Introduction
Acute pancreatitis (AP) is a common clinical
condition, with an increasing incidence over recent years (1). For example, the hospital admissions
for AP doubled in the English population between 1963 and
1998(2). Irrespective of the cause,
activation of digestive enzymes within pancreatic acinar cells is
thought to be a critical event, which can initiate and propagate
pancreatic damage during AP (3). In
addition to pancreatic injury, the pathophysiology of severe AP
(SAP) involves a systemic inflammatory response, often resulting in
distant organ dysfunction (4).
Acute lung injury (ALI) is the most common distant organ disease in
SAP and is the main cause of SAP-associated death (5). The pathological mechanism of SAP-ALI
is very complex and has not been fully elucidated. Proinflammatory
cytokines secreted by activated inflammatory cells have been
implicated in the propagation and amplification of the systemic
inflammatory response in SAP (6).
Presently, the detrimental effects of the excessive inflammatory
cascade in the pathogenesis of ALI associated with SAP have been
extensively studied (7-9).
However, the initiating mechanisms of inflammation in the lungs are
not well defined.
Previous studies have demonstrated that the
inflammatory cascade induced by sterile inflammasome activation is
a major component in a wide range of diseases, including SAP and
ALI (10,11). Molecular signaling pathways that
have been identified to contribute to sterile inflammation include
toll-like receptor (TLR) signaling pathways, specific inflammasome
complexes and IL-1 signaling pathways (12). TLR4 is an important mediator of the
inflammatory response in SAP (13-15).
Additionally, baicalin targeting TLR4 for ALI induced by AP has
been mentioned (6). IL-1β is
closely associated with SAP, and its increase is positively
correlated with disease severity (16). The pathological damage of the
pancreas, the degree of inflammation and the severity of SAP-ALI
are significantly decreased after blocking IL-1β expression in SAP
(8). Nod-like receptor protein 3
(NLRP3), an intracellular recognition receptor, is involved in
homotypic interactions and works with an adaptor protein known as
apoptosis-associated speck-like protein containing a CARD (ASC)
when triggered by a range of stimuli, including infection, tissue
damage and metabolic dysregulation (17). NLRP3 continues to recruit caspase-1
and forms an intracellular multiprotein complex known as an
inflammasome (18). Recent studies
have clarified the role of NLRP3 in the development of AP or ALI
(8,19). Aggregation of the NLRP3 inflammasome
stimulates caspase-1 and facilitates the processing and the
secretion of the proinflammatory cytokine IL-1β, which serves an
important part in inflammatory responses (20). Particularly, suppression of NLRP3
decreases neutrophil recruitment (21). Notably, the long-held belief that
neutrophils recruited to tissue injury are terminally activated to
fulfill their roles of pathogen defense, bystander tissue damage
and/or tissue repair has been challenged (22). There is emerging evidence that
neutrophils may exist in various subtypes, may have multifaceted
roles and may possess intermediate stages of activation even after
leaving the circulation (23). To
the best of our knowledge, the activation state and function of
recruited neutrophils in ALI have not been studied.
Emodin (1,3,8-trihydroxy-6-methylemodin) is an
active constituent of oriental medicinal herbs, including Rheum
officinale (24). Emodin
significantly eases pulmonary edema and improves SAP-induced ALI
(25,26). A number of studies investigating the
anti-inflammatory potential of emodin have also been reported
(27,28). However, the functional involvement
of emodin in neutrophil recruitment and IL-1β secretion through
inflammasome regulation has not been reported in SAP-induced ALI
in vivo (29). On the other
hand, dexamethasone (DEX) is an important glucocorticoid that is
vital in anti-inflammation and anti-toxicity, and can be used to
alleviate early inflammatory reactions in the lungs in SAP
(30). Several studies have
provided experimental basis for the protective role of DEX in lung
inflammation by targeting the NLRP3 inflammasome signaling pathway
(31,32). The present study aimed to
investigate the functional capacity of emodin in the improvement of
inflammation in the lungs induced by SAP and to evaluate the role
of NLRP3 inflammasomes to provide further information regarding the
clinical treatment options for ALI.
Materials and methods
Animals
A total of 40 male 6-week-old Wistar rats weighing
200-250 g were purchased from the Animal Center of Dalian Medical
University (Dalian, China). The Institutional Animal Care and Use
Committee of Dalian Medical University approved the protocols for
all the experiments (approval no. AEE19003), which were performed
in the laboratory of the Animal Center of Dalian Medical University
under the approved protocols.
Experimental process
All rats were given free food and water and kept
under standard conditions (room temperature, 22±2˚C; 12-h
light/dark cycle; relative humidity, 50-60%) and left to
acclimatize for 1 week. The mice were randomly assigned to the
following groups: i) Sham group; ii) SAP group; iii) SAP-emodin
(Emodin) group; and iv) SAP-DEX (DEX) group, with 10 rats in each
group. Pentobarbital (4%; 30-40 mg/kg) was injected
intraperitoneally in all rats for anesthesia. Retrograde infusion
treatment of 5% sodium taurocholate (1 ml/kg; Sigma-Aldrich; Merck
KGaA) into the biliary pancreatic duct was performed to induce
SAP-associated ALI in the SAP, Emodin and DEX groups. Emodin (cat.
no. E8390; Beijing Solarbio Science & Technology Co., Ltd.)
treatment (4 mg/ml; 40 mg/kg) was administered to rats via gavage 2
h after rats gained consciousness following SAP induction, as
previously described (30). DEX
(Henan Lingrui Pharmaceutical Co., Ltd.) treatment (5 mg/ml; 2
ml/kg) was intraperitoneally injected at 2 h post-SAP induction in
the DEX group as previously described (33). Rats were anesthetized using
pentobarbital (40 mg/kg) intraperitoneally before euthanasia by
exsanguination at 24 h post-modeling. Blood and lung tissues were
collected and stored at 4˚C and -80˚C, respectively, for
analysis.
