Introduction
Chronic heart failure (CHF) is commonly seen in
numerous heart disease types, characterized by impaired ventricular
filling and ejection (1). Numerous
risk factors lead to CHF, including diabetes, hypertension,
coronary heart disease and chronic renal diseases (2). Extensive research data has suggested
that diabetes mellitus contributes toward a greater risk of adverse
outcomes in CHF (3,4). Serum levels of two endogenously
generated peptides atrial natriuretic peptide (ANP) and brain
natriuretic peptide (BNP) are significantly increased in HF and
their serum content is tightly associated with CHF dysfunction
(5,6).
Unlike cell apoptosis, cell pyroptosis is a
proinflammatory form of programmed cell death that is triggered by
various pathological stimuli (7).
Pyroptosis is regularly involved in various inflammatory diseases
(8), mediated by inflammasomes and
the activation of caspase-1. Working as a protease, caspase-1
activates IL-1β, IL-18 and gasdermin D (GSDMD), transforming these
downstream molecules into their mature forms (7). NOD-, LRR- and pyrin domain-containing
protein 3 (NLRP3) and pro-caspase-1, together with other proteins,
form an inflammasome, cleaving pro-caspase-1 into active
caspase-1(9). Doxorubicin
(DOX)-mediated cardiotoxicity is tightly associated with NLRP3,
leading to cardiomyocyte pyroptosis (10). Reactive oxygen species
(ROS)-mediated NLRP3 inflammation serves an essential role during
the development of cardiomyocyte injury in diabetes (11).
Bone morphogenetic proteins (BMPs) are key
modulators for cardiac morphogenesis and development (12). BMP-mediated signaling pathway acts
through interactions with cognate Type I and Type II
serine/threonine kinase receptors (13). BMP-2 induces complete cardiogenesis
by regulating the ectopic expression of cardiac transcription
factors (14). BMP-2 promotes
survival and inhibits apoptosis of cardiac myocytes (15). Recently, BMP-2 has been identified
as a potential autocrine and paracrine factor that regulates ANP
and BNP protein synthesis (16).
Therefore, we hypothesized that BMP-2 is associated with ANP and
BNP expression in cardiomyocyte injury in diabetes. Therefore,
studies on BMP-2 would provide future possible therapeutic targets
for diabetic patients.
Materials and methods
Patients and peripheral blood
specimens
In total, 25 serum samples were collected from
patients with CHF with (19 patients were male, 6 patients were
female; age range, 60-78 years; mean age, 70.5±4.3 years) or
without type 2 diabetes mellitus (17 patients were male, 8 patients
were female; age range, 60-77 years, mean age, 73.9±3.5 years) in
Shanghai TCM-Integrated Hospital (Shanghai, China) between January
2015 and January 2017. Patients with type 1 diabetes and aged
<60 years were excluded. Another 25 serum samples were collected
from individuals during a routine examination in Shanghai
TCM-Integrated Hospital between January 2015 and January 2017 who
were diagnosed without diabetes and heart disease as controls (18
patients were male, 7 were female; age range, 65-75 years; mean
age, 70.4±3.4 years). The present study obtained written consent
from all patients for all investigation and experiments, and all
procedures were approved by the Ethics Committee of Shanghai
TCM-Integrated Hospital, Shanghai University of Traditional Chinese
Medicine (approval no. 2015-022-1) on January 10, 2015.
Cell culture and treatment
Human cardiomyocyte AC16 cell line, purchased from
EMD Millipore, were passed and cultured in a mitogen-free
Dulbecco's modified Eagle's medium supplemented with 10% fetal FBS
and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). Culture flasks were placed in a humidified atmosphere of 90%
air and 10% CO2 at 37˚C. Treatment of AC16 cells was
performed by adding recombinant human BMP-2 (rhBMP-2; 80 mg/ml;
Yamanouchi Co. Ltd.) at 37˚C for 4 h (17), followed by adding 2 µM DOX and
normal glucose (5.5 mM glucose, NG) or high glucose (33 mM glucose,
HG). Additional mannitol (27.5 mM) was included in the NG medium to
ensure that NG maintains the same osmolarity as HG medium (18).
Cell transfection
The coding sequence (CDS) of NLRP3 was synthesized
by Genewiz, Inc. and inserted into pcDNA3.1(+) to produce NLRP3
ectopic expression vector, using primers with sequences as follows:
Forward, 5'-CCCAAGCTTATGAAGATGGCAAGCACCC-3' and reverse,
5'-CGGAATTCCTACCAAGAAGGCTCAAAGACGAC-3'. AC16 cells were seeded into
a six-well plate with 1 µg pcDNA3.1(+)-NLRP3 or blank pcDNA3.1(+).
Transfections were performed using Lipofectamine® 3000
reagent following manufacturer's protocol for 4-6 h at 37˚C. The
blank pcDNA3.1(+) (vector) was included as blank control. At 24 h
following transfection, subsequent experimentation was
performed.
Cell death measurement
Pyroptotic cell death was investigated using active
caspase-1 and propidium iodide (PI) staining as previously
described (19). In brief, AC16
cells were seeded into six-well plates (5x105
cells/well) and allowed to grow until reaching 50% confluence.
Cells were then incubated with 660-YVAD-FMK (FLICA® 660
caspase-1 assay kit; ImmunoChemistry Technologies, LLC), according
to manufacturer's protocols and with 10 µl PI (Thermo Fisher
Scientific, Inc.) for 15 min and analyzed using an Accuri C6 flow
cytometer (version 1.0.264; BD Biosciences) using CellQuest Pro
software, version 3.3 (Becton Dickinson). A total of 10,000 cells
were tested for each sample.
Measurement of ROS
A fluorescent 2'-7'-dichlorodihydrofluorescein
diacetate (DCFH-DA) probe (Beyotime Institute of Biotechnology) was
used to evaluate ROS levels within the AC16 cells. In brief, AC16
cells (1x106/ml) were cultured with 10 µM DCFH-DA probe
(forward-reverse mixing once per 3 min). Following a 20-min
incubation in darkness at 37˚C, fluorescence was determined at an
excitation/emission of 485/530 nm using Accuri™ C6 flow cytometry
(BD Biosciences) and Accuri™ C6 Software (version 1.0.264; BD
Biosciences).
