Introduction
Advanced oxidation protein products (AOPPs) are
uremic toxins created during oxidative stress through the reaction
of plasma proteins with chlorinated oxidant. The AOPPs were found
in the plasma of patients with chronic renal failure in 1996 by
Witko-Sarsa et al (1). AOPPs
may serve as a novel marker of oxidative stress in uremia and
postmenopausal osteoporosis (2). It
was reported that AOPPs may induce the respiratory burst of
neutrophils and monocytes, and the damage of monocytes to produce
cytokines and endothelial cells, which is associated with disorders
of immune function and atherosclerosis in patients with chronic
renal failure (3-7).
Inflammatory bowel disease (IBD) is a common
gastrointestinal disease, which is difficult to treat. The
intestinal tract in a chronic inflammatory state often leads to
intestinal epithelial damage, which affects the quality of life of
patients (8). The serum level of
AOPPs was markedly increased in patients with IBD (9). AOPPs have been reported to deposit in
the intestinal lesions of patients with IBD and to promote the
synthesis of type I collagen in intestinal epithelial cells
(10). These studies suggest a
correlation between AOPPs and intestinal epithelial lesions.
However, in-depth studies regarding this are lacking.
Crypt epithelial cells in the intestinal tract have
a high potential to proliferate and differentiate, and serve a
major role in repairing the intestinal epithelium (11). In the present study, a sodium
hypochlorite modified method was used to produce oxidized rat serum
albumin (RSA) (12), which mimicked
the effects of AOPPs. The present study aimed to investigate the
effects of AOPPs on apoptosis and epithelial mesenchymal transition
(EMT) in rat crypt epithelial cells. Additionally, the potential
signaling pathways were determined.
Materials and methods
Preparation of AOPPs
AOPPs were prepared as previously described
(12,13). In brief, 100 mg RSA (EY-D0667; Yiyan
Biological Technology) was dissolved in 5 ml sterile water, with
73.1 µl 10% sodium hypochlorite under stirring conditions at room
temperature for 30 min, and dialyzed at 4˚C in PBS for 24 h. The
dialysate was changed every two hours, filtered and sterilized, and
kept at -20˚C for use. RSA without sodium hypochlorite modification
was weighed and dissolved in 5 ml sterile water, filtered and
sterilized, and stored at -20˚C as a control.
Treatment with AOPPs and determination
of apoptosis
Crypt epithelial IEC-6 cells were obtained from Bena
BIOTECH (bncc338482) and cultured in Dulbecco's modified Eagle's
medium supplemented with 10% FBS (HyClone; GE Healthcare Life
Sciences) in a 37˚C incubator with 5% CO2.
The optimal concentrations of AOPPs were selected
based on the aforementioned experiments. IEC-6 cells were divided
into three groups: Normal control, AOPPs and RSA groups. Three
groups of cells were collected after 2 h of treatment, and the
phosphorylation level of p65 in Akt and NF-κB was detected by
western blotting. After 72 h of treatment, the cells were collected
and the apoptotic rate was detected by flow cytometry. The
expression of E-cadherin, fibronectin, snail, slug and collagen I
associated with EMT was detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting.
The cells in the exponential growth phases were
cultured in a six-well plate. Following treatment with different
concentrations of AOPPs (100, 200 or 400 µg/ml) or RSA (100, 200 or
400 µg/ml) for 48 or 72 h, the cells were collected. The apoptotic
rate was determined by flow cytometry using Annexin V-APC and
7-aminoactinomycin D (7-AAD). The supernatant was discarded, and
the cells were washed twice with 1 ml PBS for each well. A total of
200 µl trypsin with EDTA was added into each well in a 37˚C
incubator for digestion. The cells were collected following
centrifugation (2,000 x g for 3 min at room temperature). A total
of 3 µl Annexin V-APC and 5 µl 7-AAD were added to each tube
respectively and incubated at room temperature in the dark for 10
min. Subsequently, the apoptotic rate was detected using a flow
cytometer.
