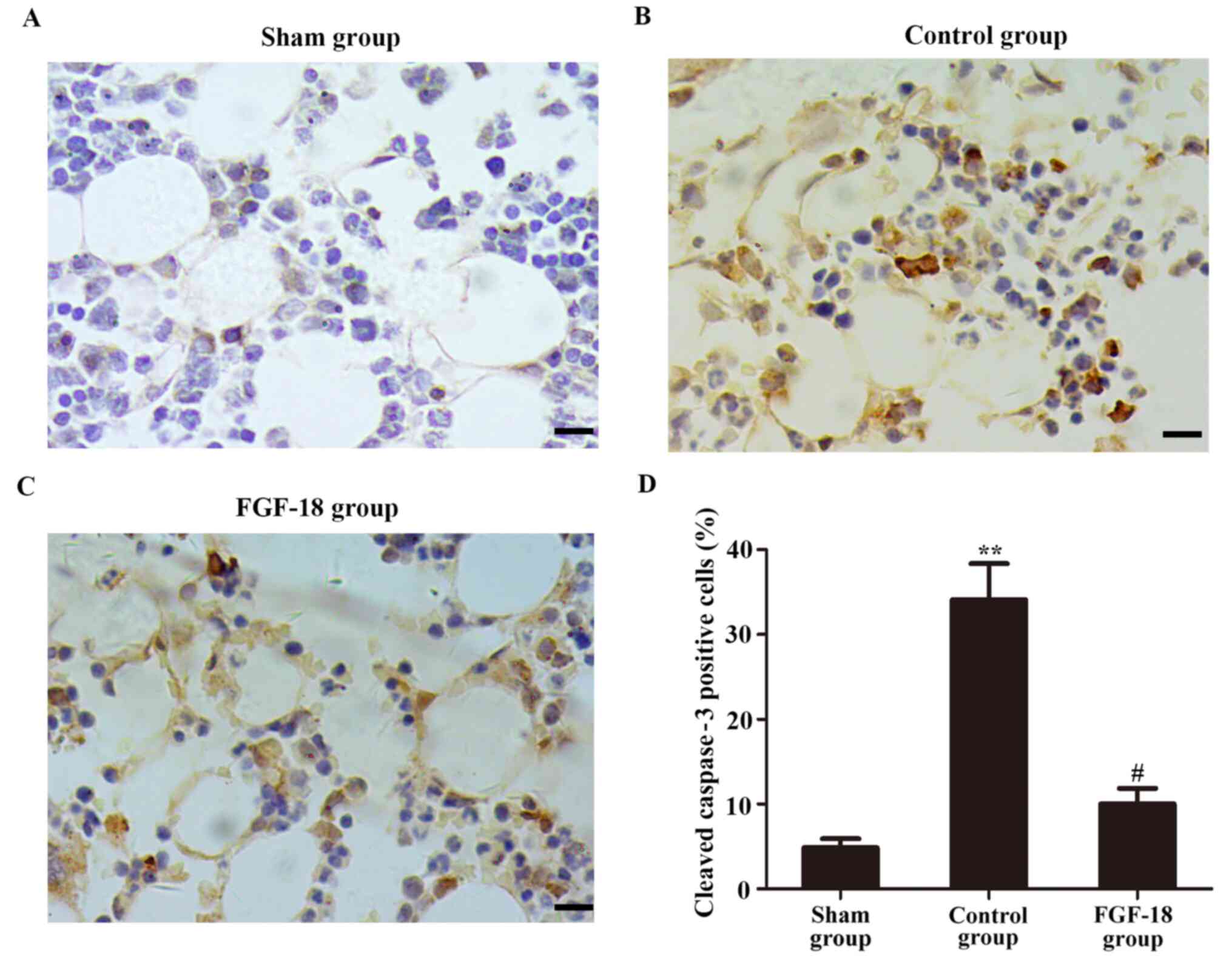
Immunohistochemical analysis of the in vivo
experiment indicated that the number of cleaved caspase-3-positive
NPs (brown positive signal) was decreased in the sham group. The
expression of cleaved caspase-3 in the control group was
significantly increased 8 weeks after surgery (Fig. 5A and B). However, the percentage of cleaved
caspase-3-positive cells in the FGF-18 group was reduced compared
with that in the control group (Fig.