ELISA
After 24 h of treatment, concentrations of IL-1β
(IL-1β Human ELISA kit; R&D Systems, Inc.) and IL-18 (IL-18
Human ELISA kit; Thermo Fisher Scientific, Inc.) in the supernatant
of AC16 cells were determined using an ELISA kit according to the
manufacturer's protocols. The concentrations of BMP-2 (Rat BMP-2
ELISA kit; Andy Gene), BNP (Rat BNP Elisa kit; Beijing Solarbio
Science & Technology Co., Ltd.), ANP (Rat ANP ELISA kit; Abcam;
cat. no. ab108797), IL-1β (IL-1β Rat ELISA kit; R&D Systems,
Inc.) and IL-18 (IL-18 Rat ELISA kit; Thermo Fisher Scientific,
Inc.) in the serum of rats were determined using an ELISA kit
(IL-1β Human ELISA kit; R&D Systems, Inc.; IL-18 Human ELISA
kit; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To determine the expression level of NLRP3, the
total RNA was extracted from the AC16 cells (1x107) and
purified using the TRIzol approach (Thermo Fisher Scientific,
Inc.). PrimeScript™ RT reagent kit (Takara Bio, Inc.) was used to
reverse transcribe total RNA into cDNA, according to the
manufacturer's protocols. RT-qPCR was then performed on ABI 9700
thermocyclers using with the SYBR™ Green PCR Master Mix reagent
(Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for RT-qPCR: 95˚C for 10 min, 40 cycles of
95˚C for 30 sec, 60˚C for 15 sec and 72˚C for 15 sec. The specific
NLRP3 primer sequences were: NLRP3 forward,
5'-TTCGGAGATTGTGGTTGGG-3' and reverse, 5'-TCAGGGAATGGCTGGTGC-3'.
GAPDH was used as an internal reference gene, and the GAPDH
specific primer sequences were as follows: GAPDH forward,
5'-AATCCCATCACCATCTTC-3' and reverse, 5'-AGGCTGTTGTCATACTTC-3'. The
relative expression of NLRP3 was calculated using the
2-ΔΔCq method (20).
Western blot analysis
Cell lysate was collected using RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA) with a protease inhibitor cocktail set
(Sigma-Aldrich; Merck KGaA) and protein concentration was
determined by a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.). The isolated proteins (30 µg) were separated by
electrophoresis in 7.5% SDS-polyacrylamide gels and transferred
onto a nitrocellulose membrane (EMD Millipore), blocked in 5%
fat-free milk overnight at 4˚C. The transferred membranes were then
incubated with primary antibodies against BMP-2 (Abcam; cat. no.
ab232401; dilution, 1:1,000), NLRP3 (Abcam; cat. no. ab210491;
dilution, 1:5,000), GSDMD-N (Abcam; cat. no. ab215203; dilution,
1:1,000), caspase-1 (Abcam; cat. no. ab207802; dilution, 1:1,000),
GAPDH (Cell Signaling Technology, Inc.; cat. no. 5174; dilution,
1:1,000) at 4˚C overnight, and horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (Beyotime Institute of
Biotechnology; cat. nos. A0208 and A0216; dilution, 1:1,000) at
37˚C for 1 h. Signal quantification was performed by an enhanced
chemiluminescence system (Bio-Rad Laboratories, Inc.). The bands
were quantified by densitometry with ImageJ software (version 1.51;
National Institutes of Health).
Animals and study design
Sprague-Dawley rats (male, 6-8 weeks old; weight,
210-230 g; n=18) were obtained from HFK Bioscience Co. Ltd. under
standard laboratory conditions. The rats were kept in the animal
facility at 25˚C (humidity, 60-70%) with a 12-h light/dark cycle
and received food and water ad libitum. All experimental
procedures were performed according to the protocols approved by
the Bioethics Committee of Shanghai TCM-Integrated Hospital,
Shanghai University of Traditional Chinese Medicine (approval no.
PZSHUTCM170823001) on August 23, 2017. To induce diabetes, all
animals were fasted for 12 h prior to receiving a single
intraperitoneal injection of streptozotocin (STZ) with a dose of 60
mg/kg (Sigma-Aldrich; Merck KGaA). Citrate buffer was used as a
vehicle and was administered to control groups (11). The diabetic rat model was considered
successful if the following observations were present: i)
Indication of hyperglycemia, with a blood glucose level >16.7
mmol/l 72 h after the injection (including 6 h of fasting); and ii)
increased food consumption and urination.
The HF injury model in vivo was generated as
previously reported (21). After 12
weeks, rats were administered with 6 equal intraperitoneal
injections of 2.5 mg/kg doxorubicin (DOX; Fresenius Kabi Oncology
Ltd.) over a period of 2 weeks. Standard cardiac function
evaluation was performed as previously reported (22,23).
The hemodynamic variables were measured by the SPR-838 rat
pressure-volume catheter as a part of the MPVS-300 pressure-volume
system (Millar). The echocardiographic variables were obtained
through a phased-array transducer probe (7.5-MHz) as connected to
the Sonos 5500 imaging system (Philips Medical Systems, Inc.).
Echocardiography images were collected following the American
Society of Echocardiography's recommendations (24). At the end of the procedure, all rats
(n=6 in each group) were sacrificed with an intravenous bolus of
140 mg/kg sodium pentobarbital (Dolethal), confirmed by the
performance of continuous involuntary breathing for 2-3 min and no
blink reflex, and their myocardial tissues samples were obtained
and subjected to apoptosis detection by terminal-deoxynucleoitidyl
transferase-mediated nick end labeling (TUNEL) (25).
Statistical analysis
A minimum sample size of 21 patients was calculated
by 80% power at a significance level of 5%. Data based on at least
three independent experiments performed in triplicate are presented
as the mean ± standard deviation and processed by statistical
tools, such as GraphPad Prism 8.0.2 (GraphPad). To determine if
data were normally distributed, the Shapiro-Wilk test was used. To
compare various parameters among different groups, the measurements
observed from each experimental group were analyzed by a one-way
analysis of variance, followed by Bonferroni correction for
multiple comparisons. Pearson correlation coefficient was used for
the correlation between BMP-2 and ANP or BNP concentration.
P<0.05 was considered to indicate a statistically significant
difference.
