Introduction
Calorie restriction has been demonstrated to
mitigate the age-associated decline of several pathophysiological
parameters and to extend the maximum lifespan in various animal
species (1-3).
Fasting is one type of calorie restriction and has been widely
practiced as a type of medical application or religious ritual.
Fasting is defined as abstinence from or reduction of food, drink
or both for a period typically lasting between 12 h and 3 weeks, in
short-term, long-term or intermittent patterns (4,5).
Intermittent fasting is an umbrella term for various diets
comprising a cycle of a period of fasting and non-fasting (6,7). In
fact, intermittent fasting has been practiced by the Muslim
population for over a thousand years in the month of Ramadan. This
usually involves 12-16 h of daily fasting by abstinence of both
drink and food for one month (8).
The health benefits of intermittent fasting have
been extensively demonstrated in animal models (9-11).
Furthermore, certain observational studies have been performed
suggesting potential benefits of reduced cancer risk and metabolic
disease associated with intermittent fasting in humans (12,13).
However, the mechanisms of health promotion by fasting have
remained largely elusive and metabolic regulation is conceivably
essential. As a state of negative energy balance, even a single
fasting interval in humans (e.g., overnight) may reduce basal
concentrations of specific metabolic biomarkers (insulin and
glucose) that are associated with chronic diseases (7). It has been indicated that long-term
dietary restriction reduces the metabolic rate and bodyweight
(14,15), while certain studies reported that
fasting improves the metabolic state due to weight loss and a
greater extent of fat burning (16,17).
In the context of Ramadan fasting, changes may range from a
reduction to a rise in bodyweight (18-20).
The liver is a central organ required for metabolic
homeostasis (21,22). The liver takes up glucose and
synthesizes glycogen and triglycerides following food intake,
releases glucose produced by glycogenolysis or gluconeogenesis and
triggers ketogenesis during fasting (23). High glucagon is produced during
fasting, which stimulates glucose release from the liver to provide
a continuous fuel supply for peripheral tissues (24,25).
Therefore, observing the alterations of small molecules by
metabolic profiling in the liver allows comprehensive analysis of
changes in several metabolic and signaling pathways and their
interactions (26,27). Previous studies had described the
health benefits of intermittent fasting (7,28,29),
but little was known about the mechanism-of-action. Furthermore,
variety types of ‘intermittent fasting’ had been studied, but many
were not relevant to human population.
It is impossible to get blood samples and liver
tissues from healthy volunteers during Ramadan fasting. The present
study mimicked the Ramadan fasting pattern in mouse models, aiming
to evaluate the effects of daily 12-h intermittent fasting for 1-2
months in mice. The focus of the experiments was on the effects and
mode of action of 12-h intermittent fasting on liver physiology and
metabolism. These are very relevant to the human population and
will add new knowledge to the field.
Materials and methods
Animal model and treatment
regimens
Male C57BL/6 mice (4-6 week-old; n=51) were
purchased from the Experimental Animal Center of Lanzhou Veterinary
Research Institute (Lanzhou, China). The animal experiments were
approved by the Laboratory Animal Ethics Committee of Northwest
Minzu University (Lanzhou, China), which is under the supervision
of the Experimental Animal Committee of Lanzhou Veterinary Research
Institute. Mice were housed individually in isolated cages (one
mouse per cage) at a standardized temperature (18-23˚C) with ~50%
humidity and a 12-h light/dark cycle. The mice were fed a standard
diet (62% carbohydrate, 26% protein and 12% fat) and had free
access to purified water. In addition, the health status of the
mice was monitored during daily feeding. Excessive weight loss (20%
of the average body weight of mice in the same period), loss of
appetite, weakness (inability to eat or drink on their own,
inability to stand or barely able stand for 24 h) and seizures
(30) were considered humane
endpoints, which did not happen in subsequent experiments.
In general, the mice were subjected to overnight
fasting prior to sacrifice. However, the present study aimed to
specifically investigate the effects of intermittent fasting.
Therefore, prior to sacrifice, the intermittent fasting group was
subjected to 12 h of fasting, while the control group was
maintained with ad libitum feeding. For the 30-day
experiment, the mice were randomly divided into two groups
(n=10/group) as follows: The ad libitum group (AL) and
intermittent fasting group (IF; Fig.
1A). In the 60-day experiment, the mice were randomly divided
into three groups (n=8-14/group), including the AL group, IF group
and the intermittent fasting followed by ad libitum feeding
group (IF&AL; Fig. 1B). In the
AL group, the mice had free access to food and water, while in the
IF group, food and water were removed for 12 h during the nighttime
(from 8:00 p.m. to 8:00 a.m.) on a daily basis (Fig. 1C) (31,32).
The food intake and bodyweight of the mice were measured every 10
days until the end of the study. All animal food was purchased from
the Double Lion Experimental Animal Feed Technology Co., Ltd. The
mice were anesthetized using 4% isoflurane United States
Pharmacopoeia (USP) 100% (1,000 ml/min; 4% isoflurane for
induction; 500 ml/min; 1.75-2.5% isoflurane for maintenance) and
then sacrificed by cervical dislocation. The livers were
immediately isolated and weighed. One portion of the liver was
fixed in 4% paraformaldehyde and the remaining liver was rapidly
frozen in liquid nitrogen and then transferred to a -80˚C
freezer.