5C).
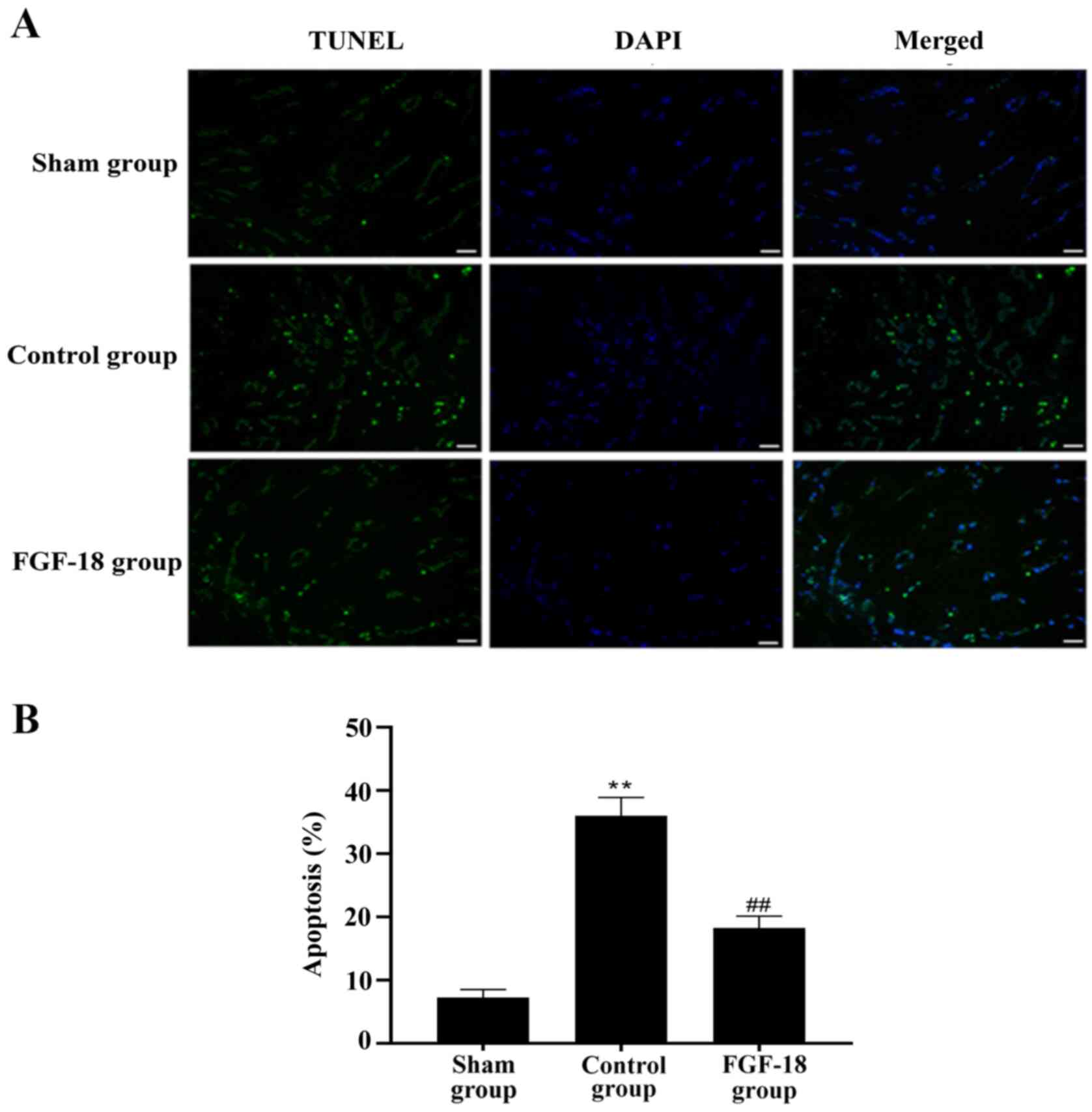
FGF-18 reduces the NP apoptosis
rate
TUNEL staining was performed to analyze the NP
apoptosis rate. The apoptosis rate of NPs was increased over time
in the control and FGF-18 groups. However, the apoptosis rate was
decreased for the FGF-18 group (Fig.