Results
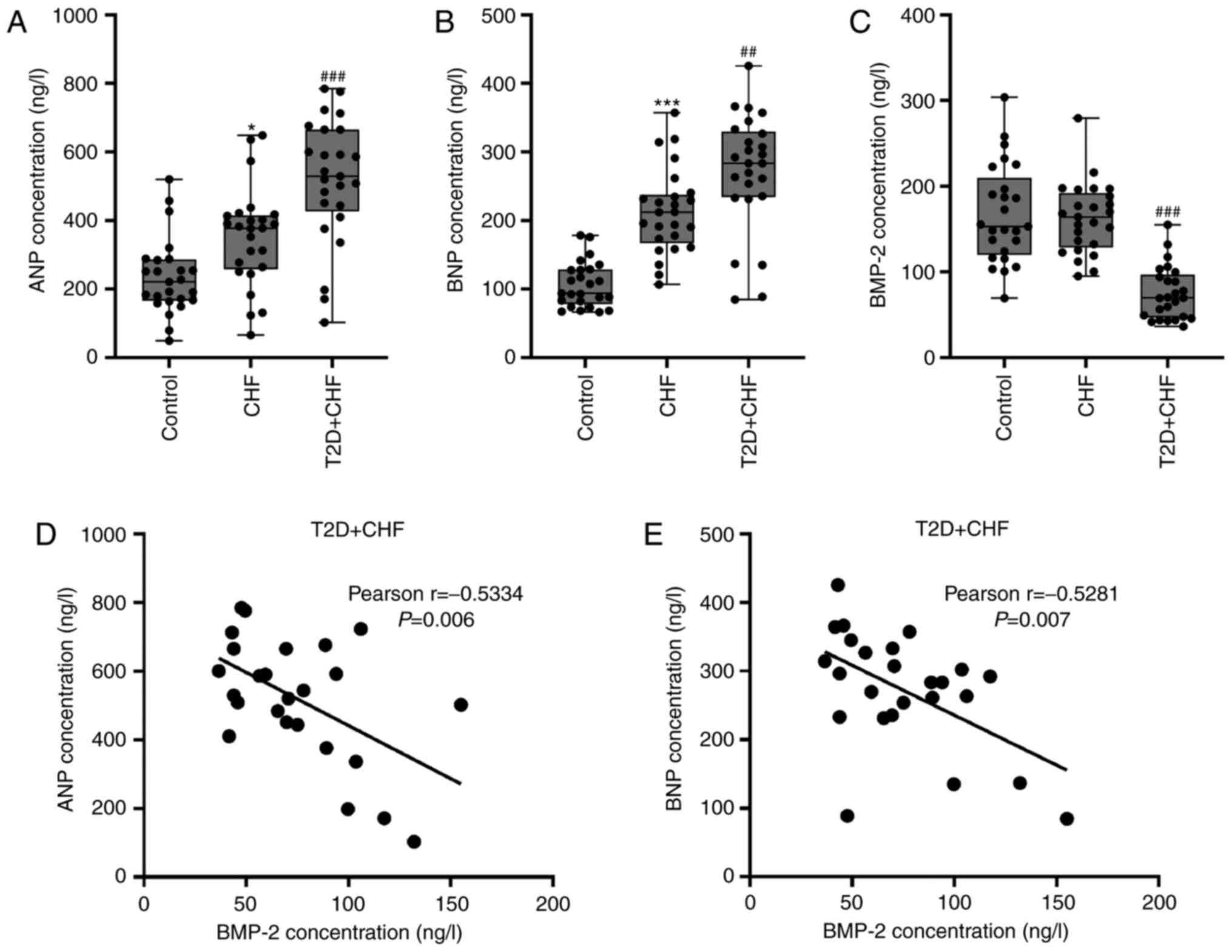
Serum levels of BMP-2 are negatively
correlated with the levels of ANP and BNP in CHF with diabetes
patients
ANP and BNP levels were first measured in serum from
patients with CHF alone and those with diabetes. Consistent with
previously published data (6,26), a
significant increase in ANP and BNP was observed in the CHF and CHF
with diabetes groups, compared with the controls (Fig. 1A and B). ANP and BNP were further increased in
the CHF with diabetes patients. By contrast, BMP-2 levels were
lower in CHF with diabetic patients than in the controls and CHF
patients (Fig. 1C). To further
investigate the association between BMP-2 and ANP or BNP levels, a
Pearson correlation analysis was performed using serum samples from
CHF patients with diabetes. The results of the present study
revealed that there were significant negative correlations between
BMP-2 levels and ANP (r=-0.5334; P=0.006; Fig. 1D) or BNP (r=-0.5281; P=0.007;
Fig. 1E). Since there was a
correlation between serum BMP-2 and ANP/BNP, the molecular
functions of BMP-2 in the pathogenesis of diabetes-associated CHF
were investigated in vitro and in vivo models.
BMP-2 reduces the NLRP3 inflammasome
activation and cell pyroptosis induced by HG and cardiac injury in
AC16 cells
Cardiac myocyte damage was induced using DOX and
cells were treated with HG. Pyroptotic cell death was investigated
with an active caspase-1 and PI staining. Consistent with
previously published results (27),
DOX significantly increased cell pyroptosis (Fig. 2A and B). HG administration further exacerbated
cell pyroptosis when combined with DOX (Fig. 2A and B). By contrast, recombinant BMP-2 markedly
decreased DOX and HG-induced cell pyroptosis (Fig. 2A and B). ROS production was measured using a
DCFH-DA probe. Similar to the cell pyroptosis results, DOX induced
ROS production, and HG exaggerated this increase in ROS (Fig. 2C and D). In the presence of BMP-2, the increase
in ROS was largely suppressed (Fig.
2C and D). Next, the amount of
ANP, BNP, BMP-2, IL-1β and IL-18 were measured using ELISA. All
were increased following DOX and HG administration, but recombinant
BMP-2 moderated these increases (Fig.
2E). Next, the protein levels of pyroptosis markers were
investigated using the Western blot analysis. Consistent with the
results of in vivo data, DOX and HG treatments caused an
increase in NLRP3, pro-caspase-1, active caspase-1 and GSDMD-N;
exogenous BMP-2 prevented DOX and HG-induced increase (Fig. 2F). Since BMP-2 inhibited an NLRP3
increase, we hypothesized that BMP-2 may affect NLRP3
signaling.