RT-qPCR
Total RNA was extracted from each group of cells
using an Ultrapure RNA Extract kit (CW0581M; CWBio). After RNA was
extracted, cDNA was synthesized according to the reverse
transcription kit (CW2569M; CWBio). cDNA was used as a template and
detected on the fluorescence quantitative PCR using UltraSYBR
Mixture (CW0957M; CWBio). The thermocycling conditions were as
follows: Initial denaturation at 95°C for 10 min,
followed by 40 cycles of PCR at 95°C for 10 sec,
60°C for 30 sec and 72°C for 30 sec. The
expression levels of E-cadherin, fibronectin, snail, slug and
collagen were detected by qPCR in each group, which were normalized
to GAPDH, as previously described (14) using the the 2-ΔΔCq method
(15). The primers are listed in
Table I.
 | Table IPrimer sequences of the genes. |
Table I
Primer sequences of the genes.
| Gene | Primer sequence
(5'-3') | Primer length,
bp | Product length,
bp | Annealing
temperature, ˚C |
|---|
| E-cadherin
forward |
FACTCTTCTCCTGGTCCTGTCA | 21 | 240 | 58.4 |
| E-cadherin
reverse |
CTCTAAGTCCTTTCTTGGTTGC | 22 | | |
| Fibronectin
forward |
CTATTTACCAACCCCAGACCC | 21 | 87 | 58.6 |
| Fibronectin
reverse |
GCATTCCCACAGAGTAGACCA | 21 | | |
| Snail
forward |
ATGAGGACAGTGGCAAAAGC | 20 | 113 | 56.9 |
| Snail
reverse |
CGGGAAGGCAATGAAGG | 17 | | |
| Slug
forward |
CAACTACAGCGAACTGGACAC | 21 | 207 | 58.2 |
| Slug
reverse |
ACACGCCCCAAAGATGAG | 18 | | |
| Collagen I
forward |
GCCTGAGCCAGCAGATTGA | 19 | 258 | 58.9 |
| Collagen I
reverse |
GCTTCTTCTCCTTGGGGTTT | 20 | | |
| GAPDH
forward |
CAACGGGAAACCCATCACCA | 20 | 96 | 62 |
| GAPDH
reverse |
ACGCCAGTAGACTCCACGACAT | 20 | | |
Western blotting
Protein was extracted using RIPA buffer (89901;
Thermo Fisher Scientific, Inc.) and the concentration was
determined using the bicinchoninic acid method (CW0014S; CWBio).
The proteins were denatured at 100˚C. A total of 20 µg protein in
each group was run on 10% sodium dodecyl sulfate-polyacrylamide
gels and transferred onto nitrocellulose membranes. The membranes
were then blocked with 5% skimmed milk for 30 min at room
temperature, followed by an incubation with the primary antibodies
at 4˚C overnight. The primary antibodies used were against
anti-E-cadherin (1:500; AF0131; Affinity Biosciences),
anti-Fibronectin (1:1,000; ab32419; Abcam), anti-Snail (1:500;
AF603; Affinity Biosciences), anti-Slug (1:500; AF4002; Affinity
Biosciences), anti-Collagen I (1:500; AF7001; Affinity
Biosciences), anti-pan-Akt (1:500; ab8805; Abcam), anti-NF-κB p65
(1:1,000; bs-0465R; BIOSS), anti-phospho-NF-κB p65 (1:500; AF2006;
Affinity) and anti-phospho-Akt (1:500; bs-2720R; BIOSS).
Subsequently, the membrane was incubated with a horseradish
peroxidase (HRP)-labeled goat anti-rabbit IgG secondary antibody
(cat. no. 65-6120; Thermo Fisher Scientific, Inc.) and a
HRP-labeled goat anti-mouse IgG secondary antibody (cat. no. 31430;
Thermo Fisher Scientific, Inc.) at room temperature for 1-2 h.
Enhanced chemiluminescence solution (cat. no. SW2010-1; Beijing
Solarbio Science & Technology Co., Ltd.) was added to the
membrane and exposed in the gel imaging system. The gray values of
antibody bands were analyzed by ‘Quantity one’ software v4.6
(Bio-Rad Laboratories, Inc.).
