Introduction
The placenta is a specialized organ that is
considered to be the vascular interface between the maternal and
fetal circulatory systems (1).
Genetic, environmental, transcriptional and epigenetic factors are
actively involved in the formation of this interface (1). Numerous studies in mice and humans
have revealed that factors such as malnutrition, smoking,
alcoholism, drug use and pollution can induce alterations in gene
expression; these alterations can lead to morphological and
physiological alterations that can produce disturbances in
placental and fetal growth, which can affect the health of the
individual in the long term (2-8).
Specifically, exposure to cigarette smoke has been
associated with alterations in placental development, such as
inhibition of trophoblast invasion, which generates placental
hypoxia (9), thickens the
trophoblastic basement membrane (10) and increases umbilical cord blood
flow resistance (11). This is
because compounds such as nicotine, polycyclic aromatic
hydrocarbons and nitrosamines, all of which are present in tobacco,
manage to cross the transplacental barrier and accumulate in the
fetal environment (12). Further to
the aforementioned alterations, exposure to cigarette smoke during
pregnancy is also considered to be a risk factor for poor pregnancy
outcomes, such as: i) Intrauterine growth restriction; ii)
premature birth (13); iii) low
birth weight (14); and iv)
diseases in early childhood, such as allergies and asthma (15).
Asthma is a phenotypically heterogeneous
inflammatory disease of the airways and is associated with
intermittent respiratory signs and symptoms, bronchial
hyperreactivity and reversible airflow obstruction (16). At the immune response level,
asthmatic individuals characteristically exhibit more Th2 type CD4
T lymphocytes (TLs), which produce a spectrum of cytokines, such as
IL-4, IL-5 and IL-13(17).
The process of cell differentiation from virgin CD4
TLs to effectors requires interactions between antigen-presenting
cells, specifically dendritic cells and CD4 TLs (18). During this interaction, signaling
pathways are activated that allow the expression of genes needed to
establish the TL profile according to the initial antigenic
stimulus (18). Therefore, for the
acquisition of the Th2 phenotype, activation of STAT6 is required,
which, along with other factors, activates the expression of GATA
binding protein 3 (GATA3) (18,19).
However, for the process of cell differentiation from virgin TLs
towards the Th1 profile, the initial expression of signal
transducer and activator of STAT1 is required. The transcription
factor STAT1 activates the expression of T-Box transcription factor
21 (T-BET), which is required for the differentiation of TLs
towards the Th1 phenotype (Fig.
S1) (20-24).
RUNX family proteins are transcription factors that
participate in processes associated with embryonic development,
such as: i) Cartilage, bone and nervous system formation; ii)
angiogenesis; iii) hematopoiesis; and iv) the immune system
response (20). RUNX1 and RUNX3
mediate the normal maturation of various components of the immune
system; specifically, it has been revealed that RUNX1 attenuates
the differentiation of Th2 TLs concomitant with the repression of
GATA3 (20). By contrast,
RUNX3 is relevant for the differentiation of TLs to the Th1
lineage, which occurs through its interaction with T-BET (21).
Data describing the effects of cigarette smoke
exposure on RUNX1 and RUNX3 gene expression are
conflicting. In a murine model, it has been reported that exposure
to nicotine and tobacco smoke during gestation significantly
decreases RUNX1 and RUNX3 expression levels in lung
tissue of 3- and 5-day-old neonatal mice (22). However, RUNX1 expression
levels in humans are increased in neonates from pregnant women
smokers, as reported in a meta-analysis study in which aberrant
expression of RUNX1 was associated with the onset and
progression of acute lymphoblastic leukemia (23). Additionally, it has been
demonstrated that the expression of RUNX transcription factors in
the placenta play fundamental roles in the formation of placental
hematopoietic stem and progenitor cells (HSPCs). Pregnancy
complications that result in preterm births differentially affect
placental HSPC localization and RUNX1 expression (24,25).
In the present study, the biological impact of
exposure to cigarette smoke during the gestation period in the gene
expression of STAT1, T-BET, GATA3, IL-4, RUNX1
and RUNX3 between placentas from women smokers and
non-smoking women was evaluated using quantitative PCR and western
blot assays. To determine whether these changes in gene expression
were associated with epigenetic mechanisms such as DNA methylation,
the global methylation levels were evaluated by ELISA.
Materials and methods
Study population
A total of 34 paraffin-embedded placentas were
obtained from 14 women smokers (median age, 28 years; age range,
19-38 years) and 20 non-smoking women (median age, 28.5 years; age
range, 18-37 years). The samples were collected between January
2013 and November 2019 at the Pathology Department of the Hospital
Universitario San Ignacio (Bogotá, Colombia) with the corresponding
clinical information. The placentas were part of a previous study,
and the subjects signed informed consent authorizing their use in
future studies.
The present study was carried out in accordance with
The Code of Ethics of the World Medical Association (Declaration of
Helsinki) and all procedures were approved by the Ethics Committee
of the Pontificia Universidad Javeriana and the Hospital
Universitario San Ignacio (approval no. FM-CIE.0224-16).
For sample selection, the corresponding medical
records were reviewed and the information routinely reported by the
patients on cigarette smoking was analyzed. Placentas from neonates
born at 29 weeks or later whose mothers reported smoking during
pregnancy or up to 1 year before pregnancy were selected according
to the criteria of the World Health Organization (26). For the controls, placentas from
neonates older than 29 weeks were selected whose mothers were: i)
Healthy before pregnancy; ii) did not develop complications during
pregnancy (except for 2 patients who presented hypertensive
disorders of pregnancy); iii) reported no history of smoking; and
iv) had healthy neonates without peripartum complications.
Chromosomal diseases and congenital malformations were used as
exclusion criteria. To describe the results, experimental cases are
placentas from mothers with a history of smoking, referred to as
‘women smokers’, and controls are placentas from non-smoking
mothers, referred to as ‘non-smoking women’.
Cell culture
Human Caucasian gastric adenocarcinoma cell line AGS
(cat. no. 89090402; Sigma-Aldrich; Merck KGaA) was cultured in
Ham's F12 Medium supplemented with 10% fetal bovine serum and 5%
antibiotics (ampicillin and streptomycin) at 37˚C in a humidified
5% CO2 atmosphere. Cells were cultured to 80% confluence
to perform the extraction of genomic DNA.
Histopathological findings
From the macroscopic findings of the 34 placentas,
the following anatomical characteristics of the umbilical cord were
considered abnormal: i) The presence of true knots; ii) abnormal
insertions into the placenta; iii) furcata (early loss of Wharton's
jelly leaving funicular vessels exposed); iv) marginal (entry of
the cord at the very edge of the placenta); and v) velamentous
(cord reaching the membranes). The following were also considered:
i) Excessive or decreased coiling (with the definition of a normal
coiling index being between 0.07 and 0.3 coils/cm); ii) the number
of umbilical vessels (defined as the abnormal presence of a single
umbilical artery); iii) the presence of retroplacental hematoma;
iv) circular weight; and v) placental weight (according to
gestational age) and its percentile.
Immunohistochemistry
Placental samples were fixed in 10% buffered
formaldehyde for 48 h, embedded in paraffin, sliced into 3-µm
sections and mounted on microscope slides. Analysis of the
immunohistochemical markers was carried out using the antibodies
listed in Table I (all Santa Cruz
Biotechnology, Inc.) and processed by the pathology department of
the Hospital Universitario San Ignacio. The paraffin-embedded
sections were rehydrated and incubated for 55 min at 20˚C in
methanol containing 10% hydrogen peroxide to block endogenous
peroxidase activity (EnVision™ FLEX kit; Dako; Agilent
Technologies, Inc.). The pretreatment of the samples was performed
using the FLEX Peroxidase-Blocking reagent (5 min at room
temperature) to facilitate the recovery of the antigen and increase
the permeability of the membrane to the antibodies (27). Immunohistochemistry of the samples
and the positive and negative controls of selected tissues was
performed at room temperature using an Autostainer Link 48 from
Dako (Agilent Technologies, Inc.). The positive reaction was
observed following incubation with the HRP-conjugated secondary
antibodies (20 min) with 3,3-diaminobenzidine according to the
manufacturer's instructions. The sections were counterstained with
Harris hematoxylin for 1 min at room temperature, dehydrated and
covered with a slide for later observation under a light
microscope. The entire slides were examined under x4 and x40
magnification. Due to the availability of tissue and reagents,
immunohistochemical analyses were performed on 28 samples,
including 14 non-smoking women and 14 women smokers.
 | Table ICharacteristics of the monoclonal
antibodies used to detect the markers of interest, and paraffin
tissues selected as a positive control for each marker. |
Table I
Characteristics of the monoclonal
antibodies used to detect the markers of interest, and paraffin
tissues selected as a positive control for each marker.
| Marker of
interest | Concentration of
primary antibody | Incubation,
min | Cat. no. | Control |
|---|
| GATA3 | 1:100 | 50 | sc-268 | Breast |
| STAT1 | 1:100 | 60 | sc-417 | Thymus |
| RUNX1 | 1:200 | 60 | sc-101146 | Epiglottis |
| RUNX3 | 1:200 | 60 | sc-101553 | Lymph node |
Anonymization was performed on the two placental
groups. Each antibody was validated by verifying the positive
control as suggested by the manufacturer's instructions. The
positive controls were tissues (breast, thymus, epiglottis and
lymph node) used routinely for diagnosis based on
immunohistochemistry in the Pathology Department of Hospital
Universitario San Ignacio, which obtained informed consent from
patients or relatives for the use of tissues from autopsies for
research. The analysis was performed discriminating between
extension and intensity, classifying each one into four categories
and assigning the corresponding grade. In the case of extension: i)
0% (grade 0); ii) <30% (grade 1); iii) 30-60% (grade 2); and iv)
>60% (grade 3). In the case of intensity: i) not expressed
(grade 0); ii) weak (grade 1); iii) moderate (grade 2); and iv)
strong (grade 3) according to Olaya-C et al (27). Each placental cell was tested, and
immunohistochemical scores are presented as the median +
interquartile range and were analyzed using the non-parametric
Mann-Whitney U test.
