Introduction
Lung cancer is the most frequent cause of
cancer-mortality worldwide (1) and
the 5-year survival rate is <17% (2). Every year, 1.8 million people are
diagnosed with lung cancer and 1.6 million people die of the
disease (3). Non-small cell lung
cancer (NSCLC) is the most common type of lung cancer and accounts
for ~85% of all cases (4). Despite
advances and improvements in surgical treatment for early-stage
NSCLC, recurrence and metastasis are still the main factors
affecting prognosis (5). The
underlying mechanisms of NSCLC metastasis remain poorly understood.
It is therefore essential to better characterize the molecular
mechanisms associated with NSCLC metastasis, which may contribute
to the identification of novel biomarkers and therapeutic targets
in NSCLC.
Forkhead box P3 (FOXP3) is a well-known specific
marker of regulatory T cells (Tregs) that has been reported to
promote tumour progression due to its immunosuppressive function
(6). Previous studies have
demonstrated that FOXP3 is expressed in numerous types of cancer
cell and has dual roles in carcinogenesis (7-15).
Increased expression of FOXP3 in tumour cells is reported to play a
protumour role in pancreatic carcinoma (8), colorectal cancer (9), thyroid carcinoma (10) and cervical cancer (11), while FOXP3 decreased expression and
antitumour role are observed in breast cancer (12), prostate cancer (13), ovarian cancer (14) and T cell acute lymphoblastic
leukaemia (T-ALL) (15). Our
previous studies have demonstrated that FOXP3 is highly expressed
in NSCLC tissues and that its increased expression is associated
with lymph node metastasis and advanced Tumor-Node-Metastasis (TNM)
stage (16,17). Furthermore, FOXP3 can promote cell
proliferation and reduce cell chemosensitivity to anticancer drugs
in NSCLC (17,18). Another study in NSCLC has reported
that FOXP3 promotes tumour growth and metastasis by upregulating
the expression levels of cyclin D1, transforming growth factor β1,
interleukin 35 and heme oxygenase-1(19). A recent study reported that FOXP3
can activate the Wnt signaling pathway to promote cell
proliferation and invasion and the induction of
epithelial-mesenchymal transition (EMT) by physically interacting
with β-catenin and transcription factor 4 (TCF4) in NSCLC (20). Although the protumour effect of
FOXP3 has been demonstrated in NSCLC, the underlying mechanisms
remain to be elucidated.
Tumour cell metastasis is a multistep process
involving several crucial events, such as local extracellular
matrix (ECM) invasion, tumour angiogenesis, EMT and abnormal
activation of signal transduction pathways (21). The Notch1 pathway is a highly
conserved signalling pathway that is involved in multiple aspects
of cancer biology, including cancer stem cells, angiogenesis and
antitumour immunity (22,23). Aberrant activation of this pathway
has been reported in many types of cancer and is associated with
tumour growth, invasion and metastasis (24). Hes family BHLH transcription factor
1 (Hes1) is the most well-characterized target gene of Notch1, and
high levels of Notch1 and Hes1 are associated with a poor prognosis
in patients with NSCLC (25,26).
However, the correlation between the Notch1/Hes1 pathway and FOXP3
in NSCLC is unclear.
The present study aimed to elucidate the potential
mechanisms involving FOXP3 in regulating the metastatic process of
NSCLC. The effects of FOXP3 on cell migratory and invasive
abilities, vascular endothelial growth factor (VEGF) expression and
EMT in NSCLC were explored. The association between FOXP3 and the
Notch1/Hes1 pathway was also investigated. The findings from the
present study may provide a novel potential therapeutic target in
NSCLC.
Materials and methods
Cell culture
The human NSCLC cell line A549 was obtained from the
American Type Culture Collection. Cells were cultured in complete
Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare
Life Sciences) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and placed at 37˚C in a humidified incubator
containing 5% CO2.
FOXP3 siRNA transfection
Small interfering (si)RNA oligonucleotides specific
to FOXP3 (si-FOXP3-1 and si-FOXP3-2) and a negative control siRNA
(si-NC) were synthesized by Guangzhou RiboBio Co., Ltd. The
sequences of the siRNAs were as follows: si-FOXP3-1,
5'-CAUGGACUACUUCAAGUUCdTdT-3'; si-FOXP3-2,
5'-GAGAGAUGGUACAGUCUCUdTdT-3'; and si-NC,
5'-UUCUCCGAACGUGUCACGUdTdT-3'. A549 cells (2.5x105) were
transfected with si-NC or si-FOXP3-1 or 2 (10 nM final
concentration) using Lipofectamine™ 2000 (cat. no. 11668019;
Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturers' protocol. After 6 h, the transfection medium was
replaced with DMEM containing 10% FBS. Transfected cells were
eventually harvested at 48 h for reverse transcription quantitative
(RT-q) PCR or at 72 h for western blotting.
RT-qPCR
Total RNA was isolated from cells using the RNAiso
Plus Kit (Takara Bio, Inc.) and cDNA was synthesized using the
PrimeScript™ RT Reagent Kit (Takara Bio, Inc.) according to the
manufacturers' instructions. Gene expression was detected using
SYBR Premix Ex Taq™ II (Takara Bio, Inc.) according to the
manufacturers' protocol on a Piko-Real 24 Real-Time PCR system
(Thermo Fisher Scientific, Inc.). The primers for FOXP3, VEGF and
GAPDH were synthesized by Sangon Biotech Co., Ltd. The sequences of
the primers are shown in Table I.