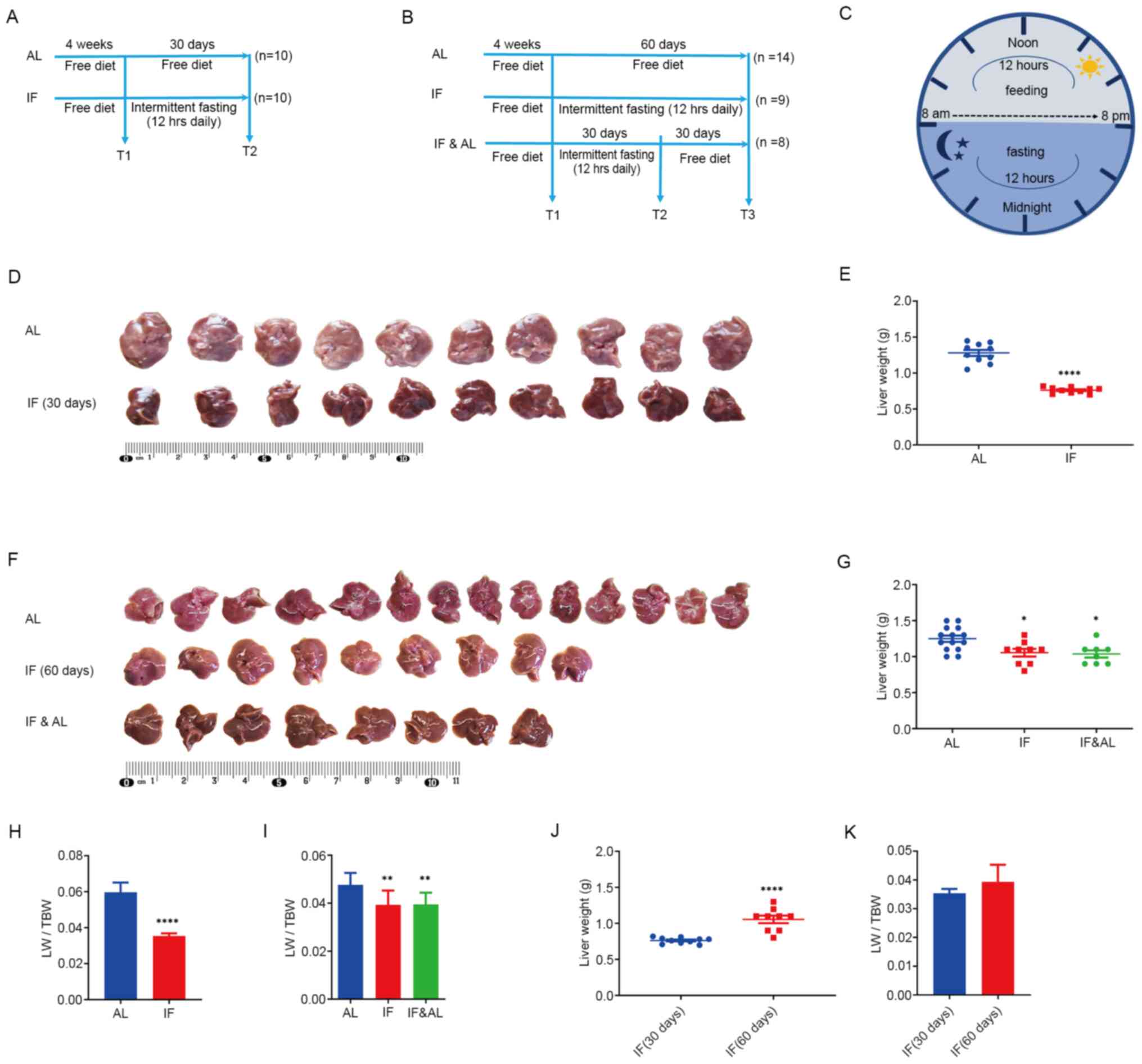 | Figure 1Effects of intermittent fasting on
mouse livers. (A) Experimental design for the first experiment (30
days), including the schedule for the AL group (n=10) and IF group
(n=10). In the AL group, mice had free access to food and water,
while in the IF group, food and water were taken away for 12 h
during the nighttime on each day. (B) Experimental design for the
second experiment (60 days), including the AL group (n=14), IF
group (n=9) and IF followed by AL (IF&AL) group (n=8). In the
AL group, mice had free access to food and water. In the IF group,
food and water were taken away for 12 h during the nighttime on
each day. In the IF&AL group, the mice were given free access
to food and water after one month of IF. (C) Schematic illustrating
the experimental design for the timing of feeding and fasting. (D)
Images of mouse livers from the first experiment. (E)
Quantification of the liver weight in the first experiment. (F)
Images of mouse livers from the first experiment. (G)
Quantification of the liver weight in the second experiment. (H and
I) Quantification of LW/TBW in (H) the first experiment and (I) the
second experiment. (J) Liver weight and (K) LW/TBW comparison
between mice fasted for 30 and 60 days. Values are expressed as the
mean ± standard error of the mean. *P<0.05,
**P<0.01, ****P<0.0001). IF,
intermittent fasting; AL, ad libitum; LW/TBW, liver
weight/total bodyweight. |
Histological examination of mouse
liver
The livers were processed in a series of stages,
including alcohol dehydration, clearing by xylene and then
embedding in paraffin. Deparaffinized sections of the liver (4 µm)
were rehydrated and stained with H&E according to standard
procedures.
Targeted metabolomics by
liquid-chromatography tandem mass spectrometry (LC-MS/MS)
The 30-day AL and 30-day IF groups were analyzed by
targeted metabolomics. Prior to sacrifice, the animals in the IF
group were subjected to 12-h fasting, while the control group was
able to feed ad libitum. The livers were immediately
isolated and rapidly stored in liquid nitrogen after sacrifice and
were finally transferred to a -80˚C refrigerator. The frozen liver
tissues were added in cold methanol/acetonitrile/H2O and
vortexed. The sample was homogenized by MP Fastprep-24 Automated
Homogenizer (MP Biomedicals) and sonicated by an Ultrasonic Liquid
Processors (Scientz JY92-II, Ningbo Scientz Biotechnology Co.,
Ltd.) in an ice-water bath (30 min each time, 2 times in total),
and the mixture was centrifuged for 20 min (14,000 x g, 4˚C).
Subsequently, the supernatant was dried in a vacuum centrifuge,
re-dissolved in acetonitrile/water (1:1, v/v) and adequately
vortexed, and centrifuged for 20 min (14,000 x, 4˚C). The final
supernatants were collected for LC-MS/MS analysis.
The LC-MS/MS analyses were performed using an UHPLC
(1290 Infinity LC; Agilent Technologies, Inc.) coupled with a QTRAP
5500 apparatus (AB Sciex LLC). For Hydrop Interaction Liquid
Chromatography separation, the samples were analyzed using an
ACQUITY UPLC BEH Amide column (Waters MS Technologies). The Quality
Control sample was set for each interval of a certain number of
experimental samples in the sample queue to detect and evaluate the
stability and repeatability of the system. In electron spray
ionization negative mode, the conditions were set as follows:
Source temperature, 450˚C; Ion Source Gas1, 45; Ion Source Gas2,
45; Curtain gas, 30; IonSapary Voltage Floating, 4,500 V; and MS/MS
Analysis mode of detection, ion pair.
Measurement of biochemical markers in
blood samples
Peripheral blood samples from the 30-day AL group
and 30-day IF group were collected to measure specific biochemical
markers. At the time of sample collection, the IF mice had fasted
for 12 h, while the control group had ad libitum access to
food. Initially, the mice were anesthetized with isoflurane USP
100% (1,000 ml/min; 4% isoflurane for induction; 500 ml/min;
1.75-2.5% isoflurane for maintenance) and subsequently, peripheral
blood was immediately collected from the orbital venous sinus. At
least 300 µl of blood (maximum 400 µl) was collected from each
mouse. The mice were sacrificed immediately by cervical dislocation
after blood collection. Mortality was further confirmed by
determining the cessation of respiratory and heart function. The
serum samples of the mice were harvested by centrifugation (440 x g
for 10 min at room temperature) and stored at -20˚C until further
analysis. Lilai Biological Co. was commissioned to measure
biochemical markers in the blood samples. The concentration levels
of glucose, total protein (TP), albumin (ALB) and globulin (GLO),
as well as the activity levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST), alkaline phosphatase (ALP),
lactate dehydrogenase (LDH) and γ-glutamyl transferase (γ-GT) were
measured using an auto-analyzer (Beckman LX-20; Beckman Coulter,
Inc.).
