Introduction
Atherosclerosis (AS), a cardiovascular disease with
complex interactions, is regarded as one of the leading causes of
morbidity and mortality worldwide (1). AS is a chronic pathophysiological
process that is initially inapparent until intimal thickening of
coronary arteries diminishes blood flow. This is accompanied by
inflammatory phenomena, such as accumulation of lipids, aggravation
of inflammatory lesions, thrombosis and fibrous hyperplasia
(2). The thickening of the artery
intima can be characterized by the deposition of low-density
lipoproteins (LDLs), and adhesion of necrotic cellular debris and
immune cells, which facilitates the formation of atherosclerotic
plaques (3,4). In the early stages of AS, endothelial
cells (ECs) and vascular smooth muscle cells (VSMCs) are activated,
inducing the expression of inflammatory mediators and adhesive
factors; these include E-selectin, P-selectin, vascular cell
adhesion molecule-1 (VCAM-1) and the glycoprotein intercellular
adhesion molecule-1 (ICAM-1) (5,6). The
migration and recruitment of platelets and inflammatory monocytes
are stimulated by C-C motif chemokine ligands (CCL), including
CCL2, CCL3 and CCL25. Following on from this, the monocytes mature
into M1 and M2 macrophages, and secrete inflammatory cytokines,
such as TNF-α, IL-1β and IL-12, which maintain inflammation and
promote chemotaxis of immune cells toward the plaque. As a
consequence of abnormal absorption of modified LDLs, macrophages
develop further into foam cells, a process induced by
platelet-released platelet factor-4 (PF4) or stromal cell-derived
factor-1 (7-9).
Therefore, the identification of expression levels of these
inflammatory mediators and chemokines has the potential to improve
the understanding of pathophysiological processes of AS, and the
regulation of related signaling pathways may contribute to the
treatment of AS (10). Homeobox
(HOX) genes are transcription factors that act during normal
development (11) and can be
categorized into four groups based on the similarity of a 61-amino
acid homeodomain, namely: i) HOXA; ii) HOXB; iii) HOXC and iv) HOXD
(12). Several studies have
demonstrated that HOX genes may participate in the regulation of
the cardiovascular system and the pathology of AS (13). Among them, HOXA9 was found to be
involved in regulating cellular processes of ECs and HOXA9
inhibition was revealed to suppress E-selectin expression in
response to inflammatory cytokines (14). Therefore, in the present study,
HOXA9 inhibition was hypothesized to promote microcirculation of
coronary arteries through downregulating PF4, E-selectin and
VCAM-1, thus revealing the pro-inflammatory role of HOXA9 in AS
rats.
Materials and methods
Modeling of AS
AS was established in Sprague Dawley (SD) rats were
intravenously injected with vitamin D3 (Sigma-Aldrich; Merck KGaA)
at a dose of 500,000 IU/kg/d for 3 days, and then orally fed with a
high-fat and high-cholesterol diet for 3 months. Rats were housed
in an environmentally controlled room (50-60% humidity, 20-25˚C)
with 12 h light/dark cycle and with free access to food and water.
The detailed feeds were purchased from ReadyDietech Co., Ltd,
according to a previous study (15). Following the intravenous vitamin D3
injection, the gross anatomy and histology of the model rats were
constantly monitored. Successful model establishment was indicated
by the occurrence of vascular intimal injury and inflammation
induced by mechanical effects, such as hypercalcemia or balloon
aortic laceration.
Tested animals grouping
Male SD rats (weight, 180-200 g; age, 4-6 weeks)
were purchased from Shanghai SLAC Laboratory Animal Center. A total
of 56 rats were randomly divided into four experimental groups
(n=14); i) AS+HOXA9-inhibitor group: AS model rats intravenously
injected with HOXA9 inhibitors (anti-HOXA9 antibody, cat. no.
SAB2108152; Sigma-Aldrich; Merck KGaA) at a dose of 100 µg/kg body
weight; ii) AS+si-HOXA9 group: AS model rats intravenously injected
with HOXA9 small interfering (si)RNA; iii) AS group: AS model rats
were intravenously injected with 100 µg/kg saline; iv) NC group:
Control rats were housed under the same conditions but fed with a
normal diet. Rats were intravenously injected with saline (100
µg/kg). For construction of the overexpression models of PF4,
E-selectin and VCAM-1, the overexpression vector of each gene was
constructed by HanBio Biotechnology Co., Ltd., via a lentivirus
expressing system. The lent-OE-PF4, lent-OE-E-selectin and
lent-OE-VCAM-1 vectors were separately injected into rats in the
AS+HOXA9-inhibitor group (n=3 for each vector) via tail vein
injection (1.0x109 TU/ml, 10 µl), whereas
lent-OE-negative control (the coding region was substituted by GFP)
was used as a control. All the tested animals were housed based on
the standard of the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (NIH Publication). Following the
aforementioned treatment for 3 months, all tested animals were
intraperitoneally anesthetized with pentobarbital (60 mg/kg) and
then sacrificed via cervical dislocation. Blood samples were
collected from the abdominal artery for subsequent analysis. The
coronary arteries and thoracic aorta samples were harvested and
stored in a frozen state using liquid nitrogen.
