Introduction
Glioma is a common primary tumor of the central
nervous system (1). The incidence
of glioma is increasing year by year, with an annual growth rate of
~1.2% (2). Glioma is a serious
threat to human life and health. Despite the emergence of treatment
regimens comprising combined surgery and chemoradiotherapy, the
prognosis of patients with malignant glioma has not been markedly
improved and the median survival time is only 12-15 months
(3,4). Since glioma usually grows and
infiltrates surrounding tissue and its boundary is not clear, it is
challenging to remove completely by surgery (5). Therefore, the use of targeted therapy
is therapeutically important (6). A
variety of targeted drugs have been used clinically or tested for
the treatment of glioma in clinical trials; however, the
development of more effective therapeutic drugs is necessary in
order to improve the prognosis of patients (7).
The antitumor effects and mechanisms of certain
anesthetics have been revealed (8).
Studies have demonstrated that anesthetics affect the apoptosis,
proliferation, metastasis and DNA demethylation of some types of
cancer cells (9-11).
Anesthetics have exhibited antitumor effects on various
malignancies. For example, studies have shown that clinical doses
of lidocaine and bupivacaine can induce the apoptosis of MCF-7
breast cancer cells, while dibucaine has a pro-apoptotic effect on
HL-60 promyelocytic leukemia cells (10,11).
In addition, procaine is able to inhibit the proliferation of
CNE-2Z nasopharyngeal carcinoma cells (12).
The p38 MAP kinase (MAPK) participates in a
signaling pathway that mediates the response of cells to cytokines
and stress (13). The involvement
of the ERK/p38 MAPK pathway in the progression of several types of
cancer, including glioma, has already been revealed (14). Several oncogenes have been shown to
affect the progression and metastasis of multiple types of cancer,
including breast, lung and gastric cancer, through this pathway,
and numerous drugs targeting the ERK/p38MAPK pathway have been
developed or are in clinical trials (15).
Strategies for the effective delivery of
anesthetics, such as procaine, into the brain are of great interest
for the treatment of glioma. Researchers have attempted to increase
the penetration of the blood-brain barrier (BBB) by drugs, and
active targeting is proving to be an attractive approach for the
enhancement of glioma-targeting ability using specific ligands
(16,17). The pentapeptide cyclic
(arginine-glycine-aspartic acid-tyrosine-lysine) (cRGDyK) is a
promising ligand (18,19) for targeting the integrin
αvβ3 receptor, which is highly expressed on
the surface of multiple types of cancer cells, including C6 glioma
and MDA-MB-231 breast cancer cells (20-22).
As the expression of integrin αvβ3 on normal
cells is limited, it is an attractive receptor for glioma
targeting. In addition, the integrin receptor is highly expressed
on cerebral capillary endothelial cells (23). Therefore, the cRGDyK peptide may
have the ability to cross the BBB and target glioma.
To verify the aforementioned functions of cRGDyK
peptide and investigate the antitumor mechanism of procaine, a
procaine-loaded liposome modified with cRGDyK (Pro/cRGDyK-L) was
developed in the present study. The ability of the cyclic peptide
to enhance the penetration of the BBB by procaine and improve
cellular uptake was investigated. Additionally, the inhibitory
effects of Pro/cRGDyK-L on cell migration, apoptosis and cell cycle
arrest were evaluated. Furthermore, whether Pro/cRGDyK-L targets
the ERK/p38 MAPK pathway and inhibits tumor growth in vivo
was analyzed.
Materials and methods
Chemistry
Synthesis of
(3S,10R,13R,17R)-10,13-dimethyl-17-((R)
-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-
tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl 4-methyl
benzenesulfonate (compound 2). To a solution of cholesterol
(compound 1; 1.00 g, 2.59 mmol) in pyridine (5 ml), a solution of
p-toluenesulfonyl chloride (0.74 g, 3.88 mmol) in pyridine
(5 ml) was added. After stirring the mixture for 10 h at 50˚C, the
solvent was removed and the residue was redissolved in ethyl
acetate (20 ml). The mixture was washed with 1 mol/l HCl and
saturated NaCl. The solvent was then removed to yield compound 2
(1.19 g, 85%), which was used for the next step without further
purification. Melting point: 127-129˚C. High-resolution mass
spectrometry (HRMS): Electrospray ionization (ESI+)
calculated for C34H52O3SNa
[M+Na]+, 563.3535; found, 563.3532. Elemental analysis:
Calculated, C, 75.51; H, 9.69; S, 5.93; found, C, 75.43; H, 9.78;
S, 6.11.
Synthesis of
23-(((3R,10R,13R,17R)-10,13-dimethyl-17-
((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)oxy)-
3,6,9,12,15,18,21-heptaoxatricosan-1-ol (compound 3). To a
solution of compound 2 (1.00 g, 1.85 mmol) in dioxane (15 ml) was
added octaethylene glycol (6.85 g, 18.49 mmol), and the reaction
mixture was refluxed for 20 h. The solvent was then removed and the
residue was redissolved in dichloromethane
(CH2Cl2; 30 ml). The mixture was washed with
saturated NaCl. After removing the CH2Cl2,
the residue was purified by chromatography to give compound 3 (0.66
g, 48%) as a colorless oil. HRMS: (ESI+) calculated for
C43H78O9Na [M+Na]+,
761.5544; found, 761.5540. Elemental analysis: Calculated, C,
69.88; H, 10.64; found, C, 69.76; H, 10.79.
Synthesis of tert-butyl 26-(((3R,10R,13R,17R)-10,
13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)oxy)-3,6,9,12,15,18,21,24-octaoxahexacosanoate
(compound 4). To a solution of compound 3 (0.50 g, 0.68 mmol)
in toluene (10 ml) was added 50% NaOH aqueous solution (5 ml) at
0˚C. Then, tert-butyl bromoacetate (0.16 ml, 1.02 mmol) and
tetra-n-butylammonium hydrogen sulfate (24 mg, 0.07 mmol)
were added to the solution and the mixture was stirred for 20 h at
ambient temperature. The organic layer was separated and washed
with saturated NaCl. After removing the solvent, the residue was
purified by chromatography to give compound 4 (0.44 g, 75%) as a
colorless oil. HRMS: (ESI+) calculated for
C49H88O11Na [M+Na]+,
875.6224; found, 875.6227. Elemental analysis: Calculated, C,
68.98; H, 10.40; found, C, 68.85; H, 10.50.
Synthesis of
26-(((3R,10R,13R,17R)-10,13-dimethyl-17- ((R)-6-methylheptan-2-yl)
-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)oxy)-
3,6,9,12,15,18,21,24-octaoxahexacosanoic acid (compound 5). To
a solution of compound 4 (1.50 g, 1.76 mmol) in toluene (20 ml) was
added p-toluenesulfonic acid (60 mg, 0.35 mmol), and the
mixture was heated to reflux for 10 h. The solvent was removed and
the residue was purified by chromatography to give compound 5 (1.23
g, 88%) in the form of a yellow oil. HRMS: (ESI+)
calculated for C45H80O11Na
[M+Na]+, 819.5598; found, 819.5595. Elemental analysis:
Calculated, C, 67.81; H, 10.12; found C, 67.89; H, 10.03.
Synthesis of 2,5-dioxopyrrolidin-1-yl
26-(((3R,10R,13R, 17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)
-2,3,4,7,8,9,10,11,12,13,14,15,16,17-
tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)oxy)-3,6,9,12,15,18,21,24-octaoxahexacosanoate
(compound 6). To a solution of compound 5 (0.80 g, 1.00 mmol)
in tetrahydrofuran (THF; 10 ml) was added
N-hydroxysuccinimide (NHS; 0.35 g, 3.00 mmol) and
N,N-dicyclohexylcarbodiimide (DCC; 0.62 g, 3.00 mmol), and
the mixture was stirred at room temperature for 20 h. After
removing the THF, the residue was purified by chromatography to
give compound 6 (0.58 g, 65%) as a yellow oil. HRMS:
(ESI+) calculated for
C49H83O13Na [M+Na]+,
916.5762; found, 916.5766. Elemental analysis: Calculated, C,
65.82; H, 9.36; N, 1.57; found, C, 65.88; H, 9.45; N, 1.42.
