Introduction
Gastric inflammatory myofibroblastic tumor (IMT) is
a rare and unique mesenchymal tumor type characterized as low-grade
malignant or borderline tumors. In recent years, the World Health
Organization (WHO) proposed the term IMT, which has gradually been
recognized by experts and scholars (1-3).
Gastric IMT occurs mostly in children and young adults (4). The most common sites are the
mesentery, omentum, posterior peritoneum and pelvic cavity,
followed by the lungs, mediastinum and head and neck (5-7),
while its occurrence in the stomach is rare. Gastric IMT mainly
manifests as a nodular mass in the stomach, which resembles a
malignant tumor and is easily misdiagnosed (8-10).
Features of gastric IMT are normally revealed by ultrasound
gastroscopy and multi-slice spiral computed tomography (11,12).
The clinicopathologic and immunohistochemical features of gastric
IMT have been reported previously, including abdominal mass,
abdominal pain, upper gastrointestinal hemorrhage; and ALK, smooth
muscle actin, and vimentin staining were observed (13,14).
However, misdiagnosis and missed diagnosis of this disease are
still common. The present study reports on the experience at our
center, focusing on histopathological diagnosis and differential
diagnosis with the aim to reduce the rates of missed diagnosis and
misdiagnosis.
Patients and methods
Patients
The present study reports on five cases of gastric
IMT, including one case encountered at the Department of Pathology
of Shenzhen Hospital of Southern Medical University (Shenzhen,
China), two cases from the Pathology Department of the 989 Hospital
of the Joint Logistics Support Department of the Chinese People's
Liberation Army (Luoyang, China), one case from the Department of
Pathology of the Third Affiliated Hospital of Zhengzhou University
(Zhengzhou, China) and one case was collected from the 990th
Hospital of the Joint Logistics Support Force of the People's
Liberation Army. These five cases of gastric IMT were definitively
diagnosed between February 2015 and February 2018. Gastric IMT was
diagnosed according to the histological diagnostic criteria of
gastric tumor pathology (10) and
the 2010 edition of the WHO digestive system tumor gastric cancer
histology classification (1). Of
the 5 cases of gastric IMT, 3 patients were male and 2 were female;
the age ranged from 12 to 41 years and the median age was 23 years.
The clinical symptoms included fatigue, epigastric fullness,
abdominal pain, melena, upper gastrointestinal bleeding and
obstruction after eating.
Sample preparation
Surgical specimens were fixed within 30 min after
excision in freshly prepared 4% formaldehyde for 8-48 h and the
ratio of stationary liquid to tissue volume was 10:1. For H&E
staining and immunohistochemical examination, six to eight pieces
of tumor tissue (conventional cutting of proximal end, distal
margin and tumor, and adjacent gastric mucosa was not used),
including the deepest infiltration point and the nearest serosal
layer, were obtained.
Immunohistochemical staining
The EnVision two-step method was used for staining.
Ready-to-use primary antibodies against vimentin (clone V9, Cat No.
kit-0019), α-smooth muscle actin (α-SMA; clone 1A4, Cat No.
kit-0006), desmin (clone D33, Cat No. MAB-0766), calponin (clone
MX023, Cat No. MAB-0712), anaplastic lymphoma kinase (ALK) p80
(polyclonal; Cat No. MAB-0281), CD34 (clone OBEnd/10; Cat No.
kit-0004), pan-cytokeratin (CKpan; clone AE1/AE3, Cat No.
kit-0009), CD117 (clone MX041, Cat No. kit-0029), anoctamin 1
(DOG1; clone SP31, Cat No. kit-0035), S-100 (clone 4C4.9, Cat No.
kit-0007) and Ki-67 (clone SP6; Cat No RMA-0542) were purchased
from Fuzhou Maixin Biotech Co., Ltd. Based on the requirements of
the primary antibody, the corresponding antigen retrieval was
performed and the steps were carried out according to the kit
instruction of each antibody. For Antigen retrieval, the slides
were placed in 10 mM sodium citrate buffer (pH 6.0) and heated
samples at 95˚C in water bath for 20 min followed by slowly cooling
to room temperature. Positive and negative controls (Fuzhou Maixin
Biotech Co., Ltd.) were established during the staining, which were
included in the respective antibody kits provided by manufacturer
(Fig. S1).
Results
Pathological features
The cases, including the patients' age and sex, as
well as tumor size, microscopic structure and immunohistochemical
staining results are summarized in Table I. In three cases, the gastric IMT
was located in the gastric antrum and in two cases, it was situated
in the gastric body. All tumors formed a bulging mass that
protruded into the gastric cavity; the submucosa and the muscularis
propria were involved in four cases and the full thickness of the
stomach wall was involved in one case. The smooth surface mucosa
was involved in three cases and surface mucosal ulcer formation
with necrosis was present in two cases. All tumors had clear
boundaries, were acapsular, nodular, had a slightly harder texture
compared with the surrounding normal regions and the cut surface
was soft and grayish-white mass. The tumor diameter ranged from 2.7
to 19.1 cm with an average tumor diameter of 5.4 cm. The mucosal
surface of the stomach cavity was smooth or ulcerated with necrosis
(Fig. 1A). Tumors were mainly
composed of spindle-shaped myofibroblasts, fibroblasts and
inflammatory cells. Histomorphology indicated that the fat fusiform
myofibroblast-fibroblasts were loosely arranged and the
inflammatory cells were diffusely or patchily distributed.
Different areas formed a diverse range of morphological structures.
