Introduction
Gastric cancer is one of the most common
malignancies, with >1 million estimated new cases and 784,000
deaths globally in 2018, which ranks the fifth among the most
diagnosed malignancies worldwide and the third most common cause of
cancer-associated mortality (1,2).
Excessive alcohol consumption, unbalanced diet and host genetic
diversity are the main risk factors inducing gastric cancer
(3). Finding the underlying
molecular mechanisms is beneficial to diagnosis and therapy of
gastric cancer, and it is urgent to identify molecular biomarkers
associated with diagnostic and therapeutic targets of gastric
cancer. A large amount of evidence has demonstrated that long
non-coding RNAs (lncRNAs) exerted essential roles in biological
processes, such as cell proliferation, migration, invasion,
apoptosis and differentiation (4,5).
Previous studies have demonstrated that nuclear-enriched abundant
transcript 1 (NEAT1) was upregulated in non-small cell lung cancer
and esophageal squamous cell carcinoma tissues compared with that
in their corresponding paracancerous tissues (6,7).
Moreover, NEAT1 has been indicated to function as a biomarker for
the diagnosis and prognosis of colorectal cancer (8). Mechanistically, NEAT1 has been
revealed to act as an oncogene and promote hepatocellular carcinoma
progression via regulating heterogeneous nuclear ribonucleoprotein
A2(9). Conversely, knockdown of
NEAT1 has been indicated to inhibit malignant glioma via targeting
miR-449b-5p and lethal-7e (10,11).
NEAT1 has also been associated with poor prognosis and has been
indicated to promote gastric cancer cell migration and invasion
(12).
microRNAs (miRNAs) consist of 21-25 nucleotides and
are endogenous non-coding RNAs that can affect the expression of
mRNA by incompletely binding to the 3'-untranslated region (3'-UTR)
of target genes (13). For example,
Liu et al (14) reported
that miR-375 enhanced cell proliferation capability by directly
targeting p53 in gastric cancer. A previous study has indicated
that downregulation of miR-124 was associated with breast cancer
progression (15). In addition,
miR-124 has been demonstrated to inhibit gastric cancer cell
proliferation, but promote apoptosis via affecting enhancer of
zeste homolog 2 and Rho-associated protein kinase 1 (16,17).
Similarly, Ma et al (18)
indicated that miR-142-5p suppressed TGF-β-induced cancer cell
growth and acted as a negative regulator of the TGF-β pathway by
targeting SMAD3. However, the mechanisms of miR-142 in inhibiting
metastasis in gastric cancer are still undetermined.
Jagged1 (JAG1) is a vital regulator of the Notch
signaling pathway, which participates in various cellular processes
(19). JAG1 overexpression has been
indicated to promote cell proliferation and inhibit apoptosis in
multiple myeloma cells (20). In
addition, JAG1 has been reported to be a target of miR-142 and
facilitate the progression of gastric cancer via regulating the
Notch signaling pathway (15,21).
In the current study, JAG1 was predicted as a target of miR-142-5p;
however, the interaction between miR-142-5p and JAG1 in gastric
cancer needs to be explored.
In the present study, NEAT1 expression was revealed
to be upregulated in gastric cancer. Loss-of-function experiments
were performed to verify the role NEAT1 in gastric cancer.
Subsequently, the association between miR-142-5p, NEAT1 and JAG1
was identified by bioinformatics analysis, and it was revealed that
NEAT1 exhibited a pro-carcinogenic role in gastric cancer
progression by functioning as a sponge to target miR-142-5p and
increase JAG1 expression.
Materials and methods
Clinical samples
A total of 33 pairs of biopsy samples used in the
present study were obtained from patients with gastric cancer who
underwent surgical resection at Yantaishan Hospital (Yantai, China)
between April 2012 and December 2017. The clinicopathological
features of these patients are presented in Table I. Inclusion criteria for patient
recruitment: i) Patients with histologically confirmed first
primary gastric cancer; ii) did not receive therapy before
admission; and iii) willing to participate in follow up. Exclusion
criteria for patients: i) Other clinical disorders (heart, liver,
kidney or lung dysfunction; blood diseases, including leukemia,
aplastic anemia, multiple myeloma, lymphoma, hemophilia and
Mediterranean anemia; neurological illnesses, including seizures,
major depression, schizophrenia, confused speech and Alzheimer
disease) except gastric cancer; ii) received prior treatment; and
iii) with history of previous malignancies. Written informed
consent was obtained from all participants prior to surgery, and
the protocols were approved by the Ethics Committee of Yantaishan
Hospital (Yantai, China) and performed in accordance with the
Declaration of Helsinki. Tumor samples and corresponding adjacent
healthy tissues >3 cm away from the primary tumors were promptly
frozen in liquid nitrogen and stored at -80˚C until total RNA was
extracted.
 | Table IClinicopathological features of
patients with gastric cancer (n=33). |
Table I
Clinicopathological features of
patients with gastric cancer (n=33).
|
Characteristics | N | % |
|---|
| Sex | | |
|
Female | 15 | 45 |
|
Male | 18 | 55 |
| Age (years) | | |
|
<50 | 16 | 48 |
|
≥50 | 17 | 52 |
| Surgical
technique | | |
|
Total
gastrectomy | 25 | 76 |
|
Partial
gastrectomy | 8 | 24 |
| Neoadjuvant
therapy | | |
|
No | 15 | 45 |
|
Yes | 18 | 55 |
| Resection
classification | | |
|
R0 | 27 | 82 |
|
R1 | 6 | 18 |
| Histopathological
response | | |
|
Minor | 18 | 55 |
|
Major | 15 | 45 |
| Clinical
T-stage | | |
|
T1 | 2 | 6 |
|
T2 | 5 | 15 |
|
T3 | 22 | 67 |
|
T4 | 4 | 12 |
| Clinical
N-stage | | |
|
N0 | 1 | 3 |
|
N1 | 6 | 18 |
|
N2 | 21 | 64 |
|
N3 | 5 | 15 |
Cell culture and transfection
The human gastric cancer cell lines AGS and MKN-45
and the human normal gastric epithelial cell line GES-1 were
purchased from China Center for Type Culture Collection. All cells
were cultured in high glucose DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified atmosphere with 95% air/5%
CO2 at 37˚C.
Specific small interfering RNA (siRNA) against NEAT1
(si-NEAT1, sense, 5'-UGAUGGAGACGGAGCUGAAUU-3' and antisense,
5'-UUCAGCUCCGUCUCCAUCAUU-3') and its negative control (NC) si-NC
(sense, 5'-UUCUCCGAACGUGUCACGU-3' and antisense,
3'-ACGUGACACGUUCGGAGAA-5'), overexpression plasmid for NEAT1 and
overexpression plasmid for JAG1 (JAG1) and their NC (pcDNA),
miR-142-5p inhibitor (in-miR-142-5p, 5'-AGUAGUGCUUUCUACUUUAUG-3')
and its NC (in-miR-NC, 5'-CGGUACGAUCGCGGCGGGAUAUC-3'), miR-142-5p
mimic (miR-142-5p, 5'-CAUAAAGUAGAAAGCACUACU-3') and its NC (miR-NC,
5'-UCACAACCUCCUAGAAAGAGUAGA-3'), as well as specific short hairpin
RNA (shRNA) against NEAT1 (sh-NEAT1, sense,
5'-CACCGGAAGGCAGGGAGAGGTAGAACGAATTCTACCTCTCCCTGCCTTCC-3' and
antisense,
5'-AAAAGGAAGGCAGGGAGAGGTAGAATTCGTTCTACCTCTCCCTGCCTTCC-3') and its
NC (sh-NC, sense,
5'-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3'
and antisense,
5'-GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3')
were purchased from Guangzhou RiboBio Co., Ltd.
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) was used to transfect the aforementioned oligonucleotides or
plasmids into AGS and MKN-45 cells at 37˚C. AGS and MKN-45 cells
were collected at 48 h post-transfection for further analyses. The
concentrations of miR-142-5p mimic and miR-142-5p inhibitor for
transfection were 60 nM. The concentrations of overexpression
plasmid for JAG1 and sh-NEAT1 for transfection were 1 µg.