Synthesis of
2-((2R,5S,8R,11R)-8-(1-(((3R,10R,13R,17R)-
10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)oxy)-26-oxo-3,6,9,12,15,18,21,24-octaoxa-
27-azahentriacontan-31-yl)-11-(3-guanidinopropyl)-
5-(4-hydroxybenzyl)-3,6,9,12,15-pentaoxo-1,4,7,10,
13-pentaazacyclopentadecan-2 -yl)acetic acid (cRGDyK-modified
ligand). To a solution of compound 6 (0.20 g, 0.22 mmol) and
cRGDyK (0.14 g, 0.22 mmol) in 10 ml dimethylformamide was added
triethylamine (0.16 ml, 1.12 mmol), and the mixture was stirred for
5 h at room temperature. The reaction was terminated by adding
saturated NaCl (50 ml) and the mixture was extracted with
CH2Cl2 three times. After removing
CH2Cl2, the residue was purified by
chromatography to provide the cRGDyK-modified ligand (0.15 g, 48%)
as a yellow oil. HRMS: (ESI+) calculated for
C72H119N9O18Na
[M+Na]+, 1,420.8571; found, 1,420.8576. Elemental
analysis: Calculated, C, 61.82; H, 8.58; N, 9.01; found, C, 61.89;
H, 8.65; N, 9.14.
Preparation and characterization of
liposomes
The procaine-loaded liposomes were prepared using
the thin film hydration method (24). The component ratio was optimized as
follows: i) conventional liposome (L), soybean phosphatidyl choline
(Shanghai Pharmaceutical Co., Ltd.)/cholesterol molar ratio 62:33;
ii) cRGDyK-modified liposome (cRGDyK-L), SPC/cholesterol/ligand
molar ratio 62:33:3. In brief, all the lipid materials were
dissolved in a mixed solvent comprising
CH2Cl2 and methanol (2:1 v/v), and then
heated to 37˚C on a rotary evaporator to remove the solvent and
form a thin film. After drying in a vacuum for 10 h, the film was
hydrated with PBS (pH 7.4) at 20˚C for 30 min and then sonicated
intermittently at 80 W for 80 sec to obtain the liposomes. An
appropriate amount of procaine (weight ratio, 1:30) or
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-carboxyfluorescein
(CFPE) (final concentration, 20 µg/ml) was added to the solution of
lipid liposomes before removing the solvent to prepare
procaine-loaded liposomes (Pro/L and Pro/cRGDyK-L) or CFPE-labeled
liposomes (CFPE/L and CFPE/cRGDyK-L).
The entrapment efficiency [EE (%)] and drug loading
efficiency [DL (%)] of procaine were determined by high performance
liquid chromatography (HPLC). The detection was performed using an
Agilent 1200 HPLC system (Agilent Technologies, Inc.) with an
Ultimate LP-C18 column (4.6x250 mm, 5 µm; Sepax Technologies, Inc.)
at 25˚C. The mobile phase comprised water and methanol (68:32 v/v)
with a flow rate of 1.0 ml/min. For testing, 10 µl
procaine-containing sample was injected and the detection
wavelength was 290 nm. The EE (%) and DL (%) were calculated
according to the following equations: EE (%) = weight of
encapsulated procaine / total weight of procaine, and DL (%) =
weight of encapsulated procaine / total weight of liposome. In
addition, the size and ζ potential of Pro/L and Pro/cRGDyK-L were
detected using a Malvern Zetasizer Nano ZS90 (Malvern
Panalytical).
Release by Pro/L and Pro/cRGDyK-L in
vitro
The release behavior of procaine from the Pro/L and
Pro/cRGDyK-L liposomes was evaluated via dialysis. The release
behavior of procaine from free procaine was also analyzed as a
control. Briefly, the liposomes or free procaine were put into a
dialysis bag (molecular weight cut-off, 8,000-14,000 Da) with 50 ml
PBS containing 0.1% Tween 80 (v/v) as release medium. The bags were
then shaken at 37˚C with gentle oscillation (45 rpm). At
predetermined time points between 0 and 48 h, 0.1 ml sample was
taken out and replaced with fresh medium. Then, the amount of
procaine was tested using the aforementioned HPLC method.
Stability of Pro/L and Pro/cRGDyK-L in
vitro
The stability of the Pro/L and Pro/cRGDyK-L
liposomes was determined by investigating turbidity variations in
the presence of fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.). In general, each of the liposome formulations
was mixed with an equal volume of FBS and then maintained at 37˚C
with continuous shaking (50 rpm). The transmittance at 750 nm was
measured between 0 and 48 h using a microplate reader (Bio-Rad
model 550; Bio-Rad Laboratories, Inc.) and was evaluated relative
to the transmittance of PBS, which was defined as 100%.
Hemolysis assays
A female BALB/c mouse (22 g; age, 4 weeks) was
anesthetized by the injection of pentobarbital sodium
intraperitoneally at a dose of 50 mg/kg. The mouse was kept in a
20˚C environment with 40-60% humidity and a 12-h light/dark cycle
and free access to food and water. Fresh mouse blood (0.1 ml) was
collected from the orbit and centrifuged at 2,000 x g for 5 min
(4˚C). The supernatant was discarded and the precipitated red blood
cells were washed with PBS until the supernatant was colorless. The
cells were then re-suspended in PBS to a concentration of 2% (w/v).
Afterwards, the Pro/L and Pro/cRGDyK-L liposomes were diluted with
PBS to prepare liposome samples with a range of lipid
concentrations (10, 25, 50, 100, 200 and 400 nM). Subsequently, the
liposome sample (0.4 ml) was mixed with the suspension of red blood
cells (0.1 ml) and the mixture was maintained at 37˚C for 2 h with
gentle shaking (50 rpm). After centrifuging at 6,000 x g for 10 min
(4˚C), the absorbance of the supernatant at 540 nm
(A540) was measured using a microplate reader. The
hemolysis rate of each sample was calculated as follows: Hemolysis
(%) = [(A540 sample - A540 negative) /
(A540 positive - A540 negative)] x100. The
absorbance of PBS and Triton X-100 mixed with the cell suspension
were defined as 0 and 100%, respectively.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
A cytotoxicity assay was performed using a standard
MTT-based colorimetric assay. In brief, bEnd.3 and C6 cells were
bought from American Type Culture Collection and cultured in
Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS at 37˚C in a humidified
incubator containing 5% CO2. The cells were seeded into
96-well plates. In each well, cells at a density of
~5x103 cells/well were cultured for 24 h. Fresh medium
with procaine, Pro/L and Pro/cRGDyK-L was applied in which the
procaine concentration ranged from 0.01 to 10 mM. The cells were
incubated for another 24 h at 37˚C, and then MTT (5 mg/ml) was
added to each well with further culturing for another 4 h at 37˚C.
Next, the cells were lysed using 200 µl DMSO and the absorbance at
490 nm (A490) was read using a microplate reader.
Survival percentages were calculated using the following equation:
Survival (%) = [(A490 treated cells) / (A490
control cells)] x100.
Transendothelial migration in an in
vitro BBB model
Millicell® Hanging Cell Culture Inserts (Corning,
Inc.) were used to build an in vitro BBB model as described
in a previous study (25). Briefly,
bEnd.3 cells were maintained in DMEM with 10% FBS and seeded on
6-well plate at a density of 1x106 cells/well and
incubated for 7 days at 37˚C. The transendothelial electric
resistance (TEER) of the BBB model was measured using a Millicell
ERS volt-ohm meter (EMD Millipore). Only the bEnd.3 monolayers with
a TEER >200 Ω were used for further experiments. In addition, C6
cells were plated on another 6-well plate. After transferring the
cell culture inserts with bEnd.3 monolayers into the plates
containing C6 cells, the cells were co-cultured for another 24 h at
37˚C. Subsequently, the CFPE-labeled liposomes (CFPE/L and
CFPE/cRGDyK-L; final concentration, 2 µg/ml) were added to the cell
culture inserts (donor chamber) of the BBB model and incubated for
4 h. After this, the bEnd.3 and C6 cells were each washed with cold
PBS three times, trypsinized and resuspended in 0.5 ml PBS. The
fluorescent intensity of the two types of cells was measured using
a flow cytometer (Cytomics FC500; Beckman Coulter, Inc.) and
corresponding software.