ELISA for inflammatory cytokines
The collected blood samples were placed at room
temperature for 10-20 min to coagulate naturally, followed by
centrifugation for 20 min (1,000 x g, 4˚C). The supernatant was
collected, and centrifugation was repeated once under the same
conditions in case of precipitation during storage. Following the
above sample pre-treatment, ELISA kits (Nanjing Jiancheng
Bioengineering Institute) were utilized to detect the levels of
inflammatory cytokines, TNF-α (cat. no. H052-1), IL-1β (cat. no.
H002), IL-12 (cat. no. H010) and CCL25 (cat. no. H458-1), as well
as LDL (cat. no. A113-1-1), very-LDL (VLDL; cat. no. H249) and
high-density lipoprotein (HDL; cat. no. A112-1-1) levels, according
to the manufacturer's protocols.
Flow cytometric analysis of M1
macrophages
The blood samples were pre-treated with 1% heparin
(Sigma-Aldrich; Merck KGaA) for anticoagulation within 30 min and
then centrifuged at 4˚C for 30 min (1,000 x g). The detection of M1
macrophages was performed using MojoSort™ human pan monocyte
isolation kit (BioLegend, Inc.) according to the manufacturer's
instructions. Blood samples were diluted with PBS and gently placed
on the separating liquid. After stratification, the second layer
was extracted and washed with PBS to obtain mononuclear cells. The
collected cells were then resuspended in a combined medium (1640
medium containing 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA), added to GM-CSF cytokines (MojoSort™) to a final
concentration of 10 ng/ml) and cultured at 37˚C in an atmosphere
containing 5% CO2 for 7 days. The M1 macrophages were
labeled with CD206-A-phycoerythrin (cat. no. SAB4700690; 1:500) and
CD163-A-fluorescein isothiocyanate (cat. no. F3668; 1:500)
(Sigma-Aldrich; Merck KGaA) and incubated at 4˚C for 30 min. After
incubation, the cells were washed twice with PBS and resuspended in
300 ml PBS. The phenotypes of cells were detected using a BD
FACSVerse™ Flow Cytometer (BD Biosciences) and analyzed using Cell
Quest Pro 5.1 (BD Biosciences) (16).
Histological evaluation and lipid
accumulation
The histological evaluation was performed for each
group based on a previously published method using hematoxylin
& eosin (H&E) staining (17). The coronary arteries and thoracic
aorta samples were fixed in 10% formalin for 12 h at 4˚C, and then
embedded in paraffin for subsequent sectioning. The 5-µm specimens
were stained with H&E for 5 min at room temperature for
histological evaluation, and then stained with oil red O for 10 min
at room temperature for lipid accumulation detection, respectively.
The lesion change was observed using a light microscope
(magnification, x20).
Immunohistochemistry
The protein expression levels of HOXA9 in coronary
arteries and thoracic aorta samples were evaluated by
immunohistochemical methods based on a previous publication
(18). The sample preparation
procedure was the same as aforementioned, after which 5-µm
specimens were embedded in paraffin. The sections were blocked with
3% H2O2 for 10 min at room temperature to
remove endogenous peroxidase. After further blocking with PBS
containing 1.5% normal horse serum (Sigma-Aldrich; Merck KGaA) at
room temperature for 45 min, the sections were incubated with the
primary antibody anti-HOXA9 (Abcam; cat. no. ab191178; 1:1,000)
overnight at 4˚C. This was followed by the addition of secondary
HRP-conjugated rabbit anti-rat IgG (cat. no. ab6734; 1:1,000) for
30 min at room temperature. The scoring was conducted according to
a previous study on an Olympus GX51 light microscope. The score was
determined according to positive staining percentage (PSP) and
calculated as follows: 0, PSP<5%; 1, PSP<5-25%; 2,
PSP<25-50%; 3, PSP<50-75%; 4, PSP>75% (18).
Western blotting
The coronary arteries and thoracic aorta samples
were primarily homogenized on ice using RIPA buffer (Sigma-Aldrich;
Merck KGaA). After being completely lysed, the sample homogenate
was centrifuged at 12,000 x g for 15 min at 4˚C. The supernatant
was preserved for protein quantification using a BCA kit and 15 µg
protein/lane was separated by polyacrylamide gel electrophoresis.
The separated proteins were subsequently transferred onto
polyvinylidene difluoride membranes at 25 V for 30 min and blocked
in 5% non-fat milk for 50 min at room temperature. The membranes
were incubated with primary antibodies overnight at 4˚C. The
primary antibodies obtained from Abcam were as follows: Anti-HOXA9
(cat. no. ab191178; 1:1,000), anti-PF4 (cat. no. ab129090;
1:1,000), anti-E-selectin (cat. no. ab18981; 1:1,000), anti-VCAM-1
(cat. no. ab134047; 1:1,000) and anti-β-actin (cat. no. ab8226;
1:1,000). After washing with TBS-Tween (0.1%) in triplicate, the
membranes were incubated with goat anti-rat IgG secondary antibody
(Abcam; cat. no. ab150165; 1:5,000) for 1 h at 37˚C. Protein bands
were detected using enhanced chemiluminescent reagent (Santa Cruz
Biotechnology, Inc.). The density of blots for proteins were
normalized to β-actin using ImageJ 2.0 software (National Institute
of Health).