RNA isolation and reverse
transcription quantitative PCR (RT-qPCR)
Total RNA was extracted from gastric cancer tissue
samples or AGS and MKN-45 cells using TRIzol® reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. cDNA for lncRNA/mRNA and miRNA was synthesized using
PrimeScript™ RT Reagent kit (Takara Biotechnology Co., Ltd.) and
TaqMan™ microRNA Reverse Transcription Kit (Thermo Fisher
Scientific, Inc.) at 37˚C for 15 min, followed by 85˚C for 15 sec.
qPCR analysis was performed using QuantiTect SYBR-Green RT-PCR Kit
(Qiagen, Inc.) on ABI 7500 HT system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) by following the thermocycling conditions:
1 cycle of 95˚C for 3 min and 40 cycles of 95˚C for 15 sec, 60˚C
for 1 min and 72˚C for 30 sec followed by 1 cycle of extension at
72˚C for 7 min, with GAPDH (for lncRNA and mRNA) or endogenous
small nuclear RNA U6 (for miRNA) as the internal control. Relative
expression was determined using the 2-ΔΔCq method
(22). The primers used are listed
as follows: miR-142-5p (forward, 5'-GCCGAGCATAAAGTAGAAAG-3';
reverse, 5'-CTCAACTGGTGTCGTGGA-3'); JAG1 (forward,
5'-GGGGCAACACCTTCAACCTC-3'; reverse, 5'-CCACGCCTCCACAAGCAAC-3');
NEAT1 (forward, 5'-AATTCTGTTACGTCATGT-3'; reverse,
5'-TTTCTAATGAGTTTAGAACTCAAAC-3'); GAPDH (forward,
5'-TCCCATCACCATCTTCCAGG-3'; reverse, 5'-GATGACCCTTTTGGCTCCC-3');
and U6 (forward, 5'-CTCGCTTCGGCAGCACA-3'; reverse,
5'-AACGCTTCACGAATTTGCGT-3').
Cell Counting Kit-8 (CCK-8) assay
AGS and MKN-45 cells were seeded into 96-well plates
(Corning, Inc.) at a density of 5.0x103 cells per well.
A total of 20 µl of CCK-8 solution were added into the wells and
the cells were incubated for 4 h at 37˚C. Subsequently, cell
viability was determined by measuring the absorbance at 450 nm on a
microplate reader (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Cell migration and invasion assay
Transwell chambers (8-µm pore size; EMD Millipore)
were used to investigate the migration and invasion of AGS and
MKN-45 cells. For the detection of migration, cells
(2x105 cells in serum-free DMEM) were placed into the
upper chamber, while the lower chamber contained high glucose DMEM
with 10% FBS for the induction of migration. After incubation at
37˚C for 48 h, the cells that passed through the membrane were
fixed with 20% methanol for 15 min at room temperature and stained
with 0.1% crystal violet for 30 min at room temperature. The
migrated cells were counted under a light microscope (Guangzhou
Micro-shot Technology Co., Ltd.) at x100 magnification. In
addition, 12.5 mg Matrigel (BD Biosciences) in 50 ml PBS was added
to the upper chamber and then incubated at 37˚C for 12 h for the
invasion assay, the other steps being the same as
aforementioned.
Dual-luciferase reporter assay
The online software LncBase v2 (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=lncBase/index)
and microT-CDS v5.0 (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index)
were employed to predict potential targets of NEAT1 and miR-142-5p,
respectively. The fragment of lncRNA NEAT1 containing the
miR-142-5p binding sites [from nucleotide (nt) 803 to 823] was
amplified using cDNA samples from AGS cells as a template with the
following primers: Forward, 5'-CGGGCTTACCAGATGACCAG-3' and reverse,
5'-CGGGCTTACCAGATGACCAG-3'. PCR was performed using the
High-Fidelity PCR Master Mix kit (Guangzhou RiboBio Co., Ltd) with
the following thermocycling conditions: 3 min initial denaturation
at 94˚C, 35 cycles of denaturation at 94˚C for 10 sec, annealing at
60˚C for 10 sec and 72˚C extension for 45 sec and a final extension
step at 72˚C for 1 min. The fragment of JAG1 3'-UTR (from nt 4127
to 5940) containing the miR-142-5p binding sites (from nt 4502 to
4522) was amplified using cDNA samples from AGS cells as a template
using the following primers: Forward, 5'-CAGCCTCTGAGGACAACACC-3'
and reverse, 5'-GACATCAAAGTCTCCCCTCCC-3'. The sequences were cloned
into pGL3 luciferase vector (Promega Corporation) to produce WT
reporters (NEAT1 WT and JAG1 3'-UTR WT). In addition, NEAT1 mutant
type (MUT) fragments and JAG1 3'-UTR MUT fragments carrying the
point mutation of miR-142-5p binding sites were utilized to produce
MUT reporters (NEAT1 MUT and JAG1 3'-UTR MUT) in the same way.
Sequence synthesis, mutations and the construction of the
luciferase reporter vector were conducted by Guangzhou RiboBio Co.,
Ltd.
The reporter vectors (1 µg/ml final concentration)
and miR-142-5p (20 nM final concentration) or miR-NC (20 nM final
concentration) were co-transfected into AGS and MKN-45 cells using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.), and 36 h
following transfection cells were lysed and luciferase activity was
detected using Dual-Luciferase Reporter Assay System (Promega
Corporation), with Renilla luciferase activity as the
transfection control.
RNA immunoprecipitation (RIP)
assay
RIP assays were performed at 24 h post-transfection
using the EZ-Magna RIP™ RNA Binding Protein
Immunoprecipitation kit (EMD Millipore) according to the
manufacturer's protocols. In brief, AGS and MKN-45 cells were lysed
using RIP buffer. Prior to incubating with equal amounts of cell
lysates (200-400 µg/200 µl per RIP reaction) overnight at 4˚C, 50
µl (500 µg) protein A/G magnetic beads (10 mg/ml) were coupled with
antibodies against IgG (5 µg per RIP reaction; cat. no. PP64B; EMD
Millipore) or argonaute2 (Ago2; 1:50, cat. no. 2897; Cell Signaling
Technology, Inc.) for 30 min at room temperature. The
immunoprecipitate pellet was then collected using the magnetic
separator by centrifugation (1,000 x g, 1 min) at room temperature,
before incubation with proteinase K at 55˚C for 30 min to remove
the proteins. The RNA was purified using phenol: Chloroform:
Isoamyl alcohol and centrifuged at 12,000 x g for 10 min at room
temperature. RNA in the aqueous phase was collected and then
precipitated with absolute ethanol at -80˚C overnight and then
analyzed by RT-qPCR.
Western blot assay
AGS and MKN-45 cells and tumor tissue samples were
lysed using RIPA buffer (Thermo Fisher Scientific, Inc.) with
protease inhibitors (Thermo Fisher Scientific, Inc.). Subsequently,
protein concentration was measured using BCA Protein Assay Kit
(Thermo Fisher Scientific, Inc.). A total of 50 µg protein/sample
were separated via 12% SDS-PAGE and transferred onto PVDF membranes
(Millipore). Subsequently, membranes were blocked with 5% nonfat
milk and incubated with primary antibodies at 4˚C overnight, and
anti-β-actin (cat. no. sc-8432; 1:2,000 dilution; Santa Cruz
Biotechnology, Inc.) served as a reference. Subsequently, the
membranes were incubated with HRP-conjugated secondary antibodies
(cat. no. sc-2005; 1:2,000 dilution; Santa Cruz Biotechnology,
Inc.). Finally, signal intensity was visualized with enhanced
chemiluminescence detection kit (Thermo Fisher Scientific, Inc.).
In addition, Image Pro Plus software v.6.0 (Media Cybernetics,
Inc.) was used to determine the protein relative expression level.
The primary antibodies were as follows: Anti-N-cadherin (cat. no.
sc-8424; 1:1,000 dilution), anti-Vimentin (cat. no. sc-6260;
1:1,000 dilution), anti-E-cadherin (cat. no. sc-8426; 1:1,000
dilution) and anti-JAG1 (cat. no. sc-390177; 1:1,000 dilution; all
from Santa Cruz Biotechnology, Inc.).
