Introduction
Stroke, also known as cerebral vascular accident, is
characterized by sudden onset and the rapid occurrence of localized
or diffuse brain dysfunction (1).
Stroke includes ischemic stroke and hemorrhagic stroke, of which
ischemic stroke accounts for 67.3-80.5% of cases, which seriously
threatens human health and creates a large social burden (1). The main treatments for ischemic stroke
are intravascular thrombolysis and mechanical thrombectomy, aiming
to restore the blood supply of ischemic brain tissue through
reperfusion (2). However, there is
often an aggravation of tissue damage called cerebral
ischemia-reperfusion injury (CIRI) when the blood flow is restored
(3,4). The mechanisms of CIRI include abnormal
signal transduction, mitochondrial damage, oxidative stress,
autophagy, inflammation and apoptosis (5,6). Thus,
avoiding the injury of CIRI is required in the early treatment of
ischemic stroke.
Tissue inhibitor of metalloproteinases (TIMP)-3 is a
member of the tissue inhibitors of metalloproteinases family, which
prevent extracellular matrix degradation by inhibiting the activity
of matrix metalloproteinases (MMPs) (7). Moreover, TIMP3 has been demonstrated
to exert a role in regulating apoptosis in tumor cell lines and
myocardial infarction, and also in inhibiting angiogenesis in
addition to targeting MMPs (8-11).
The overexpression of TIMP3 could attenuate oxidative stress in
metabolic disorders (12).
Similarly, TIMP3 also serves a notable role in ischemia-reperfusion
(I/R) injury. A previous study has demonstrated that knockout of
TIMP3 can aggravate the I/R injury of hepatocytes (13). By contrast, TIMP3-overexpression
protects myocardial I/R injury by inhibiting cardiomyocyte
apoptosis (11). However, the role
of TIMP3 in CIRI remains unknown.
Due to the nerve growth factor (NGF) receptor on the
cell membrane, PC12 cells can grow neurites and differentiate into
neurons after being induced by NGF at the physiological level.
Therefore, PC12 cells are widely used as in vitro model of
cerebral ischemia injury (14,15).
In the current study, PC12 cells were used as a cell model of
cerebral ischemia to study the effect of TIMP3 on oxygen glucose
deprivation/reoxygenation (OGD/R)-induced PC12 cell injury.
Additionally, numerous studies have indicated that the Akt pathway
mediates the occurrence and development of cerebral ischemia
injury, and AKT activity is significantly inhibited after cerebral
ischemia damage (16,17). Meanwhile, TIMP3-mediated
neuroprotection mainly depends on the activation of AKT signaling
pathway (18). The present study
aimed to investigate whether TIMP3 prevented cerebral
ischemia/reperfusion and to explore the role of TIMP3.
Materials and methods
Animals
A total of 16 male C57BL/6 mice (8-10-weeks-old;
weight, 20±2 g) were purchased from the Model Animal Research
Center of Nanjing University. The Ethics Committee of Xingtai
People's Hospital (Xingtai, China) approved all animal experiments.
All mice were kept in a climate-controlled room (temperature,
25±1˚C; relative humidity, 50-60%; free access to food and water;
12 h light/dark cycle). Mice were randomly allocated to the sham
group (n=6) or the I/R group (n=10), including two mice that died
after the transient middle cerebral artery occlusion (MCAO) with
unknown exact cause. Animal health and behavior were monitored once
a day before model construction, and once every 30 min after MCAO.
Proper anesthetic procedures were used to ensure that the mice did
not suffer unnecessarily during or after the experimental
procedure.
Models
MCAO was conducted according to a previous study
(19). In brief, the mice were
anesthetized with an intraperitoneal injection of pentobarbital
sodium (60 mg/kg) until loss of limb reflexes. Subsequently,
unilateral MCAO was carried out by introducing a 4-0 nylon thread
into the internal carotid artery after cutting the common carotid
artery. The origin of the middle cerebral artery was occluded when
a mild resistance was felt for 2 h followed by reperfusion. The
mice in the sham group underwent the same surgery without
occlusion. At 24 h after reperfusion, following sacrifice by
anesthetic overload with intraperitoneal 500 mg/kg ketamine and 50
mg/kg xylazine (no breathing and heartbeat were used to verify
mortality), the brain tissues were obtained for assays. The methods
of euthanasia conformed to the American Veterinary Medical
Association Guidelines for the Euthanasia of Animals, 2020 Edition
(20).
TTC staining
The brain tissues were kept overnight at -80˚C and
sliced into 2-mm thick sections, which were cultured with 1% TTC
for 15 min at 37˚C to visualize the infarct area. The white area
manifested infarcted brain tissues. Infarct area was measured with
computer-assisted planimetry (ImageJ version 1.57; National
Institutes of Health).
Immunohistochemistry
The brain tissues were fixed with 10% formalin at
4˚C overnight and embedded in paraffin before being cut into 5-µm
sections. After that, the sections were deparaffinized with xylene,
rehydrated in a graded alcohol series and heated until boiling in a
microwave oven at 1200w for 5 min for antigen retrieval. Endogenous
peroxidase activity was blocked with 3% H2O2
at 37˚C for 20 min. Subsequently, these sections were treated with
TIMP3 primary antibody (1:300; cat. no. 5673S; Cell Signaling
Technology, Inc.). After incubation at 4˚C for one night and
washing with PBS, the sections were cultured with a HRP
polymer-bound goat anti-rabbit secondary antibody (1:5,000; cat.
no. ab7090; Abcam) at room temperature for 1 h. These samples were
then stained with 3, 3-diaminobenzidine solution at room
temperature for 10 min and hematoxylin at room temperature for 5
min. Finally, the slides were observed using a light microscope and
the positively stained cells were counted using GraphPad Prism 6
software (GraphPad Software, Inc.).