Preparation of single-cell suspensions
of lung and flow cytometric analysis of apoptosis
After the rats were sacrificed, their lungs were
perfused through the right ventricle with 5 ml PBS. The lungs were
removed and the large airways were dissected from the peripheral
lung tissue. The peripheral lung tissue was cut into small pieces
with scissors, transferred into C-tubes (Miltenyi Biotec, Inc.) and
processed in digestion buffer (1 mg/ml of Collagenase D and 0.1
mg/ml DNase I; both dissolved in Hanks' balanced salt solution;
both from Roche Diagnostics) and a GentleMACS dissociator (Miltenyi
Biotec GmbH), according to the manufacturer's instructions.
Homogenized lungs were passed through a 40-µm nylon mesh to obtain
a single-cell suspension. The remaining red blood cells were lysed
using BD Pharm Lyse (BD Biosciences). The resultant cells were
counted using a Countess cell counter (Invitrogen; Thermo Fisher
Scientific, Inc.). For apoptosis analysis, cells were stained using
an Annexin V/FITC and PI apoptosis detection kit (Nanjing KeyGen
Biotech Co., Ltd.). Subsequently, apoptosis was detected by flow
cytometry. Briefly, cells were transferred and suspended in
Annexin-binding buffer (1x106 cells/ml), and were then
incubated with Annexin V-FITC and PI for 15 min at room temperature
in the dark and immediately detected using FACSCanto II (BD
Biosciences) and analyzed with FlowJo v10 software (FlowJo
LLC).
Histopathological analysis
Histopathological analysis was performed in
accordance with a previously published method (29). Briefly, tissue samples obtained from
the right upper lobe and from the pancreas were fixed in 4%
paraformaldehyde solution for 24 h at room temperature and then
embedded in paraffin. The tissues were sliced into 5-µm-thick
sections and subjected to hematoxylin and eosin (H&E) staining
for 5 min at room temperature (29). Histopathological assessment of the
pancreas was performed as previously described (34). The stained sections were observed
using a light microscope (Olympus Corporation) at x200
magnification and edema, acinar necrosis, leukocyte infiltration,
hemorrhage, fat necrosis and perivascular inflammation were scored
using a scale of 0-3 (with 0 representing no histological
abnormality, 1 representing slight histological abnormality, 2
representing intermediate histological abnormality, and 3
representing severe morphological deterioration) were also
assessed. The overall score was the sum of the scores of edema,
acinar necrosis, leukocyte infiltration, hemorrhage, fat necrosis
and perivascular inflammation. Assessment of the lung injury was
performed by a researcher blinded to the groups using a modified
histological scoring system as described previously (35). The pathological score was assessed
on a scale of 0-4, averaging the score of the following items: i)
Alveolar congestion; ii) thickness of the alveolar wall; iii)
aggregation of neutrophils or leukocyte infiltration in the vessel
wall or air space; and iv) hemorrhage. The scores were represented
as follows: 0 for normal lungs; 1 for mild (<25%) lung
involvement; 2 for moderate (25-50%) lung involvement; 3 for severe
(51-75%) lung involvement and 4 for extremely severe (>75%) lung
involvement. The overall score obtained was based on the addition
of all the scores and presented as the mean ± standard deviation
(with three sections from each lung using eight lungs per
group).
α-amylase (AMY), IL-1β and IL-18
analyses
All blood samples were collected through the
posterior vena cava. After centrifugation at 1,500 x g for 10 min
at 4˚C, the supernatants were collected and stored at -80˚C. Serum
levels of AMY, IL-1β and IL-18 were quantified using specific Rat
AMY2 ELISA kit (cat. no. E-EL-R2545c), Rat IL-18 ELISA kit (cat.
no. E-EL-R0567c), Rat IL-1β ELISA kit (cat. no. E-EL-R0012c; all
from Elabscience, Inc.) according to the manufacturer's protocols.
All samples were run in triplicate.
Immunohistochemical staining
Tissue samples were fixed in 4% paraformaldehyde
solution for 24 h at room temperature and then embedded in
paraffin. Sections (10-µm-thick) of rat lungs were mounted on
slides coated with poly-L-lysine. Slides were deparaffinized using
xylene and rehydrated using graded percentages of ethanol. Citrate
buffer (0.01 mol/l citric acid, pH 6.0) was used to pretreat
sections for 20 min at 95˚C. The slides were immersed in PBS
containing 3% H2O2 for 10 min at room
temperature. Tissue sections were also blocked with 10% normal goat
serum (Wuhan Servicebio Technology Co., Ltd.; cat. no. G1208) in
PBS for 30 min at room temperature and incubated with rabbit
polyclonal anti-lymphocyte antigen 6 complex locus G6D (Ly6G)
antibody (Wuhan Servicebio Technology Co., Ltd.; cat. no. GB11229;
1:100) at 4˚C overnight. Subsequently, sections were washed with
PBS, incubated with biotinylated goat anti-rabbit IgG secondary
antibody for 20 min at room temperature and treated with
3,3'-diaminobenzidine chromogen for 5 min at room temperature.
Sections were counterstained using hematoxylin for 2 min at room
temperature and observed using a light microscope at x200
magnification, and finally measured using a quantitative digital
image analysis system (Image-Pro Plus 6.0; Media Cybernetics, Inc.)
for semi-quantitative analysis. The slides were analyzed by a
researcher who was blinded to the groups of the study.
Western blot analysis
Tissue samples obtained from rat lungs were
subjected to protein extraction using a protein extraction kit
(cat. no. KGP150; Nanjing KeyGen Biotech Co., Ltd.) according to
the manufacturer's protocol. Protein concentrations were estimated
using the bicinchoninic acid procedure (Beijing Solarbio Science
& Technology Co., Ltd.). Bovine serum albumin (Beyotime
Institute of Biotechnology; cat. no. ST025) was used as the
standard. Extracted proteins (20 µg) were resuspended in the
electrophoresis sample buffer containing β-mercaptoethanol. Protein
separation was preformed via 10% SDS-PAGE (Bio-Rad Laboratories,
Inc.) and subsequently electrotransferred onto polyvinylidene
fluoride membranes (EMD Millipore). Membranes were blocked using 5%
skimmed milk in TBS with 0.1% Tween-20 (TBS-T) for 2 h at 37˚C.