In vivo experiment
All animal experiments were performed according to
the guide for the care and use of laboratory animals of the
Ministry of Science and Technology of China (23). In total, 12 4-week-old male BALB/c
nude mice (weight, 18±2 g) were purchased from The Shanghai
Experimental Animal Center and kept at a temperature 22±1˚C and
humidity 55±5%, 12-h light/dark cycle, with free access to clean
food and water. The stable AGS cell line expressing sh-NEAT1 or
sh-NC was established by transfecting AGS cells with sh-NEAT1 or
sh-NC and followed by selection with 4 µg/ml puromycin
(Sigma-Aldrich; Merck KGaA) for 4-5 weeks. Stable cell lines were
maintained in the high glucose DMEM medium with 10% FBS and 1 µg/ml
puromycin. AGS cells (5x106/100 µl PBS/mice) stably
transfected with sh-NEAT1 or sh-NC were subcutaneously injected
into the mice at right flank of nude mice. Tumor growth was
monitored with calipers by measuring the maximum (a) and minimum
(b) length of the tumor every 7 days. Tumor volume (V) was assessed
as follows: V=1/2 x ab2. All mice were sacrificed by
flowing CO2 inhalation (45% of the cage vol/min; the
heartbeat and pupils of the mice were examined to ensure death) on
day 35 after inoculation, and tumors were weighted and used for
subsequent experiments. The Institutional Animal Care and Use
Committee of Yantaishan Hospital (Yantai, China) authorized all
animal experiments.
Statistical analysis
All data are presented as the mean ± standard
deviation from ≥ three independent experiments. The difference
between two groups was examined by Student's t-test (paired
Student's t-test was used to examine statistically significant
differences between tumor and adjacent non-tumor tissues of the
same individual, while unpaired Student's t-test was used for two
unpaired groups), and differences among ≥3 groups were analyzed
using one-way ANOVA followed by Bonferroni's post hoc test using
GraphPad Prism 7 (GraphPad Inc.). P<0.05 was considered to
indicate a statistically significant difference. Survival curves of
patients with gastric cancer was performed by Kaplan-Meier curve
analysis with log-rank test. Pearson's correlation analysis was
used to determine correlation relationship.
Results
NEAT1 is highly expressed in gastric
cancer tissues and cells
The expression of NEAT1 in gastric cancer tissues
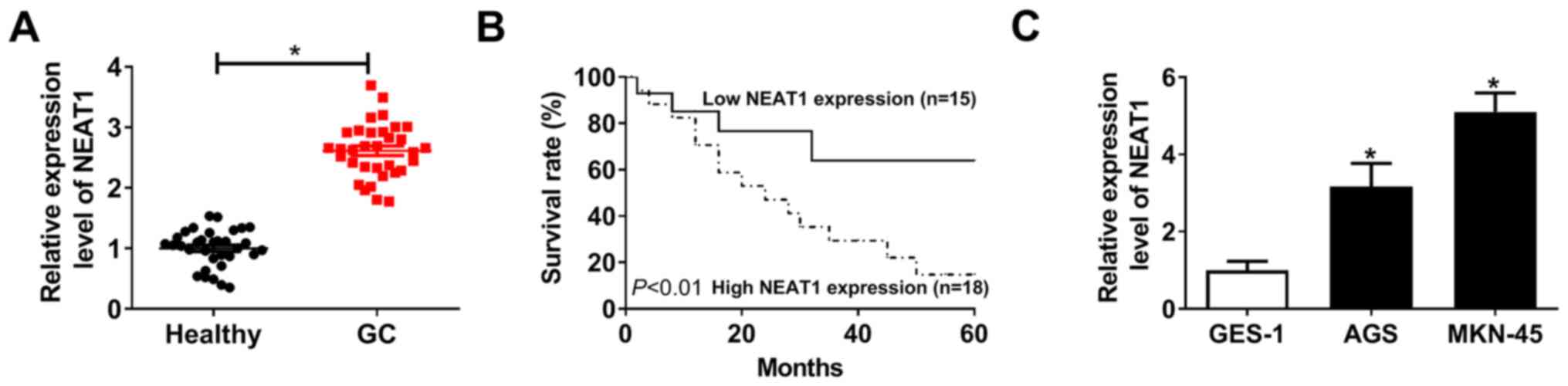
and cells was firstly examined by RT-qPCR. As presented in Fig. 1A, NEAT1 expression level was
significantly upregulated in 33 cases of gastric cancer compared
with matched healthy tissues. The data indicated that low
expression of NEAT1 (according to the median of NEAT1 level in
tumor tissues) was associated with high survival rate, while
patients with high level of NEAT1 presented low survival rate
(Fig. 1B). In addition, the
expression level of NEAT1 in human gastric cancer cell lines (AGS
and MKN-45 cells) was increased compared with the human normal
gastric epithelial cell line GES-1 (Fig. 1C). Therefore, the results indicated
that high expression of NEAT1 was associated with a poor prognosis
of gastric cancer.
NEAT1 knockdown suppresses the
proliferation, migration and invasion of gastric cancer cells
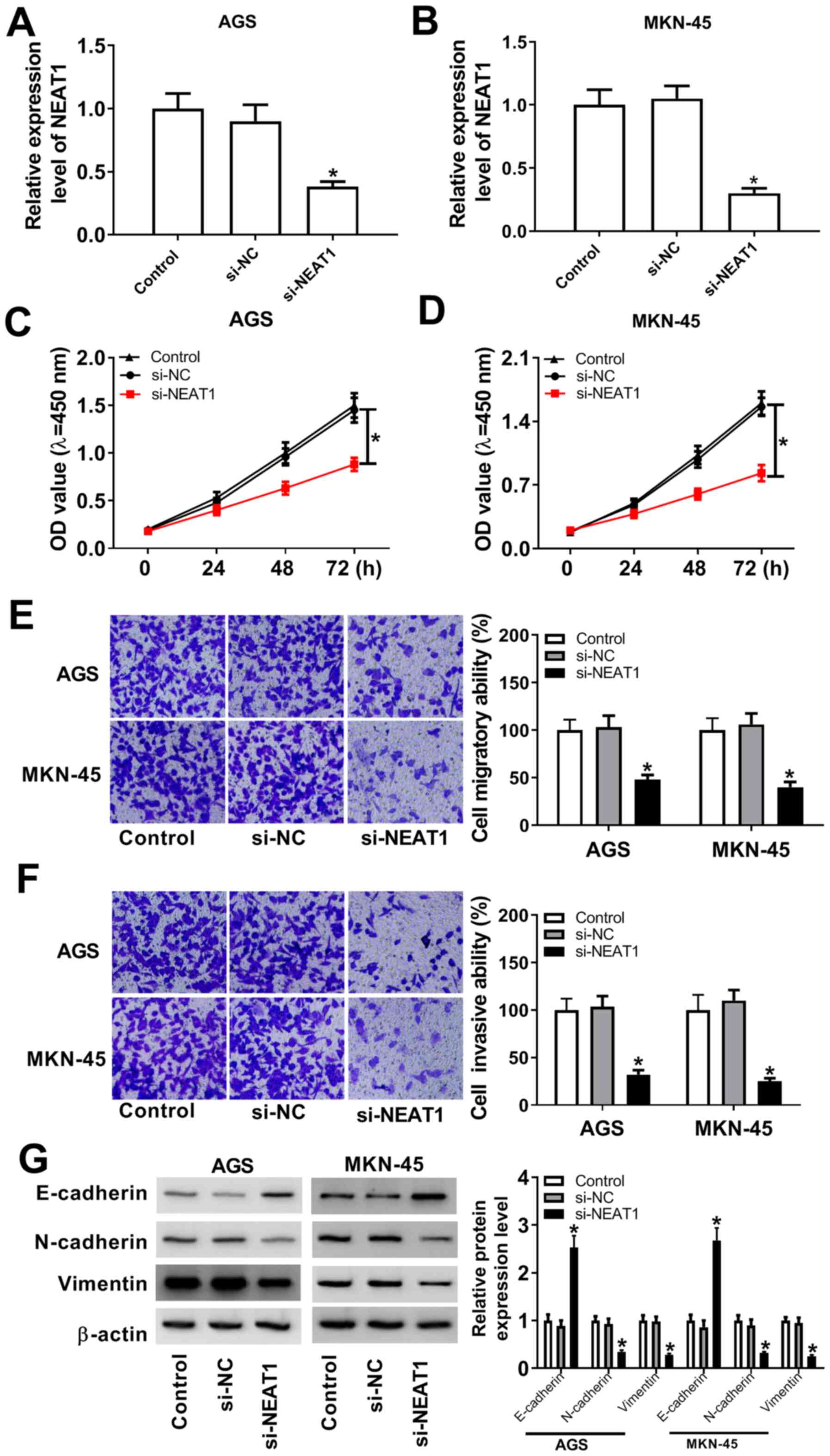
To understand the effect of NEAT1 on gastric cancer
progression, a loss-of-function experiment was conducted. The level
of NEAT1 expression was notably decreased in AGS and MKN-45 cells
transfected si-NEAT1 (Fig. 2A and
B). To explore the proliferative
ability of AGS and MKN-45 cells transfected with si-NEAT1 or si-NC,
CCK-8 assay was performed, which indicated that NEAT1 knockdown
inhibited the proliferation of gastric cancer cells (Fig. 2C and D). Gastric cancer cells transfected with
si-NEAT1 exhibited a notable reduction in the number of migrated
cells compared with the si-NC group (Fig. 2E). Consistent with the migration
assay results, NEAT1 silencing suppressed the invasion of gastric
cancer cells (Fig. 2F).
