Introduction
The etiologies of intestinal ischemia-reperfusion
(I/R) injury include artery occlusion, intestinal obstruction,
intestinal transplantation and abdominal trauma (1,2). The
intestine is a reservoir of bacteria and endotoxins; thus,
intestinal I/R injury may cause the spread of intestinal bacteria
and endotoxins, as well as secretion of various inflammatory
mediators and cytokines. Such physiological changes may induce
local intestinal injury or distant lung injury by activating
systemic inflammatory responses or may even result in multiple
organ dysfunction (3). Intestinal
I/R-induced acute lung injury, a severe life-threatening condition,
is associated with high mortality rates (~40% in Washington, USA)
(4). Currently, the mechanisms
underlying intestinal I/R-induced acute lung injury have yet to be
completely elucidated. Thus, there is an urgent need to identify
novel biomarkers and devise effective therapeutic strategies for
treating this affliction.
MicroRNAs (miRs) are single-stranded non-coding RNAs
(length, 19-23 nucleotides), and miRs that suppress expression of
target mRNAs at the post-transcriptional level by epigenetic
regulation are critical for the regulation of various processes,
including cellular proliferation, differentiation, apoptosis,
metabolism and immunity (5,6). Previous studies reported that various
miRs, including miR-29b-3p (7),
miR-381-3p (8), miR-483-5p
(9) and miR-145 are involved in the
pathogenesis of intestinal I/R injury or acute lung injury
(10); however, to the best of our
knowledge, only a few studies have examined the role of miRs in
intestinal I/R-induced acute lung injury thus far. miR-146a is one
of the key miRs involved in inflammatory responses (11,12)
and Zeng et al (13)
observed that miR-146a overexpression contributes to the
suppression of inflammatory responses during
lipopolysaccharide-induced acute lung injury. Chassin et al
(14) found that miR-146a-mediated
downregulation of interleukin 1 receptor associated kinase 1
expression alleviates small intestinal I/R-induced injury in mice
and humans; however, the role of miR-146a in the pathogenesis of
intestinal I/R-induced acute lung injury is not entirely
understood.
The NF-κB signaling pathway is closely associated
with secretion of inflammatory factors and its activation increases
the expression of cytokines and promotes I/R-induced lung injury
(15). Furthermore, intestinal
I/R-induced lung injury may occur due to activation of the NF-κB
signaling pathway (16). TNF
receptor-associated factor 6 (TRAF6) activates the NF-κB signaling
pathway, promotes the production of pro-inflammatory cytokines and
induces I/R-induced injury (17,18).
Thus, preventing inflammatory responses through TRAF6/NF-κB
signaling has been suggested as a possible treatment of intestinal
acute I/R-induced lung injury. A previous study found that miR-146a
alleviated I/R-induced injury by targeting TRAF6 and silencing the
NF-κB pathway (19); however, the
effects of miR-146a/TRAF6/NF-κB on intestinal I/R-induced lung
injury remain unclear.
The aim of the present study was to examine the role
of miR-146a in the progression of intestinal I/R-induced acute lung
injury in a mouse model. Furthermore, the effects of miR-146a
overexpression on histopathological and molecular changes
associated with intestinal I/R-induced acute lung injury were
investigated.
Materials and methods
Animals and experimental groups
Forty-two male C57BL/6 mice (age, 7-10 weeks) were
maintained at room temperature (25±2˚C) with 60% humidity under a
12-h light/dark cycle. The mice had free access to water and chow.
Before the experiments, the mice were allowed to acclimatize to the
laboratory conditions for at least two weeks. All animal
experiments adhered to the guidelines of the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (revised
1996) and were approved by the Institutional Animal Ethical
Committee of the Shenzhen Maternity and Child Healthcare Hospital,
Southern Medical University. The 42 mice were randomly assigned to
the following groups (with six per group in seven groups): Normal,
sham, model, normal-negative control (NC) and I/R-NC (negative
groups), normal-miR-146a and I/R-miR-146a group (miR-146a
overexpression groups).
Mice in the normal group were not subjected to any
procedures and the sham group mice received the same treatment as
the model group mice with the exception of superior mesenteric
artery occlusion. The model group mice were subjected to superior
mesenteric artery occlusion for 60 min, followed by reperfusion for
120 min. In the I/R-NC group mice, an adenoviral miR-NC vector was
injected into the tail vein 60 min before intestinal I/R surgery
and in the I/R-miR-146a group, an adenoviral miR-146a
overexpression vector was injected in the tail vein 60 min before
intestinal I/R surgery. In the normal-NC group mice, an adenoviral
miR-NC vector was injected into the normal mice and in the
normal-miR-146a group, an adenoviral miR-146a overexpression vector
was injected into the normal mice.
Surgical protocol
The mice were fasted for 12 h after which they were
anesthetized using pentobarbital sodium (50 mg/kg bodyweight;
intraperitoneal injection). During surgery, the mice were allowed
to breathe spontaneously. A midline laparotomy was performed to
expose the intestine, the superior mesenteric artery was exposed
and was occluded using a microvascular clamp for 60 min to elicit
intestinal ischemia. Ischemia was determined based on the lack of
pulse in the mesentery and pale coloration of the small intestine.
Subsequently, reperfusion was initiated by removing the clamp.
Reperfusion was determined based on the reoccurrence of pink
coloration of the small intestine and enhanced intestinal
peristalsis. The abdomen was temporarily covered using a sterile
plastic wrap to minimize evaporation. Reperfusion was induced for
120 min; the I/R time was set as previously described (20). Body temperature (36-38˚C) was
maintained during the procedure using heating pads. The mice were
euthanized by intraperitoneal injection with pentobarbital (140
mg/kg bodyweight) 12 h after completion of intestinal I/R surgery.
Intestinal and lung tissue samples were collected immediately,
after which they were shock-frozen in liquid nitrogen and stored in
-80˚C until analysis. Intestinal and lung tissue sections for
histopathological analysis were fixed in 10% formalin for 15 min at
25˚C.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from the tissue samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and was reverse-transcribed to cDNA using the miRNA First
Strand cDNA Synthesis kit (Sangon Biotech Co., Ltd.) for miR-146a
mRNA or using the Prime Script RT Reagent kit (Takara Biotechnology
Co., Ltd.) for TNF-α, IL-1β, IFN-γ and TGF-β1 mRNAs. RT-qPCR
analyses were performed using an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and SYBR Green qPCR
Super Mix (Invitrogen; Thermo Fisher Scientific, Inc.). The qPCR
thermocycling conditions were as follows: Denaturation at 95˚C for
10 min, followed by 40 cycles at 95˚C for 15 sec and 65˚C for 32
sec. The genes U6 and GAPDH were used for normalization of miRs and
mRNA expression, respectively. Relative expression levels of the
respective target gene were calculated according to the
2-ΔΔCq method (21).
