Introduction
DME is one of the most common causes of vision loss
in diabetic patients (1,2). The pathogenesis of DME is
multi-factorial and involves multiple pathways (3), such as poor blood sugar control
(4), high blood pressure (5), hyperlipidemia (6) and proteinuria (7), which eventually lead to the thickening
of the central retina; if untreated in time, it will cause loss of
vision. Currently, the treatment of refractory DME is still
progressing slowly (8,9).
MicroRNAs (miRNAs) are small non-coding RNAs that
regulate biological networks by regulating gene expression. In
several studies on DME, miRNAs have been found to play an important
role in the pathogenesis of DME (10,11).
Studies have shown that miR-155-5p is overexpressed in diabetic
patients (12), and can be used as
a new biological indicator for the diagnosis and evaluation of
diabetes (13).
Vascular endothelial growth factor A (VEGF-A) plays
an important role in the occurrence of DME (2,14,15),
and is the main angiogenesis promoter of endothelial cells mainly
through its homologous receptor VEGF-R1. The upregulation of
VEGF-A, inflammatory cytokines and chemokines induces pathological
changes in the vascular endothelium, triggering the destruction of
the blood-retinal barrier, causing fluid to penetrate into the
extracellular space, which is clinically manifested as macular
edema and leads to vision loss (6,16).
In vitro molecular studies demonstrated that miRNAs modulate
VEGF to promote high-glucose-induced apoptosis (17) and angiogenesis (18) of HRMECs. Qiu et al (19) confirmed that miR-21-5p inhibitor
inhibited the proliferation and angiogenesis of HRMECs induced by
high glucose. miR-199a-3p inhibits the angiogenesis of HRMECs
induced by high glucose by inhibiting VEGF (20). However, in DME, there has been no
report on the effect of miR-155-5p targeting cytokines on the
angiogenesis of HRMECs.
The present study aimed to evaluate the association
between miR-155-5p and the clinical features of DME and refractory
DME, as well as to evaluate the potential mechanism of miR-155-5p
on HRMECs induced by high glucose in vitro.
Materials and methods
Materials
Antibodies against VEGF-A (cat. no. ab214424) and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; cat. no. ab181602)
were purchased from Abcam. The miR-155-5p mimics or inhibitors, and
their negative controls, as well as primers for reverse
transcription-quantitative (RT-q)PCR were synthesized by Guangzhou
RiboBio Co., Ltd., and the miRNA sequences were as follows:
miR-155-5p mimics, 5'-UUAAUG CUAAUCGUGAUAGGGGUU-3'; miR-155-5p
negative control, 5'-GGGUUAGGUAAUGUAAUCCGUUUA-3'; miR-155-5p
inhibitor, 5'-AACCCCTATCACGATTAGCATTAA-3'; miR-155-5p inhibitor
negative control, 5'-CAGUACUUUUGU GUAGUACAA-3'. The primer
sequences for miRNAs for the RT-qPCR assay were as follows:
miR-155-5p forward, 5'-ACA CTCCAGCTGGGTTAATGCTAATCGTGATA-3';
miR-155-5p reverse, 5'-CTCAACTGGTGTCGTGGA-3'; U6 forward, 5'-CTC
GCTTCGGCAGCACA-3'; and reverse; 5'-AACGCTTCACGA ATTTGCGT-3'.
Study subjects
Between April 2020 and August 2020, 72 patients
diagnosed with DME were selected from the outpatient inpatients in
Wuhan Aier Eye hospital, including 49 males and 23 females, aged
31-71 (mean 51.17±9.98) years, with a disease course of 0.5-20
years. All patients underwent detailed medical examinations and
fundoscopy. According to the staging standard specified by the 2002
Ocular Fundus Disorders Academic Conference (21), the patients were divided into three
groups as follows: 45 cases in the DME group, including 31 males
and 14 females, with an average age of 50.72±11.48 years and an
average course of 6.45±1.73 years; 27 cases in the refractory DME
group, with 18 males and 9 females, average age of 53.55±9.63 years
and an average course of 9.25±3.36 years. The exclusion criteria
were as follows: Patients with diabetic ketoacidosis, diabetic
hyperosmolar coma, and other acute complications of diabetes;
patients with severe stress; patients with acute or chronic
infections; and patients with liver disease. The patients with
idiopathic macular hole (MH) (17 males and 5 females) served as the
control group; their age, sex ratio and body mass index (BMI) were
matched, and average age was 50.57±4.28 years. The examination of
patients with idiopathic MH excluded hypertension, heart, liver,
kidney disease and other endocrine and metabolic diseases. All
patients or their family members signed an informed consent form,
and the experiment was approved by the Aier Eye Hospital and
conformed with the guiding principles of the Declaration of
Helsinki (22).
Data measurement
On admission, the patient's height and weight were
measured, and BMI was calculated as weight/height2
(kg/m2). Medical history, age, sex, course of diabetes
and blood sugar control, among other parameters were recorded.
Fasting blood-glucose (FBG), glycated hemoglobin (HbA1c),
hemoglobin (HB), proteinuria and glycosuria were measured with the
Beckman automatic biochemical analyzer (cobas c311; Roche
Diagnostics GmbH) according to the kit's instructions.