β-actin and GAPDH were used as the loading controls. The membranes
were incubated overnight at 4˚C with the following primary
antibodies against: Cleaved caspase-3 (1:1,000; cat. no. 9661S;
Cell Signaling Technology, Inc.), Bax (cat. no. 50599-2-Ig;
ProteinTech Group, Inc.), Bcl2 (1:1,000; cat. no. 26593-1-AP;
ProteinTech Group, Inc.), NLRP3 (1:1,000; cat. no. 19771-1-AP;
ProteinTech Group, Inc.), ASC (1:1,000; cat. no. ab175449; Abcam),
cleaved caspase-1 (1:1,000; cat. no. 89332S; Cell Signaling
Technology, Inc.), intercellular adhesion molecule (ICAM-1;
1:1,000; cat. no. ab171123; Abcam), GAPDH (1:1,000; cat. no. 5174T;
Cell Signaling Technology, Inc.) and β-actin (1:500; cat. no.
AF5003; Beyotime Institute of Biotechnology). The blots were then
washed with TBS-T and incubated with HRP-conjugated goat
anti-rabbit (1:1,000; cat. no. sc-2004), mouse anti-goat (1:1,000;
cat. no. sc-2354) or goat anti-mouse IgG secondary antibody
(1:1,000; cat. no. sc-2031; all from Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Subsequently, the blots were
extensively washed with TBS-T and exposed to ECL-plus reagent
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The light emitted was analyzed on a
BioSpectrum-410 multispectral imaging system with a Chemi HR camera
410 (Bio-Rad Laboratories, Inc.). Under the transmitted ultraviolet
light, protein expression was visualized as bands and photographed.
The images were analyzed semi-quantitatively based on band
densitometry using Image Lab 4.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
SPSS 20 (IBM Corp.) software package was used for
all statistical analyses. Data were expressed as the mean ± SD from
three independent experiments. Data comparison among multiple
groups was performed using one-way ANOVA followed by Tukey's post
hoc test to determine statistically significant differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Emodin decreases the severity of
SAP-induced pancreatic and lung injury
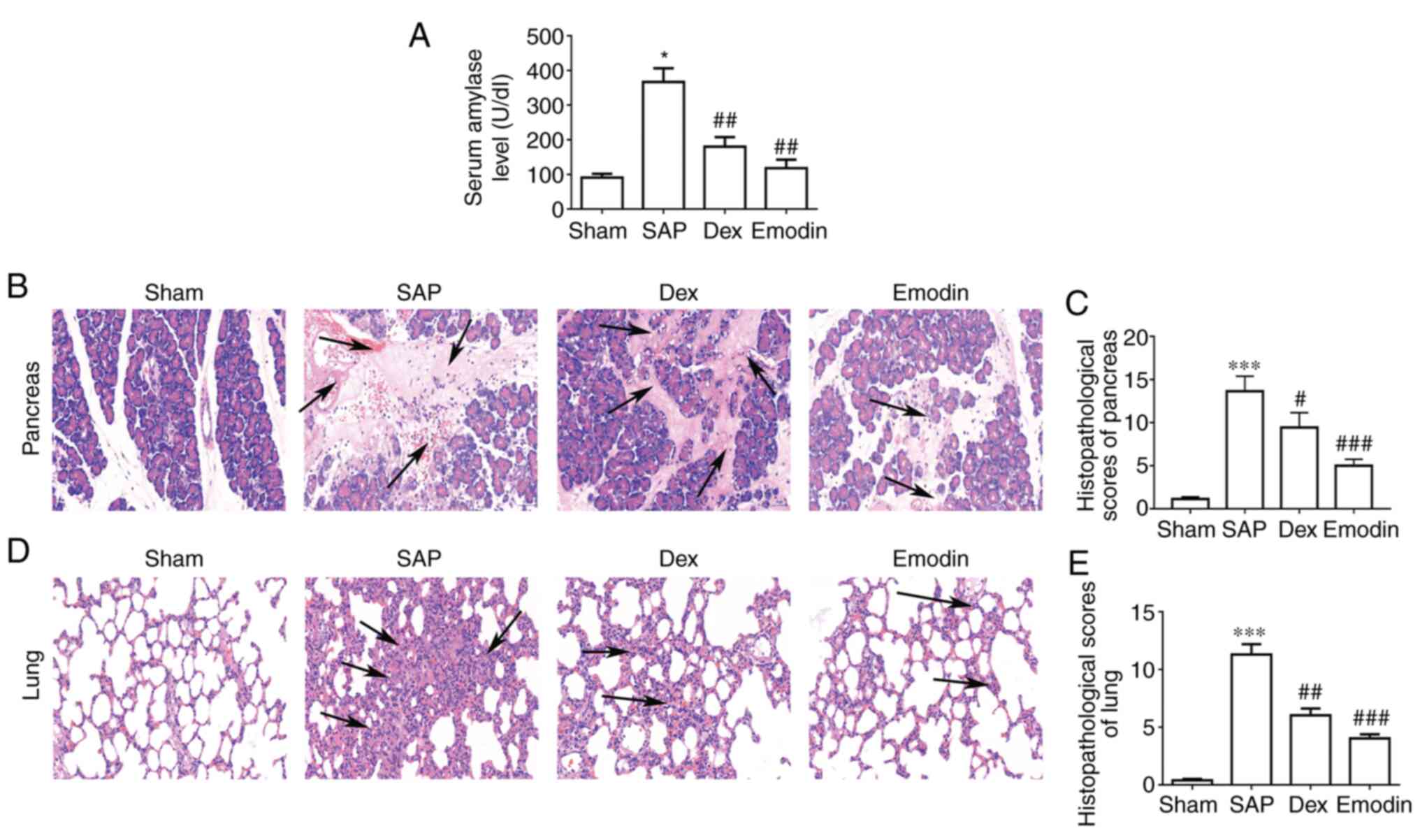
To determine the severity of SAP-induced pancreatic
and lung injury, the serum levels of AMY were measured. The SAP
group exhibited significantly increased AMY serum levels compared
with the Sham group, which could be reduced by treatment with
emodin or DEX (Fig. 1A). Using
H&E staining to analyze the pancreas, it was found that acinar
necrosis, inflammation, hemorrhage and edema occurred in SAP rats,
but these pathologies were not present in the Sham group (Fig. 1B). Furthermore, the
histopathological score of the pancreas in the SAP group was
significantly higher compared with that in the Sham group (Fig. 1C). These results confirmed that the
SAP model was successfully established in rats. Similarly, the lung
tissues of rats in the SAP group exhibited apparent alveolar wall
fracture, alveolar cavity expansion, alveolar congestion and
leukocyte infiltration, which is in contrast to the changes in the
Sham group (Fig. 1D). The overall
lung injury scores were significantly higher in the SAP group
compared with in the Sham group (Fig.
