Introduction
Postoperative cognitive dysfunction (POCD) is one of
the most common and devastating complications in patients
undergoing major surgery under general anesthesia (1). Patients with POCD generally present
with a striking decline in cognitive abilities, including memory,
concentration, attention and cognitive flexibility (2). Previous clinical studies have
indicated that POCD is closely associated with elevated healthcare
costs, extended hospitalization and increased mortality (3). While factors such as advanced age,
pre-existing cognitive impairment, lengthy surgery and general
anesthesia have been established as the major risk factors for POCD
(4), the pathogenesis and
mechanisms remain somewhat elusive.
An accumulating body of evidence suggests that POCD
shares a significant mechanical connection and commonality with
Alzheimer's disease, in which altered microglia and astrocytic
cells release extensive proinflammatory cytokines, resulting in
persistent neuroinflammation and synaptic impairment (5). The various inflammatory components of
neuroinflammation, including proinflammatory cytokines, chemokines
and glial cells, have a fundamental role in regulating the
neurogenesis process and dendric spine density in cognition-related
brain regions, such as the hippocampus (6,7).
Multiple perioperative factors, including surgical stimulation and
inhaled anesthetics, may accelerate this process by enhancing
proinflammatory factors. Overproduction of these proinflammatory
molecules results in a negative and toxic response in neurons,
including dysfunction in neurogenesis, neural plasticity and
long-term potentiation (LTP), eventually leading to cognitive
decline (8). Thus,
surgery/anesthesia-induced neuroinflammation has been indicated to
promote the incidence and development of POCD and candidate
interventions targeting aberrant neuroinflammation have aroused
global interest (9). Microglia are
considered as the innate immunity cells in the central nervous
system and their activation and M1/M2 polarization are crucial to
the process of neuroinflammation (10). Furthermore, the physiological
functions of microglia in neurogenesis and synaptic plasticity may
be dampened in neurodegenerative diseases (11). Regulating the activation and M1/M2
polarization of microglia in neuroinflammation may have a potential
therapeutic effect on cognitive ability in neurodegenerative
diseases and POCD.
Cannabinoid receptor 2 (CB2R) is a crucial
neuromodulatory target in the central nervous system and has an
important role in homeostatic control and numerous
neurodegenerative diseases. CB2R is reported to be expressed in
both microglia and neurons in healthy brains at low levels and is
markedly upregulated by microglia during pathological conditions.
Previous studies have demonstrated the vital role of CB2R in
microglial activity and polarization in the regulation of
neuroinflammatory processes (12).
Accumulated evidence suggests a critical role of CB2R in mediating
neuroinflammation, neurogenesis and neuroplasticity, and it is a
promising pharmacological target for Alzheimer's disease and POCD
(13,14). Pharmacological intervention or
genetic deletion of CB2R may affect the pathological development of
Alzheimer's disease in model mice, accompanied by attenuation of
neuroinflammation and improvement of cognitive impairment (15,16).
Another study indicated that activation of CB2R may effectively
attenuate surgery/isoflurane (Iso)-induced spatial memory
impairment by downregulating hippocampal microglial activation and
pro-inflammatory factors (17).
Bromodeoxyuridine (BrdU) immunohistochemistry has been applied for
the study of neurogenesis in the adult mammalian brain (18) and activation of CB2R was also
reported to significantly increase the BrdU+ cells in a
mouse model of stroke (19). Golgi
staining is considered as a classical method to detect dendritic
spine (20) and activation of CB2R
by minocycline increased the number of dendritic spines in the
hippocampus and enhanced learning and memory of aged mice (21). However, the mechanisms underlying
the therapeutic effect of CB2R on Iso-induced POCD model mice have
not been extensively investigated. Thus, the present study was
performed to investigate the effects of CB2R deficiency on spatial
memory, hippocampal neuroinflammation, neurogenesis and
neuroplasticity in mice with Iso-induced POCD.
Materials and methods
Animals
Five female CB2R knockout (KO) mice (aged 3 months;
weight, 25-30 g) were purchased from the Jackson Laboratory and
originally created on the background of C57BL/6J mice. Based on the
protocol of a previous study (22),
female CB2R KO mice were crossed with five male wild-type (WT)
C57BL/6J mice (aged 3 months; weight, 25-30 g; purchased from
Sipeifu Biotechnology Co.) to obtain heterozygous
CB2R+/- mice, and then the CB2R+/- mice were
bred with each other to generate littermates of CB2R+/+
(CB2R WT) and CB2R-/- (CB2R KO). All mice were
group-housed in a standard rodent unit with free access to food and
water. All mice were genotyped by PCR using the following primers:
CB2R KO, 5'-GGGGATCGATCCGTCCTGTAAGTCT-3'; CB2R WT,
(5'-GGAGTTCAACCCCATGAAGGAGTAC-3'); and CB2R common
(5'-GACTAGAGCTTTGTAAGGCGGG-3') (23). ‘Common’ refers to primers used for
both experimental types of mice. Primers for the CB2R KO mice
included CB2R KO and CB2R common, and primers for the CB2R WT mice
included CB2R WT and CB2R common.
All experiments were performed with the approval of
the Animal Care Committee of the Fourth Hospital of Hebei Medical
University (Shijiazhuang, China).
Animal groups and treatment
A total of 20 CB2R KO mice and 20 WT littermates
(CB2R WT) (aged 3 months; weight, 25-30 g) were randomly divided
into groups (n=10 in each group): CB2R WT+O2, CB2R
KO+O2, CB2R WT+Iso and CB2R KO+Iso. The level of Iso
exposure was based on previous reports indicating that 1.4% Iso for
>4 h resulted in cognitive impairment in young adult C57BL/6
mice (23). The mice from the CB2R
WT+Iso and CB2R KO+Iso groups received 1.4% Iso in 100% oxygen for
4 h using a rodent anesthesia machine (R610; RWD Life Science Co.,
Ltd.) based on previous literature (24). The mice from the CB2R
WT+O2 and CB2R KO+O2 groups received 100%
oxygen for 4 h and the temperature was maintained at 37±0.5˚C using
a heating pad during Iso treatment.
Morris water maze (MWZ) test
After a 10 day washing period between Iso treatment
and examination based on previous literature (24), the MWZ test was performed to
evaluate the spatial learning and memory capabilities of the mice,
as previously reported (25,26).
An open-field circular pool was filled with warm water (23˚C) to a
height of 30 cm and a white hidden platform (10 cm in diameter) was
submerged 1.5 cm below the water surface. A black curtain separated
the pool from its surroundings in an isolated room. An automated
video tracking system recorded the motion of each mouse and the
data were analyzed using MWZ test analysis software (version:
DigBehv-MM; Jiliang Co.). During the five consecutive training
days, each mouse performed four trials of the navigation test
daily. The escape latency to the hidden platform and swimming speed
were recorded. On the sixth day, the hidden platform was removed
and the number of times the former location of the platform was
crossed was recorded.
After the cognitive performance experiments, mice
were injected with a dose of sodium pentobarbital (50 mg/kg,
intraperitoneally) and sacrificed by cervical dislocation. Their
brains were removed from skulls and rapidly dissected into two
hemispheres. One hemisphere was post-fixed in 4% paraformaldehyde
for 24 h, incubated in 30% sucrose for 12 h and then processed for
immunofluorescence staining and BrdU immunostaining. The other
hemisphere was frozen immediately on dry ice, stored at -80˚C and
processed for RNA extraction.