Ophthalmoscopic examination
Retinal thickness was analyzed using optical
coherence tomography (OCT; RTVue XR; Optovue, Inc.) using the
software RTVue XR (version: 2018.1.0.43). Images of the fundus were
captured using Optos PLC's Panoramic Ophthalmoscope (200TX; Nikon
Corporation) with the software Optos V2®Vantage Pro
Review (version: 2.11.0.3; Nikon Corporation).
Aqueous humor (AH) sample collection
and processing
AH was collected from each patient (~50 to 100 µl)
through paracentesis. The AH samples were collected in 0.5-ml
RNA-free Eppendorf (EP) tubes, stored on ice, and further processed
within 1 h after collection. In all cases, sample collection was
non-invasive, eliminating the risk of blood or cell debris
contamination. By centrifuging the AH sample at 1,200 x g for 30
min at 4˚C, the final cell/cell debris contamination could be
prevented. Then, the supernatant was transferred to a new sterile
EP tube for RNA extraction.
Collection and processing of whole
blood samples
Firstly, 5 ml whole blood was collected from the
patients in a BD Vacutainer-EDTA tube (BD Biosciences) and
immediately mixed 5-8 times. Within 1 h after blood collection, the
blood samples were processed by centrifugation at 1,200 x g for 10
min at 4˚C; the collected plasma was centrifuged at 1,200 x g for
20 min at 4˚C to remove contaminant cells and cell debris, and then
RNA extraction was performed. Hemolyzed plasma samples were
excluded from the study.
Cell culture, transfection and
treatments
The HRMECs (cat. no. CP-H130; Procell Life Science
& Technology Co., Ltd.) were cultured at 37˚C with 5%
CO2 in complete culture medium (cat. no. CM-H130;
Procell Life Science & Technology Co., Ltd.). Once cells
achieved 60-80% confluence, they were transfected with RNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 4 h, according to the manufacturer's
instructions. Then, medium was replaced with fresh culture medium,
and cells were incubated for a further 48 h prior to RT-qPCR or
western blot analysis. The high-glucose group was pre-cultured with
30 mM glucose at 37˚C for 48 h, and the normal glucose group
(control) was pre-cultured with 5 mM glucose at 37˚C for 48 h
before experiment. The glucose concentration used in the high
glucose group was based on previous studies (23,24).
RNA extraction
Using the miRNeasy Mini kit (217004; Qiagen GmbH),
total RNA was extracted from 50 µl AH or 200 µl plasma samples. The
AH and plasma samples were centrifuged at 3000 x g for 5 min at 4˚C
to completely remove contaminant cell debris. A total of 50 µl AH
and 200 µl plasma per patient was diluted to 400 µl with RNA-free
water (Gibco; Thermo Fisher Scientific, Inc.) to avoid protein
aggregation. Then, the sample was lysed by adding three times the
volume of TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) and was finally dissolved in 10 µl RNA-free water.
RT-qPCR assay
Extracted RNA was reverse transcribed to cDNA using
a PrimeScript RT reagent kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. The RT-qPCR procedure
was performed using a SYBR Premix ExTaq II kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions in
conjunction with a 7500 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: 95˚C for 10 min; 55˚C for 2 min;72˚C for 2 min;
followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min. Target
miR-155-5p levels were normalized to those of the housekeeping gene
U6. Relative miR-155-5p expression levels were calculated using the
2-ΔΔCq method (25), and
the RT-qPCR experiment was repeated three times.
Western blotting
Transfected HRMECs were harvested by trypsin
digestion and lysed using ice-cold RIPA lysis buffer (Beyotime
Institute of Biotechnology). Lysate protein concentrations were
estimated using a BCA protein assay kit (Beijing Solarbio Science
& Technology Co., Ltd.). Equal amounts of denatured proteins
(20 µg) were resolved using 10% SDS-polyacrylamide gel
electrophoresis (Beijing Solarbio Science & Technology Co.,
Ltd.) and protein bands were transferred to a polyvinylidene
fluoride membrane (Beijing Solarbio Science & Technology Co.,
Ltd.). The membrane was blocked with 5% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) for 1 h at 22±3˚C.
Subsequently, the membranes were incubated with an anti-VEGF
antibody (1:1,000) overnight at 4˚C, rinsed, and incubated with the
horseradish peroxidase-conjugated secondary antibody (goat
anti-rabbit; 1:10,000; cat. no. ab205718; Abcam) for 2 h at 22±3˚C.
Protein bands were visualized by the addition of ELC enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.) in
conjunction with an imaging system (DNR Bio-Imaging Systems, Ltd.).
An anti-GAPDH antibody (1:10,000) was used as a loading
control.
Cell Counting Kit-8 (CCK8) assay
Briefly, a single cell suspension was prepared by
the trypsin method and inoculated in 96-well plates at a density of
3x103 cells/well. Then, 10 µl CCK-8 reagent
(Elabscience, Wuhan, China) was added at 0, 24, 48 and 72 h and
incubated for 60 min. The optical density was measured at 490 nm
using a microplate reader (multiscan MK3; Thermo Fisher Scientific,
Inc.). The CCK-8 experiment was repeated three times.
Angiogenesis assay
Transfected HRMECs were pretreated with different
concentrations of glucose (5 or 30 mM) at 37˚C for 48 h. Next,
serum-starved HRMECs (2x104 cells) were seeded onto
24-well plates coated with Matrigel (BD Biosciences) in endothelial
basal medium and incubated at 37˚C with 5% CO2 for 24 h.
