Comprehensive searches of PubMed, Medline, and Web
of Science were performed to identify all published reports on
patients with thrombosis associated with Mycoplasma
pneumoniae infection. Search terms included: ‘Mycoplasma
pneumoniae’ and ‘thrombus’ or ‘embolism’ or ‘thrombosis’ or
‘thrombotic’ or ‘thromboembolism’. Results published between
January 1970 and December 2020 were included. Only full-text,
English-language papers were included. Duplicate publications and
irrelevant topics were excluded. Data collected included the
location of thrombosis, thrombosis onset time since Mycoplasma
pneumoniae infection, laboratory examination regarding
thrombosis, and pathogenesis of thrombosis.
Certain studies and case reports have demonstrated
that patients with thrombosis secondary to Mycoplasma
pneumoniae infection were positive for anticardiolipin
antibodies, β2-glycoprotein antibodies or lupus anticoagulant
antibodies (28,32,46,51,53,54).
These aforementioned antibodies were transient and became negative
in certain patients 3-6 months after initial disease onset
(28,32,46,53,54).
Anticardiolipin antibody, β2-glycoprotein antibody and lupus
anticoagulant antibody are all antiphospholipid antibodies, which
reacts to phospholipids, phospholipid-protein complexes and
phospholipid-binding proteins (75,76).
The antiphospholipid antibodies contribute toward the formation of
a thrombus (76). Antiphospholipid
antibodies cause thrombosis through protein phosphatase 2A
activation via apolipoprotein E receptor 2, disabled-2 and src
homology domain-containing transforming protein 1 complex formation
in the endothelium (77). Patients
with thrombosis and positive antiphospholipid antibodies are also
likely to develop thrombosis again (78).
In addition to treatment with antibiotics, patients
underwent various methods to treat thrombosis associated with
Mycoplasma pneumoniae infection. In cases where the thrombus
partially detached and was almost floating in the right ventricle,
it was removed by cardiac surgery (46). Due to shape change and size
reduction of the thrombus, another patient with left ventricular
thrombus and Mycoplasma pneumoniae infection also underwent
urgent surgery (47). In another
case, an extremity deep vein thrombosis in a man with mycoplasma
pneumonia was not absorbed despite strong anticoagulant therapy,
and therefore a filter was implanted into his inferior vena cava to
prevent the thromboembolism recurring (41). Another boy with posterior cerebral
artery occlusion secondary to Mycoplasma pneumoniae
infection was treated with a low dose of aspirin (39). In other cases, thrombolytic therapy
with urokinase was performed in severe clinical condition (37,55).
Following anticoagulant treatment, the thrombus
absorption time of the majority of patients was more than 3 months,
but was 1.5 to 3 months in some others. However, in the majority of
patients, thrombus-related symptoms disappeared within 1 month
(32). However, certain patients
may also have sequelae, particularly patients with cerebral
thrombi. In one case, a boy with Mycoplasma pneumoniae
pneumonia and cerebral infarction still had poor right hand grip
power at the 6-month follow-up visit (40). In another case, an adult with
Mycoplasma pneumoniae pneumonia and cerebral infarction
slowly recovered from hemiplegia but continued to have a residual
deficit (29). Certain case reports
with cerebral infarction secondary to Mycoplasma pneumoniae
pneumonia reported that patients' neurological symptoms completely
resolved (31,34,41).