Statistical analysis
All data were statistically analyzed using SPSS 19.0
(IBM Corp.) and significant differences were determined by one-way
analysis of variance, followed by Tukey's post hoc tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
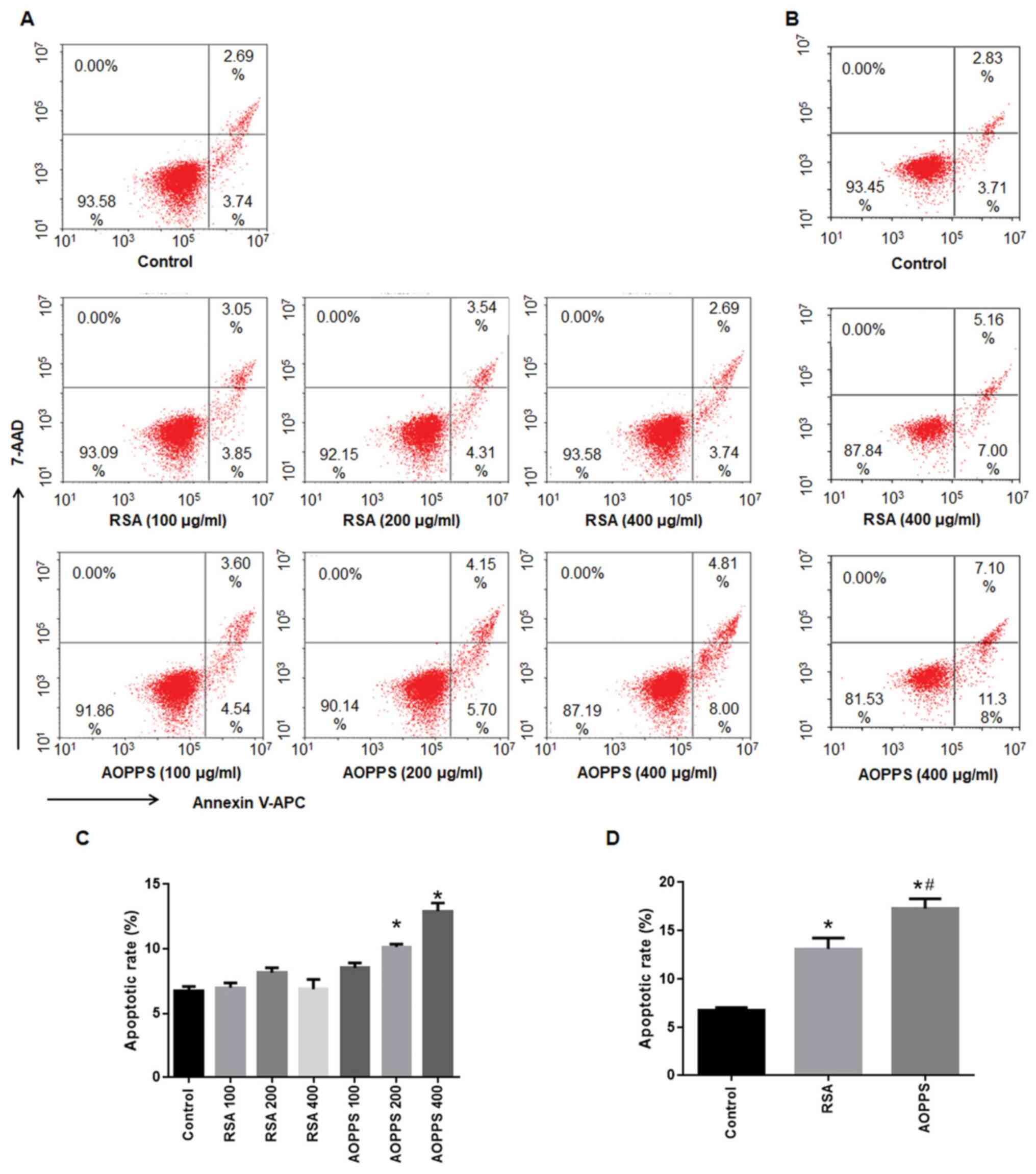
AOPPs promotes the apoptosis of IEC-6
cells
The apoptotic rates of IEC-6 cells following
treatment with AOPPs or RSA for 48 and 72 h are shown in Fig. 1. The apoptotic rate of IEC-6 cells
treated with AOPPs increased with the increased concentration of
AOPPs for 48 h (from 8.5-12.9%), while treatment with similar
concentrations of RSA for 48 h did not cause apoptosis in IEC-6
cells (from 6.5 to 8.1%; Fig. 1A
and C). By contrast, treatment with
400 µg/ml AOPPs or RSA for 72 h significantly induced apoptosis in
IEC-6 cells (compared with the normal group, P<0.05).