RNA isolation and quantitative
(q)PCR
Total RNA was extracted from paraffin-embedded
placentas with a Quick-DNA/RNA™ FFPE kit (cat. no. R1009; Zymo
Research Corp.) according to the manufacturer's protocol and RNA
quality was assessed by the optical density (OD) 260/280 nm and OD
260/230 nm ratios. For each sample, an equal amount of RNA (2 µg)
was reverse transcribed into cDNA using a ProtoScript®
First Strand cDNA Synthesis kit according to the manufacturer's
instructions (New England BioLabs, Inc.). qPCR was performed using
an SYBR-Green I Master real-time PCR kit (Roche Diagnostics) on a
LightCycler® Nano instrument (Roche Diagnostics). The
reaction conditions were as follows: Initial denaturation for 10
min at 95˚C, followed by 40 cycles of denaturation for 10 sec at
95˚C, annealing for 15 sec at 59˚C for RUNX1, 64˚C for RUNX3, 59˚C
for GATA3, 61˚C for STAT1, 62˚C for T-Bet and 60˚C for 18S, and
elongation for 20 sec at 72˚C. Data are presented as the relative
mRNA levels of the gene of interest normalized to the 18S mRNA
level, and the 2-ΔΔCq method was used to analyze the
mRNA expression of the studied genes (28). The sequences of the primers used to
amplify the genes of interest are described in Table II. Due to the RNA quality and
quality criteria for the expression analyses at the mRNA level, 23
samples, 11 from non-smoking women and 12 from women smokers, were
selected.
 | Table IIPrimer sequences used for RT-qPCR and
methylation-specific PCR. |
Table II
Primer sequences used for RT-qPCR and
methylation-specific PCR.
| A, RT-qPCR |
|---|
| Gene | Primer
sequence |
|---|
| GATA3 | FW:
5'-TGGGCTCTACTACAGCTTCACAATAT-3' |
| | RV:
5'-TTGCTAGACATTTTTCGGTTTCTG-3' |
| STAT1 | FW:
5'-ATGGCAGTCTGGCGGCTGAATT-3' |
| | RV:
5'-CCAAACCAGGCTGGCACAATTG-3' |
| RUNX1 | FW:
5'-TGCATGATAAAAGTGGCCTTGT-3' |
| | RV:
5'-CGAAGAGTAAAACGATCAGCAAAC-3' |
| RUNX3 | FW:
5'-GAGTTTCACCCTGACCATCACTGTG-3' |
| | RV:
5'-GCCCATCACTGGTCTTGAAGGTTGT-3' |
| STAT6 | FW:
5'-TGGGCCGTGGCTTCAC-3' |
| | RV:
5'-CCGGAGACAGCGTTTGGT-3' |
| T-BET | FW:
5'-GATGTTTGTGGACGTGGTCTTG-3' |
| | RV:
5'-CTTTCCACACTGCACCCACTT-3' |
| 18S | FW:
5'-ACGGACCAGAGCGAAAGCAT-3' |
| | RV:
5'-GCGGGTCATGGGATAACG-3' |
| B,
Methylation-specific PCR |
| Gene | Primer
sequence |
| β-actin | FW:
5'-TGGTGATGGAGGAGGTTTAGTAAGT-3' |
| | RV:
5'-AACCAATAAAACCACTCCTCCCTTAA-3' |
| T-BET U-met | FW:
5'-GGTTTTGTAGTATTTGTTAAGAGTGT-3' |
| | RV:
5'-CAACAAACCACTATCACTAAAATCAC-3' |
| T-BET Met | FW:
5'-GTTTTGTAGTATTCGTTAAGAGCGT-3' |
| | RV:
5'-GAACCGCTATCACTAAAATCG-3' |
Genomic DNA isolation
Genomic DNA was extracted and purified from
paraffin-embedded placentas with a Quick-DNA/RNA FFPE kit (cat. no.
R1009; Zymo Research Corp.) according to the manufacturer's
instructions. Isolation of genomic DNA consisted of three steps: i)
Deparaffinization; ii) tissue digestion; and iii) purification.
Briefly, pretreatment of paraffin-embedded tissue representative
sections was performed with the deparaffinization solution (1 min
at 55˚C), proteinase k (overnight at 55˚C) and its corresponding
buffer, followed by loading of the deparaffinized tissue sample
onto the Zymo-Spin™ IC Column provided in the kit (Zymo Research
Corp.) for consecutive washes and purification. Genomic DNA quality
was assessed by the OD 260/280 nm and OD 260/230 nm ratios. Genomic
DNA of the AGS cell line was extracted and purified with a
Quick-DNA Miniprep Plus Kit (cat. no. D4069; Zymo Research
Corp.).
Global methylation analysis
The levels of 5-methylcytosine (5-mC) were assayed
by ELISA using a 5-mC DNA ELISA kit (cat. no. D5325; Zymo Research
Corp) according to the manufacturer's protocol. Briefly, 96-well
plates were coated with 100 ng denatured DNA extracted from the
paraffin-embedded samples and were incubated at 37˚C for 1 h. A
mixture of anti-5-methylcytosine and secondary antibody conjugated
to HRP suspended in ELISA buffer was added to each well and
incubated for 1 h at 37˚C. Subsequently, the wells were washed with
the washing solution, and ELISA was developed using
3,3',5,5'-tetramethylbenzidine plus hydrogen peroxide. The
absorbance was read at 405 nm on an ELISA plate reader (Multiskan
EK; Thermolab Scientific Equipments). Determination of the 5-mC
percentage in the genomic DNA sample was performed by interpolating
the results from a standard curve of 7 methylated DNA controls with
0, 5, 10, 25, 50, 75 and 100% methylation provided by the
manufacturer.
Methylation-specific PCR
Samples from non-smokers (sample nos. 4, 13, 14, 18
and 23) and smokers (sample nos. 2, 6, 19, 25 and 27) were
evaluated by methylation-specific PCR. DNA bisulfite conversion was
carried out using an EZ DNA Methylation kit (cat. no. D5001; Zymo
Research Corp.) following the manufacturer's instructions. Briefly,
0.5-1.0 µg of genomic DNA was mixed with 130 µl of CT Conversion
Reagent prepared following the manufacturer's instructions. The
mixture was incubated in a thermocycler with 19 thermal cycles at
98˚C for 30 sec and 64˚C for 15 min. The bisulfite-converted DNA
samples were loaded onto the Zymo-Spin IC Column provided in the
kit for desulfonation and purification. The bisulfite-converted DNA
quality was analyzed by β-actin amplification and PCR was performed
using IMMOLASE™ DNA Polymerase (Bioline) on a T100™ Thermal Cycler
(Bio-Rad Laboratories, Inc.). The reaction conditions were as
follows: Activation at 95˚C for 10 min, followed by 37 cycles of
denaturation at 95˚C for 10 sec, annealing at 57˚C for U-Met T-BET
and 58˚C for Met T-BETt for 15 sec, and extension at 72˚C for 20
sec. The amplification product was visualized by electrophoresis on
a 2% agarose gel with Gel Red (cat. No. 41003; Biotium).
Additionally, for experimental validation, the positive control
(methylated DNA) and negative control (unmethylated DNA) provided
by the same manufacturer were used (cat. no. D5014; Zymo Research
Corp.) The sequences of the primers used to amplify the genes of
interest are described in Table
II.
Statistical analysis
For data analysis, the samples were categorized into
two groups: Samples of placentas from women smokers and samples of
placentas from non-smoking women. Statistically significant
differences in the expression levels of RUNX1, RUNX3, STAT1,
T-BET, IL-4 and GATA3 at the protein and mRNA
levels and methylation results were determined using the
Mann-Whitney U test as non-parametric variables of independent
samples. In all the figures, horizontal bars represent the median.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using GraphPad
Prism version 6 (GraphPad Software, Inc.).
Association between histological findings and
interest groups was evaluated by calculating raw odds ratio (OR)
with 2-sided Fisher's exact test with 95% confidence intervals
(CIs); the means and percentages were determined to summarize the
data. Qualitative variables with absolute frequencies and extreme
or aberrant data were analyzed in detail in relation to the
different variables. The hypothesis test to determine the P-value
was null hypothesis (H0), OR=1 and two-tailed
alternative hypothesis (Ha), different from OR=1. Only
variables with P<0.05 were retained in the final statistical
models. Statistical analysis was performed with Stata 14.2
(StataCorp, LLC).
