Introduction
Ulcerative colitis (UC) is a common clinical
gastrointestinal disease with unclear causes (1). UC is essentially a chronic
non-specific inflammatory disease that invades colonic mucosa
(2). It usually begins in the left
half of the colon and gradually develops throughout the colon
towards the proximal region (2,3).
Patients with UC exhibit different degrees of clinical symptoms
(4). UC is mainly associated with
infection, immunology, genetic and mental factors, among which the
infection and immunology factors predominate (5,6).
IL-6 is a multi-functional cytokine that binds to
soluble IL-6 receptor (IL-6R) to form the IL-6/IL-6R protein
complex, leading to activation of glycoprotein 130 on the surface
of the cell membrane and the upregulated phosphorylation of STAT3
protein (7,8). STAT3, a member of the STAT family, is
an important downstream protein in the IL-6 signaling chain, and it
has four isomers STAT3α, STAT3β, STAT3γ and STAT3ε (9,10).
STAT3 predominantly functions as a transcriptional factor and its
functional structural unit is composed of seven parts: N-terminal
tetramerization domain (NH2), coiled-coil domain (CCD), DNA-binding
domain (DBD), linker domain, Src homology 2 (SH2) domain, SH3
domain and C-terminal transcriptional activation domain (TAD)
(11). NH2 is primarily responsible
for tetramerization of STAT; CCD provides sites for protein
interaction; DBD specifically binds to IFN-γ palindrome sequence;
linker domain stabilizes DBD; SH2 is involved in the formation of
STAT3 dimer; and the serine in TAD activates STAT3 and regulates
gene transcription (12). The
IL-6/STAT3 signaling pathway is important in physiological and
pathological processes, such as tumors, immune diseases and
angiogenesis, presenting as important targets for clinical
treatment (13,14). Histone modification is an important
means for regulating gene transcription, in which enrichment of
H3K27ac in the promoter region is conducive to the opening of
chromatin and promotes gene transcription (15).
Intestinal epithelial cells are the basic unit of
the intestinal physical barrier and absorption function (16). The small intestine of mammalians
consists of villi and lacunae covered by stratified columnar
epithelial cells and there are endocrine cells, goblet cells and
lymphocytes among the intestinal epithelial cells (17). The ability of intestinal self-repair
is closely associated with the proliferation and migration of
intestinal epithelial cells. After injuries, intestinal epithelial
cells undergo self-repair by their own proliferation and migration
to the injured site (18). If the
intestinal epithelial cells are seriously damaged, the self-repair
of the intestinal tract becomes difficult (18). Cytokines, including IL-18, IL-10 and
IL-6, have been proven to play important roles in local
inflammation, repair and damage of tissues (19). However, the role of the IL-6/STAT3
signaling pathway on intestinal injury remains unclear. Thus, in
the present study, the effect of the IL-6/STAT3 signaling pathway
on intestinal epithelial cells was investigated at the tissue and
cellular levels.
Materials and methods
Subjects
Fifty-two patients with UC (33 males and 19 females;
mean age, 48.7±4.6 years) received treatment at The First
Affiliated Hospital of Jinzhou Medical University (Jinzhou, China)
between August 2018 and June 2019, and were included in the
experimental group. Among the 52 patients with UC, 23 were active
cases and 29 were in remission. The patients were grouped according
to the severity of the disease as follows: Mild (n=16); moderate
(n=18); and severe (n=18). During the same period, 21 healthy
subjects who underwent a physical examination at The First
Affiliated Hospital of Jinzhou Medical University were included as
the control group (14 males and 7 females; mean age, 42.6±5.4
years). None of the patients had other intestinal infections,
tumors, rheumatism or other autoimmune diseases. Fasting peripheral
blood (10 ml) was collected from all subjects and centrifuged at
4,000 x g at 4˚C for 10 min to separate plasma. All procedures
performed in the current study were approved by the Ethics
Committee of Jinzhou Medical University and written informed
consent was obtained from all patients or their families.
Cell culture
Normal human colon mucosal epithelial NCM460 cells
(American Type Culture Collection) were cultured in DMEM
supplemented with 10% fetal bovine serum (HyClone; Cytiva), 100
IU/ml penicillin and 100 IU/ml streptomycin at 37˚C, 5%
CO2 and 70% humidity. The cells were passaged every
three days and those in logarithmic growth phase were collected for
experiments.
Co-culture of intestinal epithelial
cells
NCM460 cells (1x105) were seeded in
24-well plates, cultured at 37˚C and 5% CO2, and divided
into the following groups: Negative control (NC) group; IL-6 group;
plasma group; plasma + IL-6 antibody group; and IL-6 + stattic
group. The cells in the NC group were not treated. The IL-6 group
was incubated with IL-6 recombinant protein (cat. no. abs04044;
Absin Bioscience Inc.) at 37˚C for 24 h. The plasma group was
incubated with 250 µl plasma from patients with UC for 24 h. The
plasma + IL-6 antibody group was incubated with 250 µl plasma from
patients with UC and IL-6 antibody (cat. no. abs101505; Absin
Bioscience Inc.) at 37˚C for 24 h. The IL-6 + stattic group was
incubated at 37˚C for 24 h with IL-6 recombinant protein and STAT3
signaling pathway inhibitor, stattic (cat. no. abs812053; Absin
Bioscience Inc.). The stattic was dissolved in DMSO and added to
the culture medium to reach a concentration of 10 µm. The cells
were collected for subsequent assays.
Enzyme-linked immunosorbent assay
(ELISA)
A Human IL-6 Quantikine ELISA kit (cat. no. D6050;
R&D Systems, Inc.) was used to determine the concentration of
IL-6 and the Human Haptoglobin Quantikine ELISA kit (cat. no.
DHAPG0; R&D Systems, Inc.) was used to determine the level of
zonulin. First, 50 µl RD1W diluent was added to each well of a
microplate. Then, 50 µl standards and 50 µl cell culture
supernatants were added into predefined wells before shaking and
incubation at room temperature for 2 h. After washing the plate
with cold PBS, 100 µl conjugate was added to each well before
shaking and incubation at room temperature for 2 h. After washing
the plate again with cold PBS, 100 µl substrate was added to each
well before incubation at room temperature in the dark for 30 min.
Then, 100 µl stop solution was added to each well before mixing by
shaking the plate. Within 30 min, the absorbance at 570 nm of each
well was read using a microplate reader.
Detection of intestinal epithelial
cell barrier permeability
NCM640 cells were seeded into 6-well plates at a
density of 1x107/well. Millicell® chambers
(EMD Millipore) were placed into 24-well plates. At 70% confluency,
NCM640 cells were trypsinized and seeded into the apical end of the
Millicell chambers at a density of 1x105/well (0.4 ml).
