Introduction
Coronavirus disease (COVID-19), caused by the severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently
the major public health problem worldwide (1,2).
Previous studies reported that the most common initial symptoms are
systemic, upper respiratory symptoms and cough. Lower respiratory
and gastrointestinal symptoms are less frequent and generally
appear at the late stage of the disease (3). The symptoms, if present, with the
longest duration are cough, loss of sense of smell or taste, sinus
congestion, shortness of breath upon exertion, body aches and
headache (3). A study on the time
that COVID-19 patients require to achieve a usual state of health
reported that 65 percent have returned to their usual state of
health 7 days from the date of diagnosis, whereas 35% of patients
had not returned to their usual state of health at 12-14 days after
receiving a positive test result (4). Although most infections are
self-limited, an estimated 15% of infected adults develop severe
pneumonia that requires treatment with supplemental oxygen and
hospitalization (5). However, the
number of infected patients identified as having severe infection
and requiring hospitalization varies among regions and countries,
whether due to inherent conditions in the population (6) or to the strategy used in identifying
individuals that are positive for the virus (7). In Mexico, 40.3% of confirmed cases are
estimated to require hospitalization (8).
There are numerous experimental approaches for
treating COVID-19. Initially, chloroquine appeared to be a
promising treatment, but its lack of efficacy has since been
demonstrated (9). Despite the
numerous drugs that are currently recommended, such as nonsteroidal
anti-inflammatory drugs (NSAIDs), corticoids, antivirals,
antibiotics and proinflammatory cytokine (interleukin) modulators,
no specific drug therapy has been proven to be effective against
SARS-CoV-2, yet (10). Treatment is
symptomatic and oxygen therapy is the first step in addressing
respiratory impairment (1).
Noninvasive and invasive mechanical ventilation may be necessary in
cases of respiratory failure that is refractory to oxygen therapy
(1).
COVID-19 symptomatology and manifestations depend on
the degree of immune dysregulation caused by the virus,
characterized by systemic inflammation and remote organ injury
(11,12). Viral infection is capable of
producing an excessive immune reaction in the host. In severe
cases, a reaction known as ‘cytokine storm’ occurs (1). A rapid and robust type I
IFN-orchestrated response may lead to virus clearance, given that
antiviral lymphocytes, such as natural killer (NK) cells, are
activated and expanded. Conversely, late activation of innate
immunity is usually associated with severe pathology that may lead
to pneumonia, acute respiratory distress syndrome (ARDS), septic
shock, multi-organ failure and, eventually, death (13). Different immune system alterations
come together to produce severe disease. A key factor in the
cytokine storm in COVID-19 is the elevation of monocytes, a
circulating innate immune cell type producing IL-6(14), combined with lymphocyte reduction
that limits the systemic antiviral response (15,16).
Inefficient SARS-CoV-2 clearance by alveolar macrophages may
promote excessive viral replication, leading to severe pathology
that is accompanied by increased viral shedding and, in turn, viral
transmissibility (13). In the
present study, it was postulated that administration of intravenous
and/or nebulized electrolyzed saline may aid in modulating the
body's immune response to SARS-CoV-2, reducing symptomatology and
preventing disease progression.
Electrolyzed saline is produced from a saline
solution of sodium chloride, activated by a controlled process of
electrolysis, producing reactive species of chlorine and reactive
oxygen species (ROS). Significant examples of said reactive species
are oxidant chlorine species, such as hypochlorous acid and oxidant
ROS, such as hydrogen peroxide. Molecular hydrogen (H2)
is also produced (17). ROS are
normally produced in the organism and have different physiological
functions (18). Their most
well-known activity is to control bacteria, parasites and viruses
through the activity of cells of the innate immune response,
macrophages and neutrophils that release ROS to structurally damage
the invading pathogens, thus protecting the host from infection
(19).
A series of studies have indicated that, in addition
to the primordial innate immune response, ROS are secondary
messengers in processes of exacerbated inflammation control and
tissue repair in a process known as redox signaling. Redox
signaling is ROS-dependent and the immune response varies,
according to ROS concentrations and exposure time (19-23).
Different studies have indicated that ROS are able to activate and
repair phenotypes, such as M2 macrophages and regulatory T cells,
acting as potentiators of the humoral immune response (24,25).
ROS have been indicated to mediate the communication between the
different cells of the immune system, such as polymorphonuclear
cells, neutrophils, macrophages, antigen-presenting cells, B cells
and T cells (23-26).
Specifically, hypochlorous acid may act as a coadjuvant and
adaptive immune response stimulator by modifying antigen proteins
and increasing their recognition, processing and presentation by
antigen-presenting dendritic cells (27). In addition, ROS have an important
role in later stages of B-cell activation by promoting the
sustained signaling of B-cell antigen receptors, thus favoring
antibody production (28). Numerous
studies have also suggested that H2 has beneficial
effects in diverse animal models and human diseases (29). Its oral administration in an animal
model limited the increase of IL-6 and tumor necrosis factor-alpha,
producing a potent antioxidant and anti-inflammatory effect
(30).
Therefore, the present study was designed to
randomly select patients with COVID-19 receiving usual medical care
and compare the safety and efficacy of two treatments: Usual
medical care combined with electrolyzed saline [administered
intravenously and/or through inhalation of the aerosol
(nebulization), with dose escalation] and usual medical care alone
(control).
Materials and methods
Study design
A prospective, randomized, single-blind, 2-arm,
parallel-group, open-label, phase I-II clinical trial was performed
between May and December 2020 and carried out according to the
consolidated standards of reporting trials (CONSORT) statement
guidelines for randomized controlled trials (31). The study aimed to evaluate the
safety and efficacy of electrolyzed saline for preventing disease
progression and it was approved by the ethics committee of the
School of Medicine of the Universidad de Colima (Colima, México;
April 8, 2020), and written informed consent was obtained from all
of the participants. The trial was performed in accordance with the
principles of the Declaration of Helsinki and the International
Conference on Harmonization-Good Clinical Practice guidelines. The
present clinical trial was registered in the Cuban public registry
of clinical trials (RPCEC) database (May 5, 2020; no. TX-COVID19:
RPCEC00000309).
Study subjects
The inclusion criteria were as follows: Males and
non-pregnant females aged ≥18 years, presenting with COVID-19 and a
positive diagnosis of SARS-CoV-2 by reverse
transcription-quantitative (RT-q) PCR, who had a medical
consultation due to their illness and were indicated for at-home
ambulatory treatment. Women of reproductive age, without permanent
contraceptive methods and sexually active agreed to utilize
effective non-hormonal contraceptive measures during the study
period and for at least 15 days after the final drug administration
of the study. Exclusion criteria were pregnant or breastfeeding
females and patients presenting with any of the following
conditions prior to the diagnosis of COVID-19: Cancer, ischemic
heart disease, chronic decompensated systemic disease, creatinine
1.25 times higher than the normal value or creatinine clearance
<50 milliliters/min (Cockcroft-Gault method), blood hemoglobin
<10 g/dl, drug addiction (illegal drugs) or known liver disease
with a doubling of liver function test values [aspartate
aminotransferase (AST), alanine aminotransferase (ALT), alkaline
phosphatase (ALP) or bilirubin]. In addition, the following
elimination criteria were applied: Patients who decided to drop out
of the study, patients who at any point of the study, presented
with severe toxicity (grade 3 or higher, according to the Common
Terminology Criteria for Adverse Events v5.0, US Department of
Health and Human Services) (32),
that was attributable to the administration of the experimental
drug.
The physicians participating in the project
identified candidates from primary and secondary healthcare centers
(public or private) in the Mexican states of Colima, Chiapas and
Morelos (in Colima: Regional University Hospital from the Health
Ministry of the State of Colima, Colima Hospital, General Hospital
of Zone 1 of the IMSS Colima, Medical Center Union Clinic, San
Francisco Clinic; in Chiapas: Poliforum COVID-19 Respiratory Care
Clinic; in Morelos: Private practice medical office Xochitepec).
The physicians asked the patients for their permission, once they
were at home, for the researchers to call them by telephone,
requesting their participation in the study. Prior to said phone
call, the candidates were randomly allocated to the experimental
group (electrolyzed saline + usual medical care) or the control
group (usual medical care alone). Randomization was performed using
computer-generated random allocation cards. In that manner, the
patients were directly asked to participate in one of the
non-blinded groups. The inclusion process was performed by
researchers who did not participate in the evaluation of the
results. Prior to entering the study, all of the patients were
receiving usual treatment under the care of their family physician
or specialist. When asked to participate in the study, the patients
selected for the electrolyzed saline group were told they would
receive an experimental treatment in addition to their usual
medical care, as well as have sign and symptom follow-up and
undergo certain laboratory tests. The patients receiving usual
medical care alone (control group) were asked to participate in the
study, with follow-up of signs and symptoms performed by telephone.
All of the patients were advised that they would continue to be
under the supervision of their regular physician or healthcare
institution and that the research team would in no way modify or
limit any intervention that their physician, or they themselves,
considered pertinent, such as going to the emergency service if
there were any alarming symptoms.
Neutral electrolyzed saline
The experimental treatment consisted of an aqueous
saline solution of sodium chloride, activated by a controlled
process of electrolysis (patent no. MX330845B), and thus resembled
activated saline, electrolyzed saline or electrolyzed water. It had
a neutral pH (6.0-7.5) and its active ingredient was 0.002% of
active species of chlorine and oxygen. The good manufacturing
practices for intravenous electrolyzed saline
(HOMEOSTECH®) also met the required processes for
sterile injectable products (33).
As an intravenous (IV) electrolyzed saline, its formulation was
17.12 mEq/l of sodium chloride and 0.38 mM of active species of
chlorine and oxygen. The vials utilized were 5-ml ampules, and the
name and composition were indelibly printed on each one. The
electrolyzed saline was provided by Esteripharma S.A. de C.V as an
experimental (not commercial) product.
When the randomized patient was in the electrolyzed
saline group, he or she was included in a dose escalation with
overdose control design, as has previously been reported (34). Dose level 1 consisted of
nebulizations (inhalation of the mist, produced by a nebulizer
provided with a mask for inhalation therapy). The nebulizations
were indicated 4 times a day for 10 days. They were performed by
placing 5 ml of electrolyzed saline in the nebulizer chamber
(Nebucor, type MOD. P-100; Neb S.A. de C.V.) and continuing the
nebulization until the content was used up (10-15 min). The
nebulizations were performed following the recommendations of the
American College of Allergy, Asthma and Immunology (35), the British Lung Foundation (36), the Asthma Society of Ireland
(37) and the British Thoracic
Society (38).
The IV dosing began with a dose within a safe range,
previously established in a phase I clinical trial conducted at the
Instituto Estatal de Cancerología de Colima for the treatment of
chikungunya (manuscripts in preparation; clinical trial
registration number RPCEC00000226. The initial applications were 15
ml (dose level 2) once a day for 7 days, with successive increases
to 20 ml/day (dose level 3), 30 ml/day (dose level 4), 30-40
ml/twice daily (dose level 5), 40 ml/day (dose level 6), 80 ml/day
(dose level 7) and 150 ml/day (dose level 8). All applications were
made every 24 h for 7 days or 10 days only if diarrhea, myalgia,
arthralgia or body temperature >37.5˚C was present on the
seventh day of treatment. Dose level 5 was the exception, where
applications were made every 12 h for 3 (dose level 5.1), 6 (dose
level 5.2) or 9 days (dose level 5.3) (Fig. S1). Nebulizations with electrolyzed
saline solution were always added to all IV treatment regimens. The
dose-limiting toxicity was not achieved at any dose level.