Wound healing assays
To determine the effects of the liposomal
formulations on cell migration ability, the wound healing assay was
performed. Briefly, C6 cells (confluence, 100%) treated with Pro/L
or Pro/cRGDyK-L were wounded by scraping with a pipette tip, and
the detached cells were washed away. Subsequently, the cells were
cultured with serum-free DMEM (Gibco; Thermo Fisher Scientific,
Inc.) to stimulate wound healing. Photographic images were captured
using an Olympus light microscope at 0 h and 24 h later to evaluate
the migration of the cells (cat. no. CKX53; Olympus Corporation).
The images were then analyzed by ImageJ 8.0 software (National
Institutes of Health).
Transwell assays
A total of 1x104 C6 cells treated with
either Pro/L or Pro/cRGDyK-L were seeded in the upper chamber of
Transwell plates in DMEM. The upper cells were induced to migrate
toward the bottom chamber which contained DMEM plus 10% FBS at 37˚C
for 24 h. Then, the cells were stained with 0.2% crystal violet for
20 min at 25˚C. Transwell migration was determined as the
percentage of input cells minus the percentage of cells migrating
to the medium alone (baseline). A light microscope (cat. no. IX71;
Zeiss AG) was used for imaging at a magnification of x20. The
images were then analyzed by ImageJ 8.0 software (National
Institutes of Health).
Cell cycle assays
Flow cytometric analysis was performed to determine
the cell cycle distribution of C6 cells. Following treatment with
Pro/L or Pro/cRGDyK-L (1 mM calculated as Pro) at 37˚C for 24 h,
the C6 glioma cells (4x105) were washed with PBS and
fixed with pre-cooled 70% ethanol at -20˚C for 30 min. Following
centrifugation (450 x g) at 4˚C for 5 min and washing with PBS, the
cells were stained with 500 µl propidium iodide (PI; 50 µg/ml;
Thermo Fisher Scientific, Inc.), followed by DNA content analyses
using a flow cytometer (Cytomics FC500; Beckman Coulter, Inc.) and
corresponding software. The percentage of cells in the G1, S and
G2/M phases was calculated and analyzed.
Cell apoptosis assays
Cell apoptosis was evaluated using Annexin V-FITC
and PI staining (Sangon Biotech Co., Ltd.). Following treatment of
the C6 glioma cells with Pro/L or Pro/cRGDyK-L (0-10 mM calculated
as Pro) at 37˚C for 48 h, the cells were centrifuged (200 x g) at
4˚C for 3 min and washed with PBS. Subsequently, the cells were
re-suspended in 100 µl binding buffer with 5 µl Annexin V-FITC and
incubated at room temperature for 10 min. Then, 5 µl PI solution
was added and the cells were incubated for another 5 min at room
temperature. The proportion of apoptotic cells was analyzed using a
flow cytometer (Cytomics FC500; Beckman Coulter, Inc.) and
corresponding software.
Immunoblot assay
Following treatment with Pro/L or Pro/cRGDyK-L (1 mM
calculated as Pro) at 37˚C for 24 h, and protein determination was
performed using the BCA method. Protein samples (10 µg/per lane)
were separated on 10% SDS-PAGE gels and transferred onto PVDF
membranes (250 mA, 2 h). The proteins were transferred onto PVDF
membranes, which were then blocked with 5% milk in TBS with 0.05%
Tween 20 at 25˚C for 2 h. The PVDF membranes were subsequently
treated with primary antibodies targeting the following proteins:
Phosphorylated (p-)ERK1/2 (ERK1 p-T202 + ERK2 p-T185; ab50011;
1:1,000 dilution; Abcam), ERK1/2 (ab17942; 1:1,000 dilution;
Abcam), p-p38MAPK (1:500; cat. no. ab4822; Abcam), p38MAPK (1:500;
cat. no. ab170099; Abcam) and β-actin (1:3,000; cat. no. ab8226;
Abcam) at room temperature for 2 h. The membranes were then
incubated with rabbit or mouse HRP-conjugated secondary antibodies
(both 1:5,000; cat. nos. ab6271 and ab6728; both Abcam) at room
temperature for 1 h. Protein signals were visualized via the use of
an ECL kit (Novex™ ECL Chemiluminescent Substrate Reagent kit;
Thermo Fisher Scientific, Inc.) and visualized by ImageJ 8.0
software (National Institutes of Health).
Antitumor activity in vivo
All animal experiments in this study, including the
extraction of blood from mice in the hemolysis assay, were approved
by the Laboratory Animal Ethics Committee of the Second Hospital of
Tianjin Medical University. A total of 18 female BALB/c nude mice
(4 weeks old; weight 18-20 g) were acquired from the Animal Core
Facility. For the tumor growth assay, 1x107 C6 cells
were mixed with Matrigel (BD Biosciences) in a 2:1 ratio and
subcutaneously implanted into the nude mice to induce tumors for 7
days. To detect the in vivo antitumor effects of procaine
and its liposomal formulations on glioma xenografts, the mice were
divided into 3 groups (n=6/group), and procaine, Pro/L and
Pro/cRGDyK-L were administered at a dose of 10 mg/kg, calculated as
procaine, via intravenous injection every 3 days. After 12 days of
treatment, all the mice were sacrificed by cervical dislocation,
and the tumors were isolated from the mice and weighed.
Statistical analysis
All data are presented as the mean ± standard
deviation (n=3). The statistical analyses were conducted using
GraphPad Prism 6.0 (GraphPad Software, Inc.). Unpaired Student's
t-test was used for statistical comparisons between two groups.
Statistical comparisons were performed by one-way analysis of
variance for multiple groups followed by Tukey's post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Chemistry
The synthetic pathway of the cRGDyK-modified ligand
is outlined in Fig. 1. Briefly,
cholesterol was esterified with p-toluenesulfonyl chloride
in pyridine, followed by etherification with octaethylene glycol.
Then, a Williamson etherification reaction was conducted with
tert-butyl bromoacetate in the mixed solvent of 50%
NaOH/toluene. Subsequently, the tert-butyl ester was
hydrolyzed in the presence of p-toluenesulfonic acid to
obtain the free acid, which was then reacted with NHS/DCC to form
an active ester. Finally, condensation of the peptide cRGDyK with
the active ester produced the cRGDyK-modified ligand.