Statistical analysis
The experiments were performed in triplicate and all
statistical data are expressed as the mean ± standard deviation.
Comparisons between groups were made for data analyses using
GraphPad Prism 7.0 (GraphPad Software, Inc.). Comparisons between
multiple groups were performed by one-way ANOVA followed by
Bonferroni's post hoc multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
HOXA9 inhibitors reduce the levels of
inflammatory cytokines and upregulate HDL levels in AS rats
Vascular inflammation may be an important indicator
of AS modeling in rats (19). The
successfully constructed inhibition or silencing models were
verified using western blotting (Fig.
S1). ELISAs were used to detect the levels of inflammatory
cytokines in the blood of rats. As shown in Fig. 1A-C, the levels of inflammatory
cytokines TNF-α, IL-1β and IL-12 were significantly increased in
the AS group compared with those in the normal control (NC) group.
The levels of chemokine CCL25 were also significantly upregulated
in the AS group compared with the NC group (Fig. 1D), which indicated the success of
the AS model. The levels of LDL and VLDL were increased, and HDL
was decreased in AS rats compared with the NC group, whereas
inhibition or silencing of HOXA9 demonstrated opposite effects
(Fig. 1E-G). The injection of HOXA9
inhibitors or si-HOXA9 markedly decreased the levels of these
cytokines, as well as LDL and VLDL, in AS model rats, but were
unable to completely restore them to normal levels.
