Introduction
Osteoporosis is a common systemic skeletal bone
disease that is mainly caused by age-related bone loss (1). For the treatment of osteoporosis,
inhibition of bone resorption is an important strategy to enhance
bone mineral density to slow the deterioration of the condition
(2). However, the toxicity and side
effects of a number of agents remain to be problematic. Although
bisphosphonates confer clear analgesic effects, improve bone
density and decrease the risk of fracture, they are associated with
gastrointestinal reactions (2). In
addition, teriparatide can increase the activity of osteoblasts,
but is expensive and therefore yields poor patient compliance
(2). At present, it is difficult to
achieve satisfactory clinical outcomes following the treatment of
osteoporosis (3). A novel strategy
of promoting the differentiation of osteoblasts or the regeneration
of bone tissues to slow the development of osteoporosis has been
previously proposed by using bone marrow mesenchymal stem cells
(BMSCs) (4,5). Mesenchymal stem cells are pluripotent
stem cells derived from the mesoderm and are widely distributed in
tissues, including adipose tissues, periosteum and bone marrow
(6). It has been previously
reported that BMSCs can continuously promote the formation of new
fibroblasts and bone, thereby serving a potentially beneficial role
in the reconstruction of bone (6).
Therefore, BMSCs are currently the subject of extensive
osteoporosis research due to their diverse source as they are
easily extracted from bone marrow, lack of immune rejection
(7) and have no ethical issues when
compared with embryonic stem cells (8).
As understanding on the pathophysiology of
osteoporosis deepens in the field of Traditional Chinese Medicine
(TCM), psoralen has been reported to exert an effect on the
osteogenic differentiation of BMSCs (9). Psoralen occurs naturally in the seeds
of the Psoralea corylifolia herb, which has been extensively
applied for the clinical treatment of fractures, bone defects and
osteoporosis as a herbal TCM (10).
Mechanistically, a previous study revealed that psoralen can
promote the differentiation of BMSCs into osteoblasts (11). Li et al (12) treated hFOB1.19 cells with different
concentrations (0, 5, 10, 15 and 20 µM) of psoralen and found that
psoralen stimulated the proliferation of hFOB1.19 cells in a
dose-dependent manner. The results demonstrated that the exact
mechanism may be related to the activation of the NF-κB signalling
pathway (12). In addition, Tang
et al (13) stimulated
primary mouse calvarial osteoblasts with different doses of
psoralen and found that psoralen promoted their differentiation
into osteoblasts in a dose-dependent manner (13). Psoralen upregulated the expression
of Bmp2 and Bmp4 genes, and activated the BMP
reporter gene (12xSBE-OC-Luc) (13). This indicates that the promotion of
osteoblast differentiation by psoralen may be related to BMP
signalling (13). Despite the
findings of previous studies, the exact underlying molecular
mechanism and signalling pathway mediating this process remain to
be fully elucidated.
Therefore, the present study explores the effect of
varying concentrations of psoralen on the osteogenic
differentiation of human BMSCs (hBMSCs) and investigates the
association between psoralen and the TGF-β/Smad3 pathway, with aims
of providing a reference for the development of treatment
strategies for osteoporosis.
Materials and methods
Cell culture
hBMSCs (cat. no. BNCC338194) were obtained from BeNa
Culture Collection. hBMSCs were cultured in DMEM (Thermo Fisher
Scientific, Inc.) with 100 mg/ml streptomycin and 100 U/ml
penicillin (Wuhan Boster Biological Technology, Ltd.) and 10% FBS
(Sigma-Aldrich; Merck KGaA). The cells were grown in an incubator
at 37˚C and 5% CO2. The medium was replaced every 3
days. The cells were digested using 0.05% EDTA and 0.25% trypsin
(Sigma Aldrich; Merck KGaA) and passaged when they filled ~80% of
the culture flask. hBMSCs at passage three were used in the present
study.
Treatment of cells
The hBMSCs were seeded into a 96-well plate at a
density of 1x104 cells/well and cultured in complete
medium. After 24 h the complete medium was replaced with serum-free
medium and psoralen (purity >99%; cat. no. 110739-201416; China
Food and Drug Administration) was added and dissolved in DMSO. The
concentrations used were 0.1, 1, 10 and 100 µmol/l, with each
concentration added to 6 replicate wells per experimental repeat.
The 0 µmol/l psoralen condition was used as the negative control
(NC) group. When SB431542 was required, 5 μmol of SB431542 and
different concentrations of psoralen (0.1, 1, 10 and 100 µmol/l)
were administered at the same time and co-cultured with hBMSCs at
37˚C for 3, 7 or 14 days.
Cell Counting Kit-8 (CCK-8) assay of
cell proliferation
Third-passage hBMSCs were collected and seeded into
96-well plates at a density of 1x104 cells/well and
co-cultured with different concentrations (0.1, 1, 10 and 100
µmol/l) of psoralen. Following cell culture for 12, 24, 36, 48 and
72 h at 37˚C, 10 µl CCK-8 solution (Dojindo Molecular Technologies,
Inc.) was added into each well. Cells were then incubated in the
dark at 37˚C for 2 h. Absorbance values were detected at a
wavelength of 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.). hBMSCs cultured with 0 µmol/l psoralen were
used as a NC group. A total of 6 replicate wells were established
for each group before the average values were calculated.
Cell viability analysis
Third-passage hBMSCs were seeded into 96-well plates
at 1x104 cells/well. Following cell adherence to the
culture flask, the original medium (DMEM containing 10% FBS) was
discarded and replaced with serum-free medium. Following 24 h of
incubation at 37˚C, hBMSCs were co-cultured with psoralen (0.1, 1,
10 and 100 µmol/l) for 24, 48 and 72 h at 37˚C. A total of 20 µl 5
g/l MTT was added to each well and cells were incubated at 37˚C for
4 h. Subsequently, the medium was discarded, 150 µl of DMSO was
added before shaking for 10 min and absorbance was measured using a
microplate reader at 570 nm. A total of 6 replicate wells were
established for each group and the average values were
calculated.
Alkaline phosphatase (ALP) activity
analysis
Third-passage hBMSCs were seeded into 48-well
plates. Following cell adherence to the culture flask, the DMEM was
discarded and hBMSCs were incubated with different concentrations
(0.1, 1, 10 and 100 µmol/l) of psoralen and osteogenic
differentiation medium (cat. no. RASMX-90021; Cyagen Biosciences,
Inc.) for 7 days at 37˚C. An osteogenic differentiation inducer
without any treatment was used as a NC group. The medium was
changed every 2 days. Subsequently, the cells were rinsed three
times with PBS and treated with 150 µl 0.05% Triton X-100 for 10
min at room temperature. An ALP kit (cat. no. AP0100;
Sigma-Aldrich; Merck KGaA) was used to detect the ALP activity of
hBMSCs according to the manufacturer's instructions. The wavelength
was set to 520 nm and a total of 6 replicate wells were established
in each group. The average value was calculated.