On the edematous mucus-like background, mucous vascular structures
are formed. There was infiltration of proliferating
myofibroblast-fibroblasts and numerous vascular and plasma cells,
lymphocytes and neutrophils, which was similar to granulation
tissue or reactive lesions (Fig.
1B). The proliferating spindle cells were tightly sarciniform,
arranged with varying sizes of mucin-like or collagenous areas,
which was similar to fibromatosis (Fig.
1C). In the collagenous fiber-rich area, the cell density was
low and inflammatory cell components were relatively sparse, which
was similar to a scar-like structure (Fig. 1D). The prominent areas of
myofibroblast proliferation contained numerous ganglion-like cells.
In the mucous vascular structure, mucinous or collagenous areas,
i.e., fibromatosis- or scar-like lesions, were generally <10 mm
in size and both had diffuse or patchy plasmocytes, lymphocytes and
other inflammatory cell infiltration backgrounds. Gastric IMT
infiltration involved joint corrosive invasion by spindle-shaped
myofibroblasts, fibroblasts and inflammatory cells of the smooth
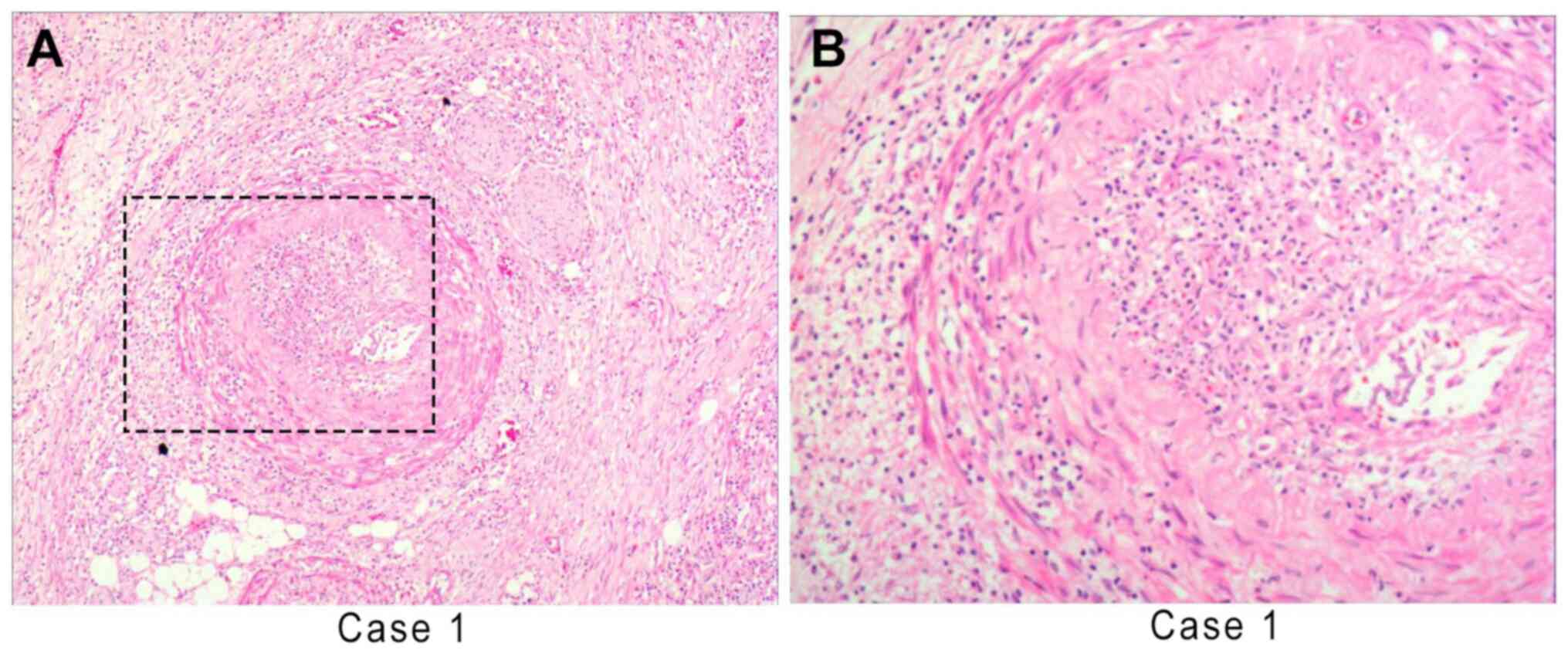
muscles of the stomach wall and adipose tissues (Fig. 2). Corrosive invasion around the
blood vessels and nerves was also able to protrude into the lumen,
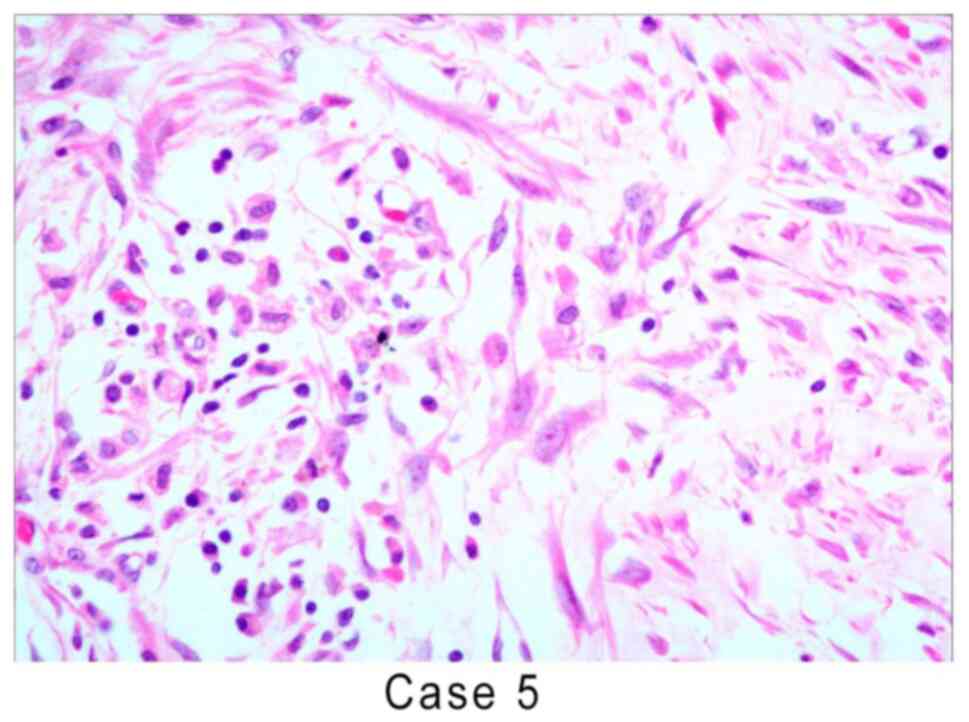
which was similar to infantile myofibroblastic disease (Fig. 3). Cytologically, the myofibroblast
and fibroblast nuclei were fat spindle-shaped with small nucleoli
and certain areas exhibited mild atypia. The myofibroblasts had
fusiform and polygonal shapes and the nucleus was vacuolated,