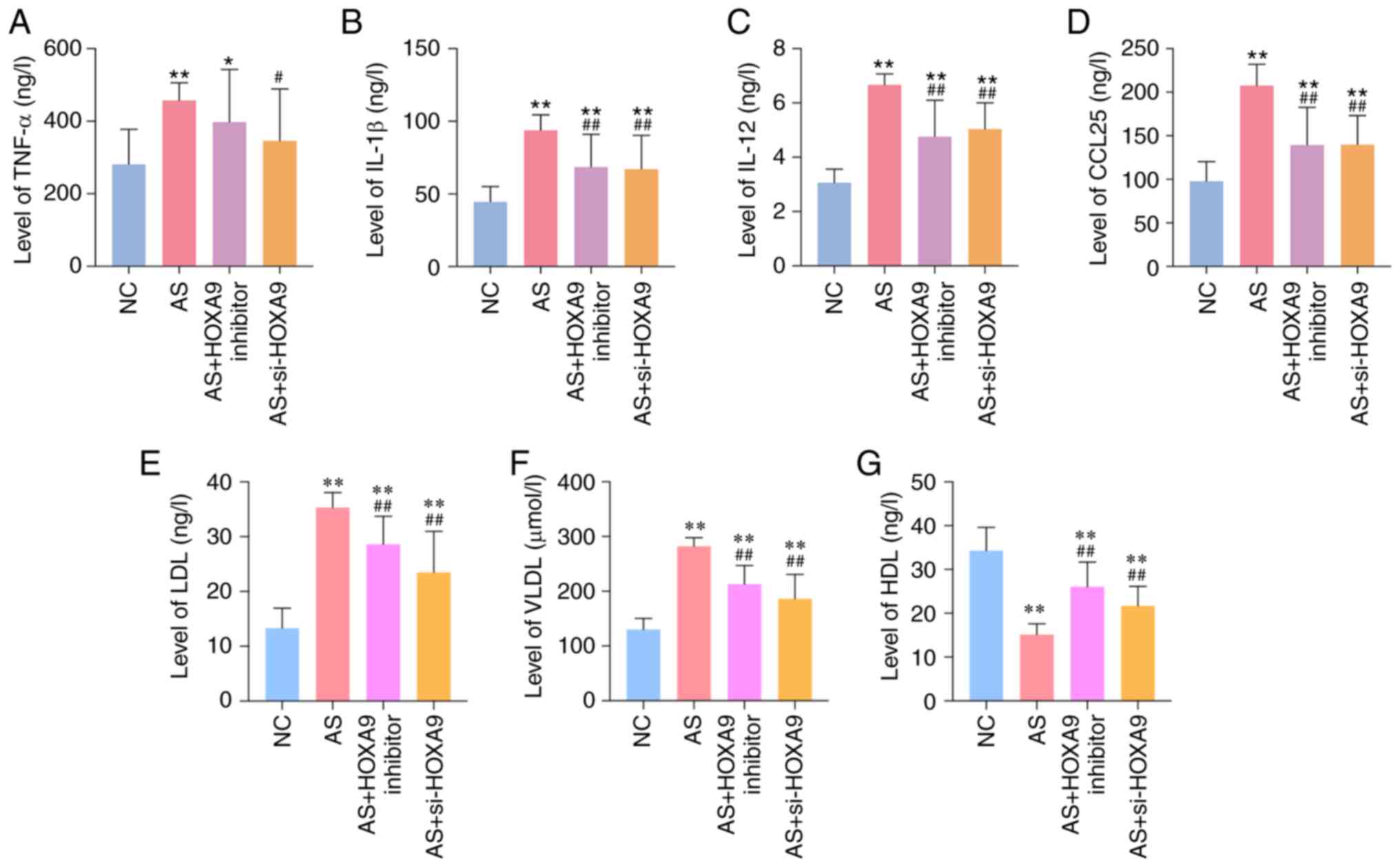 | Figure 1HOXA9 inhibitors downregulate the
levels of (A) TNF-α, (B) IL-1β, (C) IL-12, (D) CCL25, (E) LDL, (F)
VLDL and (G) HDL in blood samples of AS rats, as determined using
ELISAs. *P<0.05, **P<0.01 vs. NC group;
#P<0.05, ##P<0.01 vs. AS group. AS,
atherosclerosis; NC, normal control; si, small interfering; HOXA9,
homeobox A9; CCL25, C-C motif chemokine ligand 25; LDL, low-density
lipoprotein; HDL, high-density lipoprotein; VLDL, very-low-density
lipoprotein. |
HOXA9 inhibitors decrease the content
of M1 macrophages in AS rats
The content of M1 macrophages may directly reflect
the inflammatory status of vessels, thus acting as another
indicator of AS (20). Flow
cytometry revealed that the content of M1 macrophages in the AS
group was 73.7%, which was significantly higher than that of the NC
group (Fig. 2). The observed
increase was reversed when the rats were treated with a HOXA9
inhibitor or si-HOXA9. However, si-HOXA9 exhibited a larger
reduction in M1 macrophage content when compared with the effects
of the HOXA9 inhibitor. Taken together, these data suggested that
HOXA9 inhibitors may reduce the content of M1 macrophages in AS
rats.
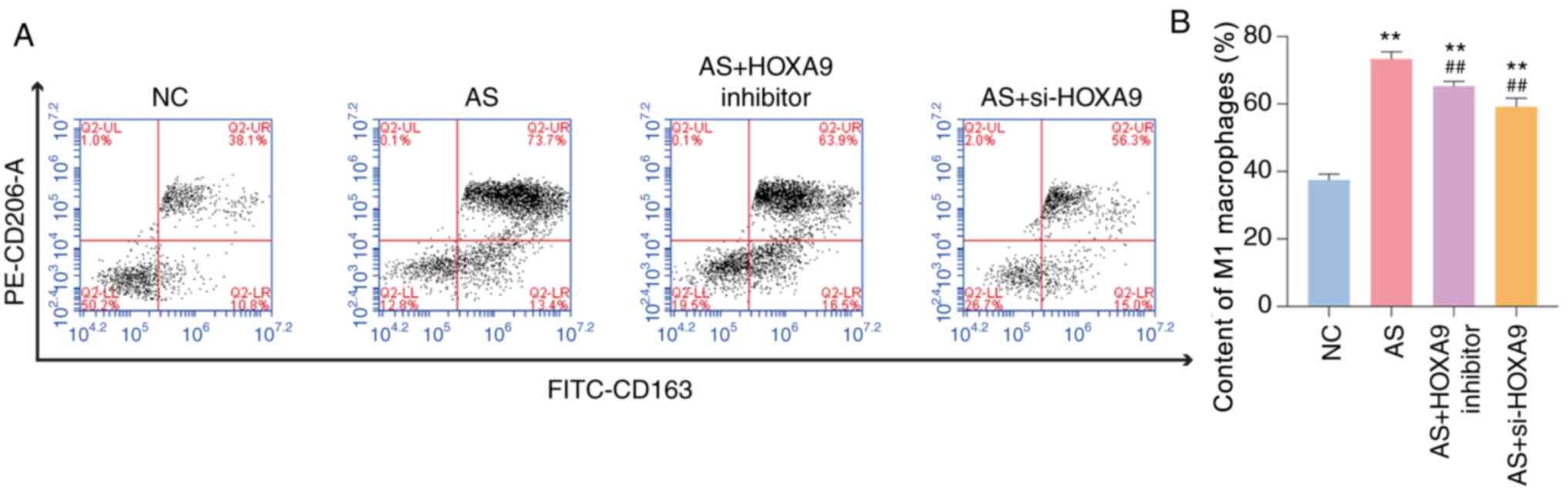 | Figure 2HOXA9 inhibitors decrease the content
of M1 macrophages in blood samples of AS rats, as determined using
flow cytometry. (A) Monocyte content in NC group, AS group, AS +
HOXA9 inhibitor group and AS + si-HOXA9 group, as detected by flow
cytometry. (B) A statistical analysis of the content of M1
macrophages. The data in Q2-UR were analyzed.