At the basolateral end, 0.6 ml DMEM was added, followed by
incubation at 37˚C and 5% CO2 for 24 h. After replacing
the medium, the cells were incubated at 37˚C for 10 days. On days
2, 5, 8 and 10, transepithelial electrical resistance (TEER) was
determined to evaluate paracellular permeability. A
Millicell-Electrical Resistance System transmembrane resistance
meter was used to analyze cell transmembrane resistance, which was
calculated as follows: Transmembrane resistance of intestinal
epithelial cells=(value of sample well-value of blank well) x
Millicell membrane area (Ω·cm2). The analysis was
performed at 37˚C in triplicate, and the mean value was taken as
the TEER. Approximately 14-21 days later, the cells formed a
compact monolayer and the TEER increased significantly, indicating
that the barrier of intestinal epithelial cells was formed and
ready for subsequent experiments.
Western blotting
After 24 h of culture, the medium was discarded, and
the cells were washed twice with cold PBS. RIPA lysis buffer and
protease inhibitor PMSF (Beyotime Institute of Biotechnology) were
added to the cells, which were lysed on ice for 5 min. Following
centrifugation at 4˚C and 12,000 x g for 10 min, the supernatant
was collected and mixed with 5X loading buffer (Beyotime Institute
of Biotechnology), followed by incubation in a boiling water bath
for 10 min. The samples (5 µl) were electrophoresed with 10%
SDS-PAGE at 100 V and transferred to a PVDF membrane at 250 mA for
1 h on ice. After blocking with 50 g/l skimmed milk at room
temperature for 1 h, the membranes were incubated with rabbit
anti-human H3K27ac (1:1,000; cat. no. 8173; Cell Signaling
Technology, Inc.), phosphorylated (p)-STAT3 (1:1,000; cat. no.
9145; Cell Signaling Technology, Inc.), STAT3 (1:1,000; cat. no.
12640; Cell Signaling Technology, Inc.) or GAPDH (1:4,000) primary
antibodies (cat. no. ab9485; Abcam) at 4˚C overnight. After washing
with TBST three times for 10 min, horseradish peroxidase-labelled
goat anti-rabbit secondary antibody (1:4,000; cat. no. ab6721;
Abcam) was added for incubation at room temperature for 1 h. After
washing with TBST three times for 10 min, the membranes were
developed with electrochemiluminescence solution (Beyotime
Institute of Biotechnology). The expression of target proteins was
calculated against GAPDH.
Fluorescein diffusion experiment
NCM640 cells (1x106) were added to a
Transwell chamber and cultured overnight. After washing the cells
with HBSS (pH 7.4) three times, the cells were incubated with HBSS
at 37˚C for 30 min. After discarding the HBSS, fluorescein (40
µg/ml) was added to the top of the Transwell chamber, followed by
incubation at 37˚C for 1 h (20).
Then, fluid from the lower chamber was collected for the
determination of absorbance using a fluorescence spectrophotometer
(excitation wavelength, 427 nm; emission wavelength, 536 nm). The
fluorescence intensity was calculated to analyze the degree of
diffusion of fluorescein (20).
Total RNA extraction
Cells (1x106) were lysed with 1 ml
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions, before extraction of total RNA.
mRNA was reverse-transcribed into cDNA using a BeyoRT™ II cDNA
First Strand Synthesis Kit (Beyotime Institute of Biotechnology)
and stored at -80˚C. The reaction system was composed of 5 µl RNA
template, 1 µl Oligo(dT)18 primer, 4 µl reaction buffer
(5X), 1 µl RNase inhibitor (20 U/µl), 1 µl dNTP Mix (10 mM each), 1
µl BeyoRT II M-MLV reverse transcriptase and 7 µl H2O.
After thorough mixing, reverse transcription (RT) was performed at
42˚C for 60 min. Then, RNAase-free H2O was added to the
RT product to reach a total volume of 50 and 5 µl was used for
subsequent quantitative PCR (qPCR).
qPCR
The expression levels of claudin (CLDN) 1 and CLDN2
mRNA were determined using a BeyoFast™ Probe qPCR kit (Beyotime
Institute of Biotechnology) with GAPDH serving as an internal
reference. The reaction system (30 µl) was composed of 5 µl cDNA,
10 µl BeyoFast Probe qPCR Mix, 1 µl upstream primer, 1 µl
downstream primer and 13 µl ddH2O. The reaction
conditions were as follows: Initial denaturation at 95˚C for 5 min;
denaturation at 95˚C for 30 sec, annealing at 60˚C for 30 sec and
elongation at 72˚C for 1 min (40 cycles); final elongation at 72˚C
for 5 min. The primer sequences were as follows: CLDN1 forward,
5'-TTGGGCTTCATTCTCGCCTT-3' and reverse, 5'-CTGGCATTGACTGGGGTCAT-3';
CLDN2 forward, 5'-CCTTGTACTTCGCTCCCCTC-3' and reverse,
5'-ACAATGCTGGCACCGACATA-3'; GAPDH forward,
5'-CGGAGTCAACGGATTTGGTCGTAT-3' and reverse,
5'-AGCCTTCTCCATGGTGGTGAAGAC-3'. The 2-ΔΔCq method
(21) was used to determine the
relative expression of CLDN1 and CLDN2 mRNA against GAPDH. Each
sample was evaluated in triplicate.
Chromatin immunoprecipitation
(ChIP)-qPCR
Co-immunoprecipitation was performed using a
SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads;
Cell Signaling Technology, Inc.) according to the manufacturer's
instructions. Two pairs of primers were designed for different
promoters of CLDN1 and CLDN2 genes. The first pair of primers for
CLDN1 were: Forward, 5'-TGCAGAGACAAGTGATGGAACGAC-3' and reverse,
5'-AAGAGCTGCAGTTTTAGGTTTAATA-3'. The second pair of primers for
CLDN1 were: Forward, 5'-TATTAAACCTAAAACTGCAGCTCTT-3' and reverse,
5'-GCTCCTGTAAGGCGTTTCACG-3'. The first pair of primers for CLDN2
were: Forward, 5'-GGCTAGGCCACTACTCTCTAGGC-3' and reverse,
5'-GCCTGCTGTTTAATACATTGCCA-3'. The second pair of primers for CLDN2
were: Forward, 5'-TCCTGGCTTTGTCCAGCTGCCA-3' and reverse,
5'-TGGCAGCTGGACAAAGCCAGGA-3'. The 2-ΔΔCq method
(21) was used to quantify H3K27ac
enrichment. qPCR was performed as aforementioned.