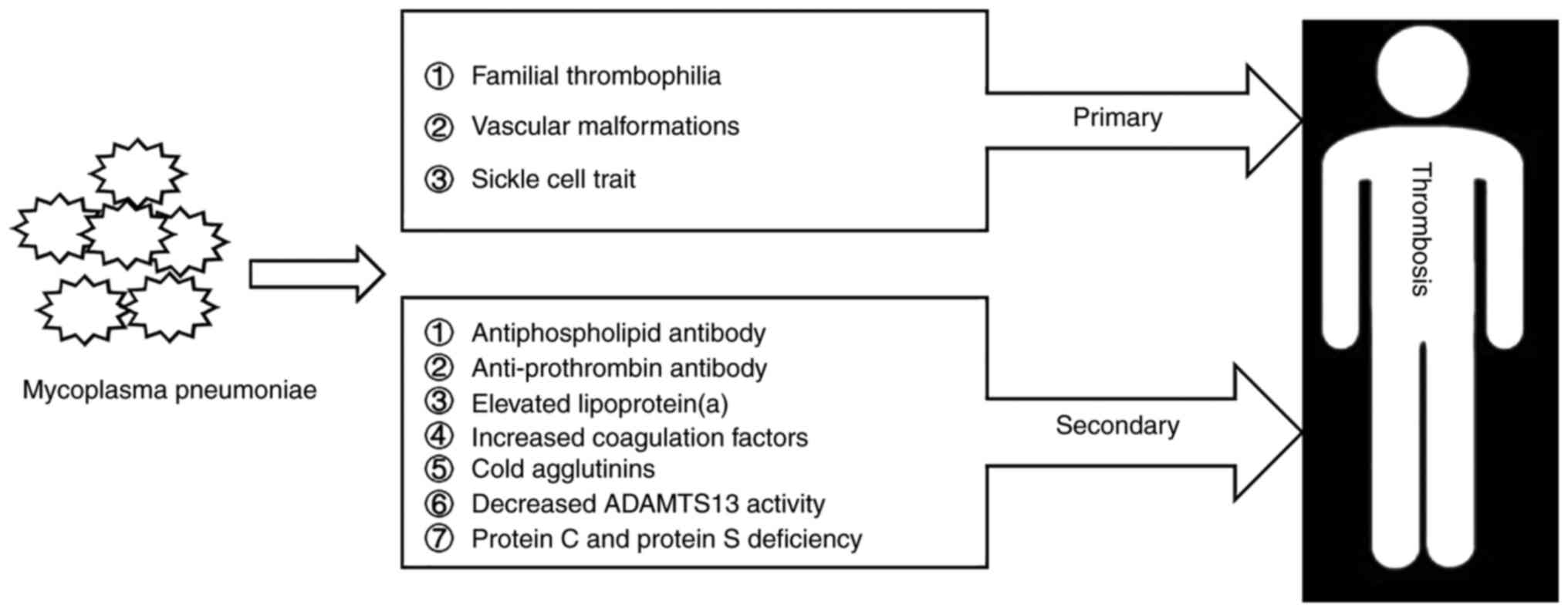
Further attention should be paid to the
extrapulmonary manifestations associated with Mycoplasma
pneumoniae infection, particularly thrombosis. The mechanism of
thrombosis in patients with Mycoplasma pneumoniae infection
includes numerous factors, and thrombi induced by Mycoplasma
pneumoniae infection may occur in any part of the body
(Fig. 1). Early diagnosis and
timely therapy may improve the prognosis of these patients.
Not applicable.
Funding: No funding was received.
Not applicable.
YL designed the study. JL wrote the manuscript.
Data authentication is not applicable for this study. Both authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Poddighe D: Mycoplasma
pneumoniae-related hepatitis in children. Microb Pathog.
139(103863)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Waites KB, Xiao L, Liu Y, Balish MF and
Atkinson TP: Mycoplasma pneumoniae from the respiratory
tract and beyond. Clin Microbiol Rev. 30:747–809. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sánchez-Vargas FM and Gómez-Duarte OG:
Mycoplasma pneumoniae-an emerging extra-pulmonary pathogen.
Clin Microbiol Infect. 14:105–117. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Katz B and Waites K: Emerging
intracellular bacterial infections. Clin Lab Med. 24:627–649.
2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rottem S: Interaction of mycoplasmas with
host cells. Physiol Rev. 83:417–432. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zlatian O, Balasoiu AT, Balasoiu M,
Cristea O, Docea AO, Mitrut R, Spandidos DA, Tsatsakis AM, Bancescu
G and Calina D: Antimicrobial resistance in bacterial pathogens
among hospitalised patients with severe invasive infections. Exp
Ther Med. 16:4499–4510. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ungureanu A, Zlatian O, Mitroi G, Drocaş
A, Ţîrcă T, Călina D, Dehelean C, Docea AO, Izotov BN, Rakitskii
VN, et al: Staphylococcus aureus colonisation in patients from a
primary regional hospital. Mol Med Rep. 16:8771–8780.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Z, Yang L, Ye J, Wang Y and Liu Y:
Monocyte subsets study in children with Mycoplasma
pneumoniae pneumonia. Immunol Res. 67:373–381. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jacobs E, Ehrhardt I and Dumke R: New
insights in the outbreak pattern of Mycoplasma pneumoniae.