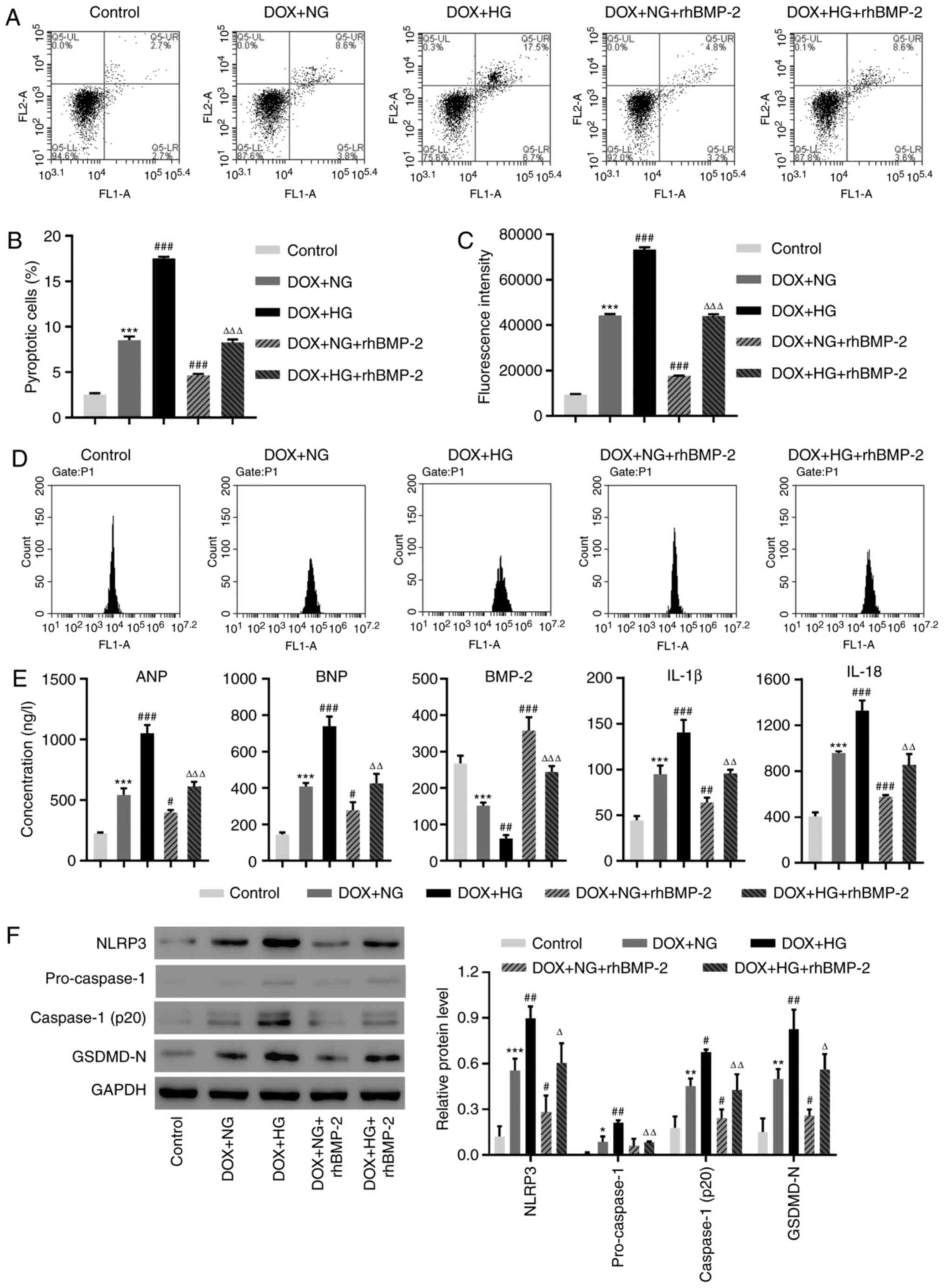 | Figure 2rhBMP-2 decreases the NLRP3
inflammasome activation and cell pyroptosis was induced by HG in
DOX-induced AC16 cells. (A and B) The cell pyroptosis and (C and D)
Reactive oxygen species production were assessed by flow cytometry.
(E) The concentrations of ANP, BNP, BMP-2, IL-1β and IL-18 were
determined by ELISA. (F) The expression of NLRP3, Caspase-1 and
GSDMD-N were measured using Western blotting.
*P<0.05, **P<0.01 and
***P<0.001, compared with the control.
#P<0.05, ##P<0.01 and
###P<0.001, compared with DOX + NG.
ΔP<0.05, ΔΔP<0.01 and
ΔΔΔP<0.001, compared with DOX + HG. rhBMP-2,
recombinant human BMP-2; NLRP3, NLR family pyrin domain-containing
3; HG, high-glucose; DOX, doxorubicin; BMP-2, bone morphogenetic
protein-2; ANP, atrial natriuretic peptide; BNP, brain natriuretic
peptide; IL, interleukin; GSDMD-N, gasdermin D; NG, no glucose. |
BMP-2 alleviates the NLRP3
inflammasome activation and cell pyroptosis, induced by
overexpressing NLRP3
NLRP3 was upregulated at transcriptional and
translational levels following overexpression in AC16cells
(Fig. 3A and B). Exogenous BMP-2 significantly
suppressed NLRP3-overexpression-induced cell pyroptosis and ROS
generation (Fig. 3C-F).
NLRP3-overexpression caused an increase in ANP, BNP, IL-1β and
IL-18. These effects were inhibited by the presence of BMP-2
(Fig. 3G). Finally, BMP-2
suppressed the increase in pyroptosis markers induced by NLRP3
(Fig. 3H). These results suggested
that BMP-2 may serve as the inhibitor of NLRP3 in pyroptosis and
inflammatory responses.