1E). These results further confirmed the establishment of
SAP-induced lung injury. Treatment with emodin and DEX markedly
decreased the injury in pancreatic and lung tissues compared with
the SAP group (Fig. 1B and D). Additionally, there was a significant
decrease in the histopathological scores in emodin or DEX group,
compared with the SAP group (Fig.
1C and E). These results
indicated that emodin and DEX had relieving effects on ALI.
Emodin and DEX decrease apoptosis in
the lungs during SAP progression
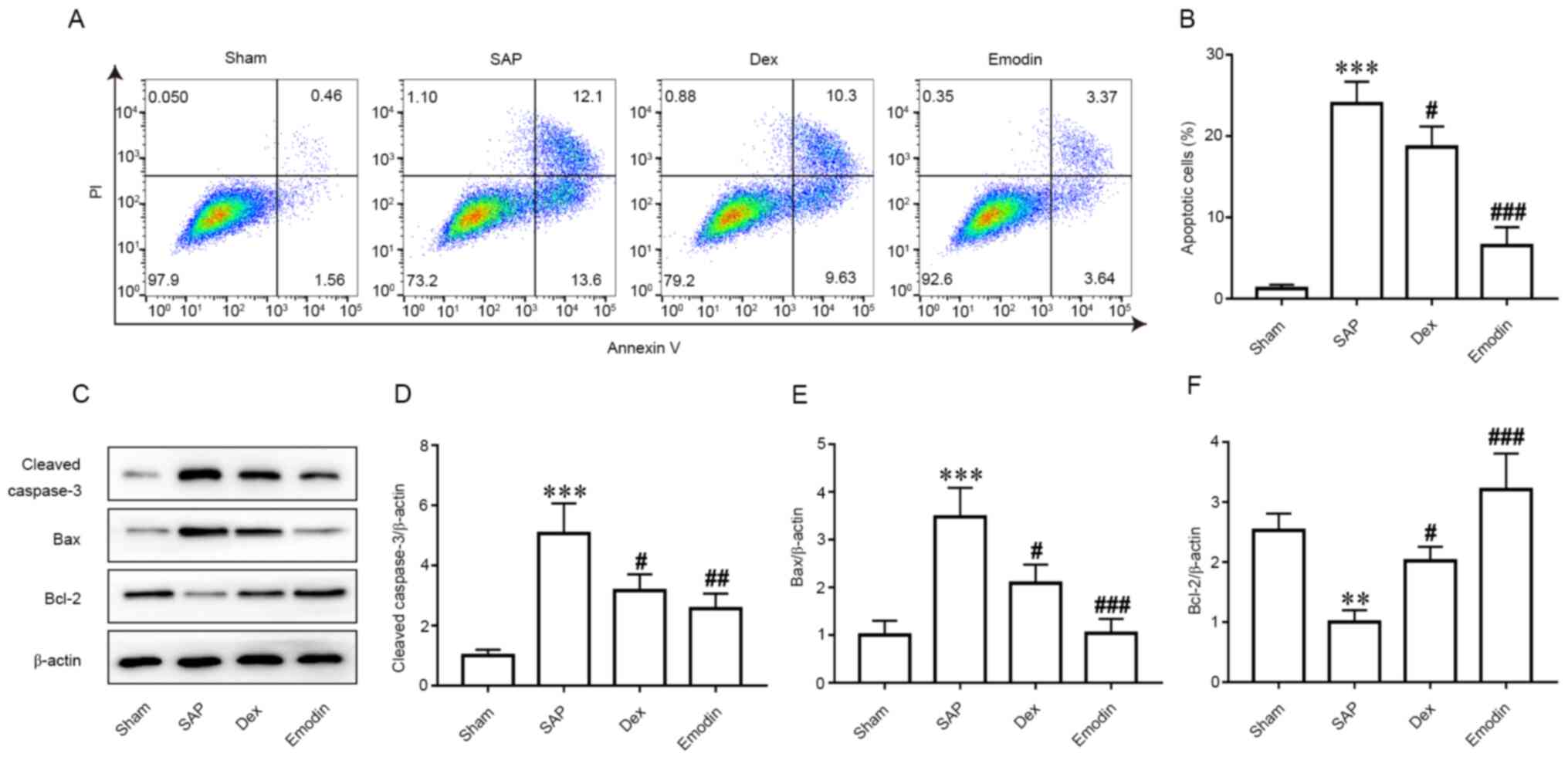
To further investigate the role of apoptosis on ALI
progression, flow cytometric analysis of apoptosis was performed in
each group (Fig. 2A). Compared with
the Sham group, the number of apoptotic cells was significantly
higher in the SAP group (Fig. 2A
and B). However, emodin and DEX
treatment significantly decreased the number of apoptotic cells
compared with the SAP group (Fig.
2A and B). In addition, the
expression levels of caspase-3, Bax and Bcl2 were evaluated.
Western blotting results revealed significantly increased
expression levels of cleaved caspase-3 and Bax, but a significant
decrease in Bcl-2 expression in the SAP group compared with in the
Sham group (Figs. 2C-F and S1). However, in emodin- and DEX-treated
rats, these changes were significantly reversed (Figs. 2C-F and S1).