Results
Clinical characterization
Within the sample group, 92.8% of women smokers were
in the age range of 18-35 years, with a median age of 26 years. In
the control group, 75% of the women classified as non-smokers were
within the same age range, with a median age of 28 years. Of the
women smokers, 50% had term births, compared with 65% of the
control group who had term births. A total of 78.6% of neonates in
the sample group were male, compared with 55% of female neonates in
the control group of non-smoking women.
In 7.2% of women smokers, intrauterine growth
restriction was recorded as a perinatal complication. An
association between low birth weight and exposure to cigarette
smoke during pregnancy was observed. Specifically, 64% of neonates
born to women smokers displayed low birth weight (Table III).
 | Table IIIClinical characteristics of patients
involved in the study. |
Table III
Clinical characteristics of patients
involved in the study.
| Clinical
characteristic | Women smokers
(n=14) | Non-smoking women
(n=20) | P-value | Odds ratio | Estimated 95%
CI |
|---|
| Maternal age, years
(%) | | | 0.38 | 0.21 | 0.02-2.19 |
|
18 | 0 (0) | 1(5) | | | |
|
19-35 | 13 (92.8) | 15(7) | | | |
|
>35 | 1 (7.2) | 4(20) | | | |
| Maternal
background, n (%) | | | 0.66 | 2.455 | 0.35-17.08 |
|
Gastrointestinal
diseases | 2 (14.5) | 0 (0) | | | |
|
Hematological
diseases | 1 (7.2) | 0 (0) | | | |
|
Malignant
diseases | 0 (0) | 1(5) | | | |
| Obstretrical
complications, n (%) | | | 0.83 | 1.3 | 0.20-7.75 |
|
Hypertensive
disorders of pregnancy | 5 (35.7) | 2(10) | | | |
|
Gestational
diabetes | 3 (21.4) | 0 (0) | | | |
|
Sexual
transmission infection | 1 (7.2) | 0 (0) | | | |
|
Premature
rupture of membranes | 0 (0) | 0 (0) | | | |
| Newborn sex, n
(%) | | | 0.10 | 0.223 | 0.047-1.052 |
|
Female | 3 (21.4) | 11(55) | | | |
|
Male | 11 (78.6) | 9(45) | | | |
| Gestational age,
weeks (%) | | | 0.60 | 0.54 | 0.13-2.17 |
|
≤37 | 7(50) | 7(35) | | | |
|
>37 | 7(50) | 13(65) | | | |
| Birth weight, g
(%) | | | 0.29 | 2.7 | 0.66-11.09 |
|
<2,500 | 9(64) | 8(40) | | | |
|
≥2,500 | 5 (35.7) | 12(60) | | | |
| Perinatal
complications, n (%) | | | 0.84 | 4.24 | 0.16-111.65 |
|
Intrauterine
growth restriction (below the 10th percentile for gestational
age) | 1 (7.2) | 0 (0) | | | |
Analysis of obstetric complications revealed that
35.7% of the women smokers had hypertensive disorders of pregnancy
compared with only 10% of non-smoking women who had hypertensive
disorders of pregnancy. In the group of women smokers, 21.4% (3
cases) developed gestational diabetes and 7.2% (1 case) had a
sexually transmitted disease. Analysis of chronic maternal
pathologies revealed 14.5% (2 cases) of women smokers had a
gastrointestinal disease (gastritis), 7.2% (1 case) of women
smokers had a history of a hematological disease (von Willebrand
disease) and 5% (1 case) of non-smoking women had a history of
cancer (treated thyroid carcinoma). There were no cases of
premature rupture of membranes in the two groups. The
histopathological findings are summarized in Table SI. Due to the data being collected
from patients in a level 4 hospital, a number of individuals (15
cases) included in this study presented comorbidities (Table III). Due to the low number of
cases for each entity, it was not possible to make groupings and
analyze their impact on the results.
Changes in the gene expression of
transcription factors are associated with the differentiation of
virgin TLs towards the Th1 and Th2 profiles
To evaluate the changes in the gene expression of
transcription factors associated with the differentiation of virgin
TLs towards the Th1 and Th2 TL profiles as a result of exposure to
cigarette smoke during pregnancy, the mRNA levels of STAT1
and GATA3 relative to 18S were determined via qPCR.
Immunohistochemical assays were performed to detect expression at
the protein level in all the samples selected for the study. The
results indicated a statistically significant increase in the
GATA3 mRNA level in placental samples from women smokers
compared with non-smoking women (Fig.
1A). In the immunohistochemical analysis, GATA3 demonstrated a
strong nuclear staining pattern, mainly in syncytiotrophoblasts and
extravillous trophoblasts of the analyzed samples, with negativity
in the middle section and positivity towards the external sections
(maternal and fetal side) of the slice (Fig. 1D and E). Fig. 1C
demonstrates the nuclear staining pattern with a high intensity in
a positive control biopsy section of breast cancer tissue. Analysis
of these pooled data numerically represented the intensity and
extent of positivity, as described in the methodology, and did not
show significant differences between the groups analyzed (Fig. 1B). Additionally, qPCR was performed
to detect RNA expression of IL-4 (Fig. S2) and the results demonstrated a
statistically significant increase in placental samples from women
smokers compared with non-smoking women.
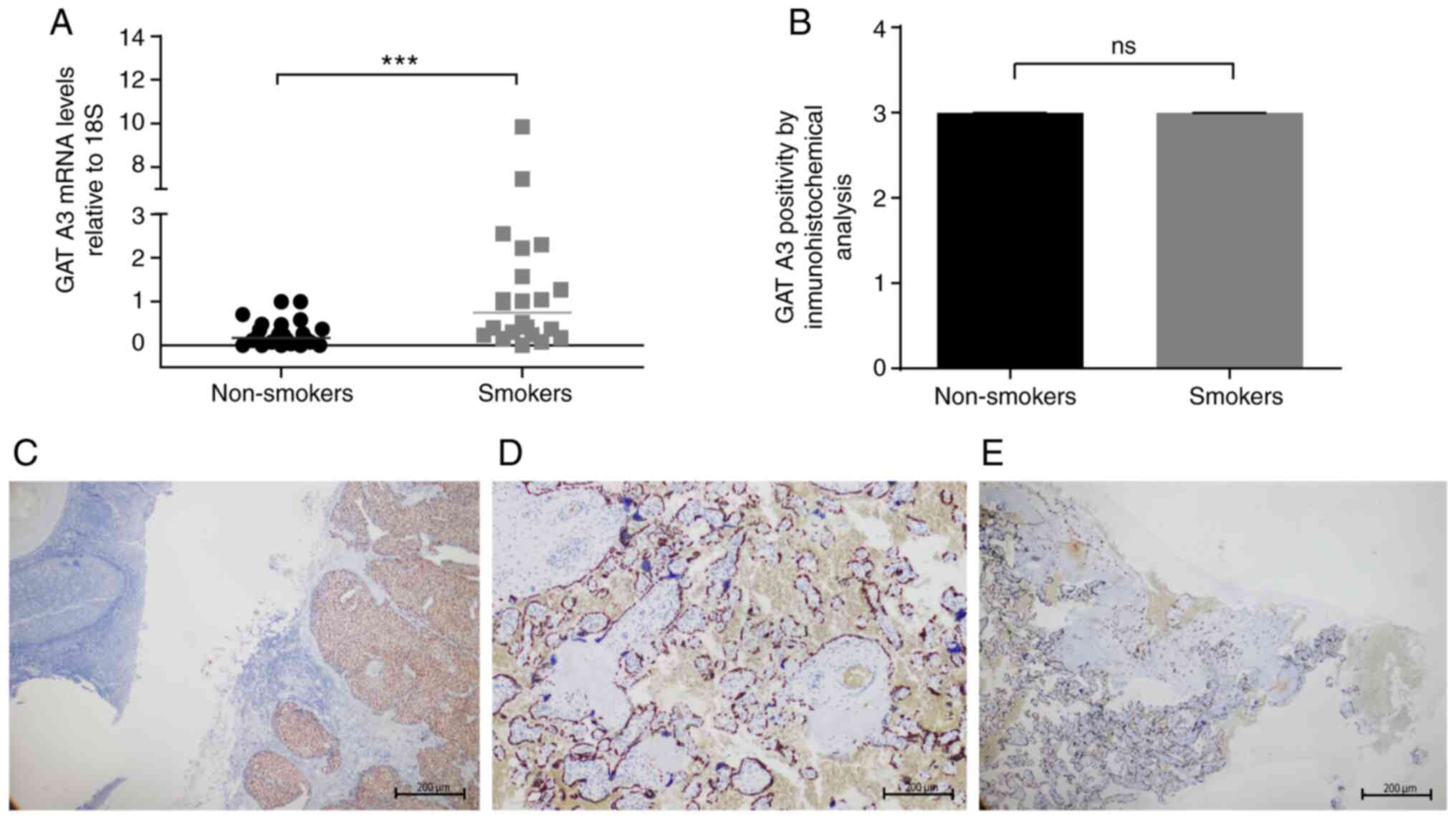 | Figure 1Analysis of the relative expression
of GATA3 at the mRNA and protein levels in the placenta. (A)
Expression levels of GATA3 relative to 18S in
placentas from non-smoking women compared with placentas from women
smokers. The horizontal bar represents the median of relative
expression. (B) Intensity of positivity of GATA3 in placental
samples from non-smoking women and women smokers. (C) Positive
control biopsy slice of breast cancer and the nuclear staining
pattern, with a strong intensity (magnification, x4). (D) Placental
slice of a non-smoking woman displaying positive nuclear staining
patterns in syncytiotrophoblasts, strong intensity, proportion
between 30 and 60% of the total observed (magnification, x10). (E)
Placental slice of a woman smoker, positive nuclear staining
pattern in syncytiotrophoblasts, strong intensity, proportion
<30% of total observed (magnification, x4). A Mann-Whitney U
test was applied. ***P<0.001. GATA3, GATA binding
protein 3; NS, not significant. |
Contrary to these findings, no changes were detected
in the analysis of STAT1 mRNA levels when comparing
placental samples from women smokers and non-smoking women (median,
186 vs. 234; Fig. 2A).