Immunofluorescence staining
Immunofluorescence staining of the hippocampal
slices was performed as described in a previous study by our group
(15). All hippocampal sections
were incubated with 3% bovine serum albumin (OriGene Technologies,
Inc.) for 20 min at room temperature (25-30˚C) to block
non-specific binding sites. The sections were then incubated with
the primary antibody, rabbit polyclonal anti-ionized
calcium-binding adaptor molecule-1 (Iba1; cat. no. ab153696; 1:500;
Abcam), for 12 h at 4˚C. The sections were then incubated with
Alexa Fluor 488-conjugated secondary antibodies (cat. no. ab150077;
1:100; Abcam) for 2 h at room temperature (25-30˚C), followed by
counterstaining with DAPI. Representative fluorescence microscopy
images of the hippocampal samples were taken and immunofluorescence
staining intensity was quantified using a Nikon Eclipse Ni-U
microscope (Nikon Corporation) at x10 magnification and image pro
plus software version 6.0 (Media Cybernetics, Inc.) by an
investigator who was blinded to the origin of the sections.
Integrated optical density of each slice from each group was
measured and presented as a percentage with CB2R WT + O2
as the control. Microglial morphology was analyzed using Imaris
software (version 8.1; Oxford Instruments) at x100 magnification to
assess the number of microglial branches.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from the hippocampal tissue
using the RNeasy mini kit (Qiagen GmbH) following the
manufacturer's protocol. RT to generate cDNA was performed using
the ReverTra Ace qPCR RT Master Mix with gDNA Remover kit (Toyobo
Co., Ltd.) according to the manufacturer's protocol. qPCR analysis
was performed using the FastStart Essential DNA Green Master kit
(Roche) according to the manufacturer's protocol. The primer
sequences of the target genes were as follows: Interleukin-6
(IL-6), 5'-TGCAAGAGACTTCCATCCAGTT-3' (forward) and
5'-GAAGTAGGGAAGGCCGTGG-3' (reverse); tumor necrosis factor-α
(TNF-α), 5'-GCACCACCATCAAGGACTC-3' (forward) and
5'-TGAGACAGAGGCAACCTGAC-3' (reverse); inducible nitric oxide
synthase (iNOS), 5'-GGCAGCCTGTGAGACCTTTG-3' (forward) and
5'-GCATTGGAAGTGAAGCGTTTC-3' (reverse); chitinase-3 like protein
(Ym1/2), 5'-CAGGGTAATGAGTGGGTTGG-3' (forward) and
5'-CACGGCACCTCCTAAATTGT-3' (reverse); GAPDH,
5'-ACTCCACTCACGGCAAATTC-3' (forward) and 5'-TCTCCATGGTGGTGAAGACA-3'
(reverse). The PCR amplification conditions were adjusted based on
the manufacturer's protocol and previous research (15): 95˚C for 10 min and 45 cycles of 95˚C
for 10 sec and 60˚C for 1 min. The expression levels of these genes
were normalized to the levels of GAPDH and presented as
2-∆∆Cq with CB2R WT+O2 as the control
(27).
BrdU injection and immunostaining
All mice received an intraperitoneal injection of
BrdU (Sigma-Aldrich; Merck KGaA) at a dose of 50 mg/kg twice a day
for five consecutive days and were sacrificed 24 h after the last
BrdU injection (28). For BrdU
staining, coronal sections were incubated in 2N HCl at 37˚C for 30
min to denature the DNA. The sections were then incubated with 1%
bovine serum albumin (cat. no. ZLI-9027; OriGene Technologies,
Inc.) for 30 min at room temperature (25-30˚C), followed by
incubation with anti-BrdU antibody (cat. no. 6326; 1:500 dilution;
Abcam) at 4˚C for 12 h. The primary antibody was detected by
subsequent incubation with the corresponding biotinylated secondary