Furthermore, the apoptotic rate in the AOPPs treatment group was
higher than that in the RSA treatment group (P<0.05; Fig. 1B and D).
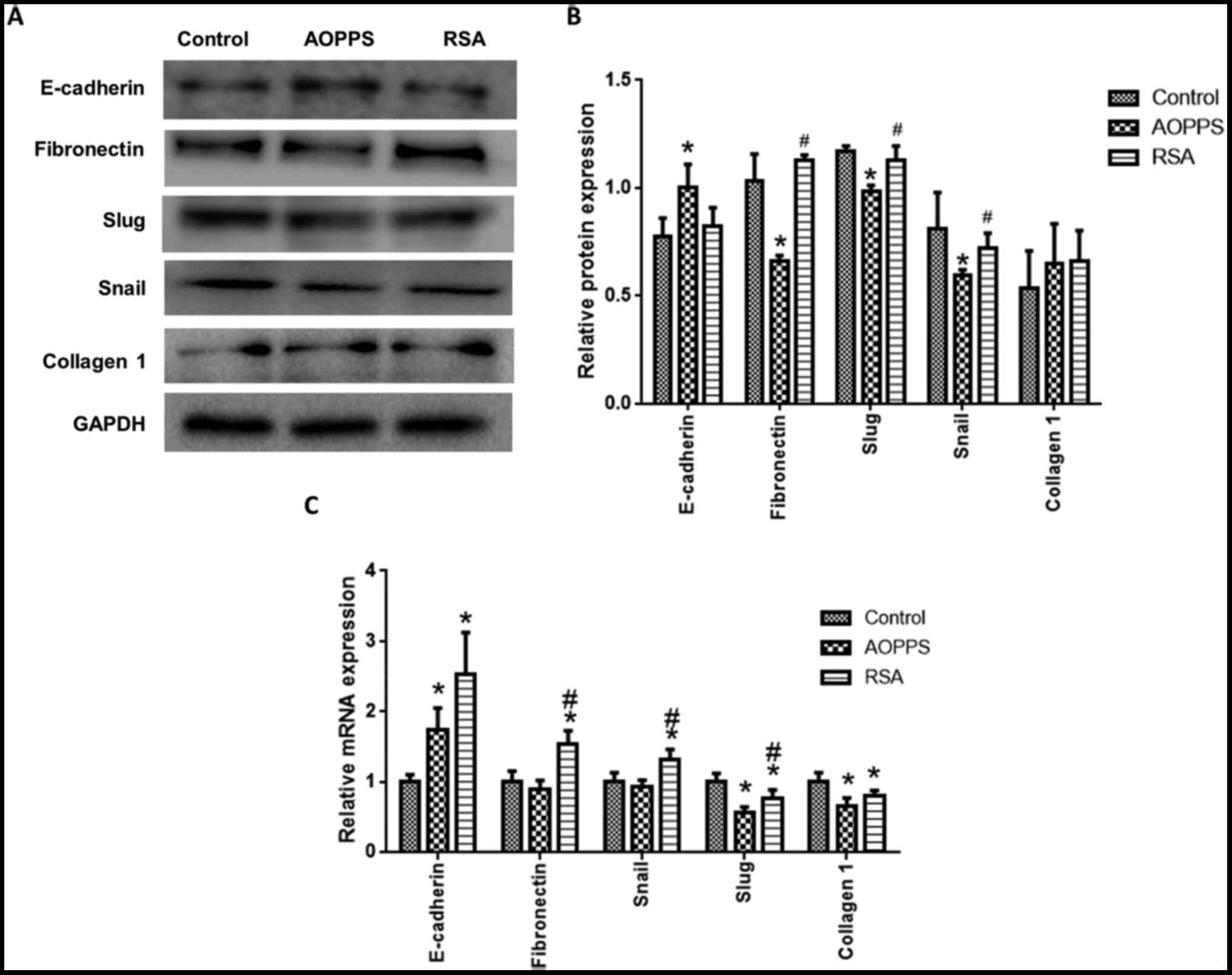
AOPPs inhibits the EMT of rat crypt
epithelial cells
EMT-related protein expression was detected in rat
crypt epithelial cells treated with AOPPs and RSA for 72 h. The
protein expression of fibronectin, snail and slug in the AOPPs
group was lower than that in the control and RSA groups, while the
expression of E-cadherin was promoted by treatment with AOPPs (vs.
