Introduction
Internal heart ischemia is the main cause of death
in most patients with heart-associated diseases (1). The restoration of the blood flow
inside the heart is a therapeutic strategy used to relieve
myocardial infarction caused by ischemia (2). However, certain studies suggested that
ischemia-reperfusion could induce additional damage to
cardiomyocytes (2,3). In addition, it was shown that
ischemia-reperfusion can lead to functional damage to the heart and
aggravate the structure of the heart muscle following a period of
time (4). These injuries eventually
lead to the injury of heart function and the development of severe
arrhythmias (5-8).
These studies have indicated that the ischemia-reperfusion process
induces severe injury in the myocardium. Furthermore, TGF-β plays a
crucial role in ventricular remodeling, cell apoptosis and the
inflammatory response (9). A study
revealed that TGF-β can improve myocardial function and inhibit
hypoxia-induced apoptosis of cardiomyocytes by alleviating
endoplasmic reticulum stress (10).
Macrophages are a type of monocyte phagocytic cells
that are present in the body. Macrophages are composed of cells
(blood monocytes and tissue macrophages) which originate from the
bone marrow (11). Monocytes in the
blood are eventually transferred to different tissues and
transformed into macrophages. Furthermore, macrophages also play a
crucial role in the occurrence, development and reduction of
inflammation (12). Macrophages
mainly exert roles in antigen presentation, immune regulation and
phagocytosis by inducing the production of various cytokines and
growth factors, which in turn affects the occurrence and
development of the inflammatory response (13,14).
In addition to these functions, macrophages are activated and
polarized into different cell types (M1 and M2) within the tissue
microenvironment (15). Macrophages
are polarized to the M1-type in the early stages of the
inflammatory response to secrete inflammatory cytokines and
associated molecules, such as TNF-α, IL-1, IL-6, reactive oxygen
species (ROS) and inducible nitric oxide synthase (iNOS). During
the late stages of inflammation, macrophages are polarized into M2
macrophages and exert anti-inflammatory, repair and fibrotic
effects through the release of cytokines, such as IL-10, IL-12,
TGF-β and Arginase-1 (16,17). A previous study revealed that long
non-coding RNA (lncRNA) activated by TGF-β (ATB) is a signaling
mediator of and can be activated by TGF-β (18). TGF-β suppresses the MyD88/NF-κB
pathway by activating the expression of lncRNA ATB to relieve the
inflammatory response of joint chondrocytes (19). However, whether macrophage cell
(M2-type)-secreted TGF-β can relieve the ischemia-reperfusion
injury of myocardial cells by enhancing the expression of lncRNA
ATB remains unclear.
Therefore, in the present study, the polarization of
macrophages was performed to generate M2-type macrophages.
Subsequently, the medium of M2-type macrophages was used to treat
cardiomyocytes, which were cultured under oxygen-glucose
deprivation/reoxygenation (OGD/R) conditions. The degree of
inflammatory injury, oxidative stress and apoptosis of these
cardiomyocytes was detected. The effects of the M2-type macrophage
secreted-TGF-β were evaluated on ischemia-reperfusion injury of
cardiomyocytes based on the results of these assays.
Materials and methods
Cell culture and treatment
Cardiomyocytes (H9c2) and macrophage cells (RAW
264.7) were obtained from American Type Culture Collection. Cells
were cultured in RPMI-1640 medium (Hyclone; Cytiva) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin-streptomycin. The cells were cultured in a
37˚C humidified atmosphere with 5% CO2 and glucose-free
balanced salt solution in the presence of 95% N2 for 6 h
to mimic hypoxia and hypoglycemic conditions. Subsequently, these
cells were cultured in RPMI-1640 medium in 95% air and 5%
CO2 at 37˚C for 12, 24 and 48 h. This process was
performed to simulate ischemia-reperfusion in vitro
(20). LY364947 (Selleck Chemicals)
is a receptor kinase I inhibitor that was added into the medium to
inhibit TGF-β function. lncRNA ATB overexpression plasmid (Oe-ATB)
and its negative control (Oe-NC) were synthesized by Shanghai
GeneChem Co., Ltd. and transfected into H9c2 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
ELISA
A TGF-β ELISA kit (Bendermed System Diagnostics;
eBioscience; Thermo Fisher Scientific, Inc.; cat. no. BMS249/4) was
used to determine the secretion of TGF-β from macrophage cells
according to the manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Subsequently, a cDNA reverse transcription kit (Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. 4368814) was used to
reverse transcribe mRNA into cDNA according to the manufacturer's
instructions. SYBR Green (Thermo Fisher Scientific, Inc.) was used
for the detection of the expression of the target genes on an ViiA™
7 Real-Time PCR System (Applied Biosystems™; Thermo Fisher
Scientific, Inc.). PCR conditions used were as follows: 95˚C
denaturation step for 30 sec and 55˚C annealing for 1 min and 72˚C
for 2 min, carried out for 26 cycles. The relative expression of
the target genes was analyzed with the 2-∆∆Cq method
(21). Primer sequences are listed
in Table I. GAPDH was used as a
reference gene for normalization of quantitative PCR data.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer
sequences |
|---|
| Mouse | F:
5'-CTCCAGGCGGTGCCTATG-3' |
| TNF-α | R:
5'-GGGCCATAGAACTGATGAGAGG-3' |
| Mouse | F:
5'-TTTGCTTCCATGCTAATGCGAAAG-3' |
| iNOS | R:
5'-GСТСТGТТGAGGТСТAAAGGСТCCG-3' |
| Mouse | F:
5'-GCACACCCACCCTGCA-3' |