Induction of OGD/R model
NGF-induced PC12 cells were obtained from the
American Type Culture Collection and cultured in Dulbecco's
Modified Eagle Medium (DMEM) with 10% fetal bovine serum (both from
Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2 for 24 h. PC12 cells were then washed with
glucose-free-Hanks' balanced salt solution (Gibco; Thermo Fisher
Scientific, Inc.) twice and placed in glucose-free DMEM (Gibco;
Thermo Fisher Scientific, Inc.) in a hypoxic incubator chamber (1%
O2; 5% CO2; 94% N2) at 37˚C for 2,
4 and 8 h. The cells were transferred to normal growth conditions
(DMEM with 10% fetal bovine serum at 37˚C with 5% CO2)
for 24 h for oxygen-glucose deprivation and reoxygenation (OGD/R)
(21). PC12 cells without OGD/R
treatment were used as the control.
Cell transfection
Recombinant adenovirus [human adenovirus (Ad) 5] of
TIMP3 (Ad: TIMP3) and empty adenovirus (human adenovirus 5, Ad5)
vector as control (Ad: Con) were purchased commercially from Hanbio
Biotechnology Co., Ltd. PC12 cells were maintained at 37˚C in a
humidified atmosphere with 5% CO2. The cells were
transfected with Ad: TIMP3 or Ad: Con (100x108 PFU/ml)
using polybrene (Sigma-Aldrich; Merck KGaA) for 48 h and treated
with or without AKT inhibitor AZD5363 (Selleck Chemicals) for
another 48 h. The cells were then subjected to the associated
experiments.
Semi-quantitative PCR and reverse
transcription-quantitative (RT-q)PCR analysis
Total RNA from brain tissues and PC12 cells was
extracted using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). The purity of RNA was used to detect the
absorbance ratio of 260/280 nm by a NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc.). Reverse
transcription of RNA was performed using a PrimeScript RT kit
(Takara Bio, Inc.). The temperature protocol used for RT was 37˚C
for 15 min and 85˚C for 15 sec, then the sample was kept at 4˚C for
immediate use or -20˚C for long-term storage. RT-PCR was used to
measure the expression of TIMP3 mRNA. The primers used in the study
were as follows: TIMP3, forward 5'-GGCAACTGTGCTGAACAGGAT-3', and
reverse, 5'-GATGGCCAGCGTGACACTT-3'; 18s ribosomal RNA forward
5'-GTTGTGCTGACGGCGCA-3' and reverse, 5'-CCGGCTTCGTGGCAGCA-3';
β-actin, forward 5'-GTAAAGACCTCTATGCCAACA-3', and reverse,
5'-GGACTCATCGTACTCCTGCT-3'.
For semi-quantitative PCR, reactions used a PCR
Master mix (cat. no. K0171; Thermo Fisher Scientific, Inc.) and
were performed on a StepOnePlus system (Applied Biosystems; Thermo
Fisher Scientific, Inc.), starting with denaturation at 94˚C for 3
min, followed by 35 cycles of 94˚C for 30 sec, 55˚C for 30 sec and
72˚C for 30 sec, with a final extension at 72˚C for 7 min.
Amplified DNA fragments were analyzed by electrophoresis on 1%
agarose gels with 5% ethidium bromide at 100 V for 30 min at room
temperature, and these ethidium bromide stained DNA fragments were
observed under UV illumination.
For RT-qPCR, the experiment was carried out using
the Power SYBR® Green PCR Master mix (cat. no. 4309155;
Thermo Fisher Scientific, Inc.) with ABI7300 detector (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction
parameters were as follows: 95˚C For 10 min, and 40 cycles at 95˚C
for 15 sec and 60˚C for 30 sec. The mRNA expression levels were
quantified using the 2-ΔΔCq method (22) and normalized to the internal
reference genes β-actin.
Western blotting
The brain tissues and PC12 cells were lysed with
lysis buffer (Cell Signaling Technology, Inc.) at 4˚C for 30 min
before centrifuging (4˚C; 14462 x g; 5 min). The total proteins
were in the supernatant. Concentrations of proteins extracted from
cerebral tissues or PC12 cells were then determined using the BCA
protein assay kit (Bio-Rad Laboratories, Inc.). After that proteins
(10 µg per lane) were separated using a 10% SDS-PAGE gel, then they
were transferred to PVDF membranes (MilliporeSigma) followed by
blocking with 5% non-fat milk (Bio-Rad Laboratories, Inc.) at room
temperature for 1 h. Next, the membrane was probed with the
following primary antibodies: TIMP3 (1:1,000; cat. no. 5673S), Bax
(1:1,000; cat. no. 5023S), Bcl-2 (1:1,000; cat. no. 15071S),
caspase-3 (1:1,000; cat. no. 9662S), AKT Thr308 (1:500; cat. no.
13038S), AKT Ser473 (1:500; cat. no. 4060S), AKT (1:1,000; cat. no.
2920S), GAPDH (1:1,000; cat. no. 5174S) and β-actin (1:2,000; cat.
no. 3700T) (all Cell Signaling Technology, Inc.). Afterwards, the
membrane was cultured with HRP-conjugated IgG anti-mouse (1:2,000;
cat. no. 7076) or anti-rabbit secondary antibodies (1:2,000; cat.
no. 7074) (both Cell Signaling Technology, Inc.) at room
temperature for 2 h and washed with TBST (0.05% Tween). Finally,
the blots were analyzed using SuperSignal West Pico PLUS
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.) and
visualized via a Chemiluminescence Imaging system (ChemiScope 3600
Mini; Clinx Science Instruments Co., Ltd). The protein bands were
quantified using GraphPad Prism 6 software (GraphPad Software,
Inc.).
