Introduction
Intrauterine adhesion (IUA; also referred to as
Asherman's syndrome) is a disease characterized by partial or
complete uterine/cervical atresia, as well as abnormal menstrual
patterns, such as amenorrhea and hypomenorrhea, and fertility
impairment, including spontaneous miscarriage, placenta accretion,
preterm delivery and intrauterine growth restriction (1-3).
Injury to the endometrium is considered the leading cause of IUA
(1-3).
At present, it is difficult to estimate the actual IUA incidence
due to underdiagnoses. However, previous studies have estimated
that 1.5% of female cases of infertility, 5-39% of those with
recurrent miscarriage and 40% with repeat dilation and curettage
for retained placental tissue are related to IUA (2,3).
To date, numerous approaches, such as adhesiolysis,
intrauterine devices, intrauterine balloon stent, anti-adhesion
barrier hyaluronic acid and carboxymethylcellulose, have been
adopted for the prevention of IUA after surgery (4). However, the rate of adhesion
recurrence has remained significantly high after hysteroscopic
adhesiolysis, while data regarding safety and efficacy of
alternative methods are currently insufficient.
Technological advancements in tissue engineering
have allowed the identification of biological materials with
crucial roles in repairing damaged tissues. For instance, the
utility of amniotic membrane (AM), a translucent membrane derived
from the placenta, which consists of monostratified epithelium
expressing few histocompatibility antigens and stroma with no blood
vessels, nerves and lymph vessels, has been documented (5). Consequently, this membrane with
anti-inflammatory, low-immunogenicity and anti-fibrotic properties
has been applied for wound healing, particularly in ophthalmology
and burns (6,7). However, this approach is still
associated with certain problems regarding storage and infection of
fresh AM. Decellularized and lyophilized amniotic membrane (DL-AM)
has been developed as an improved approach; in this material,
immunogenicity is eliminated through removal of epithelial cells
and problems related to infection and storage are prevented by
sterilization and lyophilization (8,9). In
fact, DL-AM has been successfully applied to close
pharyngocutaneous fistula (10).
Previous studies by our group indicated that DL-AM effectively
suppressed IUA by ameliorating endometrial fibrosis (11-13).
However, the underlying mechanisms of action have remained to be
elucidated.
The major pathological changes of IUA are avascular
fibrous strands joining uterine walls due to accumulation of
extracellular matrix (ECM) (1-3).
Various proteins and cytokines have been implicated in fibrosis
development. For instance, connective tissue growth factor (CTGF)
is a widely known hallmark of fibrosis across multiple tissues,
including IUA (14). Furthermore,
matrix metalloproteinases (MMPs) are a large family of
zinc-dependent endopeptidases that degrade ECM components. Of note,
disruption of the equilibrium between ECM accumulation and
degradation has been associated with fibrosis development (15).
In the present study, the efficacy of DL-AM
transplantation to inhibit endometrial fibrosis was evaluated in
damaged uteri of a rat model of IUA. It was further investigated
whether this effect was mediated via downregulation and
upregulation of CTGF and MMP-2, respectively.
Materials and methods
Ethics statement
Animal handling and experimental procedures were
performed in compliance with the guidelines approved by the
Institutional Animal Care and Use Committee (IACUC) at Nanjing
Medical University (approval no. IACUC-1912051) and the Animal
Research: Reporting of in vivo Experiments guidelines
(16). Rats were housed (3 rats per
cage) under conditions including a 12-h light/dark light-dark
cycle, temperature of 22-25˚C and relative humidity of 50-65% with
free access to food and water. All efforts were made to minimize
animal suffering. AM samples were obtained from donors who had
caesarian sections and seronegative results for syphilis, human
immunodeficiency virus, hepatitis B and hepatitis C virus. Sample
collection was performed under sterile conditions after obtainment
of written informed consent by the subjects.