Immunohistochemical analysis demonstrated a positive pattern in
extravillous trophoblasts (Fig. 2D)
and areas of strong positivity in intravillous Hofbauer cells
(Fig. 2E) in samples from women
smokers and non-smoking women. Fig.
2C demonstrates the nuclear staining pattern and intensity in a
positive control thymus tissue section. This labeling was
associated with inflammation and was not observed when H&E
staining was performed (data not shown). Analysis of these pooled
data numerically represented the intensity and extent of positivity
and did not exhibit significant differences between the analyzed
groups (Fig. 2B).
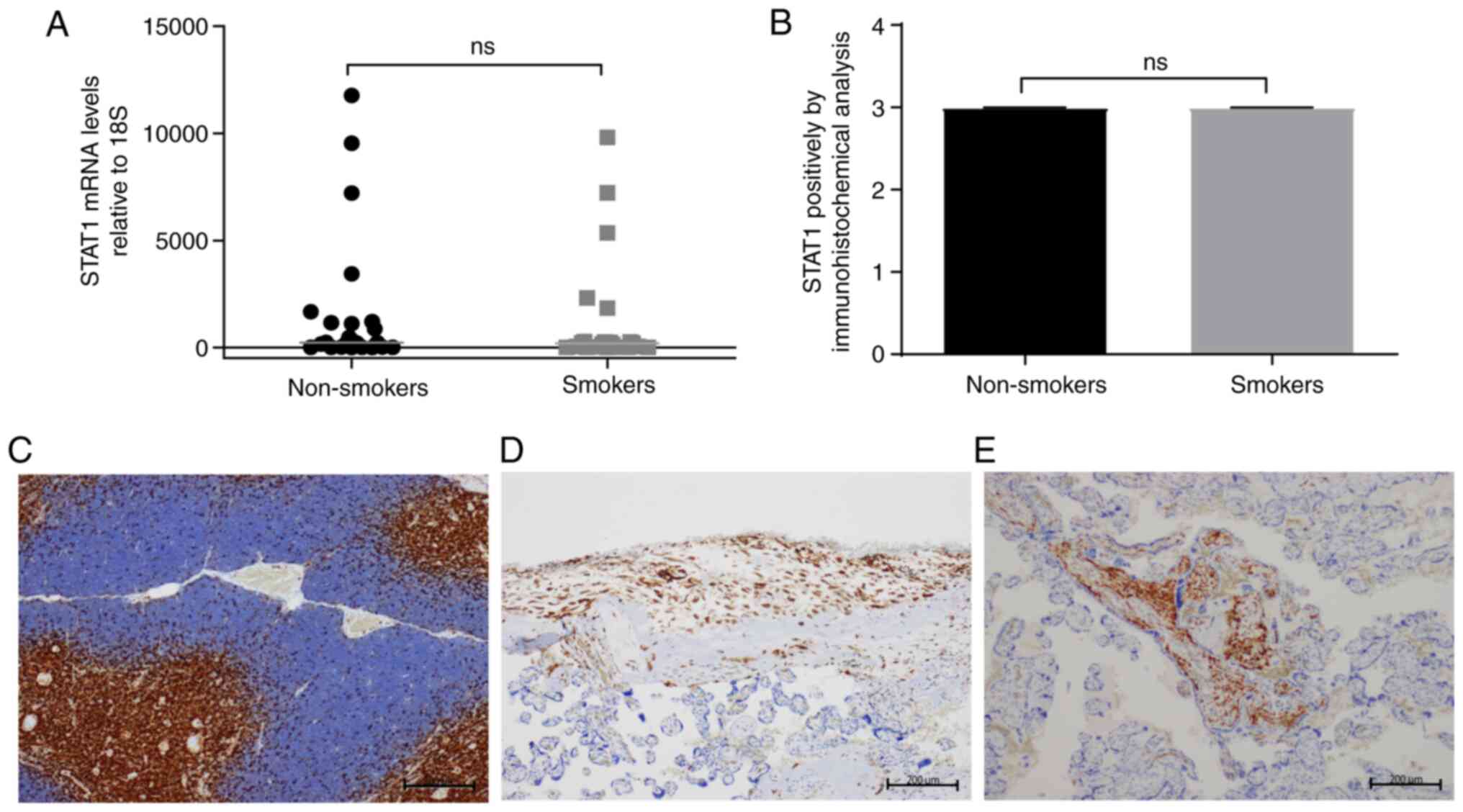 | Figure 2Analysis of the relative expression
of STAT1 at the mRNA and protein levels in the placenta. (A)
Expression levels of STAT1 relative to 18S in grouped
samples of placentas from non-smoking women compared with placentas
from women smokers. The horizontal bar represents the median of
relative expression. (B) Level of intensity of STAT1 labeling via
immunohistochemistry in samples from women smokers and non-smoking
women. (C) Positive control thymus tissue slice, nuclear staining
pattern, strong intensity (magnification, x10). (D) Placental slice
of a non-smoking woman displaying positive nuclear staining pattern
in extravillous trophoblasts and the decidua, strong intensity,
proportion >60% of the total observed (magnification, x10). (E)
Placental slice of a woman smoker, positive nuclear staining
pattern in intravillous histiocytes, strong intensity
(magnification, x10). A Mann-Whitney U statistical test was
applied. NS, not significant. |
Additionally, qPCR assays were performed for
T-BET, a gene that is translated into a master transcription
factor in the differentiation of virgin TLs towards the Th1 TL
profile. These results revealed an increase in the relative
expression of T-BET mRNA in women smokers compared with
non-smoking women (Fig. S3A).
However, it is important to highlight that in five samples of women
smokers, decreases in the mRNA expression of T-BET were
observed (Fig. S3B). To determine
whether the expression decrease was due to changes in DNA
methylation patterns, end-point methylation-specific PCR was
performed. It was revealed that 100% of the samples exhibited
hypomethylation of the promoter of the T-BET gene (Fig. S3C and D).