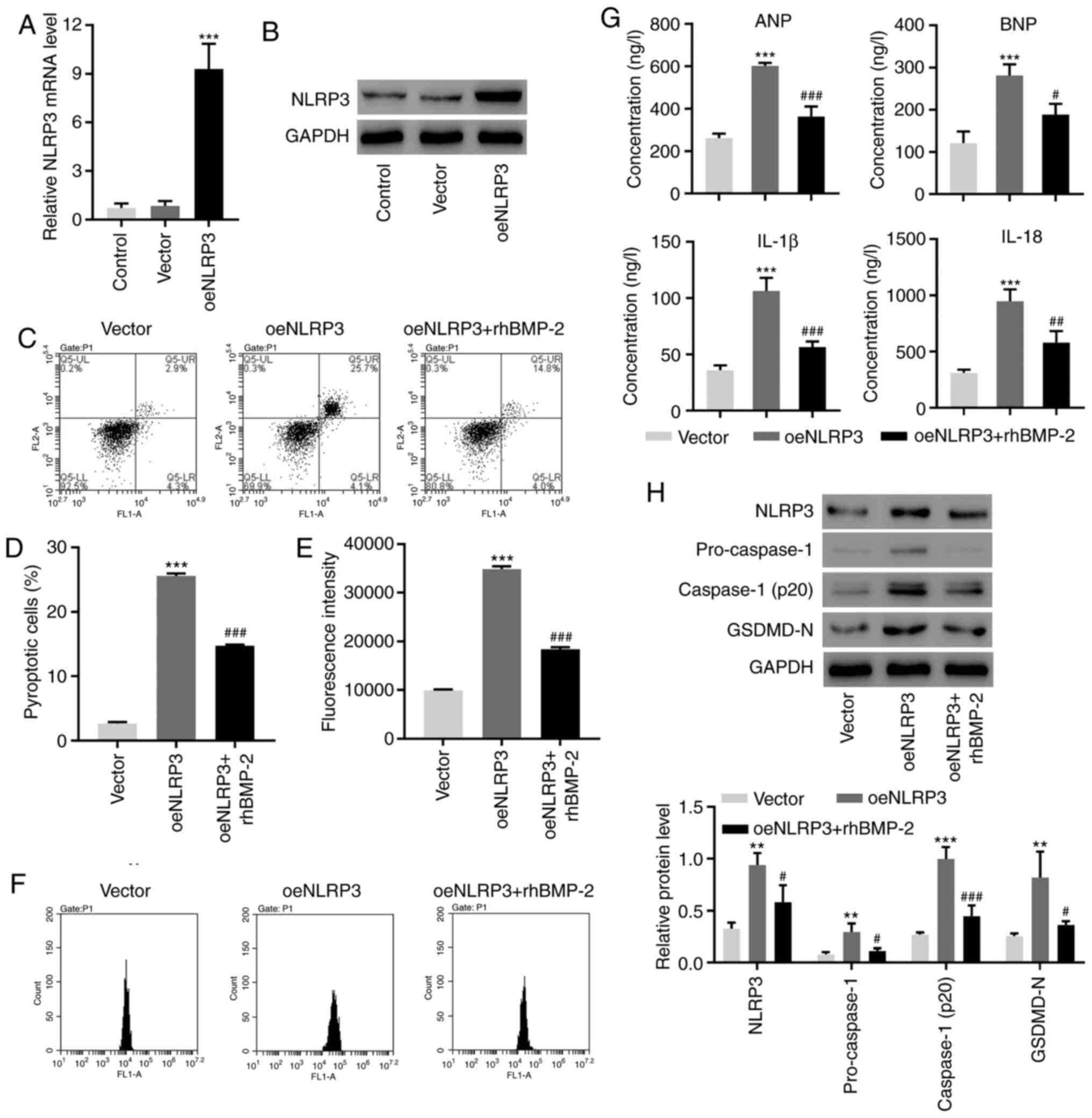 | Figure 3rhBMP-2 decreases the NLRP3
inflammasome activation and cell pyroptosis is induced by
NLRP3-overexpression in AC16 cells. The expression of NLRP3,
Caspase-1 and GSDMD-N was measured by (A) reverse
transcription-quantitative polymerase chain reaction and (B)
Western blotting. (C and D) The cell pyroptosis and (E and F)
reactive oxygen species production were assessed using flow
cytometry. (G) The concentrations of ANP, BNP, IL-1β and IL-18 were
determined using ELISA. (H) The expression of NLRP3, Caspase-1 and
GSDMD-N was measured using Western blotting. **P<0.01
and ***P<0.001, compared with the vector.
#P<0.05, ##P<0.01 and
###P<0.001, compared with oeNLRP3. rhBMP-2,
recombinant human BMP-2; NLRP3, NLR family pyrin domain-containing
3; NLRP3, NLR family pyrin domain-containing 3; GSDMD-N, gasdermin
D; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide;
IL, interleukin; oe, overexpression. |
Diabetes exaggerates DOX-induced
myocardial injuries in vivo
The present study investigated how BMP-2 contributes
toward these pathological processes in vivo. Myocardial
injury was induced in rats using DOX and induced diabetes using
STZ. The results of the present study confirmed a decline in the
end-systolic pressure, heart rate, stroke volume and ejection
fraction, as well as an increase in the end-diastolic pressure
following DOX administration (Fig.
4A). STZ exaggerated cardiotoxicity in these parameters
(Fig. 4A). These results validated
the successful establishment of myocardial damage and diabetes in
an animal model. Consistent with our patient data, ANP and BNP were
increased in the DOX group and further increased in the DOX + STZ
group (Fig. 4B). By contrast, BMP-2
was decreased in the two groups (Fig.
4B). TUNEL staining revealed an increased cell death in the
myocardium following DOX treatment and a higher increase in cell
death was observed in the DOX+STZ group (Fig. 4C and D). Western blotting confirmed the decrease
in BMP-2 (Fig. 4E), and
demonstrated that DOX administration induced increased expression
of NLRP3, pro-caspase-1, active caspase-1 and GSDMD-N (Fig. 4E). When DOX was combined with STZ,
the increase was more notable (Fig.
4E). Similar changes were found in concentrations of IL-1β and
IL-18. The two were significantly increased in DOX- and DOX +
STZ-treated animals (Fig. 4F).
These results suggested that the apoptosis and inflammasome
pathways are activated in myocardial damage and diabetes.