The following PCR primers were used: GAPDH, forward
5'-TGGCCGTGGGGCTGCCCAG-3' and reverse 5'-GGAAGGCCATGCCAGTGAGC-3';
TNF-α, forward 5'-CTAGTGGTGCCAGCCGATGG-3' and reverse
5'-GGCTCTTGACGGCAGAGAGG-3'; IL-1β, forward
5'-TGTCGGACCCATATGAGCTG-3' and reverse 5'-TCCTTTGAGGCCCAAGGCCA-3';
IFN-γ, forward 5'-TCTTCTTGGATATCTGGAGG-3' and reverse
5'-CCTGATTGTCTTTCAAGACT-3'; TGF-β1, forward
5'-GTGGAGCAACATGTGGAACT-3' and reverse 5'-AAGACAGCCACTCAGGCGT-3';
U6, forward 5'-CTCGCTTCGGCAGCACA-3' and reverse
5'-AACGCTTCACGAATTTGCGT-3'; miR-146a, forward
5'-ACACTCCAGCTGGGTGAGAACTGAATTCCA-3' and reverse
5'-CTCAACTGGTGTCGTGGA-3'.
Adenovirus-mediated overexpression of
miRs in vivo
miR-146a overexpression (Agctctgagaact
gaattccatgggttatatcaat gtcagacctgtgaaat tcagttcttcagct) and NC
(CCGGGAACTGGGGTGCGTGTGATCTCGAGATCACACGCACCCCAGTTTTTTTG) were
synthesized by Shanghai GenePharma Co., Ltd. and were combined with
the pDC316-mCMV-enhanced green fluorescent protein (EGFP) plasmid.
Subsequently, pDC316-mCMV-EGFP, pBHG and pDC316-CMV-GFP plasmids
(cat. no. PD-01-64, AdMax™ Adenovirus System; Microbix Biosystems,
Inc.) were added dropwise to the cells whilst being gently mixed,
followed by culturing for 24 h. Subsequently, the virus was
amplified over three generations, and cells were collected and
subjected to three freeze-thaw cycles at -80˚C and 37˚C, after
which they were centrifuged at 10,000 x g at 4˚C for 10 min to
separate the virus-containing supernatant. The mice were injected
in the tail vein with 100 µl solution containing adenoviral
miR-146a overexpression vector or miR NC vector (multiplicity of
infection=6x107 pfu/ml; Shanghai GeneChem Co., Ltd.) 60
min before intestinal I/R surgery. The mice were euthanized 12 h
after surgery (20). Intestinal and
lung tissue samples were excised for histopathological analysis,
measurement of mRNA and protein expression levels and to record
their wet weight.
Histopathological examination
Sections of the jejunum and of the lower lobes of
the right lungs were embedded in paraffin and cut into sections
(thickness, 5 µm). The sections were transferred to glass slides
and subjected to hematoxylin and eosin (H&E) staining for 4 h
at 25˚C. The degree of histological injury was evaluated through
blinded analysis of hand counts performed by two experienced
investigators using a light microscope (BX51 microscope; Olympus
Corporation). Histopathological scores of intestinal tissue samples
were obtained according to a previous study (22) and were based on three parameters as
follows: Severity of inflammation [based on polymorphonuclear
neutrophil infiltration: None (score=0), slight (score=1), moderate
(score=2), severe (score=3)]; depth of injury [none (score=0),
mucosal (score=1), mucosal and submucosal (score=2), transmural
(score=3)]; and crypt damage [none (score=0), basal one-third
damaged (score=1), basal two-thirds damaged (score=2), only surface
epithelium intact (score=3), entire crypt and epithelium lost
(score=4)]. The scores where then summed with a maximum possible
score of 10. Five high-magnification fields were randomly selected
and were scored to produce an average intestinal injury score for
each mouse.
Lung histological scoring was performed based on
histological changes, such as alveolar congestion, alveolar wall
edema, inflammatory cell infiltration and hemorrhage where each
standard was scored between 0 (normal) and 4 (severe) as follows:
0, No or very mild injury; 1, mild injury; 2, medium injury; 3,
severe injury; 4, very severe injury (23). The sum of all scores was processed
as a total score of lung tissue pathology. Five high-magnification
fields were randomly selected and were scored to produce an average
lung injury score for each mouse.
Lung tissue wet-to-dry (W/D) weight
ratio
The wet weight of lung tissue samples was recorded
immediately following tissue excision. The tissue samples were then
dried in an incubator at 60˚C for three days, after which the dry
weight was recorded. Lung W/D weight ratios were calculated to
assess tissue edema.
Western blotting
Intestinal and lung tissue samples were washed using
phosphate-buffered saline and were homogenized in lysis buffer
(Thermo Fisher Scientific, Inc.) containing Halt™ Protease and
Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Inc.).
Cell lysates (30 µg/lane) were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and the resolved
proteins were then transferred to a polyvinylidene fluoride
membrane. The membrane was blocked with 5% non-fat milk for 2 h at
25˚C and subsequently incubated overnight at 4˚C with the following
primary antibodies: Anti-TRAF6 [cat. no. ab33915; 1:1,000 (v/v);
Abcam], anti-p-p65 NF-κB [cat. no. ab239882; 1:500 (v/v); Abcam],
anti-p65 NF-κB [cat. no. ab32536; 1:500 (v/v); Abcam], anti-cleaved
caspase-3 [cat. no. ab214430; 1:1,000 (v/v); Abcam], anti-cleaved
caspase-9 [cat. no. ab77814; 1:1,000 (v/v); Abcam] and anti-GAPDH
[cat. no. ab181602; 1:15,000 (v/v); Abcam]. The membrane was then
washed with three times for 10 min each in TBST (0.1% Tween-20) and
was incubated with the horseradish peroxidase-conjugated Goat
Anti-Rabbit IgG H&L antibodies (cat. no. ab6721; 1:20,000
(v/v); Abcam) for 2 h at room temperature. Protein bands were
detected using a chemiluminescent peroxidase substrate, ECL
(Amersham; Cytiva). The protein band intensity was analyzed using
Quantity One software (Bio-Rad Laboratories, Inc.) and target
protein band intensities were normalized to GAPDH.