Detection of reactive oxygen species
(ROS), superoxide dismutase (SOD) and malondialdehyde (MDA)
The levels of ROS were detected using a ROS Assay
kit (cat. no. S0033S; Beyotime Institute of Biotechnology), the
level of total SOD was evaluated by Superoxide Dismutase (SOD)
assay kit (cat. no. A001-3-2; Nanjing Jiancheng Bioengineering
Institute), and the level of MDA was measured by Malondialdehyde
(MDA) assay kit (cat. no. A003-1-2; Nanjing Jiancheng
Bioengineering Institute). These kits were all used according to
the manufacturers' instructions.
TUNEL assay
PC12 cells were fixed in PBS with 4%
polyformaldehyde at room temperature for 15 min. The cells were
washed once with PBS and then, apoptosis of PC12 cells in the
different groups was detected by TUNEL via a in situ Cell
Death Detection kit (Roche Applied Science). The kit was used in
accordance with the manufacturer's protocol, and the ratio of
apoptotic to total cells was calculated under fluorescence
microscopy by observing three fields of view (magnification, x400).
After that, ImageJ software (version 1.53, National Institutes of
Health) was used to quantify the apoptosis rate.
Statistical analysis
SPSS version 20.0 statistic software (IBM Corp.) was
used to analyze the data, which was expressed as mean ± SD (unless
otherwise presented). Statistical analysis was carried out using
unpaired Student's t-test between two groups. Comparisons among two
or more groups were analyzed using one-way ANOVA followed by
Turkey's multiple comparisons post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
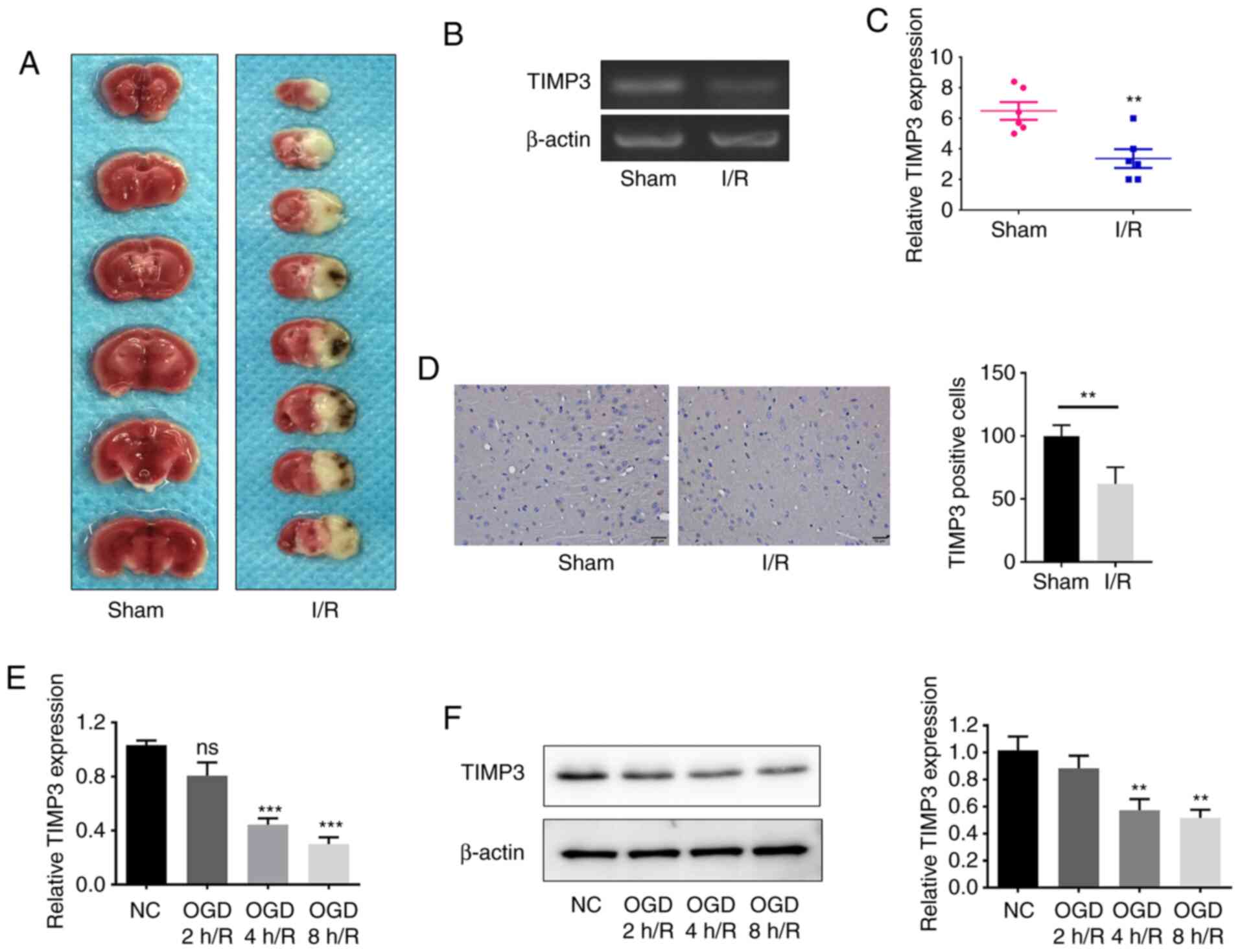
TIMP3 expression is downregulated in
OGD/R-induced PC12 cells and cerebral tissues after I/R injury
To investigate the expression difference of TIMP3
after cerebral I/R injury in vivo, a model of cerebral I/R
injury was constructed in mice. The morphology of the cerebral I/R
injury model is presented in Fig.