Calcium deposition detection
The Alizarin Red staining method (AR-S) for calcium
nodules was used to evaluate the extent of bone formation in
hBMSCs. Third-passage hBMSCs were inoculated into a 6-well plate
with different concentrations of psoralen (0.1, 1, 10 and 100
µmol/l) and osteogenic differentiation medium. Six replicate wells
were established for each concentration. An osteogenic
differentiation inducer without any treatment was used as a NC
group. The medium was replaced every 2-3 days for 2 weeks at 37˚C.
The cells were fixed with 4% formaldehyde for 15 min at room
temperature, adjusted to pH 4.2 and stained with 0.1% Alizarin Red
(Sigma-Aldrich; Merck KGaA). Cells were incubated overnight at room
temperature and subsequently washed three times with PBS. Stained
cells were photographed using a digital camera (Nikon Corporation)
under a light microscope (magnification, x100). In order to detect
calcium deposits, cells were then treated with 10% cetylpyridinium
chloride (Sigma-Aldrich; Merck KGaA) in 10 mM sodium phosphate for
15 min at room temperature. A multifunctional microplate reader
(Varioskan LUX; Thermo Fisher Scientific, Inc.) was used to measure
the absorbance value at 560 nm in each well. The experiment was
repeated three times and the average value was taken.
Reverse transcription quantitative
(RT-q)PCR
Third-passage hBMSCs were inoculated into a 6-well
plate and co-cultured with osteogenic differentiation medium and
different concentrations of psoralen (0.1, 1, 10 and 100 µmol/l) or
5 µmol SB431542 (cat. no. SF7890; Beyotime Institute of
Biotechnology) for 72 h at 37˚C. Following 72 h of co-cultivation
of hBMSCs with different concentrations of psoralen or SB431542,
RT-qPCR was used to detect the expression of osteogenic
differentiation-related genes of hBMSCs. Total RNA from the hBMSCs
was extracted using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). NanoDrop2000 was used to measure RNA
concentration and purity. PrimeScript™ RT Master Mix (cat. no.
RR036A; Takara Bio, Inc.) was used for reverse transcription
according to the manufacturer's instructions. The following
protocol was used: 37˚C for 15 min (reverse transcription reaction)
and 85˚C for 5 sec (reverse transcriptase inactivation reaction).
The cDNA obtained by reverse transcription was amplified on StepOne
Plus (Thermo Fisher Scientific, Inc.) using the SYBR™ Green PCR
Premix kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The thermocycling conditions used were as
follows: 50˚C for 2 min and 95˚C for 10 min; followed by 95˚C for
15 sec and 60˚C for 60 sec for 40 cycles; and final extension at
72˚C for 1 min. Relative mRNA levels were quantified using the
2-ΔΔCq method (14). The
ΔCqs values were obtained from Cq normalized to that of GAPDH. The
primers were synthesized by Sangon Biotech Co., Ltd. and are
displayed in Table I. The
experiment was repeated three times and the average value was
calculated.
 | Table ISequences of each primer used in the
present study. |
Table I
Sequences of each primer used in the
present study.
| Gene name | Primer pairs |
|---|
| Runx2 | F:
5'-TCTTAGAACAAATTCTGCCCTTT-3' |
| | R:
5'-TGCTTTGGTCTTGAAATCACA-3' |
| Osterix | F:
5'-AGAGATCTGAGCTGGGTAGAGG-3' |
| | R:
5'-AAGAGAGCCTGGCAAGAGG-3' |
| BMP4 | F:
5'-CTCCAAGAATGGAGGCTGTAGGAA-3' |
| | R:
5'-CCTATGAGATGGAGCAGGCAAGA-3' |
| OPN | F:
5'-ATCTCCTAGCCCCACAGAAT-3' |
| | R:
5'-CATCAGACTGGTGAGAATCATC-3' |
| TGF-β1 | F:
5'-CTGCTGACCCCCACTGATAC-3' |
| | R:
5'-AGCCCTGTATTCCGTCTCCT-3' |
| TGF-β RI | F: 5'-AAGATGACCGCT
CTGACATCA-3' |
| | R:
5'-CTTATAGACCTCAGCAAAGCGAC-3' |
| GAPDH | F:
5'-ATTTGGTCGTATTGGGCG-3' |
| | R:
5'-TGGAAGATGGTGATGGGATT-3' |
Western blot (WB) analysis
After 7 days of treatment with different
concentrations of psoralen or 5 µmol of SB431542, hBMSCs were lysed
with RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) containing protease and phosphatase inhibitors, and
centrifuged at 14,000 x g at 4˚C for 20 min. The supernatant was
the total protein and was used to determine the protein
concentration using the BCA method. Subsequently, 40 µg of the
extracted protein was added per lane and separated by SDS-PAGE on a
10% gel. Proteins were then transferred onto PVDF membranes and
blocked with 5% non-fat milk in PBS with 0.1% Tween-20 at 4˚C
overnight. The membranes were then probed using the following
primary antibodies: Osteopontin (OPN; cat. no. bs-0019P; 1:1,000;
BIOSS), bone morphogenic protein 4 (BMP4; cat. no. GP20102;
1:1,000; GlpBio Technology), runt-related transcription factor 2
(Runx2; cat. no. ab114133; 1:2,000; Abcam), Osterix (cat. no.
ab209484; 1:2,000; Abcam), TGF-β1 (cat. no. E1A0340C; 1:1,000;
EnoGene Biotech Co., Ltd.), TGF-β Receptor I (RI; cat. no.
E1A1126B-2; 1:1,000; EnoGene Biotech Co., Ltd.), Smad3 (cat. no.
E1A0031B; 1:1,000; EnoGene Biotech Co., Ltd.), phosphorylated (p-)
Smad3 (cat. no. E8ET1609-41; 1:1,000; EnoGene Biotech Co., Ltd.)
and internal reference GAPDH (cat. no. ab8245; 1:20,000; Abcam) at
room temperature for 2 h. Subsequently, the blot was incubated with
the diluted secondary antibody (1:5,000; cat. no. ab205718; Abcam)
and labelled with HRP at room temperature for 1 h, followed by a
wash step with 0.1% TBST. Protein expression was detected using a
SuperSignal™ West Femto Maximum Sensitivity Substrate kit (Roche
Applied Science). The relative value of the target protein was
calculated by comparing with the corresponding internal reference.