Furthermore, N-cadherin and Vimentin expression levels were
decreased, but E-cadherin level was increased in the si-NEAT1 group
compared with the si-NC group (Fig.
2G). Collectively, the results indicated that NEAT1 may serve
an oncogenic role by enhancing proliferation, migration and
invasion in gastric cancer cells.
NEAT1 directly interacts with
miR-142-5p
Following bioinformatics analysis using LncBase v2,
several miRNAs were predicted to serve as potential targets of
NEAT1, including miR-761, miR-101-3p, miR-9-5p, miR-181b-5p,
miR-194-5p and miR-142-5p. The expression level of these miRNAs in
healthy tissues and gastric cancer tissues was assessed, and
miR-142-5p was the most significantly decreased in gastric cancer
tissues compared with healthy tissues (Fig. S1). miR-142-5p was therefore
selected for subsequent analysis. The putative binding sites
between NEAT1 and miR-142-5p are presented in Fig. 3A. The dual-luciferase reporter assay
results indicated that miR-142-5p overexpression effectively
suppressed the luciferase activity of the NEAT1-WT reporter in AGS
and MKN-45 cells (Fig. 3B), but the
luciferase activity of the NEAT1-MUT reporter in gastric cancer
cells exhibited no significant change compared with the control
group (Fig. 3C). To further clarify
the association between NEAT1 and miR-142-5p, RIP assay was
performed to assess whether NEAT1 could interact with miR-142-5p.
miR-142-5p overexpression resulted in a significant enrichment of
NEAT1 in the Ago2 immunoprecipitation complex compared with the
miR-NC control group (Fig. 3D). To
investigate the regulatory association between NEAT1 and
miR-142-5p, the relative expression level of miR-142-5p was firstly
evaluated. miR-142-5p was indicated to be decreased in gastric
cancer tumor tissues and cells compared with that in normal tissues
and GES-1 cells (Fig. 3E and
F). A negative correlation between
NEAT1 and miR-142-5p expression level in gastric cancer tissues was
also observed (Fig. 3G).
Subsequently, RT-qPCR assay confirmed that NEAT1 expression level
was notably increased in AGS and MKN-45 cells transfected with
NEAT1 overexpression plasmid compared with control cells (Fig. 3H). miR-142-5p expression level was
significantly decreased in cells transfected with NEAT1
overexpression vector, while miR-142-5p level was elevated in the
NEAT1 knockdown group (Fig. 3I).
All these data indicated that NEAT1 directly targeted
miR-142-5p.
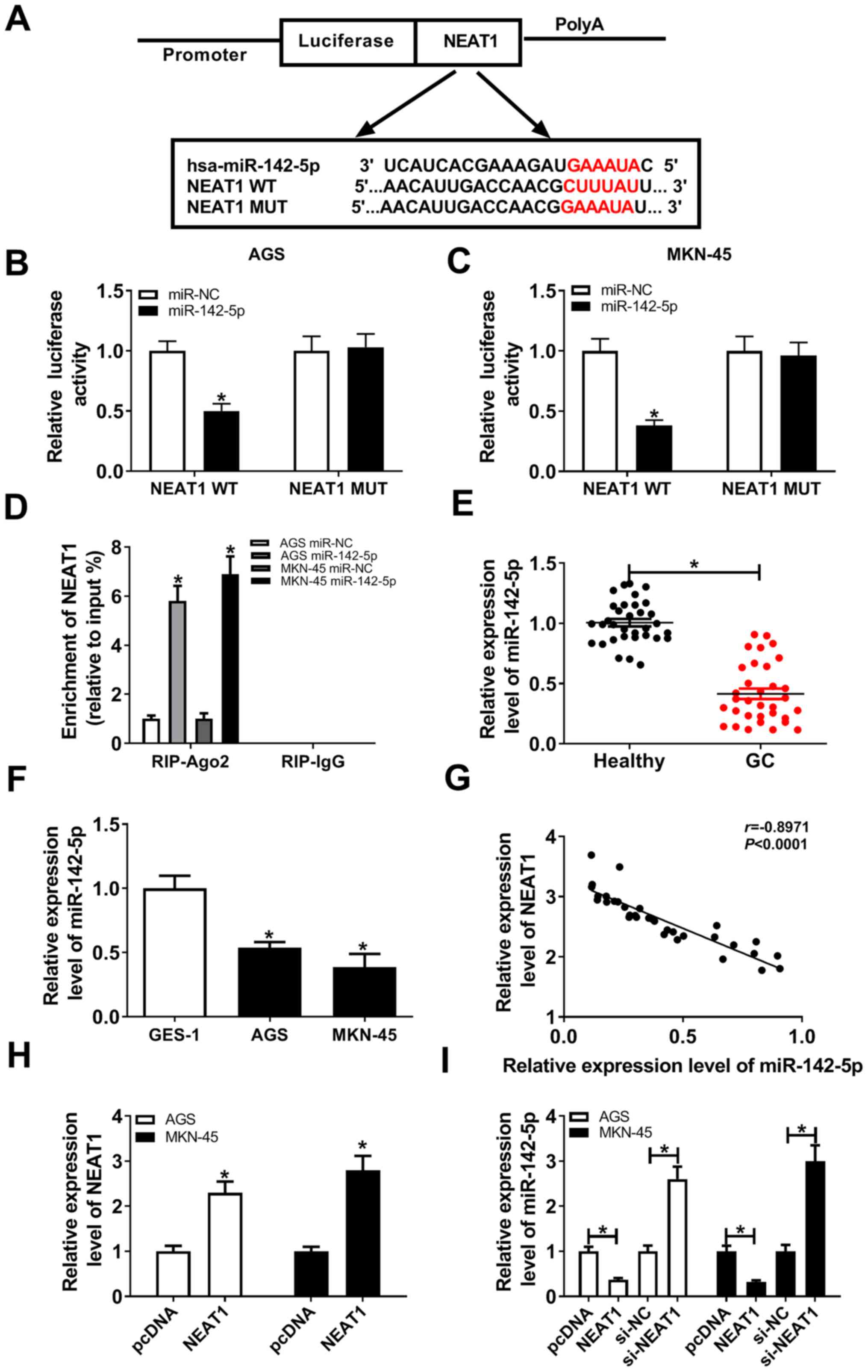 | Figure 3NEAT1 directly interacts with
miR-142-5p. (A) Putative binding sites between NEAT1 and miR-142-5p
and NEAT1 mutant sites. (B and C) Luciferase activity in AGS and
MKN-45 cells co-transfected with NEAT1-WT or NEAT1-MUT luciferase
reporter and miR-142-5p or miR-NC, respectively. (D) RIP assay in
AGS and MKN-45 cells. (E and F) Expression level of miR-142-5p in
gastric cancer tissues and cells determined by RT-qPCR. (G)
Correlation analysis of NEAT1 and miR-142-5p expression levels. (H)
Expression level of NEAT1 in AGS and MKN-45 cells transfected with
pcDNA or NEAT1 overexpression vector. (I) Expression level of
miR-142-5p in AGS and MKN-45 cells transfected with pcDNA, NEAT1,
si-NC or si-NEAT1 determined by RT-qPCR. *P<0.05.
NEAT1, nuclear-enriched abundant transcript 1; miR, microRNA; si,
small interfering; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR; RIP, RNA immunoprecipitation; WT,
wild-type; MUT, mutant; Ago2, argonaute2. |
miR-142-5p inhibitor rescues the NEAT1
silencing-mediated effects on proliferation, migration and invasion
in gastric cancer cells
The effects of NEAT1 knockdown along with miR-142-5p
silencing on proliferation, migration and invasion in gastric
cancer cells were further explored. miR-142-5p expression level was
decreased in AGS and MKN-45 cells after transfection with
miR-142-5p inhibitor, while it was increased in miR-142-5p
mimic-transfected cells compared with control cells (Fig. S2A). Silencing of miR-142-5p
reversed the reduction in cell viability in AGS and MKN-45 cells
caused by NEAT1 knockdown (Fig. 4A
and B). Similarly, Transwell assay
indicated that miR-142-5p inhibitor attenuated the NEAT1
knockdown-mediated inhibitory effects on the migration and invasion
of AGS and MKN-45 cells (Fig. 4C
and D). Moreover, NEAT1 knockdown
markedly decreased N-cadherin and Vimentin expression levels,
whereas E-cadherin was increased; however, co-transfection of
si-NEAT1 and miR-142-5p inhibitor reversed these effects in AGS and
MKN-45 cells (Fig. 4E). Taken
together, these results indicated that NEAT1 directly targeted
miR-142-5p to enhance the proliferative, migratory and invasive
capability of gastric cancer cells.