The reaction were performed as follows: 95˚C for 15 min and 40
cycles of 95˚C for 10 sec and 60˚C for 10 sec. The relative gene
expression was normalized to endogenous control and expressed as
2-ΔΔCq (27).
 | Table ISequences of the primers used for
reverse transcription quantitative PCR. |
Table I
Sequences of the primers used for
reverse transcription quantitative PCR.
| Gene | Primer sequence
(5'-3') | Genbank accession
no. | bp |
|---|
| FOXP3 | | NM_014009.3 | 167 |
|
Forward |
5'-CACAACATGCGACCCCTTTCACC-3' | | |
|
Reverse |
5'-AGGTTGTGGCGGATGGCGTTCTTC-3' | | |
| VEGF | | NM_001025368.3 | 158 |
|
Forward |
5'-GTGCCCACTGAGGAGTCCAACATC-3' | | |
|
Reverse |
5'-GAGCAAGGCCCACAGGGATTTT-3' | | |
| GAPDH | | NM_002046.3. | 107 |
|
Forward |
5'-ATGGGGAAGGTGAAGGTCG-3' | | |
|
Reverse |
5'-GGGTCATTGATGGCAACAATATC-3' | | |
Western blotting
Cells were washed with ice-cold PBS, lysed with RIPA
buffer (Beyotime Institute of Biotechnology) containing a 1 mM
protease inhibitor PMSF (Beyotime Institute of Biotechnology) on
ice for 30 min and centrifuged at 15,000 x g for 15 min at 4˚C.
Proteins (30 µg) were separated by 10% SDS-PAGE and transferred
onto PVDF membranes. Membranes were blocked with 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature
(RT) and incubated with primary antibodies (1:1,000) against FOXP3
(cat. no. 4852; Boster Biological Technology), Notch1 (cat. no.
4126; Boster Biological Technology), E-cadherin (cat. no. 0138;
Beyotime Institute of Biotechnology), vimentin (cat. no. 0318;
Beyotime Institute of Biotechnology), N-cadherin (cat. no. 0243;
Beyotime Institute of Biotechnology), Hes1 (cat. no. 2167; Beyotime
Institute of Biotechnology) and GAPDH (cat. no. 0006; Beyotime
Institute of Biotechnology) overnight at 4˚C. The membranes were
then incubated with appropriate horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:2,000; cat. no. A0208;
cat. no. A0216; Beyotime Institute of Biotechnology) for 1 h at RT.
Enhanced chemiluminescence reagent (BeyoECL Star Kit; Beyotime
Institute of Biotechnology) was used to detect the signal on the
membrane. The data were analyzed via densitometry using
VisionWorksLS 7.1 software (UVP, LLC) and normalized to expression
of the internal control GAPDH.
Transwell assay
Cell migratory and invasive abilities were assessed
in 24-well Transwell chambers containing inserts of 8.0 µm
pore size (Corning Inc.). For the invasion assay, the inserts were
precoated with 80 µl Matrigel (1:8; BD Biosciences). Cells
(5x104) were suspended in 100 µl serum-free DMEM and
seeded in the upper chamber of the chamber, whereas 500 µl
medium containing 10% FBS was added to the lower chamber to act as
a chemoattractant. Following 20 h incubation, cells on the upper
membrane surface were removed and cells that have migrated through
the membrane were fixed in 4% paraformaldehyde for 15 min at RT and
stained with haematoxylin for 5 min at RT. The number of invaded
and migrated cells was counted in five random fields using a light
microscope (BX43F; Olympus Corporation) at x400 magnification.
ELISA
Following cell transfection with siRNAs for 72 h,
the culture supernatant of A549 cells was harvested and centrifuged
at 2,000 x g for 10 min at RT. The concentrations of matrix
metalloproteinase-2 (MMP-2), MMP-9 and VEGF in the culture
supernatant were detected using ELISA kits (cat. no. EK0459; cat.
no. EK0465; cat. no. EK0539; Boster Biological Technology)
according to the manufacturers' protocol. Absorbance was read at
450 nm on a microplate reader (Tecan Group, Ltd.). The
concentrations of detected proteins were calculated according to a
standard curve.
Clinical samples
Formalin-fixed paraffin-embedded tissue samples were
obtained from 55 patients with NSCLC (average age, 60.5±9.4 years;
34 men and 21 women) who received treatment at the General Hospital
of China National Petroleum Corporation (CNPC) in Jilin between
January 2009 and December 2013. Regarding the histological subtype,
35 samples were squamous cell carcinomas and 20 were
adenocarcinomas. A total of 24 samples originated from patients who
had lymph node metastasis whereas 31 samples were free of
metastasis. According to the TNM classification, 27 samples were in
stage I, 18 samples were in stage II and 10 samples were in stage
III. The inclusion criteria included a clear pathological diagnosis
of NSCLC and no prior radiotherapy or chemotherapy. The exclusion
criteria included diagnosis with other types of cancer type and a
lack of clinicopathological data. This study was approved by the
Medical Ethics Committee of CNPC and all patients provided written
informed consent.
Immunohistochemical (IHC)
staining
Tissue sections were deparaffinized in xylene and
rehydrated in a decreasing gradient series of ethanol solutions.
Following antigen retrieval and blocking with 3% hydrogen peroxide
for 10 min at room temperature, sections were incubated with
primary antibodies against FOXP3 (1:100; cat. no. 20034; Abcam),
CD31 (1:50; cat. no. 53411; Santa) and E-cadherin (ready to use,
cat. no. 0092; ZSGB-Bio Co.,Ltd.) overnight at 4˚C. After
incubation with an HRP-conjugated secondary antibody (cat. no.
PV9000; ZSGB-Bio Co.,Ltd.) for 20 min at RT, 3,3'-diaminobenzidine
was used for chromogen detection. All sections were evaluated using
a double-blinded method. A total of five fields were observed under
light microscopy (magnification, x400) and 100 tumour cells were
counted in each section. FOXP3 and E-cadherin expression was
assessed as previously described (16). CD31-stained sections were evaluated
to determine the microvascular density (MVD) and assessed as
previously described (28).
Treatment with signalling pathway
inhibitors
N-(N-(3,5-Difluorophenacetyl)-L-alanyl)-S-phenylglycine t-butyl
ester (DAPT; cat. no. SF4139; Beyotime Institute of Biotechnology)
was used to inhibit the activity of the Notch1/Hes1 pathway in A549
cells. Briefly, cells (5x105 cells per well) in six-well
plates were incubated overnight at 37˚C and treated with or without
DAPT (10 or 20 µM) for 48 h. Cells were subsequently
collected and used for western blotting.
Statistical analysis
Statistical analysis was conducted using SPSS 17.0
(SPSS Inc.). Data were presented as the means ± standard deviation.