Statistical analysis
The results were analyzed using SPSS 18.0
statistical software (SPSS, Inc.) and data are expressed as means ±
standard deviations. All data underwent a normality test. If the
data demonstrated normal distribution and the variance was
homogeneous, differences between multiple groups were analyzed
using one-way ANOVA and Dunnett's test, while comparisons between
two groups were performed using paired or unpaired Student's
t-test. If the data were not normally distributed or the variance
was not uniform, differences between multiple groups were analyzed
using Kruskal-Wallis test and Tamhane's T2 or Dunnett's T3 method,
while comparisons between two groups were conducted using the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
IL-6 content is elevated in plasma
from patients with UC and increases with progression of the
disease
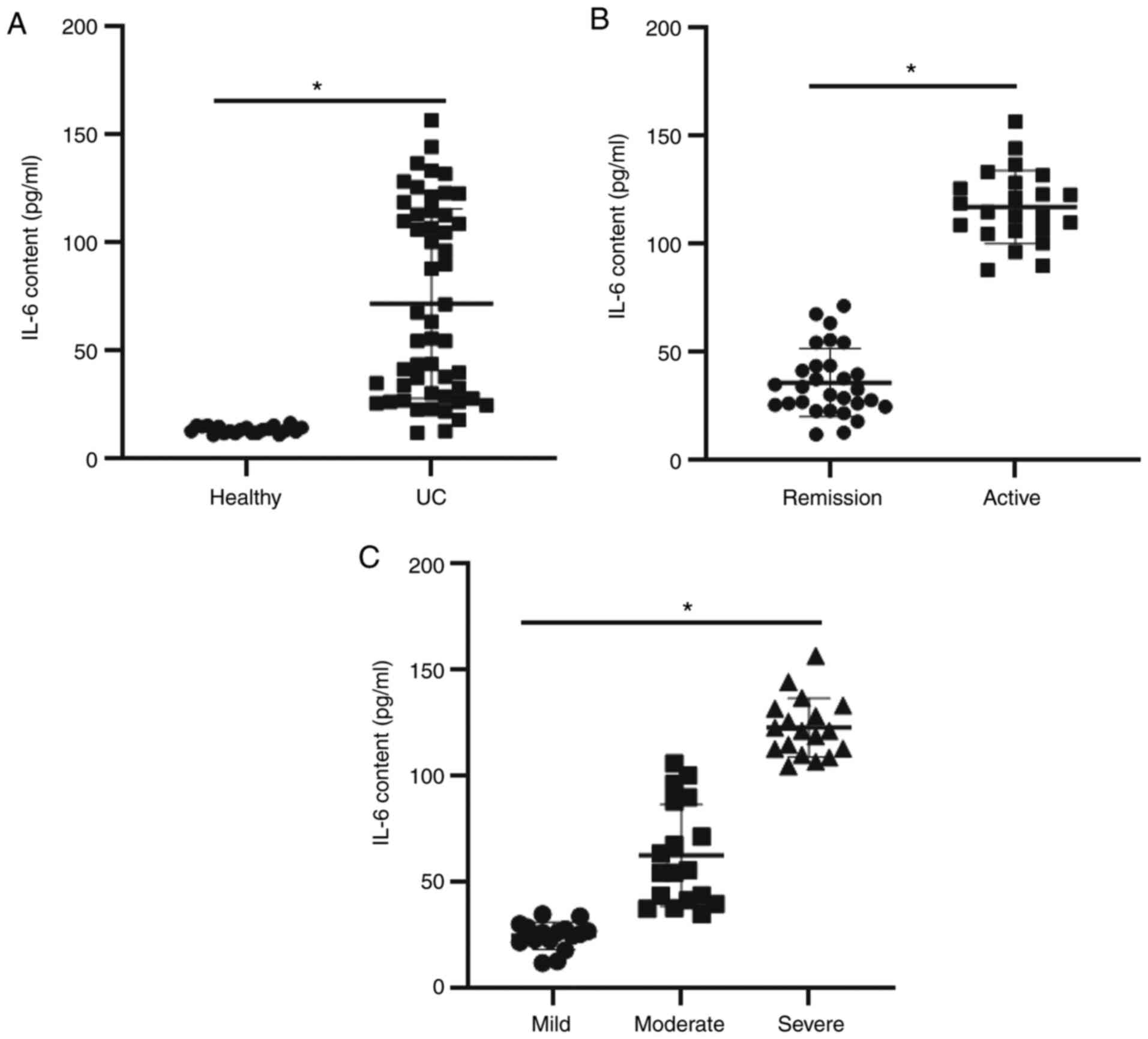
ELISA was used to evaluate the content of IL-6 in
peripheral blood. The data (Fig.
1A) indicated that IL-6 content in plasma from patients with UC
(80.5±4.6 pg/ml) was significantly higher than that of the healthy
subjects (22.6±2.7 pg/ml) (P<0.05). Furthermore, IL-6 content in
plasma from patients with active UC (110.5±5.1 pg/ml) was
significantly higher than that in patients who were in remission
(68.4±3.4 pg/ml) (P<0.05) as presented in Fig. 1B. Regarding disease severity, the
IL-6 content in plasma from patients with UC in the moderate group
(100.6±4.3 pg/ml) and severe group (118.7±6.4 pg/ml) was
significantly higher than that in the mild group (58.4±2.6 pg/ml)
(P<0.05) as presented in Fig.
1C. The results indicate that IL-6 levels are elevated in the
plasma of patients with UC and that it is increases with the
progression of the disease.
IL-6 induces intestinal epithelial
cell barrier injury
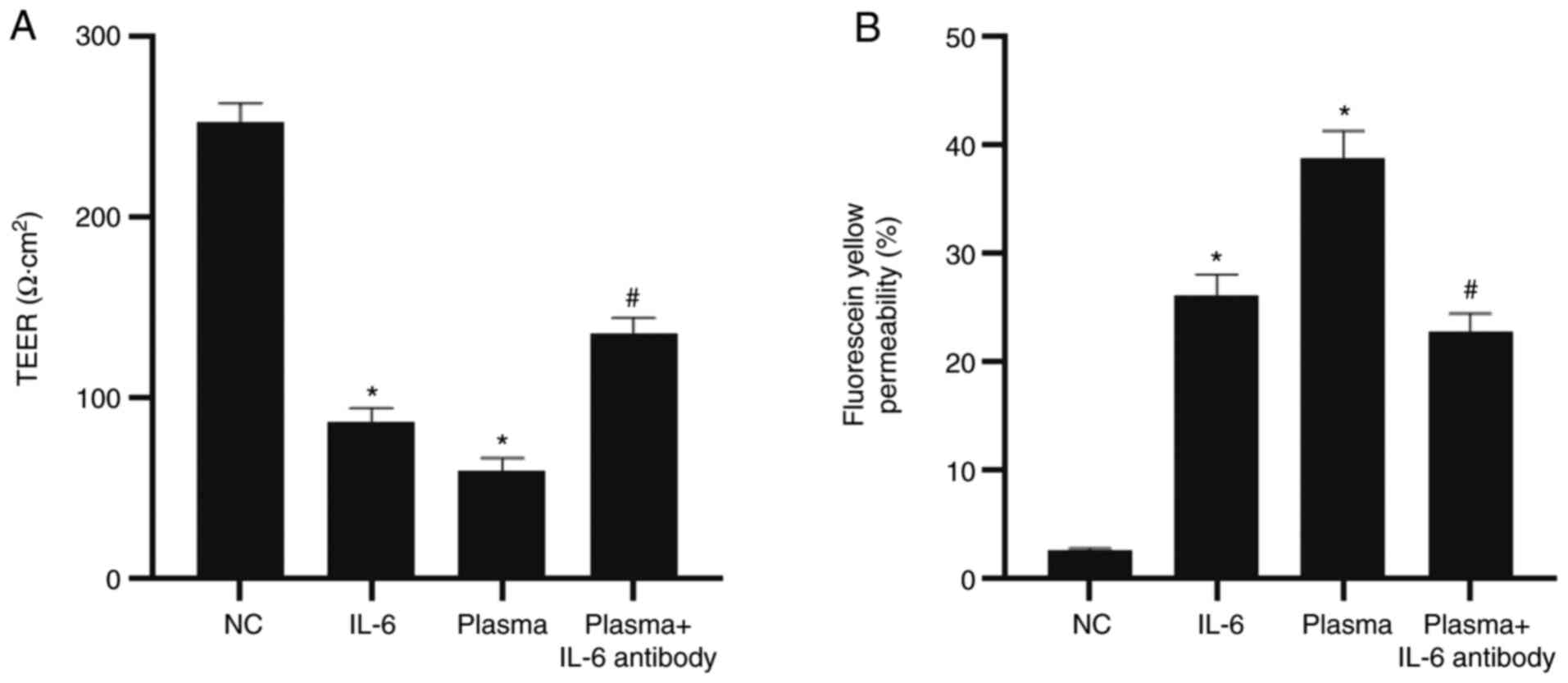
To evaluate intestinal epithelial cell barrier
permeability, the TEER was determined and Millicell inserts were
used. The data demonstrated that the TEER value of monolayer cells
in the NC group was 250 Ω·cm2. After a 12-h treatment
with IL-6, plasma from patients with UC or UC plasma containing
IL-6 antibody, the TEER values of the IL-6 group and the plasma