Changes in RUNX1 and RUNX3 gene
expression are associated with exposure to cigarette smoke during
pregnancy
Based on the roles of RUNX1 and RUNX3 in the process
of differentiation from virgin TLs towards Th1 and Th2 TLs and in
the outcome of postnatal diseases in early and late life in humans
(18), the changes in gene
expression resulting from exposure to cigarette smoke were
evaluated. To this end, the mRNA levels of RUNX1 and
RUNX3 relative to 18S were evaluated via qPCR, and
immunohistochemical assays were performed to detect protein
expression in all the samples selected for study. According to the
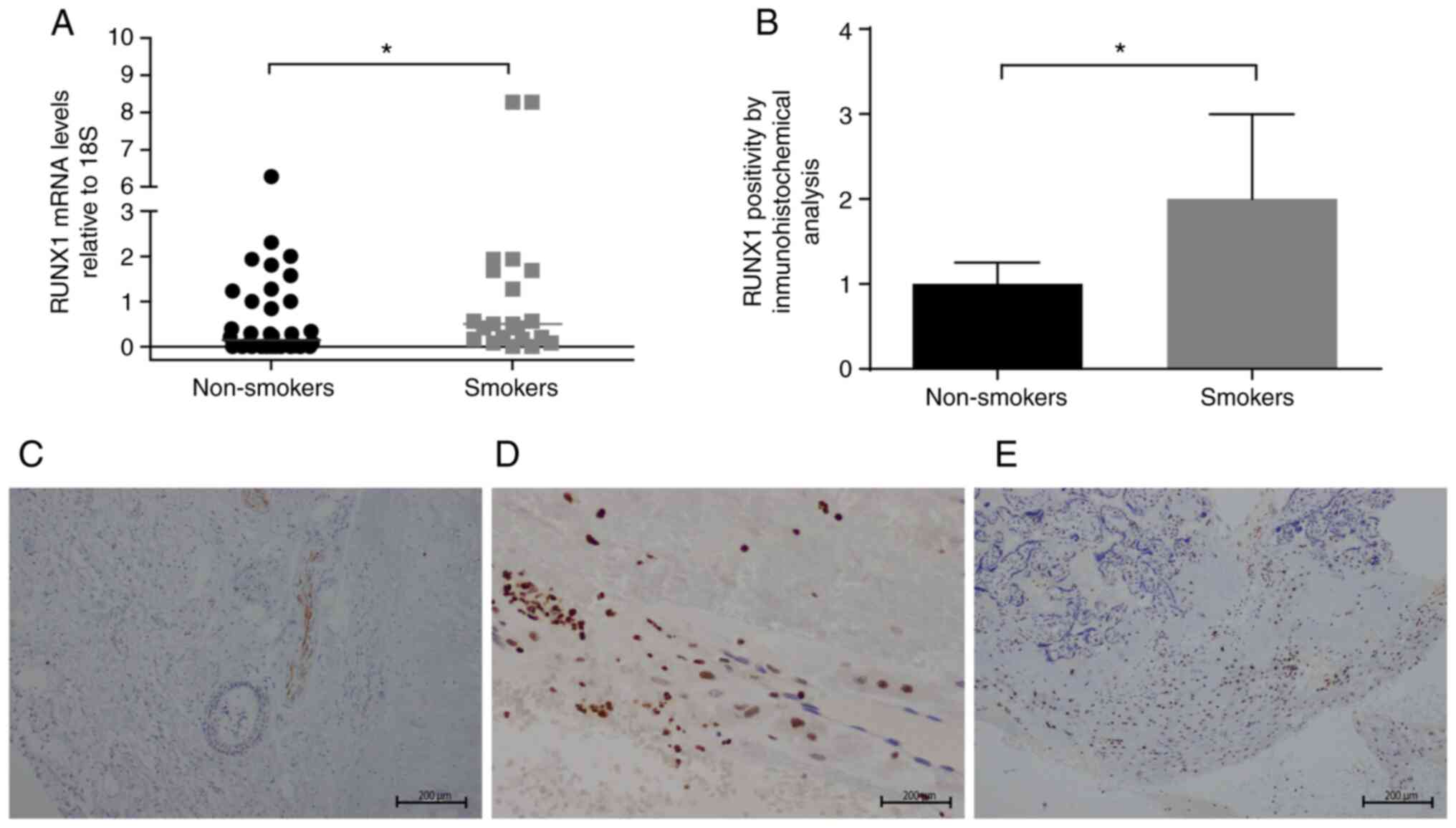
relative expression levels of mRNA, RUNX1 increased
significantly in placental samples from women smokers compared with
non-smoking women (median, 0.5 vs. 0.145; Fig. 3A). In addition, in the
immunohistochemical analysis of RUNX1, a strong nuclear staining
pattern was observed in the decidua and in chorion stromal cells
(Fig. 3D and E). Differences were observed in chorion
stromal cells, with a greater labeling intensity in placental
samples from women smokers compared with those from non-smoking
women (Figs. 3B and S4). Fig.
3C demonstrates the nuclear staining pattern with medium
intensity of the positive control epiglottis tissue section.
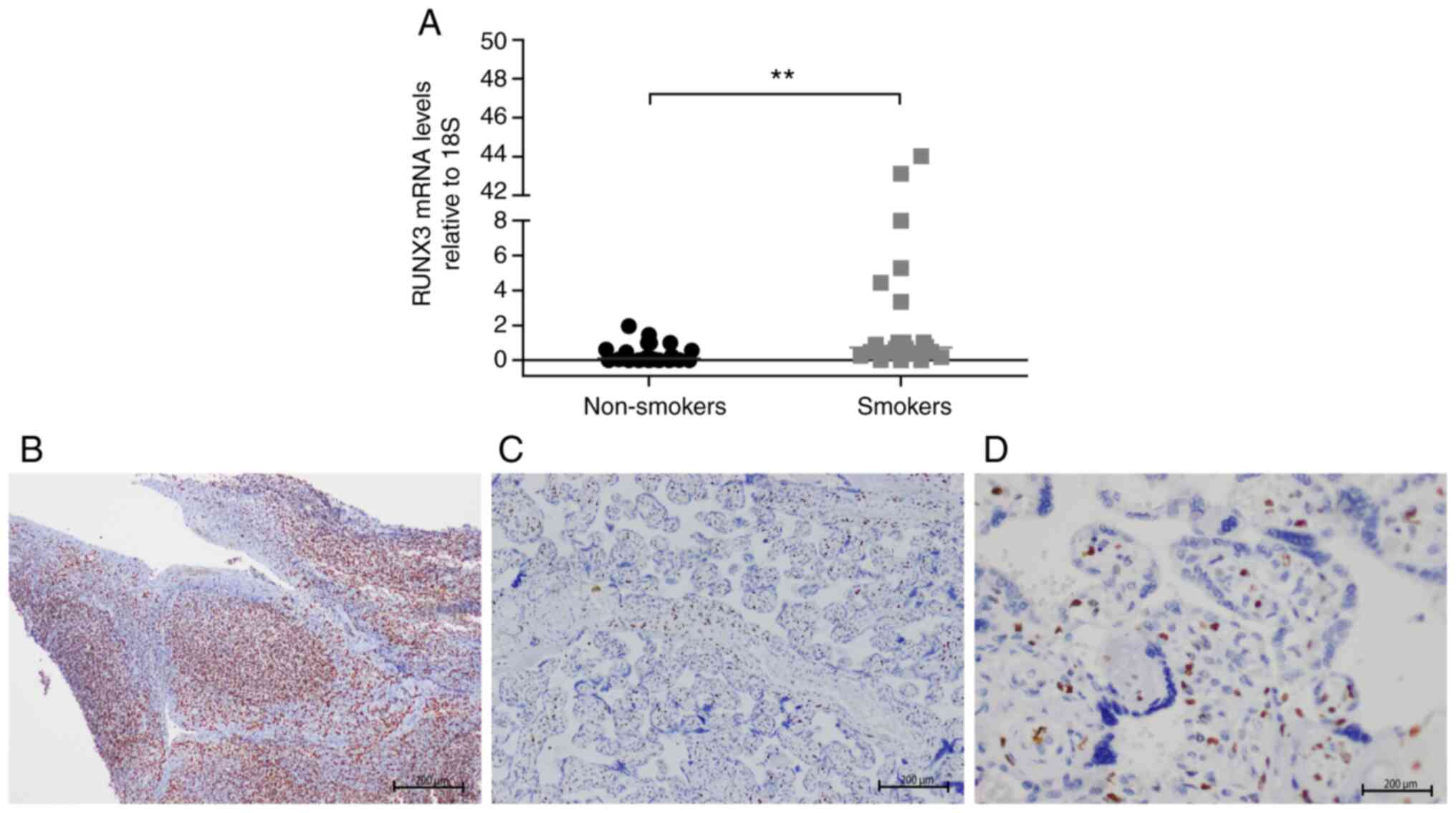
The relative expression levels of RUNX3 mRNA
revealed significant differences between groups and were higher in
placental samples from women smokers than in those from non-smoking
women (median, 0.7 vs. 0.1; Fig.
4A). However, immunohistochemical determination of the levels
of expression of RUNX3 at the protein level demonstrated a negative
pattern in all placental cells (Fig.
4C and D). Fig. 4B demonstrates the positive control
lymphoid tissue slice with a nuclear staining pattern and strong
intensity.
Changes in global placental
methylation in women smokers
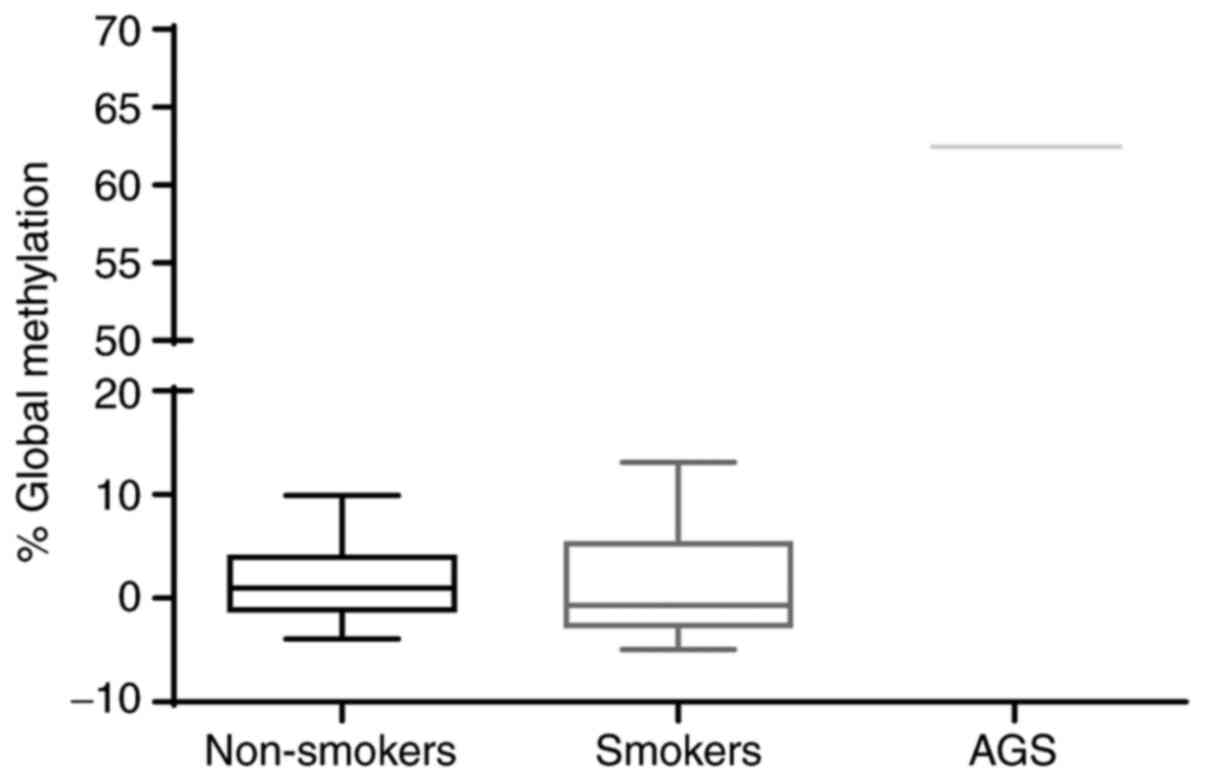
To assess whether exposure to cigarette smoke
induced changes in the overall DNA methylation level, the
methylation profiles of the extracted genomic DNA from the
paraffin-embedded tissue were analyzed by detecting the 5-mC marker
using ELISA. The results demonstrated that there were no
differences in the methylation levels detected between the
placental samples from women smokers and non-smoking women. It is
important to note that the methylation levels detected were
<15%. Although the screening kit included both positive and
negative methylation controls, a sample of genomic DNA from a human
Caucasian gastric adenocarcinoma cell line for which high levels of
overall methylation have been reported was included. In this case,
the results demonstrated a 60% methylation level, which proved the
efficiency of the kit (Fig. 5).
