|
1
|
Cascella M, Rajnik M, Cuomo A, Dulebohn SC
and Di Napoli R: Features, evaluation and treatment coronavirus
(COVID-19). In: StatPearls. Treasure Island (FL), 2020.
|
|
2
|
Caldera-Villalobos C, Garza-Veloz I,
Martínez-Avila N, Delgado-Enciso I, Ortiz-Castro Y, Cabral-Pacheco
GA and Martinez-Fierro ML: The coronavirus disease (COVID-19)
challenge in Mexico: A critical and forced reflection as
individuals and society. Front Public Health. 8(337)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
O'Keefe JB, Tong EJ, Datoo O'Keefe GA and
Tong DC: Predictors of disease duration and symptom course of
outpatients with acute covid-19: A retrospective cohort study.
medRxiv: 2020.06.05.20123471, 2020.
|
|
4
|
Tenforde MW, Kim SS, Lindsell CJ, Billig
Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS,
Smithline HA, et al: Symptom duration and risk factors for delayed
return to usual health among outpatients with COVID-19 in a
multistate health care systems network-united states, March-June
2020. MMWR Morb Mortal Wkly Rep. 69:993–998. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin
Y, Fu S, Gao L, Cheng Z, Lu Q, et al: Remdesivir in adults with
severe COVID-19: A randomised, double-blind, placebo-controlled,
multicentre trial. Lancet. 395:1569–1578. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Baqui P, Bica I, Marra V, Ercole A and van
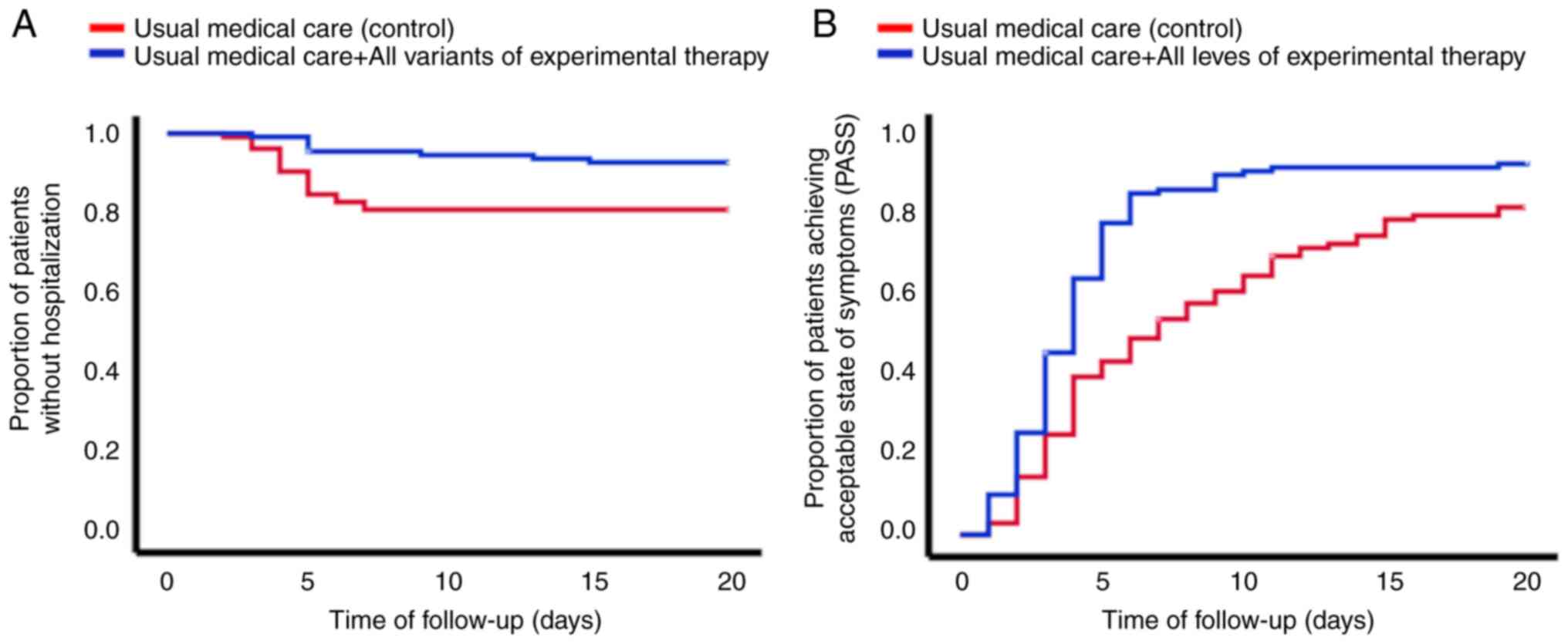
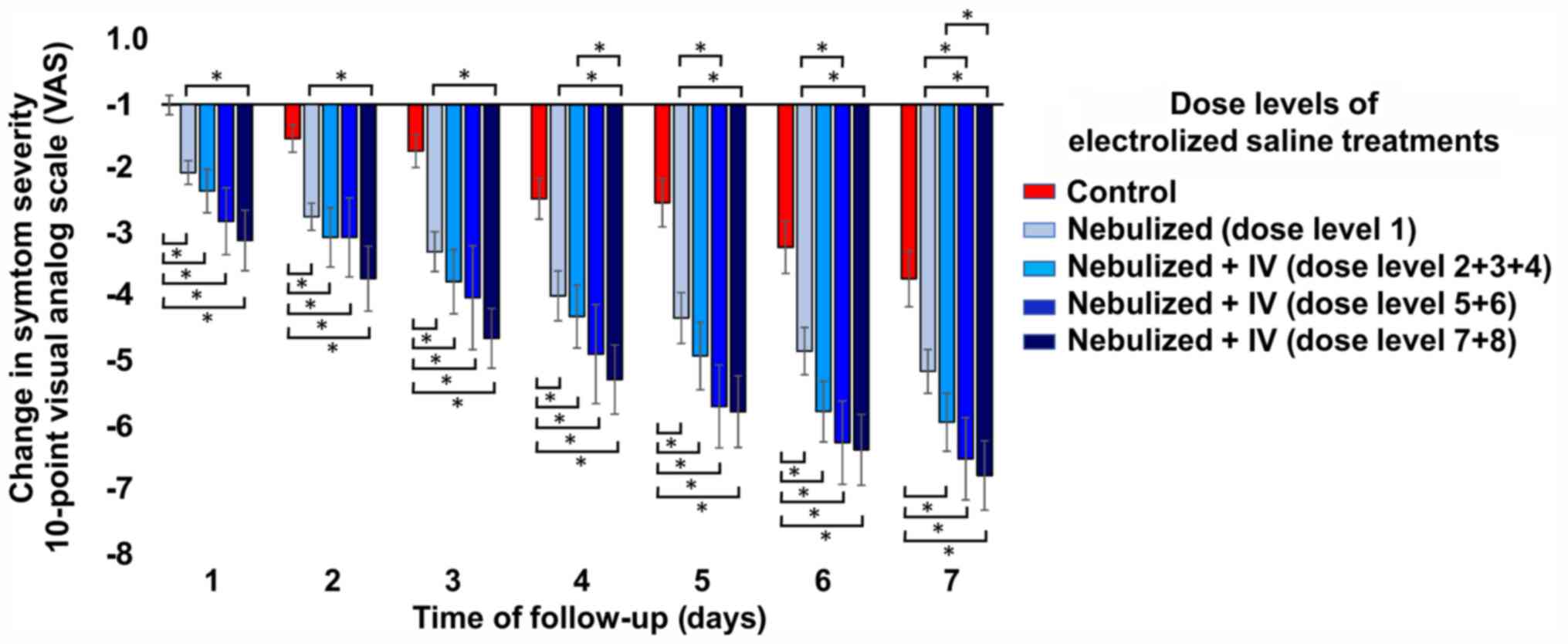
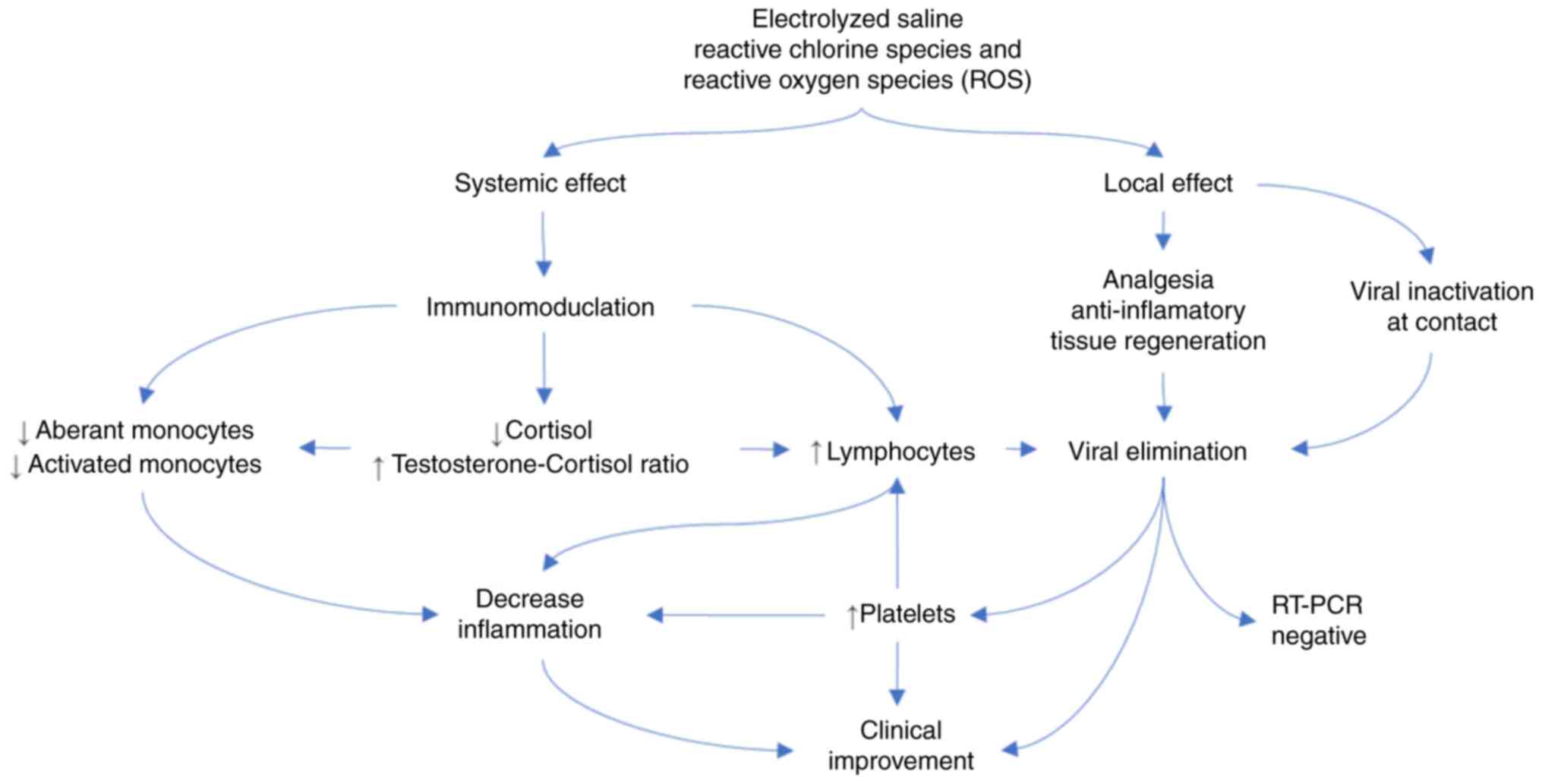
der Schaar M: Ethnic and regional variations in hospital mortality
from COVID-19 in Brazil: A cross-sectional observational study.
Lancet Glob Heal. 8:e1018–e1026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liang LL, Tseng CH, Ho HJ and Wu CY:
Covid-19 mortality is negatively associated with test number and
government effectiveness. Sci Rep. 10(12567)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Berumen J, Schmulson M, Alegre-Díaz J,
Guerrero G, Larriva-Sahd J, Olaiz G, Wong-Chew RM, Cantú-Brito C,
Ochoa-Guzmán A, Garcilazo-Ávila A, et al: Risk of infection and
hospitalization by Covid-19 in Mexico: A case-control study.
medRxiv: 2020.05.24.20104414, 2020.
|
|
9
|
Rakedzon S, Khoury Y, Rozenberg G and
Neuberger A: Hydroxychloroquine and coronavirus disease 2019: A
systematic review of a scientific failure. Rambam Maimonides Med J.