Tubular structures of HRMECs in the Matrigel were examined with a
light microscope (Olympus Corporation). The angiogenesis experiment
was repeated three times and five fields were randomly selected for
the quantitative analysis of each result.
Statistical analysis
All data are expressed as means ± standard
deviations. All statistical analyses were performed using SPSS
version 21.0 statistical analysis package (SPSS Inc.). The
male/female data were analyzed using a χ2 test. The AH,
serum and clinical characteristics of patients, the expression of
miR-155-5p under miR-155-5p mimic/inhibitor transfection, cell
proliferation, angiogenesis, and western blot data proceeded via a
one-way analysis of variance and Bonferroni post hoc test. The
difference of miR-155-5p expression before and after HG induction
was analyzed using the unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
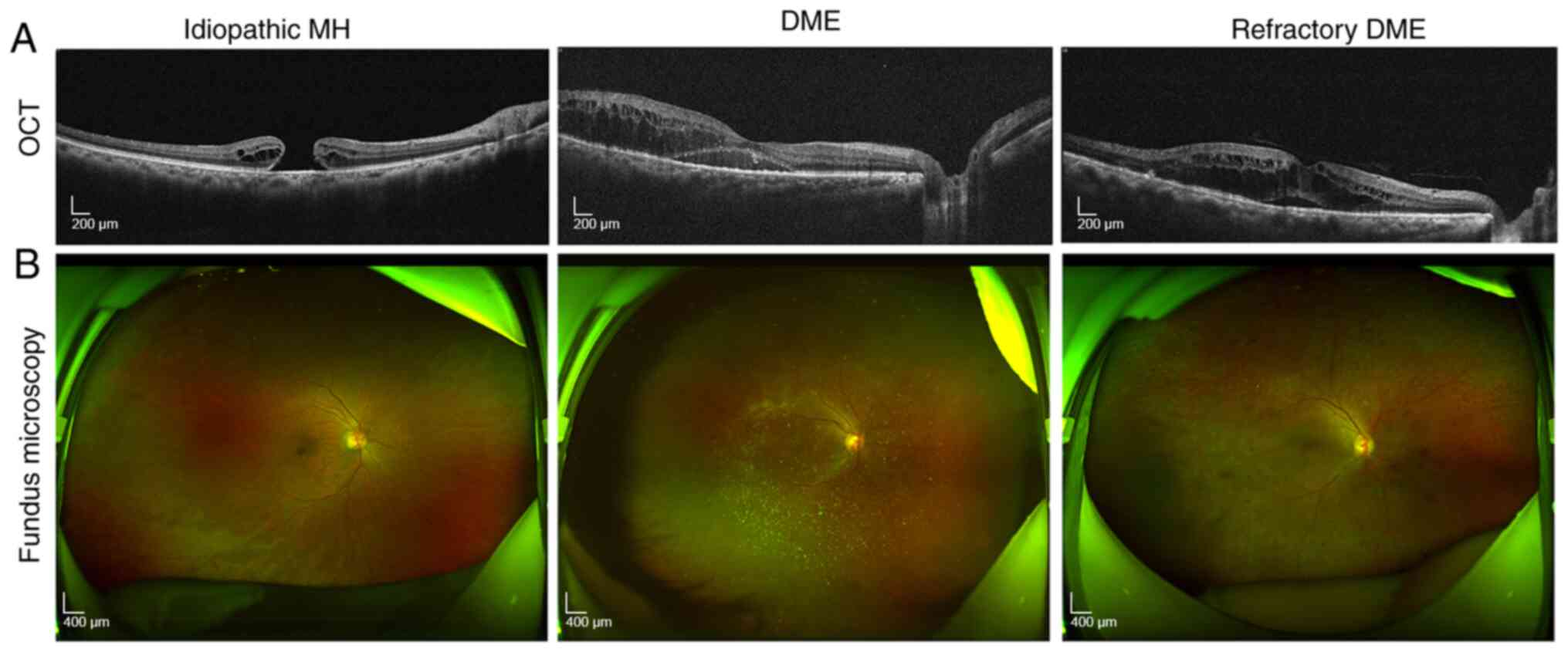
Ophthalmoscopic examination
In the present study, fundus microscopy was
performed on patients in each group. The results showed that
compared with that in the control group, patients in the DME group
had obvious macular edema and thick retina. Furthermore, compared
with disease signs in the DME group, patients with refractory DME
had bleeding on the eyeballs, as well as old laser spots remaining
from previous treatment (Fig.
1A).
Optical coherence tomography
analysis
The results showed that compared with the control
group, patients with DME had notable edema between the retinal
layers, whereas patients with refractory DME had more obvious
retinal edema. Meanwhile, patients with refractory DME have obvious
edema and dark cavities between the retinal layers (Fig. 1B).