normal, P<0.05; Fig. 2A and
B). Collagen I was not
significantly different among the groups (Fig. 2A and B). The mRNA expression of fibronectin,
snail, slug, E-cadherin and Collagen I was also detected (Fig. 2C). Consistent with the protein
expression results, AOPPs decreased slug expression and promoted
E-cadherin expression, compared with the control groups. By
contrast, AOPPs did not affect fibronectin and snail expression.
RSA treatment promoted E-cadherin, fibronectin and snail
expression, but decreased slug and collagen I expression (Fig. 2C).
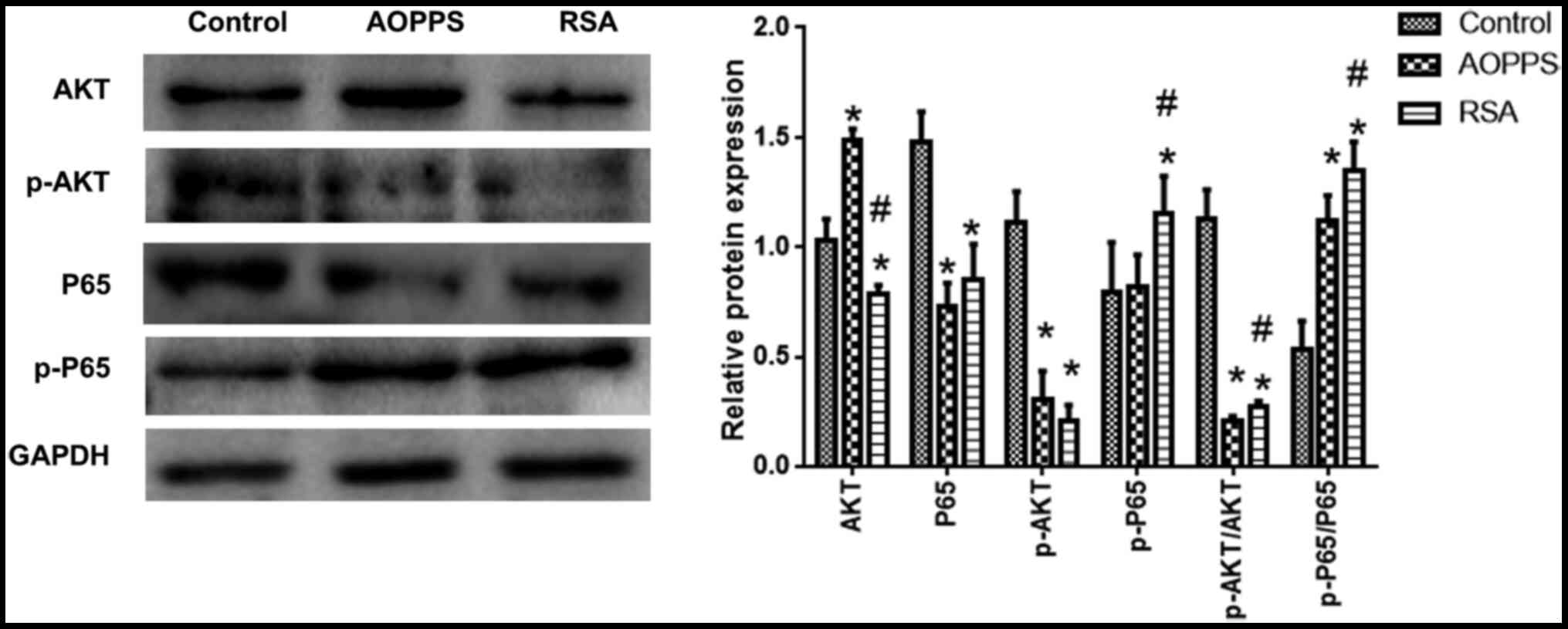
AOPPs decreases the phosphorylation of
Akt and promotes the phosphorylation of P65
The phosphorylation of Akt and NF-κB levels was
detected. Compared with the control group, Akt phosphorylation
levels in the AOPPs- and RSA-treated groups was decreased, while
NF-p65κB phosphorylation was increased (vs. normal, P<0.05). Akt
and p65 phosphorylation in the RSA treatment group was slightly
higher than that in the AOPPs treatment group (Fig. 3).
Discussion
The results of the present study suggested that
AOPPs promoted apoptosis and prohibited EMT in crypt epithelial
cells. Furthermore, it was demonstrated that AOPPs likely exhibited
these functions through the Akt and NF-κB signaling pathways. The
present study reported novel effects of AOPPs on the biological
activities of crypt epithelial cells.
It has been reported that AOPPs may increase
reactive oxygen species (ROS) production in IEC-6 cells, activate
the c-JNK signaling pathway, and induce apoptosis in IEC-6 cells
(16). The results of the present
study suggested that the apoptotic rate of IEC-6 cells treated with
AOPPs increased with the increasing concentration of AOPPs over a
48 h period. By contrast, similar concentrations of RSA treated for
48 h did not cause apoptosis in IEC-6 cells. Moreover, the
apoptotic rate of AOPPs- or RSA-treated cells at 72 h increased
compared with that of 48 h, and the apoptotic rate of the AOPPs