Densitometry was conducted using the ImageJ software (v1.8.0;
National Institutes of Health).
Statistical analysis
SPSS 21.0 software (IBM Corp.) was used for
statistical analysis. Metrological data was first evaluated for
normal distribution and data conforming to a normal distribution
were expressed as mean ± standard deviation. Mixed design ANOVA
with Bonferroni post hoc test was used to compare differences
between groups with multiple factors. One-way ANOVA with Tukey's
post hoc test was used to compare differences among groups with a
single factor. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of psoralen on cell
proliferation and viability
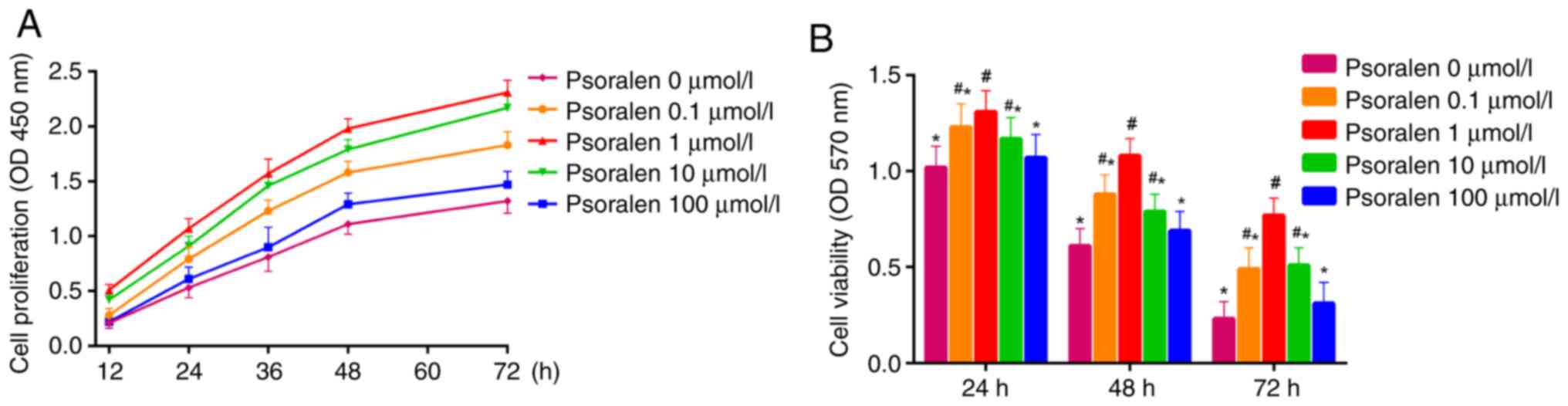
Results from the CCK-8 assay demonstrated that
elevated cell proliferation was exhibited by hBMSCs treated with
psoralen at concentrations of 0.1, 1 and 10 µmol/l, with 1 µmol/l
demonstrating the highest elevation in proliferative activity
compared with that in the NC group. There was no significant
difference in cell proliferation between concentrations of 0 and
100 µmol/l, although cell proliferation was higher at 100 µmol/l
(Fig. 1A). MTT assay revealed that
at 24, 48 and 72 h after treatment with psoralen, cell viability of
hBMSCs were increased at concentrations of 0.1, 1 and 10 µmol/l
compared with that in the NC group (Fig. 1B). The optimal concentration was
found to be 1 µmol/l. Similarly, there was no significant
difference in cell viability between 0 and 100 µmol/l. (Fig. 1B).
Effect of psoralen on the osteogenic
differentiation of hBMSCs
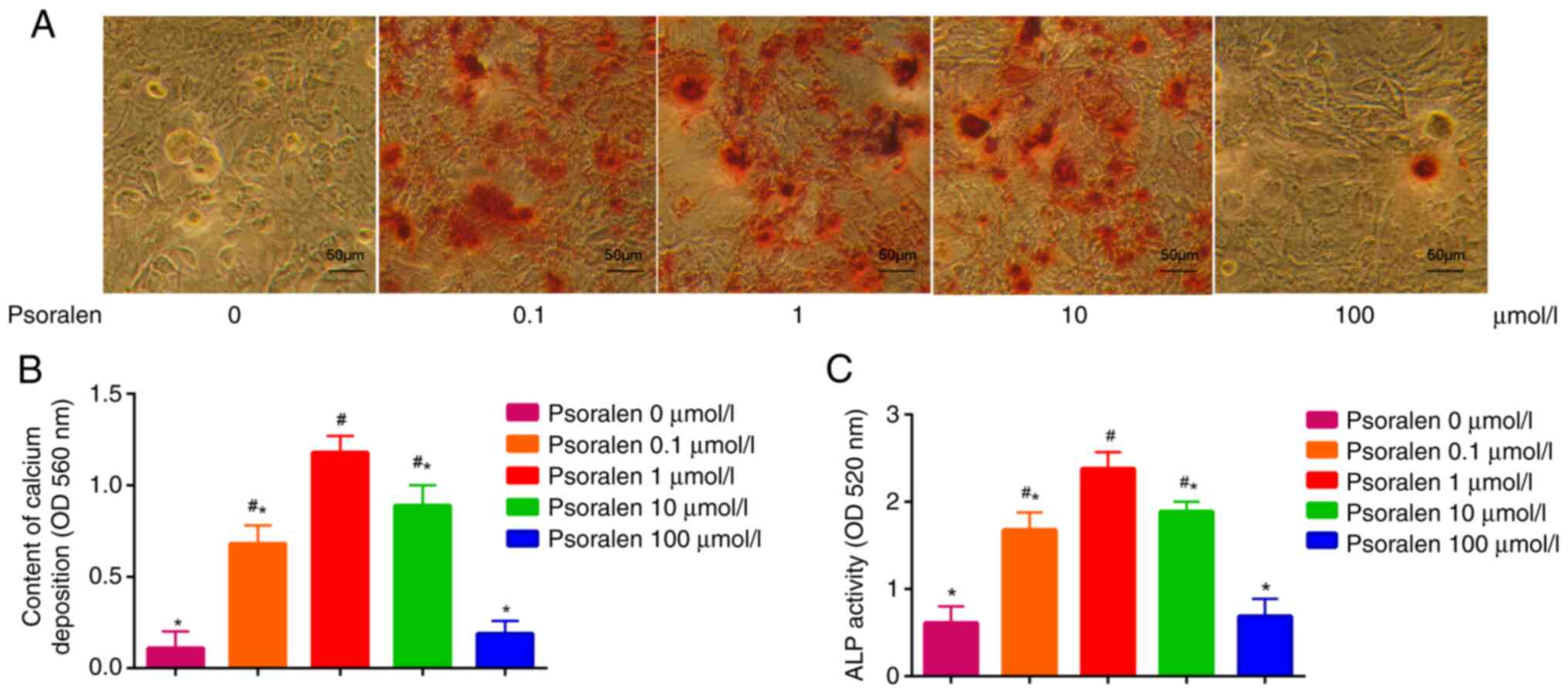
The effects of different concentrations of psoralen
on the osteogenic differentiation of hBMSCs were evaluated by
measuring ALP activity and AR-S staining of calcium deposition. The
results demonstrated that low concentrations of psoralen (0.1, 1
and 10 µmol/l) significantly promoted the formation of calcified
nodules and enhanced ALP activity in hBMSCs compared with those in
the NC cell group. However, there was no significant difference in
these two parameters tested between the high concentration (100
µmol/l) and NC groups (0 µmol/l). The most potent effect on the
osteogenic differentiation of hBMSCs was found at 1 µmol/l psoralen
(Fig. 2).