Int J Med Microbiol. 305:705–708. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Meyer Sauteur PM, van Rossum AM and Vink
C: Mycoplasma pneumoniae in children: Carriage,
pathogenesis, and antibiotic resistance. Curr Opin Infect Dis.
27:220–227. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wood PR, Hill VL, Burks ML, Peters JI,
Singh H, Kannan TR, Vale S, Cagle MP, Principe MF, Baseman JB and
Brooks EG: Mycoplasma pneumoniae in children with acute and
refractory asthma. Ann Allergy Asthma Immunol. 110:328–334 e1.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Narita M: Classification of extrapulmonary
manifestations due to Mycoplasma pneumoniae infection on the
basis of possible pathogenesis. Front Microbiol.
7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Canavan TN, Mathes EF, Frieden I and
Shinkai K: Mycoplasma pneumoniae-induced rash and mucositis
as a syndrome distinct from Stevens-Johnson syndrome and erythema
multiforme: A systematic review. J Am Acad Dermatol. 72:239–245.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuźma-Mroczkowska E, Pańczyk-Tomaszewska
M, Szmigielska A, Szymanik-Grzelak H and Roszkowska-Blaim M:
Mycoplasma pneumoniae as a trigger for Henoch-Schonlein
purpura in children. Cent Eur J Immunol. 40:489–492.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vijay A, Stendahl JC and Rosenfeld LE:
Mycoplasma pneumoniae pericarditis. Am J Cardiol.
123:1383–1384. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lan Y, Li S, Yang D, Zhou J, Wang Y, Wang
J, Xu Y and Chen Z: Clinical characteristics of Kawasaki disease
complicated with Mycoplasma pneumoniae pneumonia: A
retrospective study. Medicine (Baltimore).
99(e19987)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wandro C, Dolatshahi L and Blackall D:
Severe warm autoimmune hemolytic anemia in a 7-month-old infant
associated with a Mycoplasma pneumoniae infection. J Pediatr
Hematol Oncol. 40:e439–e441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gouveia C, Evangelista V, Almeida R and
Baptista AM: Immune thrombocytopenia associated with Mycoplasma
pneumoniae infection. Eur J Case Rep Intern Med.
5(000817)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Romero-Gómez M, Otero MA, Sánchez-Muñoz D,
Ramírez-Arcos M, Larraona JL, Suárez García E and Vargas-Romero J:
Acute hepatitis due to Mycoplasma pneumoniae infection
without lung involvement in adult patients. J Hepatol. 44:827–828.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Valdés Lacasa T, Duarte Borges MA, García
Marín A and Gómez Cuervo C: Acute pancreatitis caused by
Mycoplasma pneumoniae: An unusual etiology. Clin J
Gastroenterol. 10:279–282. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Meyer Sauteur PM, Huizinga R, Tio-Gillen
AP, Drenthen J, Unger WWJ, Jacobs E, van Rossum AMC and Jacobs BC:
Intrathecal antibody responses to GalC in Guillain-Barre syndrome
triggered by Mycoplasma pneumoniae. J Neuroimmunol.
314:13–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Coriolani G, Ferranti S, Squarci G and
Grosso S: A case of Bickerstaff encephalitis associated with
Mycoplasma pneumoniae infection. Neurol Sci. 41:1605–1606.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Siomou E, Kollios KD, Papadimitriou P,
Kostoula A and Papadopoulou Z: Acute nephritis and respiratory
tract infection caused by Mycoplasma pneumoniae: Case report
and review of the literature. Pediatr Infect Dis J. 22:1103–1106.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Poddighe D: Mycoplasma
pneumoniae-related extra-pulmonary diseases and antimicrobial
therapy. J Microbiol Immunol Infect. 53:188–189. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang TI, Chang TH, Lu CY, Chen JM, Lee PI,
Huang LM and Chang LY: Mycoplasma pneumoniae in pediatric
patients: Do macrolide-resistance and/or delayed treatment matter?