treatment group was higher than that of RSA treatment group. It can
be inferred that AOPPs deposited in intestinal lesions may inhibit
the recovery of intestinal epithelial cells, and serum albumin
alone may promote the apoptosis of intestinal epithelial cells
under the conditions of IBD and other diseases (17,18).
Therefore, it can be concluded that the common symptoms of IBD,
intestinal hemorrhage, may worsen the disease.
AOPPs may activate the TGF-β/Smad signaling pathway
by inducing ROS, which leads to the upregulation of EMT in liver
cells (19). It has also been found
that AOPPs may upregulate the inflammatory response of
osteoblast-like cells and activate the NF-κB signaling pathway, in
order to inhibit the proliferation and differentiation of
osteoblast-like cells (20). The
phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway is one
of the main pathways regulating cell proliferation and apoptosis
(21), and Akt phosphorylation
level is a key indicator reflecting the activity of the PI3K/Akt
signaling pathway (22,23). NF-κB is composed of P50 and p65
proteins, and p65 phosphorylation level is a key index reflecting
the activity of the NF-κB signaling pathway (24,25).
The present study reported that the Akt phosphorylation level
decreased and the p65 phosphorylation level increased in AOPPs- or
RSA-treated cells, which corresponded with the increase in the
apoptotic rate. However, although the apoptotic rate of cells in
the RSA treatment group was lower, the phosphorylation level of p65
was higher than that in AOPPs treatment group, which suggested that
RSA may activate the inflammatory response associated with the
NF-κB signal pathway of intestinal epithelial cells, and the
apoptosis of intestinal epithelial cells induced by AOPPs was not
only controlled by the NF-κB signaling pathway.
EMT refers to the process through which epithelial
cells change into mesenchymal cells. The mesenchymal cells produced
by EMT are mainly fibroblasts; therefore, EMT is an important
mechanism leading to tissue fibrosis (26,27).