Statistical analysis
Values were expressed as the mean ± standard error
of the mean (SEM). Comparisons were performed with the Mann-Whitney
U-test between two groups or with the Kruskal-Wallis test with
Dunn's post-hoc multiple comparisons test. P<0.05 was considered
to indicate a statistically significant difference.
Hierarchical clustering analysis was performed using
cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm)
and the Java Treeview 3.0 software (http://jtreeview.sourceforge.net). The principal
component analysis was performed using MetaboAnalyst 4.0 software.
The Euclidean distance algorithm for similarity measurement and the
average linkage clustering algorithm for clustering were selected.
The heatmap was used as a visual aid apart from the dendrogram.
Results
Effects of intermittent fasting on
food intake and bodyweight of mice
During the 30-day experiment, a significant increase
in the bodyweight of the mice was noted. The bodyweight exhibited
no significant differences between the AL and the IF at the end of
the experiment (AL vs. IF, 21.701±1.305 vs. 21.610±1.187,
respectively; mean ± SEM; n=10). However, the cumulative food
intake of AL mice was higher compared with that of the IF mice. To
confirm this result, the duration of fasting was extended to 60
days. A total of three groups were included in the 60-day
experiment as follows: The AL, IF and IF&AL (refeeding) groups.
The IF&AL group was subjected to 30-day intermittent fasting
followed by 30 days of ad libitum access to food. The
bodyweight of the mice in these three groups exhibited no
significant difference by the end of the experiment (AL vs. IF vs.
IF&AL, 26.193±1.680 vs. 26.844±1.136 vs. 26.457±1.779,
respectively; mean ± SEM; n=8-14). The bodyweight and food intakes
of three different time points were provided in Table I. Overall, the data indicated that
12-h nighttime intermittent fasting did not affect the bodyweight
of mice, although it resulted in less cumulative food intake. The
bodyweights of the mice subjected to 60-day fasting were
significantly increased compared with those at 30 days (30-day AL
vs. 60-day AL, 21.701±1.305 vs. 26.193±1.680, respectively; and
30-day IF vs. 60-day IF, 21.610±1.187 vs. 26.844±1.136,
respectively; mean ± SEM), while their weight gain was
significantly decreased. This may be explained by their food
intake, since almost all of the mice in the 60-day experiments
consumed less food than the 30-day group. A comparison of the
bodyweight and food intakes of three different time points between
the 60- and 30-day IF groups was provided in Table II. The maximum percentage of weight
loss (before and after fasting) was 7.07%.
 | Table IBodyweight and food intake of the
mice. |
Table I
Bodyweight and food intake of the
mice.
| | 30-day
experiment | 60-day
experiment |
|---|
| Item | AL (n=10) | IF (n=10) | AL (n=14) | IF (n=9) | IF&AL
(n=8) |
|---|
| Bodyweight (g) | | | | | |
|
Beginning of
experiment | 15.578±1.172 | 14.890±0.853 | 21.764±1.381 | 20.900±1.166 | 20.613±1.629 |
|
End of
experiment | 21.701±1.305 | 21.610±1.187 | 26.193±1.680 | 26.844±1.136 | 26.457±1.779 |
|
Gain of
weight (%) | 39.305 | 45.132 | 20.350 | 28.440 | 28.351 |
| Food intake per day
(g) | | | | | |
|
Beginning of
experiment | 3.667±0.343 |
3.380±0.305a | 2.729±0.580 |
3.789±0.607b | 3.325±0.486 |
|
Middle of
experiment | 3.770±0.393 |
3.219±0.238c | 3.221±0.691 | 2.889±0.389 | 2.763±0.277 |
|
End of
experiment | 3.765±0.477 |
2.721±0.393d | 3.536±0.371 |
2.433±0.424b |
2.625±0.358c |
 | Table IIBodyweight and food intake of the
mice compared between 30 and 60 days of IF. |
Table II
Bodyweight and food intake of the
mice compared between 30 and 60 days of IF.
| Item | IF (30 days,
n=10) | IF (60 days,
n=9) |
|---|
| Bodyweight (g) | | |
|
Beginning of
experiment | 14.890±0.853 |
20.900±1.166a |
|
End of
experiment | 21.610±1.187 |
26.844±1.136a |
|
Gain of
weight (%) | 45.132 | 28.440 |
| Food intake per day
(g) | | |
|
Beginning of
experiment | 3.380±0.305 | 3.789±0.607 |
|
Middle of
experiment | 3.219±0.238 | 2.889±0.389 |
|
End of
experiment | 2.721±0.393 | 2.433±0.424 |
Reduction in liver weight by
intermittent fasting
The livers were isolated, observed and weighed
following animal sacrifice. The livers of IF mice appeared smaller
than those of the mice in the AL group (Fig. 1D and F). Subsequently, the wet liver weight was
determined and the liver weight/total bodyweight (LW/TBW) ratio was
determined. The results indicated that the liver mass of IF mice
was significantly lower than that of AL mice (Fig. 1E and G) and the LW/TBW ratio of the mice in the
IF group was considerably lower than that of the AL group (Fig. 1H and I). Ad libitum refeeding for 30 days
did not recover fasting-induced loss of liver mass. The liver
weight of the mice subjected to 60-day intermittent fasting was
significantly increased compared with that of the 30-day IF mice,
while the LW/TBW ratio was not significantly altered (Fig. 1J and K). This suggested that prolonged
intermittent fasting did not alter the effects noted
previously.