1A. As demonstrated in Fig. 1B
and C, the expression levels of
TIMP3 in I/R-induced injury in mice cerebral tissues were
significantly downregulated compared with the sham group. Moreover,
immunohistochemical results indicated that the expression of
TIMP3-positive cells decreased significantly in the I/R group
(Fig. 1D). RT-qPCR and western
blotting results indicated that the TIMP3 expression in
OGD/R-induced PC12 cells was significantly downregulated in a
time-dependent manner (Fig. 1E and
F). These results demonstrated that
TIMP3 expression was downregulated in I/R injury in vivo and
in vitro.
TIMP3 inhibits OGD/R-induced PC12
apoptosis and oxidative stress
PC12 cells were transfected with Ad-TIMP3 before OGD
8h/R treatment to confirm whether overexpression of TIMP3 could
protect neurocytes from OGD/R-induced apoptosis and oxidative
stress. The expression of TIMP3 was markedly overexpressed by
Ad-TIMP3 therapy for 48 h (Fig.
2A). The number of TUNEL-positive cells in OGD/R-induced cells
significantly increased after OGD/R, but was significantly reduced
by TIMP3-overexpression (Fig. 2B).
Western blotting indicated that OGD/R increased the Bax/Bcl-2
ratio, and caspase-3 expression compared with the NC; by contrast,
this was significantly attenuated by TIMP3-overexpression (Fig. 2C). In addition, the levels of ROS
and MDA were significantly elevated in OGD/R-induced PC12 cells
compared with control cells, while this was significantly
suppressed by TIMP3-overexpression. Conversely, SOD activity
decreased significantly in PC12 cells after OGD/R treatment, and
overexpression of TIMP3 significantly elevated SOD activity in the
OGD/R-induced neurocytes (Fig. 2D).
The data revealed that TIMP3 inhibited apoptosis and oxidative
stress of OGD/R-induced PC12.
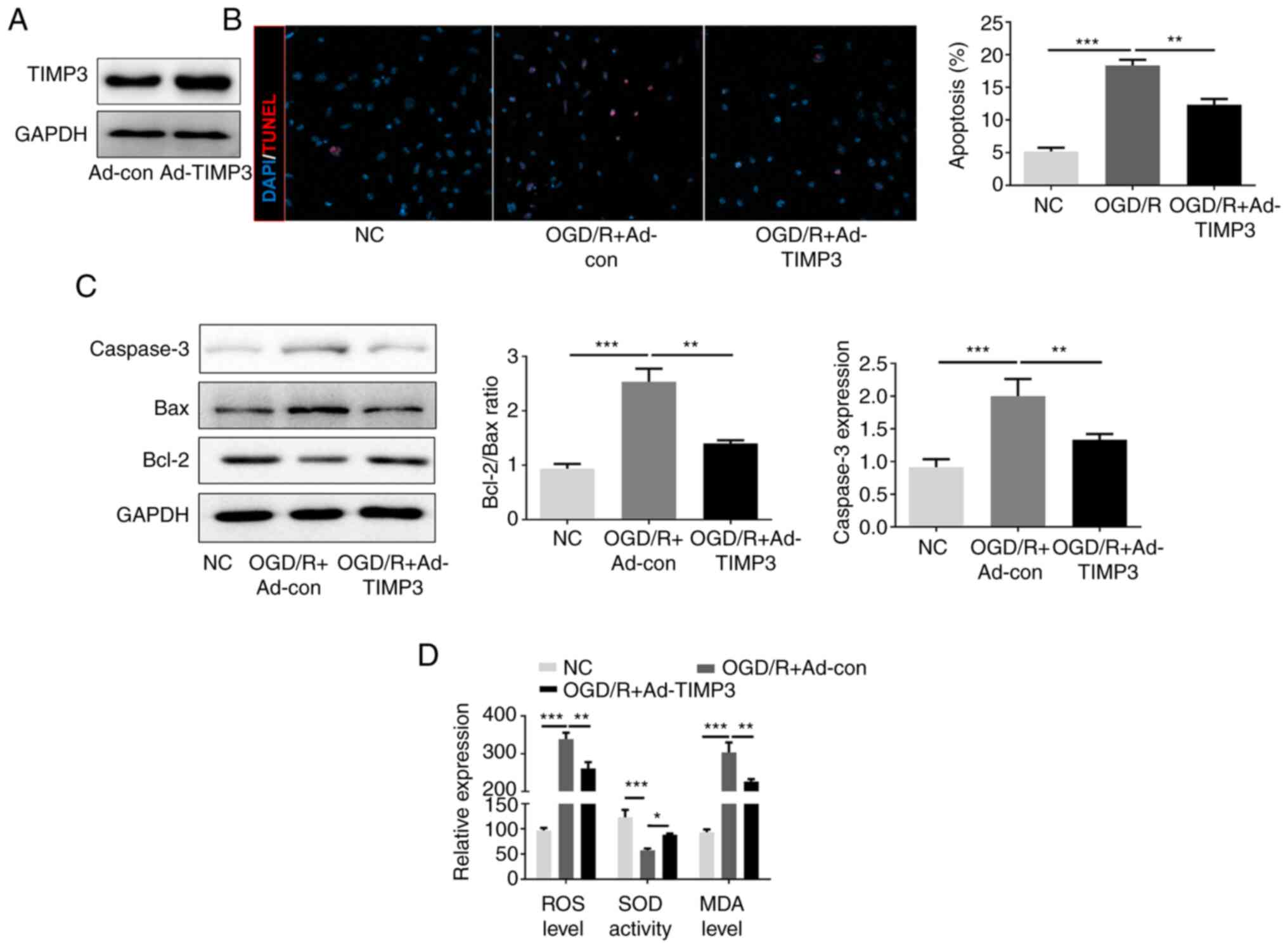 | Figure 2TIMP3 inhibits OGD/R-induced PC12