| IL-1β | R:
5'-ACCGCTTTTCCATCTTCTTCTT-3' |
| Mouse | F:
5'-TCCAGAAACCGCTATGAAGTTC-3' |
| IL-6 | R:
5'-CACCAGCATCAGTCCCAAGA-3' |
| Mouse | F:
5'-TCCACACGTCCAGAACAGTC-3' |
| Arg-1 | R:
5'-CCTTGGAAACAGAGACAGGC-3' |
| Mouse | F:
5'-CAACATACTGCTAACCGACTCCT-3' |
| IL-10 | R:
5'-TGAGGGТСТТСАGСТТСТСAС-3' |
| Mouse | F:
5'-GССАGСССАСАGТТСТАСАGС-3' |
| IL-13 | R:
5'-GAGATGTTGСTСAGСТССТСA-3' |
| Mouse | F:
5'-AGGTCGGTGTGAACGGATTTG-3' |
| GAPDH | R:
5'-TGTAGACCATGTAGTTGAGGTCA-3' |
| Rat | F:
5'-CCAACAAGGAGGAGAAGTTCC-3' |
| TNF-α | R:
5'-CTCTGCTTGGTGGTTTGCTAC-3' |
| Rat | F: 5'-GGAACCCGT
GTCTTCCTAAAG-3' |
| IL-1β | R:
5'-CTGACTTGGCAGAGGACAAAG-3' |
| Rat | F:
5'-TTGCCTTCTTGGGACTG-3' |
| IL-6 | R:
5'-CTGGCTTTGTCTTTCTTGTTA-3' |
| Rat | F:
5'-TGCTGCATATCGAGCTAAAGG-3' |
| HMGB1 | R:
5'-CCATACTGTACCAGGCAAGGT-3' |
| Rat lncRNA | F:
5'-ACAAGCTGTGCAGTCTCAGG-3' |
| ATB | R:
5'-CTAGGCCCAAAGACAATGGA-3' |
| Rat | F:
5'-ACCACAGTCCATGCCATCAC-3' |
| GAPDH | R:
5'-TCCACCACCCTGTTGCTGTA-3' |
Western blotting
RIPA buffer (Beyotime Institute of Biotechnology)
was used to extract total proteins from H9c2 cells. Subsequently,
protein concentration was determined with the BCA method (Beyotime
Institute of Biotechnology). A total of 20 µg protein was loaded
per lane. Proteins were separated with 10% SDS-PAGE (Beyotime
Institute of Biotechnology) and transferred to PVDF membranes (EMD
Millipore). The membranes were blocked with 5% skimmed milk powder
solution at room temperature for 1 h and incubated with the
following primary antibodies: TGF-β (cat. no. 3711S; Cell Signaling
Technology, Inc.), phosphorylated (p)-IκBα (cat. no. 2859; Cell
Signaling Technology, Inc.), IκBα (cat. no. 4814; Cell Signaling
Technology, Inc.), p-NF-κB (cat. no. 3033S; Cell Signaling
Technology, Inc.), NF-κB (cat. no. 8242S; Cell Signaling
Technology, Inc.), NADPH oxidase (Nox)-2 (cat. no. ab80508; Abcam),
Nox-4 (cat. no. ab109225; Abcam), Bcl-2 (cat. no. ab32124; Abcam),
Bax (cat. no. ab32503; Abcam), cleaved caspase-3 (cat. no. ab49822;
Abcam), caspase-3 (cat. no. ab44976; Abcam), IL-1β (cat. no.
ab216995; Abcam), IL-6 (cat. no. ab6672; Abcam), IL-10 (cat. no.
ab133575; Abcam), Arg-1 (cat. no. ab269541; Abcam), IL-13 (cat. no.
ab260044; Abcam) and GAPDH (cat. no. ab8245; Abcam). The following
day, membranes were washed three times with cold PBS-0.1% Tween-20
and incubated with HRP-conjugated secondary antibodies (1:10,000;
cat. no. ab205718; Abcam) for 2 h at room temperature. Protein
bands were finally developed using enhanced chemiluminescence
reagent (EMD Millipore). Densitometry was quantified with NIH
ImageJ 1.50 software (National Institutes of Health).
Apoptosis assay
Single-cell suspensions were prepared using trypsin
(Beyotime Institute of Biotechnology) and washed three times with
cold PBS to remove residual serum. Subsequently, these cells were
incubated with Annexin V and PI in the dark and washed with PBS
three times again. The apoptotic rates of these cells were detected
by MACSQuant X flow cytometer (Miltenyi Biotec, Inc.). Flow
cytometry data were analyzed with FlowJo V10 (FlowJo LLC).
Detection of malondialdehyde (MDA),
superoxide dismutase (SOD) and lactate dehydrogenase (LDH)
The production of MDA (cat. no. S0131S; Beyotime
Institute of Biotechnology), SOD (cat. no. S0086; Beyotime
Institute of Biotechnology) and LDH (cat. no. C0016; Beyotime
Institute of Biotechnology) in cardiomyocytes was measured with
commercial kits according to the manufacturer's instructions.
Statistical analysis
Data in the present study were analyzed with
GraphPad Prism 6.0 (GraphPad Software, Inc.). The data are
presented as the mean ± SD. Student's t-test or one-way ANOVA
followed by Tukey-Kramer tests were performed to compare the data
in two or multiple groups, respectively. All experiments were
independently repeated three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Polarization of macrophages under
OGD/R conditions
To determine the polarization of macrophages, RAW
246.7 cells were cultured under hypoxic conditions and subsequently
placed under normal oxygen conditions for different time periods
(12, 24 and 48 h). The levels of the M1-type macrophage markers
(TNF-α, iNOS, IL-1β and IL-6) were measured with RT-qPCR. The
results indicated that the expression levels of TNF-α, iNOS, IL-1β
and IL-6 gradually increased following reoxygenation (12 and 24 h)
(Fig. 1A). However, the expression
levels of these markers were significantly downregulated following
48 h of reoxygenation. The expression levels of the M2-type
macrophage markers (IL-10, IL-13 and Arg-1) were determined with
RT-qPCR and western blotting. The expression levels of IL-10, IL-13
and Arg-1 were increased following reoxygenation of macrophages
(Fig. 1B and C). The maximum increase of the expression
of these markers occurred at 48 h following reoxygenation.
Therefore, the data suggested that the macrophages exhibited the
characteristics of M2-type macrophages following 48 h of
reoxygenation. These macrophages were selected in subsequent
experiments as the main research cell type. TGF-β is a crucial
cytokine secreted by M2-type macrophages (22). Furthermore, a study revealed that
higher levels of TGF-β could relieve myocardial injury, which was
induced following ischemia reperfusion (10). Therefore, the protein levels of
TGF-β in these macrophages were measured. The results indicated
that TGF-β levels were significantly enhanced following 48 h of
reoxygenation compared with controls (Fig. 1D). In addition, TGF-β levels in the
cell medium of the macrophage culture were also significantly
elevated compared with controls (Fig.
1E).