antibody (cat. no. ZB-2040; 1:100; OriGene Technologies, Inc.) and
a diaminobenzidine kit (cat. no. ZLI-9017; OriGene Technologies,
Inc.) at room temperature (25-30˚C) for 1 h. Stained sections were
imaged by an investigator blinded to the treatment conditions using
an Olympus VS120 light microscope at x4 magnification (Olympus
Corporation). All BrdU+ cells in the dentate gyrus (DG)
of the hippocampus were identified and counted by an investigator
who was blinded to the origin of the section. The average numbers
of BrdU+ cells in three sections per mouse were
analyzed.
Golgi staining and spine density
analysis
Golgi staining was performed according to the
protocol of the FD Rapid Golgistain™ kit (FD NeuroTechnologies,
Inc.), as previously described (15). Mouse brain tissues were impregnated
with solutions A and B for 2 weeks (room temperature, in the dark),
followed by incubation in solution C for 1 week (4˚C, in the dark).
Next, brain tissues were cut into 100-µm coronal sections. Sections
containing the hippocampus were stained with solutions D and E
mixed with distilled water and dehydrated in a graded ethanol
series. Images were acquired using an Olympus IX-81 microscope
(Olympus Corporation) with a 60x oil lens. Dendritic spine density
in the DG of the hippocampus was measured using Imaris software
(version 8.1; Oxford Instruments) in a blinded manner.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism version 7.0 (GraphPad Software, Inc.). Values are
expressed as the mean ± standard error of mean. Results of the
escape latencies and swimming speeds from the MWZ tests were
analyzed using two-way repeated-measures ANOVA followed by Tukey's
post-hoc test. For other data, a two-way ANOVA was performed, if
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
CB2R deficiency aggravates Iso-induced
spatial cognitive impairment in adult mice
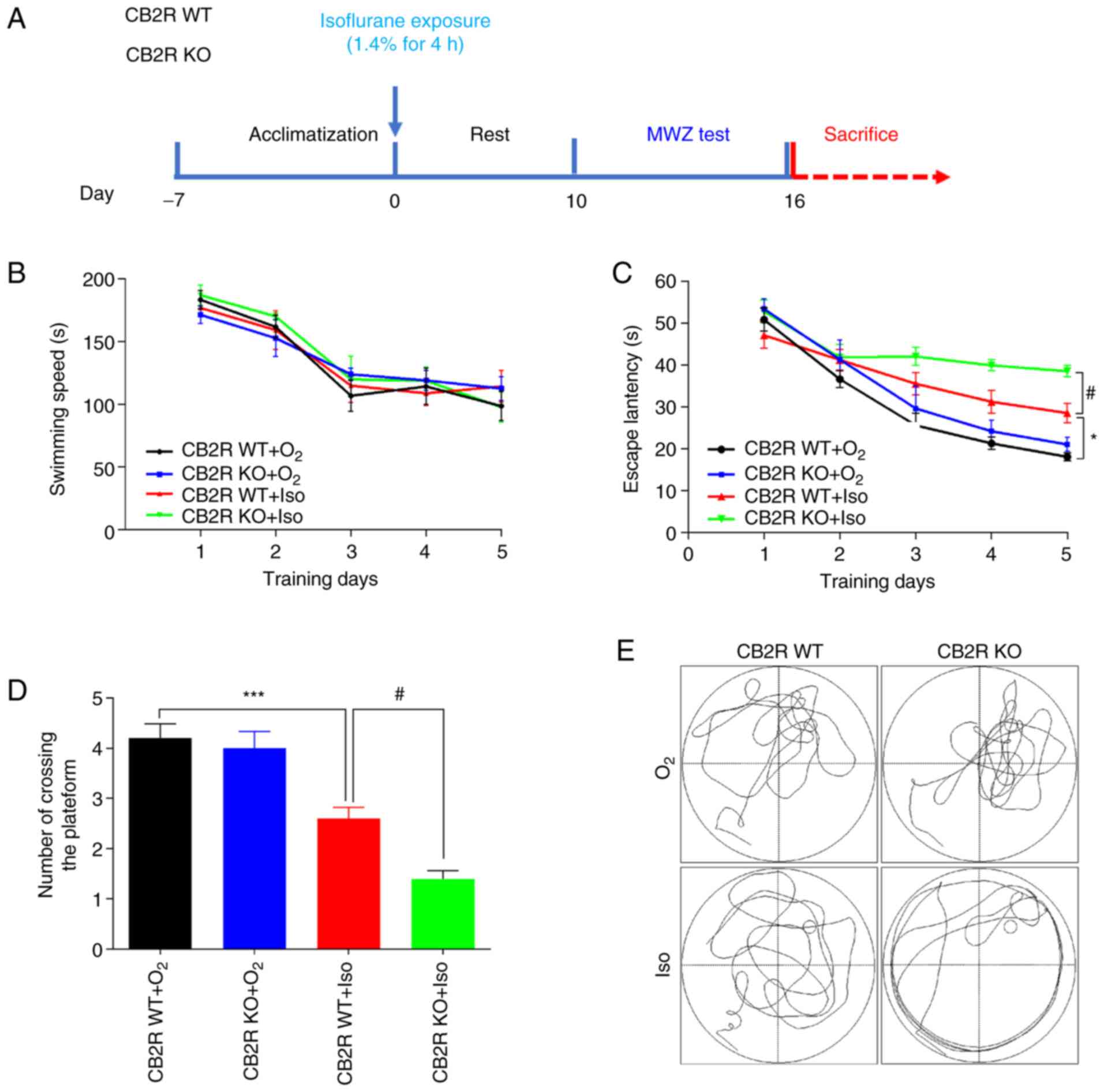
The timeline of the experimental procedures is
presented in Fig. 1A. To examine
whether CB2R deficiency affected spatial learning and memory in the
Iso-induced POCD mouse model, animals were subjected to MWZ tests.
It was revealed that Iso exposure and CB2R deficiency did not
affect the swimming speed on the five training days (Fig. 1B). The results indicated that the
CB2R WT+Iso group had a significantly longer escape latency and a
smaller number of platform crossings than the CB2R WT+O2
group (P<0.05 and P<0.001, respectively; Fig. 1C and D). Furthermore, CB2R KO+Iso mice had a
significantly longer escape latency and a smaller number of
platform crossings than the CB2R WT+Iso group (P<0.05 and
P<0.05, respectively; Fig. 1C
and D). Representative swimming
paths on the sixth day are provided in Fig. 1E. The present results indicated that
CB2R deficiency aggravated the spatial learning and memory deficits
induced by Iso exposure.
CB2R deficiency enhances Iso-induced
microglial activation and branching in the hippocampus of adult
mice
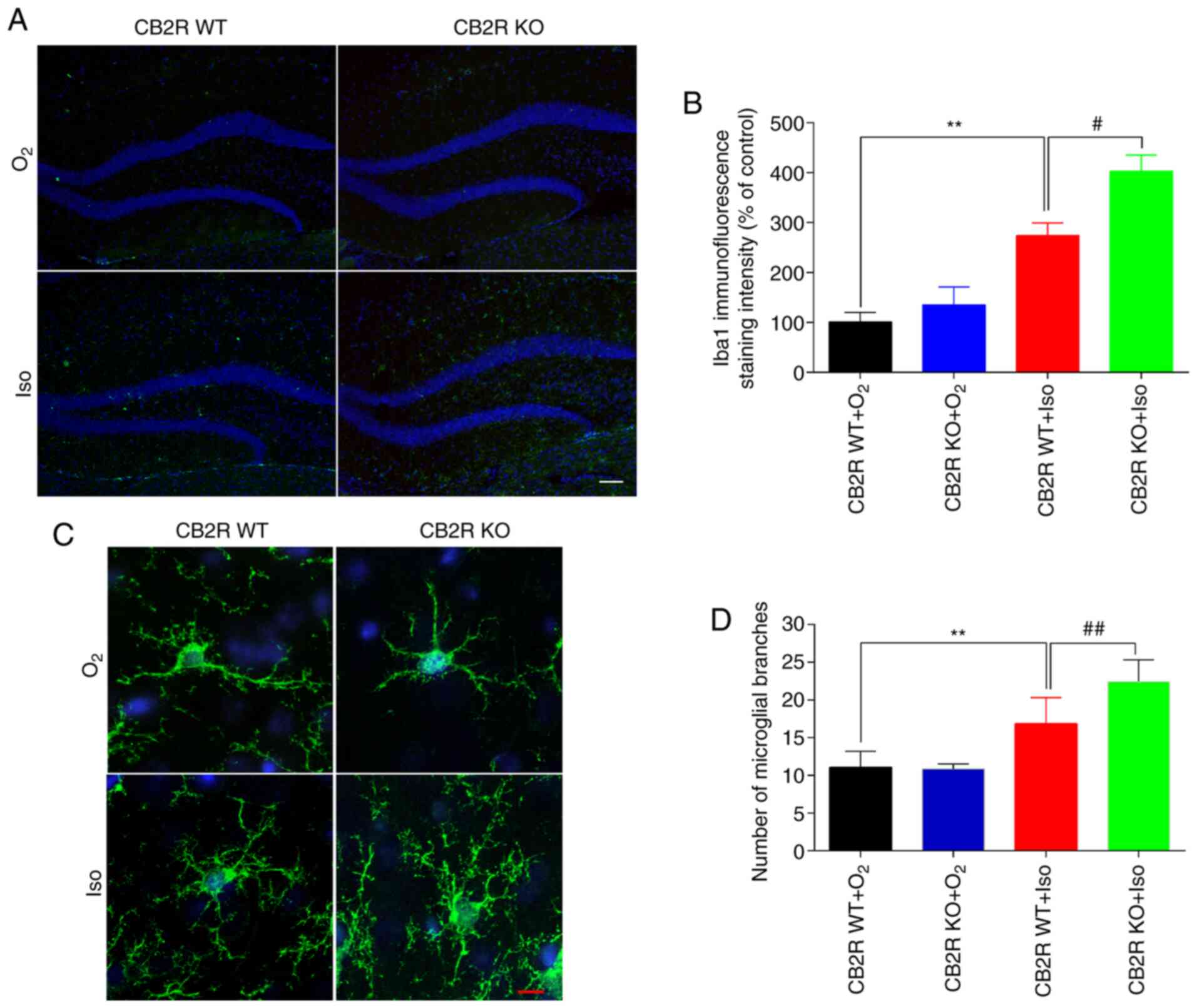
To investigate whether CB2R deficiency affected
microglial activation and morphology in the Iso-induced POCD model
mice, microglia were subjected to immunofluorescence staining for
Iba1, an efficient and specific microglial biomarker (29). The results indicated increased Iba1
immunoreactivity and a larger number of microglial branches in the
hippocampus of the CB2R WT+Iso group compared to the CB2R
WT+O2 group (P<0.01; Fig.