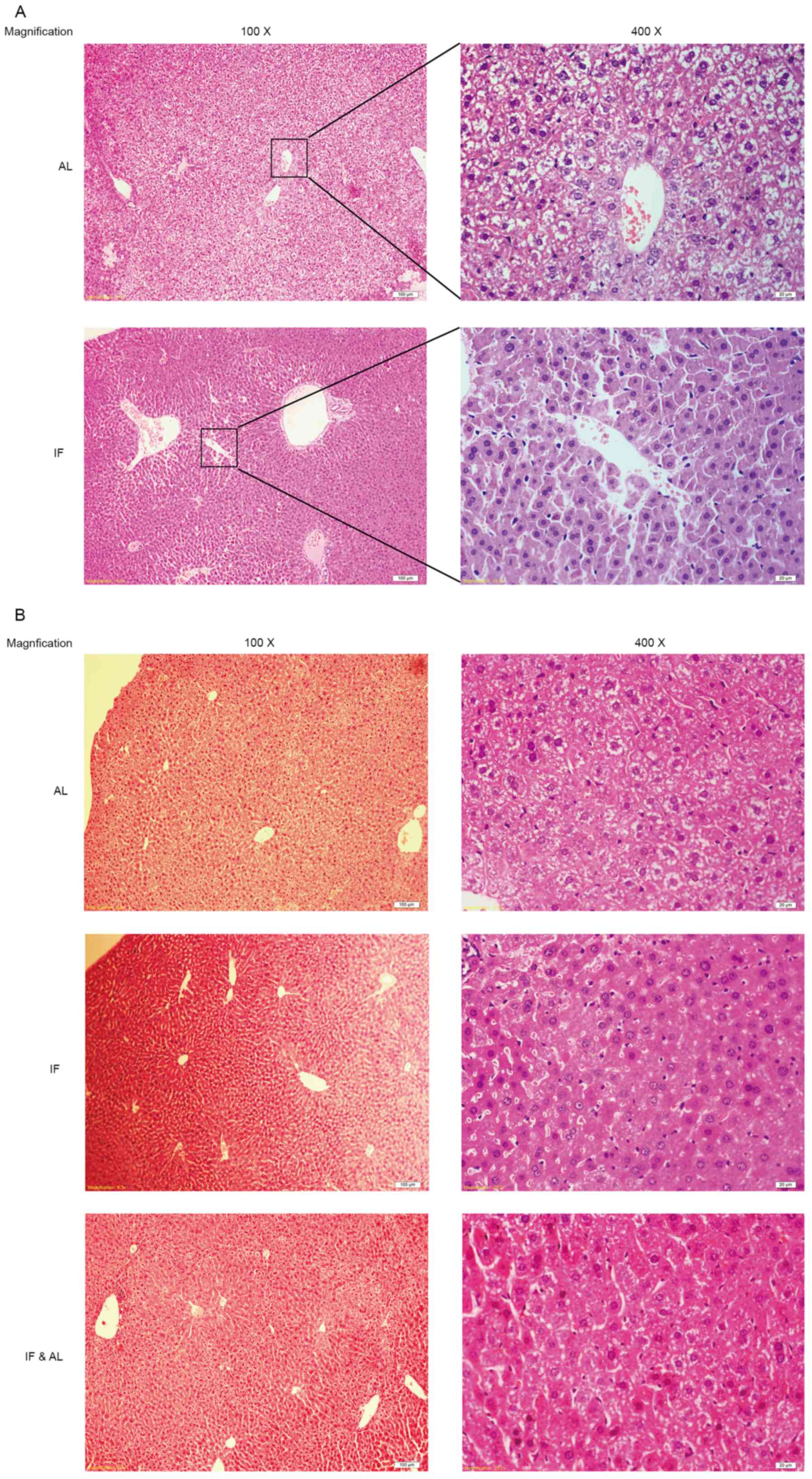
Subsequently, the morphological changes in the mouse
liver tissues were examined. Histological assessment of the liver
tissues by H&E staining indicated that the hepatocyte plates
were well developed and that the sinusoids were clearly visible in
all of the livers (Fig. 2A and
B).
Intermittent fasting alters the levels
of liver-associated biochemical markers in blood samples from
mice
Subsequently, the blood biochemical markers
associated with liver physiology or function were assessed. The
concentration levels of glucose, TP, ALB and GLO and the activity
levels of ALT, AST, ALP, LDH and γ-GT were measured. The blood
glucose levels were significantly decreased in IF mice compared
with those in AL mice (IF vs. AL, 1.391±0.914 vs. 5.726±0.648,
respectively; mean ± SEM; n=7; P<0.01; Fig. 3A). The activity levels of ALP were
significantly increased in IF mice (IF vs. AL, 171.571±13.465 vs.
152.829±17.130, respectively; mean ± SEM; n=7; P<0.05; Fig. 3B). No significant change was
observed for the other parameters tested (Fig. 3C-K). These results suggested that
intermittent fasting did not affect the liver function of mice,
whereas it was likely to regulate metabolism.
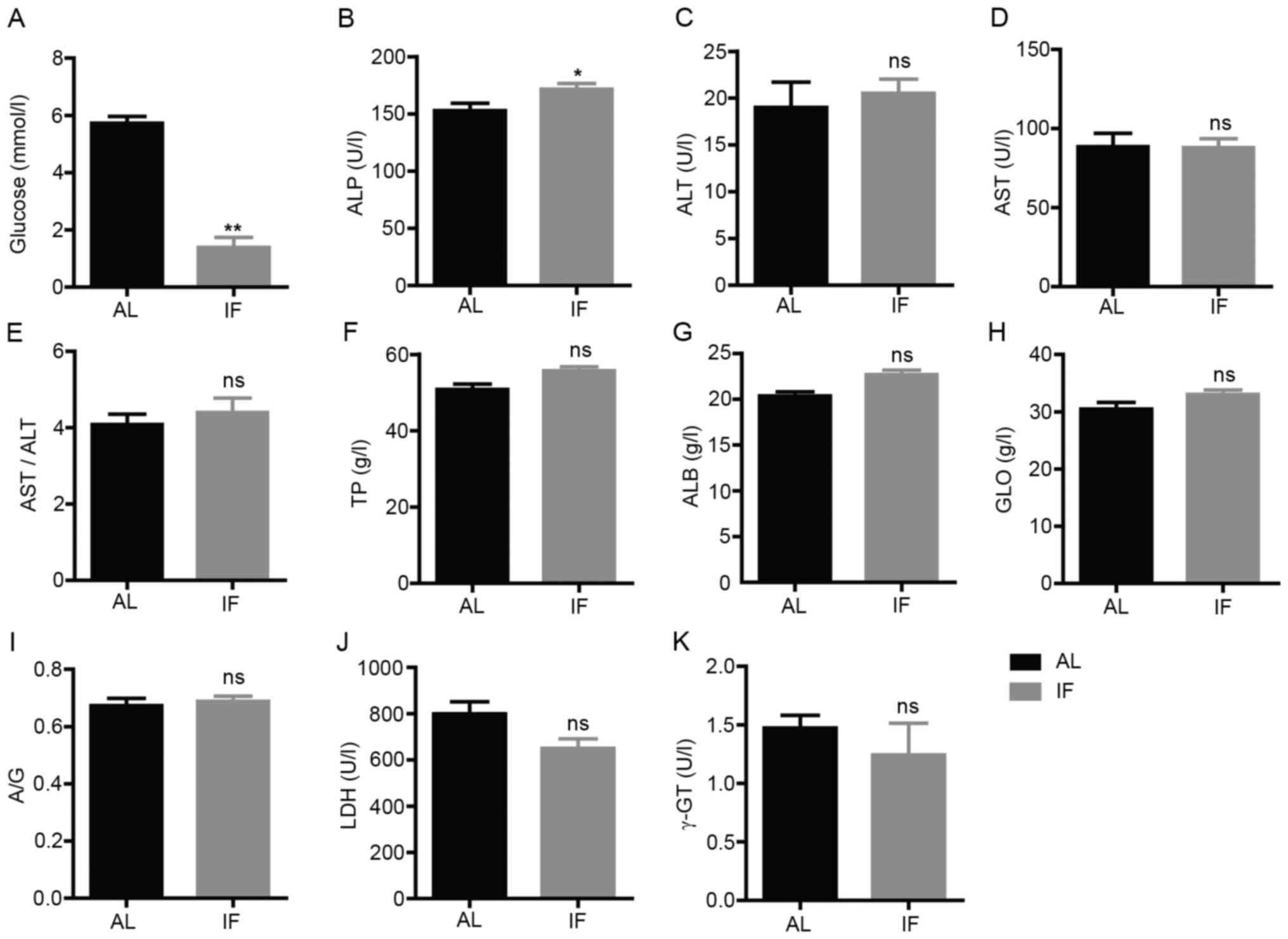 | Figure 3Levels of blood biochemical markers
at 30 days. (A) Blood glucose; (B) ALP; (C) ALT; (D) AST; (E)
AST/ALT; (F) TP; (G) ALB; (H) GLO; (I) A/G; (J) LDH; and (K) γ-GT.