The electrolyzed saline solution was diluted in
one-third of its volume with physiological saline solution (0.9% of
NaCl) (1 ml physiological solution for every 2 ml oelectrolyzed
saline), immediately prior to its application, for the case of dose
levels 2-6. The solution was administered IV as a bolus (passing it
in 1-2 min). For dose levels 7 and 8, 100 ml of normal saline
solution (0.9% of NaCl) were withdrawn from a 250-ml bottle and the
appropriate volume of electrolyzed saline for each regimen was
added, under sterile conditions using a Class-II laminar flow hood
BSL-2. The whole solution mixture was administered in 1 h, with
applications once a day using a heparinized peripheral venous
catheter for its intermittent use.
When COVID-19 symptoms of nausea, vomiting and/or
diarrhea occurred, 30 ml oral electrolyzed saline was added, 4
times a day, for as long as gastrointestinal symptoms lasted, plus
2 more days after the symptoms disappeared. The oral route of
electrolyzed saline has been shown to be safe and has been used to
treat epidemic diarrhea virus infection in preclinical trials
(39,40). In patients with oropharyngeal
ulcerations and/or intense throat irritation (causing intense
pain), the indication was to gargle with 10 ml electrolyzed saline
6 times a day and swallow the solution after gargling with it. This
was performed for the number of days necessary for the pain to
decrease to 4 or less on the 0-10 visual analog scale (VAS). The
oral pathway was indicated in 17 patients and gargling was
indicated in 25. These indications were added during the protocol
to rapidly evaluate them without compromising the original trial
outcomes, a procedure that has been considered adequate in previous
scientific reviews (41). The
indication was based on intended uses previously authorized (local
treatment of throat infections and sore) by the Mexican Federal
Commission for the Protection against Sanitary Risks (COFEPRIS) of
a similar product (Estericide Bucofaríngeo, Reg. No. 1003C2013 SSA;
Esteripharma).
Usual medical care
The patients receiving only usual medical care
continued with the usual treatment prescribed by their family
physician or specialist. Usual care is the care the targeted
patient population would be expected to receive as part of normal
practice (42). This is a valid
strategy as a reference treatment in clinical trials, including
various therapy trials against COVID-19 (43-45).
It consisted of the administration of paracetamol, NSAIDs,
steroids, azithromycin, chloroquine, ivermectin, and/or antiviral
drugs, anticoagulants, etc.; the patients were instructed to return
to the emergency service if there was respiratory difficulty or
worsening of symptomatology. The researchers did not intervene in
drug prescription or lifestyle indications (usual medical
care).
Outcome measures and follow-up
There were 3 co-primary endpoints. The first was the
number of patients with disease progression, defined as
hospitalization or death. The second primary endpoint was the
patient acceptable symptom state (PASS), defined as the value of
symptoms the patient considered to be well-being thresholds of pain
and function. In the present study, the most widely used anchoring
question to identify PASS cut-off points was incorporated, which as
follows: ‘Taking into account all your daily activities, do you
consider your current state satisfactory in relation to pain level
and functional impairment?’ with response options being ‘Yes’ or
‘No’ (46-48).
Treatment success was defined as no disease progression or a PASS
according to the answer in the affirmative test on days 1 to 20 of
follow-up. The third endpoint was the change from the baseline in
the patient overall self-assessment or the severity score, which
was determined by the response to the following question:
‘Considering all the ways in which illness and health conditions
may affect you at this time, please indicate how you are doing?’
with the response options measured on the 0-10 VAS, from ‘very
well’ (score of 0) to ‘very poorly’ (score of 10) (49). This question was validated using the
Routine Assessment of Patient Index Data 3, previously used to
determine the activity of autoimmune diseases, degenerative
diseases, such as osteoarthritis (50) and infectious diseases with a strong
component of general malaise, such as chikungunya fever (51). That endpoint is similar to the
symptom severity score (self-assessed using a 10-point VAS)
recently used in a clinical trial that evaluated the efficacy of
hydroxychloroquine in non-hospitalized patients with COVID-19,
where 0 indicated ‘no symptoms’ and 10 indicated ‘severe symptoms’
(52). The patients were also
classified at baseline according to disease severity, as directed
by the World Health Organization (WHO) interim clinical management
guidance; as mild, moderate, severe or critical disease (53). In addition, the concepts of
asymptomatic patients (0 major symptoms and 0 minor symptoms) and
pauci-symptomatic patients (0 major symptoms and 1-2 minor
symptoms) were considered, as previously defined (major symptoms:
Fever >37.8˚C and new persistent cough; minor symptoms: Hoarse
voice, non-persistent cough, sore throat, runny or stuffy nose,
shortness of breath, wheezing, headache, muscle aches, nausea
and/or vomiting and/or diarrhea and loss of sense of taste or
smell) (54).
The secondary endpoints were changes from the
baseline in different types of body pain (arthralgia, myalgia,
headache and sore throat), or more precisely, the difference from
the values at enrollment on all days of follow-up. Pain was
measured on the 0-10 VAS (55).
Intensity of pain was recorded, from ‘no pain’ (score of 0) to
‘worst pain imaginable’ (score of 10) (55,56).
Patients completed the previously validated fatigue VAS (scale of
0-10) (57), which poses the
questions of: ‘How much of a problem has unusual fatigue or
tiredness been for you today’ and was anchored from 0 (fatigue is
not a problem) to 10 (fatigue is a major problem). Daily coughing
episodes were reported by the patient on a numerical scale from 0
to 20. If there were more than 20 episodes, they were registered as
20. Dyspnea was determined once a day through the Borg scale, from
0 to 10, according to which 0 indicates no dyspnea and 10 extremely
severe dyspnea (58). Nausea,
vomiting, diarrhea, dizziness, conjunctivitis, rhinorrhea,
exanthema, skin rash and loss of sense of smell or taste were
recorded as present or absent for each day of follow-up. Adverse
events were monitored by the researchers through anamnesis and
abnormal routine laboratory test results. Follow-up was performed
for at least 20 days or until an endpoint was reached (cure or
death). Daily follow-up was suspended in the hospitalized patients,
and from the day of hospital admission, their registers were
considered lost data and were not considered in the analysis from
that day forward, with the exception of the PASS, the result of
which was reported as a negative acceptable symptom state from then
onwards. However, the general aspects of those patients were
registered, such as hospitalization and outcome (cure or
death).
Serial detection of SARS-CoV-2
In 10 patients from the experimental group treated
with electrolyzed saline, nasopharyngeal and oropharyngeal samples
were collected with swabs in 2.5 ml of viral transport medium,
immediately prior to starting treatment and on days 2, 4, 6 and 14,
and stored at -80˚C until processing. Viral RNA was isolated
utilizing TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol and SARS-CoV-2 testing was
performed through SYBR green-based reverse
transcription-quantitative (RT-q) PCR using the previously
described methodology (59). That
procedure was not performed on any of the patients in the control
group.
Evaluation of hematologic and
serologic parameters
In the experimental group, changes in hematologic
parameters were evaluated at baseline, at 48 h (day 2) and on days
4, 6, 9 and 14. The complete blood count was evaluated using Sysmex
XP-300 (Roche®) equipment, the biochemical tests for
kidney function and liver function were performed using Cobas c111
(Roche®) equipment and the serum concentration of
testosterone and cortisol were determined by immunofluorescence
with the iCHROMA (Boditech Med Inc.) equipment. The
testosterone-cortisol ratio was calculated by dividing the two
hormone levels, both expressed in nm/l (60). Thirty patients with any type of
steroidal or hormonal treatment were excluded from this analysis.
Systemic inflammation markers (erythrocyte sedimentation rate and
C-reactive protein) were also evaluated and rapid staining of blood
smears with staining kits (Hycel) were performed to quantify the
following: i) Reactive lymphocytes, also called virocytes; ii)
large granular lymphocytes, a representation of NK cells or
cytotoxic T lymphocytes; iii) activated monocytes; and iv)
monocytes with aberrant nuclei (clumped chromatin) and basophilic
cytoplasm (14,61,62).
Blinding
Only the researchers that evaluated treatment
effectiveness through the VAS, PASS and other endpoints instruments
answered by the patients, as well as those that performed the
statistical analyses, were blinded. The personnel who provided the
treatments were different from the personnel in charge of
evaluating the effectiveness of the treatments.
Sample size
The sample size calculation was based on the number
of patients that had disease progression (hospitalization or
death). Progression in 10% of patients in the experimental group
and 35% of subjects in the control group was predicted. Those
figures were based on local data from the Mexican city of Colima,
according to which 43% of confirmed patients were hospitalized,
according to health authority reports (63). A total of 32 patients from each
group were needed to reach the required statistical power (0.8)
when the statistical analysis was performed at the level of a
one-tailed alpha-value of 0.05. At the end of the study, the
statistical power for detecting a difference between two distinct
groups was calculated (one-tailed alpha=0.05), utilizing the number
of patients with disease progression, resulting in 99.2%.
Statistical analysis
Values are expressed as the mean ± standard
deviation (for data with a normal distribution), median with 25 and
75th percentiles (interquartile range) for data with a non-normal
distribution or percentages. Normality of distribution of data was
first determined using the Kolmogorov-Smirnov test and the equality
of variances was confirmed using Levene's test. Parametric data
with a normal distribution [e.g., body mass index (BMI) or age]
were compared between groups utilizing Student's t-test.
Categorical variables were compared using the Fisher's exact test
or likelihood ratio χ2 test. To compare continuous
variables with a non-normal distribution or data in ordinal scale
between two groups, the Mann-Whitney U-test was applied to
independent samples and the Wilcoxon signed-rank test was applied
to matched samples. For the oxygen saturation parameter, the change
from baseline was used to observe the absolute differences between
the evaluation periods, calculated through the value after
intervention minus the value at baseline, in each patient, which is
an acceptable manner for analyzing trial results with baseline and
after the beginning of treatment measurements (64). To test for a significant difference
in means over time in blood parameters, repeated-measures ANOVA was
used, followed by Dunnett's post-hoc test (any time-point vs.
baseline). The Jonckheere-Terpstra test was used to determine
differences in symptom severity between dose levels on different
days, followed by pairwise comparisons between groups using Dunn's
test. Kaplan-Meier analyses were performed to compare survival and
the log-rank test was applied to determine significant differences
between groups. Binary logistic regression analyses were employed
to determine the probability of hospitalization or achieving PASS
on day 5 (binomial outcome: Yes or no) with the experimental
treatment, compared with the usual medical care. Data were
summarized as relative risk (RR) with 95% confidence interval (CI)
and P-value, adjusted for age, sex, BMI, baseline of oxygen
saturation (SpO2), diabetes, hypertension, progression
time, baseline severity and other relevant variables. Binomial
regression is considered the most adequate choice for estimating
RRs in multivariate analyses (65-67).
Pearson's correlation coefficients (r) were calculated for
bivariate correlation between numeric and normally-distributed
parameters (C-reactive protein, monocytes, platelets, lymphocytes,
cortisol and testosterone-cortisol ratio); while Spearman's rank
correlation coefficients (r) were generated when any of the above
parameters was correlated with the patient symptom severity score
(ordinal scale). Significant correlations were discussed based on
the P-value.
The statistical analysis was performed using the
SPSS version 20 software (IBM Corp.), with the exception of the
number needed to treat (NNT), which was calculated using MedCalc
v17.7.2 software (MedCalc Software bvba), and sample size and
statistical power, which were calculated using the online
calculator software by HyLown Consulting LLC to compare 2
proportions: 2-sample, 1-sided (http://powerandsamplesize.com/Calculators/Compare-2-Proportions/2-Sample-1-Sided)
(68). P<0.05 was considered to
indicate statistical significance. Sample size and statistical
power were calculated for a one-tailed test. The remaining analyses
were two-tailed tests.
Results
Patients and symptoms
A total of 242 patients were randomized and
screened. Finally, 113 patients in the experimental group and 104
patients in the control group agreed to participate in the study.