**P<0.01 vs. NC group. ##P<0.01 vs. AS
group. AS, atherosclerosis; NC, normal control; si, small
interfering; HOXA9, homeobox A9; PE, phycoerythrin; FITC,
fluorescein isothiocyanate. |
HOXA9 inhibitors improve coronary
artery morphology and inhibit lipid formation in AS rats
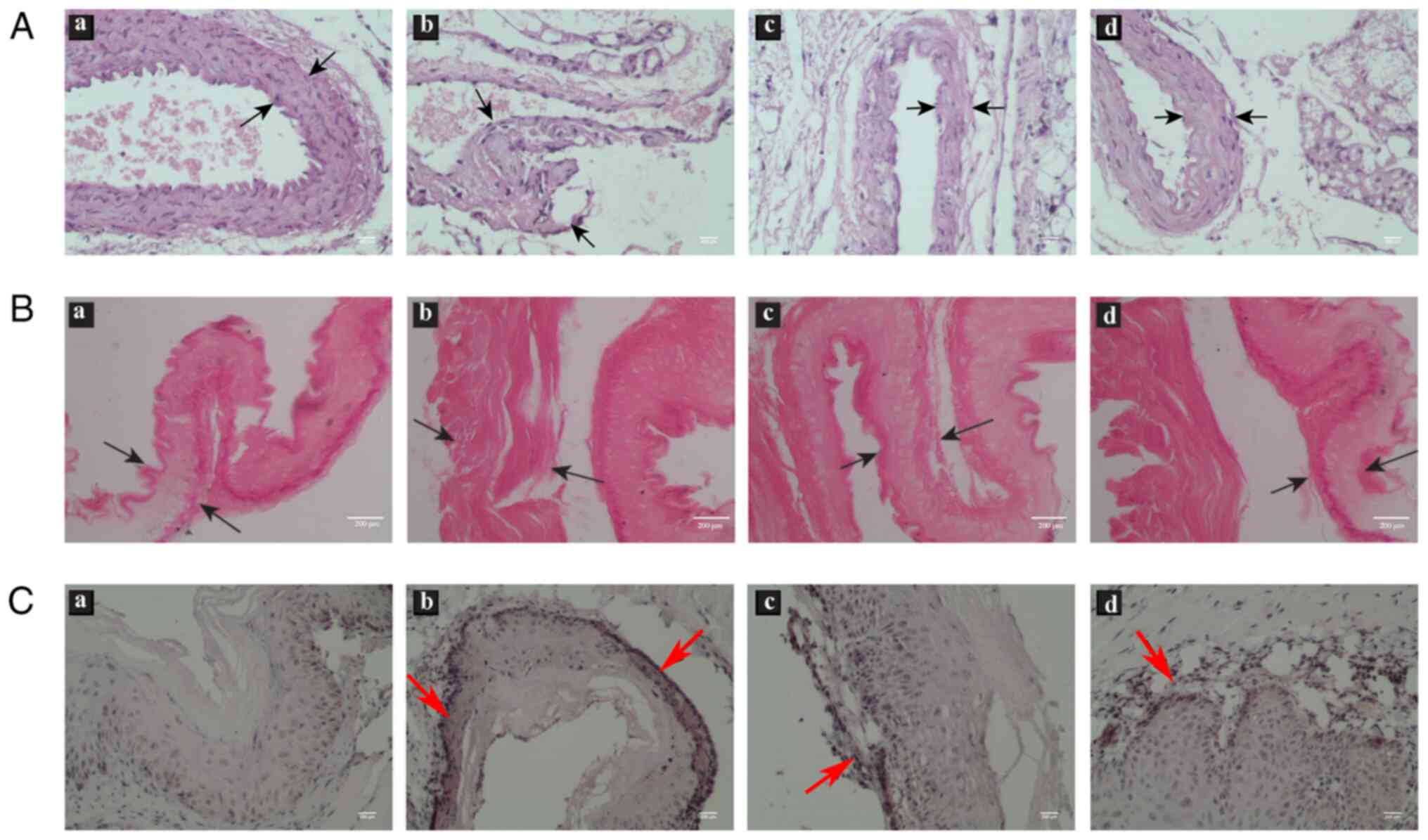
The coronary artery morphology of rats was observed
using H&E staining. In the NC group, the surface of the
vascular intima was smooth and complete, and VSMCs were arranged in
an orderly manner (Fig. 3A). In
comparison, the vascular intima was thickened and uplifted inward,
and ECs were ruptured in the AS group. After treatment with HOXA9
inhibitors or si-HOXA9, the thickness of the intima was markedly
diminished and no plaque was observed compared with that in AS rats
(Fig. 3A). Oil red O staining
revealed that the inhibition of HOXA9 markedly alleviated the lipid
formation compared with AS group (Fig.
3B). This evidence indicated that HOXA9 inhibitors may
alleviate symptoms of AS via relieving vascular injury and lipid
formation.
Expression of HOXA9 increases in
coronary arteries of AS rats
Immunohistochemical analysis was initially conducted
to examine the protein expression levels of HOXA9 in the coronary
arteries of rats. Higher levels of HOXA9 protein, localized mainly
in the wall of coronary arteries (depicted by black arrows), were
observed in the AS model rats when compared with that in the NC
group (Fig. 3C). The treatment of
rats with HOXA9 inhibitors or si-HOXA9 reduced the levels of HOXA9
protein compared with the AS group (Fig. 3C). Taken together, the qualitative
expression of HOXA9 detected by immunohistochemistry presented a
preliminary verification of the positive association between HOXA9
levels and coronary arteries morphology of AS rats.