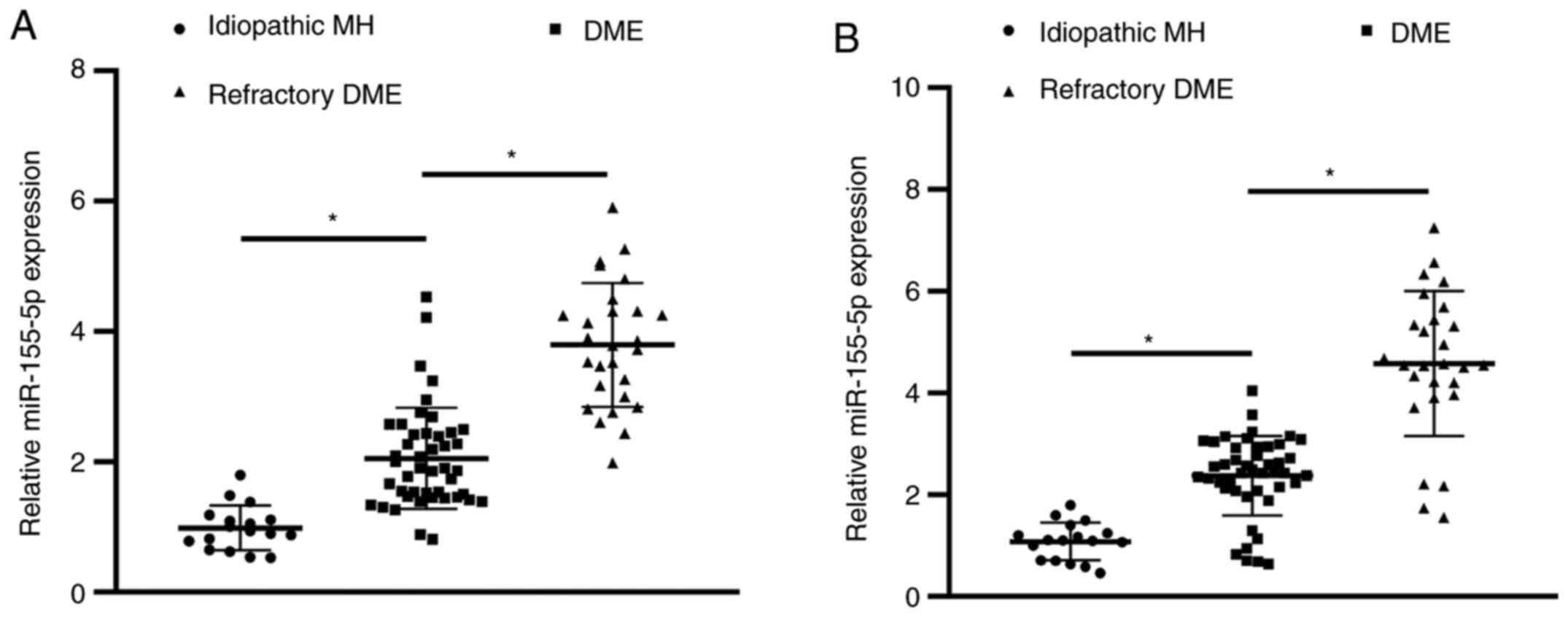
Differences in the expression of AH
and serum miR-155-5p among patients with DME
Compared with patients in the control group, the
expression of miR-155-5p in AH and serum of the DME group was
upregulated (Fig. 2A and B). The expression of miR-155-5p in AH and
serum of patients in the refractory DME group was also upregulated
compared with that in patients in the DME group.
Association between the expression
level of miR-155-5p and patient clinical characteristics
Next, the clinical characteristics of patients in
each group were analyzed, including BMI, FBG, HbA1c, HB,
intraocular pressure (IOP), proteinuria and glycosuria. The results
showed that the differences in miR-155-5p expression were
positively associated with the course of disease, BMI, FBG, HbA1C,
proteinuria and glycosuria (Fig.
3). The expression level of miR-155-5p was not significantly
different based on the age, HB, IOP and sex of the patients.
Specific data are shown in Table
SI.
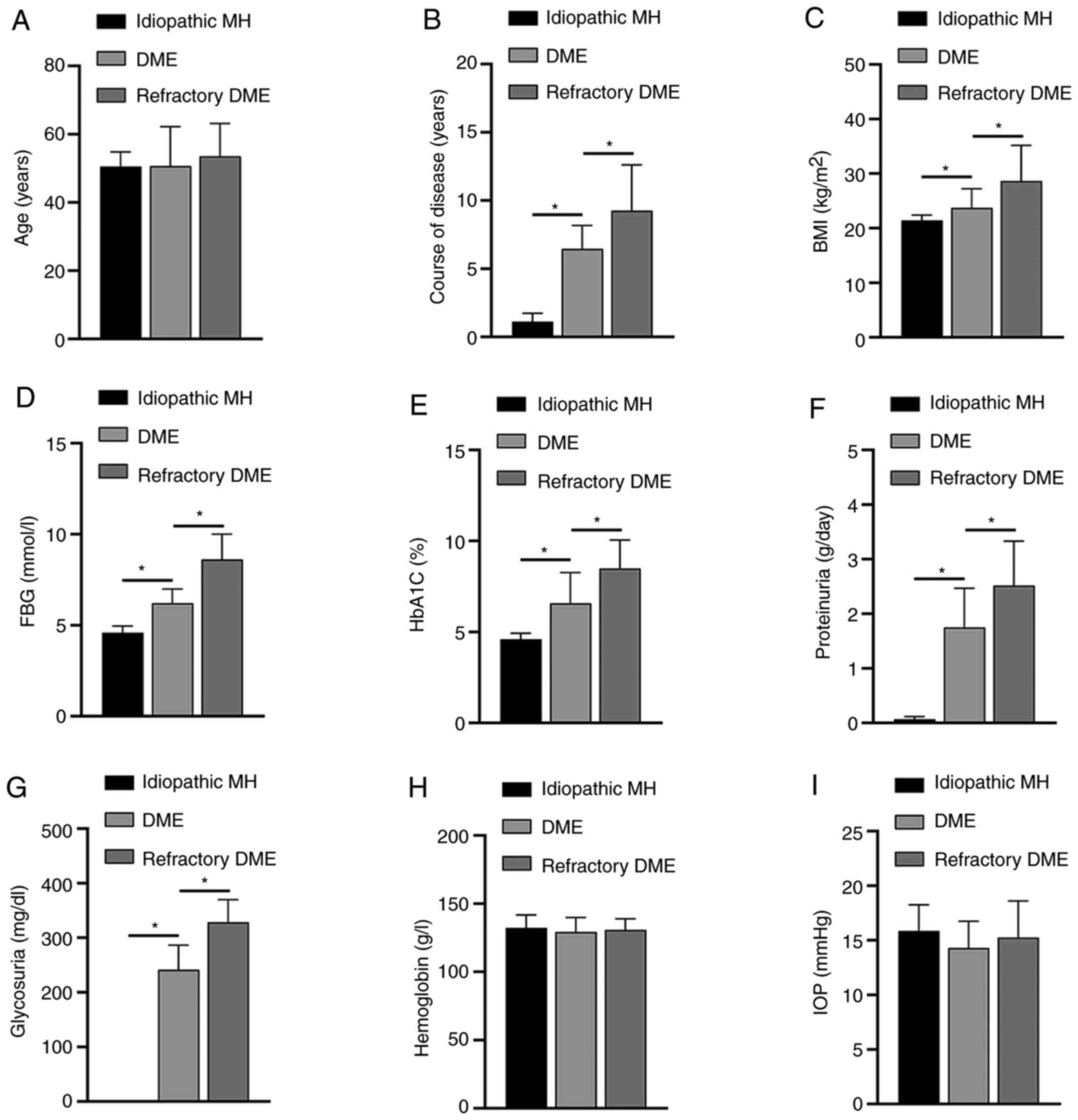 | Figure 3Association between miR-155-5p and
clinical symptoms of patients. The differences in the age (A),
course of disease (B), BMI (C), FBG (D), HbA1C (E), proteinuria
(F), glycosuria (G), HB (H) and IOP (I) in each group of patients
are shown. *P<0.05. miR, microRNA; DME, diabetic
macular edema; MH, macular hole; BMI, body mass index; FBG, fasting
blood-glucose; HbA1c, glycated hemoglobin; HB, hemoglobin; IOP,
intraocular pressure. |
High glucose induces increased
expression of miR-155-5p
Next, the potential mechanism underlying the effects
of miR-155-5p was analyzed from a molecular perspective. For this,
HRMECs were treated with high glucose in vitro. RT-qPCR
results showed that high glucose induced the upregulation of
miR-155-5p (Fig. 4A).
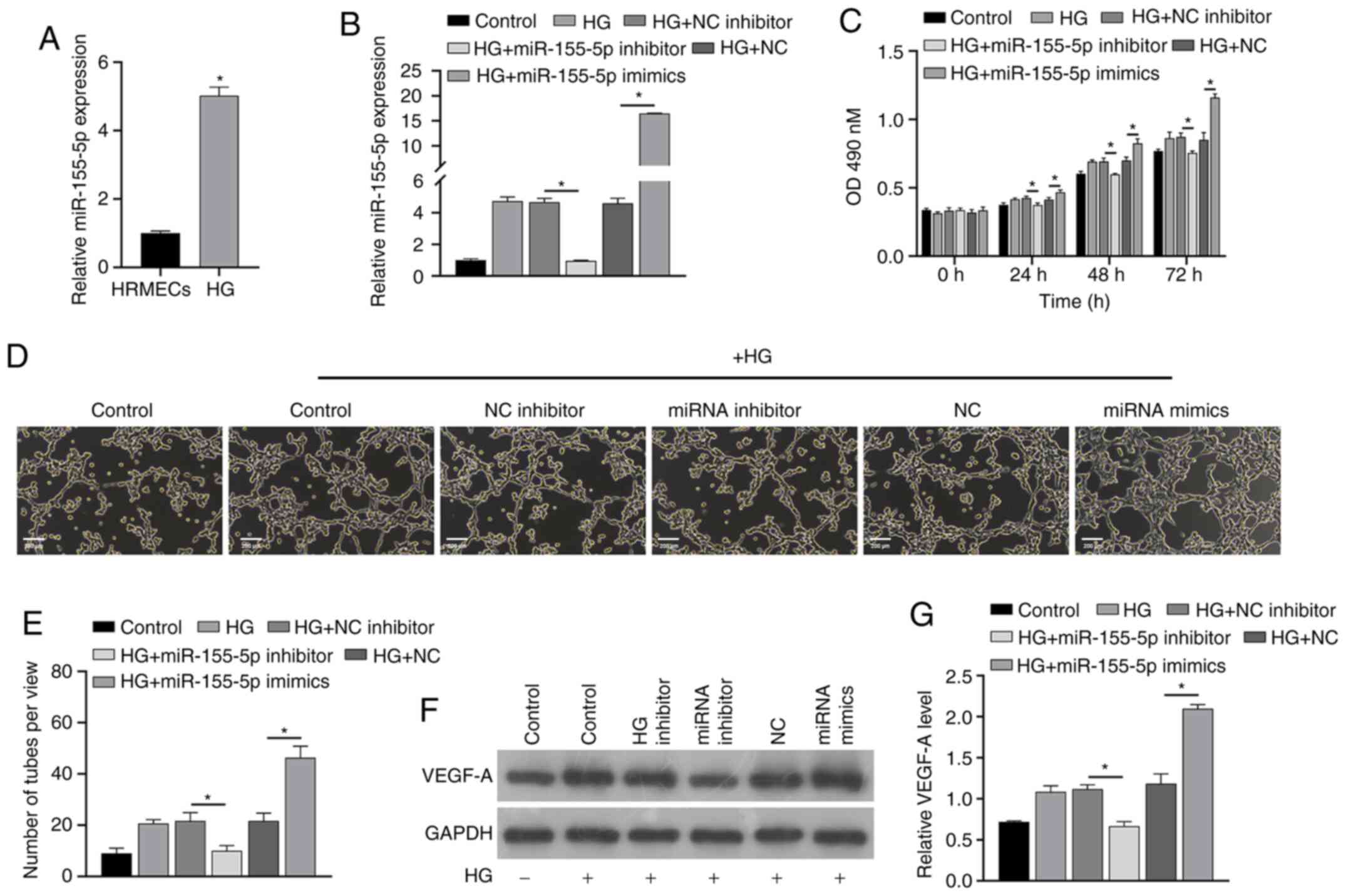 | Figure 4miR-155-5p reverses the effect of
high glucose induction. RT-qPCR (A) analysis showed that the
expression of miR-155-5p was different in HRMECs induced by high
glucose. After transfection with miRNAs, RT-qPCR (B) and Cell
Counting Kit-8 assays (C) were performed to analyze the changes in
expression of miR-155-5p and cell proliferation in each group.