11(e0025)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bchetnia M, Girard C, Duchaine C and
Laprise C: The outbreak of the novel severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2): A review of the current global
status. J Infect Public Health. 13:1601–1610. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mangalmurti N and Hunter CA: Cytokine
storms: Understanding COVID-19. Immunity. 53:19–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ucciferri C, Vecchiet J and Falasca K:
Role of monoclonal antibody drugs in the treatment of COVID-19.
World J Clin Cases. 8:4280–4285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ortiz-Prado E, Simbaña-Rivera K,
Gómez-Barreno L, Rubio-Neira M, Guaman LP, Kyriakidis NC, Muslin C,
Jaramillo AMG, Barba-Ostria C, Cevallos-Robalino D, et al:
Clinical, molecular, and epidemiological characterization of the
SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a
comprehensive literature review. Diagn Microbiol Infect Dis.
98(115094)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pence BD: Severe COVID-19 and aging: Are
monocytes the key? Geroscience. 42:1051–1061. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang W, Berube J, McNamara M, Saksena S,
Hartman M, Arshad T, Bornheimer SJ and O'Gorman M: Lymphocyte
subset counts in COVID-19 patients: A meta-analysis. Cytometry A.
97:772–776. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Henry BM, de Oliveira MHS, Benoit S,
Plebani M and Lippi G: Hematologic, biochemical and immune
biomarker abnormalities associated with severe illness and
mortality in coronavirus disease 2019 (COVID-19): A meta-analysis.
Clin Chem Lab Med. 58:1021–1028. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kono N: Superoxidized electrolyzed
solution of neutral pH and uses there of., 2015.
|
|
18
|
Li R, Jia Z and Trush MA: Defining ROS in
biology and medicine. React Oxyg Species (Apex). 1:9–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Forrester SJ, Kikuchi DS, Hernandes MS, Xu
Q and Griendling KK: Reactive oxygen species in metabolic and
inflammatory signaling. Circ Res. 122:877–902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan HY, Wang N, Li S, Hong M, Wang X and
Feng Y: The reactive oxygen species in macrophage polarization:
Reflecting its dual role in progression and treatment of human
diseases. Oxid Med Cell Longev. 2016(2795090)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
George-Chandy A, Nordström I, Nygren E,
Jonsson IM, Postigo J, Collins LV and Eriksson K: Th17 development
and autoimmune arthritis in the absence of reactive oxygen species.
Eur J Immunol. 38:1118–1126. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kuhns DB, Alvord WG, Heller T, Feld JJ,
Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, et
al: Residual NADPH oxidase and survival in chronic granulomatous
disease. N Engl J Med. 363:2600–2610. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Calabrese EJ, Giordano JJ, Kozumbo WJ,
Leak RK and Bhatia TN: Hormesis mediates dose-sensitive shifts in
macrophage activation patterns. Pharmacol Res. 137:236–249.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mills EL and O'Neill LA: Reprogramming
mitochondrial metabolism in macrophages as an anti-inflammatory
signal. Eur J Immunol. 46:13–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Prokopowicz ZM, Arce F, Biedroń R, Chiang
CL, Ciszek M, Katz DR, Nowakowska M, Zapotoczny S, Marcinkiewicz J
and Chain BM: Hypochlorous acid: A natural adjuvant that
facilitates antigen processing, cross-priming, and the induction of
adaptive immunity. J Immunol. 184:824–835. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reth M: Hydrogen peroxide as second
messenger in lymphocyte activation. Nat Immunol. 3:1129–1134.
2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Z, Xu X, Leng X, He M, Wang J, Cheng S
and Wu H: Roles of reactive oxygen species in cell signaling
pathways and immune responses to viral infections. Arch Virol.
162:603–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ge L, Yang M, Yang NN, Yin XX and Song WG:
Molecular hydrogen: A preventive and therapeutic medical gas for
various diseases. Oncotarget. 8:102653–102673. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin CP, Chuang WC, Lu FJ and Chen CY:
Anti-oxidant and anti-inflammatory effects of hydrogen-rich water
alleviate ethanol-induced fatty liver in mice. World J
Gastroenterol. 23:4920–4934. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moher D, Hopewell S, Schulz KF, Montori V,
Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M and Altman DG:
CONSORT. CONSORT 2010 explanation and elaboration: Updated
guidelines for reporting parallel group randomised trials. Int J
Surg. 10:28–55. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
33
|
Gouveia BG, Rijo P, Gonçalo TS and Reis
CP: Good manufacturing practices for medicinal products for human
use. J Pharm Bioallied Sci. 7:87–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rogatko A, Cook-Wiens G, Tighiouart M and
Piantadosi S: Escalation with overdose control is more efficient
and safer than accelerated titration for dose finding. Entropy
(Basel). 17:5288–5303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
American College of Allergy A and I: No
Title. A Messag to Asthma Suff About a Short Albuterol Metered Dose
Inhalers: 1, 2020.
|
|
36
|
British Lung Foundation: No Title. What is
Soc Shield who needs to do this? 1, 2020.
|
|
37
|
Asthma Society of Ireland: No Title.