apoptosis and oxidative stress. (A) Western blotting was used to
confirm the expression of TIMP3 by Ad-TIMP3. (B) TUNEL
immunofluorescence staining was used to examine apoptosis-positive
cells in different groups (magnification, x400). (C) Western
blotting was performed to detect the expression of apoptosis
proteins, Bax and Bcl-2. (D) ROS generation, MDA level and SOD
activity were detected after different treatments using
corresponding commercial kits. *P<0.05,
**P<0.01 and ***P<0.001 as indicated.
TIMP3, tissue inhibitor of metalloproteinases-3; OGD/R, oxygen
glucose deprivation and reoxygenation; Ad, adenovirus; ROS,
reactive oxygen species; MDA, malondialdehyde; SOD, superoxide
dismutase; NC, negative control; con, control. |
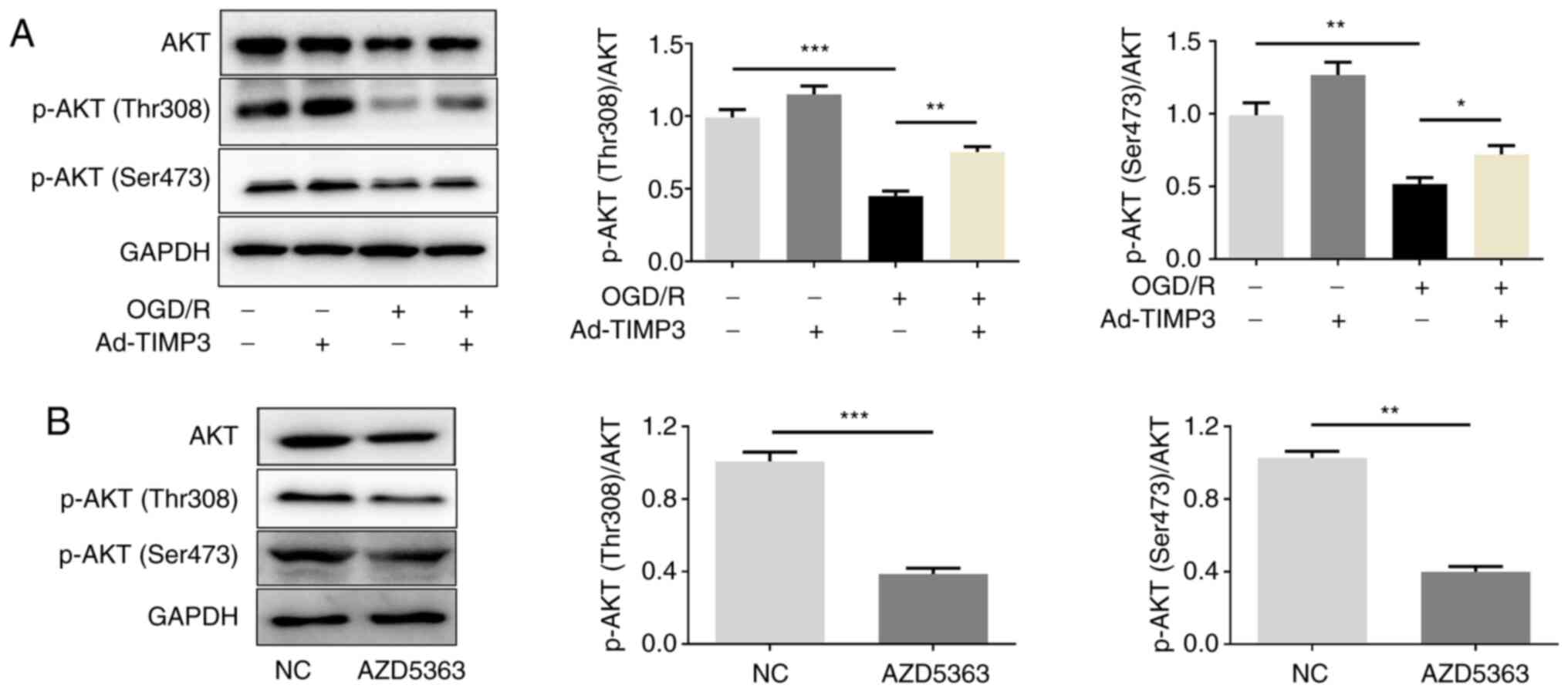
TIMP3 regulates the expression of
phosphorylated (p)-AKT
As TIMP3 was demonstrated to exert its function by
regulating the AKT pathway, the phosphorylation levels of AKT
(Thr308 and Ser473) were detected. Western blotting demonstrated
that p-AKT (Thr308 and Ser473) was significantly downregulated in
OGD/R-treated PC12 cells, which could be significantly reversed by
TIMP3-overexpression. However, TIMP3-overexpression did not
significantly affect p-AKT expression in cells without OGD/R
treatment (Fig. 3A). In addition,
in order to further conduct the rescue experiment in the following
mechanism study, the p-AKT inhibitor (AZD5363) was used to inhibit
p-AKT (Thr308 and Ser473) activity (Fig. 3B). The experiments demonstrated that
the expression of p-AKT (Thr308 and Ser473) was regulated by
TIMP3.
Overexpression of TIMP3 inhibits
OGD/R-induced PC12 apoptosis and oxidative stress via the AKT
pathway
After confirming that TIMP3 could elevate the
expression levels of p-AKT (Thr308 and Ser473) that had been
decreased by OGD/R treatment, whether the function of TIMP3 could
be mediated via p-AKT overexpression was investigated.