Statistical analysis
All statistical analyses were performed using SPSS
22.0 software (IBM Corp.). Differences between two groups were
evaluated using Student's t-test and differences between multiple
groups were tested using a one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
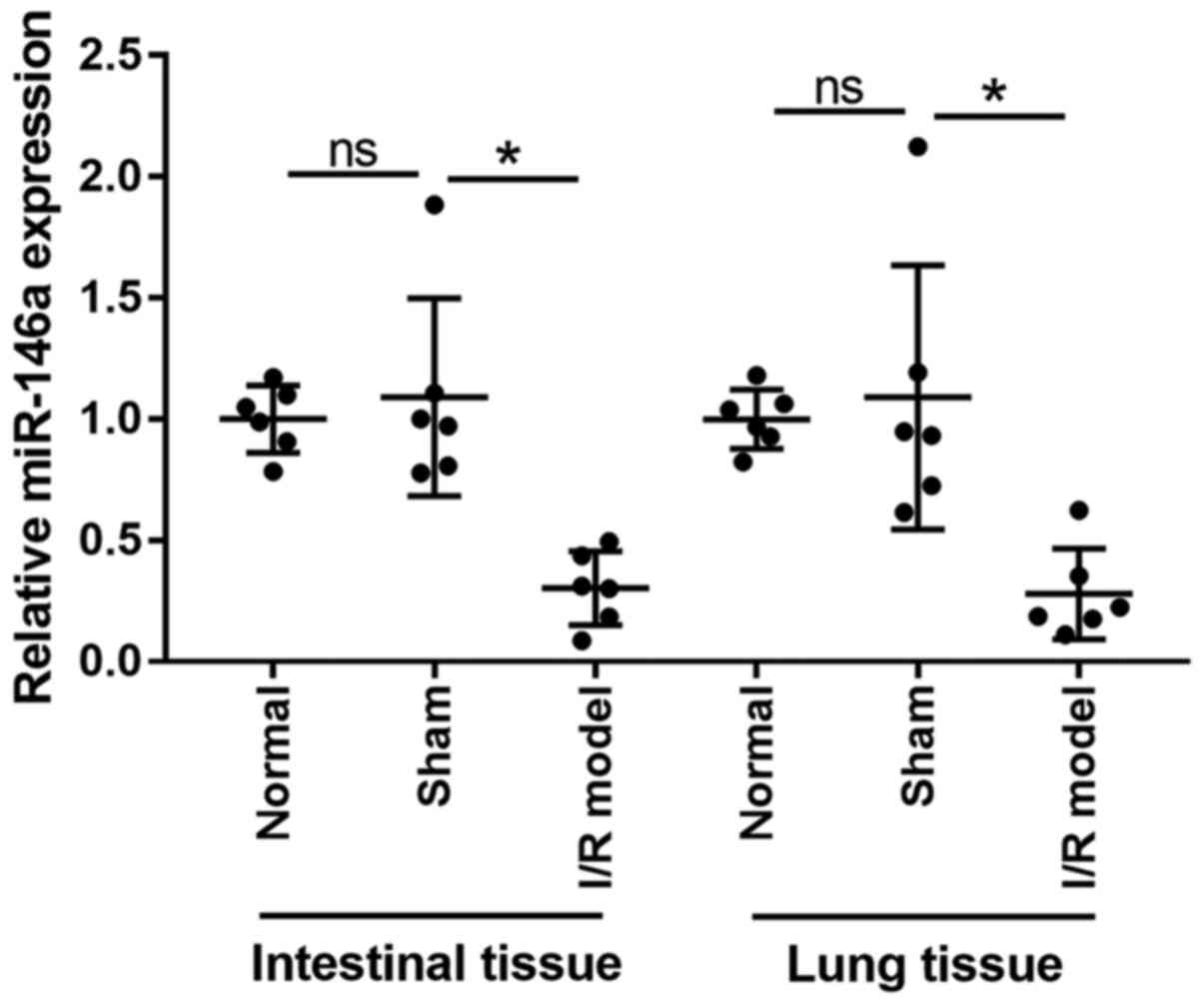
Expression levels of miR-146a are
downregulated in the lungs and intestines of mice with intestinal
I/R injury
Expression levels of miR-146a in intestinal and lung
tissue samples were analyzed using RT-qPCR (Fig. 1). miR-146a expression in the
intestines and lungs was significantly downregulated in the model
group when compared with the sham group (0.28-fold change in
intestinal tissues and 0.26-fold change in lung tissues).
Differences in miR-146a expression between the normal and the sham
group were not significant (1.09-fold change in intestinal tissues
and 1.08-fold change in lung tissues). These findings indicate that
miR-146a is involved in the development of intestinal I/R-induced
acute lung injury in mice. Additionally, no significant differences
between the normal and sham group mice were observed regarding W/D
weight ratio and IL-1β, TNF-α, IFN-γ and TGF-β1 mRNA expression
(Fig. S1A), suggesting that the
sham surgery had no significant effect.
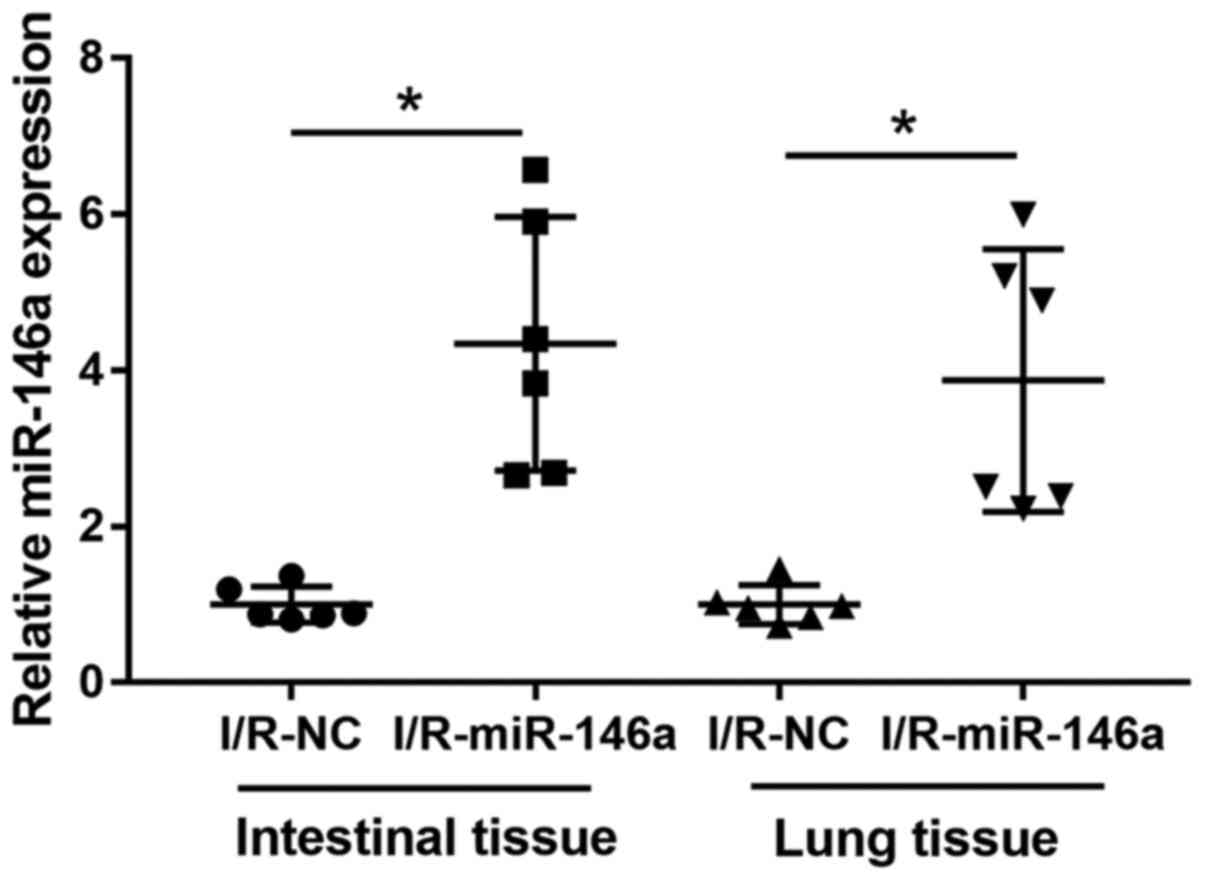
Overexpression of miR-146a during
intestinal I/R-induced injury
The role of miR-146a during intestinal I/R-induced
injury was further evaluated by injecting the mice with an
adenoviral miR-146a overexpression vector or with an adenoviral
miR-NC vector. EGFP fluorescence images of lung tissue samples
demonstrated that the adenoviral vector was successfully infected
in the lung tissue (Fig. S2).