6A). Quantification of TUNEL staining showed that the FGF-18
group exhibited significantly fewer apoptotic NPs compared with the
control group (Fig. 6B).
Discussion
The present study suggested that FGF-18
overexpression can delay the process of IVD degeneration. FGF-18
was involved in the protection of NPs against apoptosis, and may
suppress extracellular matrix degeneration and promote
extracellular matrix synthesis. The results of the in vivo
experiments indicated that FGF-18 may ameliorate the process of IVD
degeneration in a puncture-induced rabbit model. However, the exact
mechanism via which FGF-18 attenuates the apoptosis of NPs remains
unclear. Further research will be performed to determine the
detailed mechanism.
Previous studies have indicated that biological
treatments can be used to stimulate cell activity, increase the
synthesis of extracellular matrix and reverse the process of IVD
degeneration (24). For instance,
injection of bone morphogenetic protein-7 into IVDs induced an
increase in the height of IVDs (22). In addition, Nishida et al
(25) reported that the injection
of an adenovirus construct encoding TGF-β into lumbar discs may
induce proteoglycan (PG) synthesis in rabbits. Interestingly, the
gene transfer of tissue inhibitor of metalloproteinases 1, an
inhibitor of catabolic enzymes, also increased the PG content in
the pellet cultures of human IVD cells (26). FGF-18 has been identified as a
powerful anabolic growth factor involved in cartilage homeostasis
(27). The present study
hypothesized that FGF-18 can protect against IVD degeneration,
which was investigated using a lentivirus-mediated gene transfer
approach in a rabbit annular needle puncture model.
In the NP in vitro model, overexpression of
FGF-18 increased cell viability and inhibited the apoptosis induced
by TBHP. To study the effect of FGF-18 in vivo, the
transfection efficiency of FGF-18 overexpression lentivirus was
first assessed by measuring the expression of Flag protein
co-expressed with FGF-18. The data indicated that Flag was stably
expressed 8 weeks after the operation. Histological examination
indicated that local injection of FGF-18 overexpression lentivirus
into the discs delayed the process of degeneration. In normal IVDs,
anabolic factors promote the generation of PG and collagen, and
catabolic factors inhibit the synthesis of the matrix to maintain a
dynamic balance (28). A previous
study reported that extracellular matrix degradation enzymes, such
as MMPs and ADAMTSs, which are upregulated by proinflammatory
cytokines, are characteristics of IVD degeneration (29). RT-qPCR was used to detect the
expression of the catabolic indicators MMP-3 and ADAMTS-5 and
demonstrated that their mRNA levels were increased in the control
group. The expression level was decreased after FGF-18
intervention, suggesting that FGF-18 has an inhibitory effect on
the synthesis of extracellular matrix degradation enzymes in
IVD.
The apoptosis of NPs may play critical pathogenic
roles in IVD degradation (30-32).
A previous study showed that Bcl-2 overexpression in NPs prevented
apoptotic cell death under serum-starved conditions (33). Importantly, another previous study
reported that caspase-3 small interfering RNA attenuated Bcl-2
expression induced in rabbit IVD degeneration (34). These results indicate that
inhibition of apoptosis of disc NPs may mitigate disc degeneration.