TIMP3-overexpression significantly reduced the number of
TUNEL-positive cells that were elevated in OGD/R-induced neurocytes
compared with the OGD/R + Ad-control group. This effect of TIMP3 in
OGD/R-induced PC12 cells was partially but significantly reversed
after AZD5363 treatment (Fig. 4A).
Western blotting indicated that TIMP3 downregulated the expression
of Bax and upregulated the expression of Bcl-2 in OGD/R, which were
partially reversed after AZD5363 treatment (Fig. 4B). Consistent with the
aforementioned results, the anti-oxidative stress role of TIMP3 in
OGD/R-induced PC12 cells was also partially blocked by AZD5363
treatment (Fig. 4C). Thus, it is
confirmed that TIMP3 inhibits apoptosis and oxidative stress via
AKT pathway in vitro.
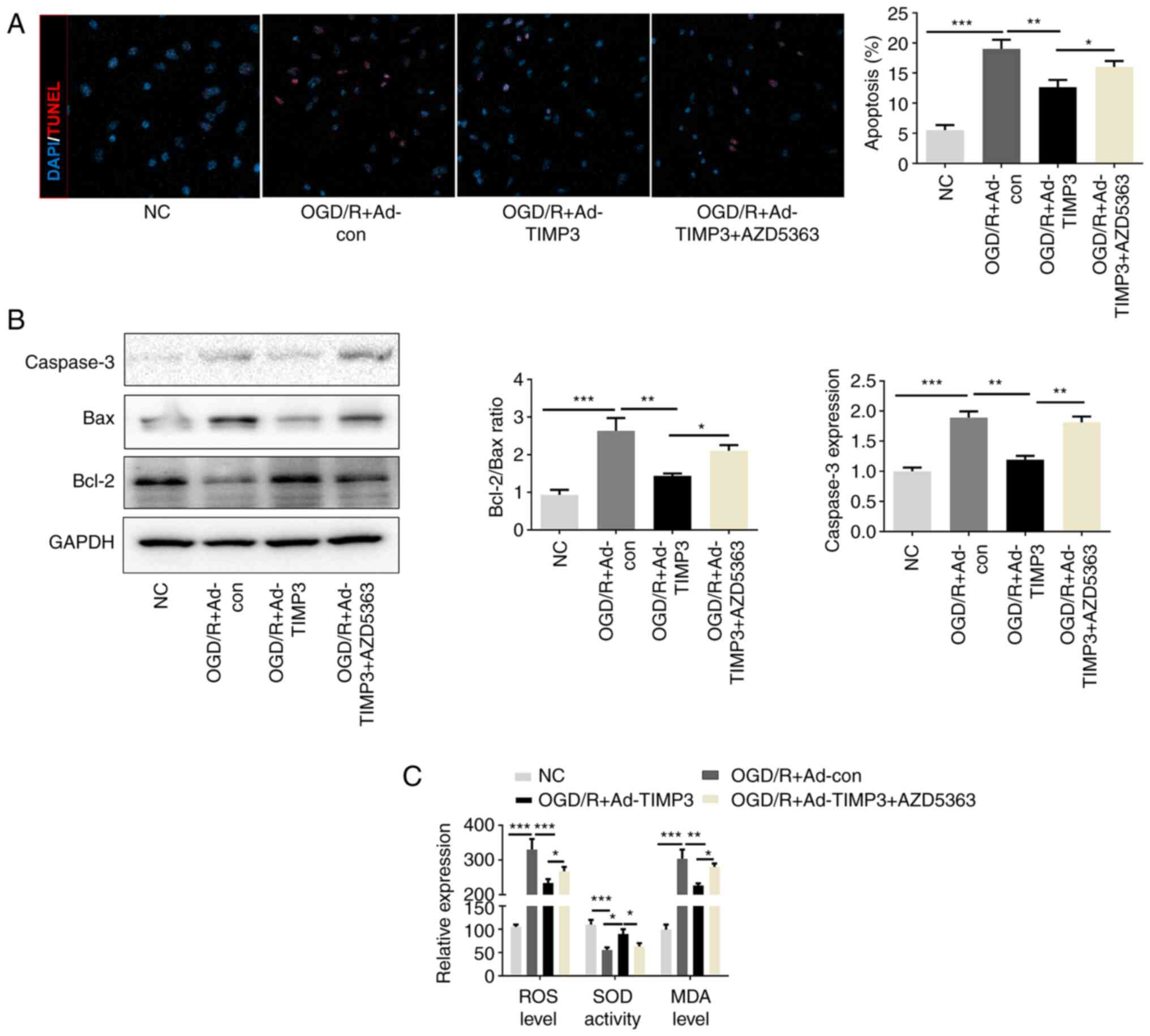 | Figure 4TIMP3 inhibits OGD/R-induced PC12
apoptosis and oxidative stress via the AKT pathway. (A) TUNEL
immunofluorescence staining was used and quantified to detect the
apoptosis-positive cells in different groups (magnification, x400).
(B) Western blotting was conducted to determine the expression
levels of apoptosis proteins Bax and Bcl-2. (C) ROS generation, MDA
level and SOD activity after different treatments were detected by
corresponding commercial kits. *P<0.05,
**P<0.01 and ***P<0.001 as indicated.
TIMP3, tissue inhibitor of metalloproteinases-3; OGD/R, oxygen
glucose deprivation and reoxygenation; ROS, reactive oxygen
species; MDA, malondialdehyde; SOD, superoxide dismutase; con,
control; Ad, adenovirus; NC, negative control. |
Discussion
CIRI is one of the important factors that aggravates
brain injury after cerebral ischemia. Reducing reperfusion injury
helps protect neurons in the ischemic area and reduces neuron
necrosis and apoptosis (2). Various
mechanisms have been demonstrated to be involved in the development
of CIRI, including apoptosis and oxidative stress (3). Thus, treatments that improve apoptosis
and oxidative stress may have potential therapeutic effects for
CIRI.