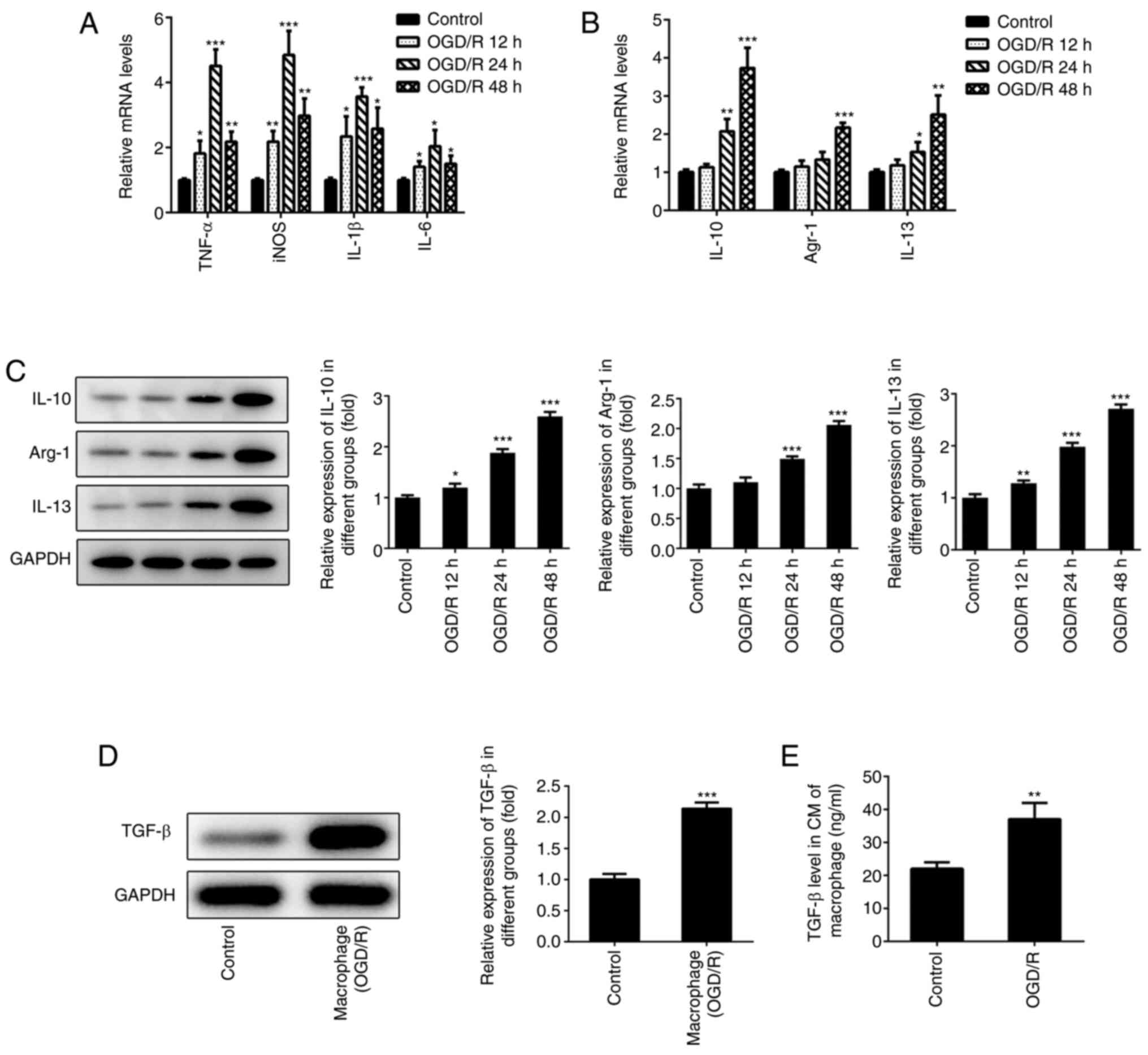 | Figure 1Polarization of macrophages under
OGD/R conditions. (A) The expression of TNF-α, IL-1β, IL-6, iNOS in
macrophages were detected using RT-qPCR. The expression of M2-type
macrophages (IL-10, Arg-1 and IL-13) were detected using (B)
RT-qPCR and (C) western blotting. (D) The expression of TGF-β in
macrophages was detected using western blotting after 48 h of
reoxygenation. (E) The expression of TGF-β in the cell medium of
macrophages was determined using ELISA after 48 h of reoxygenation.
*P<0.05, **P<0.01 and
***P<0.001 vs. control. OGD/R, oxygen-glucose
deprivation/reoxygenation; OGD/R, H9c2 cardiomyocytes under OGD/R
conditions; macrophage (OGD/R), macrophages under OGD/R conditions;
iNOS, inducible nitric oxide synthase; Arg-1, Arginase-1; RT-qPCR,
reverse transcription-quantitative PCR. |
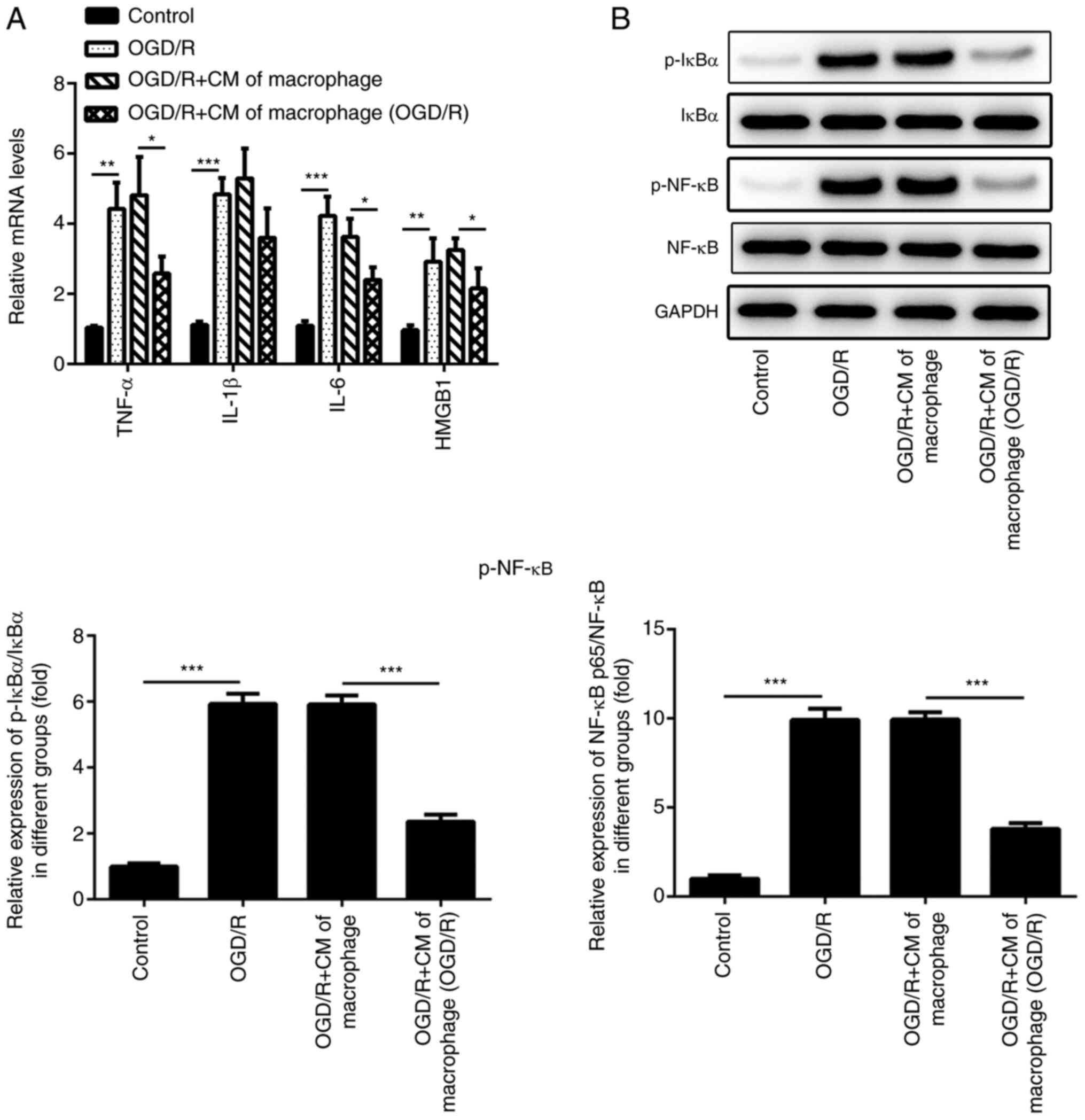
Inflammatory damage of cardiomyocytes
is relieved by M2-type macrophage culture medium
H9c2 cells were cultured under OGD/R conditions.