HOXA9 inhibitors alleviate AS damage
by downregulating PF4 and E-selectin/VCAM-1 proteins
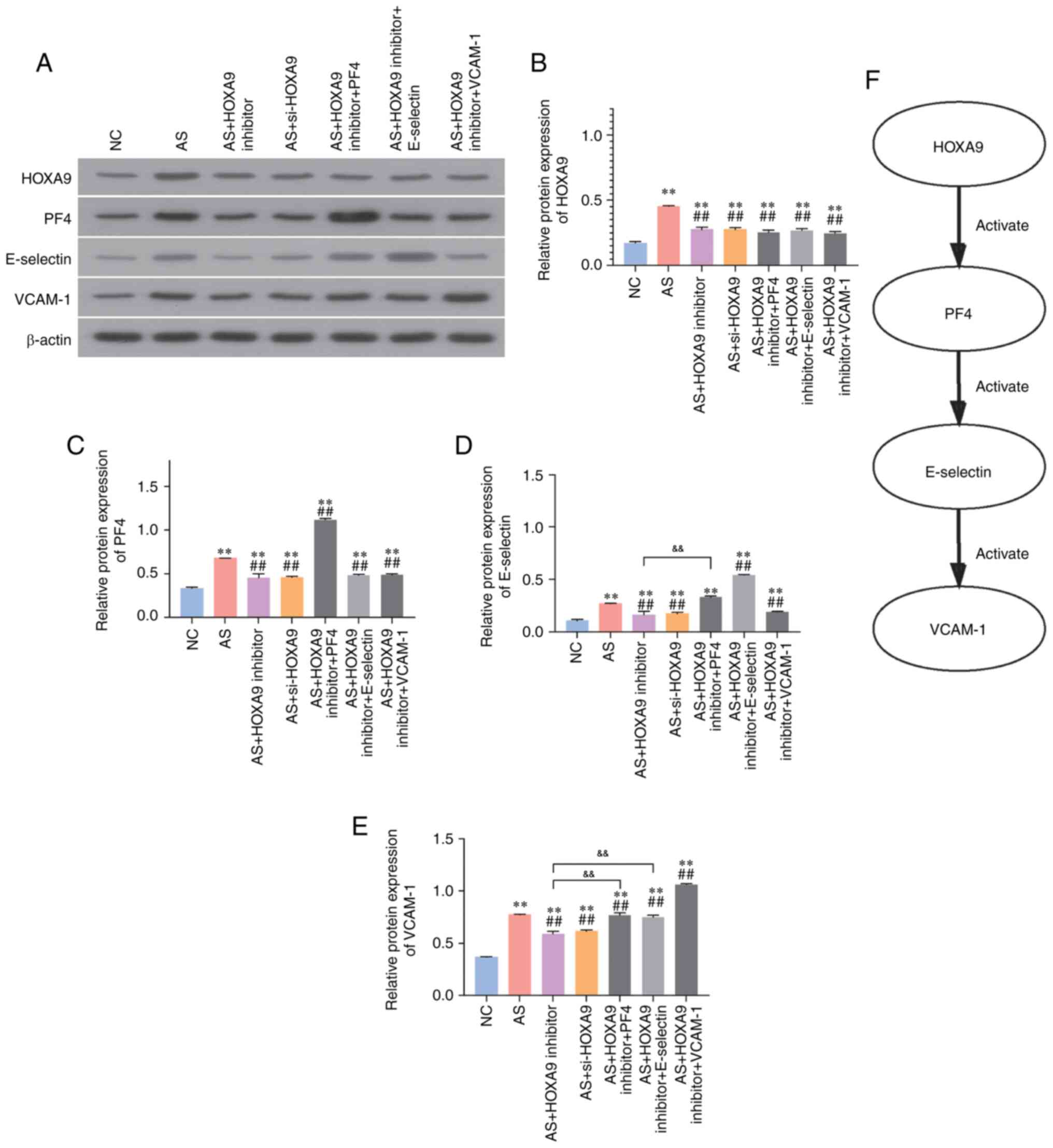
To investigate the mechanisms by which HOXA9
inhibitors alleviate AS symptoms, western blotting was employed to
detect the protein expression levels of HOXA9, PF4, E-selectin and
VCAM-1. The results revealed that the expression levels of HOXA9,
PF4, E-selectin and VCAM-1 were significantly upregulated in the AS
group compared with those in the NC group (Fig. 4). As shown in Fig. 4, the injection of HOXA9 inhibitors
or si-HOXA9 markedly decreased the expression levels of these
proteins. Overexpression of PF4 in the HOXA9 inhibitor group
significantly upregulated the expression of PF4, E-selectin and
VCAM-1. E-selectin overexpression resulted in no effects on the
levels of PF4, but increased the levels of E-selectin and VCAM-1.
Moreover, VCAM-1 overexpression upregulated VCAM-1, but had no
effects on PF4 and E-selectin. Therefore, these findings suggested
that HOXA9 inhibitors may alleviate AS damage through reducing the
levels of PF4, and subsequently downregulating E-selectin and
VCAM-1 (Fig. 4F).