RT-qPCR analysis revealed that miR-146a expression
in the intestine and lung tissue samples was significantly
upregulated in miR-1461-overexpressing mice, compared with I/R-NC
mice (4.34-fold change in intestinal tissues and 3.87-fold change
in lung tissues; Fig. 2). No
significant difference between the normal, normal-NC (normal mice
injected with the adenoviral NC vector) and normal-miR-146a (normal
mice injected with adenoviral miR-146a overexpression vector)
groups regarding W/D weight ratios and IL-1β, TNF-α, IFN-γ and
TGF-β1 mRNA expression (Fig. S1B),
suggesting that the adenoviral vector exerted no significant effect
on the normal mice.
Overexpression of miR-146a alleviated
morphological changes in intestinal and lung tissue samples of mice
with intestinal I/R-induced injury
The lungs of I/R-NC mice exhibited severe
histopathological changes, including alveolar congestion, exudate
and infiltration of inflammatory cells when compared with the lungs
of the sham group mice (6.88-fold change; Fig. 3); however, these histopathological
changes associated with intestinal I/R-induced acute lung injury
were substantially less severe in the I/R-miR-146a group mice, as
evidenced by a significantly decreased lung injury score than that
in I/R-NC group mice (0.53-fold change). The intestinal tissue
samples from the I/R-NC group mice exhibited severe intestinal
crypt injury, widespread mucosal destruction and disintegrated
intestinal villi, when compared with the sham group mice (5.67-fold
change, Fig. 3); however, miR-146a
overexpression markedly alleviated these intestinal
histopathological changes associated with intestinal I/R-induced
injury, as evidenced by a significantly decreased intestinal injury
score (0.5-fold change). Compared with the sham group, the lung
injury and intestinal score significantly increased in the
I/R-miR-146a group mice (3.63- and 2.83-fold change,
respectively).
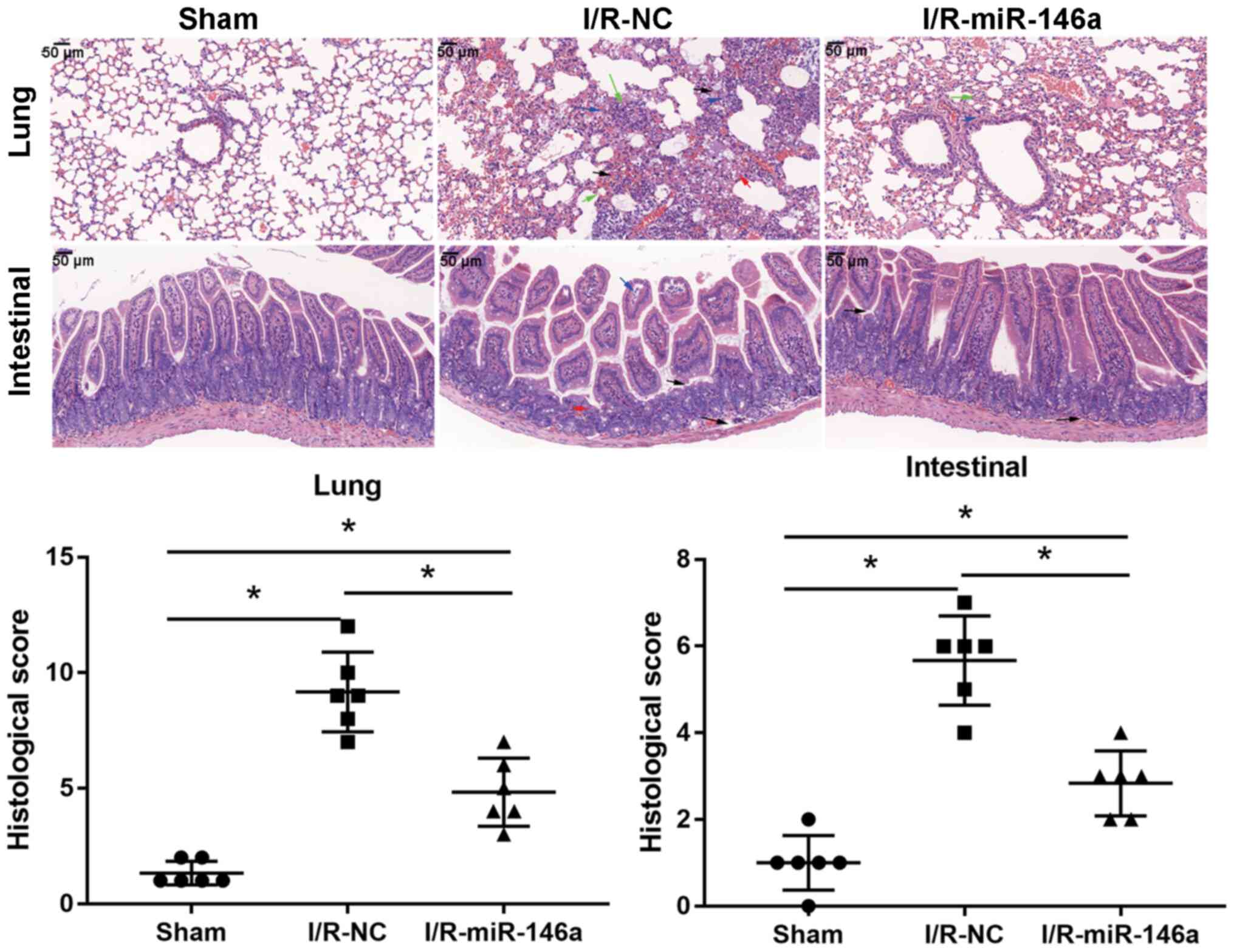 | Figure 3miR-146a overexpression alleviates
intestinal I/R-induced acute lung injury. Effects of miR-146a
overexpression on the morphology of intestinal and lung tissue
samples in an intestinal I/R-induced injury mouse model were
assessed by hematoxylin and eosin staining (magnification, x400;
n=6). In lung tissue samples: Blue arrow, inflammation (score=2);
red arrow, alveolar edema (score=2); blank arrow, hemorrhage
(score=4); green arrow, alveolar wall edema (score=4). In
intestinal tissue samples: Blue arrow, inflammation (score=2); red
arrow, crypt damage (score=2); blank arrow, mucosal destruction and
disintegrated intestinal villi (score=3). *P<0.05.