forming ganglion-like cells with large eosinophilic nucleoli
(Fig. 4). The inflammatory cell
types included plasma cells and lymphocytes, regional neutrophils,
tissue cells and multinucleated giant cells. There were 3-7 mitotic
figures per 10 high-power fields.
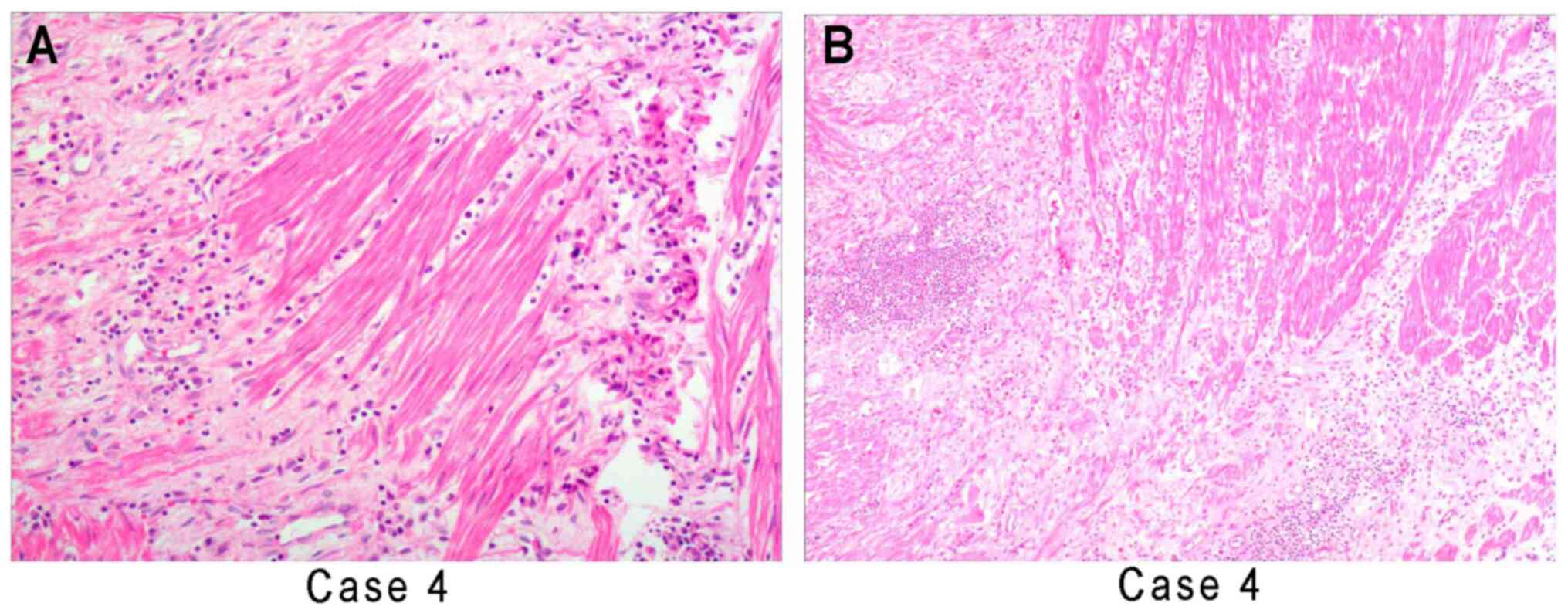
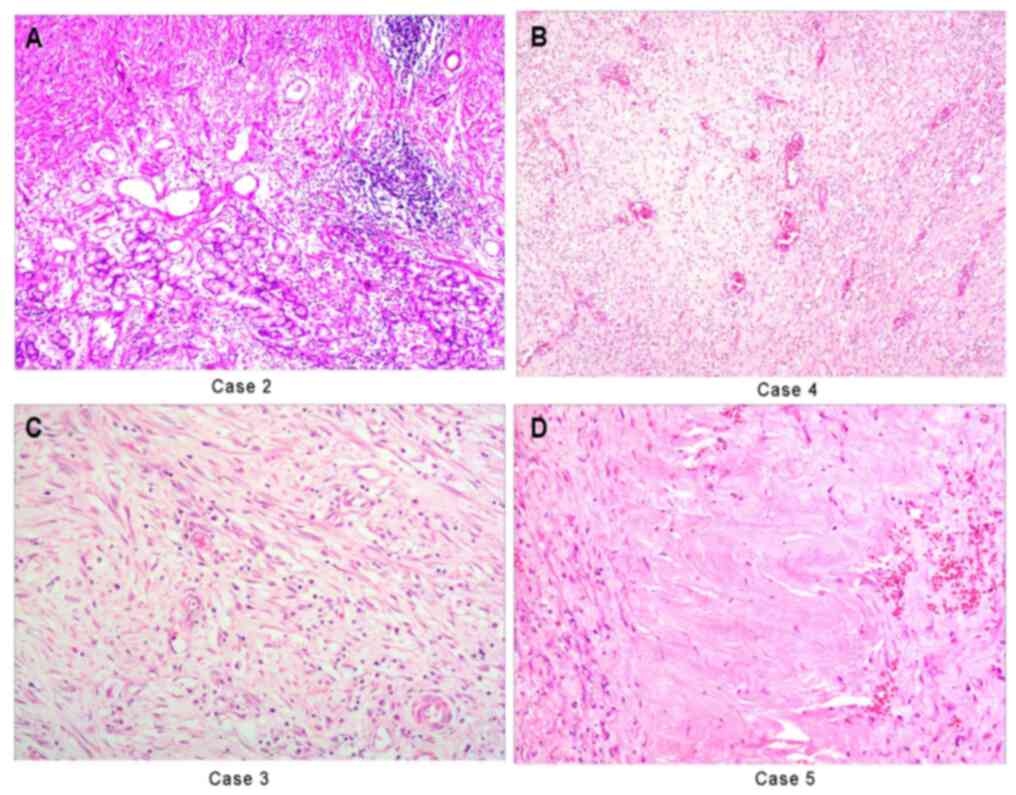 | Figure 1Histologic examination of gastric
tissue samples from case 2 stained with H&E. (A) The boundary
between the mucosa of the gastric gland and the tumor was clear,
but there was no capsule, a frequently staggered arrangement was
present and the gastric glands frequently exhibited hyperplasia
(case 2). (B) Obese or fusiform myofibroblasts are loosely
arranged, with numerous vascular and plasma cells, lymphocytes and
neutrophils infiltrating, forming mucous vascular structures, which
is similar to granulation tissue or other reactive lesions (case
4). (C) The proliferating spindle cells are tightly arranged
sarciniform and the inflammatory cells exhibit diffuse
infiltration, similar to fibromatosis (case 3). (D) Regional
collagenization and low cell density, similar to scar tissue (case
5) (magnification, x200 in A, C and D and x100 in B; scale bars, 50
µm in A, C and D and 100 µm in B). |
 | Table IClinicopathological data of five cases
of gastritis myofibroblastoma. |
Table I
Clinicopathological data of five cases
of gastritis myofibroblastoma.
| Case no. | Age (years) | Sex | Site | Tumor size (cm) | Depth | Tissue
organization | Immunophenotype | Follow-up
(months) | Outcome |
|---|
| 1 | 27 | M | Gastric antrum | 3.2x2.6x1.8 | Submucosal and
partial lamina propria | Myofibroblasts,