In the experimental group, 3 patients discontinued the
intervention, leaving this group with 110 patients for the analysis
(Fig. S1). Gender Ratio in the
analyzed patients was 101.88 male per 100 female subjects. The mean
ages of the experimental and control patients were 45.5±14.1 and
41.8±15.4 years old, respectively (P=0.073) (Table I). The major clinical
characteristics and prescribed drugs are presented in Table I, exhibiting homogeneous
characteristics between the groups (experimental vs. control) at
the beginning of the study. The symptoms at baseline were also
similar (Table SI).
 | Table IMajor clinical characteristics of the
participating subjects at the time of enrollment and usual
prescribed drugs. |
Table I
Major clinical characteristics of the
participating subjects at the time of enrollment and usual
prescribed drugs.
| Clinical
characteristic | Control
(n=104) | Experimental
(n=110) | P-value |
|---|
| Female sex (%) | 52.9 | 46.4 | 0.412a |
| Age (years) | 41.8±15.4 | 45.5±14.1 | 0.073b |
| BMI
(kg/m2) | 29.6±4.7 | 28.6±5.1 | 0.136b |
| Diabetes (%) | 15.4 | 17.3 | 0.717a |
| High blood pressure
(%) | 15.4 | 18.2 | 0.715a |
| Asthma (%) | 2.9 | 7.3 | 0.216a |
| Smoking (%) | 11.5 | 12.7 | 0.837a |
| Progression
timec | 4.1±2.6 | 4.7±3.6 | 0.142b |
| Body temperature
(˚C) | 37.3±1.0 | 37.4±0.8 | 0.718b |
|
%SpO2 | 95.1±2.8 | 94.3±3.1 | 0.077b |
| SpO2
<94% (%) | 35.6 | 41.8 | 0.400a |
| Degree of
dyspnea | 1.2±1.5 | 1.2±1.4 | 0.956b |
| Symptom
severityd | 6.8±2.2 | 6.4±2.3 | 0.153e |
| Number of
symptomsf | 8 (7-9) | 8 (6-9) | 0.109e |
| Disease severityWHO
(%) | | | 0.390g |
|
Mild | 83.7 | 76.4 | |
|
Moderate | 6.7 | 10.9 | |
|
Severe | 9.6 | 12.7 | |
| Treatments | | | |
|
Number | 2.8+1.6 | 2.7+1.5 | 0.822a |
|
Paracetamol
(%) | 56.7 | 50.0 | 0.522a |
|
NSAIDs
(%) | 57.7 | 60.0 | 0.291a |
|
Ivermectin
(%) | 9.6 | 13.6 | 0.373a |
|
Chloroquine
(%) | 7.7 | 3.6 | 0.325a |
|
Antibiotics
(%) | 45.2 | 45.5 | 0.368a |
|
Antivirals
(%) | 22.1 | 14.5 | 0.345a |
|
Antihistamines
(%) | 14.4 | 13.6 | 0.591a |
|
Steroids
(%) | 30.8 | 27.3 | 0.479a |
|
Anticoagulants
(%) | 14.4 | 11.8 | 0.430a |
|
Vitamins
(%) | 16.3 | 13.6 | 0.476a |
The clinical severity distribution of all patients
with SARS-CoV-2 infection in the present study according to the WHO
interim clinical management guidance (53) was as follows: Mild (79.9%), moderate
(8.9%) and severe (11.2%). The median reported symptom severity
score, according to a self-assessment 10-point VAS, was 7
(interquartile range, 5 to 8), and the median number of
COVID-19-compatible symptoms was 8 (interquartile range, 7 to 9
symptoms). None of the patients was asymptomatic or
pauci-symptomatic, since all of them required specialized therapy
with a health care professional. Therefore, in spite of the absence
of any clinical or imaging signs suggestive of pneumonia, the
patients were symptomatic.
Evaluation of clinical improvement and
disease progression
The results were analyzed through two data grouping
strategies. The control group (usual medical care) was compared
with the experimental group, which included all dose levels of the
experimental therapy. The other analyses compared the different
dose levels of therapy between one another and with the control
group, to determine the most efficacious therapeutic dose. In the
control group, 19.2% of the patients had disease progression
(hospitalization or death), compared with 7.3% of the patients
receiving the experimental therapy, with a statistically
significant difference in the Kaplan-Meier analysis with log-rank
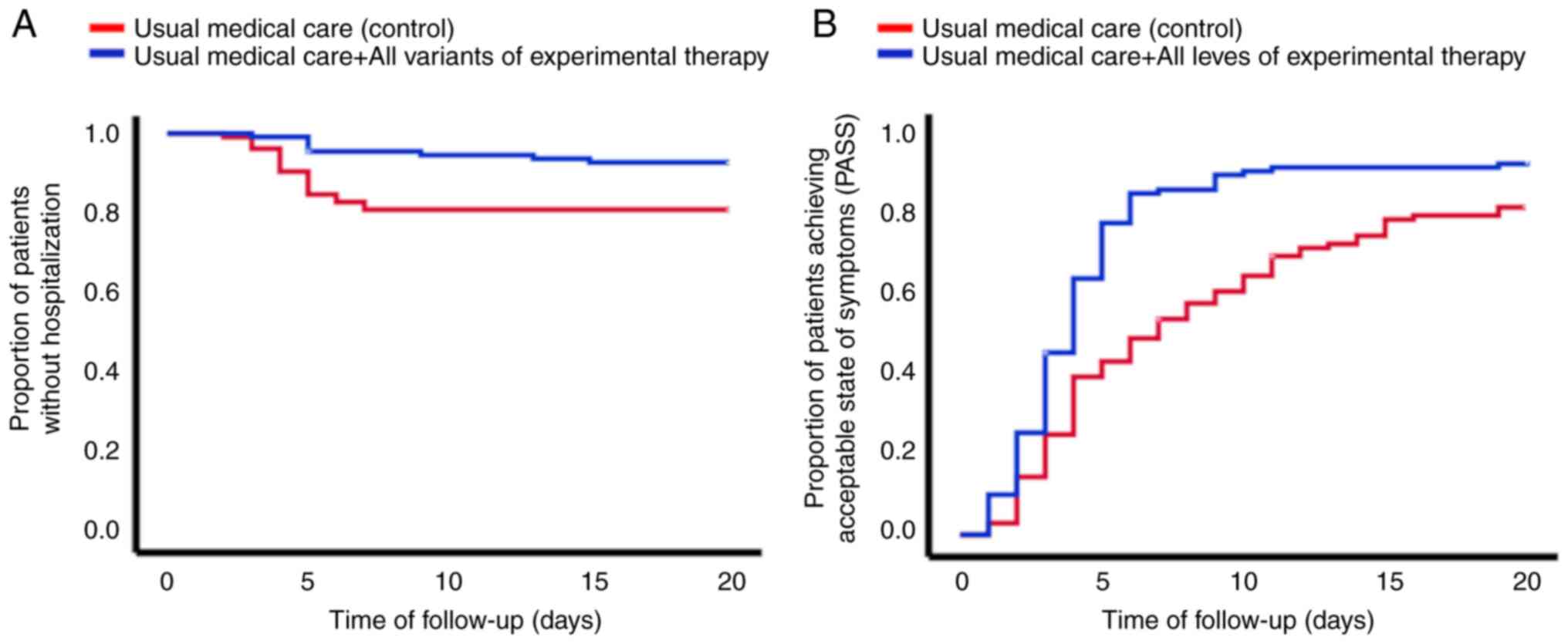
test (P=0.008). Fig. 1A shows that
the group of patients that received electrolyzed saline had fewer
hospitalizations, compared with the patients that received only the
usual medical care. Regarding only the patients that were
hospitalized, the time interval from inclusion in the study to
hospitalization was lower in the control group compared with that
in the experimental therapy group (4.5±1.3 days vs. 7.0±4.0 days,
respectively; P=0.018) (see Table
II). Death occurred in 8.7% of all the patients in the control
group and 1.8% of the patients in the experimental group (P=0.025,
Kaplan-Meier analysis with log-rank test) (Table II). Fig. 1B shows the proportion of patients
achieving PASS. The mean time to PASS in the control group was
9.0±0.6 days, compared with 5.1±0.4 days in the experimental
therapy group (P<0.001, Kaplan-Meier analysis with log-rank
test). With respect to the different treatment schemes with
electrolyzed saline, their effect on the severity of symptoms was
dose level-dependent, with IV + nebulized administration being
better than nebulized administration alone, but nebulized
administration was better than usual medical care alone (Fig. 2).
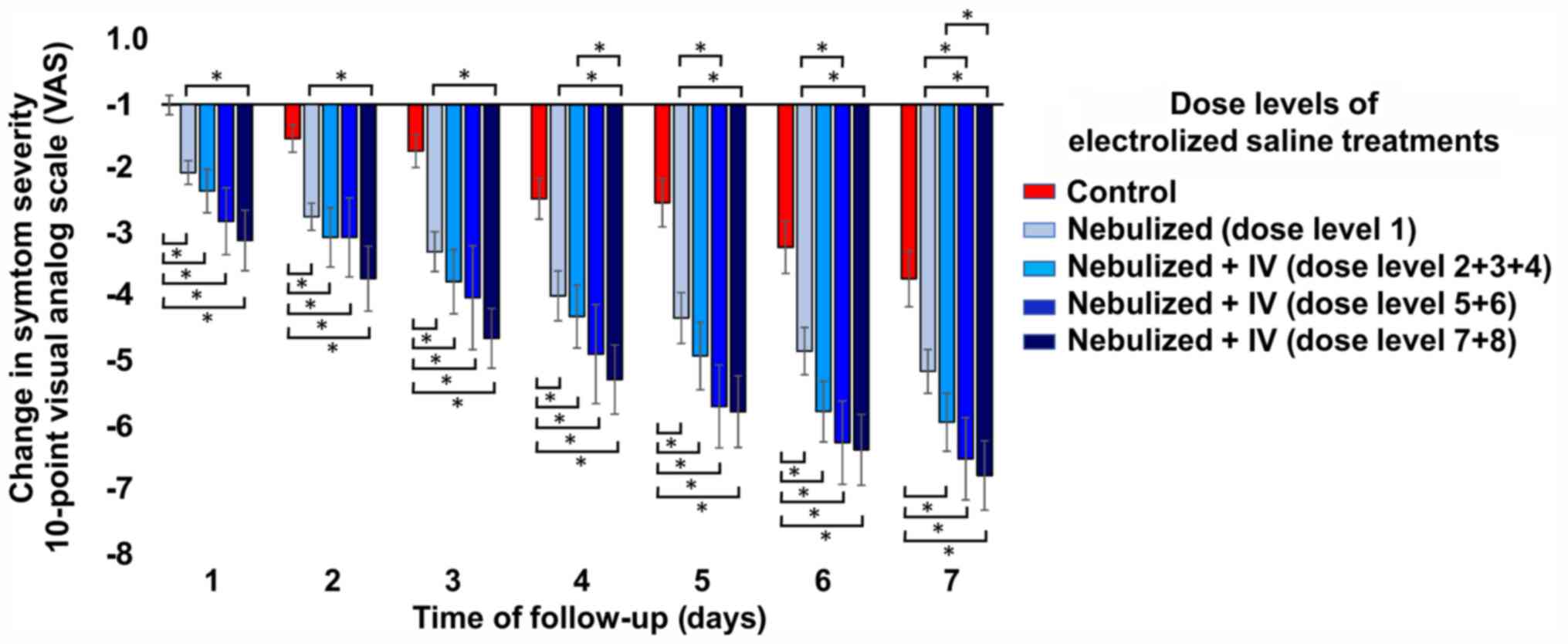 | Figure 2Change in the severity of the general
symptoms with respect to the baseline score according to the
different dose levels. All dose levels of electrolyzed saline
performed significantly better in reducing the severity of symptoms
than usual medical care alone, while the higher dose levels (dose
levels 7 + 8) were significantly better than just nebulization
(dose level 1). Compared with just nebulization, dose levels 5 + 6
only significantly reduced symptoms on days 6 and 7. Symptom
severity was determined by an overall self-assessment of the
patient using a 10-point visual analog scale, where the change is
the result of the value of a given day minus its baseline value.