Subsequently, the medium of the macrophages cultured under normal
or OGD/R conditions (48 h reoxygenation) was used to treat H9c2
cells. RT-qPCR was performed to detect the levels of the
inflammatory factors [TNF-α, IL-1β, IL-6 and high mobility group
protein B1 (HMGB1)] of H9c2 cells which were cultured with the
medium of the macrophages. The results indicated that the levels of
these inflammatory factors were significantly increased when the
H9c2 cells were cultured under OGD/R conditions (Fig. 2A). However, the mRNA expression
levels of TNF-α, IL-1β, IL-6 and HMGB1 were significantly inhibited
following treatment with the culture medium of macrophages that
were previously incubated under OGD/R conditions. Western blotting
was performed to detect the levels of inflammation-associated
proteins (p-NF-κB and p-IκBα) in H9c2 cells. Compared with the
Control group, the levels of p-NF-κB and p-IκBα were significantly
increased in OGD/R group, whereas the levels of p-NF-κB and p-IκBα
in OGD/R+CM of macrophage group were significantly inhibited
following treatment of the cells with the culture medium of the
M2-type macrophages (Fig. 2B).
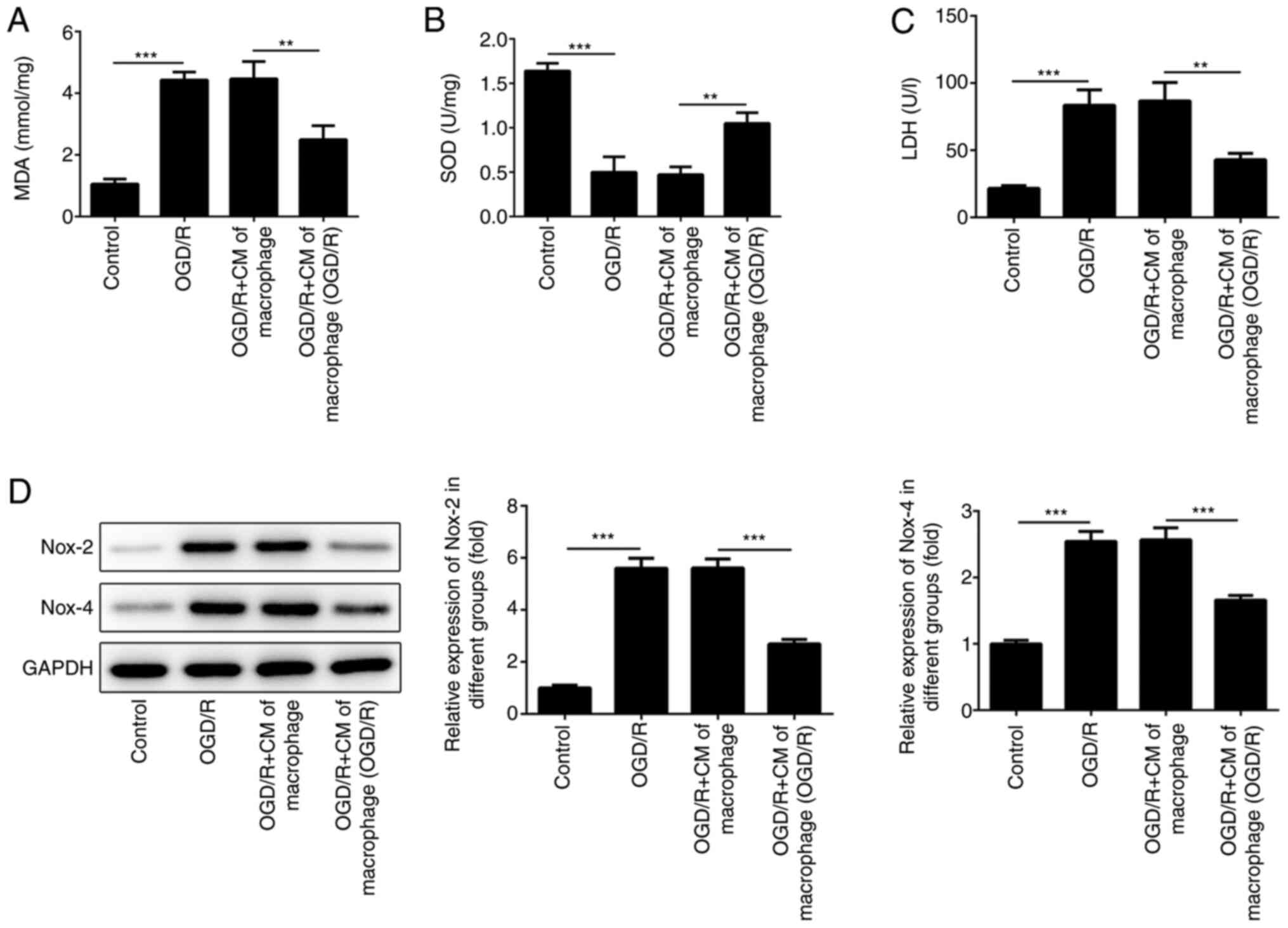
M2-type macrophage culture medium
alleviates oxidative stress injury in cardiomyocytes
Oxidative stress injury is another type of damage
which is induced following ischemia and reperfusion (23,24).