Effects of psoralen on the expression
of genes associated with osteogenic differentiation and the
TGF-β/Smad3 pathway of hBMSCs
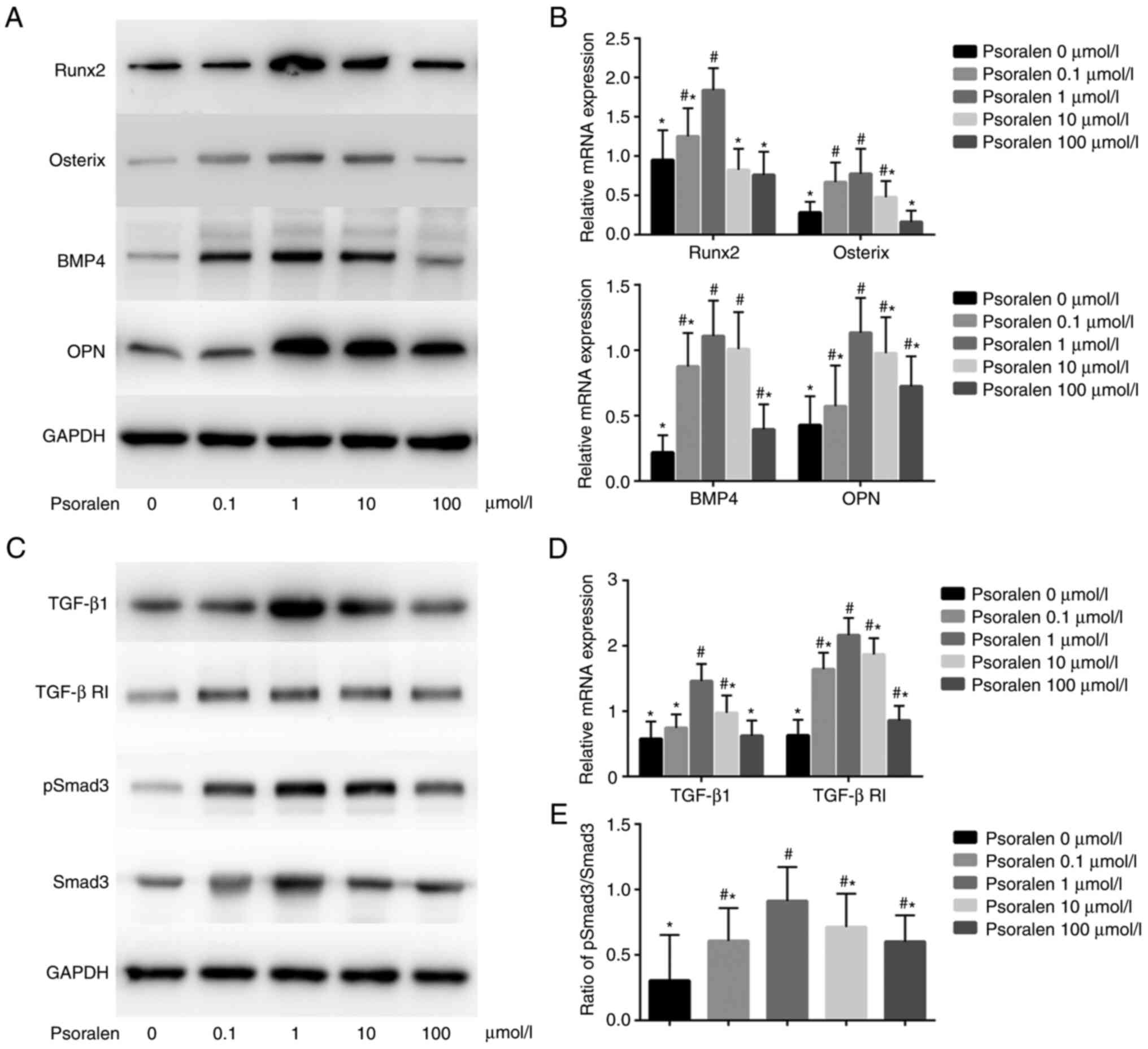
The results of qPCR and western blot analysis
revealed that low concentrations of psoralen promoted the
expression of genes associated with osteogenic differentiation.
Among these, at concentrations of 0.1, 1 and 10 µmol/l, psoralen
significantly promoted the expression of Osterix and BMP4 compared
with those in the 0 µmol/l NC group, whilst at concentrations of 1,
10 and 100 µmol/l, psoralen significantly promoted the expression
of OPN compared with those in the 0 µmol/l NC group. In terms of
Runx2, only 0.1 and 1 µmol/l of psoralen significantly promoted
expression of Runx2 compared with that in the 0 µmol/l NC group
(Fig. 3A and B). In all experimental conditions, 1
µmol/l was the optimal concentration. In addition, low
concentrations of psoralen (1 and 10 µmol/l) promoted the
expression of TGF-β1 and TGF-β RI (Fig.
3C and D), whilst all
concentrations of psoralen (0.1, 1, 10 and 100 µmol/l) promoted the
levels of p-Smad3 when compared with those in the 0 µmol/l NC group
(Fig. 3C). In addition, the
p-Smad3/Smad3 ratio was significantly increased after treatment
with psoralen compared with that in the 0 µmol/l group, with 1
µmol/l resulting in the largest increase (Fig. 3E).