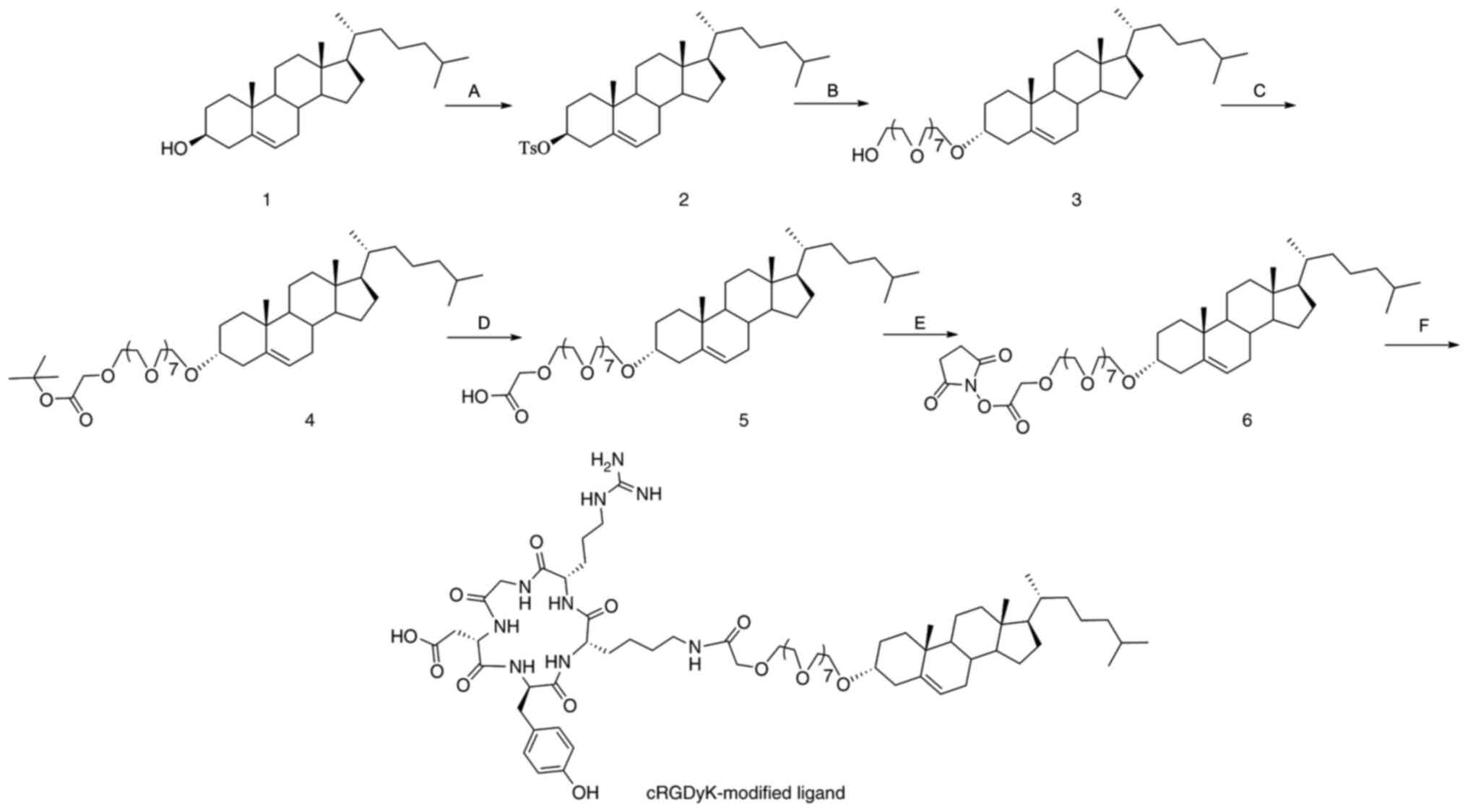 | Figure 1Synthesis of cRGDyK-modified ligand.
The reagents and conditions were as follows: (A) TsCl, pyridine,
50˚C, 10 h; (B) octaethylene glycol, dioxane, reflux, 20 h; (C)
tert-butyl bromoacetate, n-butylammonium
hydrogensulfate, 50% NaOH, toluene, room temperature, 20 h; (D)
TsOH, toluene, reflux, 10 h; (E) N-hydroxysuccinimide,
N,N-dicyclohexylcarbodiimide, tetrahydrofuran, room
temperature, 20 h; (F) cRGDyK, triethylamine,
N,N-dimethylformamide, room temperature, 5 h. Ts,
p-toluenesulfonyl. |
Characterization of liposomes
An appropriate size and uniform distribution are
critical for procaine-loaded liposomes to cross the BBB and target
glioma. The mean diameters and polydispersity index (PDI) were
detected through dynamic light scattering, and the results
indicated that the liposomes had a suitable size (107 or 114 nm)
and PDI (<0.2). Furthermore, the data in Table I also show that the EE (%) of the
liposomes was >85% and the DL (%) was ~2.8%. Also, the weak
negative ζ potential (-7.91) of Pro/cRGDyK-L should decrease
absorption by the reticuloendothelial system and the immune
response.
 | Table ICharacterization of Pro/L and
Pro/cRGDyK-L. |
Table I
Characterization of Pro/L and
Pro/cRGDyK-L.
| Liposomes | Size (nm) | PDI | EE (%) | DL (%) | ζ potential
(mV) |
|---|
| Pro/L | 107.51±8.4 | 0.195±0.052 | 85.77±3.97 | 2.81±0.34 | -4.88±0.39 |
| Pro/cRGDyK-L | 114.23±6.1 | 0.198±0.077 | 86.97±5.24 | 2.84±0.55 | -7.91±0.64 |
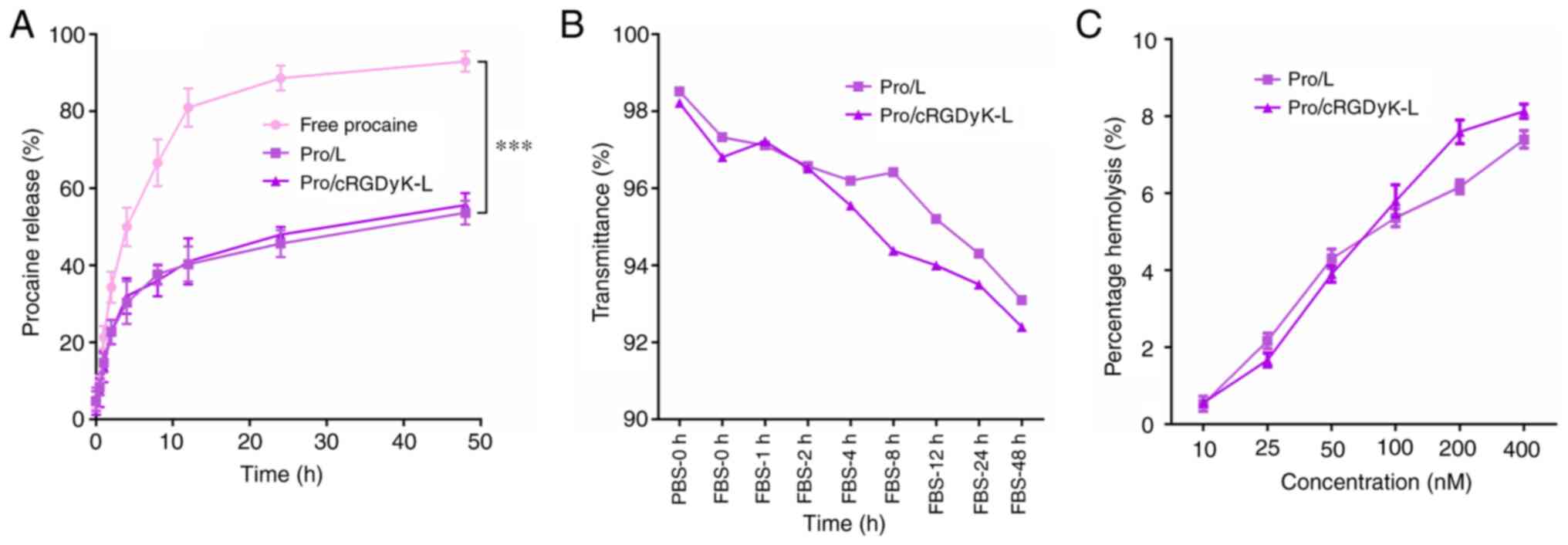
An in vitro release assay of the liposomes
was used to mimic drug release behavior in vivo and evaluate
the entrapment, membrane flexibility and integrity, and affinity of
the liposomal carrier systems. The results revealed that procaine
from free procaine exhibited fast release, with ~75% procaine being
released in 10 h (Fig. 2A).
However, the two procaine-loaded liposomes released ~40% of the
loaded procaine within 10 h, and the release rate was slow
thereafter. Neither of the two liposomes exhibited an initial burst
release pattern.
The stability of the liposomes Pro/L and
Pro/cRGDyK-L under physiological conditions is critical for their
application in vivo. In the stability assay, the
transmittances of the two liposome formulations were >90%, with
no marked variation between formulations during the 48-h culture in
FBS (Fig. 2B). The results suggest
that Pro/L and Pro/cRGDyK-L have adequate stability, which is
important for further investigation in vivo. The
hemocompatibility of Pro/L and Pro/cRGDyK-L was evaluated by a
hemolysis assay, and the results indicated that the two liposome
formulations did not induce an excessive increase in hemoglobin
release at phospholipid concentrations of ≥400 nM (Fig. 2C), which implies that they have
acceptable biosafety.