miR, microRNA; ns, not significant; I/R, ischemia/reperfusion; NC,
negative control. |
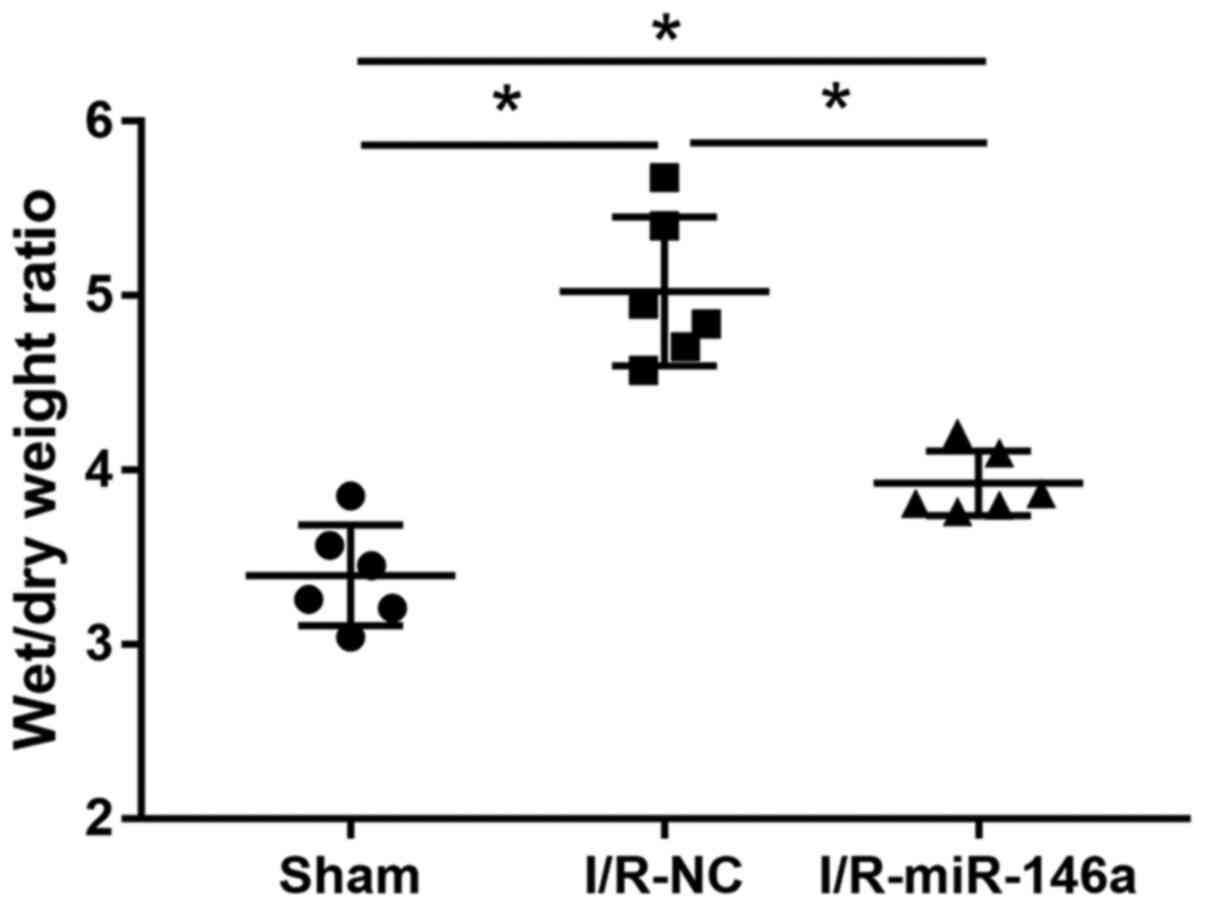
Overexpression of miR-146a alleviated
pulmonary edema in mice with intestinal I/R injury
The effect of miR-146a overexpression on lung injury
in mice with intestinal I/R-induced injury was evaluated by
examining the W/D weight ratio of lung tissue samples (Fig. 4). The W/D weight ratio of lung
tissue samples from the I/R-NC group was significantly higher than
that of the sham group lung tissue samples (1.48-fold change) and
that of the miR-146a-overexpressing lung tissue samples was
significantly lower than that of the lung tissue samples from the
I/R-NC group (0.78-fold change). The W/D weight ratio of the lung
tissue samples from the miR-146a overexpression group was
significantly higher than that of the lung tissue samples from the
sham group (1.15-fold change). There was no significant difference
in the W/D weight ratio between the normal and the sham group
(1.04-fold change).
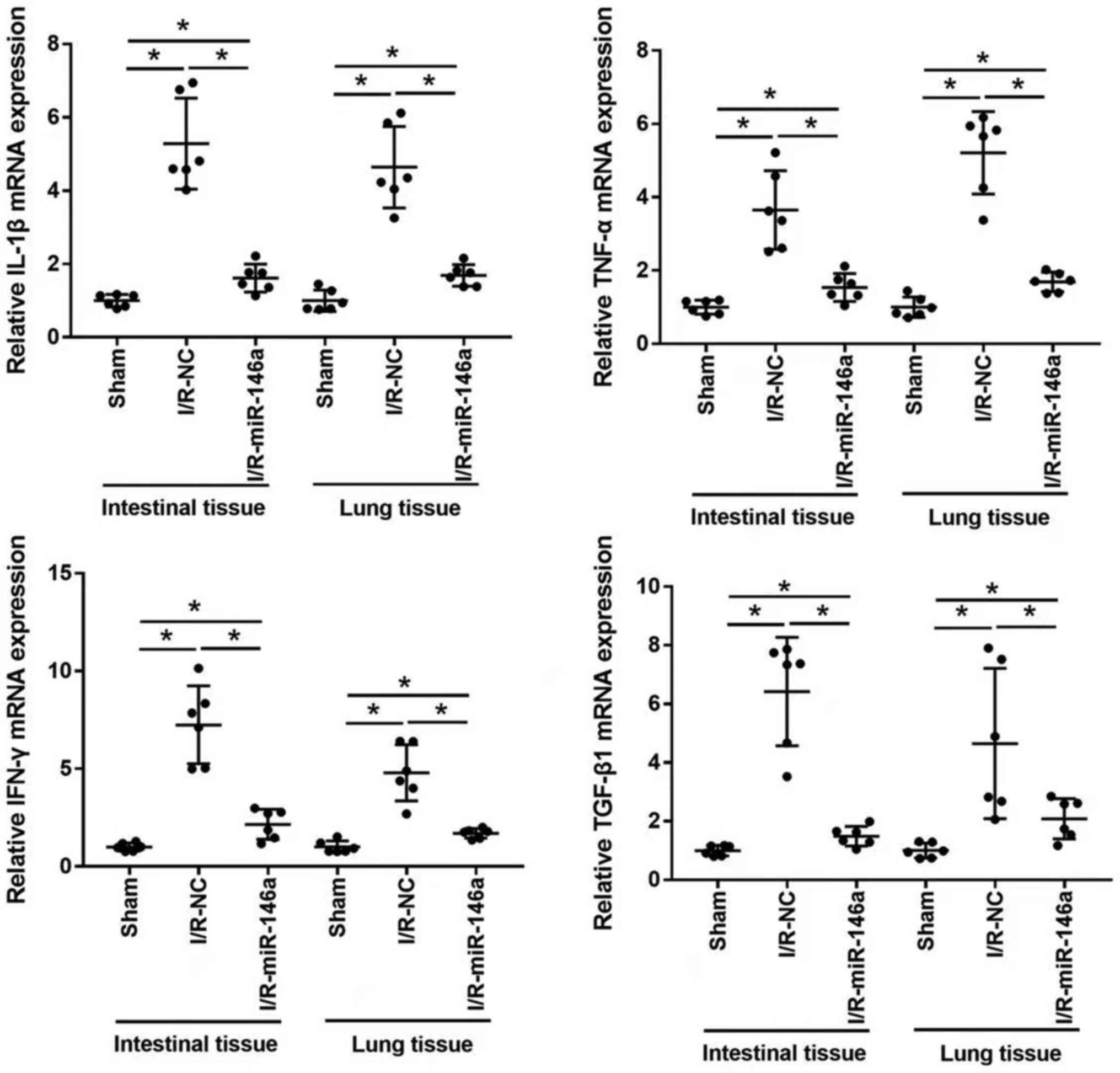
Overexpression of miR-146a
downregulated the expression of inflammatory markers in intestinal
and lung tissue samples of mice with intestinal I/R-induced
injury
The effect of miR-146a overexpression on mRNA
expression levels of proinflammatory cytokines, such as IL-1β,
TNF-α, IFN-γ and TGF-β1 in intestinal and lung tissue samples of
mice with intestinal I/R-injury was evaluated using RT-qPCR
(Fig. 5). mRNA expression levels of
IL-1β, TNF-α, IFN-γ and TGF-β1 were significantly upregulated in
the intestine and lung tissue samples from mice in the I/R-NC group
compared with those from mice in the sham group (4.98-, 4.27-,
6.84- and 6.42-fold change in intestinal tissue, respectively; and
4.71-, 6.42-, 4.79- and 5.46-fold change in lung tissue,
respectively). mRNA expression levels of IL-1β, TNF-α, IFN-γ and