The production of oxidative stress-associated enzymes was examined
in the present study. The results indicated that the culture medium
of the macrophages, which were incubated under OGD/R conditions,
could reduce the increase SOD levels induced by OGD/R (Fig. 3B). OGD/R-induced MDA and LDH levels
were also reduced in cells incubated with the culture medium of the
macrophages grown under OGD/R conditions (Fig. 3A and C). Furthermore, Nox-2 and Nox-4 has been
shown to promote ROS production and aggravate oxidative damage in
numerous types of cells (25).
Therefore, the expression levels of Nox-2 and Nox-4 were detected
in H9c2 cells using western blot analysis. The results indicated
that the levels of Nox-2 and Nox-4 were increased when H9c2 cells
were cultured under OGD/R conditions (Fig. 3D). However, the expression levels of
Nox-2 and Nox-4 were suppressed following treatment of the cells
with medium derived from M2-type macrophages.
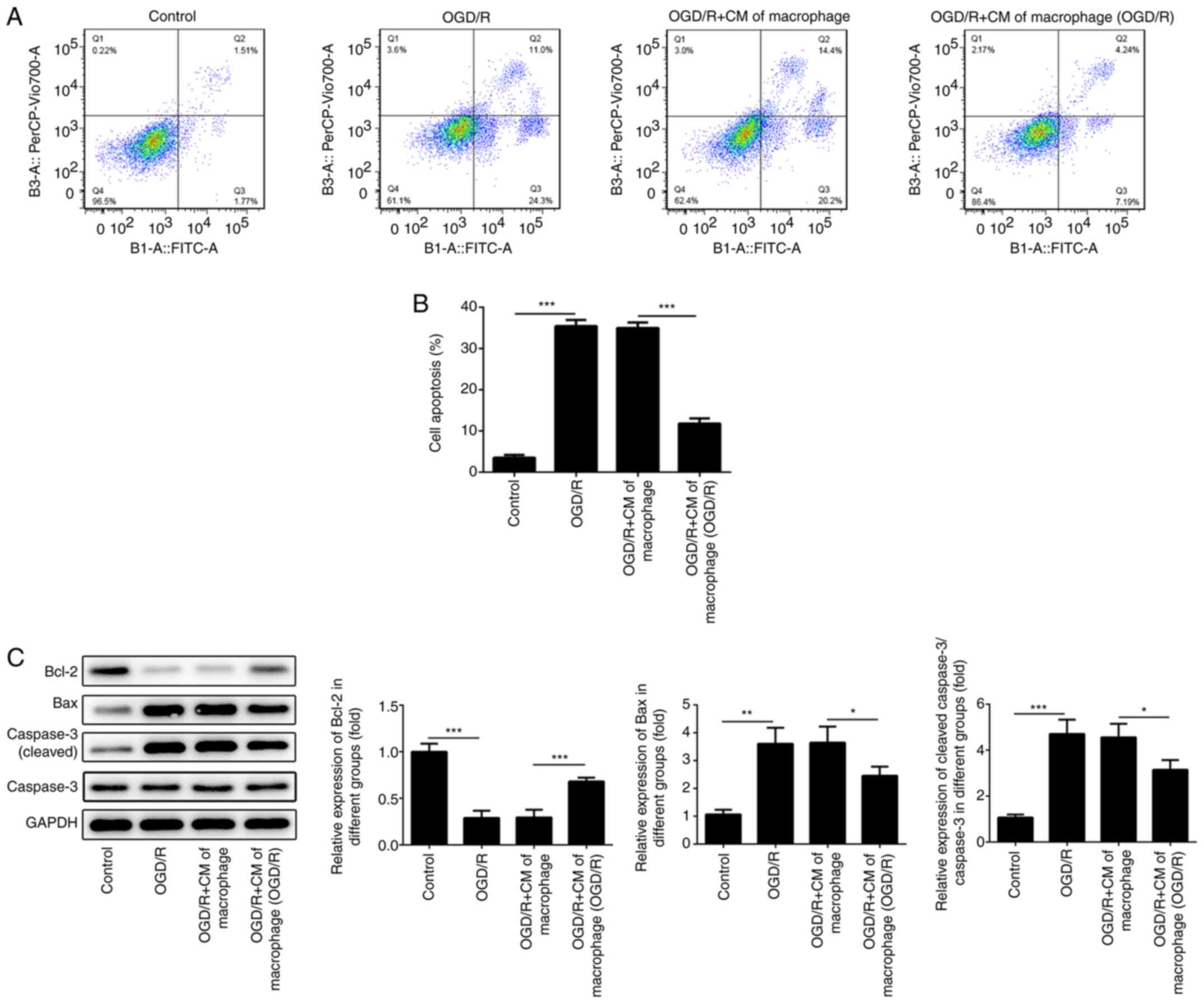
M2-type macrophage culture medium
reduces the induction of cardiomyocyte apoptosis
Ischemia-reperfusion injury induces cell apoptosis
in different tissues (26-28).
Therefore, the apoptotic rates of H9c2 cells were determined using
flow cytometry. The results indicated that the apoptotic rates of
H9c2 cells were promoted when these cells were cultured under OGD/R
conditions (Fig. 4A and B). However, the induction of H9c2 cell
apoptosis was reduced following treatment of the cells with medium
derived from macrophages cultured under OGD/R conditions.
Subsequently, the expression levels of apoptosis-associated
proteins were determined using western blotting. The expression
levels of Bcl-2 were suppressed, whereas the levels of
pro-apoptotic proteins (Bax and cleaved caspase 3) were increased
in H9c2 cells cultured under OGD/R conditions. However, the levels
of Bcl-2 were increased and the expression levels of the
pro-apoptotic proteins (Bax and cleaved caspase 3) were inhibited
in H9c2 cells following treatment with medium derived from M2-type
macrophages (Fig. 4C).
TGF-β-mediated induction of lncRNA ATB
is required for the protective effects of M2-type macrophage medium
on cardiomyocytes
It was reported that TGF-β can increase the
expression levels of lncRNA ATB, which relieves the inflammatory
response of chondrocytes (19). The
present study demonstrated that a large amount of TGF-β was
secreted by M2-type macrophages (Fig.
1D). Therefore, the expression of lncRNA ATB was determined in
H9c2 cells following treatment with LY364947. The levels of lncRNA
ATB in H9c2 cells exposed to OGD/R were decreased, while this
effect was reversed following addition of M2-type macrophage medium
(Fig. 5A). The effects of M2-type
macrophage medium were reversed following treatment with LY364947,
an inhibitor of TGF-β receptor kinase I (29). To investigate the effects of lncRNA
ATB in H9c2 cells, Oe-ATB was transfected into H9c2 cells (Fig. 5B). This cell group demonstrated
elevated expression levels of lncRNA ATB. In addition, the
anti-inflammatory effects of M2-type macrophage medium were
inhibited following LY364947 treatment. However, Oe-ATB partly
abrogated the role of LY364947 (Fig.