Angiogenesis (D and E) was analyzed in each group, and western
blotting (F and G) was performed to analyze the changes in VEGF-A
protein levels. Samples were divided into the control group, HG
group, HG+NC inhibitor group, HG+inhibitor group, HG+NC group and
HG+miR-155-5p mimic group. *P<0.05. miR/miRNA,
microRNA; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; HG, high glucose; NC negative control; VEGF,
vascular endothelial growth factor; HRMECs, human retinal
microvascular endothelial cells |
Effect of miR-155-5p on HRMECs
To confirm the effect of miR-155-5p in HRMECs,
synthetic miR-155-5p mimics/inhibitors were transfected into cells
and the effect on the cellular functions of HRMECs was analyzed.
RT-qPCR analysis showed that miRNAs were successfully constructed
(Fig. 4B). The results also showed
that high glucose promotes the proliferation and angiogenesis of
HRMECs and also upregulates the protein level of VEGF-A (Fig. 4C-G). These effects could be reversed
by a miR-155-5p inhibitor, and at the same time, these effects were
enhanced by miR-155-5p mimics. These results confirm that
miR-155-5p can regulate the proliferation and angiogenesis of
HRMECs and concomitantly affect the protein level of VEGF-A in
HRMECs.
Discussion
DME, defined as the thickening of the retina
involving or near the center of the macula, is the most common
cause of vision loss in patients affected by diabetes (26). At present, intravitreal injection of
anti-VEGF drugs is considered one of the best treatments for DME
(14,27). Early vitrectomy can also effectively
decrease the burden of treatment for diabetic patients and prevent
vision loss (28-31).
However, for some patients with intractable DME, these treatments
have not achieved long-term relief of the disease (9,32,33).
The present study analyzed the eyeball characteristics of patients
with DME and found that the retina of patients with idiopathic MH
did not have obvious edema, whereas patients with DME, in addition
to more intractable patients with DME, had obvious retinal edema.
Meanwhile, the retina of patients with refractory DME also had
symptoms of hemorrhage, and the retina was significantly heavier