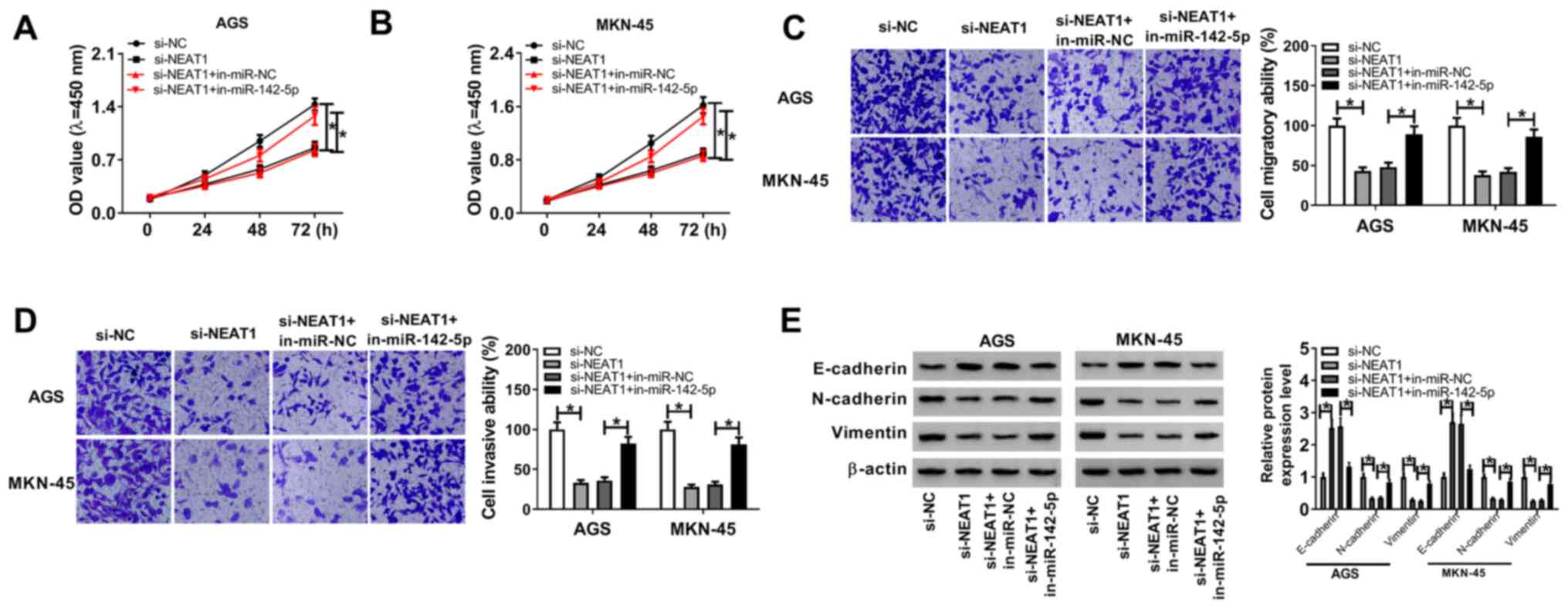 | Figure 4Effect of miR-142-5p inhibitor on the
NEAT1 knockdown-mediated effects on proliferation, migration and
invasion in gastric cancer cells. AGS and MKN-45 cells were
transfected with si-NC, si-NEAT1, si-NEAT1 + in-miR-NC or si-NEAT1
+ in-miR-142-5p. (A and B) Cell viability of transfected AGS and
MKN-45 cells at the indicated time points analyzed with Cell
Counting Kit-8 assay. (C and D) Migratory and invasive abilities of
AGS and MKN-45 cells examined by Transwell assay. (E) Protein
expression level of E-cadherin, N-cadherin and Vimentin in AGS and
MKN-45 cells post-transfection determined by western blot assay.
*P<0.05. NEAT1, nuclear-enriched abundant transcript
1; miR, microRNA; in, inhibitor; si, small interfering; NC,
negative control; OD, optical density. |
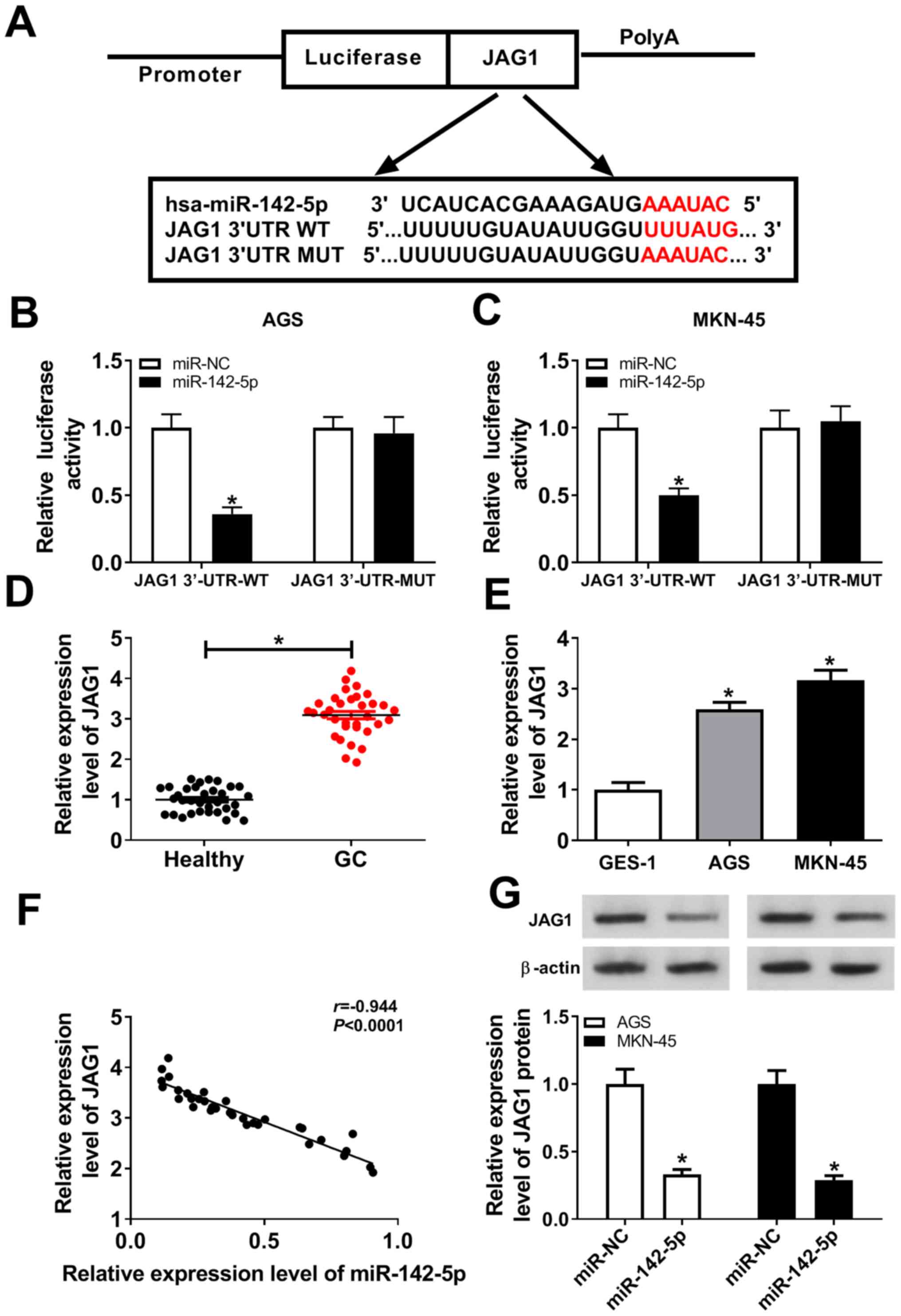
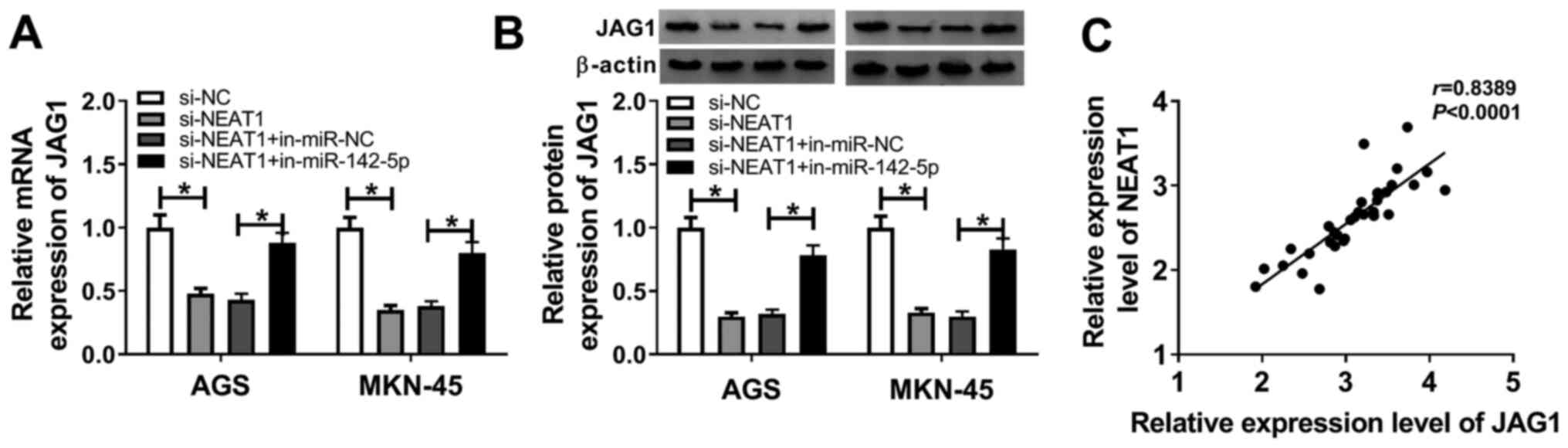
JAG1 is a target of miR-142-5p in
gastric cancer
The binding region of miR-142-5p on the 3'-UTR of
JAG1 and its mutant are presented in Fig. 5A. As indicated in Fig. 5B and C, miR-142-5p overexpression decreased the
luciferase activity of JAG1 3'-UTR WT rather than that of JAG1
3'-UTR MUT in AGS and MKN-45 cells. To further investigate whether
JAG1 was involved in the development of gastric cancer, the
expression of JAG1 in gastric cancer tissues and cells was examined
by RT-qPCR. As presented in Fig. 5D
and E, JAG1 expression level was
significantly increased in gastric cancer tissues and cells
compared with the corresponding controls. The negative correlation
between miR-142-5p and JAG1 expression level is illustrated in
Fig. 5F. A gain-of-function
experiment revealed that the protein level of JAG1 was reduced in
AGS and MKN-45 cells transfected with miR-142-5p mimic compared
with miR-NC (Fig. 5G). Thus, it was
concluded that miR-142-5p directly suppressed JAG1 expression in