Coronavirus (COVID-19) Advice: 1, 2020.
|
|
38
|
British thoracic society: No Title.
COVID-19 Inf Respir community: 1, 2020.
|
|
39
|
Watson RD: Oral administration of
electrolyzed water for treatment and prevention of PEDv in swine,
swine herds and swine confinements. US Patent 20140287065 A1. Filed
March 14, 2013; issued February 24, 2014.
|
|
40
|
Morita C, Nishida T and Ito K: Biological
toxicity of acid electrolyzed functional water: Effect of oral
administration on mouse digestive tract and changes in body weight.
Arch Oral Biol. 56:359–366. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cohen DR, Todd S, Gregory WM and Brown JM:
Adding a treatment arm to an ongoing clinical trial: A review of
methodology and practice. Trials. 16(179)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yorganci E, Evans CJ, Johnson H, Barclay
S, Murtagh FE, Yi D, Gao W, Pickles A and Koffman J: Understanding
usual care in randomised controlled trials of complex
interventions: A multi-method approach. Palliat Med. 34:667–679.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dawson L, Zarin DA, Emanuel EJ, Friedman
LM, Chaudhari B and Goodman SN: Considering usual medical care in
clinical trial design. PLoS Med. 6(e1000111)2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hinks TSC, Barber VS, Black J, Dutton SJ,
Jabeen M, Melhorn J, Rahman NM, Richards D, Lasserson D, Pavord ID
and Bafadhel M: A multi-centre open-label two-arm randomised
superiority clinical trial of azithromycin versus usual care in
ambulatory COVID-19: Study protocol for the ATOMIC2 trial. Trials.
21(718)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sterne JAC, Diaz J, Villar J, Murthy S,
Slutsky AS, Perner A, Jüni P, Angus DC, Annane D, Azevedo LCP, et
al: Corticosteroid therapy for critically ill patients with
COVID-19: A structured summary of a study protocol for a
prospective meta-analysis of randomized trials. Trials.
21(734)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kvien TK, Heiberg T and Hagen KB: Minimal
clinically important improvement/difference (MCII/MCID) and patient
acceptable symptom state (PASS): What do these concepts mean? Ann
Rheum Dis. 66 (Suppl 3):iii40–iii41. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fei JZ, Perruccio AV, Ye JY, Gladman DD
and Chandran V: The relationship between patient acceptable symptom
state and disease activity in patients with psoriatic arthritis.
Rheumatology (Oxford). 59:69–76. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Delgado-Enciso I, Valtierra-Alvarez J,
Paz-Garcia J, Preciado-Ramirez J, Soriano-Hernandez AD,
Mendoza-Hernandez MA, Guzman-Esquivel J, Cabrera-Licona A,
Delgado-Enciso J, Cortes-Bazan JL, et al: Patient-reported health
outcomes for severe knee osteoarthritis after conservative
treatment with an intra-articular cell-free formulation for
articular cartilage regeneration combined with usual medical care
vs. usual medical care alone: A randomized co. Exp Ther Med.
17:3351–3360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Coates LC, Tillett W, Shaddick G, Pincus
T, Kavanaugh A and Helliwell PS: Value of the routine assessment of
patient index data 3 in patients with psoriatic arthritis: Results
from a tight-control clinical trial and an observational cohort.
Arthritis Care Res (Hoboken). 70:1198–1205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Delgado-Enciso I, Paz-Garcia J,
Valtierra-Alvarez J, Preciado-Ramirez J, Almeida-Trinidad R,
Guzman-Esquivel J, Mendoza-Hernandez MA, Garcia-Vega A,
Soriano-Hernandez AD, Cortes-Bazan JL, et al: A phase I-II
controlled randomized trial using a promising novel cell-free
formulation for articular cartilage regeneration as treatment of
severe osteoarthritis of the knee. Eur J Med Res.