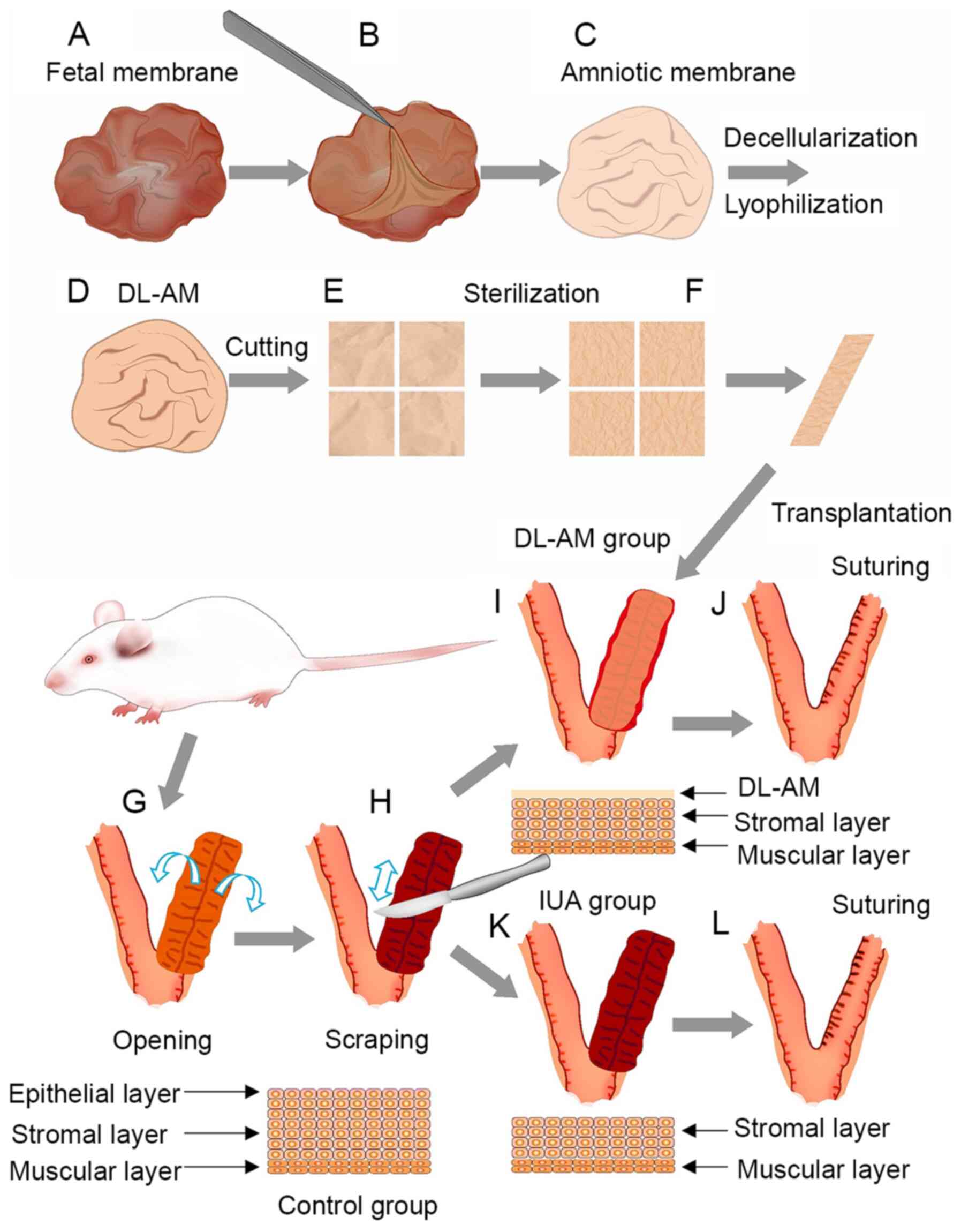
Preparation of DL-AM
First, the AM was separated from the chorion
membrane and then gently washed with sterile PBS to remove the
blood component. The clean samples were incubated with 0.2% EDTA
for 30 min, with continuous stirring for decellularization, then
dried in a lyophilizer. Samples were cut into small pieces,
measuring ~2.5x2.5 cm, sterilized under Co60 γ-ray
irradiation (25 kGy) sterilizer and then vacuum-packed for
subsequent experiments (17)
(Fig. 1).
Establishment of an IUA rat model and
DL-AM transplantation
A total of 24 Sprague Dawley rats (180-220 g;
8-week-old; female; Charles River Laboratories, Inc.) were randomly
divided into two groups: IUA (n=12) and IUA + DL-AM (n=12). Vaginal
smears of exfoliated vaginal epithelial cells were observed under
light microscopy prior to surgery. All rats were operated and
sacrificed during the anestrus period. In brief, rats were
anesthetized under pentobarbital (40 mg/kg, intraperitoneal
injection) and their Y-type uterus was exposed, with the right
uteri of each rat used as control without treatment. The left uteri
of rats in the IUA group were cut and scraped to the depth of the
stromal layer (including epithelial and at least 1/3 stromal layer
to ensure successful establishment of the IUA model) using a razor
blade. The wound was then carefully sutured and closed. For rats in
the IUA + DL-AM group, DL-AM was transplanted onto the inner
surface of the scraped uterus and the incision closed by careful
suturing (Fig. 1). Rats were
sacrificed under pentobarbital (100 mg/kg, intraperitoneal
injection) (no heartbeat and no breathing were confirmed for death)
and uteri were cut and collected 3, 7, 14 and 28 days after
surgery.
Histology and
immunohistochemistry
All samples were fixed in 4% paraformaldehyde
solution, embedded in paraffin wax and then cut into 5-µm sections.
Van Gieson staining (Beijing Solarbio Science & Technology Co.,
Ltd.) was performed to evaluate fibrosis. The percentage of
fibrotic area was defined as the ratio of endometrial fibrotic to
the whole endometrial areas (including epithelial and stromal
layers) (11). For
immunohistochemistry, sections were deparaffinized and rehydrated
using graded ethanol, then incubated with sodium citrate solution
(0.1 mM, pH 6.0) at 95-100˚C for 10 min for antigen-retrieval and
then with 3% hydrogen peroxide solution for endogenous peroxidase
activity blocking at room temperature for 15 min. The samples were
incubated with primary antibodies, namely anti-CTGF (cat. no.
bs-0843R; Bioss) and anti-MMP-2 (cat. no. bs-4599R; Bioss) diluted
at 1:200 overnight at 4˚C and then with a secondary antibody (cat.
no. TA130001; 1:100; OriGene Technologies, Inc.) matching the
respective primary antibodies at 25˚C for 1 h. Sections were then
stained with 3-3'-diaminobenzidine solution (OriGene Technologies,
Inc.) and counterstained with hematoxylin.