Emodin decreases the activation of the
NLRP3 inflammasome and suppresses neutrophil recruitment in lung
tissues
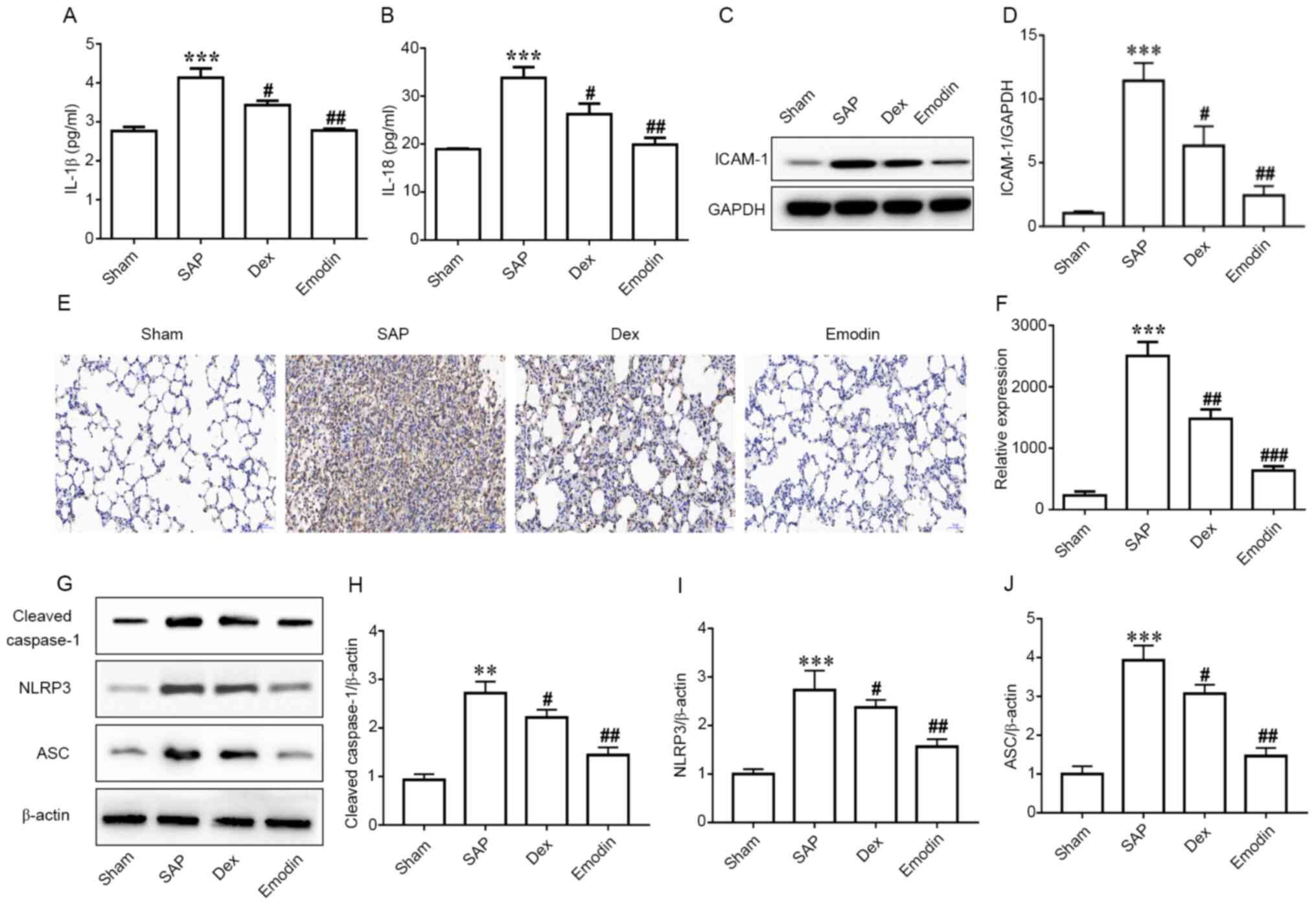
Analysis of serum samples revealed significantly
increased levels of IL-1β and IL-18 in the SAP group compared with
in the Sham group (Fig. 3A and
B). Meanwhile, treatment with
emodin and DEX significantly decreased the levels of IL-1β
(Fig. 3A) and IL-18 (Fig. 3B) compared with the SAP group. NLRP3
inflammasome is likely activated through several types of tissue
injury or pathogen-associated signatures, with outcomes such as the
autocatalytic cleavage of caspase-1, which ultimately leads to the
processing and production of pro-inflammatory cytokines,
particularly IL-1β (36). Hence,
western blotting was performed to determine NLRP3 expression. The
results revealed a significant upregulation in the expression
levels of cleaved caspase-1, NLRP3 and ASC in the SAP group
compared with in the Sham group (Figs.
3G-J and S2). Both emodin and
DEX significantly inhibited this inflammatory response (Figs. 3G-J and S2). Similarly, ICAM-1 expression was
significantly higher in the SAP group compared with in the Sham
group, and was then significantly decreased by emodin or DEX
treatment (Figs. 3C, D and S3).
Ly6G+ cell recruitment in the lungs, which is associated
with neutrophil levels (37), was
also measured, since neutrophils have been reported to serve an
important role in lung injury (38). Immunohistochemical staining results
indicated that the number of Ly6G+ cells was
significantly increased in the SAP group compared with in the Sham
group, and this was then significantly decreased by emodin or DEX
treatment (Fig. 3E and F).
Discussion
ALI is one of the most frequently observed diseases
among patients with SAP, presenting with severe systemic
complications, such as significant pulmonary edema, inflammatory
infiltration in the alveoli and hyperemia (39). Understanding the mechanisms
underlying SAP-induced ALI is important given the vital
physiological role of this organ system and its position as an
interface between the host and the environment. The respiratory
system is extremely susceptible to injury and can threaten the life
of an individual even with temporary functional impairment. It is
reported that ~20% of AP cases could lead to systemic inflammatory
response syndrome and multisystem organ injury (40). The excessive generation and release
of multiple inflammatory cytokines are considered as the
pathogenesis of SAP-induced ALI (41). Therefore, agents with
anti-inflammatory activity may be beneficial for the treatment of
SAP-induced ALI and may decrease the mortality rate of patients
with SAP.
Emodin or rhubarb is used as a traditional Chinese
medicine and has a long history of use as an anti-inflammatory
drug. Numerous studies have reported that emodin treatment can
significantly decrease the production of serum AMY and inflammatory
mediators or cytokines in lung tissues (27,40).
Additionally, DEX has been shown to act as a non-specific immune
inhibitor (42). It was reported
that its use inhibited the production of numerous inflammatory
mediators and increased the synthesis of proteins associated with
anti-inflammatory responses (43).
Its use for therapeutic intervention has been reported in
SAP-associated ALI (44). However,
DEX treatment gives rise to some adverse effects in patients,
including fungal infection, hyperglycemia, sleep insomnia and rapid
weight gain (45).