All experiments were performed ≥ three times. Differences between
groups were analysed using one-way ANOVA followed by Tukey's post
hoc test. Pearson correlation analysis was performed to assess the
correlation between FOXP3, CD31 and E-cadherin in NSCLC tissues.
P<0.05 was considered to indicate a statistically significant
difference.
Results
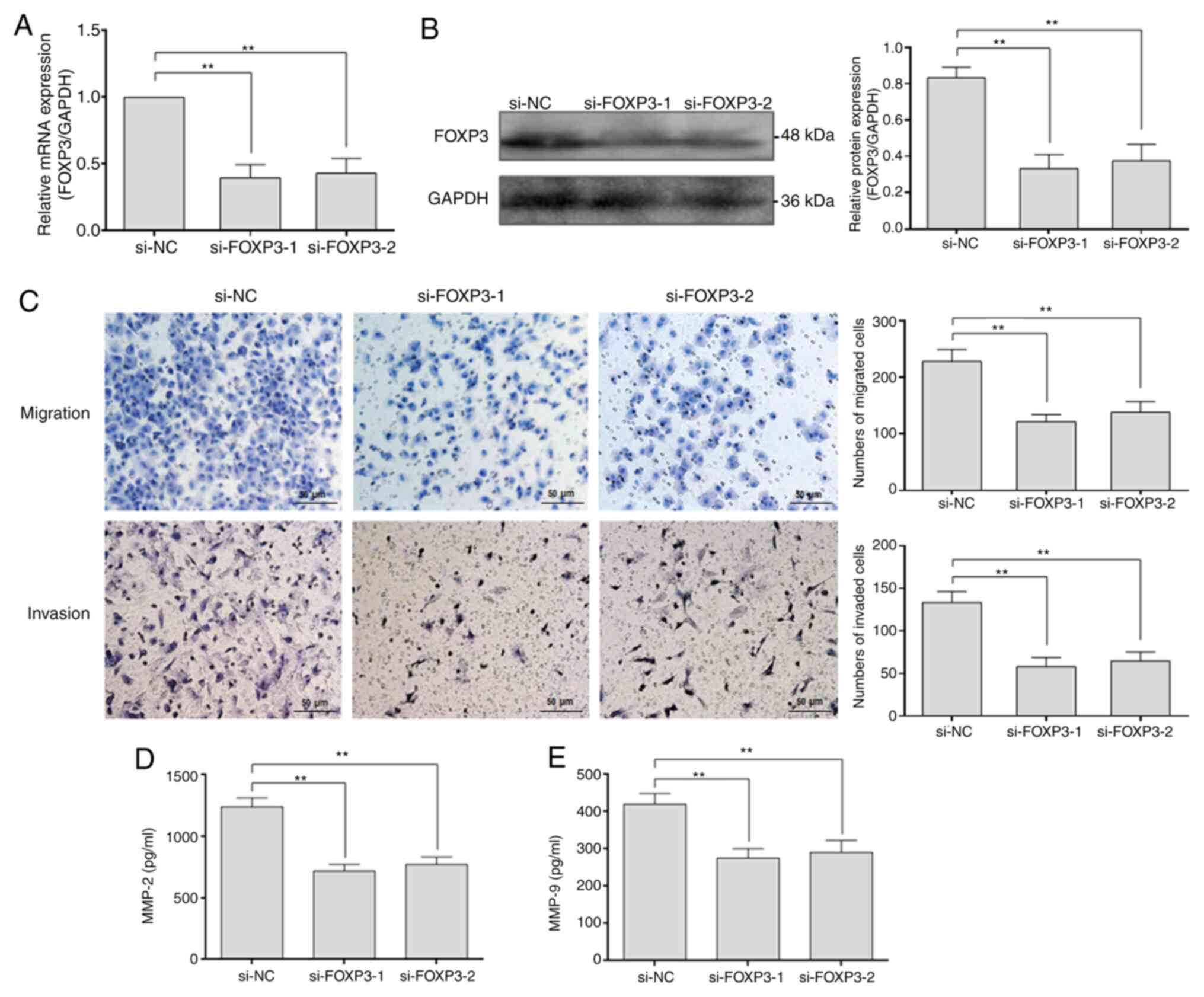
Efficiency of FOXP3 knockdown in NSCLC
cells
Our previous studies have reported that FOXP3 is
highly expressed in NSCLC cells (16,17).
To investigate the potential function and underlying mechanism of
FOXP3 in NSCLC metastasis, two FOXP3-targeted siRNAs (si-FOXP3-1
and si-FOXP3-2) were transfected into A549 cells, and the knockdown
efficiency of FOXP3 was measured by RT-qPCR and western blotting.
The results demonstrated that the mRNA and protein expression of
FOXP3 in the FOXP3-knockdown group was significantly downregulated
(P<0.01), showing an knockdown efficiency of ~60% (Fig. 1A and B).
FOXP3 knockdown inhibits the migratory
and invasive abilities of NSCLC cells
To explore the effects of FOXP3 on the migratory and
invasive abilities of NSCLC cells, Transwell assays were performed
following FOXP3 knockdown in A549 cells. The results demonstrated
that the numbers of migrated and invaded cells in the
FOXP3-knockdown group were significantly decreased compared with
the si-NC group (P<0.01; Fig.
1C), indicating that FOXP3 may enhance the migratory and
invasive abilities of NSCLC cells. Subsequently, the expression of
MMP-2 and MMP-9, which are two key molecules involved in cell
migration and invasion, was examined. The results from ELISA
demonstrated that the concentrations of MMP-2 and MMP-9 in the
culture supernatant of FOXP3-knockdown group were significantly
decreased compared with those in the culture supernatant of the
si-NC group (P<0.01; Fig. 1D and
E). These data indicated that FOXP3
may promote the migratory and invasive abilities of NSCLC cells by
increasing MMP-2 and MMP-9 secretion.