Scanning electron microscopy
Specimens were fixed in 1% glutaraldehyde at 4˚C for
24 h and then treated with 1% osmium tetroxide for 2 h. They were
then dehydrated, critical-point dried, mounted and coated with gold
prior to visualization. All specimens were observed under a
scanning electron microscope (S-3400N; Hitachi, Ltd.).
Statistical analysis
Data were analyzed using SPSS 24.0 and expressed as
the mean ± standard deviation. Comparisons among groups were
performed by one-way ANOVA and Tukey's post-hoc test. The software,
ImagePro Plus v6.0 (Media Cybernetics, Inc.), was employed to
calculate the rate of fibrotic area and evaluate the levels of CTGF
and MMP-2 expression in the groups (11-13).
The integral optical density (IOD) was defined as the sum total of
optical density for positively-stained unit areas for CTGF and
MMP-2, with high IOD values implying higher expression as
previously described as a semiquantitative method to evaluate the
expression of proteins (11-13,17,18).
The IOD value was calculated by two authors independently.
P<0.05 was considered to indicate statistical significance.
Results
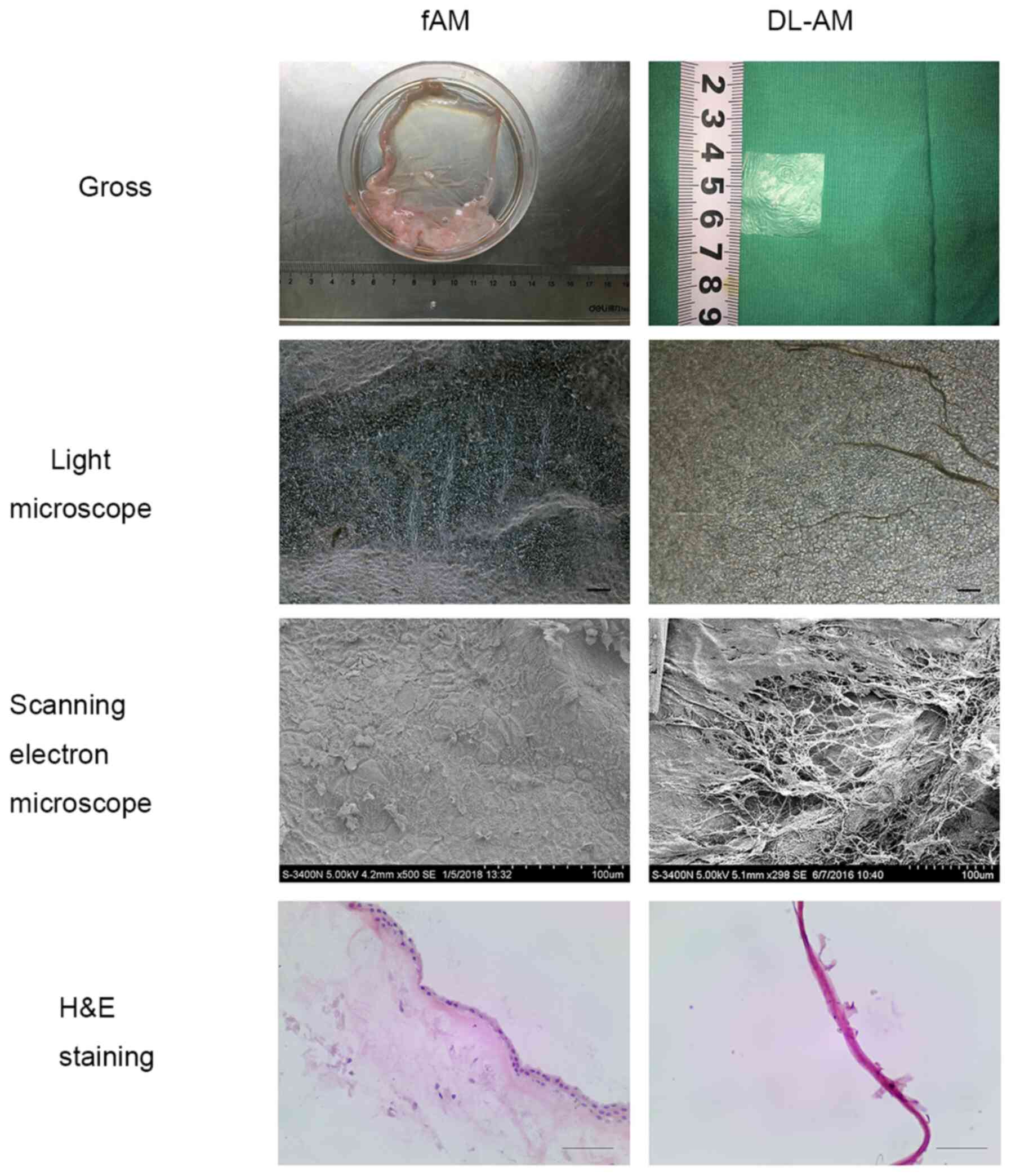
AM decellularization and
confirmation
The DL-AM appeared transparent and fragile. H&E
staining revealed monostratified cylinder cell epithelium and
stromal cells in fresh AM, but no cells on the surface of DL-AM. In
addition, light microscopy indicated no structure of epithelial
cells on the surface of DL-AM. Further examination using scanning
electron microscopy revealed only collagen fibers but no epithelial
cells in DL-AM specimens, confirming successful removal of AM
epithelial cells (Fig. 2).