Values are expressed as the mean ± standard error of the mean
(n=7). *P<0.05, **P<0.01; ns, no
significance. IF, intermittent fasting; AL, ad libitum; ALP,
alkaline phosphatase; ALT, alanine aminotransferase; ALB, albumin;
AST, aspartate aminotransferase; A/G, ALB/GLO; GLO, globulin; γ-GT,
γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; TP, total
protein. |
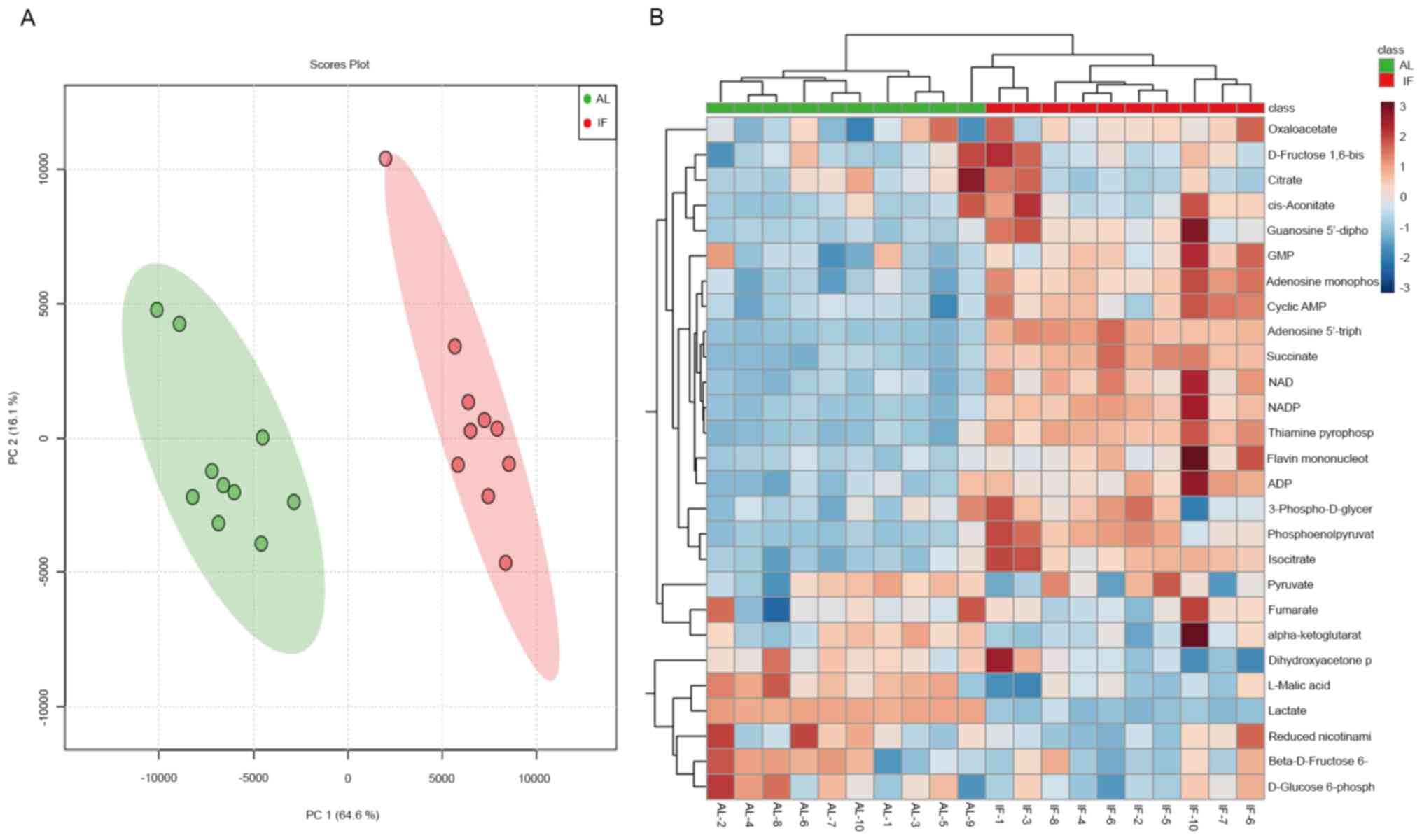
Intermittent fasting rewires liver
metabolism
To finally assess the effects of intermittent
fasting on liver metabolism, targeted metabolomics was performed by
LC-MS/MS analysis. A total of 27 metabolite molecules related to
energy metabolism (right column of Fig.
4B) were selected as targets. The principal component analysis
provided clear clustering based on the diet patterns of the groups
of mice (Fig. 4A). The distribution
of the most significantly differentially expressed metabolites was
visualized in a heatmap (Fig. 4B);
this was used to separate the AL and the IF mice. A >1.5-fold
increase was noted for 12 metabolite molecules in the IF mice
compared to those in the AL mice (Fig.
5A-L) and the differences in Fig.
5F-I were particularly significant, While the expression of the
other molecules did not exhibit any significant differences. The
molecules exhibiting increased expression included nicotinamide
adenine dinucleotide phosphate (NADP), adenosine triphosphate and
succinate, whereas the main molecule exhibiting reduced expression
was NADP. The majority of these metabolites are essentially
involved in the citric acid cycle and oxidative phosphorylation. It
was thus indicated that intermittent fasting enhanced liver
metabolism, which may explain the reduction in the liver mass of
these mice.