Discussion
In the present study, a first approximation was made
in regard to the biological impact of exposure to cigarette smoke
during gestation. Comparisons were made between the expression
levels of genes encoding the transcription factors STAT1, GATA3,
RUNX1 and RUNX3, which are involved in the process of
differentiation from virgin TLs towards Th1 and Th2 TLs. In the
current study, placentas from women who smoked during pregnancy and
placentas from women non-smokers were analyzed.
Significant increases were observed in GATA3,
IL-4, RUNX1 and RUNX3 mRNA expression levels in
placentas from women smokers compared with those from non-smoking
women. In parallel, a decrease in the expression of T-BET
was observed in 5 women smokers compared with non-smoking women.
With respect to the expression of STAT1, the results were
not statistically significant.
Early dysregulation of the newborn's immune response
is associated with the development of allergies and asthma in
childhood. In this regard, changes have been reported in the
generation and differentiation processes of virgin CD4 TLs towards
the Th1 and Th2 type profiles (29,30).
These variations are associated with genetic predispositions and
prenatal environmental exposures, such as exposure to cigarette
smoke (31). Most of the research
carried out to date evaluates the association between smoking
during pregnancy and the outcome of newborns with allergic or
asthmatic phenotypes experimentally, by determining the IgE levels
in umbilical cord blood, exacerbating the neonatal immune response
to specific antigens, and quantifying Th2 type cytokines and
changes in specific methylation levels of placental tissue as a
result of exposure to cigarette smoke (32-35).
GATA3 is the master transcription factor of Th2 lymphocyte
differentiation. This process is initiated by the binding of IL-4
to the receptor, which leads to phosphorylation and dimerization of
STAT6; STAT6 dimers are translocated into the nucleus, where they
promote GATA3 expression, favoring transcription and
translation of IL-4, a characteristic Th2 cytokine, which
also contributes to the continuous stimulation of differentiation
towards this phenotype (Fig. S1)
(36).
The increase in GATA3 expression in placentas
from women smokers compared with non-smoking women supports the
hypothesis that transcription of genes involved in the Th2
lymphocyte differentiation pathway is associated with the
predisposition to an allergic phenotype, caused by exposure to
cigarette smoke during pregnancy. This finding is consistent with
the observed increase in IL-4 RNA expression and decrease in
T-BET expression, and reflects a possible imbalance in the
response of CD4 TLs. Transcriptional activation of GATA3 may
be associated with the presence of histone covalent modifications
associated with activation, which are associated with an epigenetic
mechanism underlying the transcriptional modulation of the allergic
response (37,38). For the protein expression of GATA3
detected by immunohistochemistry, the results did not show
significant changes between the groups analyzed. However, it is
important to highlight the existence of a differential and
tissue-specific expression pattern that has not been previously
reported, with positivity towards both maternal and fetal extreme
regions.
Placental samples from 5 women smokers revealed
decreases in T-BET mRNA expression compared with those of
non-smoking women. As described above, in the differentiation
pathway towards a Th1 TL profile, T-BET expression is
induced by T cell receptor signaling (TCR) and is strongly elevated
by activation of the transcription factor STAT1, which occurs in a
positive feedback loop in response to autocrine IFNγ (39). Likewise, T-BET plays an antagonistic
role in the differentiation of Th2 lymphocytes by inhibiting
GATA3 (40). The
significantly decreased levels of T-BET and the decreasing
trend of STAT1 detected in placentas from women smokers are
in line with the elevated transcriptional levels of GATA3
and IL-4 detected in the present study. It is important to
highlight that one of the limitations of the current study is the
lack of information on the protein expression of IL-4 and IFNγ.
It has been identified that dysregulation of
T-BET expression plays a role in the immunopathogenesis of
type 1 diabetes by generating an imbalance in Th1/Th2
differentiation in peripheral blood mononuclear cells (41). The expression of T-BET has
been described as being regulated by epigenetic mechanisms, such as
DNA methylation (42). For this
reason, specific methylation PCR tests were carried out on a CpG
island of the promoter in the current study. In this way, samples
from non-smoking and smoking women were evaluated. Notably, the
results demonstrated that for all the samples analyzed, the
presence of the unmethylated condition was evident in the analyzed
region for both women smokers and non-smoking women. These results
indicated that the changes in the expression in T-BET are
independent of the DNA methylation of the analyzed region and it
would therefore be interesting to consider the existence of
additional epigenetic mechanisms for DNA methylation, such as
covalent histone modification (43). In this regard, it has been reported
that this type of mechanism is mostly affected by exposure to
solvents and toxic agents, such as tobacco and particulate matter,
among others (44,45).
RUNX1 and RUNX3 are known to be involved in T-cell
immunity. T lymphocytes differentiate into subsets with distinct
functions. In some T-cell subsets, RUNX1 and RUNX3 are equivalently
expressed and exhibit redundant activities (46). However, in other T-cell subsets,
RUNX1 and RUNX3 exert distinct functions. These differences depend
on the unique expression patterns of these proteins in a particular
T-cell subset (47). RUNX1 but not
RUNX3 is found in naive T lymphocytes, whose TCR stimulation
immediately downregulates RUNX1 protein expression; on the other
hand, Th1-committed cells express only RUNX3, whereas Th2 cells
express both RUNX1 and RUNX3(47).
RUNX1 promotes the development of Th1 lymphocytes
from virgin CD4 T lymphocytes by activating the IL-4 repressor,
leading to their transcriptional inactivation and increased
expression of IFNγ, a cytokine characteristic of the Th1 response
(48). However, it is important to
emphasize that for this process to happen, there must be a parallel
repression of GATA3(47). In the
placental samples analyzed in the current study, exposure to
cigarette smoke generated significant increases in the protein and
mRNA expression levels of RUNX1. These results are consistent with
a study in a murine model by Haley et al (22), in which changes in the expression
patterns of RUNX1 due to exposure to cigarette smoke during
pregnancy are described. Haley et al (22) report the existence of single
nucleotide polymorphisms in RUNX1 that are associated with
airway responsiveness in asthmatic children. These associations are
reported to be modified by exposure to cigarette smoke during
pregnancy, which tends to increase the expression levels of
RUNX1 (22). Other exposures
during pregnancy, such as alcohol consumption, have been associated
with the differential expression of ~304 genes identified by
microarray experiments in the placental tissue of rats (49). It has been suggested that
RUNX1 demonstrates decreased expression in placentas from
rats that consumed ethanol compared with controls (46).
In relation to RUNX3 expression, the results
of the present study revealed very low levels of gene expression in
placental samples from both women smokers and non-smoking women.
However, a significant increase in RUNX3 mRNA expression
levels was reported in women smokers compared with non-smoking
women. Despite the fact that RUNX3 has been established to be
fundamental for promoting the Th1 phenotype through the IL-4
repression induced by its interaction with T-BET (50), the significance of RUNX3 expression
in Th2 cells remains unclear (47).
It remains to be determined whether the significant increase
detected in the expression of RUNX1 and RUNX3 is
generated in response to the transcriptional increase detected for
GATA3.
Finally, to assess whether exposure to cigarette
smoke had an effect on the overall DNA methylation patterns, a
screening test was performed using a 5-mC DNA ELISA kit. For this
particular evaluation, results of the present study demonstrated no
change between the experimental groups. It is important to
highlight that during early embryonic development, the placenta
presents generalized hypomethylation compared with normal somatic
tissue, which contributes to the processes of trophoblast
differentiation and later to correct placentation (51-53).