gastric cancer.
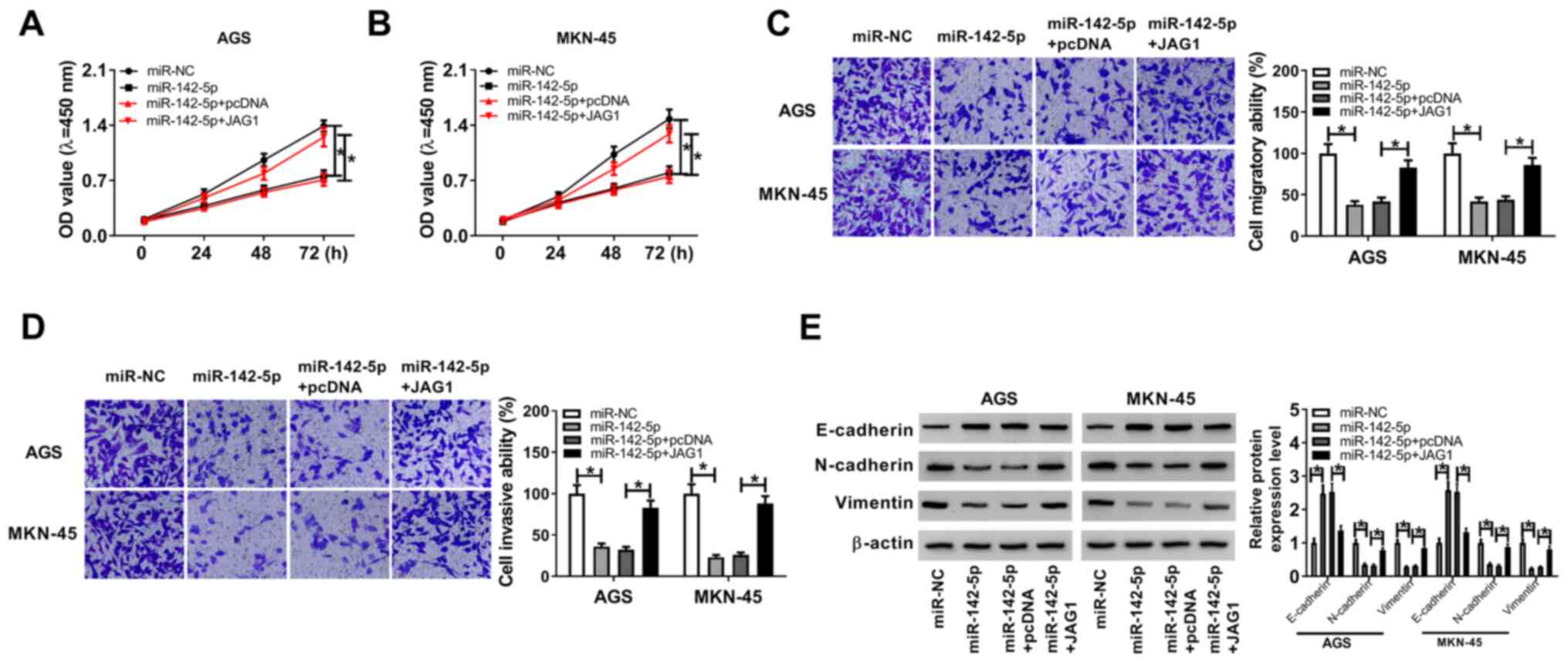
JAG1 overexpression abolishes the
effects of miR-142-5p on proliferation, migration and invasion in
gastric cancer cells
According to the aforementioned results, the
overexpression of miR-142-5p inhibited JAG1 expression in gastric
cancer cells. The expression level of JAG1 was increased in AGS and
MKN-45 cells after transfection with JAG1 overexpression plasmid
compared with the pcDNA group (Fig.
S2B). CCK-8 assay indicated that miR-142-5p overexpression
suppressed the proliferation of gastric cancer cells, and
overexpression of JAG1 abolished this effect (Fig. 6A and B). Similarly, the overexpression of JAG1
also reversed the miR-142-5p-mediated inhibitory effects on
migration and invasion in gastric cancer cells (Fig. 6C and D). Western blot assay demonstrated that
miR-142-5p decreased N-cadherin and Vimentin expression levels, but
enhanced E-cadherin level in AGS and MKN-45 cells compared with the
miR-NC group, and when co-transfected with JAG1, this effect was
reversed (Fig. 6E). Collectively,
miR-142-5p inhibited the proliferation, migration and invasion of
gastric cancer cells by targeting JAG1.
NEAT1 knockdown inhibits JAG1
expression in gastric cancer as a sponge of miR-142-5p
To elucidate the association between NEAT1 and JAG1
in gastric cancer cells, RT-qPCR and western blot assays were
conducted, which indicated that the mRNA and protein expression
levels of JAG1 were decreased in cells after NEAT1 knockdown, while
transfection with miR-142-5p inhibitor effectively attenuated the
NEAT1 silencing-induced reduced expression of JAG1 (Fig. 7A and B). A positive correlation between JAG1 and
NEAT1 expression level in gastric cancer tissues was also observed
(Fig. 7C). In conclusion, NEAT1
knockdown inhibited JAG1 expression by regulating miR-142-5p in
vitro.