Discussion
AS is a cardiovascular disease that results in high
morbidity and mortality rates. Therefore, it is necessary to study
the underlying pathological mechanisms in order to identify
potential treatments (21). The
progression of AS could be regarded as a chronic inflammatory
disease in arterial walls, where the expression levels of
pro-inflammatory cytokines and adhesive factors are upregulated. A
previous study demonstrated that microRNA-27 suppressed the lipid
accumulation and inflammatory response in apolipoprotein E-knockout
mice, suggesting that the inflammatory status in coronary arteries
could be regarded as an indicator of the progression of AS
(22).
HOXA9, a member of the HOX gene family, participates
in the differentiation of promyelocytic cells in the hematopoietic
system and is involved in cellular processes such as proliferation,
differentiation and apoptosis of ECs (23). It was reported that HOXA9-knockout
mice were deficient in the production of mature granulocytes and B
lymphocytes, and exhibited impaired T-cell differentiation with
increased apoptosis (23). To
investigate whether HOXA9 expression affects the development of AS,
the present study successfully constructed AS rat models through
oral feeding of a high-fat and high-cholesterol diet along with an
intravenous injection of vitamin D3. The results demonstrated that
inhibiting HOXA9 decreased the levels of inflammatory cytokines,
including TNF-α, IL-1β and IL-12. Consistent with the present
study, Trivedi et al (24)
demonstrated that HOXA9 participated in maintaining the inactivated
state of ECs and inhibited the expression of adhesive factors
induced by TNF-α through inhibiting nuclear factor (NF)-κB.
The present study also demonstrated that the levels
of CCL25 were downregulated following inhibition of HOXA9. As a
major mediator in macrophage chemotaxis, CCL25 has been shown to
specifically induce M1 macrophage chemotaxis, thus leading to the
further progression of AS (25).
Consistently, in the present study, the content of M1 macrophages
was increased in AS rats compared with the NC group, whereas
treatment with HOXA9 inhibitors resulted in a decrease in M1
macrophages. The observation of the morphology of coronary arteries
using H&E staining further supported these findings, suggesting
that HOXA9 inhibition alleviated the AS symptoms and improved the
blood microcirculation. In order to observe the morphology of
coronary arteries, a previous study implemented the use of a Leica
image analysis system. The size of plaques in coronary arteries of
AS rats was found to be significantly diminished using onion
extracts to suppress vascular inflammation (26).
The results of western blotting elucidated that
HOXA9 inhibitors downregulated the expression levels of PF4 and
adhesion factors E-selectin and VCAM-1. PF4 has been reported to be
upregulated in AS, and to participate in the accumulation of
monocytes and monocyte-derived cells, which can further express
inflammatory cytokines and induce expression of adhesion factors
(27). It has been demonstrated
that irbesartan attenuated TNF-α-induced expression of E-selectin,
ICAM-1 and VCAM-1 via suppression of the NF-κB pathway in human
umbilical vein endothelial cells (28). A previous study found that treatment
with β-thujaplicin counteracted AS symptoms by decreasing
E-selectin and VCAM-1(29). These
findings suggested that HOXA9 inhibitors may relieve the
progression of AS via suppression of PF4 and E-selectin/VCAM-1
protein expression, supporting the potential use of HOXA9
inhibitors in the therapy of AS.
In the present study, HOXA9 inhibition resulted in
both a decrease in the inflammatory status and an improvement in
the microcirculation of coronary arteries in AS rats, via
downregulation of PF4, E-selectin and VCAM-1 protein expression.
Thus, the inhibition of HOXA9 may have potential as a treatment
option for AS; however, further studies are needed to confirm
this.
Supplementary Material
Verification of inhibitor and
knockdown efficiency of HOXA9. Protein expression was determined
using western blotting. **P<0.01 vs. NC group. NC,
normal control; AS, atherosclerosis; si, small interfering; HOXA9,
homeobox A9.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, JG and SW conducted experiments, analyzed data
and assisted with the drafting or revision of the manuscript. SL
and JG confirm the authenticity of the raw data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Shengli Oilfield Central Hospital (approval no.
2019051).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Libby P, Lichtman AH and Hansson GK:
Immune effector mechanisms implicated in atherosclerosis: From mice
to humans. Immunity. 38:1092–1104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kwon GP, Schroeder JL, Amar MJ, Remaley AT
and Balaban RS: Contribution of macromolecular structure to the
retention of low-density lipoprotein at arterial branch points.
Circulation. 117:2919–2927. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bäck M and Hansson GK: Anti-inflammatory
therapies for atherosclerosis. Nat Rev Cardiol. 12:199–211.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Herrero-Fernandez B, Gomez-Bris R,
Somovilla-Crespo B and Gonzalez-Granado JM: Immunobiology of
atherosclerosis: A complex net of interactions. Int J Mol Sci.