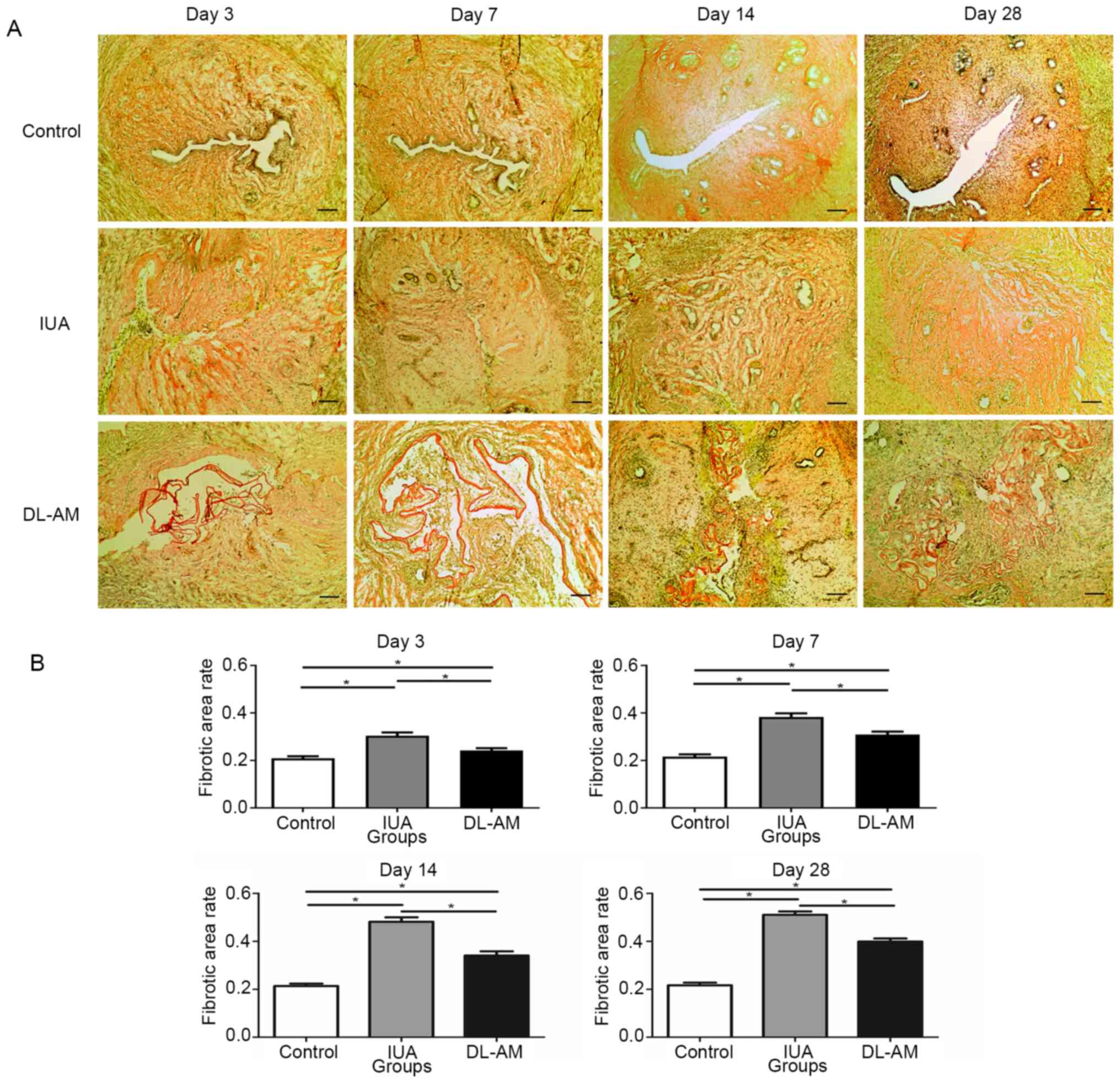
Degree of fibrosis
Van Gieson staining revealed that fibers were
stained red while non-fiber components stained yellow. Analysis of
the fibrotic area revealed higher rates in the IUA than in the
control group (P<0.05), indicating progression of IUA.
Furthermore, rats in the IUA + DL-AM group exhibited a
significantly lower fibrotic area percentage than those in the IUA
group at the same time-point (P<0.05), although this was still
higher than in the control group (P<0.05; Fig. 3).