Effect of the TGF-β RI inhibitor
SB431542 on osteogenic differentiation of hBMSCs
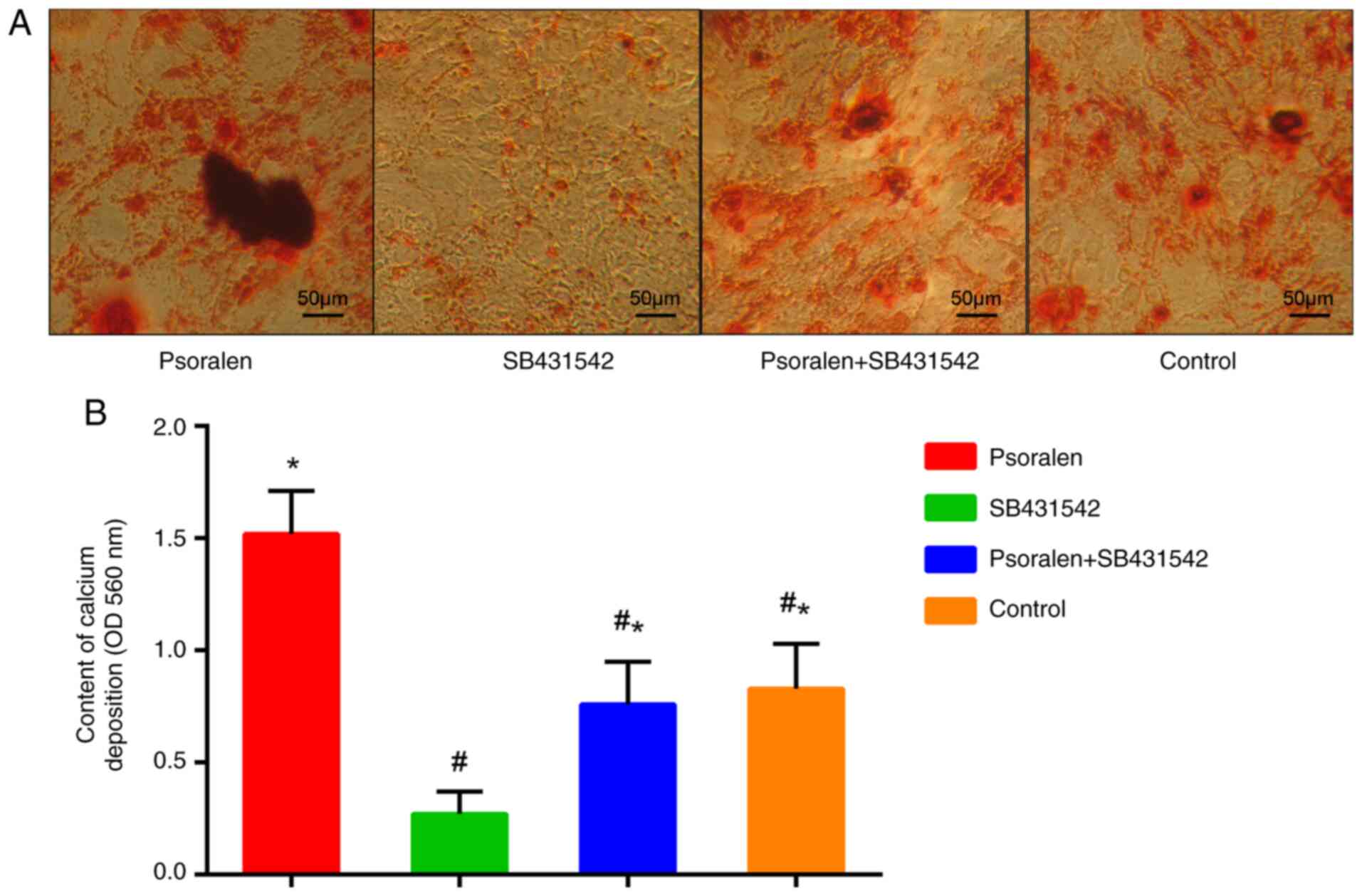
As indicated by the aforementioned results, 1 µmol/l
psoralen demonstrated the optimal effect on the osteogenic
differentiation of hBMSCs and the components of the TGF-β/Smad3
pathway. Therefore, 1 µmol/l psoralen was selected for subsequent
experiments. AR-S results revealed that psoralen promoted calcium
deposition in hBMSCs compared with all the other groups. By
contrast, SB431542 significantly inhibited the calcium deposition
of hBMSCs compared with the control group, whilst psoralen markedly
reversed this inhibitory effect (Fig.
4A and B). There was no
statistically significant difference between the control group and
the psoralen + SB431542 group.
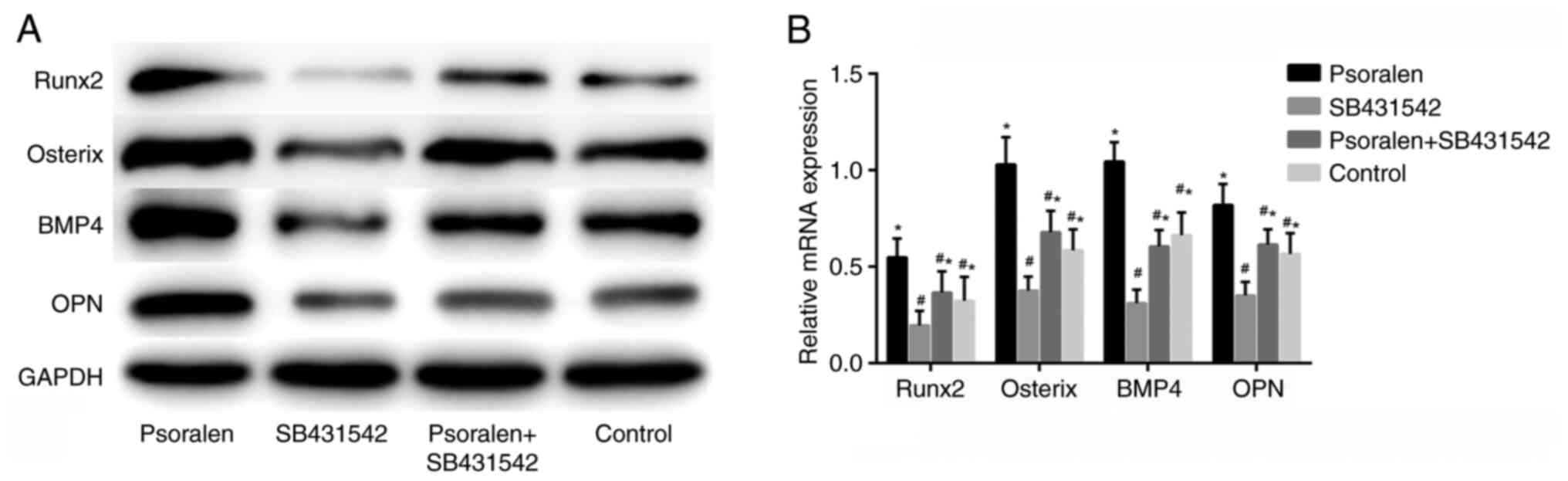
Effect of SB431542 on the expression
of osteogenic differentiation-related genes
The results of the western blot analysis and RT-qPCR
revealed that SB431542 significantly inhibited the expression of
osteogenic differentiation-related genes compared with the control
group, whilst psoralen effectively reversed the inhibitory effect
of SB431542 on the osteogenic differentiation of hBMSCs (Fig. 5A and B). There was no statistically significant
difference between those in the NC and the psoralen + SB431542
group. This suggest that the mechanism underlying psoralen
treatment in the promotion of osteogenic differentiation in hBMSCs
is closely associated with the TGF-β/Smad3 pathway.