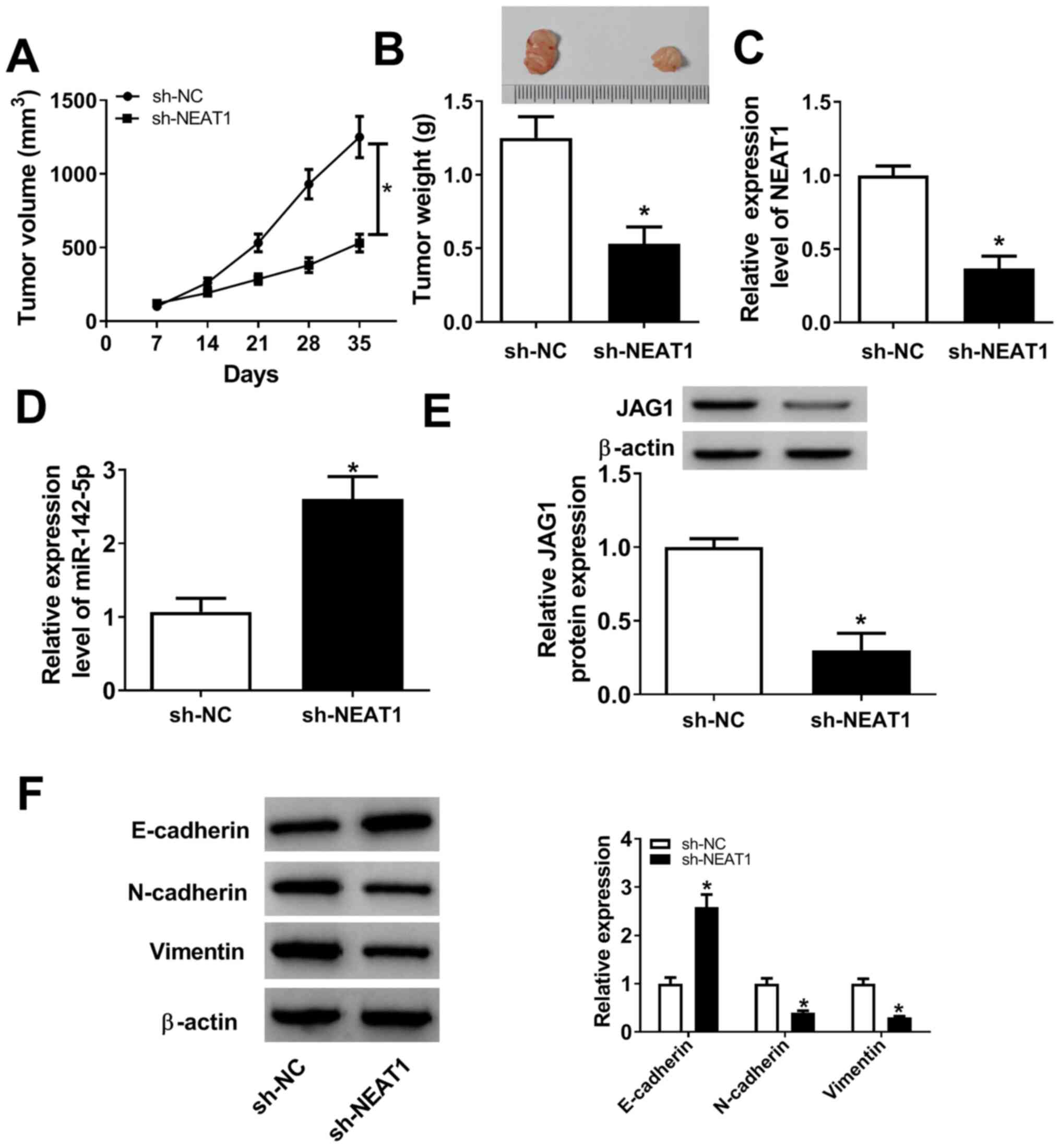
NEAT1 knockdown suppresses tumor
growth and metastasis in vivo
A xenograft tumor mouse model was established to
further investigate the functional effect of NEAT1 on tumor growth
in vivo. As presented in Fig.
8A and B, NEAT1 knockdown
resulted in an evident decrease in tumor volume and weight compared
with the sh-NC group. NEAT1 expression level was lower in the tumor
tissues of the sh-NEAT1 group compared with the sh-NC group
(Fig. 8C). Moreover, RT-qPCR assay
indicated that NEAT1 knockdown enhanced miR-142-5p expression level
in tumor tissues (Fig. 8D). In
addition, western blot analysis indicated that NEAT1 silencing
significantly decreased the protein expression level of JAG1
(Fig. 8E). In addition, analysis of
the protein levels of E-cadherin, N-cadherin and Vimentin in
xenograft tumor tissues revealed that E-cadherin expression level
was increased, while N-cadherin and Vimentin levels were decreased
in the sh-NEAT1 group compared with the sh-NC group (Fig. 8F). These results demonstrated that
NEAT1 knockdown suppressed tumor growth and metastasis in
vivo by regulating miR-142-5p and JAG1 expression.
Discussion
In recent years, lncRNAs have attracted attention
due to their potential as diagnostic and prognostic markers in
numerous cancers (24). For
example, Chen et al (25)
reported that circPVT1 was a proliferative factor and independent
prognostic biomarker for patients with gastric cancer. lncRNA
maternally expressed 3 has been indicated to regulate gastric
cancer progression via the p53 signaling pathway (26). In the present study, it was observed
that high expression of NEAT1 was associated with poor survival of
patients with gastric cancer. Functional experiments demonstrated
that NEAT1 silencing or miR-142-5p overexpression inhibited cell
migration and invasion, but these effects could be reversed by
miR-142-5p inhibitor or JAG1 overexpression, respectively.
Additionally, the current study used both AGS and MKN-45 gastric
cancer cell lines, and the results were similar in the two cell
lines, suggesting the relevance of the present results in gastric
cancer. However, certain results differed between the AGS and
MKN-45 gastric cancer cell lines. For example, the levels of NEAT1
and JAG1 were lower, but the level of miR-142-5p was higher in AGS
cells compared with that in MKN-45 cells, which may be attributed
to the different malignant potential of the two cell lines.
A previous study demonstrated that patients with
gastric cancer and high level of NEAT1 exhibited poor survival
(27). Therefore, NEAT1 may be used
as a therapeutic target and prognostic biomarker for gastric
cancer. A negative association between the high level of NEAT1 and
the five-year survival rate of patients with gastric cancer was
also observed. Fu et al (27) demonstrated that NEAT1 was
upregulated in gastric cancer tissues and cell lines, and knockdown
of NEAT1 suppressed gastric cancer cell migration and invasion
in vitro. Another study also revealed that NEAT1 knockdown
inhibited the progression of gastric cancer (28). Consistent with these studies, the
present study also demonstrated that knockdown of NEAT1 suppressed
the proliferation, migration and invasion of gastric cancer cells
in vitro, while these inhibitory effects could be abrogated
by miR-142-5p inhibitor.
A previous study has indicated that miRNAs
functioned as biomarkers, which may enhance the sensitivity and
specificity of gastric cancer diagnostic and prognostic tests
(29). Zhang et al (30) revealed that a low level of
hsa-miR-142-5p in gastric cancer was associated with high frequency
recurrence and poor survival. In the present study, miR-142-5p was
observed to be decreased in gastric cancer cells and identified as
a target of NEAT1. Further functional experiments revealed that
miR-142-5p silencing restored the NEAT1 knockdown-induced effects
on proliferation, migration and invasion in gastric cancer cells.
Shen et al (31) reported
that miR-142-3p was markedly decreased in colon cancer cells and
inhibited gene expression by binding to the 3'-UTR of the CD133. In
the current study, mechanistic experiments revealed that miR-142-5p
could bind to the 3'-UTR of JAG1.
JAG1 is one of the most important ligands of the
Notch signaling pathway (19).
Increasing evidence has suggested that dysregulation of JAG1 was
involved in the development of malignant tumors (32). Jiang et al (15) also revealed the important role of
JAG1 in gastric cancer, demonstrating that the upregulation of
miR-124 inhibited gastric cancer cell growth, migration and
invasion by regulating JAG1 expression. In the present study, the
expression level of miR-142-5p was inversely correlated with JAG1
expression level in patients with gastric cancer. Overexpression of
miR-142-5p impeded gastric cancer cell growth, migration and
invasion, which could be reversed by overexpression of JAG1.
Moreover, it was revealed that upregulation of miR-142-5p could
decrease the expression level of JAG1 in gastric cancer cells,
suggesting that miR-142-5p may inhibit gastric cancer development
via directly targeting JAG1.