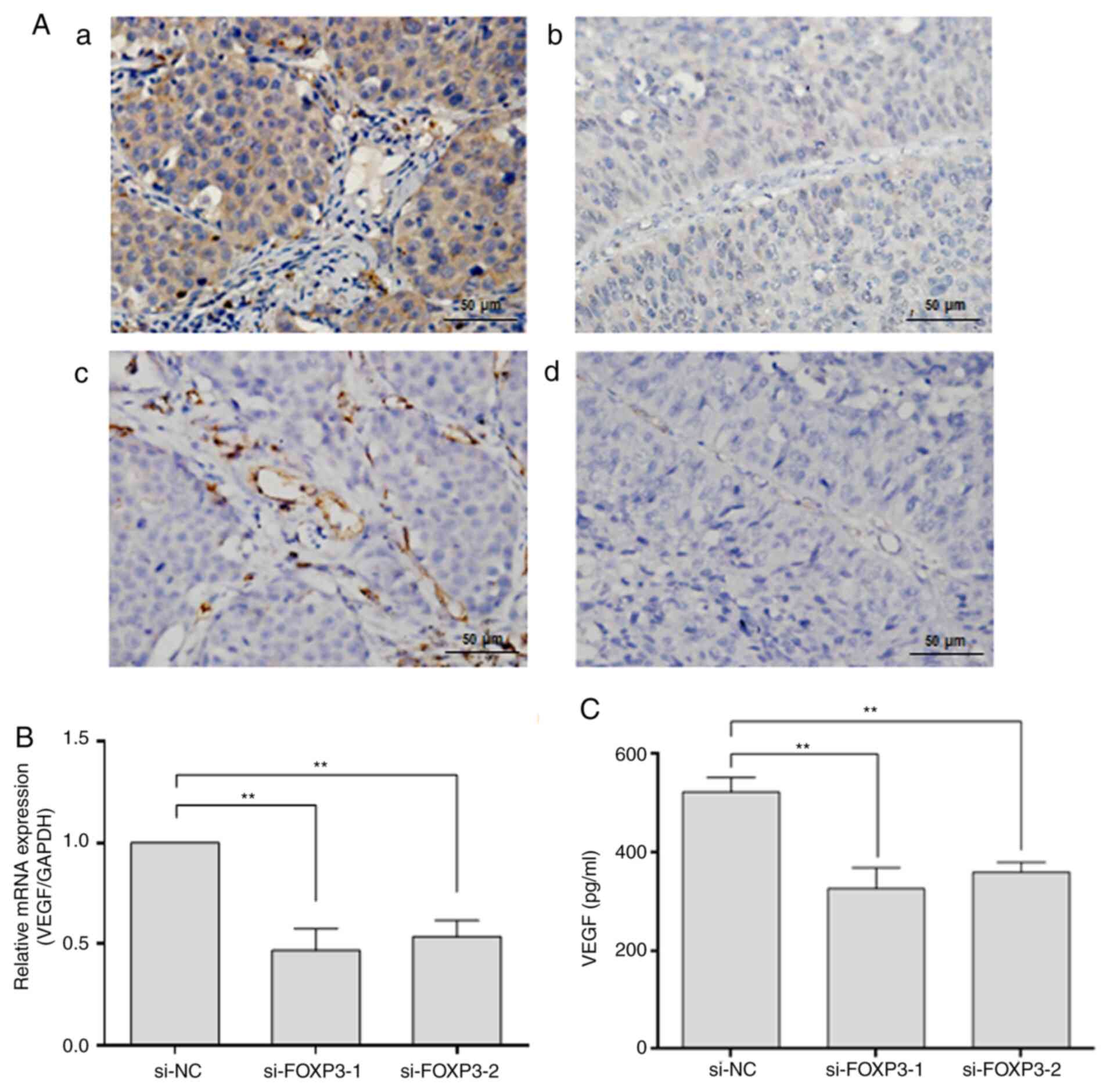
FOXP3 regulates the expression of the
angiogenic factor VEGF in NSCLC
To determine the correlation between FOXP3
expression and MVD, the expression of FOXP3 and the endothelial
cell marker CD31 was detected by IHC staining in 55 NSCLC tissue
samples. The results demonstrated that increased expression of
FOXP3 was mostly observed in samples with a higher MVD-CD31 rather
than a lower MVD-CD31 (Fig. 2A).
Pearson's correlation analysis showed a positive correlation
between FOXP3 expression and MVD-CD31 (r=0.326; P=0.015; Table II), suggesting that FOXP3 may play
a role in blood vessel formation in NSCLC tissues. Because VEGF is
one of the most important angiogenic factors, we further examined
the expression of VEGF by RT-qPCR and ELISA following FOXP3
knockdown in A549 cells. The expression of VEGF at the mRNA and
protein levels was significantly decreased in the FOXP3-knockdown
groups compared with the si-NC group (P<0.01; Fig. 2B and C), demonstrating that FOXP3 may regulate
the expression of the angiogenic factor VEGF in NSCLC cells.
 | Table IICorrelation analysis between FOXP3
and MVD-CD31 in non-small cell lung cancer tissues. |
Table II
Correlation analysis between FOXP3
and MVD-CD31 in non-small cell lung cancer tissues.