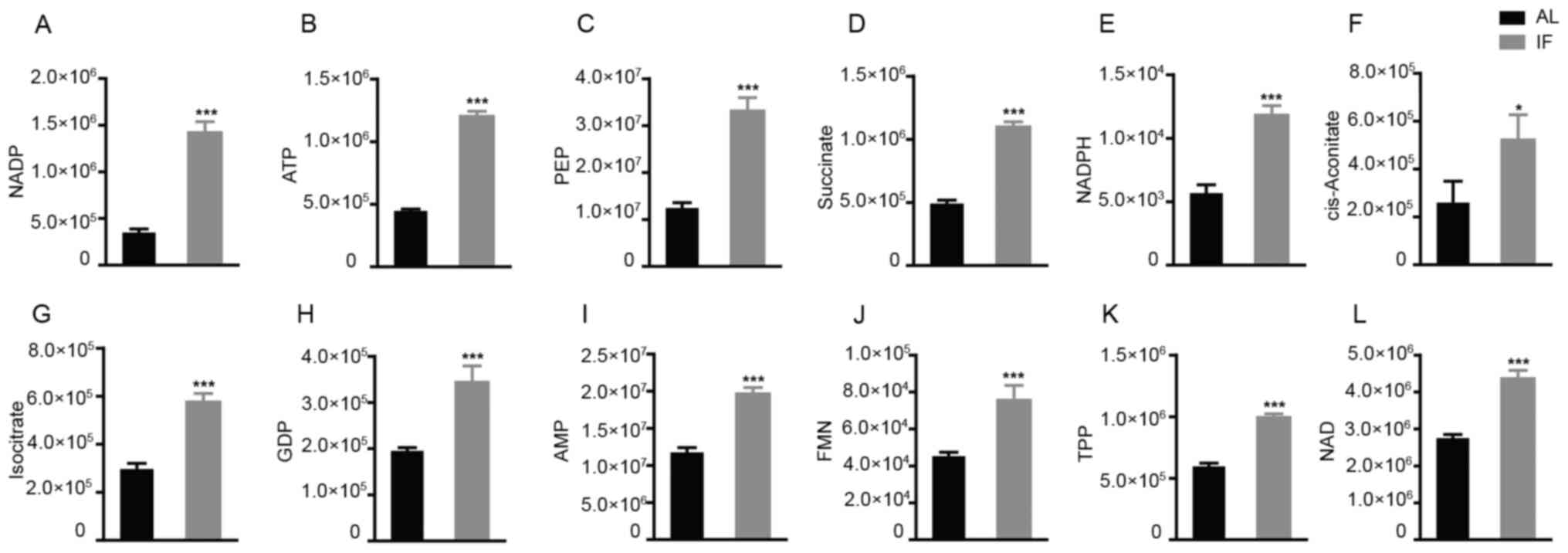 | Figure 5Among the 27 metabolites analyzed, 12
were significantly increased by intermittent fasting for 30 days.
(A) NADP; (B) ATP; (C) PEP; (D) Succinate; (E) NADPH; (F)
cis-aconitate; (G) isocitrate; (H) GDP; (I) AMP; (J) FMN; (K) TPP;
(L) NAD. Values are expressed as the mean ± standard error of the
mean (n=10). *P<0.05, ***P<0.001. IF,
intermittent fasting; AL, ad libitum; AMP, adenosine
monophosphate; ATP, adenosine triphosphate; FMN, flavin
mononucleotide; GDP, guanosine diphosphate; NADP, nicotinamide
adenine dinucleotide phosphate; NADPH, reduced NADP; PEP,
phosphoenolpyruvate; TPP, thiamine pyrophosphate. |
Discussion
Fasting, in particular intermittent fasting, has
emerged as a health-promoting diet pattern, which is supported by
evidence derived from both animal and human studies (2,28,33,34).
However, the patterns of intermittent fasting, depending on
individual practice, vary tremendously. This poses major challenges
in research with regard to experimental design and study of the
most relevant intermittent fasting regimen. In the present study,
Ramadan fasting was simulated in a mouse model involving
deprivation of both food and water, similarly to several previous
studies (35-38).
Ramadan fasting has been practiced by over one billion Muslims for
over 1,000 years and comprises daily fasting from dawn to dusk and
eating without any restriction at night (4). The period of Ramadan fasting lasts for
29-30 days yearly (4). Previous
studies have reported a modest weight loss by the end of Ramadan,
whereas the mean weight loss was regained a few weeks after Ramadan
(39,40). However, other studies have reported
weight gain following Ramadan fasting (19,20).
The present study simulated Ramadan fasting in mouse model.
However, mice naturally feed and drink during the night with high
level of physical activity, which is the opposite to humans.
Considering that the feeding habits of mice are different compared
to those of humans due to nocturnal circadian rhythms, our study in
mouse model resembled Ramadan fasting to a certain extent. The
results indicated that daily intermittent fasting for 12 h at night
for 1 month reduced blood glucose levels and enhanced liver
metabolism, although this did not affect the bodyweight of the
mice. However, it is important to note that the mouse studies have
limitations that translate to humans, including food composition
and environmental diversity.
Various studies have previously focused on the
ability of the diet to prevent disease development and progression.
A novel theorem has emerged which suggests that the time period of
eating is also important. It has been indicated that the timing of
food intake affects metabolic health and cancer development
(41-43).
A large prospective cohort study of 2,413 patients with breast
cancer reported that a short duration of fasting in the night
(<13 h per night) was associated with a 36% increased risk of
cancer recurrence (44). Therefore,
erratic feeding behaviors and/or circadian rhythms may have
detrimental health consequences (45-47).
Disruption of the circadian rhythm has been
identified as a common risk factor for obesity, metabolic
disorders, non-alcoholic fatty liver diseases and liver cancer
(48-51).
At least 10% of the liver transcriptome exhibits rhythmic gene
expression, suggesting that the circadian clock regulates a large
number of hepatic genes (52). It
has been demonstrated that chronic circadian disruption promotes
weight gain and hepatic lipid storage in mice (53,54).
This may be the reason for the loss of liver mass and the slight
bodyweight gain in the mouse model used in the present study.
Under physiological conditions, the liver serves as
the major organ for supplying energy to the body. It has been
reported that 12-24 h of fasting results in depletion of hepatic
glycogen, accompanied by a switch to a metabolic mode in which
non-hepatic glucose, fat-derived ketone bodies and free fatty acids
are used as energy sources (33).
This suggests that intermittent energy deprivation is capable of
improving metabolic health. However, the underlying mechanisms
through which intermittent energy restrictions improve the health
status have remained largely elusive. In the present study, a
number of metabolites were identified that exhibited significant
elevation in their expression levels in liver samples from mice of
the IF group. These metabolites are essential components of the
citric acid cycle, oxidative phosphorylation and glycolysis
cascades, indicating the induction of liver metabolism. This may
provide a mechanistic explanation for the effects of intermittent
fasting on liver physiology. Although the metabolic consequences of
intermittent fasting are complex and the mechanisms by which this
process benefits metabolic regulation remain elusive, previous
studies on the molecular changes occurring in the liver have
provided potential non-pharmacological approaches to improving the
health status in the general population (55,56).
Previous studies on intermittent fasting in humans
mainly focused on the therapeutic potential for different diseases,
such as cardiovascular disorders, obesity and diabetes (57-59).