J Microbiol Immunol Infect. 52:329–335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Poddighe D, Comi EV, Brambilla I, Licari
A, Bruni P and Marseglia GL: Increased total serum immunoglobulin E
in children developing Mycoplasma pneumoniae-related
extra-pulmonary diseases. Iran J Allergy Asthma Immunol.
17:490–496. 2018.PubMed/NCBI
|
|
27
|
Poddighe D and Marseglia GL: Is there any
relationship between extra-pulmonary manifestations of
Mycoplasma pneumoniae infection and atopy/respiratory
allergy in children? Pediatr Rep. 8(6395)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Flateau C, Asfalou I, Deman AL, Ficko C,
Andriamanantena D, Fontan E, Viant E, Bonnevie L and Rapp C: Aortic
thrombus and multiple embolisms during a Mycoplasma
pneumoniae infection. Infection. 41:867–873. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dowd AB, Grace R and Rees WD: Cerebral
infarction associated with Mycoplasma pneumoniae infection.
Lancet. 2(567)1987.PubMed/NCBI
|
|
30
|
Choi YH, Jeong HJ, Lee B, An HY, Lee EJ
and Park JD: Extensive and progressive cerebral infarction after
Mycoplasma pneumoniae infection. Korean J Crit Care Med.
32:211–217. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim GH, Seo WH, Je BK and Eun SH:
Mycoplasma pneumoniae associated stroke in a 3-year-old
girl. Korean J Pediatr. 56:411–415. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu J, He R, Wu R, Wang B, Xu H, Zhang Y,
Li H and Zhao S: Mycoplasma pneumoniae pneumonia associated
thrombosis at Beijing Children's hospital. BMC Infect Dis.
20(51)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ryu JS, Kim HJ, Sung IY and Ko TS:
Posterior cerebral artery occlusion after Mycoplasma
pneumoniae infection associated with genetic defect of MTHFR
C677T. J Child Neurol. 24:891–894. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sarathchandran P, Al Madani A, Alboudi AM
and Inshasi J: Mycoplasma pneumoniae infection presenting as
stroke and meningoencephalitis with aortic and subclavian aneurysms
without pulmonary involvement. BMJ Case Rep.
2018(bcr2017221831)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kang B, Kim DH, Hong YJ, Son BK, Lim MK,
Choe YH and Kwon YS: Complete occlusion of the right middle
cerebral artery associated with Mycoplasma pneumoniae
pneumonia. Korean J Pediatr. 59:149–152. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sotgiu S, Pugliatti M, Rosati G, Deiana GA
and Sechi GP: Neurological disorders associated with Mycoplasma
pneumoniae infection. Eur J Neurol. 10:165–168. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Van Dyke DC, Eldadah MK, Bale JF Jr,
Kramer M, Alexander R, Smith WL and Olivero W: Mycoplasma
pneumoniae-induced cerebral venous thrombosis treated with
urokinase. Clin Pediatr (Phila). 31:501–504. 1992.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Visudhiphan P, Chiemchanya S and Sirinavin
S: Internal carotid artery occlusion associated with Mycoplasma
pneumoniae infection. Pediatr Neurol. 8:237–239.
1992.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Antachopoulos C, Liakopoulou T, Palamidou
F, Papathanassiou D and ǎnd Youroukos S: Posterior cerebral artery
occlusion associated with Mycoplasma pneumoniae infection. J
Child Neurol. 17:55–57. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jin X, Zou Y, Zhai J, Liu J and Huang B:
Refractory Mycoplasma pneumoniae pneumonia with concomitant
acute cerebral infarction in a child: A case report and literature
review. Medicine (Baltimore). 97(e0103)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Senda J, Ito M, Atsuta N, Watanabe H,
Hattori N, Kawai H and Sobue G: Paradoxical brain embolism induced
by Mycoplasma pneumoniae infection with deep venous
thrombus. Intern Med. 49:2003–2005. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Garcia AV, Finger , et al
Thirumoorthi AS, Kadenhe-Chiweshe A, Kandel JJ: Severe
Mycoplasma pneumoniae infection requiring extracorporeal
membrane oxygenation with concomitant ischemic stroke in a child.