| | FOXP3
expression | |
|---|
| MVD-CD31
expression | Positive | Negative | Total | r | P-value |
|---|
| High | 24 | 7 | 31 | 0.326 | 0.015a |
| Low | 11 | 13 | 24 | | |
| Total | 35 | 20 | 55 | | |
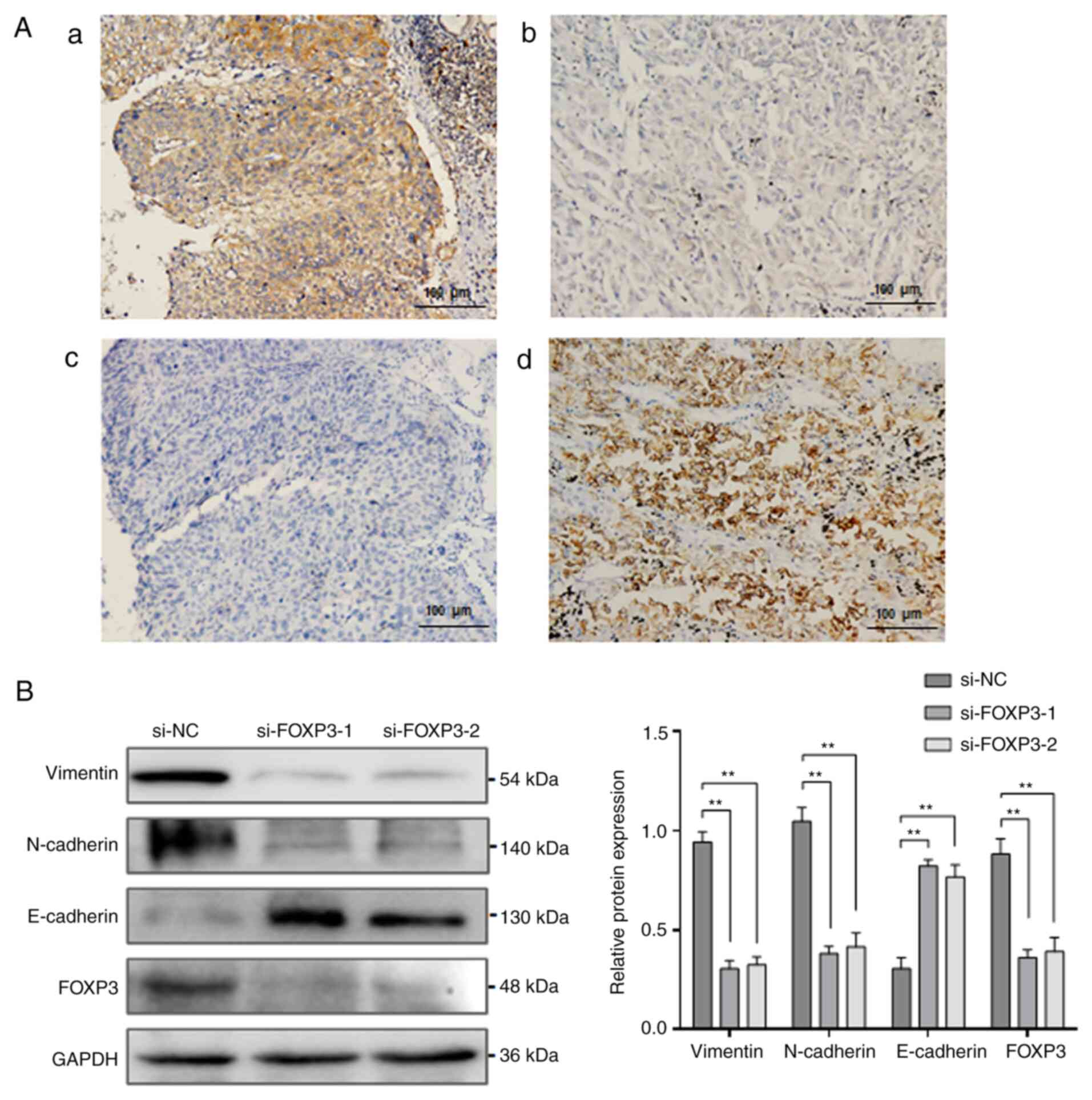
FOXP3 induces EMT in NSCLC
EMT is a key process in NSCLC invasion and
metastasis. To investigate whether FOXP3-mediated metastasis is
associated with EMT process, the epithelial marker E-cadherin was
detected in NSCLC tissue samples, and the correlation between FOXP3
and E-cadherin expression was analysed. The results from IHC
staining demonstrated that FOXP3 expression was significantly
increased, while E-cadherin expression was significantly decreased
in NSCLC tissues (Fig. 3A). A
negative correlation was reported between FOXP3 and E-cadherin
expression in the NSCLC tissue samples (r=-0.297; P=0.028; Table III), suggesting that
FOXP3-mediated metastasis may involve EMT process. To confirm the
relationship between FOXP3 and EMT, the expression of the
EMT-related proteins vimentin, N-cadherin and E-cadherin was
assessed by western blotting following FOXP3 knockdown in A549
cells. The results demonstrated that FOXP3 knockdown significantly
decreased the expression of vimentin and N-cadherin but
significantly decreased that of E-cadherin (P<0.01; Fig. 3B), indicating that FOXP3 knockdown
may reverse EMT in NSCLC cells.
 | Table IIICorrelation analysis between FOXP3
and E-cadherin in non-small cell lung cancer tissues. |
Table III
Correlation analysis between FOXP3
and E-cadherin in non-small cell lung cancer tissues.
| | FOXP3
expression | |
|---|
| E-cadherin
expression | Positive | Negative | Total | r | P-value |
|---|
| Positive | 12 | 13 | 25 | -0.297 | 0.028a |
| Negative | 23 | 7 | 30 | | |
| Total | 35 | 20 | 55 | | |
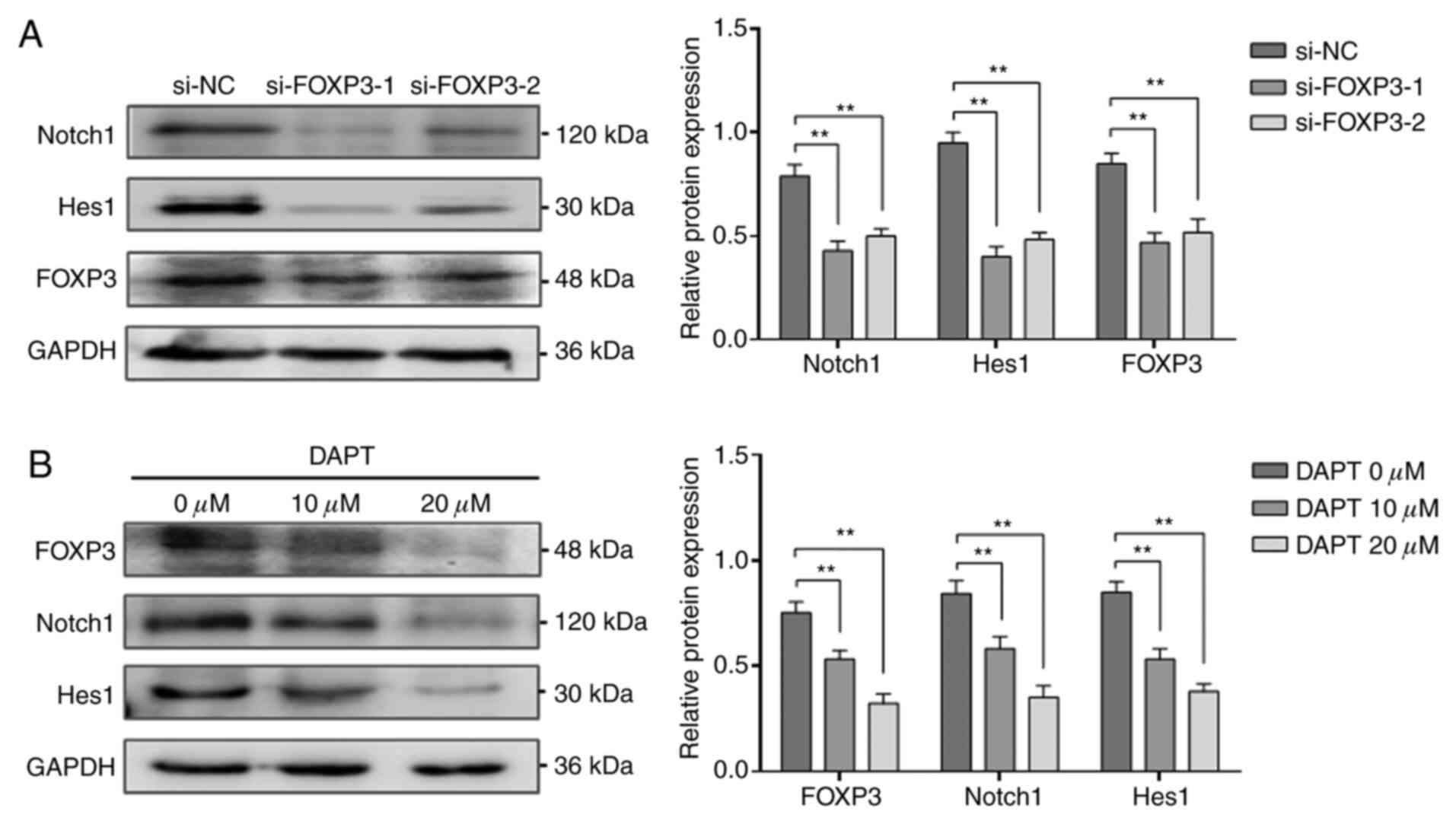
Link between FOXP3 and the Notch1/Hes1
pathway in NSCLC cells
The Notch1/Hes1 pathway is a vital signalling
pathway in cancer pathogenesis. To investigate whether the
promotion of NSCLC metastasis by FOXP3 might involve the
Notch1/Hes1 pathway, the expression of Notch1 and Hes1 was
determined by western blotting following FOXP3 knockdown in A549
cells. The results demonstrated that the expression of Notch1 and
Hes1 in the FOXP3-knockdown group was significantly decreased
compared with that in the si-NC group (P<0.01; Fig. 4A). Subsequently, the effects of
Notch1/Hes1 pathway blockade on the expression of FOXP3 in A549
cells were detected by western blotting. Following treatment with
the Notch1 pathway inhibitor DAPT (0, 10 or 20 µM), the
expression of Notch1, Hes1 and FOXP3 was significantly decreased in
a dose-dependent manner (P<0.01; Fig. 4B). These findings suggested that the
pro-metastatic effect of FOXP3 may be closely linked to the
Notch1/Hes1 pathway in NSCLC cells.
Discussion