Thus, in the present study, several apoptotic markers were
quantified to evaluate the function of FGF-18 in NPs. The results
of the western blot analysis indicated that the expression level of
Bax was significantly reduced in the FGF-18 groups compared with in
the control group, and the expression of Bcl-2 was significantly
increased. Immunohistochemical analysis of cleaved caspase-3 showed
that the percentage of cleaved caspase-3-positive cells was
significantly increased in the model. Moreover, FGF-18 treatment
reduced the expression of cleaved caspase-3. TUNEL staining
demonstrated a similar result: The apoptotic index was decreased
after FGF-18 treatment. Thus, apoptotic cell death of NPs was
rescued by FGF-18 therapy in the present rabbit model of IVD
degeneration.
The purpose of the present study was to
preliminarily explore possible effects of the application of FGF-18
for the treatment of IVD degeneration. Using in situ
hybridization technique, Ellsworth et al (14) found that FGF receptor (FGFR)18,
FGFR3IIIc and FGFR2IIIc mRNAs were localized in chondrocytes of
human articular cartilage, suggesting a potential role of FGFR2 or
FGFR3 in FGF-18-mediated human articular cartilage homeostasis. In
addition, FGFR can activate MAPK signaling pathways, with various
downstream signaling cascades specific to the subtypes activated by
FGFR (35). FGFR1-mediated MAPK
activation leads to the activation of runt-related transcription
factor 2 and ETS like-1 protein, and subsequently induces the
expression of multiple MMPs, aggrecanases and leads to chondrocyte
hypertrophy (36). FGFR3-mediated
MAPK signaling could activate a different set of the downstream
transcription factors, leading to chondroprotective effects
(37). To date, the precise
signaling pathway mediated by the FGFR3-FGF-18 axis in cartilage
homeostasis is unknown. Thus, each part of the molecular mechanism
requires investigation in vivo and in vitro. A
comparison of the expression of extracellular matrix-degrading
enzymes MMP-3 and ADAMTS-5 indicated that FGF-18 had anti-catabolic
effects in IVD degeneration. Furthermore, the present data of the
immunohistochemical analysis and TUNEL assay indicated that the
protective effects were associated with inhibition of apoptosis in
NPs.
In conclusion, the present study indicated that
TBHP-induced apoptosis can be attenuated in FGF-18-treated NPs, and
FGF-18 treatment can ameliorate puncture-induced IVD degeneration
in rabbits. These findings suggested that a therapeutic strategy of
FGF-18 application may be a promising treatment for IVD
degeneration.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the
Science-technology Program of Wenzhou Municipal Sci-Tech Bureau
(grant no. 2020Y1553).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and CL searched the literature, designed the
experiments and performed the experiments. SL analyzed, interpreted
the data and wrote the manuscript. CL revised the manuscript. SL
and CL confirmed the authenticity of all the raw data. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures and experimental operations were
approved by the Animal Care and Use Committee of Wenzhou Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Masuda K and An HS: Growth factors and the
intervertebral disc. Spine J. 4 (Suppl 6):S330–S340.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kamali A, Ziadlou R, Lang G, Pfannkuche J,
Cui S, Li Z, Richards RG, Alini M and Grad S: Small molecule-based
treatment approaches for intervertebral disc degeneration: Current
options and future directions. Theranostics. 11:27–47.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cazzanelli P and Wuertz-Kozak K: MicroRNAs
in intervertebral disc degeneration, apoptosis, inflammation, and
mechanobiology. Int J Mol Sci. 21(3601)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim MJ, Lee JH, Kim JS, Kim HY, Lee HC,
Byun JH, Lee JH, Kim NH and Oh SH: Intervertebral disc regeneration
using stem cell/growth factor-loaded porous particles with a
leaf-stacked structure. Biomacromolecules. 21:4795–4805.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cui H, Zhang J, Li Z, Chen F, Cui H, Du X,
Liu H, Wang J, Diwan AD and Zheng Z: Growth differentiation
factor-6 attenuates inflammatory and pain-related factors and
degenerated disc-induced pain behaviors in rat model. J Orthop Res.