TGF-β1 in the intestine and lungs were significantly downregulated
in miR-146a-overexpressing mice compared with I/R-NC mice (0.32-,
0.36-, 0.32- and 0.23-fold change in intestinal tissue,
respectively; and 0.36-, 0.26-, 0.35- and 0.38-fold change in lung
tissue, respectively). mRNA expression levels of IL-1β, TNF-α,
IFN-γ and TGF-β1 in the lung and intestinal tissues were
significantly higher in I/R-miR-146a group than those in sham group
(1.61-, 1.53-, 2.16- and 1.49-fold change in intestinal tissue,
respectively, and 1.69-, 1.69-, 1.70- and 2.08-fold change in lung
tissue, respectively).
Overexpression of miR-146a decreased
the expression of apoptosis-related proteins in mice with
intestinal I/R injury by inhibiting the TRAF6/NF-κB signaling
pathway
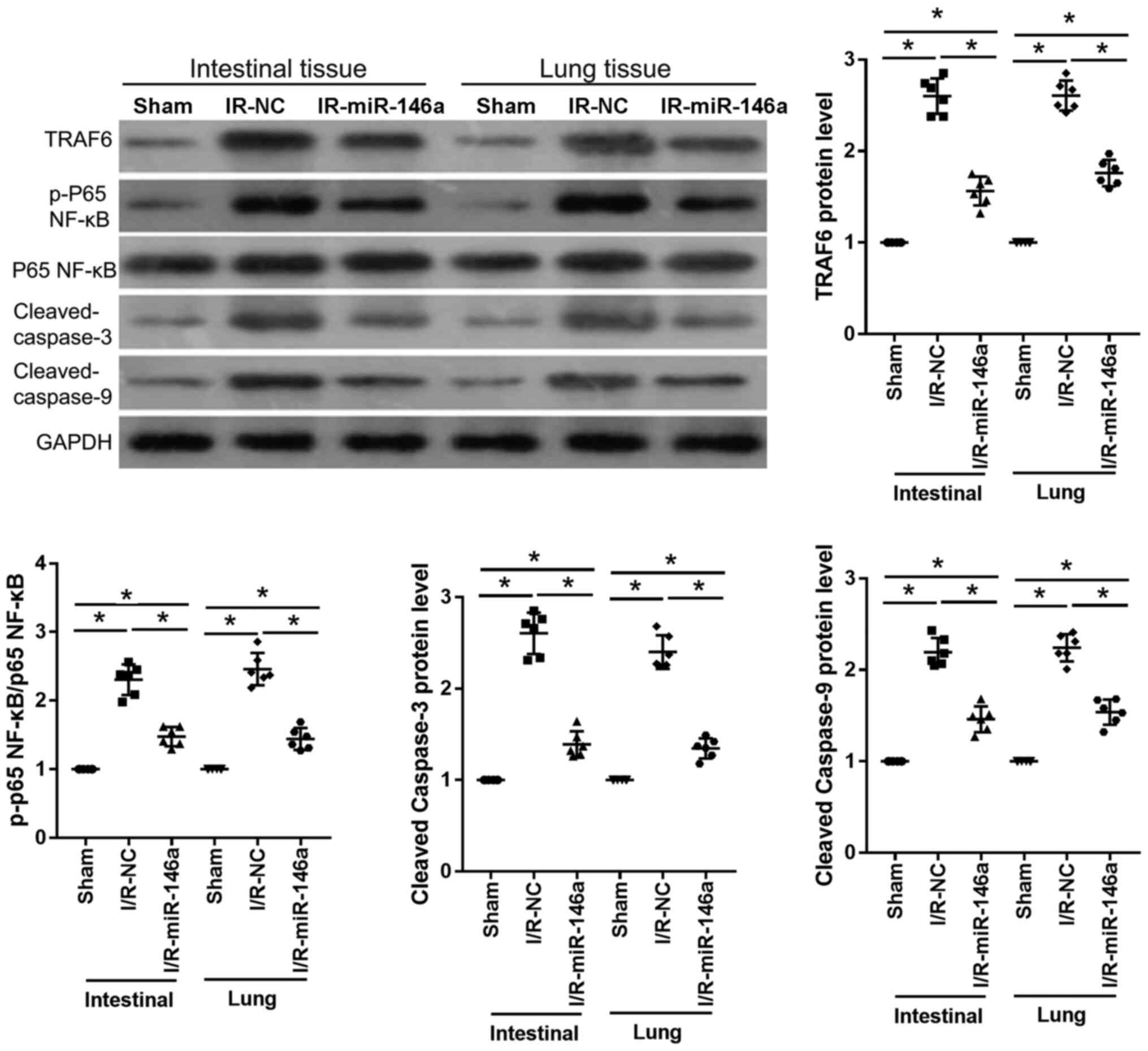
Western blotting (Fig.
6) revealed that, compared with the sham group, protein levels
of TRAF6, p-p65 NF-κB, p65 NF-κB, cleaved caspase-3 and cleaved
caspase-9 in intestinal and lung tissue samples were significantly
upregulated in the IR-NC group (2.60-, 2.31-, 2.61- and 2.19-fold
change in intestinal tissues, respectively; 2.61-, 2.46-, 2.40- and
2.24-fold change in lung tissues, respectively). And compared with
the IR-NC group, those proteins were significantly downregulated in
IR-miR-146a group mice (0.60-, 0.64-, 0.53- and 0.67-fold change in
intestinal tissues, respectively; 0.68-, 0.59-, 0.56- and 0.69-fold
change in lung tissues, respectively) as shown in Fig. 6. Protein levels of TRAF6, p-p65
NF-κB, p65 NF-κB, cleaved caspase-3 and cleaved caspase-9 in the
intestinal and lung tissue samples were significantly higher in the
IR-miR-146a group when compared with those in the sham group
(1.56-, 1.48-, 1.39- and 1.46-fold change in intestinal tissue,
respectively; and 1.76-, 1.44-, 1.35- and 1.54-fold change in lung
tissue, respectively) as presented in Fig. 6.