Previous studies from our laboratory investigated
the expression of FOXP3 in clinical NSCLC tissue samples and its
association with clinicopathological characteristics of patients
with NSCLC (16,17). The results demonstrated that FOXP3
expression is elevated in squamous cell carcinoma and
adenocarcinoma compared with normal lung tissues and positively
correlated with lymph node metastasis and TNM stage in patients
with NSCLC patients, indicating that FOXP3 may serve crucial roles
in NSCLC metastasis and progression (16,17).
In the present study, the molecular mechanisms by which FOXP3 can
promote metastasis in NSCLC tissues and cells were
investigated.
Tumour metastasis is characterized by tumour cells
acquiring the ability to migrate and invade from the primary tumour
to distant organs. In the present study, the migratory and invasive
abilities of A549 cells were significantly inhibited following
FOXP3 knockdown according to Transwell assays. Combined with our
previous studies (16,17), the results from the present study
further demonstrated that NSCLC cells with high FOXP3 expression
may have a relatively high metastatic potential. The degradation of
the ECM by MMPs is an essential initial step in tumour invasion and
metastasis (29). MMP-2 and MMP-9,
which are both type IV collagenases, have been shown to play
important roles in cell migration, invasion, angiogenesis and
metastasis of malignant tumours (30,31). A
previous study reported that FOXP3 upregulation could decrease cell
migration, invasion and MMP-2 expression in epithelial ovarian
cancer (14). In the present study,
FOXP3 knockdown significantly inhibited the secretion of MMP-2 and
MMP-9 by A549 cells, which suggested that FOXP3 may promote the
motility of NSCLC cells by increasing MMP-2 and MMP-9
secretion.
High MVD and increased expression of angiogenic
factors may increase tumour invasive and metastatic abilities
(32). In the present study, MVD
was assessed using CD31-positive vascular endothelial cells. The
results demonstrated that NSCLC tissue samples with high FOXP3
expression had a higher MVD-CD31 than those with low FOXP3
expression, and statistical analysis showed a strong correlation
between FOXP3 and MVD-CD31, indicating that FOXP3 may play a role
in blood vessel formation in NSCLC tissues. VEGF is one of the most
important angiogenic factors that can stimulate the proliferation
of vascular endothelial cells, and increasing the permeability of
the vessels and blocking the VEGF pathway can suppress tumour
angiogenesis and metastasis (33).
A previous study reported that FOXP3 expression is positively
correlated with VEGF-C which is a specific regulatory factor of
endothelial cells of blood and lymphatic vessels in cervical cancer
tissues (34). Conversely, a study
reported that FOXP3 could inhibit angiogenesis by downregulating
VEGF in breast cancer (35).