compared with that of DME patients. In addition, patients with
intractable DME had obvious edema and dark cavities between the
omentum layers.
Studies have shown that miRNA, as a biomarker of
DME, has high sensitivity and specificity (11) and is stably expressed in biological
fluids such as human AH and plasma (34,35).
Among them, overexpression of miR-155-5p has been proved by
numerous studies to play an important role in the progression of
diabetes (36,37). However, the study of miR-155-5p in
DME has not been reported. In the present study, the role of AH and
plasma miR-155-5p was investigated in DME. One of the main results
of the present study showed that the expression level of miR-155-5p
was relatively increased in the DME group and the refractory DME
group compared with that of the control group. In addition, the
expression level of miR-155-5p in the refractory DME group was
significantly higher compared with that in the DME group. The
results thus showed that the expression level of miR-155-5p is
associated with the severity of DME. Accordingly, abnormal
miR-155-5p expression is associated with the development of DME,
and the regulation of its expression might provide a potential
treatment for this disease.
Regarding the mechanism underlying the effects of
miR-155-5p on the development of DME, the association between this
marker and some clinical indicators was studied. The analysis of
the present study showed that the expression level of miR-155-5p
was positively associated with the course of disease, BMI, FBG,
HbA1C, proteinuria and glycosuria. In addition, the expression
level of miR-155-5p had no obvious association with HB, IOP and
sex. According to the clinical research data, it is necessary to
further clarify the mechanism associated with the effects of
miR-155-5p in DME. Therefore, the present study simulated
hyperglycemia conditions by exposing HRMECs to high glucose; this
significantly upregulated the expression of miR-155-5p in these
cells. High glucose in HRMECs significantly downregulated the
proliferation and angiogenesis of HRMECs and increased the level of
VEGF-A protein compared with that with normal glucose treatment.