23(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Delgado-Enciso I, Paz-Michel B, Melnikov
V, Guzman-Esquivel J, Espinoza-Gomez F, Soriano-Hernandez AD,
Rodriguez-Sanchez IP, Martinez-Fierro ML, Ceja-Espiritu G,
Olmedo-Buenrostro BA, et al: Smoking and female sex as key risk
factors associated with severe arthralgia in acute and chronic
phases of chikungunya virus infection. Exp Ther Med. 15:2634–2642.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Skipper CP, Pastick KA, Engen NW,
Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen
MF, Nicol MR, et al: Hydroxychloroquine in nonhospitalized adults
with early COVID-19: A randomized trial. Ann Intern Med.
173:623–631. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Salepci E, Turk B, Ozcan SN, Bektas ME,
Aybal A, Dokmetas I and Turgut S: Symptomatology of COVID-19 from
the otorhinolaryngology perspective: A survey of 223 SARS-CoV-2
RNA-positive patients. Eur Arch Otorhinolaryngol. 278:525–535.
2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rivett L, Sridhar S, Sparkes D, Routledge
M, Jones NK, Forrest S, Young J, Pereira-Dias J, Hamilton WL,
Ferris M, et al: Screening of healthcare workers for SARS-CoV-2
highlights the role of asymptomatic carriage in COVID-19
transmission. Elife. 9(e58728)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Englbrecht M, Tarner IH, van der Heijde
DM, Manger B, Bombardier C and Müller-Ladner U: Measuring pain and
efficacy of pain treatment in inflammatory arthritis: A systematic
literature review. J Rheumatol. (Suppl 90):3–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tubach F, Ravaud P, Baron G, Falissard B,
Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der
Heijde D and Dougados M: Evaluation of clinically relevant changes
in patient reported outcomes in knee and hip osteoarthritis: The
minimal clinically important improvement. Ann Rheum Dis. 64:29–33.
2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Khanna D, Pope JE, Khanna PP, Maloney M,
Samedi N, Norrie D, Ouimet G and Hays RD: The minimally important
difference for the fatigue visual analog scale in patients with
rheumatoid arthritis followed in an academic clinical practice. J
Rheumatol. 35:2339–2343. 2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Johnson MJ, Close L, Gillon SC,
Molassiotis A, Lee PH and Farquhar MC: Breathlessness Research
Interest Group (BRIG). Use of the modified borg scale and numerical
rating scale to measure chronic breathlessness: A pooled data
analysis. Eur Respir J. 47:1861–1864. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Meza-Robles C, Barajas-Saucedo CE,
Tiburcio-Jimenez D, Mokay-Ramírez KA, Melnikov V, Rodriguez-Sanchez
IP, Martinez-Fierro ML, Garza-Veloz I, Zaizar-Fregoso SA,
Guzman-Esquivel J, et al: One-step nested RT-PCR for COVID-19
detection: A flexible, locally developed test for SARS-CoV2 nucleic
acid detection. J Infect Dev Ctries. 14:679–684. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ferrari D, Lombardi G, Strollo M, Pontillo
M, Motta A and Locatelli M: A possible antioxidant role for vitamin
D in soccer players: A retrospective analysis of psychophysical
stress markers in a professional team. Int J Environ Res Public
Health. 17(3484)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Singh A, Sood N, Narang V and Goyal A:
Morphology of COVID-19-affected cells in peripheral blood film. BMJ
Case Rep. 13(e236117)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lüke F, Orsó E, Kirsten J, Poeck H, Grube
M, Wolff D, Burkhardt R, Lunz D, Lubnow M, Schmidt B, et al:
Coronavirus disease 2019 induces multi-lineage, morphologic changes
in peripheral blood cells. EJHaem, Jun 29, 2020 (Online ahead of
print).
|
|
63
|
El Comentario U de C: No Title. Graves,
200 pacientes Hosp con Covid-19; hay 21 intubados.
|
|
64
|
Vickers AJ: The use of percentage change
from baseline as an outcome in a controlled trial is statistically
inefficient: A simulation study. BMC Med Res Methodol.
1(6)2001.PubMed/NCBI View Article : Google Scholar
|
|
65
|
McNutt LA, Wu C, Xue X and Hafner JP:
Estimating the relative risk in cohort studies and clinical trials
of common outcomes. Am J Epidemiol. 157:940–943. 2003.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wacholder S: Binomial regression in GLIM:
Estimating risk ratios and risk differences. Am J Epidemiol.