2A-D). In addition, Iba1 immunoreactivity and the number of
microglial branches in the hippocampus of the CB2R KO+Iso group
were significantly increased in comparison with the CB2R WT+Iso
group (P<0.05; Fig. 2A-D). These
results demonstrated that CB2R deficiency facilitated Iso-induced
microglial activation and branching in the hippocampus of adult
mice.
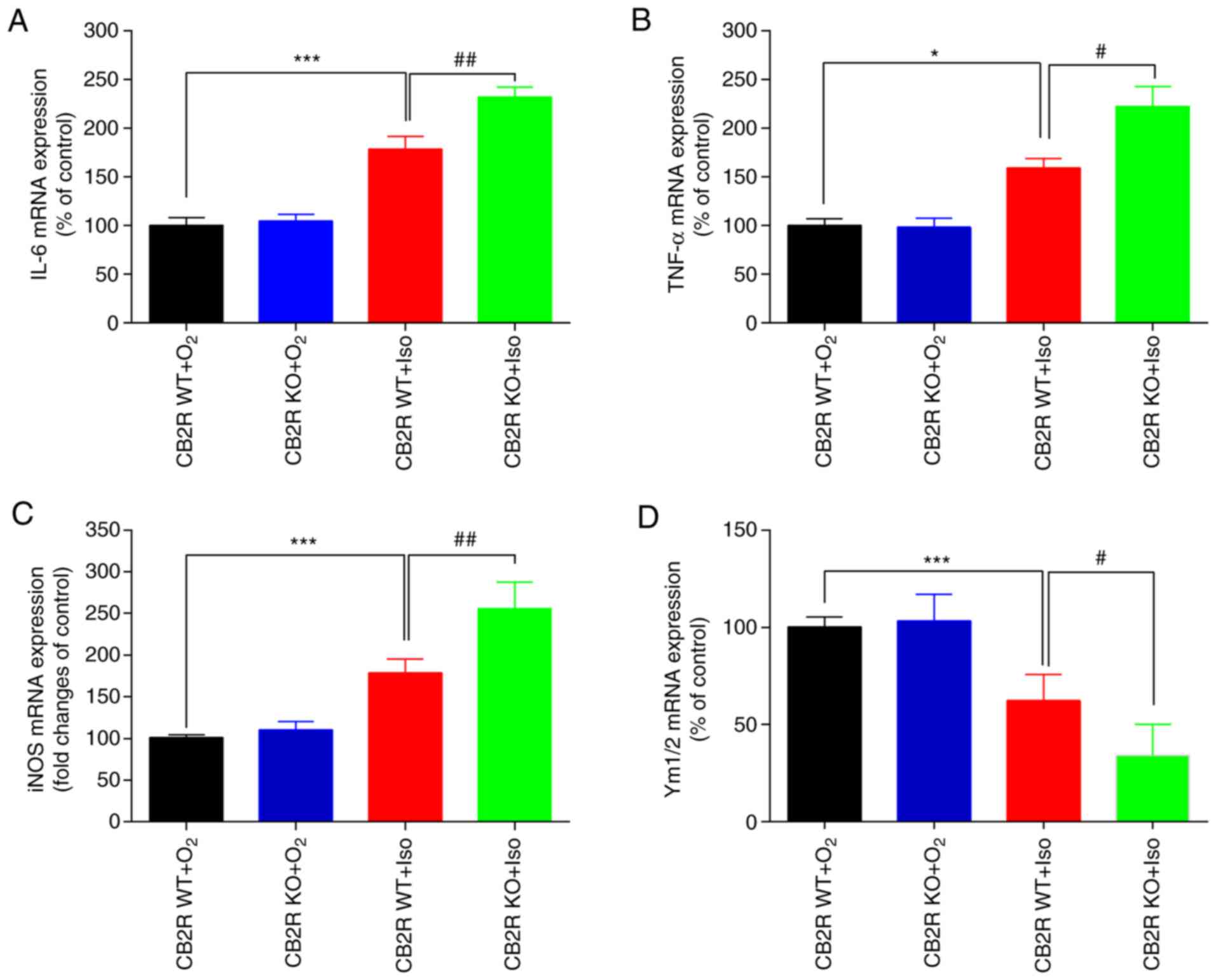
CB2 receptor deficiency promotes
microglial M1 polarization in the hippocampus of adult mice exposed
to Iso
In general, in numerous neurodegenerative diseases,
activated microglia are driven to polarize to two opposite
subtypes: M1 phenotype expressing the markers IL-6, TNF-α and iNOS
or the M2 phenotype characterized by Ym1/2 expression (10). To explore the effect of CB2R
deficiency on microglial M1/M2 polarization in an Iso-induced mouse
model of POCD, RT-qPCR analysis was performed to evaluate the mRNA
expression levels of IL-6, TNF-α, iNOS and Ym1/2. The results
indicated significant upregulation of M1-associated mRNA levels and
downregulated M2-associated mRNA levels in the CB2R WT+Iso group
compared with the CB2R WT+O2 group (all P<0.05;
Fig. 3A-D). Of note, these changes
in microglial M2/M1 polarization in the CB2R WT+Iso group were
greater than those in the CB2R KO+Iso group (all P<0.05;
Fig. 3A-D). Thus, CB2R deficiency
significantly influenced Iso-induced microglial polarization by
promoting M1 and suppressing M2 phenotype expression.