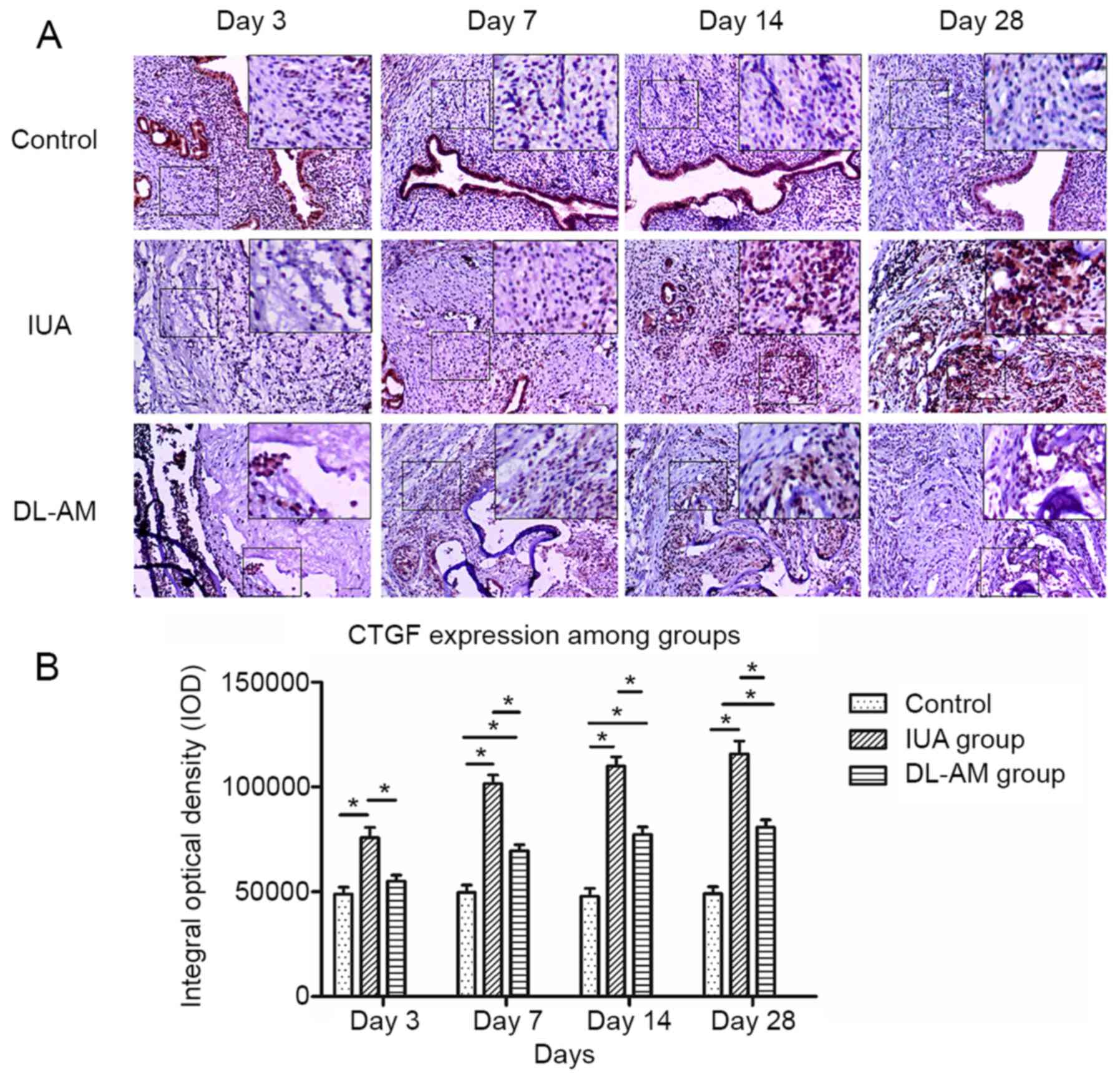
Expression of CTGF after DL-AM
transplantation
CTGF exhibited weak expression in the stromal and
epithelial layer of the control group, but this factor was
significantly upregulated after scraping (P<0.05), particularly
in the stromal layer. CTGF was significantly downregulated in the
uterus tissues at 3, 7, 14 and 28 days after DL-AM transplantation
compared with that in the IUA group at each time-point. However,
the expression was significantly higher relative to that in the
uteri of the control group (P<0.05; Fig. 4).
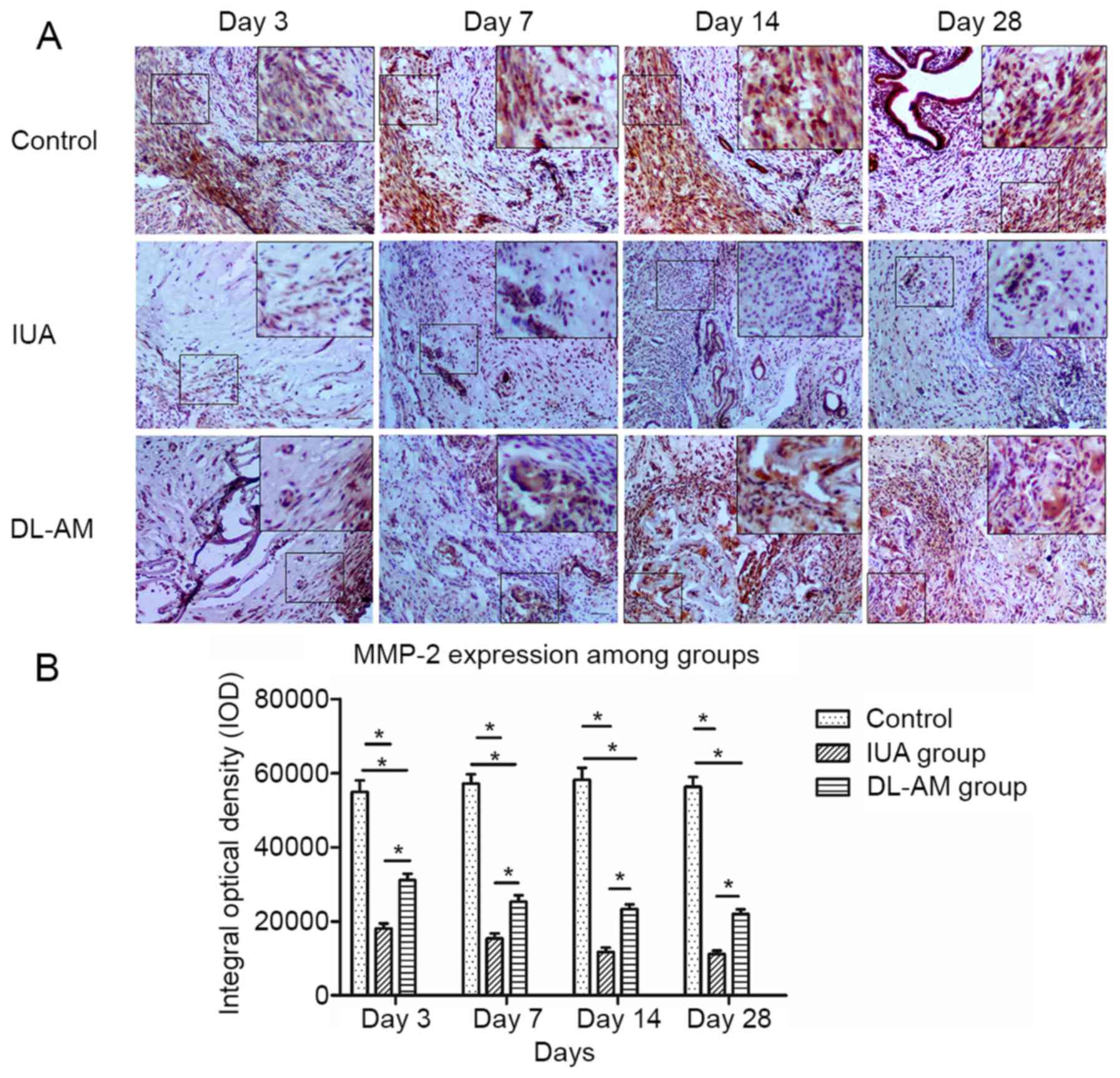
Expression of MMP-2 after DL-AM
transplantation
MMP-2 was highly expressed in control uteri,
including epithelial and stromal layers, but significantly
downregulated in scraped uteri (P<0.05). Transplantation of
DL-AM resulted in significant upregulation of MMP-2 compared with
the IUA group (P<0.05). However, the expression of MMP-2 in the
IUA + DL-AM was lower than that in the control group (P<0.05;
Fig. 5).
Discussion
The etiology of IUA has frequently been associated
with injury to the basal layer of the endometrium, particularly
during dilation and curettage. On the other hand, the incidence of
IUA has been linked to the rise in hysteroscopic surgeries and
artificial abortions (1-3).
In patients with moderate-to-severe IUA, injury to the endometrial
basal layer impairs regeneration and repair of the remaining
endometrium, thereby causing the formation of scars and adhesions
in the uterine cavity and resulting in clinical manifestations. It
is therefore imperative to elucidate the underlying mechanisms,
which may aid in the development of new strategies for treating
IUA.
In the present study, a rat model of IUA was
established via traditional endometrial scraping, to mimic the
pathogenesis and pathological changes of the disease. Successful
model establishment was confirmed after removal of endometrial
epithelial cells and disappearance of the uterine cavity.
Furthermore, the development of IUA was associated with an
increased area of fiber and a higher fibrotic area percentage,
confirming successful establishment of the IUA model.
AM is a traditional natural biomaterial that has