However, after inhibiting the expression of miR-155-5p, the effect
of high glucose was reversed. Moreover, the addition of miR-155-5p
mimics had the same effect as high sugar. The aforementioned
results confirm that miR-155-5p can regulate the cellular function
of HRMECs induced by high glucose.
The present study has limitations. Firstly, VEGF and
miR-155-5p expression vectors were not injected into the vitreous
of type 2 diabetes mellitus animals to verify the utility of
miR-155-5p. Secondly, further research on the hypothetical target
genes and associated signaling pathways of miR-155-5p were not
conducted. Finally, the number of patients was insufficient to
clearly describe the association between miR-155-5p and DME or
intractable DME, to deepen the understanding of the field. The
aforementioned deficiencies also suggest the direction of future
research. Additional experiments are intended to be conducted in
the future to verify the association between miRNA and DME or
intractable DME.
In summary, the expression level of miR-155-5p in AH
and plasma is upregulated during the development of DME and can be
used as an indicator for this disease. The expression level of
miR-155-5p is also positively associated with the course of
disease, BMI, FBG, HbA1C, proteinuria and glycosuria. The present
study provides a valuable reference for the diagnosis and treatment
of DME and refractory DME.
Supplementary Material
Demographic data of the studied
groups.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science Research
Foundation of Aier Eye Hospital Group (grant no. AF1901D5) and
Medical Research Fund of Wuhan Municipal Health Commission (grant
no. WX18D15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and TY designed the study and developed the
methodology. RZ, SW, LX, TX, CY and YL performed the experiments
and collected the data. JH, TY, RZ and SW analyzed and interpreted
the data. JH and TY drafted the original manuscript. JH and TY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Wuhan Aier Eye Hospital (permit no. 2021IRBLW01). Written informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tan GS, Cheung N, Simó R, Cheung GC and
Wong TY: Diabetic macular oedema. Lancet Diabetes Endocrinol.
5:143–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Patelli F, Radice P and Giacomotti E:
Diabetic macular edema. Dev Ophthalmol. 54:164–173. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yau JW, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Meta-Analysis for Eye Disease (META-EYE) Study Group:
Global prevalence and major risk factors of diabetic retinopathy.
Diabetes Care. 35:556–564. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fenwick EK, Xie J, Man REK, Sabanayagam C,
Lim L, Rees G, Wong TY and Lamoureux EL: Combined poor diabetes
control indicators are associated with higher risks of diabetic
retinopathy and macular edema than poor glycemic control alone.
PLoS One. 12(e0180252)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stana D, Potop V, Istrate SL, Eniceicu C,
Mihalcea AR, Paşca IG, Aqel A, Ciuluvică R and Moraru D:
Variability of diabetic macular edema in correlation with
hypertension retinopathy in patients with diabetes mellitus and
essential hypertension. Rom J Ophthalmol. 63:327–338.
2019.PubMed/NCBI
|
|
6
|
Romero-Aroca P, Baget-Bernaldiz M,
Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R and Verges R: Diabetic
Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J
Diabetes Res. 2016(2156273)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shye M, Hanna RM, Patel SS, Tram-Tran N,
Hou J, Mccannel C, Khalid M, Hanna M, Abdelnour L and Kurtz I:
Worsening proteinuria and renal function after intravitreal
vascular endothelial growth factor blockade for diabetic
proliferative retinopathy. Clin Kidney J. 13:969–980.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim JE, Pollack JS, Miller DG, Mittra RA
and Spaide RF: Isis Study Group. ISIS-DME: A prospective,
randomized, dose-escalation intravitreal steroid injection study
for refractory diabetic macular edema. Retina. 28:735–740.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hussain RM and Ciulla TA: Treatment
strategies for refractory diabetic macular edema: Switching
anti-VEGF treatments, adopting corticosteroid-based treatments, and
combination therapy. Expert Opin Biol Ther. 16:365–374.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grieco GE, Sebastiani G, Eandi CM, Neri G,
Nigi L, Brusco N, D'Aurizio R, Posarelli M, Bacci T, Benedetto E,
et al: MicroRNA Expression in the Aqueous Humor of Patients with
Diabetic Macular Edema. Int J Mol Sci. 21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chan HW, Yang B, Wong W, Blakeley P, Seah
I, Tan QS, Wang H, Bhargava M, Lin HA, Chai CH, et al: A pilot
study on MicroRNA profile in tear fluid to predict response to
anti-VEGF treatments for diabetic macular edema. J Clin Med.
9(9)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang J, Wang G, Liang Y and Zhou X:
Expression profiling and clinical significance of plasma MicroRNAs
in diabetic nephropathy. J Diabetes Res.
2019(5204394)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bai X, Luo Q, Tan K and Guo L: Diagnostic
value of VDBP and miR-155-5p in diabetic nephropathy and the
correlation with urinary microalbumin. Exp Ther Med.
20(86)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim EJ, Lin WV, Rodriguez SM, Chen A, Loya
A and Weng CY: Treatment of diabetic macular edema. Curr Diab Rep.