Discussion
Previous studies have revealed that psoralen has a
relaxant effect on smooth muscle, in addition to possessing the
ability to stimulate bone formation and induce osteoblast
differentiation without affecting cell proliferation (12,13,15).
BMSCs are abundant in bone tissues and have the potential for
multidirectional differentiation (4,5,16). In
certain conditions, BMSCs can differentiate into osteoblasts with
the increased expression of osteogenic genes, including Runx2,
Osterix and ALP (17,18). In addition, alleviation of
osteoporosis and the regeneration of osteoblasts have been
previously shown to be closely associated with the number of BMSCs
in the bone marrow and their osteogenic differentiation ability
(4-6,19).
In a previous study, psoralen was revealed to
promote the osteogenic differentiation of BMSCs via the
miR-488/Runx2 pathway (11),
however, the underlying molecular mechanism and signal pathway
remained to be fully verified. In the present study, CCK-8 and MTT
assays were used to measure cell viability and cell proliferation.
These results demonstrated that psoralen promotes the proliferation
of hBMSCs and maintains cell viability in a concentration-dependent
manner. However, at the highest concentration tested (100 µmol/l),
the result was not statistically significant when compared with 0.1
µmol/l, with the overall optimal concentration demonstrated to be 1
µmol/l.
ALP is an important marker of the osteogenic
activity of hBMSCs and is a non-specific phosphomonoesterase that
is used to determine the early osteogenic differentiation ability
of hBMSCs (20). In addition, ALP
serves an important role in hBMSC calcium deposition (21). AR-S is a method that is commonly
used for examining hBMSC calcium deposition and for determining
differentiation into advanced osteoblasts (22). Results from the present study
revealed that low concentrations of psoralen, particularly at 1
μmol/l, possessed the ability to potently promote ALP activity and
calcified nodule formation in hBMSCs, suggesting that psoralen
effectively promotes the osteogenic differentiation of hBMSCs.
Huang et al (23)
demonstrated that combination therapy with BMP-2 and psoralen
enhanced fracture healing in ovariectomized mice through increased
bone-specific ALP levels and decreased C-terminal telopeptide of
type-1 collagen (23). Since hBMSCs
are a type of adult stem cell with the potential for self-renewal
and multi-directional differentiation (24,25),
it is hypothesised that specific concentrations of psoralen can
result in the following: i) Effective promotion of the
differentiation of hBMSCs into osteoblasts; ii) an increase the
activity of ALP; and iii) promotion of the formation of calcium
mineralization nodules.
To explore the underlying mechanism of psoralen in
promoting osteogenic differentiation of hBMSCs further, RT-qPCR and
western blot analysis were performed to probe the expression of
genes associated with osteogenic differentiation, namely BMP4, OPN,
Runx2 and Osterix. BMP4 belongs to the TGF-β family that has been
reported to induce cartilage and bone formation and regulates
mesoderm induction, tooth development, limb formation and bone
fracture repair (26). OPN is also
known as secreted phosphoprotein 1, bone sialoprotein 1 and
nephropontin (27). OPN binds
tightly to hydroxyapatite and appears to form an integral part of
the mineralized matrix, which is important for cell-matrix
interactions (27). Runx2 is a
specific transcription factor that is expressed in osteoblasts and
is closely associated with the early proliferation of osteoblasts
(28). Osterix is another key
transcription factor, which is located downstream of Runx2(29). Nakashima et al (29) demonstrated that the expression of
osterix could not be detected in mice carrying a mutated version of
Runx2; while the expression of Runx2 could be detected in osterix
knockout mice (29). Subsequently,
Nishio et al (30) further
demonstrated that osterix was located downstream of the Runx2 gene
in mesenchymal cells (30). It was
revealed that overexpression of Runx2 significantly increased the
promoter activity of osterix. This up-regulation was abrogated when
the Runx2 responsive element on the osterix promoter was mutated.
It is only expressed in the bone tissue and plays a key role in the
late differentiation and maturation of osteoblasts (31). In the present study, low-dose
psoralen (1 µmol/l) effectively increased the expression of hBMSC
osteogenic markers BMP4, OPN, Runx2 and Osterix. Therefore,
specific concentrations of psoralen can activate the
differentiation of hBMSCs into osteoblasts.