The Jonckheere-Terpstra test followed by pairwise comparisons
between groups with Dunn's test indicated differences in symptom
severity between dose level groups at all measurement time-points.
*P<0.05. Groups: Control, usual medical care (n=104);
experimental groups, treatment with usual medical care plus
electrolyzed saline at different doses: 1-nebulization (n=35);
2/3/4-nebulization + IV administration of 15, 20 or 30 ml per day,
respectively (n=32); 5/6-nebulization + IV administration of 30 ml
twice a day or 40 ml once a day, respectively (n=16); and
7/8-nebulization + IV administration of 80 or 150 ml per day,
respectively (n=27). IV, intravenous. |
 | Table IIOutcomes in the experimental and
control groups of patients with Coronavirus disease 2019 according
to WHO disease severity classification. |
Table II
Outcomes in the experimental and
control groups of patients with Coronavirus disease 2019 according
to WHO disease severity classification.
| A, All
patients |
|---|
| Item | Experimental
(n=110) | Control
(n=104) | P-value |
|---|
| Days until
PASS | 5.1±0.4 | 9.0±0.6 | <0.001 |
| PASS on day 5
(%) | 79.8 | 39.4 | <0.001 |
| Hospitalized
(%) | 7.3 | 19.2 | 0.008 |
| Days to be
hospitalized | 7.0±4.0 | 4.5±1.3 | 0.018 |
| Death (%) | 1.8 | 8.7 | 0.024 |
| Days to death | 19.5±2.1 | 18.5±10.1 | 0.902 |
| B, Mild
disease |
| Item | Experimental
(n=84) | Control (n=87) | P-value |
| Days until
PASS | 4.2±0.4 | 7.2±6 | <0.001 |
| PASS on day 5
(%) | 84.5 | 46.0 | <0.001 |
| Hospitalized
(%) | 3.6 | 5.7 | 0.380 |
| Days to be
hospitalized | 6.2±3.8 | 4.0±0.7 | 0.250 |
| Death (%) | 0.0 | 2.3 | 0.257 |
| Days to death | NA | 27.5±3.5 | NA |
| C, Moderate and
severe disease |
| Item | Experimental
(n=26) | Control (n=17) | P-value |
| Days until
PASS | 7.8±1.4 | 18.5±1.0 | <0.001 |
| PASS on day 5
(%) | 65.4 | 5.9 | <0.001 |
| Hospitalized
(%) | 19.2 | 88.2 | <0.001 |
| Days to be
hospitalized | 7.8±4.3 | 4.7±1.4 | 0.025 |
| Death (%) | 7.7 | 41.2 | 0.012 |
| Days to death | 19.5±2.1 | 15.2±8.9 | 0.548 |
The multivariate analysis indicated that in patients
who received the experimental treatment, in addition to usual
medical care, the risk of becoming hospitalized was reduced by 89%
(adjusted RR=0.11, 95% CI: 0.03-0.37, P<0.001), the risk of
death was reduced by 96% (adjusted RR=0.04, 95% CI: 0.01-0.42,
P=0.007) and the probability of achieving an acceptable symptom
state on day 5 was 18-fold higher (adjusted RR=18.14, 95% CI:
7.29-45.09, P<0.001), compared to usual medical care alone. The
analysis also indicated the relationship between baseline
characteristics of patients (such as sex, age, relevant
comorbidities) and the probability of achieving an acceptable
symptom state, or being hospitalized, or dying from COVID-19. It
was observed that the presence of diabetes, advanced age or an
SpO2<94% were factors associated with an increased
risk of being hospitalized or dying from the disease (Table SII). With the experimental
treatment, the NNT to prevent hospitalization of a patient was 8.3
(95% CI: 4.7-32.6), one out of two patients treated with
electrolyzed saline achieving an acceptable symptom state on day 5
or earlier (NNT=2.4; 95% CI: 1.90-3.52).
When the patients were classified according to the
severity of their disease (Table
II), it was observed that for patients with mild disease,
treatment with electrolyzed saline significantly reduced the time
to reach an acceptable symptom state compared with usual medical
care alone (4.2±0.4 days vs. 7.2±6 days, P<0.001). Furthermore,
for patients with moderate/severe disease, electrolyzed saline
combined with usual medical care vs. usual medical care
alone achieved a large decrease in the proportion of hospitalized
patients (19% vs. 88%) and deaths (7.7% vs. 41%; Table II).
Progression of signs and symptoms
Table SI provides
an analysis of the symptoms with respect to their presence or
absence at the beginning of the study and throughout the follow-up.
The number of patients with those symptoms at baseline did not
differ between groups (except for sore throat and nausea, which
were higher in the control group). The number of patients with
fatigue, myalgia, fever, vomiting, conjunctivitis, dizziness,
anosmia and/or ageusia was significantly reduced in the
experimental group compared with the control group during the
follow-up time, but not at the baseline. This suggests that a
proportion of patients were spared of certain symptoms during their
illness due to the experimental treatment. With respect to patients
with one particular symptom, the last day from enrolment they
presented with fever (0.7±1.1 vs. 2.1±1.5, P<0.001), headache
(3.7±3.5 vs. 7.3±4.3, P<0.001), fatigue (6.0±3.8 vs. 8.6±4.3,
P<0.001), myalgia (4.4±3.5 vs. 6.0±3.4, P=0.001), retro-orbital
eye pain (2.1±2.9 vs. 3.9±3.6, P=0.001), chills (1.6±2.3 vs.
2.9±3.1, P=0.013), rhinorrhea (2.8±3.4 vs. 4.5±4.6, P<0.001),
nausea (1.8±2.5 vs. 4.4±4.5, P<0.001), vomiting (0.7±2.0 vs.
1.7±2.4, P=0.026), dizziness (1.6±2.2 vs. 3.8±4.6, P<0.001),
conjunctivitis (0.9±2.2 vs. 3.0±4.1, P<0.001), anosmia (5.7±3.2
vs. 8.9±4.8, P<0.001), ageusia (3.6±3.4 vs. 7.7±5.2, P<0.001)
or diarrhea (4.0±3.1 vs. 5.9±3.9, P=0.025) was significantly lower
in the experimental group vs. the control group (Table SI).
A quantitative analysis of the symptom severity
score (patient overall self-assessment) was performed and
score/values on various scales (10-point VAS) for fatigue,
headache, sore throat, retro-orbital eye pain, myalgia, body
temperature (degrees centigrade) and oxygen saturation
(SpO2) exhibited a significant improvement in the
experimental group at 24 h from the start of treatment (day 1) as
compared with the control group. There was also a decrease in cough
and heart rate on day 3 and arthralgia on day 9 (Table SIII).
Oral administration of electrolyzed saline to treat
gastrointestinal symptoms was indicated in 17 patients, while 25
were prescribed gargling to treat a sore throat. All of these
patients reported a reduction or disappearance of symptomatology
within 24-48 h of administration.
SARS-CoV2 detection during treatment
with electrolyzed saline
Serial virus detection in nasopharyngeal and
oropharyngeal samples at baseline and on days 2, 4, 6 and 9 was
performed in 10 patients. As presented in Table III, >50% of patients were
negative for the virus on day 4, with only a 20% of positive
patients on day 6 and 0% on day 9. In the majority of cases, the
test for the virus was negative on the days after having achieved a
PASS. Of note, patient P30 achieved a PASS on days 3-5, but
reported an unacceptable state on day 6 and a PASS on day 7 and
thereafter. This suggests that a PASS does not always accompany the
elimination of the virus (positive patients up to day 6) and that
there may be a relapse of symptoms. Patient P29 achieved a PASS on
day 2, was negative for the virus until day 6, when she was once
again positive. Patients P29 and P30 were a couple who were living
together, without implementing any physical distancing measures
during follow-up, signifying that the probable cause of positivity
on day 6 of P29 was due to transitory reinfection or contamination
derived from living with a patient still presenting with viremia
(P30).
 | Table IIISARS-CoV-2 detection over time in
nasopharyngeal samples of 10 patients in the experimental
group. |
Table III
SARS-CoV-2 detection over time in
nasopharyngeal samples of 10 patients in the experimental
group.
| | Baseline
severity | | SARS-CoV2 detection
result (days) |
|---|
| Patient
no.† | Dose level | Age (years) | Progression time
(days)a | Scoreb | WHOc | Number of
symptomsd | Days until
PASS | Baseline | 2 | 4 | 6 | 9 |
|---|
| P1-M | 1 | 45 | 3 | 3 | Mild | 5 | 4 | Pos. | Pos. | Neg. | Neg. | Neg. |
| P12-F | 2 | 48 | 1 | 8 | Severe | 7 | 3 | Pos. | Pos. | Neg. | Neg. | Neg. |
| P18-M | 3 | 46 | 3 | 9 | Mild | 8 | 5 | Pos. | Pos. | Pos. | Neg. | Neg. |
| P19-M | 3 | 18 | 2 | 2 | Mild | 3 | 2 | Pos. | Pos. | Neg. | Neg. | Neg. |
| P21-F | 4 | 29 | 3 | 5 | Mild | 7 | 3 | Pos. | Neg. | Neg. | Neg. | Neg. |
| P22-M | 4 | 34 | 6 | 10 | Moderate | 9 | 3 | Pos. | Pos. | Pos. | Neg. | Neg. |
| P29-Fe | 4 | 40 | 1 | 6 | Mild | 8 | 2 | Pos. | Neg. | Neg. | Pos. | Neg. |
| P30-M | 5 | 43 | 1 | 8 | Mild | 9 | 7 | Pos. | Pos. | Pos. | Pos. | Neg. |
| P39-M | 4 | 41 | 4 | 6 | Mild | 6 | 1 | Pos. | Neg. | Neg. | Neg. | Neg. |
| P40-F | 5 | 65 | 6 | 6 | Mild | 7 | 2 | Pos. | Pos. | Pos. | Neg. | Neg. |
| Percentage of
positivity (%) | | | | | | | | 100 | 70 | 40 | 20 | 0 |
Inflammatory and immune response
markers
The erythrocyte sedimentation rate was a parameter
that remained elevated during the entire follow-up (Table SIV), with no significant
differences between the baseline value and the 14 days of follow-up
included. This was due to the fact that the maximum value reached
by each patient exhibited marked variations over the days of
follow-up. There was a significant decrease in C-reactive protein
(CRP) 48 h after starting the treatment, with average reductions of
43 and 73% at 48 h and 4 days after the beginning of treatment,
respectively (Table SIV).
Considering the baseline CRP values and symptom severity score
(possible score of 0-10 resembling very well to very poor) as 100%
and the relative value on the subsequent days of evaluation, there
was a significant correlation between CRP and the clinical
progression of the patients (r=0.301, P<0.001). A greater
decrease in CRP was associated with a greater reduction in the
patient symptom severity score (reduced severity) (results not
shown).
In relation to the baseline level of hematopoietic
cells, there was a significant increase (within normal values) of
total leukocytes on days 6, 9 and 14. The quantity of total
lymphocytes gradually increased on days 2 and 4, until reaching
significantly elevated levels on day 6 (Table SIV). The reactive lymphocytes
exhibited a significant elevation on day 2 of follow-up, reducing
and losing its statistical significance with respect to the
baseline value on subsequent days. The quantity of large granular
lymphocytes (a representation of NK cells) began to rise gradually,
with a mean of 65±33x103/µl at baseline, until they were
significantly elevated on day 6, with 155±78x103/µl
(P=0.006), after which they began to decrease again. The quantity
of total monocytes exhibited a tendency to gradually decrease, with
no significant differences. However, the aberrant monocytes (larger
cells, with clumped chromatin and basophilic cytoplasm) decreased
significantly, with a mean of 450±357x103/µl at
baseline, to 229±232x103/µl after 48 h (P=0.003). That
decrease was sustained during the entire follow-up. The activated
monocytes exhibited no significant changes with respect to baseline
values during the follow-up. Another change was an increase in
platelets, which, although they remained within normal ranges, they
rose consistently throughout the follow-up, having significantly
high values on days 6-14 (Table
SIV).