19(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lally DR, Shah CP and Heier JS: Vascular
endothelial growth factor and diabetic macular edema. Surv
Ophthalmol. 61:759–768. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Miller K and Fortun JA: Diabetic Macular
Edema: Current understanding, pharmacologic treatment options, and
developing therapies. Asia Pac J Ophthalmol (Phila). 7:28–35.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zeng Y, Cui Z, Liu J, Chen J and Tang S:
MicroRNA-29b-3p promotes human retinal microvascular endothelial
cell Apoptosis via blocking SIRT1 in diabetic retinopathy. Front
Physiol. 10(1621)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Han N, Xu H, Yu N, Wu Y and Yu L:
MiR-203a-3p inhibits retinal angiogenesis and alleviates
proliferative diabetic retinopathy in oxygen-induced retinopathy
(OIR) rat model via targeting VEGFA and HIF-1α. Clin Exp Pharmacol
Physiol. 47:85–94. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qiu F, Tong H, Wang Y, Tao J, Wang H and
Chen L: Inhibition of miR-21-5p suppresses high glucose-induced
proliferation and angiogenesis of human retinal microvascular
endothelial cells by the regulation of AKT and ERK pathways via
maspin. Biosci Biotechnol Biochem. 82:1366–1376. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang L, Liu WX and Huang XG:
MicroRNA-199a-3p inhibits angiogenesis by targeting the
VEGF/PI3K/AKT signalling pathway in an in vitro model of diabetic
retinopathy. Exp Mol Pathol. 116(104488)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wilkinson CP, Ferris FL III, Klein RE, Lee
PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R and
Verdaguer JT: Global Diabetic Retinopathy Project Group. Proposed
international clinical diabetic retinopathy and diabetic macular
edema disease severity scales. Ophthalmology. 110:1677–1682.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mastroleo I: Post-trial obligations in the
Declaration of Helsinki 2013: Classification, reconstruction and
interpretation. Developing World Bioeth. 16:80–90. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu L, Lu Q, Chen W, Li J, Li C and Zheng
Z: Vitamin D3 Protects against diabetic retinopathy by inhibiting
high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome
pathway. J Diabetes Res. 2018(8193523)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gu C, Draga D, Zhou C, Su T, Zou C, Gu Q,
Lahm T, Zheng Z and Qiu Q: miR-590-3p inhibits pyroptosis in
diabetic retinopathy by targeting NLRP1 and inactivating the NOX4
signaling pathway. Invest Ophthalmol Vis Sci. 60:4215–4223.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bandello F, Battaglia Parodi M, Lanzetta
P, Loewenstein A, Massin P, Menchini F and Veritti D: Diabetic
macular edema. Dev Ophthalmol. 58:102–138. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Korobelnik JF, Do DV, Schmidt-Erfurth U,
Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H,
Marcus DM, et al: Intravitreal aflibercept for diabetic macular
edema. Ophthalmology. 121:2247–2254. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Michalewska Z, Stewart MW, Landers MB III,
Bednarski M, Adelman RA and Nawrocki J: Vitrectomy in the
management of diabetic macular edema in treatment-naïve patients.
Can J Ophthalmol. 53:402–407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Simunovic MP, Hunyor AP and Ho IV:
Vitrectomy for diabetic macular edema: A systematic review and
meta-analysis. Can J Ophthalmol. 49:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jackson TL, Nicod E, Angelis A, Grimaccia
F, Pringle E and Kanavos P: Pars Plana Vitrectomy For Diabetic
Macular Edema: A Systematic Review, Meta-Analysis, and Synthesis of
Safety Literature. Retina. 37:886–895. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Michalewska Z, Stewart MW, Landers MB III,
Bednarski M, Adelman RA and Nawrocki J: Response to Vitrectomy in
diabetic macular edema. Can J Ophthalmol. 54:403–404.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi MY, Jee D and Kwon JW:
Characteristics of diabetic macular edema patients refractory to
anti-VEGF treatments and a dexamethasone implant. PLoS One.
14(e0222364)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Maleki A, Stephenson AP and Hajizadeh F:
Topical interferon alpha 2b in the treatment of refractory diabetic
macular edema. J Ophthalmic Vis Res. 15:453–458. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mastropasqua R, Toto L, Cipollone F,
Santovito D, Carpineto P and Mastropasqua L: Role of microRNAs in
the modulation of diabetic retinopathy. Prog Retin Eye Res.
43:92–107. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen CF, Hua K, Woung LC, Lin CH, Chen CT,
Hsu CH, Liou SW and Tsai CY: Expression profiling of exosomal
miRNAs derived from the aqueous humor of myopia patients. Tohoku J
Exp Med. 249:213–221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang G, Wu B, Zhang B, Wang K and Wang H:
LncRNA CTBP1-AS2 alleviates high glucose-induced oxidative stress,
ECM accumulation, and inflammation in diabetic nephropathy via
miR-155-5p/FOXO1 axis. Biochem Biophys Res Commun. 532:308–314.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Assmann TS, Recamonde-Mendoza M, Puñales
M, Tschiedel B, Canani LH and Crispim D: MicroRNA expression
profile in plasma from type 1 diabetic patients: Case-control study
and bioinformatic analysis. Diabetes Res Clin Pract. 141:35–46.
2018.PubMed/NCBI View Article : Google Scholar
|