been applied in wound healing, particularly for burns (7) and ocular surface reconstruction
(19). To date, different types of
AM have been produced to circumvent difficulties regarding
infection and storage. For instance, de-epithelialization of AM has
been indicated to effectively eliminate immunogenicity of AM and
promote cell proliferation and differentiation relative to intact
AM (8,20), making it a suitable scaffold for
transplantation of other cells in tissue engineering. Although it
allows lower expression of various growth factors compared with
fresh AM (20),
de-epithelialization AM has been applied in tissue repairing,
including pericardium repairs (21)
and left ventricular remodeling (22). In the present study, DL-AM was
produced by lyophilization of de-epithelialized AM for room
temperature preservation, as well as sterilization and eliminate
potential infection. Although DL-AM has previously been reported to
treat post-laryngectomy pharyngocutaneous fistulas (10), its preventive efficacy, as well as
the underlying mechanisms of action on IUA, have remained elusive.
The present study sought to evaluate the preventative efficacy of
DL-AM on IUA by transplanting it into scraped uteri.
Previous studies have indicated that CTGF promotes
the proliferation of stromal cells and ECM accumulation in
connective tissues (23). In fact,
its high secretion across virtually all fibrotic conditions,
including the skin (24), kidney
(25) and liver (26), makes it a promising therapeutic
target for fibrosis. Pamrevlumab, a recombinant antibody that binds
to CTGF, has been applied in clinical trials (stage 3) for the
treatment of idiopathic pulmonary fibrosis (27) and was indicated to hold promise as
an alternative treatment for IUA. The results of the present study
revealed significant upregulation of CTGF in scraped uteri relative
to that in control uteri. In fact, CTGF expression appeared to
increase with the development of IUA, consistent with a previous
study (14). In the present study,
it was further observed that CTGF was downregulated after DL-AM
transplantation relative to the IUA group. However, this expression
was still significantly higher in the IUA + DL-AM than in the
control group, suggesting that the inhibitory effect of DL-AM on
CTGF expression was only partial.