The quantity of total monocytes correlated with the
CRP levels (r=0.466, P<0.001). Of note, the quantity of aberrant
monocytes correlated with the patients' overall self-assessment
score (symptom severity score; r=0.478, P=0.001), signifying that
the more the aberrant monocytes decreased, the better the patient
felt (results not shown). The gradual and significant increase of
platelets after treatment correlated with several beneficial
aspects, such as increased lymphocytes and clinical improvement of
the patients, given that the quantity of platelets correlated with
the total lymphocytes (r=0.341, P=0.004) and with the patients'
overall self-assessment score (r=-0.398, P=0.001) (results not
shown).
Testosterone and cortisol levels
The concentration of cortisol significantly
decreased on day 2. On the other hand, the testosterone
concentration increased, although there was no statistical
significance. A significant increase in the testosterone-cortisol
ratio was present on days 2 and 4 (Table SIV). The gradual and significant
decrease in cortisol after treatment correlated with the decrease
in CRP values (r=0.202, P=0.033), and with the increase in
lymphocytes (r=-0.319, P=0.001), monocytes (r=-0.251, P=0.005) and
platelets (r=-0.172, P=0.046), whereas the increase in the
testosterone-cortisol ratio correlated with the decrease in
activated monocytes (r=-0.272, P=0.019) (results not shown).
Adverse events and toxicity
A total of two patients did not tolerate the
nebulization due to a burning sensation in the throat and stopped
using it on the second day but continued with IV applications. In
addition, four patients reported transitory dizziness lasting for
10 min after the IV application of the experimental solution; this
was self-limited and managed by lying down. Furthermore, five
patients reported mild pain in the first 5 cm of the vein path
where the solution was applied after the entire treatment scheme.
This mild pain was self-limited and not accompanied by any other
signs or symptoms; it disappeared within 1 to 2 days after the end
of the treatment. No other adverse events were reported. There were
no abnormal or unexpected alterations due to COVID-19 in the serum
levels of liver enzymes (ALT, AST, lactate dehydrogenase and ALP),
bilirubin, albumin, glucose, creatinine, uric acid, urea or
complete blood count (Table
SIV).
Discussion
In ambulatory patients with COVID-19 receiving the
usual medical care, additional administration of electrolyzed
saline reduced the probability of disease progression
(hospitalization and death) by 89%, compared with ambulatory
patients treated with usual medical care alone. Different signs and
symptoms, such as fatigue, headache, sore throat, retro-orbital eye
pain, myalgia, body temperature and oxygen saturation, improved
significantly after the first 24 h of experimental therapy.
By adding neutral electrolyzed saline to the usual
medical care, it was possible to significantly reduce the time to
reach an acceptable state of symptoms in all patients, particularly
in those with mild and severe disease. The greatest benefit of the
treatment is observed in patients with moderate/severe disease,
where a major change was observed in the proportion of patients who
were hospitalized (19% vs. 88%) or died (7.7% vs. 41%), compared
with the patients under usual medical care alone. The treatment was
more effective when high doses (≥30 ml) of IV electrolyzed saline
were administered. All dose levels of electrolyzed saline were
significantly better in reducing the severity of symptoms than the
usual medical care alone and the higher dose levels (dose level 7 +
8) were significantly better than just the nebulizations (dose
level 1). The beneficial effects of the administration of
electrolyzed saline may generally be associated with the mechanisms
related to the following: i) Reduction of inflammatory processes;
and ii) elimination of the virus by the immune system and by direct
contact with the electrolyzed saline. The proposed mechanism of
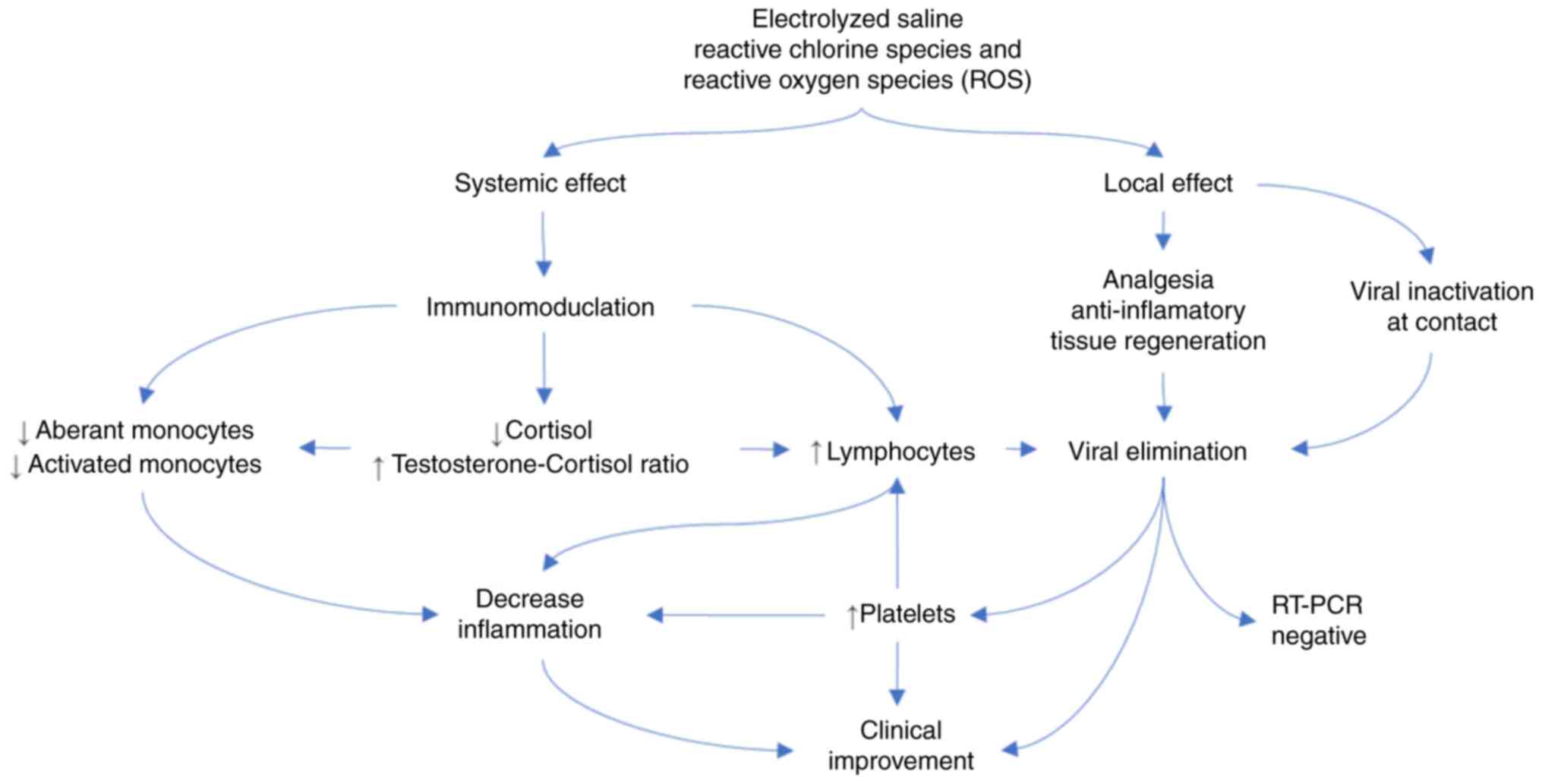
action is illustrated in Fig.
3.
The improvement of signs and symptoms correlated
with a significant reduction of systemic inflammation, with a
>40% decrease of CRP levels at 48 h after starting treatment.
There was also a correlation between CRP levels and the quantity of
monocytes. Said reduction, particularly of aberrant monocytes, was
significant at 48 h and lasted to the end of follow-up,
strengthening the hypothesis of the modulating effect of the
systemic administration of electrolyzed saline on inflammation,
reflected in the clinical improvement of the patients. In the early
stage of COVID-19, CRP levels have previously been indicated to
reflect the extent of lung lesions and disease severity, providing
an important clinical evaluation index (69). Monocytes and pulmonary monocytes
have a key early role in the progression to severe COVID-19 by
promoting a cytokine storm, ARDS and disseminated peripheral tissue
damage (14). The aberrant
monocytes that decreased after the experimental treatment were
larger than normal monocytes, with clumped chromatin and basophilic
cytoplasm (62). Morphologically
altered monocytes, particularly larger ones, are associated with a
hyperinflammatory gene expression profile and with admission to
intensive care units in patients with type 2 diabetes with
COVID-19(70). By contrast, with
the reduction in the quantity and relative percentage of aberrant
monocytes seen after the experimental treatment, the number of
normal monocytes increased. Patients with a high number of normal
monocytes have a better outcome, with earlier recovery and
discharge from hospital (71). This
result has been postulated to be relatively specific for COVID-19,
as a similar pattern in patients with other viral illnesses, such
as H1N1, influenza, HIV or hantavirus, has not been observed
(71).
In relation to improved immune function, through
the administration of electrolyzed saline, a gradual increase in
total lymphocytes and large granular lymphocytes (a representation
of NK cells) was observed, reaching a significantly elevated level
on day 6. Lymphocytes have a crucial role in virus clearance after
a viral infection. On the one hand, NK cells eliminate virally
infected cells via degranulation, receptor-mediated apoptosis and
antibody-dependent cell-mediated cytotoxicity (72). On the other hand, the humoral immune
response, primarily mediated by the production of antibodies by
plasma B cells (B lymphocyte-derived cells), has a role in the
neutralization of the virus (73).
In line with the results of the present study, the lymphocyte count
and the number of NK cells have been postulated to correlate with
disease severity and may serve as a tool for identifying patients
with a more severe clinical presentation of SARS and COVID-19
(61,69,74). A
lymphocyte count of <1.5x109/l may be useful in
predicting the severity of clinical outcomes (75). Even though T lymphocytes were not
specifically identified in the present study, the large granular
lymphocytes observed are a type of T lymphocyte (14,61,62).
Previous studies have indicated that the time of recovery of the
T-lymphocyte count was fairly consistent with the clinical course
(73). Patients with severe
disease, but who recovered, the value of T lymphocytes was reported
to begin to increase after 15 days of treatment, finally returning
to normal levels after 25 days of treatment (73). By contrast, the level of T
lymphocytes in severely ill patients and that finally deceased,
continued to fall until they succumbed to the disease (73). That behavior concurred with the
variation in the number of the large granular lymphocytes observed
in the present study, in which that special type of lymphocyte
increased on day 6 of treatment, in accordance with the clinical
improvement of the majority of patients, and began to decrease in
quantity on day 9. The speed with which the process of elevation
and reduction in those cells took place should be considered.
Another relevant aspect was the constant and
significant increase in platelets after treatment with electrolyzed
saline. Yang et al (76)
recently demonstrated an association between reduced platelets and
mortality in patients with COVID-19. Yang et al (76) correctly interpreted those results as
follows: i) A ‘higher’ platelet count for an illness as severe as
COVID-19 is unusual and likely points towards liver activation and
thrombopoietin release; ii) the lung-specific entry of SARS-CoV-2
suggests that the lung megakaryocytes, in response to liver
thrombopoietin, locally produce a large number of platelets to help
with the defense of the host; iii) the reduction of platelets in
patients with severe disease may be due to the fact that the
platelets are being consumed to form pulmonary thrombi, which
occurs when multiple efforts (including those of the platelets) to
stop the infection have not succeeded and blocking the viral
invasion has become necessary; and iv) Yang et al (76) also indicated that mortality
decreased with the increase of the platelet count, suggesting the
thrombotic process has abated and platelets are no longer consumed
into the clot. In addition, platelets also have an
anti-inflammatory function by regulating macrophage activity,
regulatory T cells and secreting pro-resolving mediators (77). All of those observations concur with
the results of the present study, according to which the increase
in platelets correlated with an increase in total lymphocytes and
clinical improvement in the patients (a lower patient overall
self-assessment score).