5C and D). Similarly, the
protective roles of M2-type macrophage medium against oxidative
stress and apoptosis were impaired by LY364947 (Fig. 5E and F), whereas Oe-ATB could reduce the harmful
effects triggered by LY364947 (Fig.
5G), indicating that TGF-β secreted by M2-type macrophage
medium could protect H9c2 cells from oxidative stress and apoptosis
via activation of lncRNA ATB.
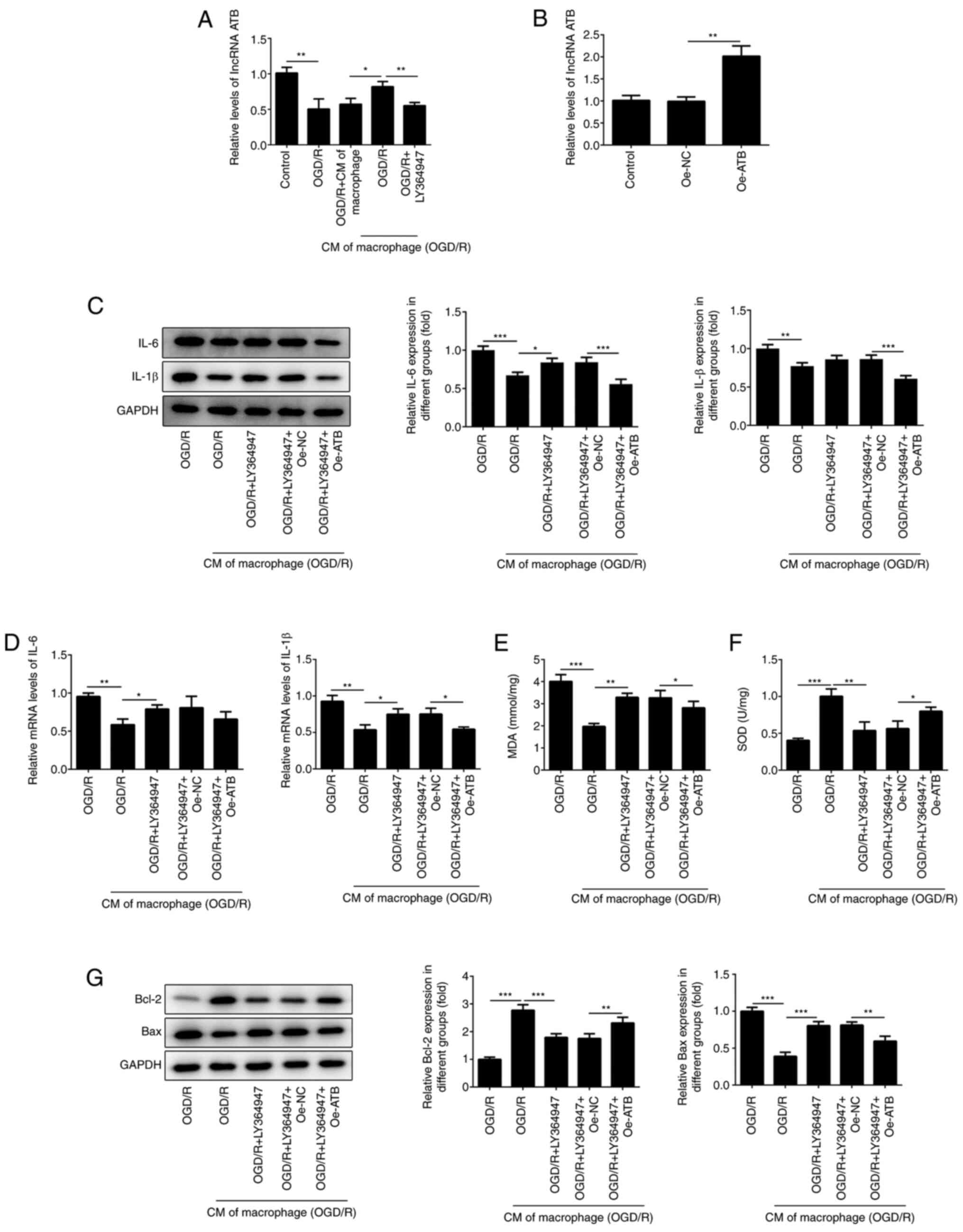 | Figure 5TGF-β-mediated induction of lncRNA
ATB is required for the protective effects of M2-type macrophage
medium on cardiomyocytes. (A) The expressions of lncRNA ATB in H9c2
cells was detected using RT-qPCR following treatment with LY364947
(5 µg/ml). (B) The levels of lncRNA ATB in H9c2 cells were
determined using RT-qPCR. The expression of IL-1β, IL-6 in H9c2
cells was detected using (C) western blotting and (D) RT-qPCR. The
levels of (E) MDA and (F) SOD in H9c2 cells were determined using
commercial kits. (G) The expression of apoptosis-related proteins
in H9c2 cells were determined using western blotting.
*P<0.05, **P<0.01 and
***P<0.001. OGD/R, oxygen-glucose
deprivation/reoxygenation; CM of macrophage, normal macrophage
culture medium; CM of macrophage (OGD/R), macrophage culture medium
under OGD/R conditions; MDA, malondialdehyde; SOD, superoxide
dismutase; lncRNA ATB, long non-coding RNA activated by TGF-β; Oe,
overexpression plasmid; NC, negative control. |
Discussion
Myocardial ischemia and reperfusion cause severe
damage to the heart tissue and in the absence of timely and
effective therapy, can lead to coronary heart disease and heart
failure (30,31). The production of excess ROS induced
by ischemia-reperfusion, the overload of calcium ions in
cardiomyocytes, myocardial contractile dysfunction and the
induction of cardiomyocyte apoptosis are considered the main
mechanisms of myocardial damage caused by ischemia-reperfusion
(32-34).
Furthermore, TGF-β plays a crucial role in ventricular remodeling,
inflammatory injury and apoptosis of cardiomyocytes (35). Previous studies have revealed that
TGF-β can manipulate myocardial fibrosis by regulating the
expression of Smad2/Smad3 and Wnt/β-catenin pathway-associated
proteins (36,37). A study suggested that higher levels
of TGF-β could alleviate the apoptosis of cardiomyocytes, which was
induced by ischemia-reperfusion, leading to the protection of the
normal function of cardiomyocytes (10). In addition, previous studies
revealed that M2-type macrophages could secrete TGF-β (38,39).