Thus, changes in this global stage have been associated with
environmental exposure, such as exposure to pollution and alcohol
consumption during the third trimester of pregnancy (49,51,54).
It has been reported that even slight changes in methylation levels
(~3%) can be considered important (52). However, there is little evidence
available in the literature from clinical and preclinical
experimental studies to support the association between exposure to
cigarette smoke and increases in overall placental methylation.
Analysis of the characteristics and clinical
outcomes of the study population allowed an association to be
established between exposure to cigarette smoke during pregnancy
and low birth weight (<2,500 g) (53). With a representative percentage of
64% in women smokers, the findings of the present study are
consistent with reports that have previously described maternal
smoking during pregnancy as a risk factor for low birth weight
(55). This association is direct
and dose-dependent according to studies demonstrating that birth
weight decreases as the number of cigarettes consumed per day
increases, with a reduction in weight from 6-10 cigarettes daily
(56). In addition, placental
pathologies such as chronic ischemia and secondary changes
associated with inadequate perfusion (57) have been identified as being
associated with both low birth weight and exposure to cigarette
smoke, highlighting the importance of an optimal environment for
fetal development and the impact of maternal smoking on
development.
In conclusion, it was found maternal smoking is
associated with low birth weight and changes in the expression of
genes that encode important transcription factors for hematopoiesis
and T lymphocyte differentiation. In the present study, 5 placental
samples exposed to cigarette smoke during pregnancy favored the
presence of transcription factors and cytokines involved in the
differentiation of TLs towards Th2 cells, such as GATA3 and IL-4.
These transcription factors predispose cells to differential
dysregulation and cause an imbalance in the Th2/Th1 ratio,
characteristic of the asthmatic phenotype with an exacerbated Th2
TL profile. In the present study, significant increases in
RUNX1 expression were also detected as a consequence of
exposure to cigarette smoke. These results were consistent with
previous scientific reports that associate this altered expression
in RUNX1 with the occurrence of hematological malignancies
in early life (23). In addition,
an analysis of the overall methylation of placental samples
demonstrated changes in methylation that were not directly
associated with exposure to cigarette smoke, but provided
additional evidence for overall placental methylation levels, for
which there are limited reports in the literature. Results of the
present study indicated the impact of exposure to cigarette smoke
on gene expression and the possible impact on health of the
individuals in the short, medium and long term. It is important to
highlight that one of the limitations of the present study is the
low number of samples. However, the results obtained open
possibilities for novel research in the area to be conducted to
demonstrate the impact of environmental exposure on maternal-fetal
health. Finally, further studies are necessary to evaluate the
asthma status of newborns from women smokers analyzed in the
present study.
Supplementary Material
Important transcription factors in the
differentiation of Th1 and Th2 lymphocytes. Modified from O'Shea
et al (58) and Wong et
al (47) and created with
Biorender. GATA3, GATA binding protein 3; p, phosphorylated; T-BET,
T-Box transcription factor 21.
Analysis of the relative expression of
IL-4 at the mRNA level in the placenta. Levels of expression of
IL-4 in samples in placentas from non-smoking women and
women smokers. The horizontal bar represents the median of relative
expression. A Mann-Whitney U statistical test was applied.
*P<0.05.
Analysis of the relative expression
and specific methylation of T-BET at the mRNA level in the
placenta. (A) Levels of expression of T-BET relative to
18S in samples of placentas from non-smoking women compared
with placentas from women smokers. (B) T-BET expression
levels relative to 18S levels in samples selected for
endpoint MSP analysis. (C) Endpoint MSP analysis of placental
samples from non-smoking women (upper bands) and women smokers
(lower bands). β-actin, T.BET U-Met (oligos that recognize the
non-methylated sequence), T-BET Met (oligos that recognize the
methylated sequence), C+ and C-. Different gels were merged, as
indicated by the vertical lines. Each vertical lane corresponds to
the same type of sample. (D) MSP statistical bars demonstrate that
0 was assigned to the unmethylated state and 1 to the methylated
state, according to the interpretation from the gel as the presence
of band or not, respectively. A Mann-Whitney U statistical test was
applied. *P<0.05, ***P<0.001. T-BET,
T-Box transcription factor 21; MSP, methylation-specific PCR;
U-Met, unmethylated; Met, methylated; C+, methylated genomic DNA
control; C-, nonmethylated genomic DNA; NS, not significant.
Analysis of RUNX1 protein expression
in the placenta. (A) Number of placental samples classified
according to the intensity of RUNX1 labeling by
immunohistochemistry of chorion stromal cells. (B) Number of
placental samples classified according to the ratio of RUNX1
labeling by immunohistochemistry of chorion stromal cells.
Histological findings of patient
samples included in the study.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Pontificia
Universidad Javeriana (grant no. PUJ 7363).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LGB performed the experiments, analyzed and
interpreted the data and wrote the paper. IM and MIZ performed some
experiments and analyzed and interpreted the data. AC, LSR and OMM
contributed reagents, materials, analysis tools or data, and
analyzed and interpreted the data. MO and AR conceived and designed
the experiments, analyzed and interpreted the data, contributed
reagents, materials, analysis tools or data, and wrote the paper.
MO and AR confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All research protocols were approved by the Ethics
Committee of the Pontificia Universidad Javeriana and the Hospital
Universitario San Ignacio (approval no. FM-CIE.0224-16). Informed
consent was obtained from patients or relatives for the use of
placental tissue and positive control tissues for research.
Patient consent for publication
The data and results presented in the present study
were anonymized and patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maltepe E and Fisher SJ: Placenta: The
forgotten organ. Annu Rev Cell Dev Biol. 31:523–552.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nugent BM and Bale TL: The omniscient
placenta: Metabolic and epigenetic regulation of fetal programming.
Front Neuroendocrinol. 39:28–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Luyten LJ, Saenen ND, Janssen BG, Vrijens
K, Plusquin M, Roels HA, Debacq-Chainiaux F and Nawrot TS: Air
pollution and the fetal origin of disease: A systematic review of
the molecular signatures of air pollution exposure in human
placenta. Environ Res. 166:310–323. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marjonen H, Toivonen M, Lahti L and
Kaminen-Ahola N: Early prenatal alcohol exposure alters imprinted
gene expression in placenta and embryo in a mouse model. PLoS One.
13(e0197461)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mikael LG, Pancer J, Jiang X, Wu Q,
Caudill M and Rozen R: Low dietary folate and
methylenetetrahydrofolate reductase deficiency may lead to
pregnancy complications through modulation of ApoAI and IFN-γ in
spleen and placenta, and through reduction of methylation
potential. Mol Nutr Food Res. 57:661–670. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thomas AE, Inagadapa PJN, Jeyapal S,
Merugu NM, Kalashikam RR and Manchala R: Maternal magnesium
restriction elevates glucocorticoid stress and inflammation in the
placenta and fetus of WNIN rat dams. Biol Trace Elem Res.
181:281–287. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wong MK, Nicholson CJ, Holloway AC and
Hardy DB: Maternal nicotine exposure leads to impaired disulfide
bond formation and augmented endoplasmic reticulum stress in the
rat placenta. PLoS One. 10(e0122295)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kalisch-Smith JI, Steane SE, Simmons DG,
Pantaleon M, Anderson ST, Akison LK, Wlodek ME and Moritz KM:
Periconceptional alcohol exposure causes female-specific
perturbations to trophoblast differentiation and placental
formation in the rat. Development. 146(dev172205)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Holloway AC, Salomon A, Soares MJ, Garnier
V, Raha S, Sergent F, Nicholson CJ, Feige JJ, Benharouga M and
Alfaidy N: Characterization of the adverse effects of nicotine on
placental development: In vivo and in vitro studies. Am J Physiol
Endocrinol Metab. 306:E443–E456. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jauniaux E and Burton GJ: The effect of
smoking in pregnancy on early placental morphology. Obstet Gynecol.
79 [5 (Pt 1)]:645–648. 1992.PubMed/NCBI
|
|
11
|
Pintican D, Poienar AA, Strilciuc S and
Mihu D: Effects of maternal smoking on human placental
vascularization: A systematic review. Taiwan J Obstet Gynecol.
58:454–459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Machado Jde B, Chatkin JM, Zimmer AR,
Goulart AP and Thiesen FV: Cotinine and polycyclic aromatic
hydrocarbons levels in the amniotic fluid and fetal cord at birth
and in the urine from pregnant smokers. PLoS One.
9(e116293)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Andres RL and Day MC: Perinatal
complications associated with maternal tobacco use. Semin Neonatol.
5:231–241. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Suzuki K, Sato M, Zheng W, Shinohara R,
Yokomichi H and Yamagata Z: Effect of maternal smoking cessation
before and during early pregnancy on fetal and childhood growth. J
Epidemiol. 24:60–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zacharasiewicz A: Maternal smoking in
pregnancy and its influence on childhood asthma. ERJ Open Res.
2:00042–2016. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
White J, Paton JY, Niven R and Pinnock H:
Guidelines for the diagnosis and management of asthma: A look at
the key differences between BTS/SIGN and NICE. Thorax. 73:293–297.