group were significantly lower than those of the NC group
(P<0.05). In addition, the TEER value of the plasma + IL-6
antibody group was significantly higher than that of the plasma
group (P<0.05; Fig. 2A). The
Millicell data showed that the permeability for fluorescein yellow
in the IL-6 group (26.5±0.65%) and plasma group (38.5±0.89%) was
significantly higher than that in the NC group (2.53±0.14%)
(P<0.05). Furthermore, the permeability for fluorescein yellow
in the plasma + IL-6 antibody group (22.7±0.71%) was significantly
lower than that in the plasma group (P<0.05; Fig. 2B). The result indicates that IL-6
induces intestinal epithelial cell barrier injury.
IL-6 regulates barrier function by
influencing the expression of tight junction-related proteins in
the epithelium
Comparable to epithelial-mesenchymal transition
markers, E-cadherin and N-cadherin, CLDN1, CLDN2 and zonulin are
markers of epithelial tight junctions. In the present study, these
molecular markers were detected to evaluate intestinal epithelial
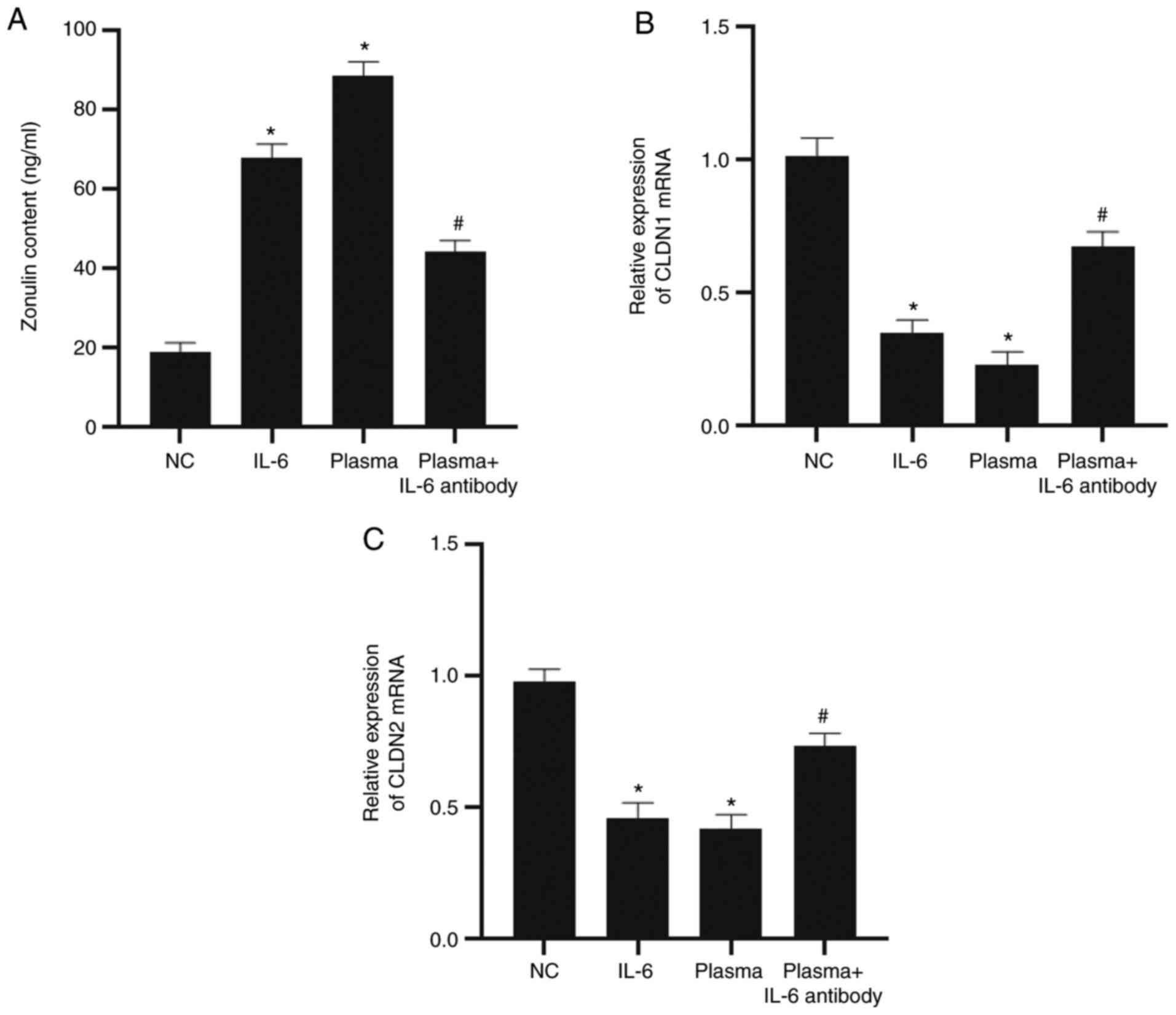
cell barrier permeability. To analyze the effect of IL-6 on the
release and expression of these monolayer epithelial cell
barrier-associated proteins, ELISA and RT-qPCR were conducted.
After stimulating NCM640 cells with IL-6 for 6 h, the zonulin
content in the culture supernatant of the cells was observed to be
significantly higher than that of the NC group (P<0.05).
Similarly, treatment with UC plasma also elevated the level of
zonulin when compared to the NC group (P<0.05). However,
addition of the IL-6 antibody reduced the content of zonulin when
compared with that of the plasma group (P<0.05; Fig. 3A). RT-qPCR demonstrated that the
mRNA expression levels of CLDN1 and CLDN2 in the IL-6 or plasma
group were significantly lower than that of the NC group
(P<0.05). However, the addition of IL-6 antibody significantly
increased the expression levels of CLDN1 and CLDN2 mRNA when
compared with the plasma group (P<0.05; Fig. 3B and C). The results suggest that IL-6 regulates
barrier function by influencing the expression of tight
junction-related proteins in the epithelium.