Cortisol and testosterone are hormones related to
immune system regulation (78). The
increase in testosterone detected in the present study (although
not statistically significant) is in agreement with the result of a
recent study reporting that low testosterone levels are associated
with immune system deficiencies and greater severity of
COVID-19(79). Likewise, low levels
of cortisol, as detected in the present study, correlated with
increased lymphocytes, which may contribute to a better antiviral
response by the body. It has recently been indicated that high
cortisol levels are associated with a greater risk of death of
patients with COVID-19(80).
Similarly, the present study reported an increase in the
testosterone-cortisol ratio on days 2 and 4 after the beginning of
treatment. This is a parameter not previously studied in patients
with COVID-19, to the best of our knowledge. This increase was
correlated with a reduction in activated monocytes, which may help
reduce the systemic inflammatory process. Monocyte activation was
abnormal and contributes to the COVID-19 cytokine storm by
releasing massive amounts of pro-inflammatory cytokines (14,81).
The influence of testosterone and cortisol on
monocytes has been previously reported. In patients with diabetes
with hypogonadism, testosterone therapy reduced inflammatory
activation of monocytes (82). It
has also been indicated that cortisol signaling through the
mineralocorticoid receptor, under oxidative stress, may promote
monocyte inflammatory activation (83,84);
thus, a reduction in cortisol would also be favoring the reduction
of activated monocytes, particularly in the context of rising
testosterone levels. Furthermore, based on the assumption that free
testosterone is a marker of anabolism, while cortisol is indicative
of catabolism, it has been suggested that an increase in the
testosterone-cortisol ratio is favorable for protein anabolism
(60,85), which may be beneficial in patients
with COVID-19.
Electrolyzed saline, also known as electrolyzed
water, has important antiseptic properties (86) and may be used directly on
contaminated tissues or fluids (87,88).
Thus, in addition to the immunomodulatory effect produced when
administered systemically, it may inactivate the new coronavirus by
degradation of the envelope and nucleocapsid proteins (89,90),
when administered locally, without dilution to the lungs and
throat, via nebulization and/or gargling, as it has been previously
demonstrated for multiple viruses (87-90).
However, the present study was the first to reveal the remarkable
immunomodulating effect of electrolyzed saline administered
systemically at the proper concentration of active species of
chlorine and oxygen, acting to control and limit COVID-19 disease.
Of note, all of the results of the present study concur with the
proposed mechanism of rapid elimination of the virus from the
respiratory tract, occurring within days, with negative virus test
results in 60 and 80% of the patients on days 4 and 6,
respectively.
Local administration of electrolyzed saline to the
throat to control pain or its oral intake to control the
gastrointestinal symptoms of nausea, vomiting or diarrhea, were
successful in reducing or eliminating said symptomatology within 24
to 48 h, which is in accordance with previous preclinical studies
(39,91). In fact, the company supplying the
product utilized in the present study (Esteripharma S.A. de C.V.)
offers products for intranasal (EsteriFlu®) and
buccopharyngeal (Estericide® Bucofaríngeo) applications,
as antiseptics that inactivate viruses and eliminate bacteria.
However, it is likely that electrolyzed saline, besides having a
direct effect on the SARS-CoV-2 virus in the throat, also has an
analgesic and regenerative effect on the epithelium at the local
level (91). The oral route for
electrolyzed saline has already been demonstrated to have no
adverse effects in preclinical trials (40). Utilized in pigs to treat porcine
epidemic diarrhea virus infection, the symptom duration in infected
pigs was markedly shortened and symptom severity was also reduced,
producing a much higher survival rate (39). The oral route for aqueous
H2, a component of electrolyzed saline, has potent local
and systemic anti-inflammatory effects, along with regulating
effects on the immune system (30),
which may be involved in the mechanism for improving
gastrointestinal symptoms.
The administration of electrolyzed saline has been
indicated to have positive regulatory effects on the immune system
in patients with COVID-19, given that its composition is similar to
that of the reactive chlorine species and ROS produced by the
immune system in mammals, which have been described as mediators
and modulators of different physiological processes. Macrophages
and neutrophils release ROS to structurally damage invasive
pathogens, thus protecting the host against infection (19). In addition, ROS have emerged as a
critical second messenger for immune system regulation and the
control of exacerbated inflammation or tissue repair via processes
of redox signaling (19-23).
Evidence of a direct impact of ROS on the life
cycles of viruses is scarce and controversial. Numerous lines of
evidence suggest that marked signs of increased production of ROS
accompany all respiratory viral infections, which are associated
with potentially pathologic processes including cytokine
production, inflammation and cell death (92). However, none of the published data
are based on direct measurement of ROS levels, but rather on their
indirect determination (e.g. quantification of oxidated
metabolites, which although is an accepted technique to evaluate
ROS concentration, it continues to be an indirect determination)
(92). In accordance with the
results of the present study, the view that ROS contribute to the
suppression of certain respiratory infections through the induction
of innate immune responses, including T-cell receptor signaling and
T-cell activation, is posited (92).
Examples of mechanisms that support the
administration of ROS as beneficial in the fight against viral
infections are as follows: i) Influenza virus enhances interferon
λ1 (IL29) and λ2/3 (IL28A/IL28B) production via ROS (93). ROS scavenging or suppression of ROS
production leads to the inhibition of IFNλ synthesis and secretion,
and in turn, the enhancement of viral replication (92); ii) signal transducers and activators
of transcription (STAT) activation has been indicated to be a
relevant event in the response against different viruses (94). ROS formation is involved in STAT
activation and the subsequent interferon regulatory factor 1
(IRF-1) and IRF-7 gene expression (95). IRF-1 has been indicated to have a
role in shaping innate and adaptive antiviral immunity by inducing
the expression of IFN-stimulated genes and mediating signals
downstream of IFN-γ (95),
contributing to the clinical improvement of patients with viral
infection (96).
Antioxidant therapies are also known to ameliorate
and improve disease outcomes (92).
Since electrolyzed saline also contains small amounts of molecular
H2, additional antiviral and anti-inflammatory effects,
associated to antioxidant mechanisms, may be expected (97). Treatment with molecular antioxidants
reduces intracellular levels of influenza virus polymerase,
providing a possible mechanism of viral titer reduction in response
to antioxidant treatment (98).
Additionally, it has been demonstrated that small antioxidant
molecules, specifically molecular hydrogen, produce
anti-inflammatory effects over multiple COVID-19 target organs,
such as the lung, kidney, liver and brain, when compromised by
acute and/or chronic diseases (97,99-102).
For instance, a study suggested that intraperitoneal administration
of hydrogen-rich saline to rats with ischemia/reperfusion-induced
acute kidney injury, prevented fibrosis damage and improved renal
function (99). The use of a
hydrogen-enriched solution during hemodialysis therapies in
patients diminished pro-inflammatory markers and prevented
complications related to oxidative stress (100). For the case of the benefits
observed in damaged lung, a study performed in mice with chronic
lung injury induced by hypoxia/re-oxygenation, demonstrated that
inhalation of molecular hydrogen attenuated preexistent lung
injuries (101). When 20 patients
with asthma or Chronic Obstructive Pulmonary Disease inhaled a 2.4%
hydrogen-containing steam mixed gas (for 45 min), attenuation of
their inflammatory airway status (decrease of selected
pro-inflammatory biomarkers) was observed (102).
In the present study, it was demonstrated that the
neutral electrolyzed saline administered is an effective
alternative therapy to improve the health and/or prognosis of
patients with COVID-19. The different mechanisms of action were
also discussed. However, more specific studies regarding each
possible mode of action may be performed in order to clearly
understand how the electrolyzed saline helps control COVID-19.
The present study had several limitations. First of
all, the study was not placebo-controlled and the patients were not
blinded. Blood samples were not collected from the control group,
preventing the comparison between groups in terms of the
progression of the different hematologic and biochemical
parameters. There was a correlation between the clinical evaluation
and the different laboratory parameters in the experimental group,
leading to the supposition that the less favorable clinical
conditions in the control group may also be accompanied by equally
unfavorable laboratory parameters, but this was not confirmed. In
addition, a higher number of inflammation and coagulation markers
should be included in future studies, as well as molecular
phenotyping of the blood cell strains. Studies with a larger number
of patients, both hospitalized and ambulatory, receiving the most
effective dose determined in the present study, are also required
to confirm the present results.
In conclusion, IV or nebulized administration of
electrolyzed saline markedly reduced the symptomatology and risk of
disease progression in ambulatory patients with COVID-19. Its
administration was well-tolerated and there were no important
adverse effects. The treatment effect was mediated by the reduction
of inflammation and the apparently increased antiviral immune
response, induced by the active species of oxygen and chlorine from
the electrolyzed saline that appeared to mimic the effect of
physiologic ROS. Further studies are required to confirm those
results.
Supplementary Material
CONSORT 2010 flow diagram displaying
the number of patients screened, included, eliminated and analyzed.
IV, intravenous.
Proportion of patients presenting with
the major signs and symptoms of Coronavirus disease 2019 (%).
Binary logistic regression analysis to
evaluate the relationship between multiple predictor variables and
achieving an acceptable symptom state, or for becoming hospitalized
or die from COVID-19.
Progression of signs and symptoms over
time in the control and experimental groups of patients.
Laboratory parameters of the patients
in the experimental group during follow-up: An analysis comparing
baseline values vs. days 2, 4, 6, 9 and 14 was performed.
Acknowledgements
The authors wish to thank Dr Carlos Salazar Silva
(Department of Molecular Medicine, School of Medicine, University
of Colima, Colima, Mexico) for performing administrative activities
necessary for the project and Gusti Gould (freelance translator
Spanish to English, Guadalajara, Mexico) for the English language
editing of the manuscript.
Funding
Funding: The methodology for serial SARS-CoV-2 detection was
funded by the Consejo Estatal de Ciencia y Tecnología del Estado de
Colima (grant no. 1, Convocatoria Desafío COVID-19). Esteripharma
S.A. de C.V. provided support in the form of salaries for authors
BPM and ACL. The sponsors had no role in the study design, data
collection and analysis or the decision to publish the
manuscript.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IDE, JGE, VM and JPG designed the study and wrote
the manuscript. IDE, BAPM and JPG conceived the novel application
of electrolyzed saline. CEBS, KAMR, CMR, RLF, MDM, EMZ, JATV, OGDE,
JDE, MWG, HRGS, PMD, VM, PJMD, HPM, JMJV and LGG visited the
ambulatory patients and administered their medication. CEBS, KAMR,
DAMG, CMR, JATV, AEHR, IPRS, DTJ, IGV and VOR performed the
biochemical and molecular analyses. VM, FEG, FRL, MJHM, JGE, LMBR,
SAZF, PJMD, HPM, JMJV, FGA, LDL, HPGS, MAMH and EPDC performed the
clinical evaluations of the patients. EBN, MLMF, MRF, GGS, CRMP and
IDE designed and performed the statistical analysis. BAPM and ACL
coordinated the production and quality control processes of the
experimental therapeutic product. EJDM coordinated and authorized
the recruitment of patients at the INSABI Poliforum hospital. JDE
was the administrative coordinator of the clinical trial. JDE and
JGE checked and approved the authenticity of the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study (registered 2020-01-05) was approved by
the ethics committee of the School of Medicine of the Universidad
de Colima (Colima, Mexico) and written informed consent was
obtained from all participants. All procedures performed in the
present protocol were in accordance with the Declaration of
Helsinki and the clinical trial was registered as TX-COVID19:
RPCEC00000309 in the RPCEC database (05/05/2020).
Patient consent for publication
Not applicable.
Competing interests
BAPM and ACL declare that they work for the company
Esteripharma, who provided the neutral electrolyzed saline
administered in this trial. The company owns a patent for the
synthesis of the electrolyzed saline, but had no role in the study
design, data collection and analysis or decision to publish the
manuscript. Those authors did not participate in the study design,
data collection or data analyses. The other authors declare that
they have no competing interests.