TIMP3 is a member of the TIMP family of proteins,
which are characterized as inhibitors of MMPs (7). However, with increasing research, the
role of TIMP3 in regulating apoptosis and alleviating oxidative
stress has attracted much attention (11,12).
For example, Liu et al (11)
revealed that TIMP3 upregulation could protect against cardiac I/R
injury by inhibiting myocardial apoptosis. In the present study,
the expression of TIMP3 was significantly decreased in the cerebral
tissues after I/R injury and in OGD/R-induced PC12 cells. The
results indicated that TIMP3 may have a pathological role in
CIRI.
Mitochondrial dysfunction and oxidative stress are
responsible for neuronal damage, which eventually leads to a
cascade of apoptosis (23). Brain
injury is associated with an increase of apoptosis, which is
characterized by the increased expression of Bax and the decreased
expression of Bcl-2; meanwhile, inhibition of apoptosis has been
demonstrated to attenuate neuronal damage in CIRI (24). The overproduction of ROS and the
weakening of the antioxidant mechanism are notable pathological
events in the process of CIRI (25). The increase of ROS leads to the
oxidation of lipids, proteins and nucleic acids, which changes the
function of cells (26). MDA is one
of the essential products of membrane lipid peroxidation, thus, its
expression reflects the degree of oxidative stress (26). Therefore, the clearance of ROS is
particularly important in CIRI. SOD has a significant scavenging
effect on ROS produced in the process of oxidation (26). In the present study, overexpression
of TIMP3 reduced the number of TUNEL-positive cells and attenuated
the Bax/Bcl-2 ratio in OGD/R-induced PC12 cells. In addition,
overexpression of TIMP3 could suppress the levels of ROS and MDA,
and also elevate SOD activity in the OGD/R-induced neurocytes.
These data indicated that overexpression of TIMP3 could protect
neurocytes from OGD/R-induced apoptosis and oxidative stress in
vitro. The effect of TIMP3 in cerebral tissues after I/R injury
in vivo would need to be further investigated to confirm
these results.
The AKT pathway serves a notable role in regulating
CIRI (27); TIMP3 exerts its
function by regulating the AKT pathway (18). The results of the current study
indicated that TIMP3-overexpression could reverse the
downregulation of p-AKT (Thr308 and Ser473) in OGD/R-treated PC12
cells. Further rescue studies indicated that the anti-apoptosis and
anti-oxidative stress role of TIMP3 in OGD/R-induced PC12 cells
could be partially abolished after AZD5363 treatment. This
demonstrated that TIMP3 attenuated CIRI apoptosis and oxidative
stress in neurocytes by regulating the AKT pathway. However, the
specific binding molecules and their sites of binding to TIMP3 to
modify the phosphorylation of AKT in I/R injury were unknown, which
will be investigated further in the future. It has been previously
demonstrated that the AKT pathway is activated in hypoxia-induced
neuronal injury, which is contrary to the findings of the present
study (28). These inconsistent
results may be caused by the different models applied. Continuous
lack of glucose oxygen was possibly a factor in AKT
phosphorylation. Thus, the opposite effect on AKT activity would be
observed under glucose and oxygen-glucose deprivation
condition.
In conclusion, the present study demonstrated that
TIMP3 exerted anti-apoptotic and anti-oxidative stress roles in
cerebral I/R injury and provided a potential novel therapeutic
target for the treatment of CIRI.
Acknowledgements
Not applicable.
Funding
Funding: The work was supported by The Xingtai Science and
Technology Support Plan Project (grant no. 2018ZC191).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM, YZ and FG proposed the project, aims and
objectives. LM, YZ, DL and XS conducted the experiments and
collected the data. LM, YZ, XH, XC and FG analyzed the results and
wrote the manuscript. FG submitted the manuscript. LM and FG
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted according to
the ethical guidelines of The Xingtai People's Hospital and the
‘3R’ principle (Replacement, Reduction, and Refinement). All
efforts were made to minimize animal suffering. The present study
was approved by The Ethics Committee of Xingtai People's Hospital
(Xingtai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bosetti F, Koenig JI, Ayata C, Back SA,
Becker K, Broderick JP, Carmichael ST, Cho S, Cipolla MJ, Corbett
D, et al: Translational stroke research: Vision and opportunities.
Stroke. 48:2632–2637. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dorado L, Millan M and Davalos A:
Reperfusion therapies for acute ischemic stroke: An update. Curr
Cardiol Rev. 10:327–335. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nishijima Y, Akamatsu Y, Weinstein PR and
Liu J: Collaterals: Implications in cerebral ischemic diseases and
therapeutic interventions. Brain Res. 1623:18–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang J, Fang X, Zhou Y, Deng X, Lu Y, Li
J, Li S, Wang B and Xu R: The possible damaged mechanism and the
preventive effect of monosialotetrahexosylganglioside in a rat
model of cerebral ischemia-reperfusion injury. J Stroke Cerebrovasc
Dis. 24:1471–1478. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liang G, Shi B, Luo W and Yang J: The
protective effect of caffeic acid on global cerebral
ischemia-reperfusion injury in rats. Behav Brain Funct.