IL-6 regulates the barrier function of
intestinal epithelial cells via STAT3
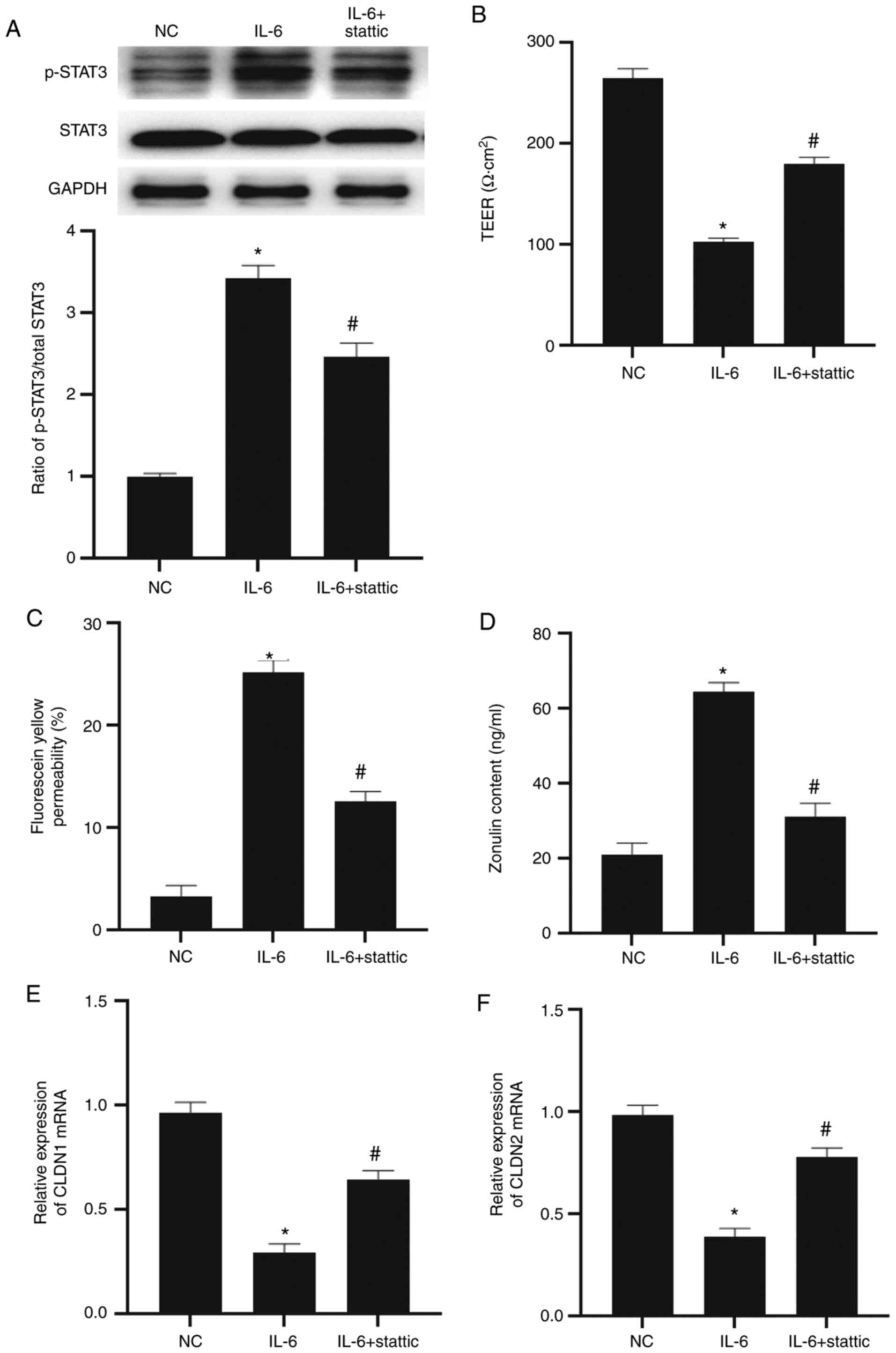
To evaluate the effect of STAT3 on the barrier
function of intestinal epithelial cells, NCM640 cells were treated
with stattic to inhibit the STAT3 signaling pathway. Western
blotting indicated that the level of p-STAT3 in the IL-6 group was
significantly higher than that in the NC group (P<0.05) and the
level of p-STAT3 in the IL-6 + stattic group was significantly
lower than in the IL-6 group (P<0.05; Fig. 4A). The TEER value in the IL-6 group
was significantly lower than that in the NC group (P<0.05),
while that in the IL-6 + stattic group was significantly higher
than that in the IL-6 group (P<0.05; Fig. 4B). In addition, fluorescein yellow
permeability in the IL-6 group was significantly higher than that
in the NC group (P<0.05), while that in the IL-6 + stattic group
was significantly lower than that in the IL-6 group (P<0.05;
Fig. 4C). ELISA showed that zonulin
content in the culture supernatant of NCM640 cells of the IL-6
group was significantly higher than that in the NC group
(P<0.05), while that in the IL-6 + stattic group was
significantly lower than that in the IL-6 group (P<0.05;
Fig. 4D). RT-qPCR showed that CLDN1
and CLDN2 mRNA expression levels in the IL-6 group were
significantly lower than those in the NC group (P<0.05), and
that CLDN1 and CLDN2 mRNA expression levels in the IL-6 + stattic
group were significantly higher than in the IL-6 group (P<0.05;
Fig. 4E and F). The results indicate that IL-6
regulates the barrier function of intestinal epithelial cells via
STAT3.