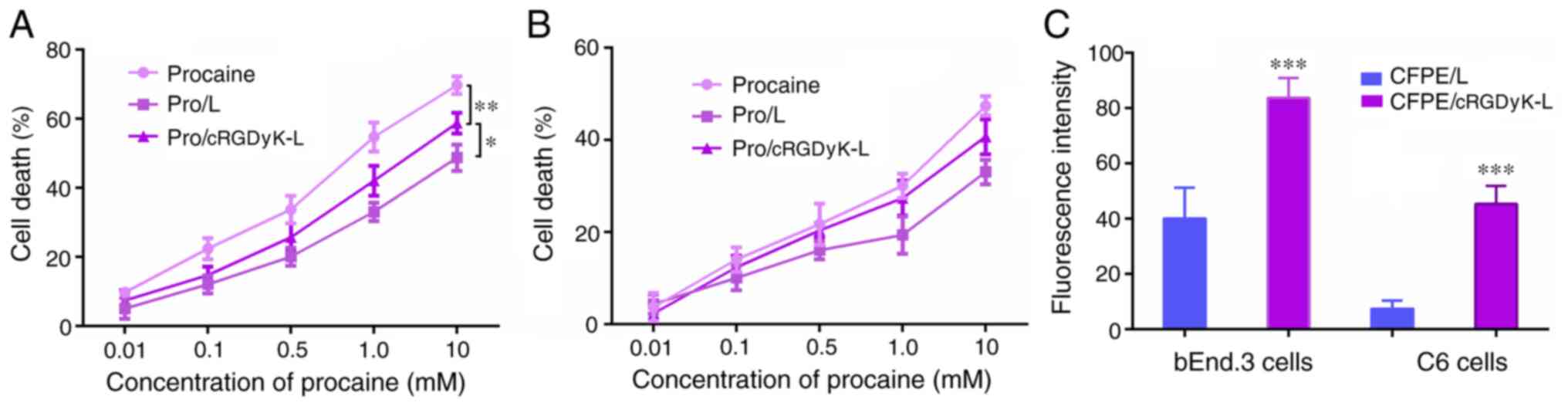
MTT assay
The cytotoxicity of the liposomes to bEnd.3 and C6
cells was evaluated using an MTT assay. As shown in Fig. 3A, free procaine exhibited a greater
cytotoxic effect than the procaine-loaded liposomes on the C6
cells, because the free drugs could directly enter the cells by
passive diffusion, without a drug-release process. In addition,
Pro/cRGDyK-L had a stronger antiproliferative effect than Pro/L,
presumably due to the ability of cRGDyK peptide to enhance cellular
uptake. The inhibitory effects of the procaine formulations on
bEnd.3 cells were also evaluated (Fig.
3B). The results revealed that the formulations had a weaker
effect on the survival of the endothelial cells, which indicated
the specific antitumor effects of procaine.
Transendothelial migration in a BBB
model
The bEnd.3 cells were used to establish a BBB model
in vitro. The bEnd.3 cells on the Transwell membrane in the
donor chamber and the underlying layer of C6 cells on the plate
from the acceptor chamber were collected, and the fluorescence
intensity was measured using flow cytometry. The results revealed
that the bEnd.3 monolayer on the Transwell membrane internalized
liposomes by cellular uptake (Figs.
3C and S1). The CFPE/cRGDyK-L
formulation induced a significant increase in the fluorescence
intensity of the C6 cells after penetrating the bEnd.3 monolayer
when compared with the CFPE/L formulation, which indicates that
cRGDyK-L was transported across the bEnd.3 monolayer and delivered
to the C6 cells. These results suggest that the transport of
liposomes across the BBB model was enhanced when they were modified
with cRGDyK peptide.
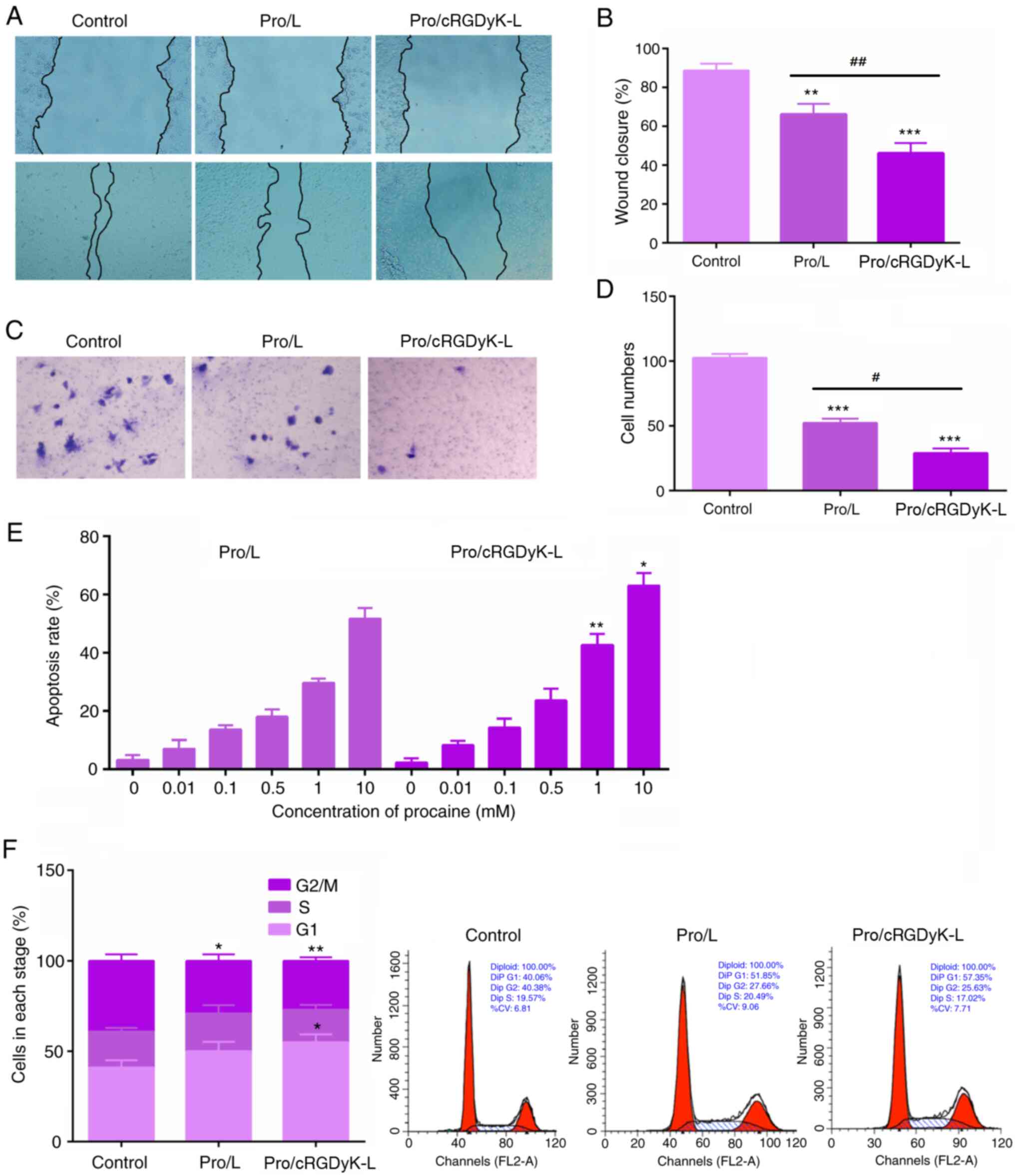
Wound closure and Transwell
assays
Wound closure and Transwell assays were performed to
investigate the effects of Pro/L and Pro/cRGDyK-L on C6 cell
migration-. The results revealed that the Pro/cRGDyK-L formulation
significantly suppressed the migration of C6 cells (Fig. 4A and B) in the wound healing assay compared with
that in the control group. Similarly, the Transwell assay results
confirmed that Pro/cRGDyK-L significantly inhibited the migration
of C6 cells, with a stronger inhibitory effect than the Pro/L
formulation (Fig. 4C and D). These results indicate that the
suppressive effect of Pro/cRGDyK-L on glioma cell migration was
superior to that of Pro/L.