Pediatr Pulmonol. 48:98–101. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Garcia Tirado A, Jimenez-Rolando B, Noval
S and Martinez Bermejo A: Cortical Blindness in a Child Secondary
to Mycoplasma pneumoniae Infection. J Stroke Cerebrovasc
Dis. 26:e12–e13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fu M, Wong KS, Lam WW and Wong GW: Middle
cerebral artery occlusion after recent Mycoplasma pneumoniae
infection. J Neurol Sci. 157:113–115. 1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Socan M, Ravnik I, Bencina D, Dovc P,
Zakotnik B and Jazbec J: Neurological symptoms in patients whose
cerebrospinal fluid is culture- and/or polymerase chain
reaction-positive for Mycoplasma pneumoniae. Clin Infect
Dis. 32:E31–E35. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
46
|
Nagashima M, Higaki T, Satoh H and Nakano
T: Cardiac thrombus associated with Mycoplasma pneumoniae
infection. Interact Cardiovasc Thorac Surg. 11:849–851.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Oeser C, Andreas M, Rath C, Habertheuer A
and Kocher A: Left ventricular thrombus in a patient with cutaneous
T-cell lymphoma, hypereosinophilia and Mycoplasma pneumoniae
infection-a challenging diagnosis: A case report. J Cardiothorac
Surg. 10(21)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pachet A, Dumestre-Perard C, Moine M,
Marlu R, Rubio A and Bost-Bru C: Splenic infarction associated with
transient anti-prothrombin antibodies is a rare manifestation of
acute Mycoplasma pneumoniae infection. Arch Pediatr.
26:483–486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li Y, Pattan V, Syed B, Islam M and Yousif
A: Splenic infarction caused by a rare coinfection of Epstein-Barr
virus, cytomegalovirus, and Mycoplasma pneumoniae. Pediatr
Emerg Care. 30:636–637. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Park SJ, Lee YM, Lee CH, Cho JH and Lee
JH: A case of splenic infarction possibly attributable to
Mycoplasma pneumoniae infection without accompanying
pneumonia. J Infect Chemother. 18:945–947. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Witmer CM, Steenhoff AP, Shah SS and
Raffini LJ: Mycoplasma pneumoniae, splenic infarct, and
transient antiphospholipid antibodies: A new association?
Pediatrics. 119:e292–e295. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu J, Zhao F, Lu J, Xu H, Liu H, Tang X,
Yang H, Zhang J and Zhao S: High Mycoplasma pneumoniae loads
and persistent long-term Mycoplasma pneumoniae DNA in lower
airway associated with severity of pediatric Mycoplasma
pneumoniae pneumonia. BMC Infect Dis. 19(1045)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen Y, Huang P, Chen Q, Lin Z and Tian W:
Two separated thrombi in deep veins associated with pulmonary
embolism after Mycoplasma pneumoniae infection: A case in
adolescent female. Transl Pediatr. 2:198–201. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Graw-Panzer KD, Verma S, Rao S, Miller ST
and Lee H: Venous thrombosis and pulmonary embolism in a child with
pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc.
101:956–958. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Joo CU, Kim JS and Han YM: Mycoplasma
pneumoniae induced popliteal artery thrombosis treated with
urokinase. Postgrad Med J. 77:723–724. 2001.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Creagh MD, Roberts IF, Clark DJ and
Preston FE: Familial antithrombin III deficiency and Mycoplasma
pneumoniae pneumonia. J Clin Pathol. 44:870–871.
1991.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kalicki B, Sadecka M, Wawrzyniak A,
Kozinski P, Dziekiewicz M and Jung A: Absence of inferior vena cava
in 14-year old boy associated with deep venous thrombosis and
positive Mycoplasma pneumoniae serum antibodies-a case
report. BMC Pediatr. 15(40)2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Caeiro Alves F, Aguiar R, Pessegueiro P
and Pires C: Thrombotic microangiopathy associated with
Mycoplasma pneumoniae infection. BMJ Case Rep.
2018(bcr2017222582)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Godron A, Pereyre S, Monet C, Llanas B and
Harambat J: Hemolytic uremic syndrome complicating Mycoplasma
pneumoniae infection. Pediatr Nephrol. 28:2057–2060.
2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bar Meir E, Amital H, Levy Y, Kneller A,
Bar-Dayan Y and Shoenfeld Y: Mycoplasma-pneumoniae-induced
thrombotic thrombocytopenic purpura. Acta Haematol. 103:112–115.