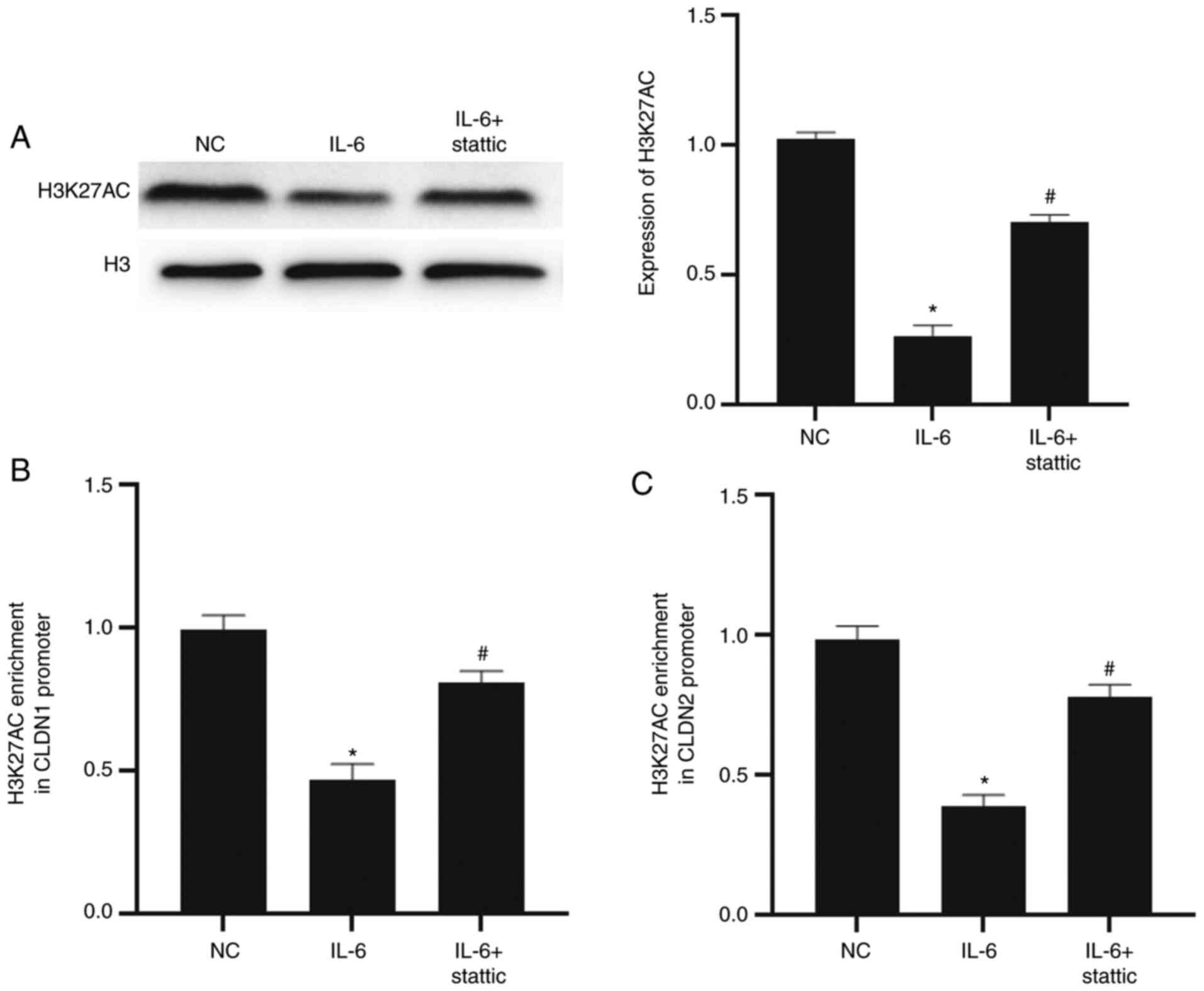
IL-6/STAT3 signaling pathway regulates
the transcription of CLDN1 and CLDN2 by affecting the enrichment of
histone H3K27ac in their promoter regions
To evaluate the effect of the IL-6/STAT3 signaling
pathway on the H3K27ac level in NCM640 cells and H3K27ac enrichment
on the promoter regions of CLDN1 and CLDN2, western blotting and
ChIP-qPCR were conducted. The data showed that the H3K27ac level in
cells of the IL-6 group was significantly lower than that in the NC
group (P<0.05) and that the H3K27ac level in the IL-6 + stattic
group was significantly higher than that in the IL-6 group
(P<0.05; Fig. 5A). The
enrichment of H3K27ac in the promoter regions of CLDN1 and CLDN2 in
the IL-6 group was significantly lower than in the NC group
(P<0.05 for both), while that of the IL-6 + stattic group was
significantly higher than the IL-6 group (P<0.05 for both;
Fig. 5B and C). The results suggest that the IL-6/STAT3
signaling pathway regulates the transcription of CLDN1 and CLDN2 by
affecting the enrichment of histone H3K27ac in their promoter
regions.
Discussion
Injury of the intestinal epithelial barrier is the
predominant pathological change in UC and its main characteristics
include intestinal epithelial cell apoptosis, normal intestinal
epithelial cell barrier damage, infiltration of subcutaneous
lymphocytes of the intestine and cytokine expression, leading to
damage of the intestinal epithelium (22,23).
As many cytokines are found in the peripheral blood and intestinal
tissue of patients with UC, the roles of these cytokines in
intestinal epithelial damage have become a notable research
topic.
IL-6 plays important regulatory roles in
cardiovascular diseases, lipid metabolism, mitochondrial activity,
and the occurrence and development of tumors (24,25).
IL-6 can be synthesized and secreted by almost all stromal cells
and immune cells, such as T cells, macrophages and epithelial tumor
cells (26,27). IL-6 also plays important roles at
different stages of inflammation. For example, significant
quantities of IL-6 are secreted by monocytes and macrophages at the
early stage of infectious inflammation and participates in host
immune defense (28). Furthermore,
IL-6 participates in humoral immune regulation by regulating B cell
functions (29). The expression of
IL-6 in the peripheral blood of patients with UC is significantly
upregulated and the concentration of IL-6 is negatively correlated
with the response of patients to infliximab (30). In the present study, the level of
IL-6 in the peripheral blood of patients with UC was observed to be
significantly elevated and was higher in patients with active UC
when compared with those who were in remission. In addition, the
expression level of IL-6 in patients with moderate or severe UC was
higher than that in patients with mild UC. These results indicate
that the IL-6 expression level was positively associated with the
progression of UC.
Intestinal barrier damage is the main pathological
change in UC (31). The intestinal
barrier includes normal flora, a mucus layer, intestinal epithelial
cell layer and intestinal immune system, among which the intestinal
epithelial cells are particularly important in the composition and
maintenance of the intestinal barrier (32). The degree of intestinal barrier
damage can be reflected by the TEER value, the diffusion efficiency
of fluorescein yellow and the release of zonulin (33). TEER is a simple and authoritative
method of evaluating the permeability of monolayer epithelial cells
(34). In the present study, it was
found that IL-6 and plasma from patients with UC significantly
decreased the TEER value and promoted the diffusion of fluorescein
yellow and the release of zonulin, whilst reducing the expression
of tight junction proteins CLDN1 and CLDN2 at the molecular level.
After adding the IL-6 antibody, the TEER value, fluorescein yellow
diffusion, zonulin release and the expression levels of CLDN1 and
CLDN2 in the plasma group were significantly reversed. These
results indicated that IL-6 promoted intestinal epithelial cell
damage in patients with UC. As a downstream transducer of IL-6,
STAT3 exerts important biological functions in many types of
disease, such as tumors, autoimmune diseases and pulmonary fibrosis
(35,36). In the present study, the addition of
STAT3 inhibitor recovered the damage of intestinal epithelial cells
induced by IL-6, suggesting that IL-6 exerted its biological
functions via STAT3.