Cell cycle and apoptosis detection
assays
The effects of Pro/L and Pro/cRGDyK-L on the cell
cycle and apoptosis of C6 glioma cells were investigated using flow
cytometry. Notably, the results revealed that Pro/cRGDyK-L
stimulated cell apoptosis in a concentration-dependent manner
(Figs. 4E and S2). Interestingly, comparison with
control cells revealed that Pro/L led to cell cycle arrest while
treatment with Pro/cRGDyK-L resulted in increased cell cycle arrest
at the G1 phase (Fig. 4F). These
results indicate Pro/cRGDyK-L significantly stimulates glioma cell
cycle arrest and apoptosis.
Pro/cRGDyK-L exhibits antitumor
activity in glioma by targeting the ERK/p38MAPK pathway
The previous results demonstrate that Pro/cRGDyK-L
can suppress the proliferation and migration of glioma cells and
stimulate their apoptosis. By performing immunoblot assays, it was
observed that the phosphorylation level of ERK was significantly
decreased by treatment with Pro/cRGDyK-L and Pro/L, with
Pro/cRGDyK-L exhibiting a stronger effect than Pro/L (Fig. 5A and B). Additionally, the results revealed that
Pro/L treatment inhibited the phosphorylation of p38MAPK, and
Pro/cRGDyK-L treatment decreased p38MAPK phosphorylation levels
compared with those in the Pro/L treatment group (Fig. 5A and C). Therefore, it appears that Pro/cRGDyK-L
exhibits antitumor activity in glioma by targeting the ERK/p38MAPK
pathway.
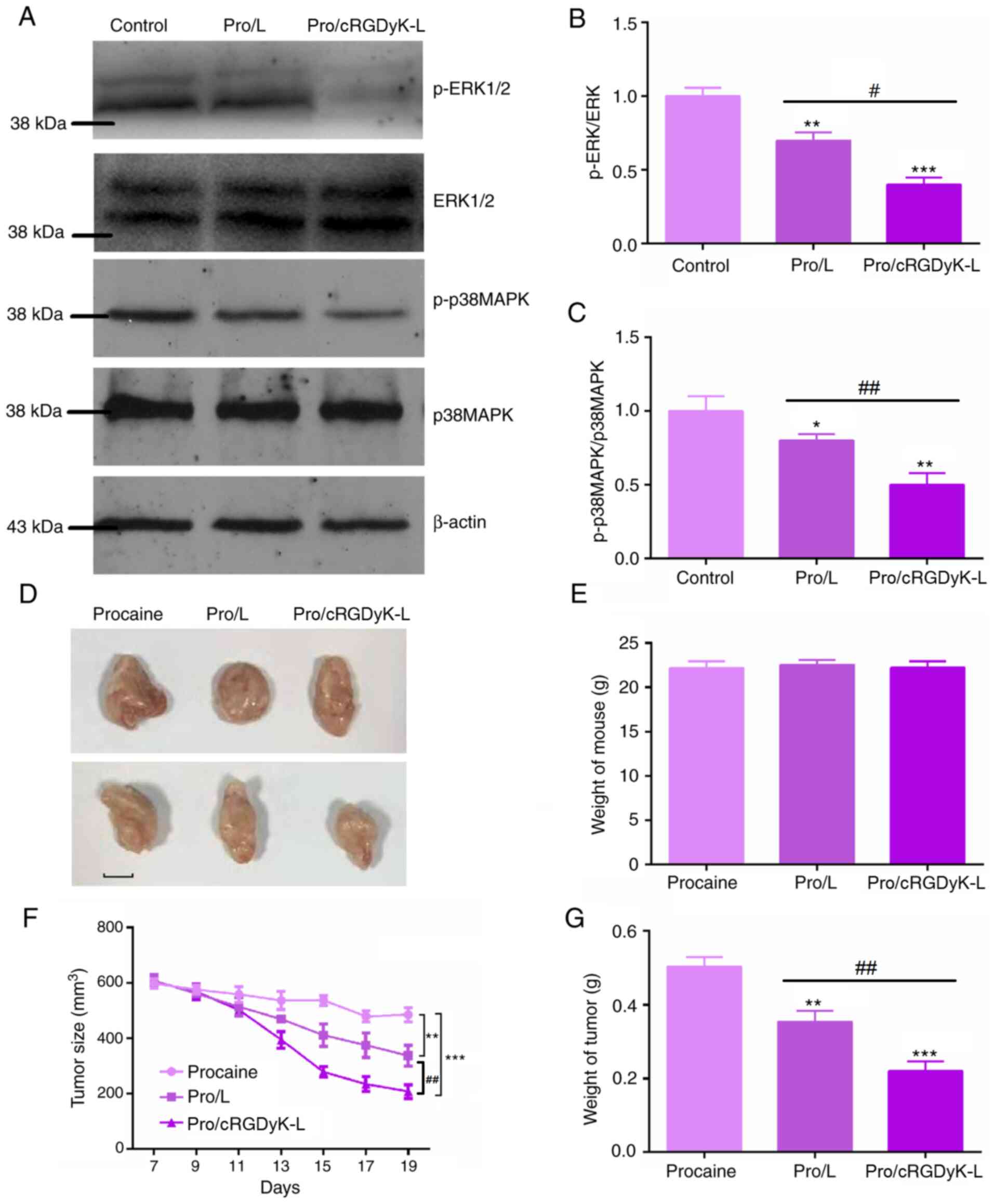 | Figure 5Mechanism of procaine-loaded
liposomes in glioma cells and antitumor effects in vivo. (A)
Western blotting assays showing the protein levels of p-ERK1/2,
ERK1/2, p-p38MAPK, and p38MAPK following the treatment of C6 cells
with procaine-loaded liposomes. Quantitative analysis of (B)
p-ERK1/2/ERK1/2 and (C) p-p38MAPK/p38MAPK ratios. (D)
Representative images of tumors formed from C6 cells in nude mice
and treated with procaine formulations (n=5/group). Scale bar, 5
mm. (E) Weights of the mice were measured. (F) Tumor growth curves
of mice in the control and liposomal treatment groups are shown.
(G) Weights of the tumors were measured. Results are presented as
the mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001 vs. Control;
#P<0.05, ##P<0.01 vs. Pro/L. Pro,
procaine; L, conventional liposome; cRGDyK-L, cRGDyK-modified
liposome; p-, phosphorylated. |
The antitumor activity of Pro/cRGDyK-L was also
confirmed in vivo. The findings from the xenograft assay
demonstrate the effectiveness of this targeted therapy. The tumors
observed in mice from the Pro/cRGDyK-L treatment group were smaller
than those in the other two groups. Representative image of the
tumors from each group are shown in Fig. 5D. Although there was no significant
different in the weight of the mice in the three groups (Fig. 5E), the tumor volume (Fig. 5F) and weight (Fig. 5G) of the mice treated with
Pro/cRGDyK-L were significantly reduced compared with those in the
control group according to the tumor growth curves and tumor weight
measurements, further demonstrating the antitumor effect of
Pro/cRGDyK-L.
Discussion
Gliomas are the most common primary craniocerebral
malignancies caused by cancerous changes in glial cells (26). The incidence rate of glioma among
intracranial tumors is 35.2-61.0% (1). Notably, the incidence and recurrence
rates of glioma are high, and the cure rate is low (27). However, due to the existence of the
BBB, the invasion and metastasis of glioma is extremely low
(27). However, existing
therapeutic drugs are not adequate to meet clinical needs due to
the BBB blocking their uptake into the brain (28). Therefore, it is urgently necessary
to develop a novel strategy for targeted drug delivery to treat
this particular malignancy.
Procaine is a type of local anesthetic (29). Procaine is used for local, regional
and neuraxial anesthesia, and despite the emergence of several new
anesthetic drugs, it remains a widely used anesthetic (30). Interestingly, procaine has also been
revealed to have antitumor effects (31). A previous study indicated that
procaine may serve as a specific DNA methylation inhibitor with
antitumor effects in gastric cancer (31). Additionally, in other studies,
procaine suppressed the proliferation and motility of colon cancer
cells by suppressing the ERK/MAPK/FAK axis (32), and exhibited an inhibitory effect on
osteosarcoma (33). These studies,
together with the findings of the present study, indicate the
potential role of procaine in tumor therapy.