References
|
1
|
Cascella M, Rajnik M, Cuomo A, Dulebohn SC
and Di Napoli R: Features, evaluation and treatment coronavirus
(COVID-19). In: StatPearls. Treasure Island (FL), 2020.
|
|
2
|
Caldera-Villalobos C, Garza-Veloz I,
Martínez-Avila N, Delgado-Enciso I, Ortiz-Castro Y, Cabral-Pacheco
GA and Martinez-Fierro ML: The coronavirus disease (COVID-19)
challenge in Mexico: A critical and forced reflection as
individuals and society. Front Public Health. 8(337)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
O'Keefe JB, Tong EJ, Datoo O'Keefe GA and
Tong DC: Predictors of disease duration and symptom course of
outpatients with acute covid-19: A retrospective cohort study.
medRxiv: 2020.06.05.20123471, 2020.
|
|
4
|
Tenforde MW, Kim SS, Lindsell CJ, Billig
Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS,
Smithline HA, et al: Symptom duration and risk factors for delayed
return to usual health among outpatients with COVID-19 in a
multistate health care systems network-united states, March-June
2020. MMWR Morb Mortal Wkly Rep. 69:993–998. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin
Y, Fu S, Gao L, Cheng Z, Lu Q, et al: Remdesivir in adults with
severe COVID-19: A randomised, double-blind, placebo-controlled,
multicentre trial. Lancet. 395:1569–1578. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Baqui P, Bica I, Marra V, Ercole A and van
der Schaar M: Ethnic and regional variations in hospital mortality
from COVID-19 in Brazil: A cross-sectional observational study.
Lancet Glob Heal. 8:e1018–e1026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liang LL, Tseng CH, Ho HJ and Wu CY:
Covid-19 mortality is negatively associated with test number and
government effectiveness. Sci Rep. 10(12567)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Berumen J, Schmulson M, Alegre-Díaz J,
Guerrero G, Larriva-Sahd J, Olaiz G, Wong-Chew RM, Cantú-Brito C,
Ochoa-Guzmán A, Garcilazo-Ávila A, et al: Risk of infection and
hospitalization by Covid-19 in Mexico: A case-control study.
medRxiv: 2020.05.24.20104414, 2020.
|
|
9
|
Rakedzon S, Khoury Y, Rozenberg G and
Neuberger A: Hydroxychloroquine and coronavirus disease 2019: A
systematic review of a scientific failure. Rambam Maimonides Med J.
11(e0025)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bchetnia M, Girard C, Duchaine C and
Laprise C: The outbreak of the novel severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2): A review of the current global
status. J Infect Public Health. 13:1601–1610. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mangalmurti N and Hunter CA: Cytokine
storms: Understanding COVID-19. Immunity. 53:19–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ucciferri C, Vecchiet J and Falasca K:
Role of monoclonal antibody drugs in the treatment of COVID-19.
World J Clin Cases. 8:4280–4285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ortiz-Prado E, Simbaña-Rivera K,
Gómez-Barreno L, Rubio-Neira M, Guaman LP, Kyriakidis NC, Muslin C,
Jaramillo AMG, Barba-Ostria C, Cevallos-Robalino D, et al:
Clinical, molecular, and epidemiological characterization of the
SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a
comprehensive literature review. Diagn Microbiol Infect Dis.
98(115094)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pence BD: Severe COVID-19 and aging: Are
monocytes the key? Geroscience. 42:1051–1061. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang W, Berube J, McNamara M, Saksena S,
Hartman M, Arshad T, Bornheimer SJ and O'Gorman M: Lymphocyte
subset counts in COVID-19 patients: A meta-analysis. Cytometry A.
97:772–776. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Henry BM, de Oliveira MHS, Benoit S,
Plebani M and Lippi G: Hematologic, biochemical and immune
biomarker abnormalities associated with severe illness and
mortality in coronavirus disease 2019 (COVID-19): A meta-analysis.
Clin Chem Lab Med. 58:1021–1028. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kono N: Superoxidized electrolyzed
solution of neutral pH and uses there of., 2015.
|
|
18
|
Li R, Jia Z and Trush MA: Defining ROS in
biology and medicine. React Oxyg Species (Apex). 1:9–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Forrester SJ, Kikuchi DS, Hernandes MS, Xu
Q and Griendling KK: Reactive oxygen species in metabolic and
inflammatory signaling. Circ Res. 122:877–902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan HY, Wang N, Li S, Hong M, Wang X and
Feng Y: The reactive oxygen species in macrophage polarization:
Reflecting its dual role in progression and treatment of human
diseases. Oxid Med Cell Longev. 2016(2795090)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
George-Chandy A, Nordström I, Nygren E,
Jonsson IM, Postigo J, Collins LV and Eriksson K: Th17 development
and autoimmune arthritis in the absence of reactive oxygen species.
Eur J Immunol. 38:1118–1126. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kuhns DB, Alvord WG, Heller T, Feld JJ,
Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, et
al: Residual NADPH oxidase and survival in chronic granulomatous
disease. N Engl J Med. 363:2600–2610. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Calabrese EJ, Giordano JJ, Kozumbo WJ,
Leak RK and Bhatia TN: Hormesis mediates dose-sensitive shifts in
macrophage activation patterns. Pharmacol Res. 137:236–249.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mills EL and O'Neill LA: Reprogramming
mitochondrial metabolism in macrophages as an anti-inflammatory
signal. Eur J Immunol. 46:13–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Prokopowicz ZM, Arce F, Biedroń R, Chiang
CL, Ciszek M, Katz DR, Nowakowska M, Zapotoczny S, Marcinkiewicz J
and Chain BM: Hypochlorous acid: A natural adjuvant that
facilitates antigen processing, cross-priming, and the induction of
adaptive immunity. J Immunol. 184:824–835. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reth M: Hydrogen peroxide as second
messenger in lymphocyte activation. Nat Immunol. 3:1129–1134.
2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Z, Xu X, Leng X, He M, Wang J, Cheng S
and Wu H: Roles of reactive oxygen species in cell signaling
pathways and immune responses to viral infections. Arch Virol.
162:603–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ge L, Yang M, Yang NN, Yin XX and Song WG:
Molecular hydrogen: A preventive and therapeutic medical gas for
various diseases. Oncotarget. 8:102653–102673. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin CP, Chuang WC, Lu FJ and Chen CY:
Anti-oxidant and anti-inflammatory effects of hydrogen-rich water
alleviate ethanol-induced fatty liver in mice. World J
Gastroenterol. 23:4920–4934. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moher D, Hopewell S, Schulz KF, Montori V,
Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M and Altman DG:
CONSORT. CONSORT 2010 explanation and elaboration: Updated
guidelines for reporting parallel group randomised trials. Int J
Surg. 10:28–55. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
33
|
Gouveia BG, Rijo P, Gonçalo TS and Reis
CP: Good manufacturing practices for medicinal products for human
use. J Pharm Bioallied Sci. 7:87–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rogatko A, Cook-Wiens G, Tighiouart M and
Piantadosi S: Escalation with overdose control is more efficient
and safer than accelerated titration for dose finding. Entropy
(Basel). 17:5288–5303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
American College of Allergy A and I: No
Title. A Messag to Asthma Suff About a Short Albuterol Metered Dose
Inhalers: 1, 2020.
|
|
36
|
British Lung Foundation: No Title. What is
Soc Shield who needs to do this? 1, 2020.
|
|
37
|
Asthma Society of Ireland: No Title.
Coronavirus (COVID-19) Advice: 1, 2020.
|
|
38
|
British thoracic society: No Title.
COVID-19 Inf Respir community: 1, 2020.
|
|
39
|
Watson RD: Oral administration of
electrolyzed water for treatment and prevention of PEDv in swine,
swine herds and swine confinements. US Patent 20140287065 A1. Filed
March 14, 2013; issued February 24, 2014.
|
|
40
|
Morita C, Nishida T and Ito K: Biological
toxicity of acid electrolyzed functional water: Effect of oral
administration on mouse digestive tract and changes in body weight.
Arch Oral Biol. 56:359–366. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cohen DR, Todd S, Gregory WM and Brown JM:
Adding a treatment arm to an ongoing clinical trial: A review of
methodology and practice. Trials. 16(179)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yorganci E, Evans CJ, Johnson H, Barclay
S, Murtagh FE, Yi D, Gao W, Pickles A and Koffman J: Understanding
usual care in randomised controlled trials of complex
interventions: A multi-method approach. Palliat Med. 34:667–679.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dawson L, Zarin DA, Emanuel EJ, Friedman
LM, Chaudhari B and Goodman SN: Considering usual medical care in
clinical trial design. PLoS Med. 6(e1000111)2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hinks TSC, Barber VS, Black J, Dutton SJ,
Jabeen M, Melhorn J, Rahman NM, Richards D, Lasserson D, Pavord ID
and Bafadhel M: A multi-centre open-label two-arm randomised
superiority clinical trial of azithromycin versus usual care in
ambulatory COVID-19: Study protocol for the ATOMIC2 trial. Trials.
21(718)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sterne JAC, Diaz J, Villar J, Murthy S,
Slutsky AS, Perner A, Jüni P, Angus DC, Annane D, Azevedo LCP, et
al: Corticosteroid therapy for critically ill patients with
COVID-19: A structured summary of a study protocol for a
prospective meta-analysis of randomized trials. Trials.
21(734)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kvien TK, Heiberg T and Hagen KB: Minimal
clinically important improvement/difference (MCII/MCID) and patient
acceptable symptom state (PASS): What do these concepts mean? Ann
Rheum Dis. 66 (Suppl 3):iii40–iii41. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fei JZ, Perruccio AV, Ye JY, Gladman DD
and Chandran V: The relationship between patient acceptable symptom
state and disease activity in patients with psoriatic arthritis.
Rheumatology (Oxford). 59:69–76. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Delgado-Enciso I, Valtierra-Alvarez J,
Paz-Garcia J, Preciado-Ramirez J, Soriano-Hernandez AD,
Mendoza-Hernandez MA, Guzman-Esquivel J, Cabrera-Licona A,
Delgado-Enciso J, Cortes-Bazan JL, et al: Patient-reported health
outcomes for severe knee osteoarthritis after conservative
treatment with an intra-articular cell-free formulation for
articular cartilage regeneration combined with usual medical care
vs. usual medical care alone: A randomized co. Exp Ther Med.
17:3351–3360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Coates LC, Tillett W, Shaddick G, Pincus
T, Kavanaugh A and Helliwell PS: Value of the routine assessment of
patient index data 3 in patients with psoriatic arthritis: Results
from a tight-control clinical trial and an observational cohort.
Arthritis Care Res (Hoboken). 70:1198–1205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Delgado-Enciso I, Paz-Garcia J,
Valtierra-Alvarez J, Preciado-Ramirez J, Almeida-Trinidad R,
Guzman-Esquivel J, Mendoza-Hernandez MA, Garcia-Vega A,
Soriano-Hernandez AD, Cortes-Bazan JL, et al: A phase I-II
controlled randomized trial using a promising novel cell-free
formulation for articular cartilage regeneration as treatment of
severe osteoarthritis of the knee. Eur J Med Res.
23(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Delgado-Enciso I, Paz-Michel B, Melnikov
V, Guzman-Esquivel J, Espinoza-Gomez F, Soriano-Hernandez AD,
Rodriguez-Sanchez IP, Martinez-Fierro ML, Ceja-Espiritu G,
Olmedo-Buenrostro BA, et al: Smoking and female sex as key risk
factors associated with severe arthralgia in acute and chronic
phases of chikungunya virus infection. Exp Ther Med. 15:2634–2642.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Skipper CP, Pastick KA, Engen NW,
Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen
MF, Nicol MR, et al: Hydroxychloroquine in nonhospitalized adults
with early COVID-19: A randomized trial. Ann Intern Med.