ECM accumulation is a common phenomenon during the
development of IUA. MMPs have a pivotal role in ECM degradation
compared to CTGF. Although MMP-2 has been indicated to have a
crucial role in fibrogenesis, its pattern of expression in fibrotic
tissues remains controversial. Certain studies have reported that
MMP-2 is upregulated (28-30),
while others have demonstrated its downregulation in fibrosis
(31-33).
The results of the present study indicated that MMP-2 was
significantly downregulated in scraped relative to control uteri,
while DL-AM transplantation partially induced its upregulation,
thus facilitating ECM degradation and inhibiting the development of
fibrosis.
Overall, the present results are consistent with
those of previous studies and further affirm the preventive
efficacy of DL-AM on IUA. However, there were still significant
differences between scraped uteri with DL-AM and control uteri with
regards to the expression of CTGF and MMP-2. Furthermore, no
evidence of endometrial epithelium regeneration and restoration of
the uterine cavity was found, indicating that DL-AM only has
limited efficacy, which necessitates the development of alternative
methods to help regenerate endometrial epithelium.
In conclusion, a rat model of IUA was successfully
generated and used to reveal that CTGF and MMP-2 are upregulated
and downregulated, respectively, during IUA progression relative to
normal uteri. DL-AM transplantation resulted in downregulation of
CTGF, while the expression levels of MMP-2 were higher than those
in the IUA group. Taken together, these results indicated that
DL-AM is able to prevent endometrial fibrosis by suppressing CTGF
and upregulating MMP-2 expression.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Research Project of
Jiangsu Commission of Health (grant no. H201404).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC wrote the manuscript and performed the
experiments. YZ and YS acquired the data and assisted in analyzing
the data. TJ and HD designed the experiments, supervised the study
and were responsible for confirming the authenticity of the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Animal handling and experimental procedures were
performed in compliance with the guidelines approved by the
Institutional Animal Care and Use Committee at Nanjing Medical
University (approval no. IACUC-1912051) and the Animal Research:
Reporting of in vivo Experiments guidelines (16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu D, Wong YM, Cheong Y, Xia E and Li TC:
Asherman syndrome-one century later. Fertil Steril. 89:759–779.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Westendorp IC, Ankum WM, Mol BW and Vonk
J: Prevalence of Asherman's syndrome after secondary removal of
placental remnants or a repeat curettage for incomplete abortion.
Hum Reprod. 13:3347–3350. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vancaillie TG and Garad R: Asherman's
syndrome. Aust Nurs J. 20:34–36. 2013.PubMed/NCBI
|
|
4
|
Conforti A, Alviggi C, Mollo A, De Placido
G and Magos A: The management of Asherman syndrome: A review of
literature. Reprod Biol Endocrinol. 11(118)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fairbairn NG, Randolph MA and Redmond RW:
The clinical applications of human amnion in plastic surgery. J
Plast Reconstr Aesthet Surg. 67:662–675. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dua HS, Gomes JAP, King AJ and Maharajan
VS: The amniotic membrane in ophthalmology. Surv Ophthalmol.
49:51–77. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fraser JF, Cuttle L, Kempf M, Phillips GE,
Hayes MT and Kimble RM: A randomised controlled trial of amniotic
membrane in the treatment of a standardised burn injury in the
merino lamb. Burns. 35:998–1003. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gholipourmalekabadi M, Sameni M,
Radenkovic D, Mozafari M, Mossahebi-Mohammadi M and Seifalian A:
Decellularized human amniotic membrane: How viable is it as a
delivery system for human adipose tissue-derived stromal cells?
Cell Prolif. 49:115–121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wilshaw SP, Kearney JN, Fisher J and
Ingham E: Production of an acellular amniotic membrane matrix for
use in tissue engineering. Tissue Eng. 12:2117–2129.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kakabadze Z, Mardaleishvili K, Loladze G,
Javakhishvili I, Chakhunasvili K, Karalashvili L, Sukhitashvili N,
Chutkerashvili G, Kakabadze A and Chakhunasvili D: Clinical
application of decellularized and lyophilized human amnion/chorion
membrane grafts for closing post-laryngectomy pharyngocutaneous
fistulas. J Surg Oncol. 113:538–543. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen X, Sun J, Li X, Mao L, Cui L and Bai
W: Transplantation of oral mucosal epithelial cells seeded on
decellularized and lyophilized amniotic membrane for the
regeneration of injured endometrium. Stem Cell Res Ther.
10(107)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen X, Sun J, Li X, Mao L, Zhou Y, Cui L
and Bai W: Antifibrotic effects of decellularized and lyophilized
human amniotic membrane transplant on the formation of intrauterine
adhesion. Exp Clin Transplant. 17:236–242. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen X and Zhou YF: Preventive effects of
transplantation of oral mucosal epithelial cells seeded on a
decellularized amniotic membrane in a model of intrauterine
adhesion. Int J Clin Exp Pathol. 11:1510–1519. 2018.PubMed/NCBI
|
|
14
|
Xue X, Chen Q, Zhao G, Zhao JY, Duan Z and
Zheng PS: The overexpression of TGF-β and CCN2 in intrauterine
adhesions involves the NF-κB signaling pathway. PLoS One.