2000.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Cameron D, Welsby P and Turner M:
Thrombotic thrombocytopenic purpura due to Mycoplasma
pneumoniae. Postgrad Med J. 68:393–394. 1992.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Byrnes JR and Wolberg AS: Red blood cells
in thrombosis. Blood. 130:1795–1799. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li T, Yu H, Hou W, Li Z, Han C and Wang L:
Evaluation of variation in coagulation among children with
Mycoplasma pneumoniae pneumonia: A case-control study. J Int
Med Res. 45:2110–2118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Connors JM: Thrombophilia testing and
venous thrombosis. N Engl J Med. 377:1177–1187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Narita M: Pathogenesis of extrapulmonary
manifestations of Mycoplasma pneumoniae infection with
special reference to pneumonia. J Infect Chemother. 16:162–169.
2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Higuchi ML, Sambiase N, Palomino S,
Gutierrez P, Demarchi LM, Aiello VD and Ramires JA: Detection of
Mycoplasma pneumoniae and Chlamydia pneumoniae in ruptured
atherosclerotic plaques. Braz J Med Biol Res. 33:1023–1026.
2000.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Higuchi Mde L, Reis MM, Sambiase NV,
Palomino SA, Castelli JB, Gutierrez PS, Aiello VD and Ramires JA:
Coinfection with Mycoplasma pneumoniae and Chlamydia
pneumoniae in ruptured plaques associated with acute myocardial
infarction. Arq Bras Cardiol. 81:1–22. 2003.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Cho A, Ragi SD, Oh JK, Lima de Carvalho JR
Jr, Ryu J, Yang BY and Tsang SH: Sequential multiple retinal vein
occlusions and transient ischemic attack in MTHFR polymorphism and
protein S deficiency. Mol Genet Genomic Med.
8(e1273)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gatt A and Makris M: Hyperhomocysteinemia
and venous thrombosis. Semin Hematol. 44:70–76. 2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Cheng Z, Jiang X, Pansuria M, Fang P, Mai
J, Mallilankaraman K, Gandhirajan RK, Eguchi S, Scalia R, Madesh M,
et al: Hyperhomocysteinemia and hyperglycemia induce and potentiate
endothelial dysfunction via µ-calpain activation. Diabetes.
64:947–959. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ospina-Romero M, Cannegieter SC, den
Heijer M, Doggen CJM, Rosendaal FR and Lijfering WM:
Hyperhomocysteinemia and risk of first venous thrombosis: The
influence of (Unmeasured) confounding factors. Am J Epidemiol.
187:1392–1400. 2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Muszbek L, Bereczky Z, Kovács B and
Komáromi I: Antithrombin deficiency and its laboratory diagnosis.
Clin Chem Lab Med. 48 (Suppl 1):S67–S78. 2010.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Pabinger I and Thaler J: How I treat
patients with hereditary antithrombin deficiency. Blood.
134:2346–2353. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Patnaik MM and Moll S: Inherited
antithrombin deficiency: A review. Haemophilia. 14:1229–1239.
2008.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wang D, Lv W, Zhang S and Zhang J:
Advances in the research on anticardiolipin antibody. J Immunol
Res. 2019(8380214)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Habe K, Wada H, Matsumoto T, Ohishi K,
Ikejiri M, Matsubara K, Morioka T, Kamimoto Y, Ikeda T, Katayama N,
et al: Presence of antiphospholipid antibody is a risk factor in
thrombotic events in patients with antiphospholipid syndrome or
relevant diseases. Int J Hematol. 97:345–350. 2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Sacharidou A, Chambliss KL, Ulrich V,
Salmon JE, Shen YM, Herz J, Hui DY, Terada LS, Shaul PW and Mineo
C: Antiphospholipid antibodies induce thrombosis by PP2A activation
via apoER2-Dab2-SHC1 complex formation in endothelium. Blood.
131:2097–2110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ortel TL, Meleth S, Catellier D, Crowther
M, Erkan D, Fortin PR, Garcia D, Haywood N, Kosinski AS, Levine SR,
et al: Recurrent thrombosis in patients with antiphospholipid
antibodies and an initial venous or arterial thromboembolic event:
A systematic review and meta-analysis. J Thromb Haemost.