In the present study, a cRGDyK-cholesterol
derivative was designed and prepared, and used as a ligand in a
liposomal drug delivery system. The procaine-loaded liposome
Pro/cRGDyK-L was characterized for particle size, ζ potential,
encapsulation efficiency, release profile, stability and hemolysis
in vitro, and the results indicated the liposome had
superior properties.
The present study also demonstrated that the
Pro/cRGDyK-L formulation significantly enhanced the ability of
procaine to penetrate the BBB and improve cellular uptake, and
further revealed that it had strong effects on cell migration,
induced apoptosis and led to cell cycle arrest. Notably, the
present study also indicated that Pro/cRGDyK-L exhibited superior
antitumor effects by targeting the ERK/p38MAPK pathway and
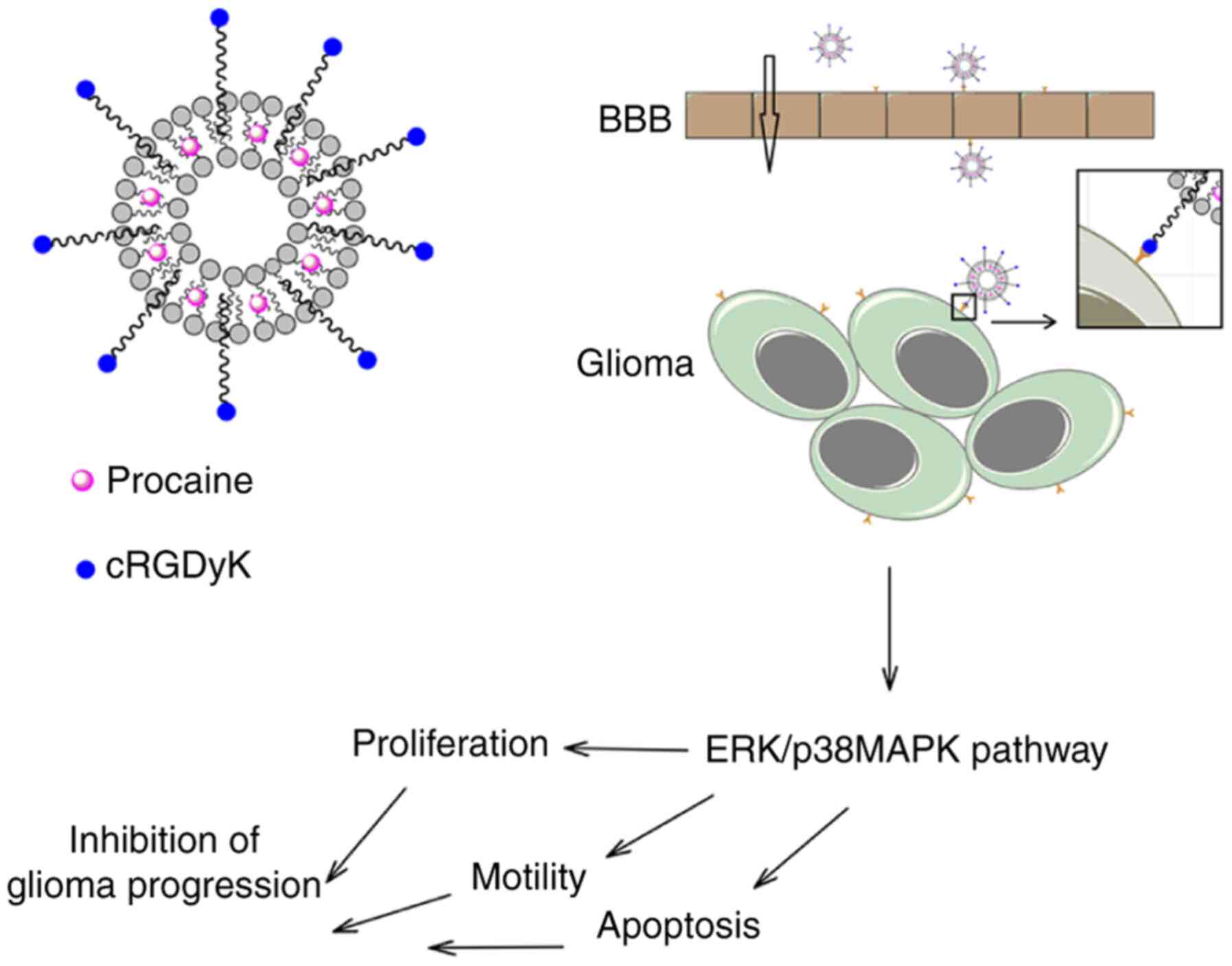
inhibited tumor growth in vivo, as illustrated by the scheme
in Fig. 6.
Tumor angiogenesis is an important factor that
affects the prognosis of patients with tumors. During tumor growth,
endothelial cells play a major role in the formation of new blood
vessels that feed the tumor with nutrients (34). The results of the present study
demonstrate that Pro/cRGDyK-L has an important effect on
endothelial cells and thus may influence tumor angiogenesis and
further affect tumor development.
In conclusion, with the aim of developing an
efficient drug delivery system targeting glioma to improve the
antitumor effect of procaine and explore its antitumor mechanism, a
novel cRGDyK-cholesterol derivative was designed and synthesized
for use in liposomes. The cyclic peptide markedly increase the
ability of procaine to penetrate a model of the BBB and improved
its cellular uptake. Additionally, the Pro/cRGDyK-L formulation
exhibited strong inhibitory effects on cell migration, and also
induced apoptosis and cell cycle arrest. The results further
confirmed the superior antitumor effects of Pro/cRGDyK-L in
vivo, which were mediated via targeting the ERK/p38MAPK
pathway. Therefore, cRGDyK is indicated to be an effective ligand
for BBB and glioma targeting, and the procaine-loaded liposome
formulation Pro/cRGDyK-L exerts antitumor effects via targeting
ERK/p38MAPK pathway and thereby suppresses tumor growth in
mice.
Supplementary Material
Representative flow cytometry plots
showing the uptake of CFPE-labeled liposomes by bEnd-3 and C6
cells. CFPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-carboxyfluorescein;
L, conventional liposome; cRGDyk-L, cRGDyk-modified liposome.
Representative flow cytometry plots
showing the apoptosis of C6 cells stimulated by Pro/L and
Pro/cRGDyk-L at different procaine concentrations. Pro, procaine;
L, conventional liposome; cRGDyk-L, cRGDyk-modified liposome.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Science & Technology
Development Fund of Tianjin Education Commission for Higher
Education (grant no. 2020KJ174).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL, JG, CY and YW carried out the molecular biology
experiments and drafted the manuscript. DL, JG, CY, BL, JS and MY
participated in the design of the study and performed the
statistical analysis. DL, HW and YL conceived the study,
participated in its design and coordination and helped to draft the
manuscript. All authors read and approved the final manuscript. DL
and YL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Laboratory Animal Ethics Committee of the Second
Hospital of Tianjin Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Comba A, Dunn PJ, Kish PE, Kadiyala P,
Kahana A, Castro MG and Lowenstein PR: Laser Capture
Microdissection of Glioma Subregions for Spatial and Molecular
Characterization of Intratumoral Heterogeneity, Oncostreams, and
Invasion. J Vis Exp 158: 10.3791/60939, 2020.
|
|
2
|
Zhang G, Ju H, Huang P, Yue P, Wan F and
Dou C: Advances in gene therapy and viral therapy for glioma. J
Clin Neurosurg. 18:237–240. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lawrie TA, Gillespie D, Dowswell T, Evans
J, Erridge S, Vale L, Kernohan A and Grant R: Long-term
neurocognitive and other side effects of radiotherapy, with or
without chemotherapy, for glioma. Cochrane Database Syst Rev.