The results of the present study revealed serious
lung injury occurring in SAP rats compared with in Sham rats.
Characteristically, more severe pulmonary edema and
histopathological alterations were accompanied by increased
apoptosis and inflammatory mediator production. Treatment with
emodin or DEX significantly alleviated injury in lung and
pancreatic tissues and suppressed inflammation. Notably, treatment
with emodin exhibited improved protective effects on lung injury
caused by AP compared with DEX. Therefore, the present results
suggested that emodin may be a better alternative to DEX with fewer
side effects.
The transmigration of neutrophils through
endothelial cells serves important roles in the acute phase of
pulmonary inflammation (46).
Activated neutrophils are attracted by pro-inflammatory cytokines
to the site of injury; subsequently, neutrophils react with
excessive pro-inflammatory cytokine release and oxidative burst,
which in turn further aggravates the overall cellular inflammatory
response and lung tissue injury (47). Conversely, a decrease of neutrophil
transmigration has been described to limit lung injury (47). The present study reported that
neutrophil infiltration was attenuated after treatment with emodin,
suggesting a protective role of emodin in ALI. This suggested that
neutrophils may be important for ALI management.
However, the molecular mechanism involving
neutrophil recruitment into the lungs remains unclear. A number of
studies have indicated that NLRP3 serves a key role in neutrophil
recruitment, which has been demonstrated in different experimental
models. For example, a murine model of gout revealed that NLRP3 was
capable of mediating neutrophil recruitment (48). In another mice model where hepatic
ischemia/reperfusion injury was induced, NLRP3 was reported to
regulate chemokine-mediated roles and neutrophil recruitment,
contributing to liver injury (49).
The NLRP3 inflammasome improved the response to inflammation by
promoting neutrophil infiltration (21). A previous study by Gao et al
(29) reported that emodin could
alleviate acute pancreatitis-associated lung injury by inhibiting
NLPR3 inflammasome activation via Nrf2/HO-1 signalling. In line
with the aforementioned study, the present study reported that
emodin treatment inhibited NLRP3 and decreased neutrophil
recruitment, thereby contributing to the protective effects of
emodin treatment on ALI in a SAP model. Furthermore, the present
study further demonstrated that suppression of apoptosis was
involved in the benefical effect of emodin.
Although the present study did not fully examine the
mechanisms by which NLRP3 initiates neutrophil recruitment into the
lungs, it was speculated that the IL-1β signaling pathway may be
potentially involved. An important role of NLRP3 is cleaving IL-1β
into its active secretable forms (50). IL-1β and IL-1-receptor (IL-1R)
signaling is important for triggering the expression of adhesion
molecules, which are required for neutrophil attachment to vascular
endothelial cells for further infiltration (51). Recently, studies have demonstrated
that the IL-1β signaling pathway is involved in initiating
neutrophil recruitment in infectious inflammation, as well as
initiating neutrophil recruitment in response to sterile
inflammation (52,53). In a focal hepatic necrosis mice
model, mice that were administered with IL-1β blocking antibodies
exhibited a similar decrease in neutrophil accumulation (54). In addition, the number of
neutrophils induced to the necrotic areas was significantly
decreased in NLRP3-deficient mice compared with in wild-type mice
(54). These observations suggested
the potential involvement of the IL-1β signaling pathway in
NLRP3-induced neutrophil recruitment. However, whether the IL-1β
signaling pathway is also involved in ALI induced by AP requires
further investigation. The results of the present study revealed
that IL-1β serum levels and ICAM-1 expression in the lung were
significantly increased following SAP. However, following emodin or
DEX administration, a significant decrease in the IL-1β and ICAM-1
levels was observed, together with decreases in neutrophil
infiltration. Overall, IL-1β may be one of the signaling pathways
downstream of NLRP3, and further research is required to determine
the involvement of the IL-1β signaling pathway in recruiting
NLRP3-induced neutrophils. A limitation of the present study was
that only male rats were used and not female rats. The reason for
this was to maintain consistency with our previous studies as well
as with other studies (29,55). However, we plan to undertake similar
experiments in female rats in future experiments to ascertain
whether the effect is similar.
In conclusion, the present study demonstrated that
emodin and DEX treatment prevented lung and pancreatic injury in a
rat model of SAP-induced ALI. Inhibition of NLRP3 inflammasome and
IL-1β seems to be an important factor in preventing neutrophil
recruitment in the lungs. The current findings supported the notion
of NLRP3 inflammasome-induced neutrophil recruitment, which may be
a potential target for preventing SAP-induced ALI. Moreover, the
present study suggested that emodin may be used as an alternative
agent to DEX for SAP-ALI treatment.
Supplementary Material
Two replicate validation of cleaved
caspase-3, Bax and Bcl2 expression by western blot analyses. SAP,
severe acute pancreatitis; Dex, dexamethasone.
Two replicate validation of NLRP3, ASC
and cleaved caspase-1 expression by western blot analyses. NLRP3,
Nod-like receptor protein 3; ASC, apoptosis-associated speck-like
protein containing a CARD; SAP, severe acute pancreatitis; Dex,
dexamethasone.
Two replicate validation of ICAM-1
expression by western blot analyses. SAP, severe acute
pancreatitis; Dex, dexamethasone; ICAM-1, intercellular adhesion
molecule 1.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Fund of China (grant no. 81573751) and the Natural Science
Foundation Guidance Plan of Liaoning Province (grant no.
2019-ZD-0919).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC, QY and NJ conceived and designed the
experiments. NJ, ZL, YL and LJ established the animal model and
performed sample collection and validation experiments. NJ, QY and
GZ analyzed the data and drafted the manuscript. HC edited the
manuscript. HC and GZ secured funding for the study. NJ and HC
confirmed the authenticity of all the raw data. All authors
critically reviewed, read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments in the present study were
approved by the Institutional Animal Care and Use Committee of
Dalian Medical University (Dalian, China; approval no.