CB2 receptor deficiency aggravates
neurogenesis damage in the hippocampus of adult mice exposed to
Iso
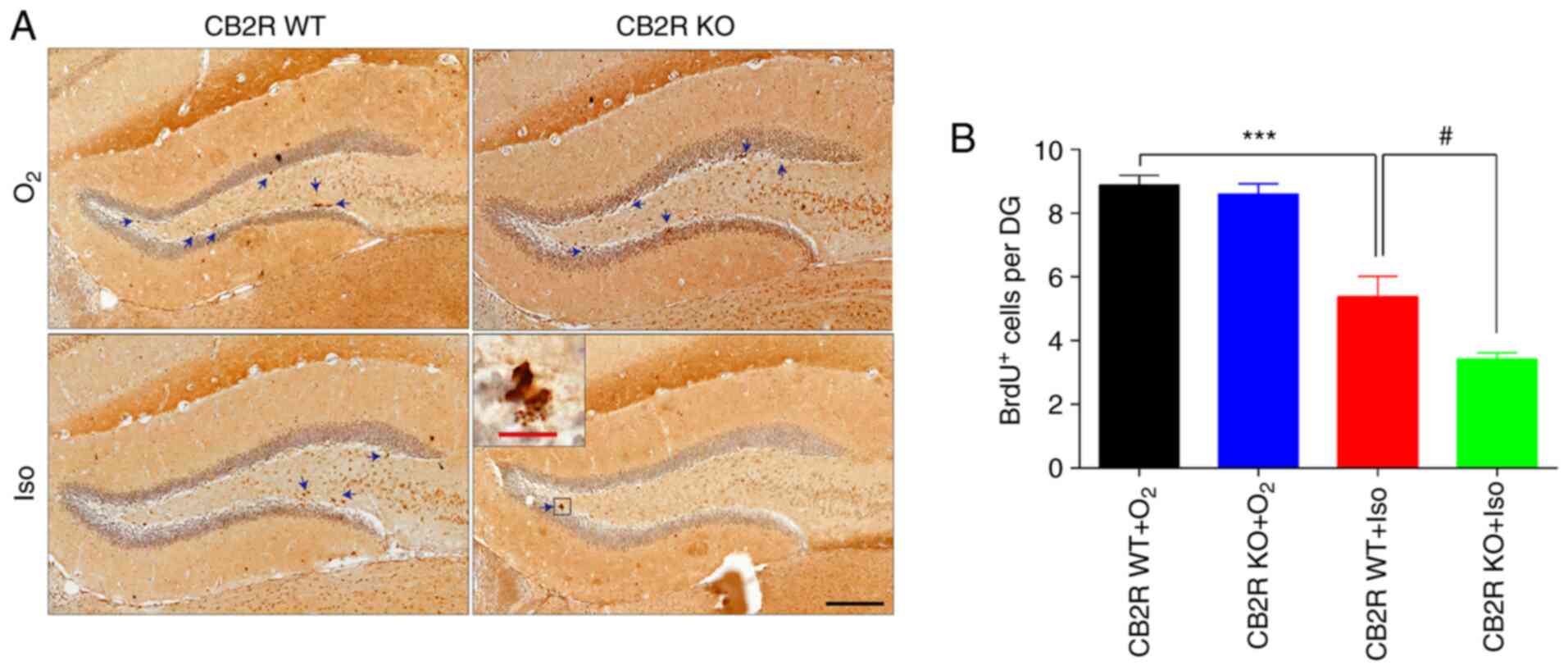
Inhibition of hippocampal neurogenesis has been
demonstrated to be a vital hallmark of the POCD model (30). To investigate the effect of CB2R
deficiency on neurogenesis in an Iso-induced POCD mouse model, BrdU
immunostaining was performed to label proliferating neural
progenitor cells. The results revealed that CB2R WT+Iso mice had
significantly fewer BrdU+ cells in the hippocampus
compared with the CB2R WT+O2 group (P<0.001; Fig. 4A and B). Furthermore, the number of
BrdU+ cells in CB2R KO+Iso mice was significantly lower
than that in CB2R WT+Iso mice (P<0.05; Fig. 4A and B). These results indicated that CB2R
deficiency enhanced Iso-induced neurogenesis damage in the
hippocampus of adult mice.
CB2 receptor deficiency reduces
dendritic complexity in the hippocampus of adult mice exposed to
Iso
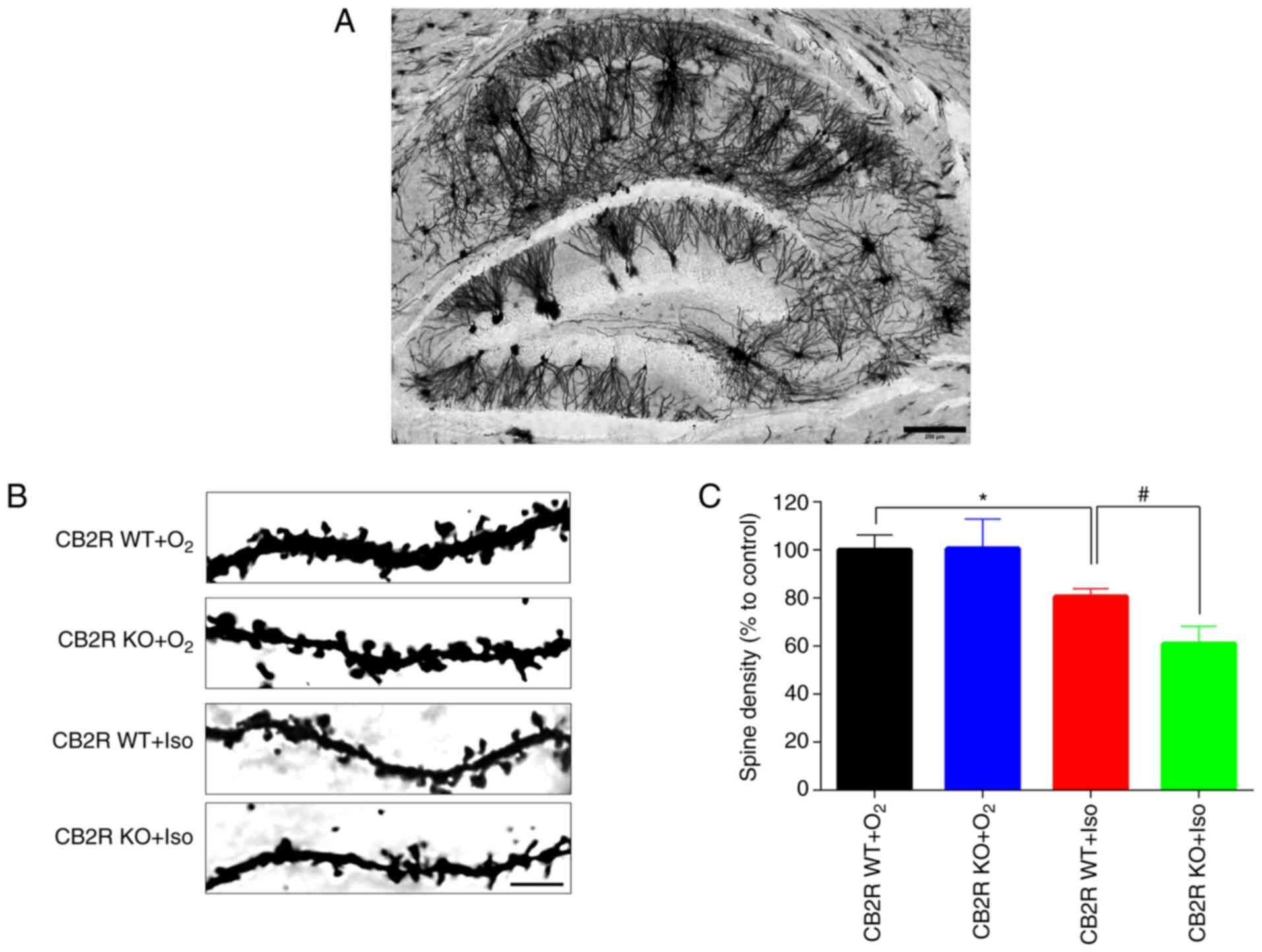
Aberrant synaptic plasticity has been reported to be
closely related to Iso-induced POCD (31). In Fig.
5A, Golgi staining demonstrated that neurons in the whole brain
section from the control mice were interlaced, thus making neuronal
changes in the whole brain section difficult to analyze. Thus,
Golgi staining was performed to detect the spine density in the
hippocampal DG region in the different groups. The results
indicated that spine density in hippocampal DG neurons of CB2R
WT+Iso mice was significantly lower than that of CB2R
WT+O2 mice (P<0.05; Fig.
5B and C). In addition, a
marked reduction in spine density was determined in the hippocampal
DG region of CB2R KO+Iso mice compared with that in CB2R WT+Iso
mice (P<0.05; Fig. 5B and
C). These results indicated that
CB2R deficiency significantly enhanced the Iso-induced spine
density reduction and dendritic complexity in the hippocampus of
adult mice.