Finally, the biological functions of NEAT1 were
further investigated on tumor growth in vivo, and the
results indicated that NEAT1 silencing suppressed tumor growth and
metastatic potential in vivo via regulating the
miR-142-5p/JAG1 axis. However, there are also certain limitations
to the present study. The number of clinical samples was low, which
may lead to an inaccurate result. Moreover, the effect of the
NEAT1/miR-142-5p/JAG1 axis on the Notch signaling pathway has not
been explored. In addition, whether the NEAT1/miR-142-5p/JAG1 axis
could regulate drug resistance in gastric cancer also requires
further elucidation.
To conclude, increased NEAT1 and JAG1 expression
levels, as well as decreased miR-142-5p level were observed in
gastric cancer tissues compared with neighboring healthy tissues.
Functionally, NEAT1 contributed to gastric cancer progression via
targeting miR-142-5p to regulate JAG1 expression in vitro
and in vivo, which indicated that NEAT1 and miR-142-5p may
serve as promising therapeutic targets for the treatment of gastric
cancer.
Supplementary Material
Expression of miRNAs in healthy and GC
tissues. miRNA expression level in GC tissues and the control group
determined by reverse transcription-quantitative PCR.
*P<0.05. miRNA/miR, microRNA; GC, gastric
cancer.
Expression of miR-142-5p and JAG1 in
gastric cancer cells. Transfection efficiency of (A) miR-142-5p
inhibitor/mimic and (B) overexpression vector of JAG1 determined by
reverse transcription-quantitative PCR in AGS and MKN-45 cells.
*P<0.05. miR, microRNA; NC, negative control; in,
inhibitor; JAG1, jagged1.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ: Conceptualization. LC and QW: Acquisition of
data. YZ and FZ: Analysis of data and data curation. YZ and FZ:
Confirming the authenticity of the raw data. All authors: Read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Yantaishan Hospital (Yantai, China), and a written
informed consent form was obtained from each patient. The
Institutional Animal Care and Use Committee of Yantaishan Hospital
(Yantai, China) authorized all animal experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Du X, Cheng Z, Wang YH, Guo ZH, Zhang SQ,
Hu JK and Zhou ZG: Role of Notch signaling pathway in gastric
cancer: A meta-analysis of the literature. World J Gastroenterol.
20:9191–9199. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dey BK, Mueller AC and Dutta A: Long
non-coding RNAs as emerging regulators of differentiation,
development, and disease. Transcription. 5(e944014)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pan LJ, Zhong TF, Tang RX, Li P, Dang YW,
Huang SN and Chen G: Upregulation and clinicopathological
significance of long non-coding NEAT1 RNA in NSCLC tissues. Asian
Pac J Cancer Prev. 16:2851–2855. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Y, Chen D, Gao X, Li X and Shi G:
LncRNA NEAT1 regulates cell viability and invasion in esophageal
squamous cell carcinoma through the miR-129/CTBP2 axis. Dis
Markers. 2017(5314649)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu Y, Yang L, Zhao J, Li C, Nie J, Liu F,
Zhuo C, Zheng Y, Li B, Wang Z and Xu Y: Nuclear-enriched abundant
transcript 1 as a diagnostic and prognostic biomarker in colorectal
cancer. Mol Cancer. 14(191)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mang Y, Li L, Ran J, Zhang S, Liu J, Li L,
Chen Y, Liu J, Gao Y and Ren G: Long noncoding RNA NEAT1 promotes
cell proliferation and invasion by regulating hnRNP A2 expression
in hepatocellular carcinoma cells. Onco Targets Ther. 10:1003–1016.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhen L, Yun-Hui L, Hong-Yu D, Jun M and
Yi-Long Y: Long noncoding RNA NEAT1 promotes glioma pathogenesis by
regulating miR-449b-5p/c-Met axis. Tumour Biol. 37:673–683.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gong W, Zheng J, Liu X, Ma J, Liu Y and
Xue Y: Knockdown of NEAT1 restrained the malignant progression of
glioma stem cells by activating microRNA let-7e. Oncotarget.
7:62208–62223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen X, Kong J, Ma Z, Gao S and Feng X: Up
regulation of the long non-coding RNA NEAT1 promotes esophageal
squamous cell carcinoma cell progression and correlates with poor
prognosis. Am J Cancer Res. 5:2808–2815. 2015.PubMed/NCBI
|
|
13
|
Tafrihi M and Hasheminasab E: miRNAs:
Biology, biogenesis, their web-based tools, and databases.
Microrna. 8:4–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Xing R, Zhang X, Dong W, Zhang J,
Yan Z, Li W, Cui J and Lu Y: miR-375 targets the p53 gene to
regulate cellular response to ionizing radiation and etoposide in
gastric cancer cells. DNA Repair (Amst). 12:741–750.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jiang L, Lin T, Xu C, Hu S, Pan Y and Jin
R: miR-124 interacts with the Notch1 signalling pathway and has
therapeutic potential against gastric cancer. J Cell Mol Med.
20:313–322. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie
Y, Li S, Tang G, Tang H and He X: MicroRNA-124 inhibits
proliferation and induces apoptosis by directly repressing EZH2 in
gastric cancer. Mol Cell Biochem. 392:153–159. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu CB, Li QL, Hu JF, Zhang Q, Xie JP and
Deng L: miR-124 inhibits growth and invasion of gastric cancer by
targeting ROCK1. Asian Pac J Cancer Prev. 15:6543–6546.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ma Z, Liu T, Huang W, Liu H, Zhang HM, Li
Q, Chen Z and Guo AY: MicroRNA regulatory pathway analysis
identifies miR-142-5p as a negative regulator of TGF-β pathway via
targeting SMAD3. Oncotarget. 7:71504–71513. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Grochowski CM, Loomes KM and Spinner NB:
Jagged1 (JAG1): Structure, expression, and disease associations.
Gene. 576:381–384. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jia CM, Tian YY, Quan LN, Jiang L and Liu
AC: miR-26b-5p suppresses proliferation and promotes apoptosis in
multiple myeloma cells by targeting JAG1. Pathol Res Pract.
214:1388–1394. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao HJ, Ji Q, Yang L, Li RT, Zhang C and
Hou JM: In vivo and in vitro effects of microRNA-124 on human
gastric cancer by targeting JAG1 through the Notch signaling
pathway. J Cell Biochem. 119:2520–2534. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ministry of Science and Technology of the
People's Republic of China. Guide for the care and use of
laboratory animals. Ministry of Science and Technology of the
People's Republic of China, Beijing, People's Republic of China (In
Chinese).
|
|
24
|
Chandra Gupta S and Nandan Tripathi Y:
Potential of long non-coding RNAs in cancer patients: From
biomarkers to therapeutic targets. Int J Cancer. 140:1955–1967.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen J, Li Y, Zheng Q, Bao C, He J, Chen
B, Lyu D, Zheng B, Xu Y, Long Z, et al: Circular RNA profile
identifies circPVT1 as a proliferative factor and prognostic marker
in gastric cancer. Cancer Lett. 388:208–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wei GH and Wang X: lncRNA MEG3 inhibit
proliferation and metastasis of gastric cancer via p53 signaling
pathway. Eur Rev Med Pharmacol Sci. 21:3850–3856. 2017.PubMed/NCBI
|
|
27
|
Fu JW, Kong Y and Sun X: Long noncoding
RNA NEAT1 is an unfavorable prognostic factor and regulates
migration and invasion in gastric cancer. J Cancer Res Clin Oncol.
142:1571–1579. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang J, Zhao B, Chen X, Wang Z, Xu H and
Huang B: Silence of long noncoding RNA NEAT1 inhibits malignant
biological behaviors and chemotherapy resistance in gastric cancer.
Pathol Oncol Res. 24:109–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu HS and Xiao HS: MicroRNAs as potential
biomarkers for gastric cancer. World J Gastroenterol.
20:12007–12017. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui
J, Liu Y, Gao Z, Li J, Shen L and Lu Y: Combination of hsa-miR-375
and hsa-miR-142-5p as a predictor for recurrence risk in gastric
cancer patients following surgical resection. Ann Oncol.
22:2257–2266. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shen WW, Zeng Z, Zhu WX and Fu GH:
miR-142-3p functions as a tumor suppressor by targeting CD133,
ABCG2, and Lgr5 in colon cancer cells. J Mol Med (Berl).
91:989–1000. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li D, Masiero M, Banham AH and Harris AL:
The notch ligand JAGGED1 as a target for anti-tumor therapy. Front
Oncol. 4(254)2014.PubMed/NCBI View Article : Google Scholar
|