39:959–970. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hodgkinson T, Shen B, Diwan A, Hoyland JA
and Richardson SM: Therapeutic potential of growth differentiation
factors in the treatment of degenerative disc diseases. JOR Spine.
2(e1045)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Clouet J, Fusellier M, Camus A, Le Visage
C and Guicheux J: Intervertebral disc regeneration: From cell
therapy to the development of novel bioinspired endogenous repair
strategies. Adv Drug Deliv Rev. 146:306–324. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
An JL, Zhang W, Zhang J, Lian LC, Shen Y
and Ding WY: Vitamin D improves the content of TGF-β and IGF-1 in
intervertebral disc of diabetic rats. Exp Biol Med (Maywood).
242:1254–1261. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen S, Liu S, Ma K, Zhao L, Lin H and
Shao Z: TGF-β signaling in inter-ertebral disc health and disease.
Osteoarthritis Cartilage. 27:1109–1117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu Z, Zhou X and Chen G: Expression and
mechanism of interleukin 1 (IL-1), interleukin 2 (IL-2),
interleukin 8 (IL-8), BMP, fibroblast growth factor 1 (FGF1), and
insulin-like growth factor (IGF-1) in lumbar disc herniation. Med
Sci Monit. 25:984–990. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ellman MB, An HS, Muddasani P and Im HJ:
Biological impact of the fibroblast growth factor family on
articular cartilage and intervertebral disc homeostasis. Gene.
420:82–89. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ellman MB, Yan D, Ahmadinia K, Chen D, An
HS and Im HJ: Fibroblast growth factor control of cartilage
homeostasis. J Cell Biochem. 114:735–742. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Häckel S, Zolfaghar M, Du J, Hoppe S,
Benneker LM, Garstka N, Peroglio M, Alini M, Grad S, Yayon A and Li
Z: Fibrin-hyaluronic acid hydrogel (RegenoGel) with fibroblast
growth factor-18 for in vitro 3D culture of human and bovine
nucleus pulposus cells. Int J Mol Sci. 20(5036)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ellsworth JL, Berry J, Bukowski T, Claus
J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond
F, et al: Fibroblast growth factor-18 is a trophic factor for
mature chondrocytes and their progenitors. Osteoarthritis
Cartilage. 10:308–320. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moore EE, Bendele AM, Thompson DL, Littau
A, Waggie KS, Reardon B and Ellsworth JL: Fibroblast growth
factor-18 stimulates chondrogenesis and cartilage repair in a rat
model of injury-induced osteoarthritis. Osteoarthritis Cartilage.
13:623–631. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Muresanu C, Somasundaram SG, Vissarionov
SV, Gavryushova LV, Nikolenko VN, Mikhaleva LM, Kirkland CE and
Aliev G: Hypothetical role of growth factors to reduce
intervertebral disc degeneration significantly through trained
biological transformations. Curr Pharm Des: Oct 18, 2020 (Epub
ahead of print).
|
|
17
|
Yue B, Lin Y, Ma X, Xiang H, Qiu C, Zhang
J, Li L and Chen B: Survivin-TGFB3-TIMP1 gene therapy via
lentivirus vector slows the course of intervertebral disc
degeneration in an in vivo rabbit model. Spine (Phila Pa 1976).
41:926–934. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mobasheri A and Richardson SM: Cell and
gene therapy for spine regeneration: Mammalian protein production
platforms for overproduction of therapeutic proteins and growth
factors. Neurosurg Clin N Am. 31:131–139. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US), Washington, DC,
1996.
|
|
20
|
Giry-Laterrière M, Verhoeyen E and Salmon
P: Lentiviral vectors. Methods Mol Biol. 737:183–209.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Masuda K, Aota Y, Muehleman C, Imai Y,
Okuma M, Thonar EJ, Andersson GB and An HS: A novel rabbit model of
mild, reproducible disc degeneration by an anulus needle puncture:
Correlation between the degree of disc injury and radiological and
histological appearances of disc degeneration. Spine (Phila Pa
1976). 30:5–14. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Imai Y, Okuma M, An HS, Nakagawa K, Yamada
M, Muehleman C, Thonar E and Masuda K: Restoration of disc height
loss by recombinant human osteogenic protein-1 injection into
intervertebral discs undergoing degeneration induced by an
intradiscal injection of chondroitinase ABC. Spine (Phila Pa 1976).