The IL-6/STAT3 signaling pathway is important in
intestinal inflammation and colon cancer. For example, inhibition
of the IL-6/STAT3 signaling pathway improves intestinal injury in
acute enteritis (37). In addition,
the IL-6/STAT3/suppressor of cytokine signaling-3 (SOCS3) signaling
pathway plays an important role in the carcinogenesis of enteritis
(38). Another study demonstrated
that the expression imbalance of the IL-6/STAT3/SOCS3 signaling
pathway exists in the occurrence of tumors associated with UC or
enteritis (39). The conclusions of
the aforementioned studies concur with the results of the present
study. Notably, the present study also found that the permeability
of cells in the IL-6 rescue group was only partially restored. This
indicates the existence of an underlying mechanism independent of
IL-6, which regulates intestinal epithelial barrier permeability.
For example, recombinant IL-17A-dependent regulation of the tight
junction protein occludin during epithelial injury impacts
permeability and maintains barrier integrity (40). Future studies are required to
investigate potential factors in the regulation of epithelial
barrier permeability.
H3K27ac is an important histone modification. When
H3K27ac is enriched in promoter regions, it promotes the opening of
chromatin, so that transcriptional factors can combine with
promoter region sequences to initiate downstream gene transcription
(41). In the present study, the
data showed that CLDN1 and CLDN2 were negatively regulated by the
IL-6/STAT3 signaling pathway. As STAT3 is a transcription factor
responsible for initiating gene transcription, the present study
speculated that there were other factors that affected the
expression of CLDN1 and CLDN2. It was discovered that the
IL-6/STAT3 signaling pathway directly inhibited the expression of
H3K27ac in intestinal epithelial cells. Using ChIP-qPCR, the
present study demonstrated that IL-6 reduced the enrichment of
H3K27ac in the promoter regions of CLDN1 and CLDN2. This indicated
that the IL-6/STAT3 signaling pathway affected the barrier function
of intestinal epithelial cells by downregulating the expression
level of H3K27ac.
Thus, the present study demonstrates that the
expression level of IL-6 in the peripheral blood of patients with
UC is increased significantly and is positively associated with the
development of UC. Furthermore, the IL-6/STAT3 signaling pathway
affects the function of the intestinal barrier by affecting the
H3K27ac level in intestinal epithelial cells. However, there were
certain limitations of the present study; only a single cell line,
NCM460 was used. Although the NCM460 cell line is one of the most
commonly used normal intestinal epithelial cell lines, the
influence of the IL-6/STAT3 signaling pathway on NCM460 cells does
not represent its impact on intestinal epithelial cells as a whole.
Therefore, the scope of our work will be expanded further to verify
this result on other cell lines in future studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and YJ contributed to the design of the study.
YL, YJ and TC performed the experiments. YL, YJ and JZ analyzed the
data. YL interpreted results and prepared the manuscript. YL and YJ
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Jinzhou Medical University.
Written informed consent was obtained from all patients or their
families.
Patient consent for publication
Written informed consents for publication of any
associated data and accompanying images were obtained from all
patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sahu P, Bopanna S, Kedia S and Ahuja V:
Risk of colorectal cancer in Asian patients with ulcerative
colitis. J Gastroenterol Hepatol. 35(1451)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wakai M, Hayashi R, Tanaka S, Naito T,
Kumada J, Nomura M, Takigawa H, Oka S, Ueno Y, Ito M and Chayama K:
Serum amyloid A is a better predictive biomarker of mucosal healing
than C-reactive protein in ulcerative colitis in clinical
remission. BMC Gastroenterol. 20(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Akiyama S, Traboulsi C, Rai V and Rubin
DT: Re: 18F-FDG PET-MR enterography in predicting
histological active disease using the Nancy index in ulcerative
colitis: A randomized controlled trial. Eur J Nucl Med Mol Imaging.
47(2247)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Biemans VBC, Sleutjes JAM, de Vries AC,
Bodelier AGL, Dijkstra G, Oldenburg B, Löwenberg M, van Bodegraven
AA, van der Meulen-de Jong AE, de Boer NKH, et al: Tofacitinib for
ulcerative colitis: Results of the prospective dutch initiative on
crohn and colitis (ICC) registry. Aliment Pharmacol Ther.
51:880–888. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Longhi MS and Kokkotou E: Lnc-ing RNA
expression with disease pathogenesis: MALAT1 and ANRIL in
Ulcerative Colitis. Dig Dis Sci. 65:3061–3063. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rozich JJ, Holmer A and Singh S: Effect of
lifestyle factors on outcomes in patients with inflammatory bowel
diseases. Am J Gastroenterol. 115:832–840. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Itsuji T, Tonomura H, Ishibashi H, Mikami
Y, Nagae M, Takatori R, Tanida T, Matsuda KI, Tanaka M and Kubo T:
Hepatocyte growth factor regulates HIF-1α-induced nucleus pulposus
cell proliferation through MAPK-, PI3K/Akt-, and STAT3-mediated
signaling. J Orthop Res 2020 (Epub ahead of print).
|
|
8
|
Zhao C, Yang L, Zhou F, Yu Y, Du X, Xiang
Y, Li C, Huang X, Xie C, Liu Z, et al: Feedback activation of EGFR
is the main cause for STAT3 inhibition-irresponsiveness in
pancreatic cancer cells. Oncogene. 39:3997–4013. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Samiea A, Yoon JSJ, Cheung ST, Chamberlain
TC and Mui AL: Interleukin-10 contributes to PGE2 signalling
through upregulation of EP4 via SHIP1 and STAT3. PLoS One.
15(e0230427)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi S, Song L, Liu Y and He Y: Activation
of CREB protein with tabersonine attenuates STAT3 during
atherosclerosis in apolipoprotein E-Deficient mice. Dose Response.