123:174–184. 1986.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Diaz-Quijano FA: A simple method for
estimating relative risk using logistic regression. BMC Med Res
Methodol. 12(14)2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
HyLown Consulting LLC: Power and sample
size HomeCalculators compare 2 proportions: 2-Sample, 1-Sided.
Calculator: 1, 2013.
|
|
69
|
Wang L: C-reactive protein levels in the
early stage of COVID-19. Med Mal Infect. 50:332–334.
2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Alzaid F, Julla JB, Diedisheim M, Potier
C, Potier L, Velho G, Gaborit B, Manivet P, Germain S, Vidal-Trecan
T, et al: Monocyte class switch and hyperinflammation characterise
severe COVID-19 in type 2 diabetes. medRxiv: 2020.06.02.20119909,
2020.
|
|
71
|
Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang
Y, Qian F, Dai T, Zhang T, Lai Y, et al: COVID-19 infection induces
readily detectable morphological and inflammation-related
phenotypic changes in peripheral blood monocytes, the severity of
which correlate with patient outcome. medRxiv: 2020.03.24.20042655,
2020.
|
|
72
|
McKechnie JL and Blish CA: The innate
immune system: Fighting on the front lines or fanning the flames of
COVID-19? Cell Host Microbe. 27:863–869. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
He R, Lu Z, Zhang L, Fan T, Xiong R, Shen
X, Feng H, Meng H, Lin W, Jiang W and Geng Q: The clinical course
and its correlated immune status in COVID-19 pneumonia. J Clin
Virol. 127(104361)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
National Research Project for SARS,
Beijing Group. The involvement of natural killer cells in the
pathogenesis of severe acute respiratory syndrome. Am J Clin
Pathol. 121:507–511. 2004.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhao Q, Meng M, Kumar R, Wu Y, Huang J,
Deng Y, Weng Z and Yang L: Lymphopenia is associated with severe
coronavirus disease 2019 (COVID-19) infections: A systemic review
and meta-analysis. Int J Infect Dis. 96:131–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y
and Shang Y: Thrombocytopenia and its association with mortality in
patients with COVID-19. J Thromb Haemost. 18:1469–1472.
2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Margraf A and Zarbock A: Platelets in
inflammation and resolution. J Immunol. 203:2357–2367.
2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Taneja V: Sex hormones determine immune
response. Front Immunol. 9(1931)2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Pozzilli P and Lenzi A: Commentary:
Testosterone, a key hormone in the context of COVID-19 pandemic.
Metabolism. 108(154252)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tan T, Khoo B, Mills EG, Phylactou M,
Patel B, Eng PC, Thurston L, Muzi B, Meeran K, Prevost AT, et al:
Association between high serum total cortisol concentrations and
mortality from COVID-19. Lancet Diabetes Endocrinol. 8:659–660.
2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Merad M and Martin JC: Pathological
inflammation in patients with COVID-19: A key role for monocytes
and macrophages. Nat Rev Immunol. 20:355–362. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Bateman L, Rao P, Jones TH and Kelly DM:
SAT-049 testosterone therapy reduces inflammatory activation of
human monocytes in hypogonadal type-2 diabetic men as a potential
mechanism to improve atherosclerosis. J Endocr Soc. 4 (Suppl
1):SAT–049. 2020.
|
|
83
|
Milush J: Role of monocyte oxidative
stress and mineralocorticoid receptor signaling on cardiovascular
disease and persistent inflammation in antiretroviral-treated HIV+
persons. Grantome 1, 2015.
|
|
84
|
Gomez-Sanchez E and Gomez-Sanchez CE: The
multifaceted mineralocorticoid receptor. Compr Physiol. 4:965–994.
2014.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Uchida MC, Bacurau RFP, Navarro F, Pontes
FL Jr, Tessuti VD, Moreau RL, Rosa LFBPC and Aoki MS: Alteração da
relação testosterona: Cortisol induzida pelo treinamento de força
em mulheres. Rev Bras Med Esporte. 10:165–168. 2004.
|
|
86
|
Block MS and Rowan BG: Hypochlorous acid:
A review. J Oral Maxillofac Surg. 78:1461–1466. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Montesinos-Peña NE, Hernández-Valencia M,
Delgado-Enciso I, Herrera-Leal A and Paz-Michel BA: Evaluation of
an antiseptic gel of intravaginal application for multitreated
patients for infectious cervicovaginitis. Ginecol Obs Mex.