However, whether FOXP3 regulates VEGF in NSCLC remains unclear. In
the present study, FOXP3 knockdown significantly decreased VEGF
mRNA and protein expression in A549 cells, which indicated that
FOXP3 may upregulate VEGF expression and subsequently facilitate
the invasion and metastasis of NSCLC cells.
EMT is a key event in local invasion and distant
metastasis in human cancers, and this transformation is
characterized by epithelial cells losing epithelial cell markers,
such as the cell adhesion molecule E-cadherin, and acquiring a
mesenchymal phenotype, characterized by vimentin and N-cadherin
expression (36). Previous studies
demonstrated that cancer cells with EMT phenotype might exhibit
highly invasive and metastatic abilities that lead to an elevated
rate of distant metastases and a worse prognosis in patients with
cancer, including breast, colorectal and lung cancer (37-39).
Loss or downregulation of E-cadherin expression is a major hallmark
of EMT, which results in reduced cell-cell connections and enhanced
cellular mobility. In the present study, the correlation between
the expression of FOXP3 and E-cadherin in NSCLC tissues was
analysed. The results revealed a negative correlation between the
two proteins, indicating that FOXP3-mediated metastases may involve
EMT process in NSCLC. This hypothesis was further confirmed by
results showing that FOXP3 knockdown reversed the EMT phenotype in
A549 cells. This finding was supported by a previous study from
Yang et al (20) who
demonstrated that FOXP3 could enhance the function of β-catenin and
TCF4 in order to activate EMT-related molecules, such as snail and
slug, leading to the induction of EMT in human NSCLC cell lines.
The results on NSCLC tissues and cells from the present study
further confirmed that FOXP3 might facilitate the invasion and
metastasis of NSCLC cells via EMT induction.
The Notch1/Hes1 pathway plays a critical role in
tumorigenesis and promotes angiogenesis and EMT in many types of
malignant tumour, including NSCLC (24,40,41).
It has been reported that blocking the Notch1 pathway can
downregulate FOXP3 expression in T-ALL and melanoma cells (15,42).
Combined with the aforementioned results and the pro-metastatic
effect of FOXP3 demonstrated in the present study, we hypothesized
that FOXP3 and the Notch1/Hes1 pathway may be closely related in
NSCLC. This hypothesis was confirmed by results showing that FOXP3
knockdown significantly decreased the activity of the Notch1/Hes1
pathway and that Notch1/Hes1 pathway blockade significantly
downregulated the expression of FOXP3 in a dose-dependent manner.
These findings therefore suggested a possible interaction between
FOXP3 and the Notch1/Hes1 pathway and revealed that the
pro-metastatic effect of FOXP3 may be linked to the Notch1/Hes1
pathway in NSCLC cells.
This study presented some limitations. Firstly, this
study was performed using only one cell line. Secondly, the
molecular mechanism underlying the interaction between FOXP3 and
the Notch1 pathway in NSCLC progression was not determined and
requires further investigation.
In summary, the results from the present study
demonstrated that FOXP3 may facilitate the invasion and metastasis
of NSCLC cells via regulating VEGF, EMT and the Notch1/Hes1
pathway. This study provided novel mechanistic insights into the
role of FOXP3 in NSCLC metastasis and suggested that FOXP3 may be
considered as a promising therapeutic target in NSCLC.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Technology Research
Projects of the Education Department of Jilin Province (grant no.
JJKH20180353KJ), the Health Department of Jilin Province (grant no.
2020J017) and the Science and Technology Department of Jilin
Province (grant nos. 20190201220JC and YDZJ202101ZYTS089).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL, HW, HF, CH, YP and XG performed the experiments.
CL and XG designed the experiments. CL and XG confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the General Hospital of China National Petroleum
Corporation (approval no. 2018031). Patients or their family
members were fully informed of the study details and provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Souza MC, Cruz OG and Vasconcelos AG:
Factors Associated with disease-specific survival of patients with
non-small cell lung cancer. J Bras Pneumol. 42:317–325.
2016.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
5
|
Zinner R, Visseren-Grul C, Spigel DR and
Obasaju C: Pemetrexed clinical studies in performance status 2
patients with non-small cell lung cancer (Review). Int J Oncol.
48:13–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Togashi Y, Shitara K and Nishikawa H:
Regulatory T cells in cancer immunosuppression-implications for
anticancer therapy. Nat Rev Clin Oncol. 16:356–371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Triulzi T, Tagliabue E, Balsari A and
Casalini P: FOXP3 expression in tumor cells and implications for
cancer progression. J Cell Physiol. 228:30–35. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hinz S, Pagerols-Raluy L, Oberg HH,
Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C,
Ungefroren H, Saeger HD, et al: Foxp3 expression in pancreatic
carcinoma cells as a noveral mechanism of immune evasion in cancer.
Cancer Res. 67:8344–8350. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim M, Grimmig T, Grimm M, Lazariotou M,
Meier E, Rosenwald A, Tsaur I, Blaheta R, Heemann U, Germer CT, et
al: Expression of Foxp3 in colorectal cancer but not in Treg cells
correlates with disease progression in patients with colorectal
cancer. PLoS One. 8(e53630)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chu R, Liu SY, Vlantis AC, van Hasselt CA,
Ng EK, Fan MD, Ng SK, Chan AB, Du J, Wei W, et al: Inhibition of
Foxp3 in cancer cells induces apoptosis of thyroid cancer cells.
Mol Cell Endocrinol. 399:228–234. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zeng C, Yao Y, Jie W, Zhang M, Hu X, Zhao
Y, Wang S, Yin J and Song Y: Up-regulation of Foxp3 participates in
progression of cervical cancer. Cancer Immunol Immunother.
62:481–487. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zuo T, Wang L, Morrison C, Chang X, Zhang
H, Li W, Liu Y, Wang Y, Liu X, Chan MW, et al: FOXP3 is an X-linked
breast cancer suppressor gene and an important repressor of the
HER-2/ErbB2 oncogene. Cell. 129:1275–1286. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang L, Liu R, Li W, Chen C, Katoh H, Chen
GY, McNally B, Lin L, Zhou P, Zuo T, et al: Somatic single hits
inactivate the X-linked tumor suppressor FOXP3 in the prostate.