18(1559325820912067)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim SH, Hong JH, Yang WK, Geum JH, Kim HR,
Choi SY, Kang YM, An HJ and Lee YC: Herbal combinational medication
of glycyrrhiza glabra, agastache rugosa containing glycyrrhizic
acid, tilianin inhibits neutrophilic lung inflammation by affecting
CXCL2, Interleukin-17/STAT3 signal pathways in a murine model of
COPD. Nutrients. 12(926)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao T, Jin F, Xiao D, Wang H, Huang C,
Wang X, Gao S, Liu J, Yang S and Hao J: IL-37/STAT3/HIF-1α negative
feedback signaling drives gemcitabine resistance in pancreatic
cancer. Theranostics. 10:4088–4100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang CQ, Ma LL, Lv ZD, Feng F, Chen Z and
Liu ZD: Polydatin induces apoptosis and autophagy via STAT3
signaling in human osteosarcoma MG-63 cells. J Nat Med. 74:533–544.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu CI, Cheng CI, Kang YF, Chang PC, Lin
IP, Kuo YH, Jhou AJ, Lin MY, Chen CY and Lee CH: Hispidulin
inhibits neuroinflammation in lipopolysaccharide-activated BV2
microglia and attenuates the activation of Akt, NF-κB, and STAT3
pathway. Neurotox Res. 38:163–174. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chandra V, Bhattacharyya S, Schmiedel BJ,
Madrigal A, Gonzalez-Colin C, Fotsing S, Crinklaw A, Seumois G,
Mohammadi P, Kronenberg M, et al: Promoter-interacting expression
quantitative trait loci are enriched for functional genetic
variants. Nat Genet. 53:110–119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Song M, Yang Q, Zhang F, Chen L, Su H,
Yang X, He H, Liu F, Zheng J, Ling M, et al: Hyodeoxycholic acid
(HDCA) suppresses intestinal epithelial cell proliferation through
FXR-PI3K/AKT pathway, accompanied by alteration of bile acids
metabolism profiles induced by gut bacteria. FASEB J. 34:7103–7117.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Du G, Xiong L, Li X, Zhuo Z, Zhuang X, Yu
Z, Wu L, Xiao D, Liu Z, Jie M, et al: Peroxisome elevation induces
stem cell differentiation and intestinal epithelial repair. Dev
Cell. 53:169–184.e11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pearce SC, Weber GJ, van Sambeek DM,
Soares JW, Racicot K and Breault DT: Intestinal enteroids
recapitulate the effects of short-chain fatty acids on the
intestinal epithelium. PLoS One. 15(e0230231)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tkáčiková Ľ, Mochnáčová E, Tyagi P,
Kiššová Z and Bhide M: Comprehensive mapping of the cell response
to E. coli infection in porcine intestinal epithelial cells
pretreated with exopolysaccharide derived from Lactobacillus
reuteri. Vet Res. 51(49)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin CW, Wang Y, Challa P, Epstein DL and
Yuan F: Transscleral diffusion of ethacrynic acid and sodium
fluorescein. Mol Vis. 13:243–251. 2007.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wei F, Lang Y, Shen Q, Xu L, Cheng N, Chu
Y, Lyu H and Chen F: Osteopontin-loaded PLGA nanoparticles enhance
the intestinal mucosal barrier and alleviate inflammation via the
NF-κB signaling pathway. Colloids Surf B Biointerfaces.
190(110952)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang S, Wu J, Wang F, Wang H, Wu Z, Wu S
and Bao W: Expression pattern analysis of antiviral genes and
inflammatory cytokines in PEDV-Infected porcine intestinal
epithelial cells. Front Vet Sci. 7(75)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
St Paul M, Saibil SD, Lien SC, Han S,
Sayad A, Mulder DT, Garcia-Batres CR, Elford AR, Israni-Winger K,
Robert-Tissot C, et al: IL6 Induces an IL22+
CD8+ T-cell subset with potent antitumor function.
Cancer Immunol Res. 8:321–333. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim JH, Kim WS and Park C: Interleukin-6
mediates resistance to PI3K-pathway-targeted therapy in lymphoma.
BMC Cancer. 19(936)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nilsson AM, Tufvesson E, Hesselstrand R,
Olsson P, Wollmer P and Mandl T: Increased B-cell activating
factor, interleukin-6, and interleukin-8 in induced sputum from
primary Sjögren's syndrome patients. Scand J Rheumatol. 48:149–156.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou J, Jiang Y, Zhao J, Zhang H, Fu J,
Luo P, Ma Y, Zou D, Gao H, Hu J, et al: Dp44mT, an iron chelator,
suppresses growth and induces apoptosis via RORA-mediated
NDRG2-IL6/JAK2/STAT3 signaling in glioma. Cell Oncol (Dordr).
43:461–475. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sureda A, Martorell M, Bibiloni MDM,
Bouzas C, Gallardo-Alfaro L, Mateos D, Capó X, Tur JA and Pons A:
Effect of free fatty acids on inflammatory gene expression and
hydrogen peroxide production by ex vivo blood mononuclear cells.
Nutrients. 12(146)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rezaee D, Bandehpour M, Kazemi B and
Salehi M: Role of intrauterine administration of transfected
peripheral blood mononuclear cells by GM-CSF on embryo implantation
and pregnancy rate in mice. Mol Hum Reprod. 26:101–110.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nishida Y, Hosomi S, Watanabe K, Watanabe
K, Yukawa T, Otani K, Nagami Y, Tanaka F, Taira K, Kamata N, et al:
Serum interleukin-6 level is associated with response to infliximab
in ulcerative colitis. Scand J Gastroenterol. 53:579–585.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ding S, Song Y, Brulois KF, Pan J, Co JY,
Ren L, Feng N, Yasukawa LL, Sánchez-Tacuba L, Wosen JE, et al:
Retinoic acid and lymphotoxin signaling promote differentiation of
human intestinal M cells. Gastroenterology. 159:214–226.e1.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang JC, Chen P, Zhang C, Khalil MM,
Zhang NY, Qi DS, Wang YW and Sun LH: Yeast culture promotes the
production of aged laying hens by improving intestinal digestive
enzyme activities and the intestinal health status. Poult Sci.
99:2026–2032. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Domenech J, Hernández A, Demir E, Marcos R
and Cortés C: Interactions of graphene oxide and graphene
nanoplatelets with the in vitro Caco-2/HT29 model of intestinal
barrier. Sci Rep. 10(2793)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xia ZY, Luo C, Liu BW, Bian XQ, Li Y, Pang
AM, Xu YH, Tan HM and Zhao YH: Shengui Sansheng Pulvis maintains
blood-brain barrier integrity by vasoactive intestinal peptide
after ischemic stroke. Phytomedicine. 67(153158)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu J, Gao FX, Wang C, Qin M, Han F, Xu T,
Hu Z, Long Y, He XM, Deng X, et al: IL-6 and IL-8 secreted by
tumour cells impair the function of NK cells via the STAT3 pathway
in oesophageal squamous cell carcinoma. J Exp Clin Cancer Res.
38(321)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Babiuch K, Kuśnierz-Cabala B, Kęsek B,
Okoń K, Darczuk D and Chomyszyn-Gajewska M: Evaluation of
proinflammatory, NF-kappaB dependent cytokines: IL-1α, IL-6, IL-8,
and TNF-α in tissue specimens and saliva of patients with oral
squamous cell carcinoma and oral potentially malignant disorders. J
Clin Med. 9(867)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li L, Shen A, Chu J, Sferra TJ,
Sankararaman S, Ke X, Chen Y and Peng J: Pien Tze Huang ameliorates
DSS-induced colonic inflammation in a mouse colitis model through
inhibition of the IL-6/STAT3 pathway. Mol Med Rep. 18:1113–1119.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen YY, Ma ZB, Xu HY, Shi LJ, Li DY, Sun
LY, Yin XH, Sang GY, Xu D, Tang YH, et al: IL-6/STAT3/SOCS3
signaling pathway playing a regulatory role in ulcerative colitis
carcinogenesis. Int J Clin Exp Med. 8:12009–12017. 2015.PubMed/NCBI
|
|
39
|
Li Y, de Haar C, Chen M, Deuring J,
Gerrits MM, Smits R, Xia B, Kuipers EJ and van der Woude CJ:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF,
Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan
TK, et al: Interleukin-23-Independent IL-17 production regulates
intestinal epithelial permeability. Immunity. 43:727–738.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang T, Zhang Z, Dong Q, Xiong J and Zhu
B: Histone H3K27 acetylation is dispensable for enhancer activity
in mouse embryonic stem cells. Genome Biol. 21(45)2020.PubMed/NCBI View Article : Google Scholar
|