87:454–466. 2019.
|
|
88
|
Cabello GC, Rosete ODP and Manjarrez ZME:
Efecto de una solución electrolizada de superoxidación con pH
neutro sobre la infección del virus de influenza A en células MDCK.
Rev Inst Nal Enf Resp Mex. 22:280–287. 2009.
|
|
89
|
Takeda Y, Uchiumi H, Matsuda S and Ogawa
H: Acidic electrolyzed water potently inactivates SARS-CoV-2
depending on the amount of free available chlorine contacting with
the virus. Biochem Biophys Res Commun. 530:1–3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Moorman E, Montazeri N and Jaykus LA:
Efficacy of neutral electrolyzed water for inactivation of human
norovirus. Appl Environ Microbiol. 83:e00653–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
García JP, Maldonado RA, Díaz RI, Muñiz J
and Rodríguez HA: Sustitución del uso de solución salina fi
siológica como irrigante en el manejo de pacientes sépticos y
quirúrgicos por solución electrolizada. Rev Mex Cir Bucal
Maxilofac. 7:46–52. 2011.
|
|
92
|
Khomich OA, Kochetkov SN, Bartosch B and
Ivanov AV: Redox biology of respiratory viral infections. Viruses.
10(392)2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kim HJ, Kim CH, Ryu JH, Kim MJ, Park CY,
Lee JM, Holtzman MJ and Yoon JH: Reactive oxygen species induce
antiviral innate immune response through IFN-λ regulation in human
nasal epithelial cells. Am J Respir Cell Mol Biol. 49:855–865.
2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Delgado-Enciso I, López-Lemus AU,
Valcarcel-Gamiño AJ, Rodriguez-Sanchez IP, Valle-Reyes S,
Martinez-Fierro ML, Melnikov V, Guzmán-Esquivel J, Vaca-Paniagua F,
Valdez-Velazquez LL, et al: Dengue virus-1 NS5 genetic variant
associated with a severe clinical infection: Possible reduction of
the innate immune response by inhibition of interferon type 1 and
the Janus kinase-signal transducer and activator of transcription
signaling pathway. Int J Mol Med. 41:2263–2269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Liu T, Castro S, Brasier AR, Jamaluddin M,
Garofalo RP and Casola A: Reactive oxygen species mediate
virus-induced STAT activation: Role of tyrosine phosphatases. J
Biol Chem. 279:2461–2469. 2004.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Nair S, Poddar S, Shimak RM and Diamond
MS: Interferon regulatory factor 1 protects against chikungunya
virus-induced immunopathology by restricting infection in muscle
cells. J Virol. 91:e01419–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Hong Y, Chen S and Zhang JM: Hydrogen as a
selective antioxidant: A review of clinical and experimental
studies. J Int Med Res. 38:1893–1903. 2010.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Pyo CW, Shin N, Jung K, Choi JH and Choi
SY: Alteration of copper-zinc superoxide dismutase 1 expression by
influenza A virus is correlated with virus replication. Biochem
Biophys Res Commun. 450:711–716. 2014.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Chen J, Zhang H, Hu J, Gu Y, Shen Z, Xu L,
Jia X, Zhang X and Ding X: Hydrogen-rich saline alleviates kidney
fibrosis following AKI and retains klotho expression. Front
Pharmacol. 8(499)2017.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Nakayama M, Kabayama S and Ito S: The
hydrogen molecule as antioxidant therapy: Clinical application in
hemodialysis and perspectives. Ren Replace Ther. 2(23)2016.
|
|
101
|
Chen M, Zhang J, Chen Y, Qiu Y, Luo Z,
Zhao S, Du L and Tian D: Hydrogen protects lung from
hypoxia/re-oxygenation injury by reducing hydroxyl radical
production and inhibiting inflammatory responses. Sci Rep.
8(8004)2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang ST, Bao C, He Y, Tian X, Yang Y,
Zhang T and Xu KF: Hydrogen gas (XEN) inhalation ameliorates airway
inflammation in asthma and COPD patients. QJM. 113:870–875.
2020.PubMed/NCBI View Article : Google Scholar
|