Discussion
POCD is one of the most urgent concerns for patients
and clinicians worldwide, as it significantly increases medical
costs, morbidity and mortality. Therefore, extensive research has
been performed to investigate its underlying etiology and potential
therapeutic targets. A large and growing body of evidence supports
the theory that CB2R has a vital neuroprotective role in cognitive
dysfunction diseases via modulation of microglia-associated
neuroinflammation (32). The
present study investigated the effects of CB2R deficiency on
Iso-induced spatial cognition impairment in adult mice. The results
indicated that CB2R KO mice with Iso exposure had a significantly
poorer performance in the MWZ test, more pronounced changes in
neuroinflammation parameters and more severe neurogenesis and
neuroplasticity damage in the hippocampus than WT mice, indicating
that CB2R deficiency made adult mice more vulnerable to the
neurotoxicity of Iso, as summarized in the schematic in Fig. 6.
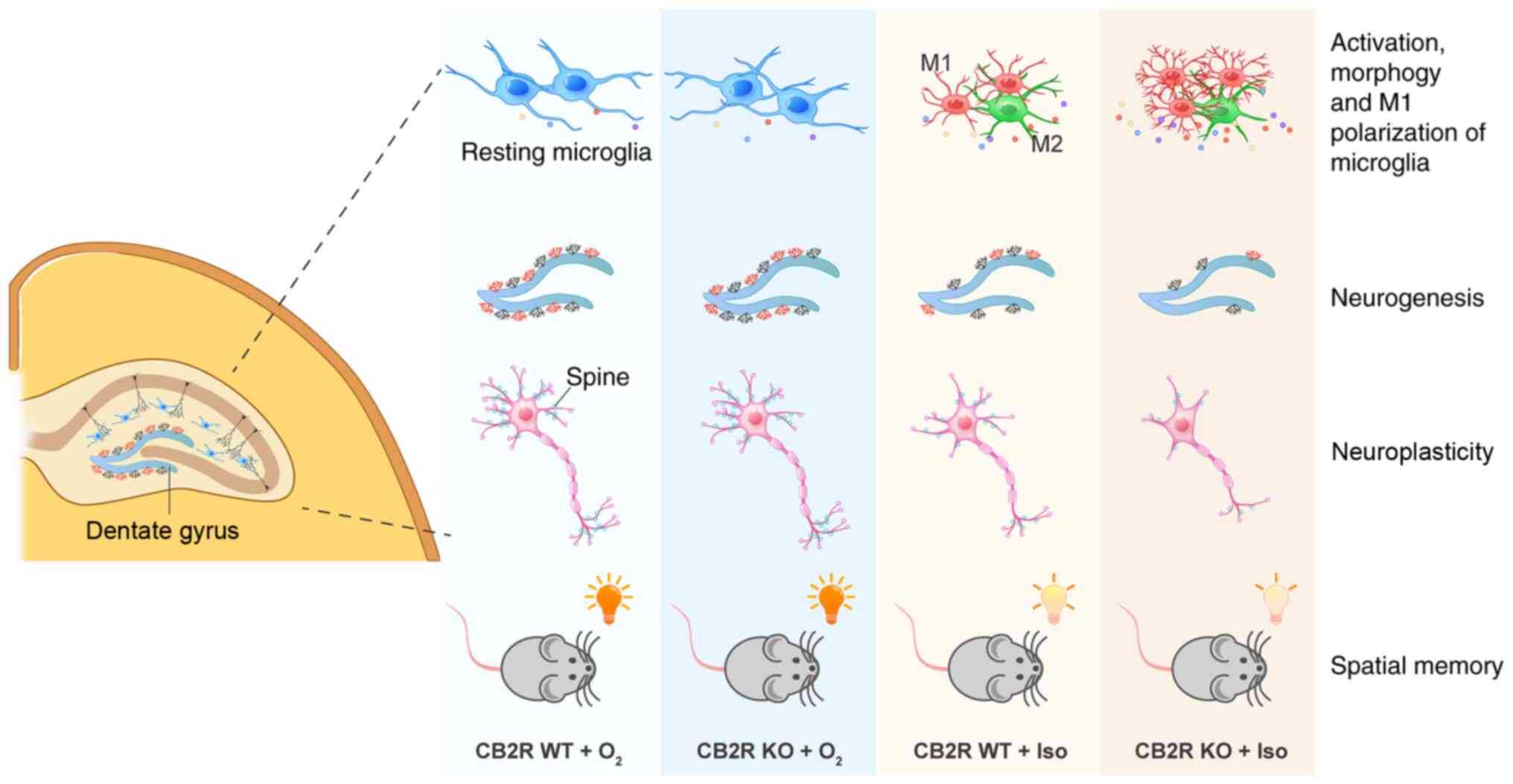 | Figure 6Schematic representation of the
effects of CB2R deficiency on Iso-induced cognitive impairment. Iso
exposure leads to spatial memory impairment of adult mice,
accompanied by alterations in the activation, morphology and M1
polarization of microglia, BrdU+ neurogenesis and spine
density in the hippocampus. CB2R deficiency makes adult mice more
vulnerable to Iso neurotoxicity, as indicated by more significant
injury in terms of neuroinflammation, neurogenesis and
neuroplasticity after Iso exposure. BrdU, 5-bromodeoxyuridine;
CB2R, cannabinoid receptor 2; WT, wild-type; KO, knockout; Iso,
isoflurane. |
The MWZ test, a classical tool used to monitor
hippocampus-related spatial learning and memory ability, has been
widely applied in laboratory research (33). The results suggested that CB2R KO
mice without Iso exposure had a similar cognitive score in the MWZ
test compared with control mice and that Iso-exposed CB2R KO mice
had a poorer performance in the MWZ test compared with Iso-exposed
WT mice. However, previous studies on the effect of CB2R deficiency
on cognition and memory were inconclusive. Li and Kim (23) reported that CB2R KO mice displayed
impaired long-term contextual fear memory but enhanced spatial
working memory. Schmöle et al (26) indicated that CB2R deletion enhanced
spatial learning of 6-month-old CB2R KO and APP/PS1 (double
transgenic mice)/CB2R KO mice in the MWZ test. Aso et al
(34) reported that CB2R deficiency
did not significantly affect memory impairment in a two-object
recognition test of 3-month- and 6-month-old APP/PS1/CB2R KO mice.
Of note, the present results also suggested that CB2R deficiency in
the absence of Iso exposure did not affect the cognitive
performance. These independent studies imply that in the absence of
Iso, CB2Rs alone have diverse, or even opposite roles, in
regulating different types of cognition and memory, depending on
the distinct disease stage. Moreover, combined surgery, anesthetic
treatment or other injury factors and the effect of CB2 receptor
deficiency on cognitive function requires further study.