32:1197–1205. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Choi UY, Joshi HP, Payne S, Kim KT, Kyung
JW, Choi H, Cooke MJ, Kwon SY, Roh EJ, Sohn S, et al: An injectable
hyaluronan-methylcellulose (HAMC) hydrogel combined with Wharton's
Jelly-derived mesenchymal stromal cells (WJ-MSCs) promotes
degenerative disc repair. Int J Mol Sci. 21(7391)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nishida K, Kang JD, Gilbertson LG, Moon
SH, Suh JK, Vogt MT, Robbins PD and Evans CH: Modulation of the
biologic activity of the rabbit intervertebral disc by gene
therapy: An in vivo study of adenovirus-mediated transfer of the
human transforming growth factor beta 1 encoding gene. Spine (Phila
Pa 1976). 24:2419–2425. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vo NV, Hartman RA, Yurube T, Jacobs LJ,
Sowa GA and Kang JD: Expression and regulation of
metalloproteinases and their inhibitors in intervertebral disc
aging and degeneration. Spine J. 13:331–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie Y, Zinkle A, Chen L and Mohammadi M:
Fibroblast growth factor signalling in osteoarthritis and cartilage
repair. Nat Rev Rheumatol. 16:547–564. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine (Phila
Pa 1976). 31:2151–2161. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chung SA, Khan SN and Diwan AD: The
molecular basis of intervertebral disk degeneration. Orthop Clin
North Am. 34:209–219. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Heyde CE, Tschoeke SK, Hellmuth M,
Hostmann A, Ertel W and Oberholzer A: Trauma induces apoptosis in
human thoracolumbar intervertebral disc. BMC Clin Pathol.
6(5)2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rannou F, Lee TS, Zhou RH, Chin J, Lotz
JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A and Shyy JY:
Intervertebral disc degeneration: The role of the mitochondrial
pathway in annulus fibrosus cell apoptosis induced by overload. Am
J Pathol. 164:915–924. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lin X and Lin Q: μiRNA-495-3p attenuates
TNF-α induced apoptosis and inflammation in human nucleus pulposus
cells by targeting IL5RA. Inflammation. 43:1797–1805.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sudo H and Minami A: Regulation of
apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2
overexpression. J Orthop Res. 28:1608–1613. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yamada K, Sudo H, Iwasaki K, Sasaki N,
Higashi H, Kameda Y, Ito M, Takahata M, Abumi K, Minami A, et al:
Caspase 3 silencing inhibits biomechanical overloade induced
intervertebral disk degeneration. Am J Pathol. 184:753–764.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mao P, Cohen O, Kowalski KJ, Kusiel JG,
Buendia-Buendia JE, Cuoco MS, Exman P, Wander SA, Waks AG, Nayar U,
et al: Acquired FGFR and FGF alterations confer resistance to
estrogen receptor (ER) targeted therapy in ER+
metastatic breast cancer. Clin Cancer Res. 26:5974–5989.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yan D, Chen D and Im HJ: Fibroblast growth
factor-2 promotes catabolism via FGFR1-Ras-Raf-MEK1/2-ERK1/2 axis
that coordinates with the PKCδ pathway in human articular
chondrocytes. J Cell Biochem. 13:2856–2865. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Davidson D, Blanc A, Filion D, Wang H,
Plut P, Pfeffer G, Buschmann MD and Henderson JE: Fibroblast growth
factor (FGF) 18 signals through FGF receptor 3 to promote
chondrogenesis. J Biol Chem. 280:20509–20515. 2005.PubMed/NCBI View Article : Google Scholar
|