AEE19003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Donnelly PE and Winch DE: Acute
pancreatitis. N Engl J Med. 376(597)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Goldacre MJ and Roberts SE: Hospital
admission for acute pancreatitis in an English population, 1963-98:
Database study of incidence and mortality. BMJ. 328:1466–1469.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grupp K, Bonk S, Poppe A, Wodack K, Reeh
M, Gocht A, Mann O, Izbicki JR and Bachmann K: Cholecystokinin-8
treatment reduces acinar necrosis and edema of pigs with induced
pancreatitis. Asian J Surg. 43:272–277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Granger J and Remick D: Acute
pancreatitis: Models, markers, and mediators. Shock. 24 (Suppl
1):S45–S51. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pastor CM, Matthay MA and Frossard JL:
Pancreatitis-associated acute lung injury: New insights. Chest.
124:2341–2351. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li Z, Xia X, Zhang S, Zhang A, Bo W and
Zhou R: Up-regulation of Toll-like receptor 4 was suppressed by
emodin and baicalin in the setting of acute pancreatitis. Biomed
Pharmacother. 63:120–128. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gukovskaya AS, Gukovsky I, Algül H and
Habtezion A: Autophagy, inflammation, and immune dysfunction in the
pathogenesis of pancreatitis. Gastroenterology. 153:1212–1226.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fu Q, Zhai Z, Wang Y, Xu L, Jia P, Xia P,
Liu C, Zhang X, Qin T and Zhang H: NLRP3 deficiency alleviates
severe acute pancreatitis and pancreatitis-associated lung injury
in a mouse model. Biomed Res Int. 2018(1294951)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang M, Chen XM, Du XG, Cao FF, Vijaya
Luxmi S and Shen Q: Continuous blood purification ameliorates
endothelial hyperpermeability in SAP patients with MODS by
regulating tight junction proteins via ROCK. Int J Artif Organs.
36:700–709. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tian X, Sun H, Casbon AJ, Lim E, Francis
KP, Hellman J and Prakash A: NLRP3 inflammasome mediates dormant
neutrophil recruitment following sterile lung injury and protects
against subsequent bacterial pneumonia in mice. Front Immunol.
8(1337)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hoque R, Farooq A, Ghani A, Gorelick F and
Mehal WZ: Lactate reduces liver and pancreatic injury in Toll-like
receptor- and inflammasome-mediated inflammation via GPR81-mediated
suppression of innate immunity. Gastroenterology. 146:1763–1774.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dolinay T, Kim YS, Howrylak J, Hunninghake
GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L,
Nakahira K, et al: Inflammasome-regulated cytokines are critical
mediators of acute lung injury. Am J Respir Crit Care Med.
185:1225–1234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Johnson GB, Brunn GJ and Platt JL: Cutting
edge: An endogenous pathway to systemic inflammatory response
syndrome (SIRS)-like reactions through Toll-like receptor 4. J
Immunol. 172:20–24. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hietaranta A, Mustonen H, Puolakkainen P,
Haapiainen R and Kemppainen E: Proinflammatory effects of
pancreatic elastase are mediated through TLR4 and NF-kappaB.
Biochem Biophys Res Commun. 323:192–196. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Y, Zhou ZG, Zhang J, Chen YD, Li HG,
Gao HK, Wang R and Hu TZ: Microcirculatory detection of Toll-like
receptor 4 in rat pancreas and intestine. Clin Hemorheol Microcirc.
34:213–219. 2006.PubMed/NCBI
|
|
16
|
Watanabe T, Kudo M and Strober W:
Immunopathogenesis of pancreatitis. Mucosal Immunol. 10:283–298.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cai X, Chen J, Xu H, Liu S, Jiang QX,
Halfmann R and Chen ZJ: Prion-like polymerization underlies signal
transduction in antiviral immune defense and inflammasome
activation. Cell. 156:1207–1222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jin HZ, Yang XJ, Zhao KL, Mei FC, Zhou Y,
You YD and Wang WX: Apocynin alleviates lung injury by suppressing
NLRP3 inflammasome activation and NF-κB signaling in acute
pancreatitis. Int Immunopharmacol. 75(105821)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Baroja-Mazo A, Martín-Sánchez F, Gomez AI,
Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe
J, Ruiz-Ortiz E, Antón J, et al: The NLRP3 inflammasome is released
as a particulate danger signal that amplifies the inflammatory
response. Nat Immunol. 15:738–748. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Ma Q, Chen S, Hu Q, Feng H, Zhang JH and
Tang J: NLRP3 inflammasome contributes to inflammation after
intracerebral hemorrhage. Ann Neurol. 75:209–219. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chou RC, Kim ND, Sadik CD, Seung E, Lan Y,
Byrne MH, Haribabu B, Iwakura Y and Luster AD:
Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a
murine model of inflammatory arthritis. Immunity. 33:266–278.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mayadas TN, Cullere X and Lowell CA: The
multifaceted functions of neutrophils. Annu Rev Pathol. 9:181–218.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kuo YC, Tsai WJ, Meng HC, Chen WP, Yang LY
and Lin CY: Immune reponses in human mesangial cells regulated by
emodin from Polygonum hypoleucum Ohwi. Life Sci. 68:1271–1286.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu C, Zhang J, Liu J, Li Z, Liu Z, Luo Y,
Xu Q, Wang M, Zhang G, Wang F and Chen H: Proteomic analysis
reveals the protective effects of emodin on severe acute
pancreatitis induced lung injury by inhibiting neutrophil proteases
activity. J Proteomics. 220(103760)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu J, Huang B, Wang Y, Tong C, Xie P, Fan
R and Gao Z: Emodin ameliorates acute lung injury induced by severe
acute pancreatitis through the up-regulated expressions of AQP1 and
AQP5 in lung. Clin Exp Pharmacol Physiol. 43:1071–1079.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu Y, Tu X, Lin G, Xia H, Huang H, Wan J,
Cheng Z, Liu M, Chen G, Zhang H, et al: Emodin-mediated protection
from acute myocardial infarction via inhibition of inflammation and
apoptosis in local ischemic myocardium. Life Sci. 81:1332–1338.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ding Y, Zhao L, Mei H, Zhang SL, Huang ZH,
Duan YY and Ye P: Exploration of Emodin to treat
alpha-naphthylisothiocyanate-induced cholestatic hepatitis via
anti-inflammatory pathway. Eur J Pharmacol. 590:377–386.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gao Z, Sui J, Fan R, Qu W, Dong X and Sun
D: Emodin protects against acute pancreatitis-associated lung
injury by inhibiting nlpr3 inflammasome activation via Nrf2/HO-1
signaling. Drug Des Devel Ther. 14:1971–1982. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu C, Luo Y, Ntim M, Quan W, Li Z, Xu Q,
Jiang L, Zhang J, Shang D, Li L, et al: Effect of emodin on long
non-coding RNA-mRNA networks in rats with severe acute
pancreatitis-induced acute lung injury. J Cell Mol Med.