The neuroinflammatory hypothesis is considered one
of the leading mechanisms of POCD, based on which various promising
candidates for the prevention and treatment of POCD have been
developed (9). Strategies targeting
microglia to reduce the development of POCD may be feasible and
viable (35). Iso exposure or
surgery leads to post-operative cognitive dysfunction in aged
rodents, accompanied by upregulated protein levels of
pro-inflammatory cytokines TNF-α, IL-1β, interferon-γ and microglia
marker Iba-1 in the hippocampus (36). Thus, immunofluorescence staining and
RT-qPCR were performed in the present study to determine the effect
of CB2R deficiency on microglial activity and mRNA expression of
M1/M2 microglial phenotype markers in the whole hippocampus. The
results indicated the presence of spatial cognitive impairment
accompanied by significant alterations of activation, morphology
and M1 polarization of microglia in the hippocampus of Iso-exposed
CB2R WT mice compared with control mice. CB2R KO mice without Iso
exposure had a similar expression of Iba1 and M1 polarization
compared with control mice and Iso-exposed CB2R KO mice had more
expression of Iba1 and M1 polarization compared with Iso-exposed WT
mice. This may be interpreted as CB2R deficiency alone not being
able to induce the activation of microglia, while it enhanced
Iso-induced microglial activation and spatial cognitive impairment
in the MWM test. These results are consistent with those of
previous studies. Nent et al (37) demonstrated that hyperreactive
neuropathic pain responses in CB2R KO mice were associated with
increased immunostaining of microglial marker Iba1 in the spinal
cord. Amenta et al (38)
indicated that blockade or deletion of CB2Rs expanded the
post-traumatic inflammatory responses in controlled cortical impact
model mice, as evidenced by substantial upregulation of iNOS
expression and aberrant activation of resident microglia. Sun et
al (17) reported that
selective activation of CB2R effectively attenuated
Iso/surgery-induced hippocampal memory loss in adult mice by
downregulating microglia-associated CD11b expression and
proinflammatory factors in the hippocampus and medial prefrontal
cortex. In an experimental germinal matrix hemorrhage rat model,
activation of CB2R by JWH133 downregulated neuroinflammation by
promoting microglial M1-to-M2 polarization through the cyclic
adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling
pathway (39). The underlying
relationship of CB2R deletion, the cAMP/PKA pathway and microglial
polarization in the pathological process of POCD requires
validation in further experiments. All of these results highlight
that the neuroprotective effects of CB2R were associated with the
modulation of microglial neuroinflammation in POCD.
In addition to neuroinflammation, neurogenesis and
neuroplasticity are considered crucial mechanisms for the
endogenous cannabinoid system to exert regulatory roles in numerous
neurocognitive disorders (40).
CB2R has been linked to the regulation of adult neurogenesis in the
mammalian brain. The largest rates of neurogenesis were observed in
the subgranular zone of hippocampal DG and in the subventricular
zone (41). Thus, in the present
study, BrdU immunostaining was performed to detect the neurogenesis
ability in hippocampal DG. The results indicated that CB2R
deficiency did not alter BrdU+ neuronal populations in
the hippocampus compared to control mice but significantly enhanced
the suppression of neurogenesis induced by Iso exposure. This is in
accordance with the results of previous studies. Mensching et
al (41) determined that adult
CB2R-deficient mice had a stable level of proliferation in the
hippocampus. Bravo-Ferrer et al (19) revealed that blockade and deletion of
CB2R deteriorated the number of new neurons in the peri-infarct
cortex after stroke.
Dendritic spines are post-synaptic structures at a
majority of excitatory synapses in the mammalian brain. The number
and size of dendritic spines are closely related to cognitive
function in different neurological diseases (42). Iso exposure was reported to lead to
neuroplastic deficit. Neonatal Iso exposure was indicated to
contribute to the decline of dendritic spine densities in the
hippocampal DG of juvenile mice accompanied by cognitive deficits
in an object recognition test (43). Thus, Golgi staining was performed to
detect the dendritic spine destiny in hippocampal DG. In other
studies, deletion of CB2R led to alterations of various targets
involved in the hippocampal neuroplasticity of mice, including
synaptophysin, synaptic transmission, LTP function and dendritic
complexity (22,44). However, the present results
indicated no difference in spine density between CB2R WT and CB2R
KO mice, although the changes after Iso exposure were significantly
different. It is possible that CB2R deficiency may be more relevant
in feeble conditions in which neurogenesis and neuroplasticity are
severely impaired by Iso or traumatic injury.
There are certain limitations to the present study.
RT-qPCR experiments were performed to explore the impact of CB2R
deficiency on microglial phenotype changes in Iso-induced POCD mice
based on a previous study by our group (15). It may be better to detect the
expression of M1/M2 microglial phenotypes markers through different
tests, including western blot or flow cytometry.
In conclusion, the present study indicated that CB2R
deficiency aggravated spatial cognitive impairment in the
Iso-induced POCD mouse model. This was partly explained by the
aggravated neuroinflammatory reactivity of microglia and enhanced
injury to neurogenesis and neuroplasticity in the hippocampus.
These results further suggest that CB2R is a promising
pharmacological target for Iso-induced POCD; however, further
research is required to demonstrate its validity.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and JPS were responsible for designing the study,
performing the experiment, collecting the data and writing the
manuscript. JGS and YS were responsible for designing the study,
performing the experiment, and collecting the data and reviewing
the manuscript. HJ was responsible for providing experimental ideas
and reviewing the manuscript. CL and HJ were responsible for the
confirming the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed with the approval of
the Animal Care Committee of the Fourth Hospital of Hebei Medical
University (Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evered LA and Silbert BS: Postoperative
cognitive dysfunction and noncardiac surgery. Anesth Analg.
127:496–505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hovens IB, Schoemaker RG, van der Zee EA,
Heineman E, Izaks GJ and van Leeuwen BL: Thinking through
postoperative cognitive dysfunction: How to bridge the gap between
clinical and pre-clinical perspectives. Brain Behav Immun.
26:1169–1179. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rundshagen I: Postoperative cognitive
dysfunction. Dtsch Arztebl Int. 111:119–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Monk TG, Weldon BC, Garvan CW, Dede DE,
van der Aa MT, Heilman KM and Gravenstein JS: Predictors of
cognitive dysfunction after major noncardiac surgery.
Anesthesiology. 108:18–30. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kapila AK, Watts HR, Wang T and Ma D: The
impact of surgery and anesthesia on post-operative cognitive
decline and Alzheimer's disease development: Biomarkers and
preventive strategies. J Alzheimers Dis. 41:1–13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Spangenberg EE and Green KN: Inflammation
in Alzheimer's disease: Lessons learned from microglia-depletion
models. Brain Behav Immun. 61:1–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sung PS, Lin PY, Liu CH, Su HC and Tsai
KJ: Neuroinflammation and neurogenesis in Alzheimer's disease and
potential therapeutic approaches. Int J Mol Sci.
21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd
S, Lewis M, Marriott A, Moore EM, Morris G, Page RS, et al:
Post-operative cognitive dysfunction: An exploration of the
inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev.
84:116–133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Safavynia SA and Goldstein PA: The Role of
neuroinflammation in postoperative cognitive dysfunction: Moving
from hypothesis to treatment. Front Psychiatry.
9(752)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tang Y and Le W: Differential roles of M1
and M2 microglia in neurodegenerative diseases. Mol Neurobiol.
53:1181–1194. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Santos LE, Beckman D and Ferreira ST:
Microglial dysfunction connects depression and Alzheimer's disease.
Brain Behav Immun. 55:151–165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Komorowska-Müller JA and Schmöle AC: CB2
Receptor in microglia: The guardian of self-control. Int J Mol Sci.
22(19)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aso E and Ferrer I: CB2 Cannabinoid
receptor as potential target against Alzheimer's disease. Front
Neurosci. 10(243)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Basavarajappa BS, Shivakumar M, Joshi V
and Subbanna S: Endocannabinoid system in neurodegenerative
disorders. J Neurochem. 142:624–648. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li C, Shi J, Wang B, Li J and Jia H: CB2
cannabinoid receptor agonist ameliorates novel object recognition
but not spatial memory in transgenic APP/PS1 mice. Neurosci Lett.