Signal transduction by the TGF-β superfamily of
proteins serves an important role in the regulation of cell
proliferation, differentiation and development in many biological
systems (32). Signal transduction
starts with the ligand-induced oligomerization of serine/threonine
receptor kinases and the phosphorylation of cytoplasmic signal
transduction molecules Smad2 and Smad3(33). The carboxyl terminus of Smad
proteins is phosphorylated by an activated receptor, causing it to
bind to the common signal transduction factor Smad4 and
transporting it into the nucleus (34). Activated Smad proteins regulate
various biological processes by binding to transcription factors,
such as BMP, leading to the transcriptional regulation of cell
states (35). Once bound to the
corresponding receptors, transforming growth factors, activin,
TGF-β and BMP will cause Smad to be phosphorylated, thereby
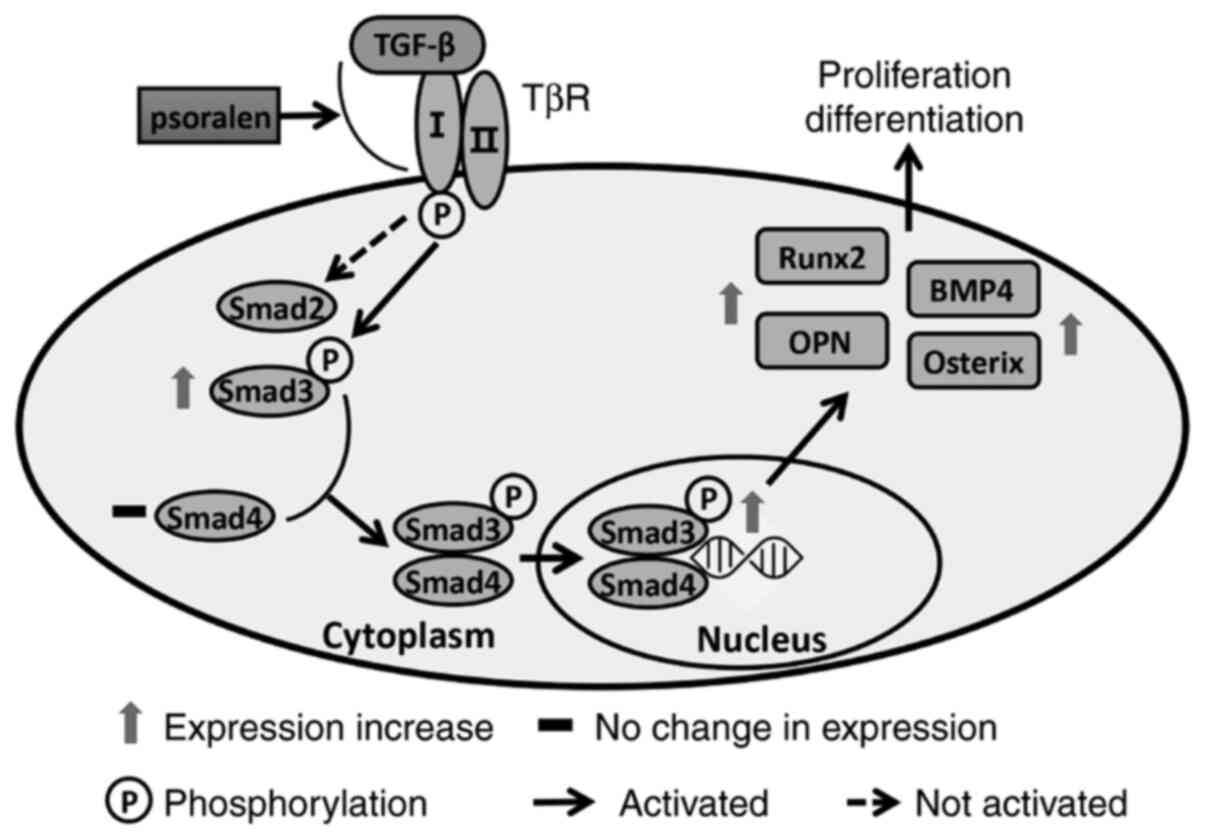
activating the TGF-β/Smad signalling pathway (36). In the present study, psoralen (1
µmol/l) significantly promoted the activation of the TGF-β/Smad3
signalling pathway and mediated the strongest effect on the
promotion of osteoblast differentiation. It was therefore
hypothesised that psoralen promotes the binding of TGF-β to the
TGF-β RI, which subsequently stimulates RI-mediated Smad3
signaling. Thus, Smad3 is activated in the nucleus which mediates
the transcription of specific genes and induces the differentiation
of hBMSCs into osteoblasts (Fig.
6). This then strengthens the activity of osteoblasts and
promotes the mineralization of the extracellular matrix.
To test this hypothesis, 5 mol/l TGF-β RI inhibitor
SB431542(37) was applied to the
hBMSCs. The results of the present study demonstrated that SB431542
effectively inhibited the formation of calcified nodules in hBMSCs
and the expression of genes associated with osteogenic
differentiation. Furthermore, psoralen reversed the inhibitory
effect of SB431542 on the osteogenic differentiation of hBMSCs.
This further suggests that psoralen can promote the differentiation
of hBMSCs into osteoblasts by activating the TGF-β/Smad3 pathway.
Combining this with findings from previous studies (11), this indicate that psoralen regulates
the expression of Runx2 through the TGF-β/Smad3 pathway, thereby
regulating the osteogenic differentiation of BMSCs.
In conclusion, the present study suggest that
psoralen promotes hBMSC cell proliferation, maintain cell viability
and increase the expression of genes associated with osteogenic
differentiation in hBMSCs, which may be closely associated with the
activation of the TGF-β/Smad3 pathway. However, the present study
has some limitations, such as the blocking of TGF-β RI alone and
not the Smad pathway, which may lead to insufficient results. A
combination of blocking both TGF-β RI and Smad would more
accurately prove that psoralen promotes the differentiation of
hBMSCs into osteoblasts through the TGF-β/Smad3 pathway. In
addition, it remains unclear how psoralen activates the binding of
TGF-β to the receptor, therefore the precise molecular mechanism
underlying this phenomenon remains to be fully elucidated and is
therefore a matter for future research.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation (grant nos. 82004387 and 81804047) and Natural
Science Foundation of Guangdong Province (grant no.
2018A030313694).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QH, YH and JL conceived and designed the
experiments; YH, LL and TJ performed the experiments; HS and XC
contributed to the statistical analysis of the data. YH and QH
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xie Y, Wang J, Wang L, Zhu Y, Lei L, Wan
T, Liao X, Liang B, Pang G, Miyamoto A, et al: Research trends in
osteoporosis in Asian countries and regions in the last 20 years.
Arch Osteoporos. 15(130)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Föger-Samwald U, Dovjak P, Azizi-Semrad U,
Kerschan-Schindl K and Pietschmann P: Osteoporosis: Pathophysiology
and therapeutic options. EXCLI J. 19:1017–1037. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim Y, Tian Y, Yang J, Huser V, Jin P,
Lambert CG, Park H, You SC, Park RW, Rijnbeek PR, et al:
Comparative safety and effectiveness of alendronate versus
raloxifene in women with osteoporosis. Sci Rep.
10(11115)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu Y, Wang H, Dou H, Tian B, Li L, Jin L,
Zhang Z and Hu L: Bone regeneration capacities of alveolar bone
mesenchymal stem cells sheet in rabbit calvarial bone defect. J
Tissue Eng. 11(2041731420930379)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong R, Bai Y, Dai J, Deng M, Zhao C, Tian
Z, Zeng F, Liang W, Liu L and Dong S: Engineered scaffolds based on
mesenchymal stem cells/preosteoclasts extracellular matrix promote
bone regeneration. J Tissue Eng.
11(2041731420926918)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Safarova Y, Umbayev B, Hortelano G and
Askarova S: Mesenchymal stem cells modifications for enhanced bone
targeting and bone regeneration. Regen Med. 15:1579–1594.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chow L, Johnson V, Regan D, Wheat W, Webb
S, Koch P and Dow S: Safety and immune regulatory properties of
canine induced pluripotent stem cell-derived mesenchymal stem
cells. Stem Cell Res (Amst). 25:221–232. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
King NM and Perrin J: Ethical issues in
stem cell research and therapy. Stem Cell Res Ther.