10(e0146159)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. J Physiol. 598:3793–3801.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fan B, Jin XH, Shi Y, Zhu H, Zhou W, Tu W
and Ding L: Expression and significance of TIMP-3, PACAP and VIP in
vaginal wall tissues of patients with stress urinary incontinence.
Exp Ther Med. 13:624–628. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu J, Luo Y, Yang XF, Yang MX, Yang J,
Yang XS, Zhou J, Gao F, He LT and Xu J: Effects of perinatal
exposure to nonylphenol on delivery outcomes of pregnant rats and
inflammatory hepatic injury in newborn rats. Braz J Med Biol Res.
49(e5647)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chávez-García C, Jiménez-Corona A,
Graue-Hernández EO, Zaga-Clavellina V, García-Mejía M,
Jiménez-Martínez MC and Garfias Y: Ophthalmic indications of
amniotic membrane transplantation in Mexico: An eight years
amniotic membrane bank experience. Cell Tissue Bank. 17:261–268.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koizumi N, Rigby H, Fullwood NJ, Kawasaki
S, Tanioka H, Koizumi K, Kociok N, Joussen AM and Kinoshita S:
Comparison of intact and denuded amniotic membrane as a substrate
for cell-suspension culture of human limbal epithelial cells.
Graefes Arch Clin Exp Ophthalmol. 245:123–134. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Francisco JC, Correa Cunha R, Cardoso MA,
Baggio Simeoni R, Mogharbel BF, Picharski GL, Silva Moreira
Dziedzic D, Guarita-Souza LC and Carvalho KA: Decellularized
amniotic membrane scaffold as a pericardial substitute: An in vivo
study. Transplant Proc. 48:2845–2849. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Roy R, Haase T, Ma N, Bader A, Becker M,
Seifert M, Choi YH, Falk V and Stamm C: Decellularized amniotic
membrane attenuates postinfarct left ventricular remodeling. J Surg
Res. 200:409–419. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lian N and Li T: Growth factor pathways in
hypertrophic scars: Molecular pathoenesis and therapeutic
implications. Biomed Pharmacother. 84:42–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
He T, Quan T, Shao Y, Voorhees JJ and
Fisher GJ: Oxidative exposure impairs TGF-β pathway via reduction
of type II receptor and SMAD3 in human skin fibroblasts. Age
(Dordr). 36(9623)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sharma A, Thakur R, Lingaraju MC and Kumar
D, Mathesh K, Telang AG, Singh TU and Kumar D: Betulinic acid
attenuates renal fibrosis in rat chronic kidney disease model.
Biomed Pharmacother. 89:796–804. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Paradis V, Dargere D, Vidaud M, De
Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R,
Ratziu V and Bedossa P: Expression of connective tissue growth
factor in experimental rat and human liver fibrosis. Hepatology.
30:968–976. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sgalla G, Franciosa C, Simonetti J and
Richeldi L: Pamrevlumab for the treatment of idiopathic pulmonary
fibrosis. Expert Opin Investig Drugs. 29:771–777. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hafez MM, Hamed SS, El-Khadragy MF, Hassan
ZK, Al Rejaie SS, Sayed-Ahmed MM, Al-Harbi NO, Al-Hosaini KA,
Al-Harbi MM, Alhoshani AR, et al: Effect of ginseng extract on the
TGF-β1 signaling pathway in CCl4-induced liver fibrosis
in rats. BMC Complement Altern Med. 17(45)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Knittel T, Mehde M, Grundmann A, Saile B,
Scharf JG and Ramadori G: Expression of matrix metalloproteinases
and their inhibitors during hepatic tissue repair in the rat.
Histochem Cell Biol. 113:443–453. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guan S, Liu Q, Han F, Gu W, Song L, Zhang
Y, Guo X and Xu W: Ginsenoside Rg1 ameliorates cigarette
smoke-induced airway fibrosis by suppressing the TGF-β1/Smad
pathway in vivo and in vitro. Biomed Res Int.
2017(6510198)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang M, Hu X, Li S, Lu C, Li J, Zong Y,
Qi W and Yang H: Hepatoprotective effects of ethyl pyruvate against
CCl4-induced hepatic fibrosis via inhibition of TLR4/NF-κB
signaling and up-regulation of MMPs/TIMPs ratio. Clin Res Hepatol
Gastroenterol. 42:72–81. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zuo WL, Zhao JM, Huang JX, Zhou W, Lei ZH,
Huang YM, Huang YF and Li HG: Effect of bosentan is correlated with
MMP-9/TIMP-1 ratio in bleomycin-induced pulmonary fibrosis. Biomed
Rep. 6:201–205. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhao D, Wang Y, Du C, Shan S, Zhang Y, Du
Z and Han D: Honokiol alleviates hypertrophic scar by targeting
transforming growth factor-β/Smad2/3 signaling pathway. Front
Pharmacol. 8(206)2017.PubMed/NCBI View Article : Google Scholar
|