18:2274–2286. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zohoury N, Bertolaccini ML,
Rodriguez-Garcia JL, Shums Z, Ateka-Barrutia O, Sorice M, Norman GL
and Khamashta M: Closing the serological gap in the
antiphospholipid syndrome: the value of ‘non-criteria’
antiphospholipid antibodies. J Rheumatol. 44:1597–1602.
2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sciascia S, Sanna G, Murru V, Roccatello
D, Khamashta MA and Bertolaccini ML: Anti-prothrombin (aPT) and
anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the
risk of thrombosis in the antiphospholipid syndrome. A systematic
review. Thromb Haemost. 111:354–364. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ho WK and Rigano J: Prevalence of
autoantibodies directed against prothrombin in unprovoked venous
thromboembolism. J Thromb Thrombolysis. 49:446–450. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Berentsen S: Complement activation and
inhibition in autoimmune hemolytic anemia: Focus on cold agglutinin
disease. Semin Hematol. 55:141–149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Habib A, Kunzelmann C, Shamseddeen W,
Zobairi F, Freyssinet JM and Taher A: Elevated levels of
circulating procoagulant microparticles in patients with
beta-thalassemia intermedia. Haematologica. 93:941–942.
2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Zhou Z, Behymer M and Guchhait P: Role of
extracellular hemoglobin in thrombosis and vascular occlusion in
patients with sickle cell anemia. Anemia.
2011(918916)2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Rother RP, Bell L, Hillmen P and Gladwin
MT: The clinical sequelae of intravascular hemolysis and
extracellular plasma hemoglobin: A novel mechanism of human
disease. JAMA. 293:1653–1662. 2005.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Skuza AA, Polak M and Undas A: Elevated
lipoprotein(a) as a new risk factor of cerebral venous sinus
thrombosis: Association with fibrin clot properties. J Thromb
Thrombolysis. 47:8–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Anglés-Cano E, de la Peña Díaz A and Loyau
S: Inhibition of fibrinolysis by lipoprotein(a). Ann N Y Acad Sci.
936:261–275. 2001.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Feric NT, Boffa MB, Johnston SM and
Koschinsky ML: Apolipoprotein(a) inhibits the conversion of
Glu-plasminogen to Lys-plasminogen: A novel mechanism for
lipoprotein(a)-mediated inhibition of plasminogen activation. J
Thromb Haemost. 6:2113–2120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Rouy D, Grailhe P, Nigon F, Chapman J and
Anglés-Cano E: Lipoprotein(a) impairs generation of plasmin by
fibrin-bound tissue-type plasminogen activator. In vitro studies in
a plasma milieu. Arterioscler Thromb. 11:629–638. 1991.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Chew RR, Lim AH and Toh D: Congenital
absence of inferior vena cava: An under recognised cause of
unprovoked venous thromboembolism. QJM. 111:117–118.
2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Gökçe Ş, Keskin G, Yaşar ŞK, Arslan AT,
Cerit Z, Koska Öİ and Aydoğdu S: A case of May-Thurner Syndrome: An
old anomaly but, a new suggestion: A case report. Malawi Med J.
31:230–232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ho AMH, Chung AD and Mizubuti GB: A
hairdresser's painful swollen left leg: Artery compresses vein in
May-Thurner syndrome. Lance. 394(e33)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Yang S, You R, Wu W, Wei Z, Hong M and
Peng Z: Dural arteriovenous fistula complicated with cerebral
venous sinus thrombosis. World Neurosurg. 134:348–352.
2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Carqueja IM, Sousa J and Mansilha A:
Vascular malformations: Classification, diagnosis and treatment.
Int Angiol. 37:127–142. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Jensen CT, Chahin A, Amin VD, Khalaf AM,
Elsayes KM, Wagner-Bartak N, Zhao B, Zhou S and Bedi DG:
Qualitative slow blood flow in lower extremity deep veins on
doppler sonography: Quantitative assessment and preliminary
evaluation of correlation with subsequent deep venous thrombosis
development in a tertiary care oncology center. J Ultrasound Med.
36:1867–1874. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Roggério A, Sambiase NV, Palomino SA, de
Castro MA, da Silva ES, Stolf NG and de Lourdes Higuchi M:
Correlation of bacterial coinfection versus matrix
metalloproteinase 9 and tissue inhibitor of metalloproteinase 1
expression in aortic aneurysm and atherosclerosis. Ann Vasc Surg.