fibroblasts and inflammatory cells constituted diverse
morphologies; significant mucovascular structures and fibroid-like
lesions were formed. | Positive for vimentin
and SMA; weakly positive for CKpan, desmin, CD34, ALK; negative for
S-100, DOG1 and CD117. | 38 | No recurrence |
| 2 | 15 | F | Gastric antrum | 5.0x4.3x2.8 | Submucosal and
partial lamina propria | Comprised obese
myofibroblasts, fibroblasts and inflammatory cells; ganglion-like
cells with larger eosinophils were prominent; corrosive invasion of
the smooth muscles of the gastric wall. | Positive for vimentin
and SMA; weakly positive for CKpan, desmin, and CD34; negative for
ALK, S-100, DOG1 and CD117. | 42 | No recurrence |
| 3 | 12 | M | Gastric antrum | 4.5x3.7x1.5 | Submucosal and
partial lamina propria | Myofibroblasts,
fibroblasts and inflammatory cells constituted diverse
morphologies; markedly myxoid or collagenized areas formed
scar-like lesions. | Positive for vimentin
and SMA; weakly positive for CKpan, desmin, and CD34; negative for
S-100, ALK, DOG1 and CD117. | 36 | Local recurrence |
| 4 | 41 | M | Gastric body | 6.1x4.6x3.5 | Submucosal and
partial lamina propria | Myofibroblasts,
fibroblasts and inflammatory cells; prominently larger eosinophilic
ganglion-like cells. | Positive for vimentin
and SMA; weakly positive for CKpan, desmin, CD34, and ALK; negative
for S-100, DOG1 and CD117. | 12 | No recurrence |
| 5 | 22 | F | Gastric body | 6.8x5.4x4.7 | Full stomach
wall | Obese myofibroblasts,
fibroblasts and inflammatory cells constituted varied morphologies;
corrosive invasion of the smooth muscle, blood vessels, nerves and