Discussion
The present results demonstrate that miR-146a
expression was significantly downregulated in the intestinal and
lung tissue samples of an intestinal I/R-induced injury mouse
model. Overexpression of miR-146a alleviated histopathological
changes in lung and intestinal tissue samples, reduced pulmonary
edema and downregulated the expression of inflammatory and
apoptotic markers in the intestinal and lung tissue samples of mice
with intestinal I/R-induced injury. Moreover, miR-146a
overexpression suppressed apoptotic responses in intestinal and
lung tissue samples of mice with intestinal I/R-induced lung injury
by inhibiting the TRAF6/NF-κB signaling pathway.
Intestinal I/R, a severe clinical condition that
leads to local intestinal damage or injury to distant organs
including the lungs is associated with high morbidity and mortality
rates (24). The pathological
mechanism underlying intestinal I/R-induced acute lung injury is
complex and is currently not completely understood. miRs are
associated with the pathogenesis of acute lung injury (25) and Kong et al (26) found that miR-216a alleviates
lipopolysaccharide-induced acute lung injury by regulating the
Janus kinase 2/STAT3 and NF-κB signaling pathways. He et al
(27) observed that miR-146a
expression was downregulated in the plasma of patients with
mesenteric ischemia, in IEC-6 cells and in small intestinal tissues
of ischemia and I/R rat models. In addition to causing local
damage, intestinal I/R contributes to distant organ injury and the
lungs are the most vulnerable organ in this respect. Consistent
with the results of He et al (27), the present study observed that
miR-146a expression was significantly downregulated in the
intestinal and lung tissue samples of mice with intestinal
I/R-induced injury, suggesting that miR-146a is associated with the
development of intestinal I/R-induced acute lung injury in
mice.
Furthermore, the present study investigated the role
of miR-146a in mice with intestinal I/R-induced injury.
Histopathological changes, such as acute lung injury and intestinal
injury were markedly ameliorated in tissue samples of
miR-146a-overexpressing mice, compared with those of I/R-NC mice.
Additionally, the lung W/D weight ratio was significantly lower in
miR-146a-overexpressing mice than that in I/R-NC mice. These
results indicate that miR-146a overexpression protects mice against
intestinal I/R-induced acute lung injury. Previous studies reported
that miR-146a, a key mediator of inflammatory responses, is
involved in the pathogenesis of intestinal I/R-induced injury
(11); for example, Chassin et
al (14) observed that miR-146a
suppresses inflammatory response and alleviates I/R-induced small
intestine injury in mice and humans by inhibiting interleukin 1
receptor associated kinase 1 expression.
Proinflammatory cytokines play a key role in local
intestinal I/R-induced lung injury (28) and TNF-α, IL-1β, IFN-γ and TGF-β1
activities are frequently used to assess the degree of inflammation
during intestinal I/R-induced injury. A previous study suggested
that TNF-α, IL-1β and IL-6 are the key inflammatory mediators during
intestinal I/R-induced acute lung injury (29). In the present study, mRNA expression
levels of TNF-α, IL-1β, IFN-γ and TGF-β1 in the lung and intestinal
tissue samples were significantly downregulated in
miR-146a-overexpressing mice, compared with I/R-NC mice. This
suggests that miR-146a overexpression alleviates intestinal
I/R-induced acute lung injury in mice partly by reducing the
secretion of proinflammatory cytokines. Lung epithelium and
endothelium injuries also contribute to the development of acute
lung injury, which is associated with the loss of cells through
apoptosis (30). A previous study
demonstrated the role of miR-146 in regulating apoptosis and
pathogenesis of intestinal I/R (27). In the current study, the role of
miR-146a in regulating apoptotic responses in a mouse model of
intestinal I/R-induced injury was assessed by evaluating expression
levels of the apoptosis markers, cleaved caspase-3 and cleaved
caspase-9 in intestinal and lung tissue samples. Protein expression
levels of TRAF6, p-p65 NF-κB, p65 NF-κB, cleaved caspase-3 and
cleaved caspase-9 in intestinal and lung tissues were significantly
downregulated in miR-146a-overexpressing mice when compared with
I/R-NC mice. These results suggest that miR-146a overexpression may
attenuate apoptotic responses in intestinal and lung tissues by
inhibiting the TRAF6/NF-κB signaling pathway.
Furthermore, the adenoviral miR-146a overexpression
vector was injected in the tail vein of mice 60 min before
intestinal I/R surgery, suggesting that miR-146a inhibits the acute
lung injury process. These findings suggested that pretreatment
with the adenoviral miR-146a overexpression vector acted as a
preventive measure for treating intestinal I/R-induced acute lung
injury. Hence, pretreatment with the adenoviral miR-146a
overexpression vector was only suitable for planned intestinal I/R
surgery in clinical practice. However, whether miR-146a treatment
improves lung injury induced by intestinal ischemia requires
further investigation, which is a potential limitation of the
present study. Hence, miR-146a treatment may not be applicable to
unplanned intestinal I/R surgery in clinical practice.
In conclusion, the present study demonstrated that
miR-146a was significantly downregulated in the intestine and lungs
of an intestinal I/R-induced mouse model. miR-146a overexpression
alleviated intestinal I/R-induced acute lung injury in mice through
modulation of inflammatory responses and apoptosis. Thus, miR-146a
may serve as a potential target to prevent intestinal I/R-induced
acute lung injury.
Supplementary Material
Wet/dry weight ratio and inflammation
were evaluated in intestinal and lung tissue samples. Wet/dry
weight ratio and relative expression levels of IL?1β, TNF-α, INF-γ
and TGF-β1 were analyzed in (A) the normal and sham groups and (B)
the normal, normal-NC and normal-miR-146a groups (n=6). miR,
microRNA; ns, not significant; I/R, ischemia/reperfusion; NC,
negative control.
Representative EGFP fluorescence
images of lung tissue samples demonstrating that the adenoviral
vector was successfully infected in the lung tissue. I/R,
ischemia/reperfusion; NC, negative control; miR, microRNA; EGFP,
enhanced green fluorescent protein.
Acknowledgements
Not applicable.
Funding
Funding: The study was financially supported by the Foundation
of Shenzhen Maternity and Child Healthcare Hospital (grant no.