20(5293)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barrett TJ: Macrophages in atherosclerosis
regression. Arterioscler Thromb Vasc Biol. 40:20–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Endothelial barrier and its abnormalities in cardiovascular
disease. Front Physiol. 6(365)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chistiakov DA, Melnichenko AA, Myasoedova
VA, Grechko AV and Orekhov AN: Mechanisms of foam cell formation in
atherosclerosis. J Mol Med (Berl). 95:1153–1165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bakogiannis C, Sachse M, Stamatelopoulos K
and Stellos K: Platelet-derived chemokines in inflammation and
atherosclerosis. Cytokine. 122(154157)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iademarco MF, Barks JL and Dean DC:
Regulation of vascular cell adhesion molecule-1 expression by IL-4
and TNF-alpha in cultured endothelial cells. J Clin Invest.
95:264–271. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cantile M, Schiavo G, Terracciano L and
Cillo C: Homeobox genes in normal and abnormal vasculogenesis. Nutr
Metab Cardiovasc Dis. 18:651–658. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Duboule D and Morata G: Colinearity and
functional hierarchy among genes of the homeotic complexes. Trends
Genet. 10:358–364. 1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gorski DH and Walsh K: The role of
homeobox genes in vascular remodeling and angiogenesis. Circ Res.
87:865–872. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bandyopadhyay S, Ashraf MZ, Daher P, Howe
PH and DiCorleto PE: HOXA9 participates in the transcriptional
activation of E-selectin in endothelial cells. Mol Cell Biol.
27:4207–4216. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pang J, Xu Q, Xu X, Yin H, Xu R, Guo S,
Hao W, Wang L, Chen C and Cao JM: Hexarelin suppresses high lipid
diet and vitamin D3-induced atherosclerosis in the rat. Peptides.
31:630–638. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng X and Hu X: Effects of rosuvastatin
on hypertension with carotid atherosclerosis and its influence on
peripheral macrophage polarization. Chinese Journal of Clinical
Pharmacology and Therapeutics. 22:1035–1039. 2017.
|
|
17
|
Wang J, Hou J, Lin C, Fu J, Ren J, Li L,
Guo H, Han X, Wang B and Liu J: Shuangshen ningxin capsule, a
traditional Chinese medicinal preparation, alleviates myocardial
ischemia through autophagy regulation. Evid Based Complement
Alternat Med. 2015(581260)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu B, Geerts D, Bu Z, Ai J, Jin L, Li Y,
Zhang H and Zhu G: Regulation of endometrial receptivity by the
highly expressed HOXA9, HOXA11 and HOXD10 HOX-class homeobox genes.
Hum Reprod. 29:781–790. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun X, He S, Wara AKM, Icli B, Shvartz E,
Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, et al:
Systemic delivery of microRNA-181b inhibits nuclear factor-κB
activation, vascular inflammation, and atherosclerosis in
apolipoprotein E-deficient mice. Circ Res. 114:32–40.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shirai T, Hilhorst M, Harrison DG, Goronzy
JJ and Weyand CM: Macrophages in vascular inflammation-From
atherosclerosis to vasculitis. Autoimmunity. 48:139–151.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nitsa A, Toutouza M, Machairas N, Mariolis
A, Philippou A and Koutsilieris M: Vitamin D in cardiovascular
disease. In Vivo. 32:977–981. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xie W, Li L, Zhang M, Cheng HP, Gong D, Lv
YC, Yao F, He PP, Ouyang XP, Lan G, et al: MicroRNA-27 prevents
atherosclerosis by suppressing lipoprotein lipase-induced lipid
accumulation and inflammatory response in apolipoprotein E knockout
mice. PLoS One. 11(e0157085)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Calvo KR, Sykes DB, Pasillas M and Kamps
MP: Hoxa9 immortalizes a granulocyte-macrophage colony-stimulating
factor-dependent promyelocyte capable of biphenotypic
differentiation to neutrophils or macrophages, independent of
enforced meis expression. Mol Cell Biol. 20:3274–3285.
2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Trivedi CM, Patel RC and Patel CV:
Homeobox gene HOXA9 inhibits nuclear factor-kappa B dependent
activation of endothelium. Atherosclerosis. 195:e50–e60.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xuan W, Qu Q, Zheng B, Xiong S and Fan GH:
The chemotaxis of M1 and M2 macrophages is regulated by different
chemokines. J Leukoc Biol. 97:61–69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li W, Tang C, Jin H and Du J: Effects of
onion extract on endogenous vascular H2S and adrenomedulin in rat
atherosclerosis. Curr Pharm Biotechnol. 12:1427–1439.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Domschke G and Gleissner CA: CXCL4-induced
macrophages in human atherosclerosis. Cytokine.
122(154141)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiang Y, Jiang LL, Maimaitirexiati XM,
Zhang Y and Wu L: Irbesartan attenuates TNF-α-induced ICAM-1,
VCAM-1, and E-selectin expression through suppression of NF-κB
pathway in HUVECs. Eur Rev Med Pharmacol Sci. 19:3295–3302.
2015.PubMed/NCBI
|
|
29
|
Shih MF, Pan KH, Liu CC, Shen CR and
Cherng JY: Treatment of β-thujaplicin counteracts
di(2-ethylhexyl)phthalate (DEHP)-exposed vascular smooth muscle
activation, inflammation and atherosclerosis progression. Regul
Toxicol Pharmacol. 92:333–337. 2018.PubMed/NCBI View Article : Google Scholar
|