11(18)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Siroli L, Braschi G, de Jong A, Kok J,
Patrignani F and Lanciotti R: Transcriptomic approach and membrane
fatty acid analysis to study the response mechanisms of Escherichia
coli to thyme essential oil, carvacrol, 2-(E)-hexanal and citral
exposure. J Appl Microbiol. 125:1308–1320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Di Gregoli K, Mohamad Anuar NN, Bianco R,
White SJ, Newby AC, George SJ and Johnson JL: MicroRNA-181b
controls atherosclerosis and aneurysms through regulation of TIMP-3
and elastin. Circ Res. 120:49–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): An ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shen B, Jiang Y, Chen YR, Zheng HC, Zeng
W, Li YY, Yin A and Nie Y: Expression and inhibitory role of TIMP-3
in hepatocellular carcinoma. Oncol Rep. 36:494–502. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kubatka P, Uramova S, Kello M, Kajo K,
Kruzliak P, Mojzis J, Vybohova D, Adamkov M, Jasek K, Lasabova Z,
et al: Antineoplastic effects of clove buds (Syzygium
aromaticum L.) in the model of breast carcinoma. J Cell Mol
Med. 21:2837–2851. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu H, Jing X, Dong A, Bai B and Wang H:
Overexpression of TIMP3 protects against cardiac
ischemia/reperfusion injury by inhibiting myocardial apoptosis
through ROS/Mapks pathway. Cell Physiol Biochem. 44:1011–1023.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Menghini R, Casagrande V, Menini S, Marino
A, Marzano V, Hribal ML, Gentileschi P, Lauro D, Schillaci O,
Pugliese G, et al: TIMP3 overexpression in macrophages protects
from insulin resistance, adipose inflammation, and nonalcoholic
fatty liver disease in mice. Diabetes. 61:454–462. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fujii T, Duarte S, Lee E, Ke B, Busuttil
RW and Coito AJ: Tissue inhibitor of metalloproteinase 3 deficiency
disrupts the hepatocyte E-cadherin/β-catenin complex and induces
cell death in liver ischemia/reperfusion injury. Liver Transpl.
26:113–126. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Wang G, Wang T, Hu Y, Wang J, Wang Y,
Zhang Y, Li F, Liu W, Sun Y, Yu B and Kou J: NMMHC IIA triggers
neuronal autophagic cell death by promoting F-actin-dependent ATG9A
trafficking in cerebral ischemia/reperfusion. Cell Death Dis.
11(428)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou Z, Xu N, Matei N, McBride DW, Ding Y,
Liang H, Tang J and Zhang JH: Sodium butyrate attenuated neuronal
apoptosis via GPR41/Gβγ/PI3K/Akt pathway after MCAO in rats. J
Cereb Blood Flow Metab. 41:267–281. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Y, Guo S, Liu W, Jin T, Li X, He X,
Zhang X, Su H, Zhang N and Duan C: Silencing of SNHG12 enhanced the
effectiveness of MSCs in alleviating ischemia/reperfusion injuries
via the PI3K/AKT/mTOR signaling pathway. Front Neurosci.
13(645)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Feng H, Hu L, Zhu H, Tao L, Wu L, Zhao Q,
Gao Y, Gong Q, Mao F, Li X, et al: Repurposing antimycotic
ciclopirox olamine as a promising anti-ischemic stroke agent. Acta
Pharm Sin B. 10:434–446. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gibb SL, Zhao Y, Potter D, Hylin MJ, Bruhn
R, Baimukanova G, Zhao J, Xue H, Abdel-Mohsen M, Pillai SK, et al:
TIMP3 attenuates the loss of neural stem cells, mature neurons and
neurocognitive dysfunction in traumatic brain injury. Stem Cells.
33:3530–3544. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bu X, Zhang N, Yang X, Liu Y, Du J, Liang
J, Xu Q and Li J: Proteomic analysis of cPKCβII-interacting
proteins involved in HPC-induced neuroprotection against cerebral
ischemia of mice. J Neurochem. 117:346–356. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Leary S, Anthony R, Grandin T, Greenacre
C, Gwaltney-Brant S, Ann McCrackin M, Meyer R, Miller D, Shearer J,
Turner T and Yanong R: AVMA guidelines for the euthanasia of
animals: 2020 Edition*. AVMA, 2020.
|
|
21
|
Liu X, Li M, Hou M, Huang W and Song J:
MicroRNA-135a alleviates oxygen-glucose deprivation and
reoxygenation-induced injury in neurons through regulation of
GSK-3β/Nrf2 signaling. J Biochem Mol Toxicol: e22159, 2018. doi:
10.1002/jbt.22159.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li P, Shen M, Gao F, Wu J, Zhang J, Teng F
and Zhang C: An antagomir to microRNA-106b-5p ameliorates cerebral
ischemia and reperfusion injury in rats via inhibiting apoptosis
and oxidative stress. Mol Neurobiol. 54:2901–2921. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Woodruff TM, Thundyil J, Tang SC, Sobey
CG, Taylor SM and Arumugam TV: Pathophysiology, treatment, and
animal and cellular models of human ischemic stroke. Mol
Neurodegener. 6(11)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lewen A, Matz P and Chan PH: Free radical
pathways in CNS injury. J Neurotrauma. 17:871–890. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu X, Qing Wang, Cui Y, Li X and Yang H:
In-depth transcriptomic and proteomic analyses of the hippocampus
and cortex in a rat model after cerebral ischemic injury and repair
by Shuxuetong (SXT) injection. J Ethnopharmacol.
249(112362)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Barialai L, Strecker MI, Luger AL, Jäger
M, Bruns I, Sittig ACM, Mildenberger IC, Heller SM, Delaidelli A,
Lorenz NI, et al: AMPK activation protects astrocytes from
hypoxia-induced cell death. Int J Mol Med. 45:1385–1396.
2020.PubMed/NCBI View Article : Google Scholar
|