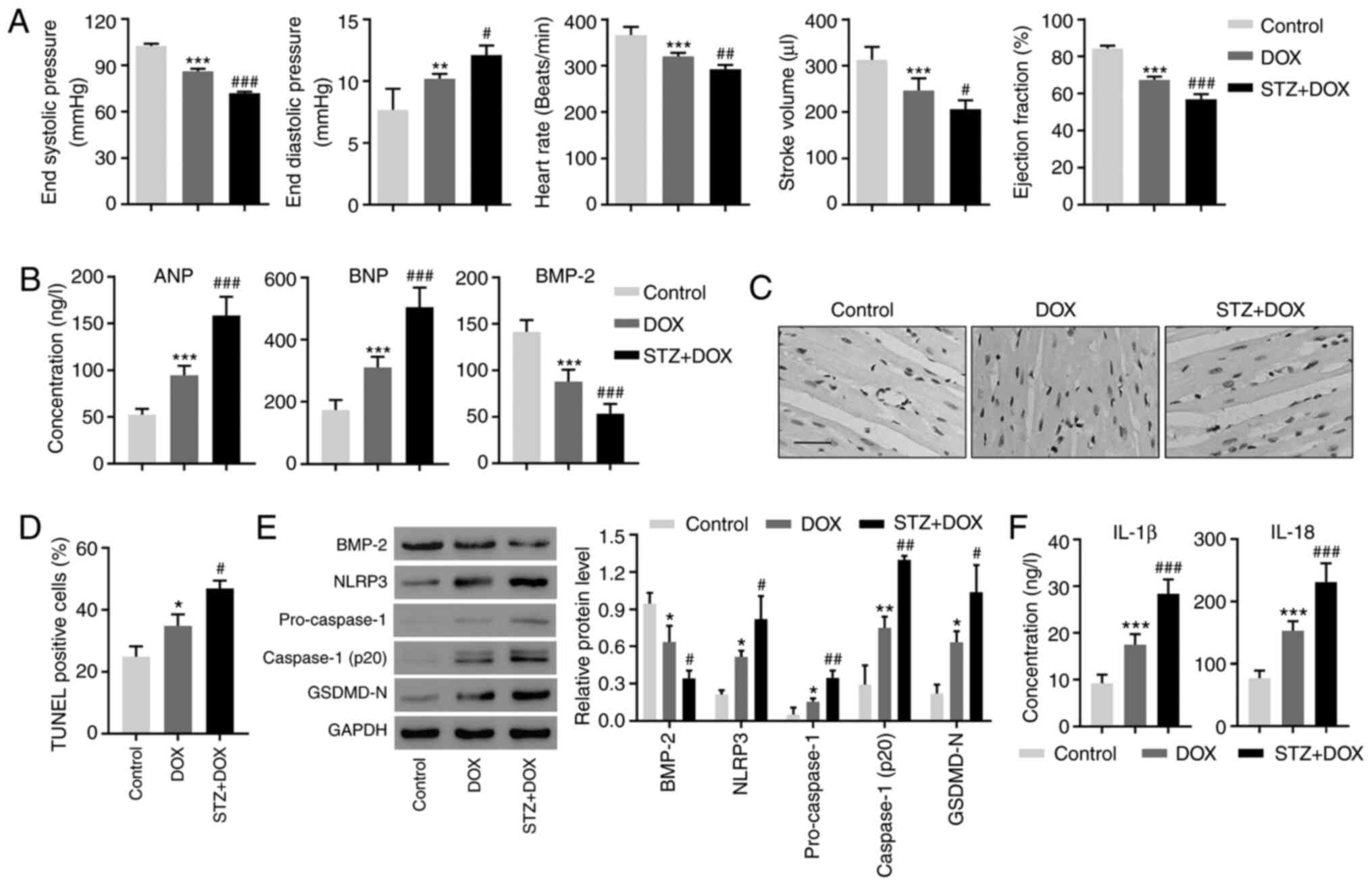 | Figure 4Diabetes aggravates the degree of
DOX-induced myocardial injury in rats. (A) Cardiac functional
parameters were measured. (B) The peripheral blood levels of ANP,
BNP and BMP-2 were determined using ELISA. (C) Representative
photomicrographs of TUNEL-stained myocardium. Scale bar, 50 µm. (D)
Quantization of TUNEL-positive cells. (E) The expression of BMP-2,
NLRP3, Caspase-1 and GSDMD-N in myocardium were measured using
Western blotting. (F) The peripheral blood levels of IL-1β and
IL-18 were determined using ELISA. *P<0.05,
**P<0.01 and ***P<0.001, compared with
the control. #P<0.05, ##P<0.01 and
###P<0.001, compared with DOX. DOX, doxorubicin; ANP,
atrial natriuretic peptide; BNP, brain natriuretic peptide; BMP-2,
bone morphogenetic protein-2; NLRP3, NLR family pyrin
domain-containing 3; GSDMD-N, gasdermin D; IL, interleukin. |
Discussion
The present study first demonstrated that BMP-2 is
negatively associated with ANP and BNP content in CHF patients with
diabetes. Consistent with patient data, a similar association
between BMP-2 and natriuretic peptides was also observed in animal
models and cultured cells. The results of the present study
confirmed that BMP-2 antagonizes the activation of the NLRP3
inflammasome. In addition, BMP-2 inhibits the expression and
release of pyroptosis-related factors, including NLRP3, active
caspase-1, IL-1β, IL-18 and GSDMD-N, in diabetic
cardiomyopathy.
BMPs regulate the proper development of the heart.
BMP-2, BMP-4 and BMP-5 are localized to the myocardium, and each
has distinct functions (28).
Bmp2 deletion is embryonically lethal due to severe defects
in cardiogenesis (29). BMP-2 is
necessary and sufficient to specify cardiac progenitors during the
development of the atria and ventricles (30). The dysregulation of BMPs and ROS in
CHF and diabetes has been well characterized (31). However, how BMP-2 suppresses excess
ROS damage remains unclear since the changes between BMP-2 and ROS
has only been reported in an ethanol-induced abnormal cardiogenesis
(32). BMP-2 is upregulated in
atherosclerotic lesions under oxidative stress, inflammation and
hyperglycemia (33). Consistent
with these results, it was found that overexpressing BMP-2
significantly decreased ROS generation and inflammation responses.
The analysis on clinical data suggested that diabetic hearts have
higher sensitivity to various injuries, mainly due to the increased
ROS production, activating inflammatory cascades in the
NLRP3-mediated pyroptosis (11).
This unique pyroptosis is a proinflammatory form of regulated
necrosis and caspase 1-dependent cell death. The initiation of
pyroptosis occurs through the inflammatory caspases. Crosstalk
between proinflammatory cytokines and ROS has been indicated in
hyperglycemia- and pyroptosis-caused pathological processes in
diabetic cardiomyopathy (34). BMP
activity inhibits inflammation in myocardial infarction (35). Consistent with these data, the
results of the present study provided compelling evidence that
BMP-2 suppresses NLRP3-mediated pyroptosis in diabetic hearts.
Diabetes also promotes the DOX-induced decrease in heart rate. The
decreased heart rate induced by DOX was also observed in the study
by Jafarinezhad et al (36),
which demonstrated increased serum troponin I, QT interval and QRS
complex in rats treated with DOX. The increment of serum troponin
Ilevels following DOX is a strong predictor of ventricular
dysfunction and poor cardiac outcome in rats and humans (37,38).
Ji et al (39) indicated
that NF-κB may be responsible for the transcriptional regulation of
the TNNI1 gene, coding troponin I. These data suggested that
diabetes may promote a DOX-induced decrease in the heart rate
through the NF-κB signaling pathway. This should be further
investigated in future studies. The molecular mechanisms by which
BMP-2 regulates NLRP3 inflammasomes via ROS remain under
investigation. BMP-2 was previously reported to antagonize
BMP-4-induced cardiomyocyte apoptosis, and BMP-2 alone did not
elicit apoptosis (15). The results
of the present study demonstrated that BMP-2 alone was sufficient
to exhibit protective effects against cell death in
cardiomyocytes.