Cancer Cell. 16:336–346. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang HY and Sun H: Up-regulation of Foxp3
inhibits cell proliferation, migration and invasion in epithelial
ovarian cancer. Cancer Lett. 287:91–97. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luo X, Tan H, Zhou Y, Xiao T, Wang C and
Li Y: Notch1 signaling is involved in regulating Foxp3 expression
in T-ALL. Cancer Cell Int. 13(34)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fu HY, Li C, Yang W, Gai XD, Jia T, Lei YM
and Li Y: FOXP3 and TLR4 protein expression are correlated in
non-small cell lung cancer: Implications for tumor progression and
escape. Acta Histochem. 115:151–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li C, Sun L, Jiang R, Wang P, Xue H, Zhan
Y and Gai X: Downregulation of FOXP3 inhibits cell proliferation
and enhances chemosensitivity to cisplatin in human lung
adenocarcinoma. Pathol Res Pract. 213:1251–1256. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li C, Yang W, Gai X, Zhang Y, Li Y and Fu
H: Foxp3 overexpression decreases sensitivity to chemotherapy in
mouse Lewis lung cancer cells. Mol Med Rep. 6:977–982.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y, Li D, Yang W, Fu H, Liu Y and Li Y:
Overexpression of the transcription factor FOXP3 in lung
adenocarcinoma sustains malignant character by promoting G1/S
transition gene CCND1. Tumour Biol. 37:7395–7404. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang S, Liu Y, Li MY, Ng CSH, Yang SL,
Wang S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor
growth and metastasis by activating Wnt/β-catenin signaling pathway
and EMT in non-small cell lung cancer. Mol Cancer.
16(124)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Takebe N, Miele L, Harris PJ, Jeong W,
Bando H, Kahn M, Yang SX and Ivy SP: Targeting notch Hedgehog, and
Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin
Oncol. 12:445–464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang Z, Qi Y, Lai N, Zhang J, Chen Z, Liu
M, Zhang W, Luo R and Kang S: Notch1 signaling in melanoma cells
promoted tumor-induced immunosuppression via upregulation of
TGF-β1. J Exp Clin Cancer Res. 37(1)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Teoh SL and Das S: Notch signalling
pathways and their importance in the treatment of cancers. Curr
Drug Targets. 19:128–143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zou B, Zhou XL, Lai SQ and Liu JC: Notch
signaling and non-small cell lung cancer. Oncol Lett. 15:3415–3421.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S,
Chen Y and Wu K: Meta-analysis reveals the correlation of Notch
signaling with non-small cell lung cancer progression and
prognosis. Sci Rep. 5(10338)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta DeltaC (T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li C, Ma X, Tan C, Fang H, Sun Y and Gai
X: IL-17F expression correlates with clinicopathologic factors and
biological markers in non-small cell lung cancer. Pathol Res Pract.
215(152562)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang YQ, Jaganath IB, Manikam R and
Sekaran SD: Phyllanthus spp. exerts anti-angiogenic and
anti-metastatic effects through inhibition on matrix
metalloproteinase enzymes. Nutr Cancer. 67:783–795. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci.
17(868)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao L, Niu H, Liu Y, Wang L, Zhang N,
Zhang G, Liu R and Han M: LOX inhibition downregulates MMP-2 and
MMP-9 in gastric cancer tissues and cells. J Cancer. 10:6481–6490.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Giatromanolaki A, Lyberakidis G,
Lyratzopoulos N, Koukourakis MI, Sivridis E and Manolas C:
Angiogenesis and angiogenic factor expression in thyroid cancer. J
BUON. 15:357–361. 2010.PubMed/NCBI
|
|
33
|
Sadremomtaz A, Kobarfard F, Mansouri K,
Mirzanejad L and Asghari SM: Suppression of migratory and
metastatic pathways via blocking VEGFR1 and VEGFR2. J Recept Signal
Transduct Res. 38:432–441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tang J, Yang Z, Wang Z, Li Z, Li H, Yin J,
Deng M, Zhu W and Zeng C: Foxp3 is correlated with VEGF-C
expression and lymphangiogenesis in cervical cancer. World J Surg
Oncol. 15(173)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li X, Gao Y, Li J, Zhang K, Han J, Li W,
Hao Q, Zhang W, Wang S, Zeng C, et al: FOXP3 inhibits angiogenesis
by downregulating VEGF in breast cancer. Cell Death Dis.
9(744)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsubakihara Y and Moustakas A:
Epithelial-mesenchymal transition and metastasis under the control
of transforming growth factor beta. Int J Mol Sci.
19(3672)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li P, Sun T, Yuan Q, Pan G, Zhang J and
Sun D: The expressions of NEDD9 and E-cadherin correlate with
metastasis and poor prognosis in triple-negative breast cancer
patients. Onco Targets Ther. 9:5751–5759. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
He X, Chen Z, Jia M and Zhao X:
Downregulated E-cadherin expression indicates worse prognosis in
Asian patients with colorectal cancer: Evidence from meta-analysis.
PLoS One. 8(e70858)2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ancel J, Dewolf M, Deslée G, Nawrocky-Raby
B, Dalstein V, Gilles C and Polette M: Clinical impact of the
epithelial-mesenchymal transition in lung cancer as a biomarker
assisting in therapeutic decisions. Cells Tissues Organs. 1–19.
2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
40
|
Yang MH, Zang YS, Huang H, Chen K, Li B,
Sun GY and Zhao XW: Arsenic trioxide exerts anti-lung cancer
activity by inhibiting angiogenesis. Curr Cancer Drug Targets.
14:557–566. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gao YP, Li Y, Li HJ and Zhao B: LncRNA
NBR2 inhibits EMT progression by regulating Notch1 pathway in
NSCLC. Eur Rev Med Pharmacol Sci. 23:7950–7958. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Skarmoutsou E, Bevelacqua V, D' Amico F,
Russo A, Spandidos DA, Scalisi A, Malaponte G and Guarneri C: FOXP3
expression is modulated by TGF-β1/NOTCH1 pathway in human melanoma.
Int J Mol Med. 42:392–404. 2018.PubMed/NCBI View Article : Google Scholar
|