The present study aimed to explore the physiological and
biochemical changes occurring in the liver following intermittent
fasting in healthy mice. A fasting pattern was adopted, which
simulated Ramadan fasting to a certain extent. Therefore, the
results may provide an understanding of the physiological effects
of intermittent fasting on the general population. However, the
mechanisms by which intermittent fasting provides therapeutic
benefits require further investigation using specific disease
models. A limitation of the present study was that liver
metabolomics were only performed for the 30-day fasting group,
mainly due to financial limitations. It would be worthwhile to
perform this assay for the 60-day fasting group in future
studies.
In conclusion, the present study indicated that
intermittent fasting reduces liver weight and rewires liver
metabolism in mice. This may partially contribute to the health
benefits of fasting and provides approaches for therapeutic
intervention in chronic diseases, such as non-alcoholic fatty liver
disease. However, the mechanisms through which intermittent fasting
regulates liver metabolism remain to be further investigated.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81972281); the Program for
Changjiang Scholars and Innovative Research Teams in University of
the Ministry of Education, China (grant no. IRT_17R88) Medical
Scientific Research, the foundation of Guangdong Province (grant
no. A2015297).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM, YW, LL and QS performed the experiments. JM and
YC analyzed the data and wrote the manuscript. WA, LG, ZM, ZQ, QP
and KC designed the project and discussed the results. QP and KC
supervised the research and edited the manuscript. JM and KC
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal study was performed with the approval of
the Laboratory Animal Ethics Committee of Northwest Minzu
University (Lanzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Cabo R, Carmona-Gutierrez D, Bernier M,
Hall MN and Madeo F: The search for antiaging interventions: From
elixirs to fasting regimens. Cell. 157:1515–1526. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Longo VD and Panda S: Fasting, circadian
rhythms, and time-restricted feeding in healthy lifespan. Cell
Metab. 23:1048–1059. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mitchell SJ, Bernier M, Mattison JA, Aon
MA, Kaiser TA, Anson RM, Ikeno Y, Anderson RM, Ingram DK and de
Cabo R: Daily fasting improves health and survival in male mice
independent of diet composition and calories. Cell Metab.
29:221–228.e3. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lessan N and Ali T: Energy metabolism and
intermittent fasting: The Ramadan perspective. Nutrients.
11(1192)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Patterson RE, Laughlin GA, LaCroix AZ,
Hartman SJ, Natarajan L, Senger CM, Martínez ME, Villaseñor A,
Sears DD, Marinac CR and Gallo LC: Intermittent fasting and human
metabolic health. J Acad Nutr Diet. 115:1203–1212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harney DJ, Hutchison AT, Hatchwell L,
Humphrey SJ, James DE, Hocking S, Heilbronn LK and Larance M:
Proteomic analysis of human plasma during intermittent fasting. J
Proteome Res. 18:2228–2240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Patterson RE and Sears DD: Metabolic
effects of intermittent fasting. Annu Rev Nutr. 37:371–393.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ahmad S and Chowdhury TA: Fasting during
Ramadan in people with chronic kidney disease: A review of the
literature. Ther Adv Endocrinol Metab.
10(2042018819889019)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weindruch R and Sohal RS: Seminars in
medicine of the Beth Israel deaconess medical center. Caloric
intake and aging. N Engl J Med. 337:986–994. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Baumeier C, Kaiser D, Heeren J, Scheja L,
John C, Weise C, Eravci M, Lagerpusch M, Schulze G, Joost HG, et
al: Caloric restriction and intermittent fasting alter hepatic
lipid droplet proteome and diacylglycerol species and prevent
diabetes in NZO mice. Biochim Biophys Acta. 1851:566–576.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Belkacemi L, Selselet-Attou G, Bulur N,
Louchami K, Sener A and Malaisse WJ: Intermittent fasting
modulation of the diabetic syndrome in sand rats. III. Post-mortem
investigations. Int J Mol Med. 27:95–102. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Horne BD, Muhlestein JB and Anderson JL:
Health effects of intermittent fasting: Hormesis or harm? A
systematic review. Am J Clin Nutr. 102:464–470. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jane L, Atkinson G, Jaime V, Hamilton S,
Waller G and Harrison S: Intermittent fasting interventions for the
treatment of overweight and obesity in adults aged 18 years and
over: A systematic review protocol. JBI Database System Rev
Implement Rep. 13:60–68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nair KS, Woolf PD, Welle SL and Matthews
DE: Leucine, glucose, and energy metabolism after 3 days of fasting
in healthy human subjects. Am J Clin Nutr. 46:557–562.
1987.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Müller MJ, Enderle J, Pourhassan M, Braun
W, Eggeling B, Lagerpusch M, Glüer CC, Kehayias JJ, Kiosz D and
Bosy-Westphal A: Metabolic adaptation to caloric restriction and
subsequent refeeding: The minnesota starvation experiment
revisited. Am J Clin Nutr. 102:807–819. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mansell PI and Macdonald IA: The effect of
starvation on insulin-induced glucose disposal and thermogenesis in
humans. Metabolism. 39:502–510. 1990.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Webber J and Macdonald IA: The
cardiovascular, metabolic and hormonal changes accompanying acute
starvation in men and women. Br J Nutr. 71:437–447. 1994.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Haouari M, Haouari-Oukerro F, Sfaxi A, Ben
Rayana MC, Kâabachi N and Mbazâa A: How Ramadan fasting affects
caloric consumption, body weight, and circadian evolution of
cortisol serum levels in young, healthy male volunteers. Horm Metab
Res. 40:575–577. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kul S, Savaş E, Öztürk ZA and Karadağ G:
Does Ramadan fasting alter body weight and blood lipids and fasting
blood glucose in a healthy population? A meta-analysis. J Relig
Health. 53:929–942. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sadeghirad B, Motaghipisheh S, Kolahdooz
F, Zahedi MJ and Haghdoost AA: Islamic fasting and weight loss: A
systematic review and meta-analysis. Public Health Nutr.
17:396–406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Charlton MR: Protein metabolism and liver
disease. Baillieres Clin Endocrinol Metab. 10:617–635.
1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lawrence YA, Guard BC, Steiner JM,
Suchodolski JS and Lidbury JA: Untargeted metabolomic profiling of
urine from healthy dogs and dogs with chronic hepatic disease. PLoS
One. 14(e0217797)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Besse-Patin A, Jeromson S,
Levesque-Damphousse P, Secco B, Laplante M and Estall JL: PGC1A
regulates the IRS1:IRS2 ratio during fasting to influence hepatic
metabolism downstream of insulin. Proc Natl Acad Sci USA.
116:4285–4290. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Moore MC, Coate KC, Winnick JJ, An Z and
Cherrington AD: Regulation of hepatic glucose uptake and storage in
vivo. Adv Nutr. 3:286–294. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ramnanan CJ, Edgerton DS, Kraft G and
Cherrington AD: Physiologic action of glucagon on liver glucose
metabolism. Diabetes Obes Metab. 13 (Suppl 1):S118–S125.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gibney MJ, Walsh M, Brennan L, Roche HM,
German B and van Ommen B: Metabolomics in human nutrition:
Opportunities and challenges. Am J Clin Nutr. 82:497–503.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hollywood K, Brison DR and Goodacre R:
Metabolomics: Current technologies and future trends. Proteomics.