adipose tissue of the gastric wall. | Positive for vimentin
and SMA; weakly positive for CKpan, desmin, CD34, and ALK; negative
for S-100, DOG1 and CD117. | 34 | No recurrence |
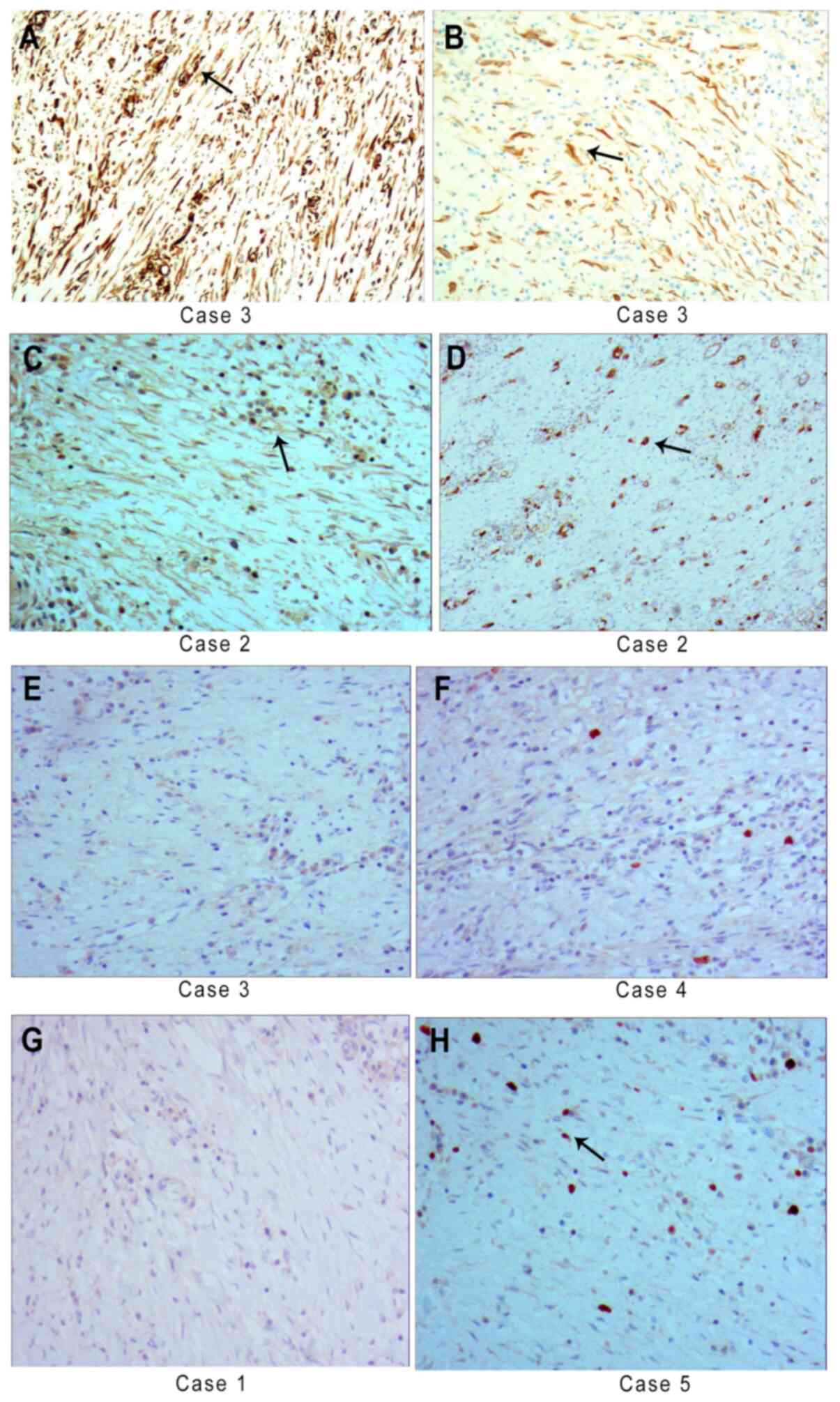
Immunohistochemical staining
All five cases had positive but diffuse vimentin
(Fig. 5A) and SMA expression and
CKpan (Fig. 5B), desmin and
calponin expression were partly positive. Furthermore, three cases
had focal positive expression of ALK (Fig. 5C). Focal positive expression of CD34
was detected in two cases (Fig.
5D). The cases were negative for S-100 (Fig. 5E), DOG1 (Fig. 5F) and CD117 expression (Fig. 5G). Ki-67-positive expression was
present in 5-15 per 100 cells in one slide (Fig. 5H). Positive and negative control
images are provided in Fig.
S1.
Follow-up
The patients were followed up by telephone and the
follow-up time was 12-42 months. One patient had local recurrence
at the primary tumor site at 3 years and 6 months after local
resection of the stomach. This patient underwent a second
resection. The other four cases had no recurrence.
Discussion
IMT was first reported in two cases of benign
pulmonary spindle cell tumor in 1939(11). In 1954, it was proposed that
pulmonary spindle cell proliferation was a post-inflammation tumor,
which was later termed inflammatory pseudotumor and became a
disease classification (15). After
the 1980s, it was determined that inflammatory pseudotumor is
closely related to certain actual tumors (16). The terms plasma cell granuloma,
fibrinous xanthogranuloma, mucinous hamartoma, pseudosarcoma and
inflammatory pseudosarcomatoid myofibroblast sarcoma have been used
to describe the disease (11).