FYB2017015).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL, MX and YTL conceived and designed the study. HW
and XQ developed the methodology. GL, MX, HW and XQ conducted the
experiments and collected the data. GL, MX, XW, YL and JS analyzed
and interpreted the data. GL drafted the manuscript and MX and YTL
revised the manuscript. GL and MX confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments conducted adhered to the
guidelines of the National Institutes of Health Guide for the Care
and Use of Laboratory Animals (revised 1996) and were approved by
the Institutional Animal Ethical Committee of the Shenzhen
Maternity and Child Healthcare Hospital, Southern Medical
University (Shenzen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou J, Zimmermann K, Krieg T, Soltow M,
Pavlovic D, Cerny V and Lehmann C: Adenosine receptor activation
improves microcirculation in experimental intestinal
ischemia/reperfusion. Clin Hemorheol Microcirc. 59:257–265.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu MC, Brennan FH, Lynch JP, Mantovani S,
Phipps S, Wetsel RA, Ruitenberg MJ, Taylor SM and Woodruff TM: The
receptor for complement component C3a mediates protection from
intestinal ischemia-reperfusion injuries by inhibiting neutrophil
mobilization. Proc Natl Acad Sci USA. 110:9439–9444.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao W, Gan X, Su G, Wanling G, Li S, Hei
Z, Yang C and Wang H: The interaction between oxidative stress and
mast cell activation plays a role in acute lung injuries induced by
intestinal ischemia-reperfusion. J Surg Res. 187:542–552.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Clark EA, Kalomoiris S, Nolta JA and
Fierro FA: Concise review: MicroRNA function in multipotent
mesenchymal stromal cells. Stem Cells. 32:1074–1082.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dai Y, Mao Z, Han X, Xu Y, Xu L, Yin L, Qi
Y and Peng J: MicroRNA-29b-3p reduces intestinal
ischaemia/reperfusion injury via targeting of TNF
receptor-associated factor 3. Br J Pharmacol. 176:3264–3278.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu L, Yao J, Li Z, Zu G, Feng D, Li Y,
Qasim W, Zhang S, Li T, Zeng H and Tian X: miR-381-3p knockdown
improves intestinal epithelial proliferation and barrier function
after intestinal ischemia/reperfusion injury by targeting Nurr1.
Cell Death Dis. 9(411)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leng C, Sun J, Xin K, Ge J, Liu P and Feng
X: High expression of miR-483-5p aggravates sepsis-induced acute
lung injury. J Toxicol Sci. 45:77–86. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cao X, Zhang C, Zhang X, Chen Y and Zhang
H: MiR-145 negatively regulates TGFBR2 signaling responsible for
sepsis-induced acute lung injury. Biomed Pharmacother. 111:852–858.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dai Y, Jia P, Fang Y, Liu H, Jiao X, He JC
and Ding X: miR-146a is essential for lipopolysaccharide
(LPS)-induced cross-tolerance against kidney ischemia/reperfusion
injury in mice. Sci Rep. 6(27091)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang S, Hu Y, Deng S, Deng J, Yu X, Huang
G, Kawai T and Han X: miR-146a regulates inflammatory cytokine
production in porphyromonas gingivalis
lipopolysaccharide-stimulated B cells by targeting IRAK1 but not
TRAF6. Biochim Biophys Acta Mol Basis Dis. 1864:925–933.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zeng Z, Gong H, Li Y, Jie K, Ding C, Shao
Q, Liu F, Zhan Y, Nie C, Zhu W and Qian K: Upregulation of miR-146a
contributes to the suppression of inflammatory responses in
LPS-induced acute lung injury. Exp Lung Res. 39:275–282.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chassin C, Hempel C, Stockinger S, Dupont
A, Kübler JF, Wedemeyer J, Vandewalle A and Hornef MW:
MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and
human small intestine against ischemia/reperfusion injury. EMBO Mol
Med. 4:1308–1319. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lv N and Li X: Isoflurane suppresses lung
ischemia-reperfusion injury by inactivating NF-κB and inhibiting
cell apoptosis. Exp Ther Med. 20(74)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu J, Chen T, Lei P, Tang X and Huang P:
Exosomes released by bone marrow mesenchymal stem cells attenuate
lung injury induced by intestinal ischemia reperfusion via the
TLR4/NF-κB pathway. Int J Med Sci. 16:1238–1244. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen CH, Lin JY, Chang YL, Wu SY, Peng CK,
Wu CP and Huang KL: Inhibition of NKCC1 modulates alveolar fluid
clearance and inflammation in ischemia-reperfusion lung injury via
TRAF6-mediated pathways. Front Immunol. 9(2049)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu X, Cao H, Li J, Wang B, Zhang P, Zhang
XD, Liu Z, Yuan H and Zhan Z: Autophagy induced by DAMPs
facilitates the inflammation response in lungs undergoing
ischemia-reperfusion injury through promoting TRAF6 ubiquitination.
Cell Death Differ. 24:683–693. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He L, Wang Z, Zhou R, Xiong W, Yang Y,
Song N and Qian J: Dexmedetomidine exerts cardioprotective effect
through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the
NF-κB pathway. Biomed Pharmacother. 133(110993)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhu Q, He G, Wang J, Wang Y and Chen W:
Pretreatment with the ALDH2 agonist Alda-1 reduces intestinal
injury induced by ischaemia and reperfusion in mice. Clin Sci
(Lond). 131:1123–1136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma Y, Guan Q, Bai A, Weiss CR, Hillman CL,
Ma A, Zhou G, Qing G and Peng Z: Targeting TGF-beta1 by employing a
vaccine ameliorates fibrosis in a mouse model of chronic colitis.
Inflamm Bowel Dis. 16:1040–1050. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Huang X, Hu S, He H and Meng Z:
Dexmedetomidine attenuates lipopolysaccharide induced acute lung
injury in rats by inhibition of caveolin-1 downstream signaling.
Biomed Pharmacother. 118(109314)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kerzmann A, Haumann A, Boesmans E, Detry O
and Defraigne JO: Acute mesenteric ischemia. Rev Med Liege.
73:300–303. 2018.PubMed/NCBI(In French).
|
|
25
|
Yang Y, Yang F, Yu X, Wang B, Yang Y and
Zhou X, Cheng R, Xia S and Zhou X: miR-16 inhibits NLRP3
inflammasome activation by directly targeting TLR4 in acute lung
injury. Biomed Pharmacother. 112(108664)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kong F, Sun Y, Song W, Zhou Y and Zhu S:
MiR-216a alleviates LPS-induced acute lung injury via regulating
JAK2/STAT3 and NF-κB signaling. Hum Cell. 33:67–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y,
Zhang C and Zhou X: MiR-146a protects small intestine against
ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB
pathway. J Cell Physiol. 233:2476–2488. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu Q, He G, Wang J, Wang Y and Chen W:
Protective effects of fenofibrate against acute lung injury induced
by intestinal ischemia/reperfusion in mice. Sci Rep.
6(22044)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
de Lima FM, Villaverde AB, Albertini R,
Corrêa JC, Carvalho RLP, Munin E, Araújo T, Silva JA and Aimbire F:
Dual Effect of low-level laser therapy (LLLT) on the acute lung
inflammation induced by intestinal ischemia and reperfusion: Action
on anti- and pro-inflammatory cytokines. Lasers Surg Med.
43:410–420. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen G, Zhang Z, Cheng Y, Xiao W, Qiu Y,
Yu M, Sun L, Wang W, Du G, Gu Y, et al: The canonical Notch
signaling was involved in the regulation of intestinal epithelial
cells apoptosis after intestinal ischemia/reperfusion injury. Int J
Mol Sci. 15:7883–7896. 2014.PubMed/NCBI View Article : Google Scholar
|