Macrophages are a type of autoimmune cells in the body, which can
alleviate the inflammatory damage caused in various tissues of the
body by secreting diverse cytokines. In addition, macrophages are
polarized and transformed into M1- and M2-type macrophages
(40). Furthermore, M1- and M2-type
macrophages secrete different cytokines and protect the tissues
from the induction of the inflammatory response (15). However, whether TGF-β secreted by M2
macrophages can relieve cardiomyocyte injury induced by
ischemia-reperfusion is unclear. In the present study, macrophages
were cultured under OGD/R conditions. However, the expression
levels of M1 and M2 macrophage markers differed according to the
different time periods (12, 24 and 48 h) of reoxygenation. The
expression levels of M2 macrophage markers (IL-10, Arg-1 and IL-13)
were the highest following 48 h of reoxygenation. The levels of
TGF-β were also higher in these macrophages following 48 h of
reoxygenation. Therefore, these macrophages were considered M2
macrophages and were used for subsequent experiments.
lncRNAs are non-coding RNAs consisting of >200
nucleotides. lncRNAs participate in the regulation of several
physiological activities in different types of cells (41,42). A
previous study revealed that lncRNA ATB is the signal transmitting
medium of TGF-β (17). lncRNA ATB
activated by TGF-β relieves the inflammatory injury of chondrocytes
by suppressing the expression levels of NF-κB and MyD88(19). According to these findings, the
expression levels of lncRNA ATB in cardiomyocytes were increased
following treatment of macrophage culture medium, which were
cultured under OGD/R conditions. These results indicated that TGF-β
secreted by M2 macrophages could increase the expression levels of
lncRNA ATB in cardiomyocytes.
Given that inflammatory damage is the main symptom
of ischemia and reperfusion injury (43,44),
the levels of inflammatory factors in the cardiomyocytes cultured
under OGD/R conditions were examined. The results indicated that
the expression levels of inflammatory factors (TNF-α, IL-1β, IL-6,
HMGB1, p-IκBα and NF-κB p65) were inhibited in cardiomyocytes
following treatment of cells with medium derived from M2
macrophages cultured under OGD/R conditions. Previous studies
revealed that ischemia and reperfusion could also induce oxidative
damage and apoptosis of cells (45,46).
In the present study, the induction of oxidative stress and
apoptosis in cardiomyocytes were relieved following treatment of
the cells with culture medium derived from M2 macrophages, which
were cultured under OGD/R conditions. The results of the present
study indicated that TGF-β secreted by M2 macrophages alleviated
OGD/R-induced inflammatory damage, oxidative stress, and apoptosis
by upregulating the expression levels of lncRNA ATB in
cardiomyocytes. However, there are limitations in the present
study. Firstly, TGF-β could induce myocardial fibrosis and
remodeling, which adversely affects the heart according to previous
literature (47,48). Hence, the effects of TGF-β on
myocardial fibrosis while protecting cardiomyocytes from
ischemia-reperfusion injury should be further investigated.
Secondly, the specific role and mechanisms of anti-inflammatory
factors secreted by macrophages in myocardial ischemia-reperfusion
injury have not been fully elucidated, and the experimental
contents of the study need to be repeated with soluble TGF-β alone.
These will be investigated in future studies.
In conclusion, the present study demonstrated the
effects of TGF-β secreted by M2 macrophages on OGD/R-induced injury
of cardiomyocytes. The present study suggested that macrophages may
be the source of TGF-β in the process of myocardial ischemic
injury. TGF-β alleviated OGD/R-induced inflammatory damage,
oxidative injury and apoptosis of cardiomyocytes via suppressing
the NF-κB signaling pathway by activating lncRNA ATB, which will
provide a basis for clinical treatment of myocardial ischemic
injury targeting the inflammatory response.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by a grant from
the Baotou Medical College Natural Science Fund Sailing Project
(grant no. BYJJ-YF201718), Baotou Science and Technology Plan
Project (grant no. WSJJ2017027), Inner Mongolia Autonomous Region
Natural Science Fund Project (grant nos. 2018MS08145 and
2014MS0812) and the Baotou Medical College Natural Science Fund
Sailing Project (grant no. BYJJ-YF201687).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL and WSX acquired the data. XWL and ZW analyzed
the data. JY and TZ contributed to the design of the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou M, Bao Y, Li H, Pan Y, Shu L, Xia Z,
Wu D, Lam KSL, Vanhoutte PM, Xu A, et al: Deficiency of adipocyte
fatty-acid-binding protein alleviates myocardial
ischaemia/reperfusion injury and diabetes-induced cardiac
dysfunction. Clin Sci (Lond). 129:547–559. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lejay A, Fang F, John R, Van JAD, Barr M,
Thaveau F, Chakfe N, Geny B and Scholey JW: Ischemia reperfusion
injury, ischemic conditioning and diabetes mellitus. J Mol Cell
Cardiol. 91:11–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang S, Wang C, Yan F, Wang T, He Y, Li H,
Xia Z and Zhang Z: N-acetylcysteine attenuates diabetic myocardial
ischemia reperfusion injury through inhibiting excessive autophagy.
Mediators Inflamm. 2017(9257291)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xia KP, Cao HM and Shao CZ: Protective
effect of notoginsenoside R1 in a rat model of myocardial ischemia
reperfusion injury by regulation of Vitamin D3 upregulated protein
1/NF-κB pathway. Pharmazie. 70:740–744. 2015.PubMed/NCBI
|
|
5
|
Dressing A, Graeter Z and Bardutzky J:
Safe intravenous thrombolysis after traumatic cardiopulmonary
resuscitation with Rib fractures: A case report. Case Rep Neurol.
9:156–160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pagel PS, Sethi P, Freed JK, Boettcher BT
and Hossein Almassi G: A rare complication of cardiopulmonary
resuscitation after mitral valve replacement. J Cardiothorac Vasc
Anesth. 31:770–772. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosoff PM and Schneiderman LJ: Response to
open peer commentaries on ‘Irrational Exuberance: Cardiopulmonary
resuscitation as Fetish’. Am J Bioeth. 17:W1–W3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sarma S, Bucuti H, Chitnis A, Klacman A
and Dantu R: Real-time mobile device-assisted chest compression
during cardiopulmonary resuscitation. Am J Cardiol. 120:196–200.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dobaczewski M, Chen W and Frangogiannis
NG: Transforming growth factor (TGF)-beta signaling in cardiac
remodeling. J Mol Cell Cardiol. 51:600–606. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Zong L and Wang X: TGF-β improves
myocardial function and prevents apoptosis induced by
anoxia-reoxygenation, through the reduction of endoplasmic
reticulum stress. Can J Physiol Pharmacol. 94:9–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lu CH, Yeh DW, Lai CY, Liu YL, Huang LR,
Lee AY, Jin SC and Chuang TH: USP17 mediates macrophage-promoted
inflammation and stemness in lung cancer cells by regulating
TRAF2/TRAF3 complex formation. Oncogene. 37:6327–6340.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cochain C and Zernecke A: Macrophages in
vascular inflammation and atherosclerosis. Pflugers Arch.