27:964–971. 2013.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Hellwig K, Hoffmann L, Rother U, Meyer A,
Lang W and Schmid A: Eligibility of endovascular repair for
popliteal artery aneurysms according the instructions for use. Ann
Vasc Surg. 67:370–375. 2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wehbe E, Abou Antoun S and Peery WH: An
unusual complication of sickle cell trait: Intraureter thrombus.
Int Urol Nephrol. 42:517–518. 2010.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Ali JM, Besser M, Goddard M, Abu-Omar Y,
Catarino P, Bhagra S and Berman M: Catastrophic sickling crisis in
patient undergoing cardiac transplantation with sickle cell trait.
Am J Transplant. 19:2378–2382. 2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Kumar R, Kapoor R, Singh J, Das S, Sharma
A, Yanamandra U and Nair V: Splenic infarct on exposure to extreme
high altitude in individuals with sickle trait: A single-center
experience. High Alt Med Biol. 20:215–220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Tripette J, Hardy-Dessources MD, Romana M,
Hue O, Diaw M, Samb A, Diop S and Connes P: Exercise-related
complications in sickle cell trait. Clin Hemorheol Microcirc.
55:29–37. 2013.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Eichner ER: Sickle cell considerations in
athletes. Clin Sports Med. 30:537–549. 2011.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Vincent L, Féasson L, Oyono-Enguéllé S,
Banimbek V, Denis C, Guarneri C, Aufradet E, Monchanin G, Martin C,
Gozal D, et al: Remodeling of skeletal muscle microvasculature in
sickle cell trait and alpha-thalassemia. Am J Physiol Heart Circ
Physiol. 298:H375–H384. 2010.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Faёs C, Martin C, Chirico EN, Féasson L,
Oyonno-Enguelle S, Dubouchaud H, Francina A, Thiriet P, Pialoux V
and Messonnier L: Effect of α-thalassaemia on exercise-induced
oxidative stress in sickle cell trait. Acta Physiol (Oxf).
205:541–550. 2012.PubMed/NCBI View Article : Google Scholar
|
|
105
|
South K and Lane DA: ADAMTS-13 and von
Willebrand factor: A dynamic duo. J Thromb Haemost. 16:6–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Ono T, Mimuro J, Madoiwa S, Soejima K,
Kashiwakura Y, Ishiwata A, Takano K, Ohmori T and Sakata Y: Severe
secondary deficiency of von Willebrand factor-cleaving protease
(ADAMTS13) in patients with sepsis-induced disseminated
intravascular coagulation: Its correlation with development of
renal failure. Blood. 107:528–534. 2006.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Levi M, Scully M and Singer M: The role of
ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost.
16:646–651. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Chen J and Chung DW: Inflammation, von
Willebrand factor, and ADAMTS13. Blood. 132:141–147.
2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Bernardo A, Ball C, Nolasco L, Moake JF
and Dong JF: Effects of inflammatory cytokines on the release and
cleavage of the endothelial cell-derived ultralarge von Willebrand
factor multimers under flow. Blood. 104:100–106. 2004.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Wypasek E and Undas A: Protein C and
protein S deficiency-practical diagnostic issues. Adv Clin Exp Med.
22:459–467. 2013.PubMed/NCBI
|
|
111
|
Dinarvand P and Moser KA: Protein C
deficiency. Arch Pathol Lab Med. 143:1281–1285. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Padda IS, Patel P and D Citla Sridhar D:
Protein S and C. In: StatPearls. Treasure Island (FL), StatPearls
Publishing Copyright ©. 2020, StatPearls Publishing LLC, 2020.
|
|
113
|
Girolami A, Cosi E, Ferrari S and Girolami
B: Heparin, coumarin, protein C, antithrombin, fibrinolysis and
other clotting related resistances: Old and new concepts in blood
coagulation. J Thromb Thrombolysis. 45:135–141. 2018.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Mirijello A, La Marca A, D'Errico MM,
Curci S, Vendemiale G, Grandone E and De Cosmo S: Venous
thromboembolism during Mycoplasma pneumoniae infection: Case
report and review of the literature. Eur Rev Med Pharmacol Sci.
24:10061–10068. 2020.PubMed/NCBI View Article : Google Scholar
|