In the present study, the effects of AOPPs on the EMT of intestinal
epithelial cells were analyzed by detecting the expression of
E-cadherin, fibronectin, snail, slug and collagen I (28,29).
The results of the present study suggested that the expression of
E-cadherin in IEC-6 cells treated with AOPPs was higher, while
fibronectin was lower than that in the control group. These data
indicated that AOPPs decreased the EMT of IEC-6 cells. In addition,
transcription factors promoting EMT phenotype transformation,
including snail and slug, were also lower in APPPs-treated cells.
These results further suggested that AOPPs prohibited EMT, unlike
RSA. The present study reported that the mRNA expression of
EMT-related genes was inconsistent with the protein expression.
These data suggested that AOPPs regulated the EMT at the
translation level. Considering that the fibrosis of skin tissue
will form scars, which is helpful for skin healing and has a
certain protective effect, the inhibition of EMT of intestinal
epithelial cells by AOPPs may be one of the reasons for IBD
intestinal bleeding (30).
In conclusion, AOPPs promote apoptosis and inhibit
the EMT of rat crypt epithelial cells, which may be associated with
the inhibition of Akt phosphorylation and the promotion of p65
phosphorylation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Fujian Natural
Science Foundation (grant no. 2016J01504).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, JTZ, XYW, HXH and LXH performed the experiments
and analyzed the data. YZ, LXH and CQZ designed the study and wrote
the manuscript. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Witko-Sarsat V, Friedlander M,
Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers
P and Descamps-Latscha B: Advanced oxidation protein products as a
novel marker of oxidative stress in uremia. Kidney Int.
49:1304–1313. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu Q, Zhong ZM, Pan Y, Zeng JH, Zheng S,
Zhu SY and Chen JT: Advanced oxidation protein products as a novel
marker of oxidative stress in postmenopausal osteoporosis. Med Sci
Monit. 21:2428–2432. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Witko-Sarsat V, Gausson V, Nguyen AT,
Touam M, Drüeke T, Santangelo F and Descamps-Latscha B:
AOPP-induced activation of human neutrophil and monocyte oxidative
metabolism: A potential target for N-acetylcysteine treatment in
dialysis patients. Kidney Int. 64:82–91. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Witko-Sarsat V, Friedlander M, Nguyen Khoa
T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers
P, Drüeke T and Descamps-Latscha B: Advanced oxidation protein
products as novel mediators of inflammation and monocyte activation
in chronic renal failure. J Immunol. 161:2524–2532. 1998.PubMed/NCBI
|
|
5
|
Kaneda H, Taguchi J, Ogasawara K, Aizawa T
and Ohno M: Increased level of advanced oxidation protein products
in patients with coronary artery disease. Atherosclerosis.
162:221–225. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou LL, Cao W, Xie C, Tian J, Zhou Z,
Zhou Q, Zhu P, Li A, Liu Y, Miyata T, et al: The receptor of
advanced glycation end products plays a central role in advanced
oxidation protein products-induced podocyte apoptosis. Kidney Int.
82:759–770. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Z, Yao X, Jiang W, Li W, Zhu S, Liao
C, Zou L, Ding R and Chen J: Advanced oxidation protein products
induce microglia-mediated neuroinflammation via MAPKs-NF-κB
signaling pathway and pyroptosis after secondary spinal cord
injury. J Neuroinflammation. 17(90)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Derkacz A, Olczyk P and Komosinska-Vassev
K: Diagnostic markers for nonspecific inflammatory bowel diseases.
Dis Markers. 2018(7451946)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Krzystek-Korpacka M, Neubauer K, Berdowska
I, Boehm D, Zielinski B, Petryszyn P, Terlecki G, Paradowski L and
Gamian A: Enhanced formation of advanced oxidation protein products
in IBD. Inflamm Bowel Dis. 14:794–802. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Balmus IM, Ciobica A, Trifan A and Stanciu
C: The implications of oxidative stress and antioxidant therapies
in inflammatory bowel disease: Clinical aspects and animal models.