The diminished effectiveness of natriuretic peptides
is an important component of the CHF pathogenesis, impairing volume
overload, vasoconstriction and patient prognoses (26). ANP exhibits various biological
functions, not only in the myocardium but also by mediating
crosstalk between the myocardium and epicardium (40). Changes in BMP signaling and ANP/BNP
were reported in cardiovascular hypertrophy (41). However, the association between
BMP-2 and ANP or BNP in CHF patients with diabetes was not
previously studied. To the best of our knowledge, the present study
was the first to report that BMP-2 is negatively correlated with
ANP and BNP levels in CHF patients with diabetes. The other
important aspect of ANP and BNP is their blunted renal response in
CHF patients (23). The
compensatory neurohormonal, local renal effectors and how BMP-2 is
involved in vasoconstrictor systems, including the
renin-angiotensin-aldosterone system, require future study. A
previous study has proven that rhBMP2 administration may stimulate
osteogenesis (42). Since an
increased expression of BMP2 and inactivation of NLRP3 inflammasome
may inhibit diabetic cardiomyopathy in vitro and in
vivo, the administration of rhBMP2 or NLRP3 inflammasome
inhibitors alone or in combination may therefore be potential
treatments for diabetic cardiomyopathy. Since some nod-like
receptors, including NLRP1, NLRP3, NLRP6, NLRP7, NLRP12 and NLRC4,
and AIM2 and Pyrin may also form inflammasome, whether the
anti-inflammatory effect by BMP-2 associated with these
inflammasomes require further investigation. Furthermore, NLRP3 was
diffused across the cytosol under basal conditions. However, it
formed multiple small puncta upon the NLRP3 activator nigericin
treatment (43), and the protection
of rhBMP-2 in cardiomyocytes against DOX-induced NLRP3 inflammasome
activation by means of NLRP3 immunofluorescence would be further
confirmed in a future study.
In summary, the results of the present study
demonstrated that BMP-2 negatively regulated ANP and BNP content in
diabetic cardiomyopathy. BMP-2 suppresses pyroptotic cell death
induced by ROS-mediated NLRP3 inflammasomes via caspase-1 cascades.
Future studies investigating other pathways and renal responses
will further elucidate the molecular mechanisms underlying CHF and
diabetes.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Scientific Research
Project of Science and Technology Commission of Shanghai
Municipality (grant no. 18401900400) and the Scientific Research
Project of Shanghai Hongkou District Health Commission (grant no.
1802-02).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JMZ, RQY, FZW and CYX were involved in experimental
designs and drafting of the manuscript. LQ, XRW and YJF performed
the experiments. JMZ, RQY and CYX confirm the authenticity of all
the raw data. YFL, YP and CYX acquired, analyzed and interpreted
the data and involved in writing, review and editing the
manuscript, as well as supervision. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by Shanghai
TCM-Integrated Hospital, Shanghai University of Traditional Chinese
Medicine (approval no. 2015-022-1), and written informed consent
was obtained from each patient. Animal experiments were approved by
the Animal Research Ethics Committee of Shanghai TCM-Integrated
Hospital, Shanghai University of Traditional Chinese Medicine
(approval no. PZSHUTCM170823001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan H, Anker SD, Januzzi JL Jr, McGuire
DK, Sattar N, Woerle HJ and Butler J: Heart failure epidemiology in
patients with diabetes mellitus without coronary heart disease. J
Card Fail. 25:78–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hunt SA, Abraham WT, Chin MH, Feldman AM,
Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K,
et al: ACC/AHA 2005 Guideline Update for the Diagnosis and
Management of Chronic Heart Failure in the Adult: A report of the
American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (Writing Committee to Update the 2001
Guidelines for the Evaluation and Management of Heart Failure):
Developed in collaboration with the American College of Chest
Physicians and the International Society for Heart and Lung
Transplantation: Endorsed by the Heart Rhythm Society. Circulation.
112:e154–e235. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dunlay SM, Givertz MM, Aguilar D, Allen
LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich
CL, et al: Type 2 diabetes mellitus and heart failure, a scientific
statement from the American heart association and heart failure
society of America. J Card Fail. 25:584–619. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bertoni AG, Hundley WG, Massing MW, Bonds
DE, Burke GL and Goff DC Jr: Heart failure prevalence, incidence,
and mortality in the elderly with diabetes. Diabetes Care.
27:699–703. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cullan A, Grover M and Hitchcock K: FPIN's
clinical inquiries: Brain natriuretic peptide for ruling out heart
failure. Am Fam Physician. 83:1333–1334. 2011.PubMed/NCBI
|
|
6
|
Kalra PR, Anker SD and Coats AJ: Water and
sodium regulation in chronic heart failure: The role of natriuretic
peptides and vasopressin. Cardiovasc Res. 51:495–509.
2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Broz P: Immunology: Caspase target drives
pyroptosis. Nature. 526:642–643. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Danelishvili L and Bermudez LE: Analysis
of pyroptosis in bacterial infection. Methods Mol Biol. 1004:67–73.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tavakolizadeh J, Roshanaei K, Salmaninejad
A, Yari R, Nahand JS, Sarkarizi HK, Mousavi SM, Salarinia R,
Rahmati M, Mousavi SF, et al: MicroRNAs and exosomes in depression:
Potential diagnostic biomarkers. J Cell Biochem. 119:3783–3797.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M,
Meng Q, Zhou B, Leng Y and Xia ZY: NLRP3 inflammasome
activation-mediated pyroptosis aggravates myocardial
ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev.
2017(9743280)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang J, Greene SB and Martin JF: BMP
signaling in congenital heart disease: New developments and future
directions. Birth Defects Res A Clin Mol Teratol. 91:441–448.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Miyazono K, Kamiya Y and Morikawa M: Bone
morphogenetic protein receptors and signal transduction. J Biochem.