6:4716–4723. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
de Cabo R and Mattson MP: Effects of
intermittent fasting on health, aging, and disease. N Engl J Med.
381:2541–2551. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Golbidi S, Daiber A, Korac B, Li H, Essop
MF and Laher I: Health benefits of fasting and caloric restriction.
Curr Diab Rep. 17(123)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Johnson EL: Seizures and epilepsy. Med
Clin North Am. 103:309–324. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo ZV, Hires SA, Li N, O'Connor DH,
Komiyama T, Ophir E, Huber D, Bonardi C, Morandell K, Gutnisky D,
et al: Procedures for behavioral experiments in head-fixed mice.
PLoS One. 9(e88678)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tucci V, Hardy A and Nolan PM: A
comparison of physiological and behavioural parameters in C57BL/6J
mice undergoing food or water restriction regimes. Behav Brain Res.
173:22–29. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Longo VD and Mattson MP: Fasting:
Molecular mechanisms and clinical applications. Cell Metab.
19:181–192. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wegman MP, Guo MH, Bennion DM, Shankar MN,
Chrzanowski SM, Goldberg LA, Xu J, Williams TA, Lu X, Hsu SI, et
al: Practicality of intermittent fasting in humans and its effect
on oxidative stress and genes related to aging and metabolism.
Rejuvenation Res. 18:162–172. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Manzanero S, Erion JR, Santro T, Steyn FJ,
Chen C, Arumugam TV and Stranahan AM: Intermittent fasting
attenuates increases in neurogenesis after ischemia and reperfusion
and improves recovery. J Cereb Blood Flow Metab. 34:897–905.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Farooq N, Priyamvada S, Arivarasu NA,
Salim S, Khan F and Yusufi AN: Influence of Ramadan-type fasting on
enzymes of carbohydrate metabolism and brush border membrane in
small intestine and liver of rat used as a model. Br J Nutr.
96:1087–1094. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mohany M, Ashton N, Harrath AH, Nyengaard
JR, Alomar SY and Alwasel S: A new model for fetal programming:
Maternal Ramadan-type fasting programs nephrogenesis. J Dev Orig
Health Dis. 9:287–298. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Salim S, Farooq N, Priyamvada S, Asghar M,
Khundmiri SJ, Khan S, Khan F and Yusufi AN: Influence of
Ramadan-type fasting on carbohydrate metabolism, brush border
membrane enzymes and phosphate transport in rat kidney used as a
model. Br J Nutr. 98:984–990. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fernando HA, Zibellini J, Harris RA,
Seimon RV and Sainsbury A: Effect of Ramadan fasting on weight and
body composition in healthy non-athlete adults: A systematic review
and meta-analysis. Nutrients. 11(478)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sethi BK and Nagesh VS: Weight management
in Ramadan. J Pak Med Assoc. 65 (5 Suppl 1):S54–S56.
2015.PubMed/NCBI
|
|
41
|
Hutchison AT and Heilbronn LK: Metabolic
impacts of altering meal frequency and timing-does when we eat
matter? Biochimie. 124:187–197. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mattson MP, Allison DB, Fontana L, Harvie
M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer
FA, et al: Meal frequency and timing in health and disease. Proc
Natl Acad Sci USA. 111:16647–16653. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Marinac CR, Natarajan L, Sears DD, Gallo
LC, Hartman SJ, Arredondo E and Patterson RE: Prolonged nightly
fasting and breast cancer risk: Findings from NHANES (2009-2010).
Cancer Epidemiol Biomarkers Prev. 24:783–789. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Marinac CR, Nelson SH, Breen CI, Hartman
SJ, Natarajan L, Pierce JP, Flatt SW, Sears DD and Patterson RE:
Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol.
2:1049–1055. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gill S and Panda S: A smartphone App
reveals erratic diurnal eating patterns in humans that can be
modulated for health benefits. Cell Metab. 22:789–798.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Manoogian ENC and Panda S: Circadian
rhythms, time-restricted feeding, and healthy aging. Ageing Res
Rev. 39:59–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jain Gupta N and Khare A: Disruption in
daily eating-fasting and activity-rest cycles in Indian adolescents
attending school. PLoS One. 15(e0227002)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen K, Ma J, Jia X, Ai W, Ma Z and Pan Q:
Advancing the understanding of NAFLD to hepatocellular carcinoma
development: From experimental models to humans. Biochim Biophys
Acta Rev Cancer. 1871:117–125. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fu L and Kettner NM: The circadian clock
in cancer development and therapy. Prog Mol Biol Transl Sci.
119:221–282. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kim CW, Yun KE, Jung HS, Chang Y, Choi ES,
Kwon MJ, Lee EH, Woo EJ, Kim NH, Shin H and Ryu S: Sleep duration
and quality in relation to non-alcoholic fatty liver disease in
middle-aged workers and their spouses. J Hepatol. 59:351–357.
2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maury E: Off the clock: From circadian
disruption to metabolic disease. Int J Mol Sci.
20(1597)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hunter AL and Ray DW: Circadian clock
regulation of hepatic energy metabolism regulatory circuits.
Biology (Basel). 8(79)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Christie S, Vincent AD, Li H, Frisby CL,
Kentish SJ, O'Rielly R, Wittert GA and Page AJ: A rotating light
cycle promotes weight gain and hepatic lipid storage in mice. Am J
Physiol Gastrointest Liver Physiol. 315:G932–G942. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fleet T, Stashi E, Zhu B, Rajapakshe K,
Marcelo KL, Kettner NM, Gorman BK, Coarfa C, Fu L, O'Malley BW and
York B: Genetic and environmental models of circadian disruption
link SRC-2 function to hepatic pathology. J Biol Rhythms.
31:443–460. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bertholdt L, Gudiksen A, Jessen H and
Pilegaard H: Impact of skeletal muscle IL-6 on regulation of liver
and adipose tissue metabolism during fasting. Pflugers Arch.
470:1597–1613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Marinho TS, Ornellas F, Barbosa-da-Silva
S, Mandarim-de-Lacerda CA and Aguila MB: Beneficial effects of
intermittent fasting on steatosis and inflammation of the liver in
mice fed a high-fat or a high-fructose diet. Nutrition. 65:103–112.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Johnstone A: Fasting for weight loss: An
effective strategy or latest dieting trend? Int J Obes (Lond).
39:727–733. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Barnosky AR, Hoddy KK, Unterman TG and
Varady KA: Intermittent fasting vs daily calorie restriction for
type 2 diabetes prevention: A review of human findings. Transl Res.
164:302–311. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Carter S, Clifton PM and Keogh JB: The
effects of intermittent compared to continuous energy restriction
on glycaemic control in type 2 diabetes; a pragmatic pilot trial.
Diabetes Res Clin Pract. 122:106–112. 2016.PubMed/NCBI View Article : Google Scholar
|