Since the initial case report, numerous cases have been accumulated
in recent years, which may be utilized for exploring the clinical
manifestations and pathological features of IMT. The WHO soft
tissue tumor pathology and genetic classification defines it as a
mesenchymal tumor composed of differentiated myofibroblastic
spindle cells with numerous inflammatory cells, with low-grade
malignant or borderline tumor characteristics (1). The present study reported on five
cases of gastric IMT and indicated that the tumor formed a bulging
mass protruding into the gastric cavity, involving the stomach
wall. The tumor was acapsular, nodular and the cut surface was soft
and grayish-white mass. The tumor diameters ranged between 2.7 and
19.1 cm with an average tumor diameter of 5.4 cm, similar to that
reported in a previous study (4.5-8 cm) (13). However, the large amount of mature
bone-like tissue reported in a case study was not observed
(14). S-100, CD21, CD34, CD35,
CD68 and CD117 were negative in all IMTs (13); CD117, CD34, DOG1, CK, S100,
epithelial membrane antigen (EMA) and desmin were all negative in a
previous case report (14).
However, CD34 exhibited partial positive expression in the present
study. ALK was positive in the present study, similar to the result
reported in the previous case study (14).
A total of six histopathological diagnostic key
points may be proposed: i) The tumor is acapsular and nodular and
the cut surface is soft and grayish-white mass; ii) the fat
fusiform myofibroblast-fibroblasts are loosely arranged and the
inflammatory cells are diffusely or patchily distributed, forming a
diverse morphological structure; iii) in the mucous vascular
structure, mucinous or collagenous areas, or fibromatosis- or
scar-like lesions, are generally <10 mm in size and both have
diffuse or patchy plasma cells, lymphocytes and other inflammatory
cell infiltration backgrounds; iv) corrosive invasion of the smooth
muscles of the stomach wall, blood vessels, nerves, and adipose
tissues is present; v) cytologically, the myofibroblast and
fibroblast nuclei are wide spindle-shaped, with small nucleoli, and
certain areas exhibit mild atypia. In certain regions,
myofibroblasts form fusiform and polygonal shapes and the nucleus
is vacuolated, forming ganglion-like cells with large eosinophilic
nucleoli; vi) immunophenotype: Positive but diffuse vimentin and
SMA diffuse expression; CKpan, desmin, calponin and CD34 partial
positive expression; 50% of cases are positive for ALK
expression.
The etiology of gastric IMT remains elusive and it
may originate from stem cells differentiated into myofibroblasts in
the gastric mesenchyme. Associated pathogenic factors include
surgery, trauma, inflammation, abnormal repair or unique infection
(17). Genetically, the tumors are
heterogeneous; 50-70% of pediatric or young patients have clonal
cytogenetic rearrangements; the affected chromosome is 2p23 and the
ALK gene on this fragment fuses with various partner genes, such as
the gene for tropomyosin 3 (TPM3), TPM4, or Ran-binding protein 2,
whereas these changes are not common in patients aged >40 years
(18). The ALK fusion gene results
in activation and overexpression of the tyrosine kinase domain at
the C-terminus of the ALK protein, which is limited to neoplastic
myofibroblasts. Immunohistochemical staining of the ALK protein
C-terminus is the most effective means of determining the presence
of ALK gene rearrangement in IMT. In gastric IMT,
immunohistochemical staining indicated positive ALK expression in
50-60% of cases (19). In the
present study, two patients were ALK-negative and both were >35
years old, which is consistent with previous studies (20,21).
Gastric IMT has no specific clinical manifestations,
but has unique morphological features on histopathology. Therefore,
the pathological diagnosis should be differentiated from benign and
malignant spindle cell tumors and tumor-like lesions:
I) Inflammatory fibrous polyps, which are
mesenchymal hyperplasia composed of a mixture of spindle cells,
small blood vessels and inflammatory cells (particularly
eosinophils). The age at onset is usually 60-75 years. The average
diameter is 1.5 cm and it is a non-pedunculated, hard polypoid
mass. Histologically, inflammatory fibrous polyps are composed of
loose connective tissue; the major component is fusiform
fibroblasts mixed with varying numbers of inflammatory cells and
hyperplastic thin-walled blood vessels, with regional edema or
mucoid background. The inflammatory cells are mainly lymphocytes
and eosinophils, and at times, eosinophils are the main component
of inflammation, frequently surrounding the blood vessels.
Occasionally, hyperplastic mesenchymal cells surround the small
blood vessels and medium-sized blood vessels to form a
concentric-circular structure. Immunohistochemical staining
indicates positive vimentin and CD34 expression and negative SMA
and ALK expression (22).
II) Gastrointestinal stromal tumor (GIST) is the
most common type of mesenchymal tumor of the stomach. It is more
common in older patients with a median age of 60-65 years. GIST may
occur anywhere in the stomach, from the smallest adherent nodules
to large complex masses intracavity and outside of the cavity. GIST
has numerous histological types, most of which are spindle-shaped
cell types. A small number of epithelial-like cell types and hybrid
spindle epithelial-like cells may be present in a small proportion
of cases and certain special forms have sarcomatoid
characteristics, accompanied by numerous nuclear heterotypes and
mitotic phases, which are pleomorphic forms. Most GISTs are
positive for CD117 and DOG1 expression and certain GISTs are
positive for CD34 and S-100 expression. By contrast, CD117 and DOG1
expression is negative in gastric IMT and in certain cases, ALK
expression is present (23,24).