147:35–51. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zheng M, Zhu J, Lv T, Liu L, Sun H and
Tian J: Bone morphogenetic protein-2 enhances the expression of
cardiac transcription factors by increasing histone H3 acetylation
in H9c2 cells. Mol Med Rep. 7:953–958. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu J, Sun B, Huo R, Wang YC, Yang D, Xing
Y, Xiao XL, Xie X and Dong DL: Bone morphogenetic protein-2
antagonizes bone morphogenetic protein-4 induced cardiomyocyte
hypertrophy and apoptosis. J Cell Physiol. 229:1503–1510.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tokola H, Rysä J, Pikkarainen S, Hautala
N, Leskinen H, Kerkelä R, Ilves M, Aro J, Vuolteenaho O, Ritvos O
and Ruskoaho H: Bone morphogenetic protein-2-a potential
autocrine/paracrine factor in mediating the stretch activated
B-type and atrial natriuretic peptide expression in cardiac
myocytes. Mol Cell Endocrinol. 399:9–21. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Izumi M, Masaki M, Hiramoto Y, Sugiyama S,
Kuroda T, Terai K, Hori M, Kawase I and Hirota H: Cross-talk
between bone morphogenetic protein 2 and leukemia inhibitory factor
through ERK 1/2 and Smad1 in protection against doxorubicin-induced
injury of cardiomyocytes. J Mol Cell Cardiol. 40:224–233.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Panahi G, Pasalar P, Zare M, Rizzuto R and
Meshkani R: High glucose induces inflammatory responses in HepG2
cells via the oxidative stress-mediated activation of NF-κB, and
MAPK pathways in HepG2 cells. Arch Physiol Biochem. 124:468–474.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wree A, Eguchi A, McGeough MD, Pena CA,
Johnson CD, Canbay A, Hoffman HM and Feldstein AE: NLRP3
inflammasome activation results in hepatocyte pyroptosis, liver
inflammation, and fibrosis in mice. Hepatology. 59:898–910.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ahmad S, Panda BP, Kohli K, Fahim M and
Dubey K: Folic acid ameliorates celecoxib cardiotoxicity in a
doxorubicin heart failure rat model. Pharm Biol. 55:1295–1303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Malka A, Ertracht O, Bachner-Hinenzon N,
Reiter I and Binah O: The cardioprotective efficacy of TVP1022
against ischemia/reperfusion injury and cardiac remodeling in rats.
Pharmacol Res Perspect. 4(e00272)2016.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Ma H, Kong J, Wang YL, Li JL, Hei NH, Cao
XR, Yang JJ, Yan WJ, Liang WJ, Dai HY and Dong B:
Angiotensin-converting enzyme 2 overexpression protects against
doxorubicin-induced cardiomyopathy by multiple mechanisms in rats.
Oncotarget. 8:24548–24563. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Arias T, Chen J, Fayad ZA, Fuster V,
Hajjar RJ and Chemaly ER: Comparison of echocardiographic
measurements of left ventricular volumes to full volume magnetic
resonance imaging in normal and diseased rats. J Am Soc
Echocardiogr. 26:910–918. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu X, Philip JL, Razzaque MA, Lloyd JW,
Muller CM and Akhter SA: High-molecular-weight polyethylene glycol
inhibits myocardial ischemia-reperfusion injury in vivo. J Thorac
Cardiovasc Surg. 149:588–593. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Diez J: Chronic heart failure as a state
of reduced effectiveness of the natriuretic peptide system:
Implications for therapy. Eur J Heart Fail. 19:167–176.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Meng L, Lin H, Zhang J, Lin N, Sun Z, Gao
F, Luo H, Ni T, Luo W, Chi J and Guo H: Doxorubicin induces
cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional
stabilization of NLR family pyrin domain containing 3. J Mol Cell
Cardiol. 136:15–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cai CL, Zhou W, Yang L, Bu L, Qyang Y,
Zhang X, Li X, Rosenfeld MG, Chen J and Evans S: T-box genes
coordinate regional rates of proliferation and regional
specification during cardiogenesis. Development. 132:2475–2487.
2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rivera-Feliciano J and Tabin CJ: Bmp2
instructs cardiac progenitors to form the heart-valve-inducing
field. Dev Biol. 295:580–588. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sánchez-de-Diego C, Valer JA,
Pimenta-Lopes C, Rosa JL and Ventura F: Interplay between BMPs and
reactive oxygen species in cell signaling and pathology.
Biomolecules. 9(534)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li S, Wang G, Gao LR, Lu WH, Wang XY,
Chuai M, Lee KK, Cao L and Yang X: Autophagy is involved in
ethanol-induced cardia bifida during chick cardiogenesis. Cell
Cycle. 14:3306–3317. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hruska KA, Mathew S and Saab G: Bone
morphogenetic proteins in vascular calcification. Circ Res.
97:105–114. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li X, Du N, Zhang Q, Li J, Chen X, Liu X,
Hu Y, Qin W, Shen N, Xu C, et al: MicroRNA-30d regulates
cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic
cardiomyopathy. Cell Death Dis. 5(e1479)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sanders LN, Schoenhard JA, Saleh MA,
Mukherjee A, Ryzhov S, McMaster WG Jr, Nolan K, Gumina RJ, Thompson
TB, Magnuson MA, et al: BMP antagonist gremlin 2 limits
inflammation after myocardial infarction. Circ Res. 119:434–449.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jafarinezhad Z, Rafati A, Ketabchi F,
Noorafshan A and Karbalay-Doust S: Cardioprotective effects of
curcumin and carvacrol in doxorubicin-treated rats: Stereological
study. Food Sci Nutr. 7:3581–3588. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Reagan WJ, York M, Berridge B, Schultze E,
Walker D and Pettit S: Comparison of cardiac troponin I and T,
including the evaluation of an ultrasensitive assay, as indicators
of doxorubicin-induced cardiotoxicity. Toxicol Pathol.
41:1146–1158. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
El-Sayed el SM, Mansour AM and
Abdul-Hameed MS: Thymol and carvacrol prevent doxorubicin-induced
cardiotoxicity by abrogation of oxidative stress, inflammation, and
apoptosis in rats. J Biochem Mol Toxicol. 30:37–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ji GG, Shu JT, Zhang M, Ju XJ, Shan YJ,
Liu YF and Tu YJ: Transcriptional regulatory region and DNA
methylation analysis of TNNI1 gene promoters in Gaoyou duck
skeletal muscle (Anas platyrhynchos domestica). Br Poult Sci.
60:202–208. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Milano S, Carmosino M, Gerbino A, Svelto M
and Procino G: Hereditary nephrogenic diabetes insipidus:
Pathophysiology and possible treatment. An update. Int J Mol Sci.
18(2385)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Usher MG, Duan SZ, Ivaschenko CY, Frieler
RA, Berger S, Schütz G, Lumeng CN and Mortensen RM: Myeloid
mineralocorticoid receptor controls macrophage polarization and
cardiovascular hypertrophy and remodeling in mice. J Clin Invest.
120:3350–3364. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yao J, Liu Z, Ma W, Dong W, Wang Y, Zhang
H, Zhang M and Sun D: Three-dimensional coating of SF/PLGA coaxial
nanofiber membranes on surfaces of calcium phosphate cement for
enhanced bone regeneration. ACS Biomater Sci Eng. 6:2970–2984.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen J and Chen ZJ: PtdIns4P on dispersed
trans-Golgi network mediates NLRP3 inflammasome activation. Nature.
564:71–76. 2018.PubMed/NCBI View Article : Google Scholar
|