5(85)2014.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Yang Z, Huang JH, Liu SF, Zhao YJ, Shen
ZY, Wang YJ and Bian Q: The osteoprotective effect of psoralen in
ovariectomy-induced osteoporotic rats via stimulating the
osteoblastic differentiation from bone mesenchymal stem cells.
Menopause. 19:1156–1164. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yuan X, Bi Y, Yan Z, Pu W, Li Y and Zhou
K: Psoralen and Isopsoralen Ameliorate Sex Hormone
Deficiency-Induced Osteoporosis in Female and Male Mice. BioMed Res
Int. 2016(6869452)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang Y, Hou Q, Su H, Chen D, Luo Y and
Jiang T: miR-488 negatively regulates osteogenic differentiation of
bone marrow mesenchymal stem cells induced by psoralen by targeting
Runx2. Mol Med Rep. 20:3746–3754. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li F, Li Q, Huang X, Wang Y, Ge C, Qi Y,
Guo W and Sun H: Psoralen stimulates osteoblast proliferation
through the activation of nuclear factor-κB-mitogen-activated
protein kinase signaling. Exp Ther Med. 14:2385–2391.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tang DZ, Yang F, Yang Z, Huang J, Shi Q,
Chen D and Wang YJ: Psoralen stimulates osteoblast differentiation
through activation of BMP signaling. Biochem Biophys Res Commun.
405:256–261. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wong RW and Rabie AB: Effect of psoralen
on bone formation. J Orthop Res. 29:158–164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Z, Shi Y, Chen W, Wei H and Shang J:
Mesenchymal stem cells repair bone marrow damage of aging rats and
regulate autophagy and aging genes. Cell Biochem Funct. 38:792–800.
2020.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Xu C, Liu H, He Y, Li Y and He X:
Endothelial progenitor cells promote osteogenic differentiation in
co-cultured with mesenchymal stem cells via the MAPK-dependent
pathway. Stem Cell Res Ther. 11(537)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang S, Sun S, He J and Shen L: NT-3
promotes osteogenic differentiation of mouse bone marrow
mesenchymal stem cells by regulating the Akt pathway. J
Musculoskelet Neuronal Interact. 20:591–599. 2020.PubMed/NCBI
|
|
19
|
Wu Y, Tang Y, Zhang X, Chu Z, Liu Y and
Tang C: MMP-1 promotes osteogenic differentiation of human bone
marrow mesenchymal stem cells via the JNK and ERK pathway. Int J
Biochem Cell Biol. 129(105880)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
O'Grady S and Morgan MP: Deposition of
calcium in an in vitro model of human breast tumour calcification
reveals functional role for ALP activity, altered expression of
osteogenic genes and dysregulation of the TRPM7 ion channel. Sci
Rep. 9(542)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang J, Zhang W, Dai J, Wang X and Shen
SG: Overexpression of Dlx2 enhances osteogenic
differentiation of BMSCs and MC3T3-E1 cells via direct upregulation
of Osteocalcin and Alp. Int J Oral Sci.
11(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li J, Wang X, Yang F, Yuan J, Cui Q, Nie F
and Zhang J: Matrine enhances osteogenic differentiation of bone
marrow-derived mesenchymal stem cells and promotes bone
regeneration in rapid maxillary expansion. Arch Oral Biol.
118(104862)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang K, Wu G, Zou J and Peng S:
Combination therapy with BMP-2 and psoralen enhances fracture
healing in ovariectomized mice. Exp Ther Med. 16:1655–1662.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tuan RS, Boland G and Tuli R: Adult
mesenchymal stem cells and cell-based tissue engineering. Arthritis
Res Ther. 5:32–45. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Pereira RF, Halford KW, O'Hara MD, Leeper
DB, Sokolov BP, Pollard MD, Bagasra O and Prockop DJ: Cultured
adherent cells from marrow can serve as long-lasting precursor
cells for bone, cartilage, and lung in irradiated mice. Proc Natl
Acad Sci USA. 92:4857–4861. 1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Choi J, Bae T, Byambasuren N, Park SH, Jo
CH, Kim D, Hur JK and Hwang NS: CRISPR-Cpf1 activation of
endogenous BMP4 gene for osteogenic differentiation of
umbilical-cord-derived mesenchymal stem cells. Mol Ther Methods
Clin Dev. 17:309–316. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Carvalho MS, Cabral JM, da Silva CL and
Vashishth D: Synergistic effect of extracellularly supplemented
osteopontin and osteocalcin on stem cell proliferation, osteogenic
differentiation, and angiogenic properties. J Cell Biochem.
120:6555–6569. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Narayanan A, Srinaath N, Rohini M and
Selvamurugan N: Regulation of Runx2 by MicroRNAs in osteoblast
differentiation. Life Sci. 232(116676)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nishio Y, Dong Y, Paris M, O'Keefe RJ,
Schwarz EM and Drissi H: Runx2-mediated regulation of the zinc
finger Osterix/Sp7 gene. Gene. 372:62–70. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fu X, Li Y, Huang T, Yu Z, Ma K, Yang M,
Liu Q, Pan H, Wang H, Wang J, et al: Runx2/Osterix and Zinc Uptake
Synergize to Orchestrate Osteogenic Differentiation and Citrate
Containing Bone Apatite Formation. Adv Sci (Weinh).
5(1700755)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Thatcher JD: The TGF-beta signal
transduction pathway. Sci Signal. 3(tr4)2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Frick CL, Yarka C, Nunns H and Goentoro L:
Sensing relative signal in the Tgf-β/Smad pathway. Proc Natl Acad
Sci USA. 114:E2975–E2982. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Si J, Yang B, Yu J, Li Y and Gao P: Effect
of MiR-21 on pulmonary arterial hypertension via the TGF-β1/Smad2
signal pathway. Minerva Med. 111:181–183. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xiao YT, Xiang LX and Shao JZ: Bone
morphogenetic protein. Biochem Biophys Res Commun. 362:550–553.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hudnall AM, Arthur JW and Lowery JW:
Clinical relevance and mechanisms of antagonism between the BMP and
activin/TGF-β signaling pathways. J Am Osteopath Assoc.
116:452–461. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou HQ, Liu MS, Deng TB, Xie PB, Wang W,
Shao T, Wu Y and Zhang P: The TGF-β/Smad pathway inhibitor SB431542
enhances the antitumor effect of radiofrequency ablation on bladder
cancer cells. OncoTargets Ther. 12:7809–7821. 2019.PubMed/NCBI View Article : Google Scholar
|