25:1851–1866. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guan M, Ma H, Fan X, Chen X, Miao M and Wu
H: Dexamethasone alleviate allergic airway inflammation in mice by
inhibiting the activation of NLRP3 inflammasome. Int
Immunopharmacol. 78(106017)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoshida K, Okamura H, Hiroshima Y, Abe K,
Kido JI, Shinohara Y and Ozaki K: PKR induces the expression of
NLRP3 by regulating the NF-κB pathway in Porphyromonas
gingivalis-infected osteoblasts. Exp Cell Res. 354:57–64.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu
L, Wang YX, Wang GY and Xu CM: Therapeutic effect of Qingyi
decoction in severe acute pancreatitis-induced intestinal barrier
injury. World J Gastroenterol. 21:3537–3546. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu Q, Gui P, Yao S and Xiang H: Expression
of integrin alpha v beta 6 in rats with ventilator-induced lung
injury and the attenuating effect of synthesized peptide S247. Med
Sci Monit. 14:BR41–BR48. 2008.PubMed/NCBI
|
|
36
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khomtchouk KM, Joseph LI, Khomtchouk BB,
Kouhi A, Massa S, Xia A, Koliesnik I, Pletzer D, Bollyky PL and
Santa Maria PL: Treatment with a neutrophil elastase inhibitor and
ofloxacin reduces P. aeruginosa burden in a mouse model of chronic
suppurative otitis media. NPJ Biofilms Microbiomes.
7(31)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xu Y, Lu B, Zhang N, Liang Y, Gao Y, Ye X
and Liu W: Neutrophil extracellular traps are not produced in
pediatric patients with one-lung ventilation: A prospective,
single-center, observational study. Transl Pediatr. 9:775–783.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Browne GW and Pitchumoni CS:
Pathophysiology of pulmonary complications of acute pancreatitis.
World J Gastroenterol. 12:7087–7096. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gao Z, Xu J, Sun D, Zhang R, Liang R, Wang
L and Fan R: Traditional Chinese medicine, Qing Ying Tang,
ameliorates the severity of acute lung injury induced by severe
acute pancreatitis in rats via the upregulation of aquaporin-1. Exp
Ther Med. 8:1819–1824. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang H, Neuhöfer P, Song L, Rabe B,
Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius
H, et al: IL-6 trans-signaling promotes pancreatitis-associated
lung injury and lethality. J Clin Invest. 123:1019–1031.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Janowitz T, Kleeman S and Vonderheide RH:
Reconsidering dexamethasone for antiemesis when combining
chemotherapy and immunotherapy. Oncologist. 26:269–273.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pontes-Quero GM, Benito-Garzón L, Pérez
Cano J, Aguilar MR and Vázquez-Lasa B: Modulation of inflammatory
mediators by polymeric nanoparticles loaded with anti-inflammatory
drugs. Pharmaceutics. 13(290)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sugiyama Y, Kato S, Abe M, Mitsufuji S and
Takeuchi K: Different effects of dexamethasone and the nitric oxide
synthase inhibitor L-NAME on caerulein-induced rat acute
pancreatitis, depending on the severity. Inflammopharmacology.
13:291–301. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Buzatto AZ, Malkawi A, Sabi EM, Mujamammi
AH, Li L and Abdel Rahman AM: Tissue lipidomic alterations induced
by prolonged dexamethasone treatment. J Proteome Res. 20:1558–1570.
2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Grommes J and Soehnlein O: Contribution of
neutrophils to acute lung injury. Mol Med. 17:293–307.
2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Faller S, Hausler F, Goeft A, von Itter
MA, Gyllenram V, Hoetzel A and Spassov SG: Hydrogen sulfide limits
neutrophil transmigration, inflammation, and oxidative burst in
lipopolysaccharide-induced acute lung injury. Sci Rep.
8(14676)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Amaral FA, Costa VV, Tavares LD, Sachs D,
Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni
DS, et al: NLRP3 inflammasome-mediated neutrophil recruitment and
hypernociception depend on leukotriene B(4) in a murine model of
gout. Arthritis Rheum. 64:474–484. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Inoue Y, Shirasuna K, Kimura H, Usui F,
Kawashima A, Karasawa T, Tago K, Dezaki K, Nishimura S, Sagara J,
et al: NLRP3 regulates neutrophil functions and contributes to
hepatic ischemia-reperfusion injury independently of inflammasomes.
J Immunol. 192:4342–4351. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fann DY, Lee SY, Manzanero S, Chunduri P,
Sobey CG and Arumugam TV: Pathogenesis of acute stroke and the role
of inflammasomes. Ageing Res Rev. 12:941–966. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Garlanda C, Dinarello CA and Mantovani A:
The interleukin-1 family: Back to the future. Immunity.
39:1003–1018. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Crother TR, Porritt RA, Dagvadorj J,
Tumurkhuu G, Slepenkin AV, Peterson EM, Chen S, Shimada K and
Arditi M: Autophagy limits inflammasome during chlamydia pneumoniae
infection. Front Immunol. 10(754)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sun Y, Abbondante S, Karmakar M, de Jesus
Carrion S, Che C, Hise AG and Pearlman E: Neutrophil caspase-11 is
required for cleavage of caspase-1 and secretion of IL-1β in
aspergillus fumigatus infection. J Immunol. 201:2767–2775.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
McDonald B, Pittman K, Menezes GB, Hirota
SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA and Kubes P:
Intravascular danger signals guide neutrophils to sites of sterile
inflammation. Science. 330:362–366. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhou W, McCollum MO, Levine BA and Olson
MS: Role of platelet-activating factor in pancreatitis-associated
acute lung injury in the rat. Am J Pathol. 140:971–979.
1992.PubMed/NCBI
|