173:623–631. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Salepci E, Turk B, Ozcan SN, Bektas ME,
Aybal A, Dokmetas I and Turgut S: Symptomatology of COVID-19 from
the otorhinolaryngology perspective: A survey of 223 SARS-CoV-2
RNA-positive patients. Eur Arch Otorhinolaryngol. 278:525–535.
2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rivett L, Sridhar S, Sparkes D, Routledge
M, Jones NK, Forrest S, Young J, Pereira-Dias J, Hamilton WL,
Ferris M, et al: Screening of healthcare workers for SARS-CoV-2
highlights the role of asymptomatic carriage in COVID-19
transmission. Elife. 9(e58728)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Englbrecht M, Tarner IH, van der Heijde
DM, Manger B, Bombardier C and Müller-Ladner U: Measuring pain and
efficacy of pain treatment in inflammatory arthritis: A systematic
literature review. J Rheumatol. (Suppl 90):3–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tubach F, Ravaud P, Baron G, Falissard B,
Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der
Heijde D and Dougados M: Evaluation of clinically relevant changes
in patient reported outcomes in knee and hip osteoarthritis: The
minimal clinically important improvement. Ann Rheum Dis. 64:29–33.
2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Khanna D, Pope JE, Khanna PP, Maloney M,
Samedi N, Norrie D, Ouimet G and Hays RD: The minimally important
difference for the fatigue visual analog scale in patients with
rheumatoid arthritis followed in an academic clinical practice. J
Rheumatol. 35:2339–2343. 2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Johnson MJ, Close L, Gillon SC,
Molassiotis A, Lee PH and Farquhar MC: Breathlessness Research
Interest Group (BRIG). Use of the modified borg scale and numerical
rating scale to measure chronic breathlessness: A pooled data
analysis. Eur Respir J. 47:1861–1864. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Meza-Robles C, Barajas-Saucedo CE,
Tiburcio-Jimenez D, Mokay-Ramírez KA, Melnikov V, Rodriguez-Sanchez
IP, Martinez-Fierro ML, Garza-Veloz I, Zaizar-Fregoso SA,
Guzman-Esquivel J, et al: One-step nested RT-PCR for COVID-19
detection: A flexible, locally developed test for SARS-CoV2 nucleic
acid detection. J Infect Dev Ctries. 14:679–684. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ferrari D, Lombardi G, Strollo M, Pontillo
M, Motta A and Locatelli M: A possible antioxidant role for vitamin
D in soccer players: A retrospective analysis of psychophysical
stress markers in a professional team. Int J Environ Res Public
Health. 17(3484)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Singh A, Sood N, Narang V and Goyal A:
Morphology of COVID-19-affected cells in peripheral blood film. BMJ
Case Rep. 13(e236117)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lüke F, Orsó E, Kirsten J, Poeck H, Grube
M, Wolff D, Burkhardt R, Lunz D, Lubnow M, Schmidt B, et al:
Coronavirus disease 2019 induces multi-lineage, morphologic changes
in peripheral blood cells. EJHaem, Jun 29, 2020 (Online ahead of
print).
|
|
63
|
El Comentario U de C: No Title. Graves,
200 pacientes Hosp con Covid-19; hay 21 intubados.
|
|
64
|
Vickers AJ: The use of percentage change
from baseline as an outcome in a controlled trial is statistically
inefficient: A simulation study. BMC Med Res Methodol.
1(6)2001.PubMed/NCBI View Article : Google Scholar
|
|
65
|
McNutt LA, Wu C, Xue X and Hafner JP:
Estimating the relative risk in cohort studies and clinical trials
of common outcomes. Am J Epidemiol. 157:940–943. 2003.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wacholder S: Binomial regression in GLIM:
Estimating risk ratios and risk differences. Am J Epidemiol.
123:174–184. 1986.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Diaz-Quijano FA: A simple method for
estimating relative risk using logistic regression. BMC Med Res
Methodol. 12(14)2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
HyLown Consulting LLC: Power and sample
size HomeCalculators compare 2 proportions: 2-Sample, 1-Sided.
Calculator: 1, 2013.
|
|
69
|
Wang L: C-reactive protein levels in the
early stage of COVID-19. Med Mal Infect. 50:332–334.
2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Alzaid F, Julla JB, Diedisheim M, Potier
C, Potier L, Velho G, Gaborit B, Manivet P, Germain S, Vidal-Trecan
T, et al: Monocyte class switch and hyperinflammation characterise
severe COVID-19 in type 2 diabetes. medRxiv: 2020.06.02.20119909,
2020.
|
|
71
|
Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang
Y, Qian F, Dai T, Zhang T, Lai Y, et al: COVID-19 infection induces
readily detectable morphological and inflammation-related
phenotypic changes in peripheral blood monocytes, the severity of
which correlate with patient outcome. medRxiv: 2020.03.24.20042655,
2020.
|
|
72
|
McKechnie JL and Blish CA: The innate
immune system: Fighting on the front lines or fanning the flames of
COVID-19? Cell Host Microbe. 27:863–869. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
He R, Lu Z, Zhang L, Fan T, Xiong R, Shen
X, Feng H, Meng H, Lin W, Jiang W and Geng Q: The clinical course
and its correlated immune status in COVID-19 pneumonia. J Clin
Virol. 127(104361)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
National Research Project for SARS,
Beijing Group. The involvement of natural killer cells in the
pathogenesis of severe acute respiratory syndrome. Am J Clin
Pathol. 121:507–511. 2004.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhao Q, Meng M, Kumar R, Wu Y, Huang J,
Deng Y, Weng Z and Yang L: Lymphopenia is associated with severe
coronavirus disease 2019 (COVID-19) infections: A systemic review
and meta-analysis. Int J Infect Dis. 96:131–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y
and Shang Y: Thrombocytopenia and its association with mortality in
patients with COVID-19. J Thromb Haemost. 18:1469–1472.
2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Margraf A and Zarbock A: Platelets in
inflammation and resolution. J Immunol. 203:2357–2367.
2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Taneja V: Sex hormones determine immune
response. Front Immunol. 9(1931)2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Pozzilli P and Lenzi A: Commentary:
Testosterone, a key hormone in the context of COVID-19 pandemic.
Metabolism. 108(154252)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tan T, Khoo B, Mills EG, Phylactou M,
Patel B, Eng PC, Thurston L, Muzi B, Meeran K, Prevost AT, et al:
Association between high serum total cortisol concentrations and
mortality from COVID-19. Lancet Diabetes Endocrinol. 8:659–660.
2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Merad M and Martin JC: Pathological
inflammation in patients with COVID-19: A key role for monocytes
and macrophages. Nat Rev Immunol. 20:355–362. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Bateman L, Rao P, Jones TH and Kelly DM:
SAT-049 testosterone therapy reduces inflammatory activation of
human monocytes in hypogonadal type-2 diabetic men as a potential
mechanism to improve atherosclerosis. J Endocr Soc. 4 (Suppl
1):SAT–049. 2020.
|
|
83
|
Milush J: Role of monocyte oxidative
stress and mineralocorticoid receptor signaling on cardiovascular
disease and persistent inflammation in antiretroviral-treated HIV+
persons. Grantome 1, 2015.
|
|
84
|
Gomez-Sanchez E and Gomez-Sanchez CE: The
multifaceted mineralocorticoid receptor. Compr Physiol. 4:965–994.
2014.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Uchida MC, Bacurau RFP, Navarro F, Pontes
FL Jr, Tessuti VD, Moreau RL, Rosa LFBPC and Aoki MS: Alteração da
relação testosterona: Cortisol induzida pelo treinamento de força
em mulheres. Rev Bras Med Esporte. 10:165–168. 2004.
|
|
86
|
Block MS and Rowan BG: Hypochlorous acid:
A review. J Oral Maxillofac Surg. 78:1461–1466. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Montesinos-Peña NE, Hernández-Valencia M,
Delgado-Enciso I, Herrera-Leal A and Paz-Michel BA: Evaluation of
an antiseptic gel of intravaginal application for multitreated
patients for infectious cervicovaginitis. Ginecol Obs Mex.
87:454–466. 2019.
|
|
88
|
Cabello GC, Rosete ODP and Manjarrez ZME:
Efecto de una solución electrolizada de superoxidación con pH
neutro sobre la infección del virus de influenza A en células MDCK.
Rev Inst Nal Enf Resp Mex. 22:280–287. 2009.
|
|
89
|
Takeda Y, Uchiumi H, Matsuda S and Ogawa
H: Acidic electrolyzed water potently inactivates SARS-CoV-2
depending on the amount of free available chlorine contacting with
the virus. Biochem Biophys Res Commun. 530:1–3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Moorman E, Montazeri N and Jaykus LA:
Efficacy of neutral electrolyzed water for inactivation of human
norovirus. Appl Environ Microbiol. 83:e00653–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
García JP, Maldonado RA, Díaz RI, Muñiz J
and Rodríguez HA: Sustitución del uso de solución salina fi
siológica como irrigante en el manejo de pacientes sépticos y
quirúrgicos por solución electrolizada. Rev Mex Cir Bucal
Maxilofac. 7:46–52. 2011.
|
|
92
|
Khomich OA, Kochetkov SN, Bartosch B and
Ivanov AV: Redox biology of respiratory viral infections. Viruses.
10(392)2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kim HJ, Kim CH, Ryu JH, Kim MJ, Park CY,
Lee JM, Holtzman MJ and Yoon JH: Reactive oxygen species induce
antiviral innate immune response through IFN-λ regulation in human
nasal epithelial cells. Am J Respir Cell Mol Biol. 49:855–865.
2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Delgado-Enciso I, López-Lemus AU,
Valcarcel-Gamiño AJ, Rodriguez-Sanchez IP, Valle-Reyes S,
Martinez-Fierro ML, Melnikov V, Guzmán-Esquivel J, Vaca-Paniagua F,
Valdez-Velazquez LL, et al: Dengue virus-1 NS5 genetic variant
associated with a severe clinical infection: Possible reduction of
the innate immune response by inhibition of interferon type 1 and
the Janus kinase-signal transducer and activator of transcription
signaling pathway. Int J Mol Med. 41:2263–2269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Liu T, Castro S, Brasier AR, Jamaluddin M,
Garofalo RP and Casola A: Reactive oxygen species mediate
virus-induced STAT activation: Role of tyrosine phosphatases. J
Biol Chem. 279:2461–2469. 2004.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Nair S, Poddar S, Shimak RM and Diamond
MS: Interferon regulatory factor 1 protects against chikungunya
virus-induced immunopathology by restricting infection in muscle
cells. J Virol. 91:e01419–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Hong Y, Chen S and Zhang JM: Hydrogen as a
selective antioxidant: A review of clinical and experimental
studies. J Int Med Res. 38:1893–1903. 2010.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Pyo CW, Shin N, Jung K, Choi JH and Choi
SY: Alteration of copper-zinc superoxide dismutase 1 expression by
influenza A virus is correlated with virus replication. Biochem
Biophys Res Commun. 450:711–716. 2014.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Chen J, Zhang H, Hu J, Gu Y, Shen Z, Xu L,
Jia X, Zhang X and Ding X: Hydrogen-rich saline alleviates kidney
fibrosis following AKI and retains klotho expression. Front
Pharmacol. 8(499)2017.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Nakayama M, Kabayama S and Ito S: The
hydrogen molecule as antioxidant therapy: Clinical application in
hemodialysis and perspectives. Ren Replace Ther. 2(23)2016.
|
|
101
|
Chen M, Zhang J, Chen Y, Qiu Y, Luo Z,
Zhao S, Du L and Tian D: Hydrogen protects lung from
hypoxia/re-oxygenation injury by reducing hydroxyl radical
production and inhibiting inflammatory responses. Sci Rep.
8(8004)2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang ST, Bao C, He Y, Tian X, Yang Y,
Zhang T and Xu KF: Hydrogen gas (XEN) inhalation ameliorates airway
inflammation in asthma and COPD patients. QJM. 113:870–875.
2020.PubMed/NCBI View Article : Google Scholar
|