Saudi J Gastroenterol. 22:3–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gunther C, Neumann H, Neurath MF and
Becker C: Apoptosis, necrosis and necroptosis: Cell death
regulation in the intestinal epithelium. Gut. 62:1062–1071.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Witko-Sarsat V, Nguyen-Khoa T, Jungers P,
Drueke TB and Descamps-Latscha B: Advanced oxidation protein
products as a novel molecular basis of oxidative stress in uraemia.
Nephrol Dial Transplant. 14 (Suppl 1):S76–S78. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marsche G, Frank S, Hrzenjak A, Holzer M,
Dirnberger S, Wadsack C, Scharnagl H, Stojakovic T, Heinemann A and
Oettl K: Plasma-advanced oxidation protein products are potent
high-density lipoprotein receptor antagonists in vivo. Circ Res.
104:750–757. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu G, Li J, He L, Wang X and Hong X:
MPTP-induced changes in hippocampal synaptic plasticity and memory
are prevented by memantine through the BDNF-TrkB pathway. Br J
Pharmacol. 172:2354–2368. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun S, Xie F, Zhang Q, Cui Z, Cheng X,
Zhong F, He K and Zhou J: Advanced oxidation protein products
induce hepatocyte epithelial-mesenchymal transition via a
ROS-dependent, TGF-beta/Smad signaling pathway. Cell Biol Int.
41:842–853. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schulzke JD, Ploeger S, Amasheh M, Fromm
A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M and
Fromm M: Epithelial tight junctions in intestinal inflammation. Ann
N Y Acad Sci. 1165:294–300. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Low D, Mino-Kenudson M and Mizoguchi E:
Recent advancement in understanding colitis-associated
tumorigenesis. Inflamm Bowel Dis. 20:2115–2123. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu X, Sun S, Xie F, Ma J, Tang J, He S and
Bai L: Advanced oxidation protein products induce
epithelial-mesenchymal transition of intestinal epithelial cells
via a PKC delta-mediated, redox-dependent signaling pathway.
Antioxid Redox Signal. 27:37–56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhong ZM, Bai L and Chen JT: Advanced
oxidation protein products inhibit proliferation and
differentiation of rat osteoblast-like cells via NF-kappaB pathway.
Cell Physiol Biochem. 24:105–114. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang SJ, Song ZJ, Wang XC, Zhang ZR, Wu SB
and Zhu GQ: Curculigoside facilitates fear extinction and prevents
depression-like behaviors in a mouse learned helplessness model
through increasing hippocampal BDNF. Acta Pharmacol Sin.
40:1269–1278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3' kinase/AKT pathways. Oncogene.
24:7443–7454. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Huber MA, Beug H and Wirth T:
Epithelial-mesenchymal transition: NF-kappaB takes center stage.
Cell Cycle. 3:1477–1480. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song Z, Shen F, Zhang Z, Wu S and Zhu G:
Calpain inhibition ameliorates depression-like behaviors by
reducing inflammation and promoting synaptic protein expression in
the hippocampus. Neuropharmacology. 174(108175)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ma C, Huang S, Xu L, Tian L, Yang Y and
Wang J: Transcription co-activator P300 activates Elk1-aPKC-iota
signaling mediated epithelial-to-mesenchymal transition and
malignancy in hepatocellular carcinoma. Oncogenesis.
9(32)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gasiule S, Dreize N, Kaupinis A, Ražanskas
R, Čiupas L, Stankevičius V, Kapustina Ž, Laurinavičius A, Valius M
and Vilkaitis G: Molecular insights into miRNA-driven resistance to
5-fluorouracil and oxaliplatin chemotherapy: miR-23b modulates the
epithelial-mesenchymal transition of colorectal cancer cells. J
Clin Med. 8(2115)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Roda G, Sartini A, Zambon E, Calafiore A,
Marocchi M, Caponi A, Belluzzi A and Roda E: Intestinal epithelial
cells in inflammatory bowel diseases. World J Gastroenterol.
16:4264–4271. 2010.PubMed/NCBI View Article : Google Scholar
|