2018.
|
|
17
|
Renauld JC: New insights into the role of
cytokines in asthma. J Clin Pathol. 54:577–589. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wilson CB, Rowell E and Sekimata M:
Epigenetic control of T-helper-cell differentiation. Nat Rev
Immunol. 9:91–105. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Afkarian M, Sedy JR, Yang J, Jacobson NG,
Cereb N, Yang SY, Murphy TL and Murphy KM: T-bet is a STATI-induced
regulator for IL-12R expression in naïve CD4+ T cells.
Nat Immunol. 3:549–557. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Komine O, Hayashi K, Natsume W, Watanabe
T, Seki Y, Seki N, Yagi R, Sukzuki W, Tamauchi H, Hozumi K, et al:
The Runx1 transcription factor inhibits the differentiation of
naive CD4+ T cells into the Th2 lineage by repressing GATA3
expression. J Exp Med. 198:51–61. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Djuretic IM, Levanon D, Negreanu V, Groner
Y, Rao A and Ansel KM: Transcription factors T-bet and Runx3
cooperate to activate Ifng and silence Il4 in T helper type 1
cells. Nat Immunol. 8:145–153. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Haley KJ, Lasky-Su J, Manoli SE, Smith LA,
Shahsafaei A, Weiss ST and Tantisira K: RUNX transcription factors:
Association with pediatric asthma and modulated by maternal
smoking. Am J Physiol Lung Cell Mol Physiol. 301:L693–L701.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rumrich IK, Viluksela M, Vähäkangas K,
Gissler M, Surcel HM and Hänninen O: Maternal smoking and the risk
of cancer in early life-A meta-analysis. PLoS One.
11(e0165040)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ponder KL, Bárcena A, Bos FL, Gormley M,
Zhou Y, Ona K, Kapidzic M, Zovein AC and Fisher SJ: Preeclampsia
and inflammatory preterm labor alter the human placental
hematopoietic niche. Reprod Sci. 23:1179–1192. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ottersbach K and Dzierzak E: The placenta
as a haematopoietic organ. Int J Dev Biol. 54:1099–1106.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
WHO: Children's environmental health.
|
|
27
|
Olaya-C M, Fritsch M and Bernal JE:
Immunohistochemical protein expression profiling of growth- and
apoptotic-related factors in relation to umbilical cord length.
Early Hum Dev. 91:291–297. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liao SY, Liao TN, Chiang BL, Huang MS,
Chen CC, Chou CC and Hsieh KH: Decreased production of IFN gamma
and increased production of IL-6 by cord blood mononuclear cells of
newborns with a high risk of allergy. Clin Exp Allergy. 26:397–405.
1996.PubMed/NCBI
|
|
30
|
Spinozzi F, Agea E, Russano A, Bistoni O,
Minelli L, Bologni D, Bertotto A and de Benedictis FM: CD4+IL13+ T
lymphocytes at birth and the development of wheezing and/or asthma
during the 1st year of life. Int Arch Allergy Immunol. 124:497–501.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Noakes PS, Holt PG and Prescott SL:
Maternal smoking in pregnancy alters neonatal cytokine responses.
Allergy. 58:1053–1058. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Novakovic B, Yuen RK, Gordon L,
Penaherrera MS, Sharkey A, Moffett A, Craig JM, Robinson WP and
Saffery R: Evidence for widespread changes in promoter methylation
profile in human placenta in response to increasing gestational age
and environmental/stochastic factors. BMC Genomics.
12(529)2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Devereux G, Barker RN and Seaton A:
Antenatal determinants of neonatal immune responses to allergens.
Clin Exp Allergy. 32:43–50. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nabavi M, Ghorbani R, Asadi AM and
Faranoush M: Factors associated with cord blood IgE levels. Asian
Pacific J Allergy Immunol. 31:157–162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Singh SP, Gundavarapu S, Peña-Philippides
JC, Rir-Sima-ah J, Mishra NC, Wilder JA, Langley RJ, Smith KR and
Sopori ML: Prenatal secondhand cigarette smoke promotes Th2
polarization and impairs goblet cell differentiation and airway
mucus formation. J Immunol. 187:4542–4552. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Maier E, Duschl A and Horejs-Hoeck J:
STAT6-dependent and -independent mechanisms in Th2 polarization.
Eur J Immunol. 42:2827–2833. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Deaton AM, Webb S, Kerr AR, Illingworth
RS, Guy J, Andrews R and Bird A: Cell type-specific DNA methylation
at intragenic CpG islands in the immune system. Genome Res.
21:1074–1086. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Onodera A, Kokubo K and Nakayama T: The
Interplay between Transcription Factors and Epigenetic
Modifications in Th2 Cells. In: Gene Expression and Regulation in
Mammalian Cells-Transcription From General Aspects. InTech,
2018.
|
|
39
|
Weaver CT, Hatton RD, Mangan PR and
Harrington LE: IL-17 family cytokines and the expanding diversity
of effector T cell lineages. Annu Rev Immunol. 25:821–852.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream
JH, O'Shea JJ and Strober W: T-bet regulates Th1 responses through
essential effects on GATA-3 function rather than on IFNG gene
acetylation and transcription. J Exp Med. 203:755–766.
2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Vaseghi H, Hossein MH and Jadali Z:
T-helper cell type-1 transcription factor T-Bet Is Down-regulated
in type 1 diabetes. Iran J Allergy Asthma Inmmunol. 15:386–393.
2016.PubMed/NCBI
|
|
42
|
Miller SA and Weinmann AS: Molecular
mechanisms by which T-bet regulates T-helper cell commitment.
Immunol Rev. 238:233–246. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yankulov K: Book review: Epigenetics
(second edition, eds. Allis, Caparros, Jenuwein, Reinberg). Front
Genet. 6(315)2015.
|
|
44
|
Zheng Y, Sanchez-Guerra M, Zhang Z, Joyce
BT, Zhong J, Kresovich JK, Liu L, Zhang W, Gao T, Chang D, et al:
Traffic-derived particulate matter exposure and histone H3
modification: A repeated measures study. Environ Res. 153:112–119.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zong D, Liu X, Li J, Ouyang R and Chen P:
The role of cigarette smoke-induced epigenetic alterations in
inflammation. Epigenetics Chromatin. 12(65)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Rosenberg MJ, Wolff CR, El-Emawy A,
Staples MC, Perrone-Bizzozero NI and Savage DD: Effects of moderate
drinking during pregnancy on placental gene expression. Alcohol.
44:673–690. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wong WF, Kohu K, Chiba T, Sato T and
Satake M: Interplay of transcription factors in T-cell
differentiation and function: The role of Runx. Immunology.
132:157–164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Naoe Y, Setoguchi R, Akiyama K, Muroi S,
Kuroda M, Hatam F, Littman DR and Taniuchi I: Repression of
interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to
the Il4 silencer. J Exp Med. 204:1749–1755. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Loke YJ, Muggli E, Nguyen L, Ryan J,
Saffery R, Elliott EJ, Halliday J and Craig JM: Time- and
sex-dependent associations between prenatal alcohol exposure and
placental global DNA methylation. Epigenomics. 10:981–991.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kohu K, Ohmori H, Wong WF, Onda D, Wakoh
T, Kon S, Yamashita M, Nakayama T, Kubo M and Satake M: The Runx3
transcription factor augments Th1 and down-modulates Th2 phenotypes
by interacting with and attenuating GATA3. J Immunol.
183:7817–7824. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maghbooli Z, Hossein-nezhad A, Adabi E,
Asadollah-Pour E, Sadeghi M, Mohammad-Nabi S, Zakeri Rad L, Malek
Hosseini AA, Radmehr M, Faghihi F, et al: Air pollution during
pregnancy and placental adaptation in the levels of global DNA
methylation. PLoS One. 13(e0199772)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Reichetzeder C, Dwi Putra SE, Pfab T,
Slowinski T, Neuber C, Kleuser B and Hocher B: Increased global
placental DNA methylation levels are associated with gestational
diabetes. Clin Epigenetics. 8(82)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
WHO|Care of the preterm and
low-birth-weight newborn. WHO, 2018.
|
|
54
|
Ehrlich M, Gama-Sosa MA, Huang LH, Midgett
RM, Kuo KC, McCune RA and Gehrke C: Amount and distribution of
5-methylcytosine in human DNA from different types of tissues or
cells. Nucleic Acids Res. 10:2709–2721. 1982.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zheng W, Suzuki K, Tanaka T, Kohama M and
Yamagata Z: Okinawa Child Health Study Group. Association between
maternal smoking during pregnancy and low birthweight: Effects by
maternal age. PLoS One. 11(0146241)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kataoka MC, Carvalheira APP, Ferrari AP,
Malta MB, de Barros Leite Carvalhaes MA and de Lima Parada CMG:
Smoking during pregnancy and harm reduction in birth weight: A
cross-sectional study. BMC Pregnancy Childbirth.
18(67)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nigam J, Misra V, Singh P, Singh P,
Chauhan S and Thakur B: Histopathological study of placentae in low
birth weight babies in India. Ann Med Health Sci Res. 4 (Suppl
2):S79–S83. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
O'Shea JJ, Lahesmaa R, Vahedi G, Laurence
A and Kanno Y: Genomic views of STAT function in CD4 + T helper
cell differentiation. Nat Rev Immunol. 11:239–250. 2011.PubMed/NCBI View Article : Google Scholar
|