8(CD013047)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eulitz J, Troost EGC, Raschke F, Schulz E,
Lutz B, Dutz A, Löck S, Wohlfahrt P, Enghardt W, Karpowitz C, et
al: Predicting late magnetic resonance image changes in glioma
patients after proton therapy. Acta Oncol. 58:1536–1539.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang Z, Du Y, Sun Q, Peng Y, Wang R, Zhou
Y, Wang Y, Zhang C and Qi X: Albumin-Based Nanotheranostic Probe
with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging
and Phototherapy for Glioma. ACS Nano. 14:6191–6212.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Patel D, Wairkar S and Yergeri MC: Current
Developments in Targeted Drug Delivery Systems for Glioma. Curr
Pharm Des. 26:3973–3984. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ran D, Zhou J, Chai Z, Li J, Xie C, Mao J,
Lu L, Zhang Y, Wu S, Zhan C, et al: All-stage precisional glioma
targeted therapy enabled by a well-designed D-peptide.
Theranostics. 10:4073–4087. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang D, Yang T, Liu J, Liu Y, Xing N, He
J, Yang J and Ai Y: Propofol Inhibits the Migration and Invasion of
Glioma Cells by Blocking the PI3K/AKT Pathway Through miR-206/ROCK1
Axis. OncoTargets Ther. 13:361–370. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li C, Xia M, Wang H, Li W, Peng J and
Jiang H: Propofol facilitates migration and invasion of oral
squamous cell carcinoma cells by upregulating SNAI1 expression.
Life Sci. 241(117143)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chang YC, Liu CL, Chen MJ, Hsu YW, Chen
SN, Lin CH, Chen CM, Yang FM and Hu MC: Local anesthetics induce
apoptosis in human breast tumor cells. Anesth Analg. 118:116–124.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Arita K, Utsumi T, Kato A, Kanno T,
Kobuchi H, Inoue B, Akiyama J and Utsumi K: Mechanism of
dibucaine-induced apoptosis in promyelocytic leukemia cells
(HL-60). Biochem Pharmacol. 60:905–915. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou H, Xu M, Luo G and Zhang Y: Effects
of procaine on human nasopharyngeal carcinoma cell strain CNE-2Z.
Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 21:1118–1121.
2007.PubMed/NCBI(In Chinese).
|
|
13
|
Peifer C, Kinkel K, Abadleh M, Schollmeyer
D and Laufer S: From five- to six-membered rings:
3,4-diarylquinolinone as lead for novel p38MAP kinase inhibitors. J
Med Chem. 50:1213–1221. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang J, Zhang JN, Chen WL, Wang GS, Mao Q,
Li SQ, Xiong WH, Lin YY, Ge JW, Li XX, et al: Effects of AQP5 gene
silencing on proliferation, migration and apoptosis of human glioma
cells through regulating EGFR/ERK/ p38 MAPK signaling pathway.
Oncotarget. 8:38444–38455. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rasheduzzaman M, Yin H and Park SY:
Cardiac glycoside sensitized hepatocellular carcinoma cells to
TRAIL via ROS generation, p38MAPK, mitochondrial transition, and
autophagy mediation. Mol Carcinog. 58:2040–2051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao Y, Qu B, Wu X, Li X, Liu Q, Jin X,
Guo L, Hai L and Wu Y: Design, synthesis and biological evaluation
of brain targeting l-ascorbic acid prodrugs of ibuprofen with
‘lock-in’ function. Eur J Med Chem. 82:314–323. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Singh I, Swami R, Jeengar MK, Khan W and
Sistla R: p-Aminophenyl-α-D-mannopyranoside engineered lipidic
nanoparticles for effective delivery of docetaxel to brain. Chem
Phys Lipids. 188:1–9. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qiu L, Hu Q, Cheng L, Li L, Tian C, Chen
W, Chen Q, Hu W, Xu L, Yang J, et al: cRGDyK modified pH responsive
nanoparticles for specific intracellular delivery of doxorubicin.
Acta Biomater. 30:285–298. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan ZQ, Li JZ, Liu Y, Chen WL, Yang SD,
Zhang CG, Zhu WJ, Zhou XF, Liu C and Zhang XN: Systemic delivery of
micelles loading with paclitaxel using
N-succinyl-palmitoyl-chitosan decorated with cRGDyK peptide to
inhibit non-small-cell lung cancer. Int J Pharm. 492:141–151.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao Z, Zhao Y, Xie C, Chen C, Lin D, Wang
S, Lin D, Cui X, Guo Z and Zhou J: Dual-active targeting liposomes
drug delivery system for bone metastatic breast cancer: Synthesis
and biological evaluation. Chem Phys Lipids.
223(104785)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qiu Y, Yu Q, Liu Y, Tang J, Wang X, Lu Z,
Xu Z and He Q: Dual Receptor Targeting Cell Penetrating Peptide
Modified Liposome for Glioma and Breast Cancer Postoperative
Recurrence Therapy. Pharm Res. 35(130)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang Y, Zhao Z, Xie C and Zhao Y:
Dual-targeting liposome modified by glutamic hexapeptide and folic
acid for bone metastatic breast cancer. Chem Phys Lipids.
228(104882)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Belhadj Z, Ying M, Cao X, Hu X, Zhan C,
Wei X, Gao J, Wang X, Yan Z and Lu W: Design of Y-shaped targeting
material for liposome-based multifunctional glioblastoma-targeted
drug delivery. J Control Release. 255:132–141. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao Z, Chen C, Xie C and Zhao Y: Design,
synthesis and evaluation of liposomes modified with dendritic
aspartic acid for bone-specific targeting. Chem Phys Lipids.
226(104832)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yue H, Xie K, Ji X, Xu B, Wang C and Shi
P: Vascularized neural constructs for ex-vivo reconstitution of
blood-brain barrier function. Biomaterials.
245(119980)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Radünz M, Hackbart HCDS, Bona NP, Pedra
NS, Hoffmann JF, Stefanello FM and Da Rosa Zavareze E:
Glucosinolates and phenolic compounds rich broccoli extract:
Encapsulation by electrospraying and antitumor activity against
glial tumor cells. Colloids Surf B Biointerfaces.
192(111020)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Delgado-López PD and Martín-Alonso J:
Prophylactic anticonvulsant therapy in high-grade glioma: A
systematic review and meta-analysis of longitudinal studies.
Neurocirugia (Astur : Engl Ed). 31:268–278. 2020.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
28
|
Lübtow MM, Oerter S, Quader S, Jeanclos E,
Cubukova A, Krafft M, Haider MS, Schulte C, Meier L, Rist M, et al:
In Vitro Blood-Brain Barrier Permeability and Cytotoxicity of an
Atorvastatin-Loaded Nanoformulation Against Glioblastoma in 2D and
3D Models. Mol Pharm. 17:1835–1847. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao X, Qi T, Yang M, Zhang W, Kong C, Hao
M, Wang Y, Zhang H, Yang B, Yang J, et al: Synthesis of dual
functional procaine-derived carbon dots for bioimaging and
anticancer therapy. Nanomedicine (Lond). 15:677–689.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dunaway SB, Maxwell CL, Tantalo LC, Sahi
SK and Marra CM: Neurosyphilis Treatment Outcomes After Intravenous
Penicillin G Versus Intramuscular Procaine Penicillin Plus Oral
Probenecid. Clin Infect Dis. 71:267–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li YC, Wang Y, Li DD, Zhang Y, Zhao TC and
Li CF: Procaine is a specific DNA methylation inhibitor with
anti-tumor effect for human gastric cancer. J Cell Biochem.
119:2440–2449. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li C, Gao S, Li X, Li C and Ma L: Procaine
Inhibits the Proliferation and Migration of Colon Cancer Cells
Through Inactivation of the ERK/MAPK/FAK Pathways by Regulation of
RhoA. Oncol Res. 26:209–217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ying B, Huang H, Li H, Song M, Wu S and
Ying H: Procaine Inhibits Proliferation and Migration and Promotes
Cell Apoptosis in Osteosarcoma Cells by Upregulation of
MicroRNA-133b. Oncol Res. 25:1463–1470. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiang W and Wang M: New insights into the
immunomodulatory role of exosomes in cardiovascular disease. Rev
Cardiovasc Med. 20:153–160. 2019.PubMed/NCBI View Article : Google Scholar
|