III) Primary invasive fibromatosis of the stomach is
composed of well-differentiated fibroblasts, with varying amounts
of collagen fibers, a single cell component and a small number of
inflammatory cells; strong invasion and destruction of surrounding
tissues is present, with the characteristics of directional
invasion and destruction of smooth muscle, blood vessels and nerve
tissue. Immunohistochemical staining is positive for vimentin, SMA
and β-catenin; in particular, β-catenin expression is positive in
the nuclei; CKpan, CD34, S-100, desmin and ALK are not expressed
(25).
IV) Malignant solitary fibrous tumor (SFT) of the
stomach is composed of no fixed tissue, the histological features
are irregular distribution of areas scarce and rich in tumor cells
and there is denser scar-like collagen fiber deposition and
branching vascular peripheral cell tumor-like vascular separation
(hemangiopericytoma-like area). Gastric malignant SFT cells are
abundant, tumor cells are at least moderately to severely
heterotypic and there is tumor tissue necrosis. Lymph node
metastasis occurs in gastric malignant SFT. The immunophenotype is
positive for CD99, CD34, Bcl-2 and vimentin in tumor cells; in
addition, focal weak positive expression of CKpan, EMA, SMA, S-100
and desmin is present. ALK, CD68, CD163, CD21, CD23, β-catenin,
CD117 and DOG1 are not expressed (26).
V) In schwannomas, the nuclei are arranged in
palisades. Immunophenotypically, S-100 expression is positive and
glial fibrillary acidic protein is usually expressed, while CD117,
ALK, desmin and SMA expression are negative.
VI) Synovial sarcoma, a malignant mesenchymal tumor
type, exhibits varying degrees of epithelioid differentiation, with
characteristic chromosomal translocation t (X;18) (p11;q11),
resulting in synovial sarcoma (SS)-specific, SS18-SSX gene
fusion (27). The immunophenotype
is CK and EMA positive expression.
In conclusion, gastric IMT is rare, with unique
histopathological changes and corrosive invasion of the smooth
muscle of the stomach wall, blood vessels, nerves and adipose
tissue. It should be differentiated from spindle cell tumors and
tumor-like lesions, where a frequent and long-term follow-up
strategy should be established to improve the diagnostic rate and
to reduce the rates of missed diagnosis and misdiagnosis. In the
present study, no western blot or PCR analysis was used to support
the immunohistochemical data, which is a limitation of the study.
As ~50% of conventional IMTs overexpress ALK, the ALK level
detected by western blot and/or PCR analysis should aid the
diagnosis of IMT, although being ALK-negative is not an indicator
to exclude IMT. In the future, novel biomarkers should be developed
to aid the diagnosis of this disease.
Supplementary Material
Images of
immunohistochemistry-positive and negative controls.
Photomicrographs of paired, stained tissue samples with (left
column) and without (right column) antibodies (hematoxylin
counterstain, all x200 magnification). Immunostaining include
antibodies against vimentin for (A) fibrous tissue and (B)
epithelial tissue of parathyroid gland. CKpan staining for (C)
epithelial tissue of adenocarcinoma and (D) fibrous tissue of
hemangioma. ALK staining for (E) inflammatory myofibroblastic tumor
tissue and (F) schwannoma. CD34 for (G) solitary fibrous tumor
tissue and (H) lymphatic tissue of lymphoma. S-100 for (I)
gastrointestinal stromal tumor tissue and (J) colonic mucosal
tissue. Anoctamin-1 for (K) gastrointestinal stromal tumor tissue
and (L) tumor tissue of leiomyoma. CD117 for (M) gastrointestinal
stromal tumor tissue and (N) tumor tissue of leiomyoma. Ki-67 for
(O) lymphatic tissue and (P) tumor tissue of leiomyoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW, SW and LS conceived the study, participated in
its design and coordination, wrote the manuscript and secured
funding. LS, CZ, and TY performed the experiments. LS, TY, PW, SW
and CZ collected the data and performed the data analysis and the
follow-up of the patients. YW, SW and LS checked and approved the
authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from the subjects; for
minors, the guardians of the patients provided informed consent for
their child to be included in this study. This study was approved
by the Institutional Review Board at Shenzhen Hospital of Southern
Medical University (Shenzhen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Odze RD, Lam AK, Ochiai A and Washington
MK: WHO classification of tumours of the digestive system. Lyon:
International Agency for Research on Cancer, 2019.
|
|
2
|
Theilen TM, Soerensen J, Bochennek K,
Becker M, Schwabe D, Rolle U, Klingebiel T and Lehrnbecher T:
Crizotinib in ALK+ inflammatory myofibroblastic
tumors-Current experience and future perspectives. Pediatr Blood
Cancer. 65(65)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raggio B and Chheda N: Inflammatory
myofibroblastic tumor of the epiglottis excised with a carbon
dioxide laser: Case report and literature review. Ear Nose Throat
J. 97:E31–E33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yutaka Y, Sato T, Matsushita K, Aiba H,
Muranishi Y, Sakaguchi Y, Sugiura T, Okada M, Nakamura T and Date
H: Pulmonary inflammatory myofibroblastic tumor in children: A case
report and brief review of literature. Semin Thorac Cardiovasc
Surg. 30:230–237. 2018.
|
|
5
|
Kube S, Vokuhl C, Dantonello T, Scheer M,
Hallmen E, Feuchtgruber S, Escherich G, Niggli F, Kuehnle I, von
Kalle T, et al: Inflammatory myofibroblastic tumors-A retrospective
analysis of the Cooperative Weichteilsarkom Studiengruppe. Pediatr
Blood Cancer. 65(e27012)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hu J and Chen TB: Inflammatory
myofibroblastic tumor of the paranasal sinus: Three cases report.
Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 31:722–724.
2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
7
|
Sagar AES, Jimenez CA and Shannon VR:
Clinical and Histopathologic correlates and management strategies
for inflammatory myofibroblastic tumor of the lung. A case series
and review of the literature. Med Oncol. 35(102)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hayashi M, Kawakubo H, Mayanagi S,
Nakamura R, Suda K, Wada N and Kitagawa Y: Gastric inflammatory
myofibroblastic tumor treated with combined laparoscopic and
endoscopic gastric wedge resection: A case report. World J Surg
Oncol. 16(161)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jadhav M, Harvi R, Patil R and Kittur S:
Inflammatory myofibroblastic tumor of the stomach presenting as an
Exophytic Mass-A diagnostic dilemma. Turk Patoloji Derg.
35:151–156. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y: Gastric Cancer Pathology
[M]//Chunfang Gao, Yangkun Wang. Digestive System Oncology. 1st
Edition, Beijing, People's Military Medical Press, pp296-404,
2012.
|
|
11
|
Strianese D, Tranfa F, Finelli M, Iuliano
A, Staibano S and Mariniello G: Inflammatory myofibroblastic tumor
of the orbit: A clinico-pathological study of 25 cases. Saudi J
Ophthalmol. 32:33–39. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee JE, Choi SY, Lee HK, Yi BH, Lee MH,
Lee S, Lee SJ, Lee J and Jeong WK: Computed tomographic features of
inflammatory myofibroblastic tumour of the stomach in adult
patients: An analysis of five multicentre cases with literature
review. J Med Imaging Radiat Oncol. 62:769–776. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shi H, Wei L, Sun L and Guo A: Primary
gastric inflammatory myofibroblastic tumor: A clinicopathologic and
immunohistochemical study of 5 cases. Pathol Res Pract.
206:287–291. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cheng B, Yang C, Liu Z, Liu L and Zhou L:
Primary gastric inflammatory myofibroblastic tumor: A case report.
Medicine (Baltimore). 97(e13423)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pettinato G, Manivel JC, De Rosa N and
Dehner LP: Inflammatory myofibroblastic tumor (plasma cell
granuloma). Clinicopathologic study of 20 cases with
immunohistochemical and ultrastructural observations. Am J Clin
Pathol. 94:538–546. 1990.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Coindre JM: Histologic classification of
soft tissue tumors (WHO, 1994). Ann Pathol. 14:426–427.
1994.PubMed/NCBI(In French).
|
|
17
|
Lemale J, Boudjemaa S, Parmentier B, Ducou
Le Pointe H, Coulomb A and Dainese L: A pseudotumoral lesion
revealing Meckel's diverticulum. Arch Pediatr. 23:1157–1160.
2016.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
18
|
Rao N, Iwenofu H, Tang B, Woyach J and
Liebner DA: Inflammatory myofibroblastic tumor driven by novel
NUMA1-ALK fusion responds to ALK inhibition. J Natl Compr Canc
Netw. 16:115–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fan J, Yang M, Huang B, Wang Z, Luo D,
Zhang J, Zhang P, Shi H, Li Y and Nie X: ALK expressed in a
gastrointestinal stromal tumor harboring PDGFRA p. D842V mutation:
A case report. Diagn Pathol. 15(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mariño-Enríquez A, Wang WL, Roy A,
Lopez-Terrada D, Lazar AJ, Fletcher CD, Coffin CM and Hornick JL:
Epithelioid inflammatory myofibroblastic sarcoma: An aggressive
intra-abdominal variant of infammatory myofibroblatic tumor with
nuclear membrane or perinuclear ALK. Am J Surg Pathol. 35:135–144.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Koh J, Lee KL, Lee MS, Ahn HS and Chang
MS: Gastric inverted hyperplastic polyp with inflammatory
myofibroblastic tumor-like stroma, mimicking GI stromal tumor.
Gastrointest Endosc. 89:433–435. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Klingbeil KD, Balaban A, Fertig RM, Gamret
AC, Gong Y, Torres C and Satahoo SS: Inflammatory fibroid polyp of
the gastric antrum presenting as hypovolemic shock: Case report and
literature review. Intractable Rare Dis Res. 6:304–309.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lacka DE and Nasierowska-Guttmejer A:
Fibromatosis-immunohistochemical evaluation, differential diagnosis
from gastrointestinal tumors, and other mesenchymal tumours. Prz
Gastroenterol. 14:79–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rowe SP and Fishman EK: Cinematic
rendering of neurofibromatosis type I gastrointestinal stromal
tumors. Radiology. 291(298)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang YK, Jiang B, Yang YC, Wang SN, Li YY,
Meng NL, Yuan XT, Jiang RD and Li ZG: Gastric aggressive
fibromatosis: Report of a case and review of the literature. Int J
Clin Exp Pathol. 12:372–377. 2019.PubMed/NCBI
|
|
26
|
Voth E, Serio S, Gross J, Singh A, Dietz N
and Nandipati K: Solitary fibrous tumor of the stomach with
high-grade sarcomatous dedifferentiation. J Surg Case Rep.
2018(rjy307)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Olsen G, Beal EW, Pfeil S and Dillhoff M:
Primary gastric synovial sarcoma mimicking a gastrointestinal
stromal tumor (GIST): Gastric synovial sarcoma. J Gastrointest
Surg. 22:1450–1451. 2018.PubMed/NCBI View Article : Google Scholar
|