707(134286)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schmöle AC, Lundt R, Toporowski G, Hansen
JN, Beins E, Halle A and Zimmer A: Cannabinoid receptor
2-deficiency ameliorates disease symptoms in a mouse model with
Alzheimer's disease-like pathology. J Alzheimers Dis. 64:379–392.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun L, Dong R, Xu X, Yang X and Peng M:
Activation of cannabinoid receptor type 2 attenuates
surgery-induced cognitive impairment in mice through
anti-inflammatory activity. J Neuroinflammation.
14(138)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taupin P: BrdU immunohistochemistry for
studying adult neurogenesis: Paradigms, pitfalls, limitations, and
validation. Brain Res Brain Res Rev. 53:198–214. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bravo-Ferrer I, Cuartero MI, Zarruk JG,
Pradillo JM, Hurtado O, Romera VG, Díaz-Alonso J, García-Segura JM,
Guzmán M, Lizasoain I, et al: Cannabinoid Type-2 receptor drives
neurogenesis and improves functional outcome after stroke. Stroke.
48:204–212. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mancuso JJ, Chen Y, Li X, Xue Z and Wong
ST: Methods of dendritic spine detection: From Golgi to
high-resolution optical imaging. Neuroscience. 251:129–140.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jiang Y, Liu Y, Zhu C, Ma X, Ma L, Zhou L,
Huang Q, Cen L, Pi R and Chen X: Minocycline enhances hippocampal
memory, neuroplasticity and synapse-associated proteins in aged C57
BL/6 mice. Neurobiol Learn Mem. 121:20–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y and Kim J: Deletion of CB2
cannabinoid receptors reduces synaptic transmission and long-term
potentiation in the mouse hippocampus. Hippocampus. 26:275–281.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Y and Kim J: CB2 Cannabinoid receptor
knockout in mice impairs contextual long-term memory and enhances
spatial working memory. Neural Plast. 2016(9817089)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu J, Wang P, Zhang X, Zhang W and Gu G:
Effects of different concentration and duration time of isoflurane
on acute and long-term neurocognitive function of young adult
C57BL/6 mouse. Int J Clin Exp Pathol. 7:5828–5836. 2014.PubMed/NCBI
|
|
25
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schmöle AC, Lundt R, Ternes S, Albayram Ö,
Ulas T, Schultze JL, Bano D, Nicotera P, Alferink J and Zimmer A:
Cannabinoid receptor 2 deficiency results in reduced
neuroinflammation in an Alzheimer's disease mouse model. Neurobiol
Aging. 36:710–719. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen B, Bromley-Brits K, He G, Cai F,
Zhang X and Song W: Effect of synthetic cannabinoid HU210 on memory
deficits and neuropathology in Alzheimer's disease mouse model.
Curr Alzheimer Res. 7:255–261. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zöller T, Attaai A, Potru PS, Ruß T and
Spittau B: Aged Mouse cortical microglia display an activation
profile suggesting immunotolerogenic functions. Int J Mol Sci.
19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hem S, Albite R, Loresi M, Rasmussen J,
Ajler P, Yampolsky C, Chabot JD, Gerszten PC and Goldschmidt E:
Pathological changes of the hippocampus and cognitive dysfunction
following frontal lobe surgery in a rat model. Acta Neurochir
(Wien). 158:2163–2171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qiu LL, Pan W, Luo D, Zhang GF, Zhou ZQ,
Sun XY, Yang JJ and Ji MH: Dysregulation of BDNF/TrkB signaling
mediated by NMDAR/Ca2+/calpain might contribute to
postoperative cognitive dysfunction in aging mice. J
Neuroinflammation. 17(23)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Contino M, Capparelli E, Colabufo NA and
Bush AI: Editorial: The CB2 cannabinoid system: A new strategy in
neurodegenerative disorder and neuroinflammation. Front Neurosci.
11(196)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bromley-Brits K, Deng Y and Song W: Morris
water maze test for learning and memory deficits in Alzheimer's
disease model mice. J Vis Exp. 53(2920)2011.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Aso E, Andrés-Benito P, Carmona M,
Maldonado R and Ferrer I: Cannabinoid receptor 2 participates in
amyloid-β processing in a mouse model of Alzheimer's disease but
plays a minor role in the therapeutic properties of a
cannabis-based medicine. J Alzheimers Dis. 51:489–500.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feng X, Valdearcos M, Uchida Y, Lutrin D,
Maze M and Koliwad SK: Microglia mediate postoperative hippocampal
inflammation and cognitive decline in mice. JCI Insight.
2(e91229)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang HL, Liu H, Xue ZG, Liao QW and Fang
H: Minocycline attenuates post-operative cognitive impairment in
aged mice by inhibiting microglia activation. J Cell Mol Med.
20:1632–1639. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nent E, Nozaki C, Schmöle AC, Otte D and
Zimmer A: CB2 receptor deletion on myeloid cells enhanced
mechanical allodynia in a mouse model of neuropathic pain. Sci Rep.
9(7468)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Amenta PS, Jallo JI, Tuma RF, Hooper DC
and Elliott MB: Cannabinoid receptor type-2 stimulation, blockade,
and deletion alter the vascular inflammatory responses to traumatic
brain injury. J Neuroinflammation. 11(191)2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tao Y, Li L, Jiang B, Feng Z, Yang L, Tang
J, Chen Q, Zhang J, Tan Q, Feng H, et al: Cannabinoid receptor-2
stimulation suppresses neuroinflammation by regulating microglial
M1/M2 polarization through the cAMP/PKA pathway in an experimental
GMH rat model. Brain Behav Immun. 58:118–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cassano T, Calcagnini S, Pace L, De Marco
F, Romano A and Gaetani S: Cannabinoid receptor 2 signaling in
neurodegenerative disorders: From pathogenesis to a promising
therapeutic target. Front Neurosci. 11(30)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mensching L, Djogo N, Keller C, Rading S
and Karsak M: Stable adult hippocampal neurogenesis in cannabinoid
receptor CB2 deficient mice. Int J Mol Sci. 20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang K, Lian N, Ding R, Guo C, Dong X, Li
Y, Wei S, Jiao Q, Yu Y and Shen H: Sleep deprivation aggravates
cognitive impairment by the alteration of hippocampal neuronal
activity and the density of dendritic spine in isoflurane-exposed
mice. Front Behav Neurosci. 14(589176)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Schaefer ML, Perez PJ, Wang M, Gray C,
Krall C, Sun X, Hunter E, Skinner J and Johns RA: Neonatal
isoflurane anesthesia or disruption of postsynaptic density-95
protein interactions change dendritic spine densities and cognitive
function in juvenile mice. Anesthesiology. 133:812–823.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
García-Gutiérrez MS, Ortega-Álvaro A,
Busquets-García A, Pérez-Ortiz JM, Caltana L, Ricatti MJ, Brusco A,
Maldonado R and Manzanares J: Synaptic plasticity alterations
associated with memory impairment induced by deletion of CB2
cannabinoid receptors. Neuropharmacology. 73:388–396.
2013.PubMed/NCBI View Article : Google Scholar
|