469:485–499. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Essandoh K, Li Y, Huo J and Fan GC:
MiRNA-mediated macrophage polarization and its potential role in
the regulation of inflammatory response. Shock. 46:122–131.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang M, Hutter G, Kahn SA, Azad TD,
Gholamin S, Xu CY, Liu J, Achrol AS, Richard C, Sommerkamp P, et
al: Anti-CD47 treatment stimulates phagocytosis of glioblastoma by
M1 and M2 polarized macrophages and promotes M1 polarized
macrophages in vivo. PLoS One. 11(e0153550)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun YY, Li XF, Meng XM, Huang C, Zhang L
and Li J: Macrophage phenotype in liver injury and repair. Scand J
Immunol. 85:166–174. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sarie Y, Takafumi S, Takeshi M, Yutaro M,
Norio I, Kazuki F, Saiko MN, Shuhei N, Shuji K, Hiroyuki M, et al:
Inhibition of local macrophage growth ameliorates focal
inflammation and suppresses atherosclerosis. Arterioscler Thromb
Vasc Biol. 38:994–1006. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-beta promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ying H, Wang Y, Gao Z and Zhang Q: Long
non-coding RNA activated by transforming growth factor beta
alleviates lipopolysaccharide-induced inflammatory injury via
regulating microRNA-223 in ATDC5 cells. Int Immunopharmacol.
69:313–320. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhi W, Li K, Wang H, Lei M and Guo Y:
Melatonin elicits protective effects on OGD/R-insulted H9c2 cells
by activating PGC-1α/Nrf2 signaling. Int J Mol Med. 45:1294–1304.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Italiani P and Boraschi D: From monocytes
to M1/M2 macrophages: Phenotypical vs. functional differentiation.
Front Immunol. 5(514)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Higashida K, Kim SH, Jung SR, Asaka M,
Holloszy JO and Han DH: Effects of resveratrol and SIRT1 on PGC-1α
activity and mitochondrial biogenesis: A reevaluation. PLoS Biol.
11(e1001603)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen Y, Li Y, Xu H, Li G, Ma Y and Pang
YJ: Morin mitigates oxidative stress, apoptosis and inflammation in
cerebral ischemic rats. Afr J Tradit Complement Altern Med.
14:348–355. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lou Z, Wang AP, Duan XM, Hu GH, Song GL,
Zuo ML and Yang ZB: Upregulation of NOX2 and NOX4 mediated by TGF-β
signaling pathway exacerbates cerebral ischemia/reperfusion
oxidative stress injury. Cell Physiol Biochem. 46:2103–2113.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Takeda T: A pathomorphological study on
damage and repair process of tubuli after renal ischemia. Nihon
Jinzo Gakkai Shi. 38:493–501. 1996.PubMed/NCBI(In Japanese).
|
|
27
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956.
1996.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Du C, Hu R, Csernansky CA, Hsu CY and Choi
DW: Very delayed infarction after mild focal cerebral ischemia: A
role for apoptosis? J Cereb Blood Flow Metab. 16:195–201.
1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang XL and Huang C: Difference of
TGF-β/Smads signaling pathway in epithelial-mesenchymal transition
of normal colonic epithelial cells induced by tumor-associated
fibroblasts and colon cancer cells. Mol Biol Rep. 46:2749–2759.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alleman RJ, Tsang AM, Ryan TE, Patteson
DJ, McClung JM, Spangenburg EE, Shaikh SR, Neufer PD and Brown DA:
Exercise-induced protection against reperfusion arrhythmia involves
stabilization of mitochondrial energetics. Am J Physiol Heart Circ
Physiol. 310:H1360–H1370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Arsenian M: Potential cardiovascular
applications of glutamate, aspartate, and other amino acids. Clin
Cardiol. 21:620–624. 1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Prasad A, Stone GW, Holmes DR and Gersh B:
Reperfusion injury, microvascular dysfunction, and
cardioprotection: The ‘dark side’ of reperfusion. Circulation.
120:2105–2112. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Marunouchi T and Tanonaka K: Cell death in
the cardiac myocyte. Biol Pharm Bull. 38:1094–1097. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Działo E, Tkacz K and Błyszczuk P:
Crosstalk between the TGF-β and WNT signalling pathways during
cardiac fibrogenesis. Acta Biochim Pol. 65:341–349. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khalil H, Kanisicak O, Prasad V, Correll
RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, et al:
Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac
fibrosis. J Clin Invest. 127:3770–3783. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Zhang F, Wang H, Wang X, Jiang G, Liu H,
Zhang G, Wang H, Fang R, Bu X, Cai S and Du J: TGF-β induces
M2-like macrophage polarization via SNAIL-mediated suppression of a
pro-inflammatory phenotype. Oncotarget. 7:52294–52306.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu L, Fu X, Chen X, Han X and Dong P: M2
macrophages induce EMT through the TGF-β/Smad2 signaling pathway.
Cell Biol Int. 41:960–968. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Milich LM, Ryan CB and Lee JK: The origin,
fate, and contribution of macrophages to spinal cord injury
pathology. Acta Neuropathol. 137:785–797. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346.
2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen Z, Ding T and Ma CG: Dexmedetomidine
(DEX) protects against hepatic ischemia/reperfusion (I/R) injury by
suppressing inflammation and oxidative stress in NLRC5 deficient
mice. Biochem Biophys Res Commun. 493:1143–1150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sharma AK, Charles EJ, Zhao Y, Narahari
AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA,
Ravichandran KS, et al: Pannexin-1 channels on endothelial cells
mediate vascular inflammation during lung ischemia-reperfusion
injury. Am J Physiol Lung Cell Mol Physiol. 315:L301–L312.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Han SJ, Choi HS, Kim JI, Park JW and Park
KM: IDH2 deficiency increases the liver susceptibility to
ischemia-reperfusion injury via increased mitochondrial oxidative
injury. Redox Biol. 14:142–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu Y, Shi B, Li Y and Zhang H: Protective
effect of Luteolin against renal ischemia/reperfusion injury via
modulation of pro-inflammatory cytokines, oxidative stress and
apoptosis for possible benefit in kidney transplant. Med Sci Monit.
23:5720–5727. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Huo L, Shi W, Chong L, Wang J, Zhang K and
Li Y: Asiatic acid inhibits left ventricular remodeling and
improves cardiac function in a rat model of myocardial infarction.
Exp Ther Med. 11:57–64. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016.PubMed/NCBI View Article : Google Scholar
|