Introduction
Oral lichen planus (OLP) is a chronic inflammatory
disorder affecting 1-3% of the population, with a higher prevalence
in women (women to men ratio 1.4:1) (1). Oral lesions of lichen planus may
accompany cutaneous lesions or they may be the only sign of the
disease. They are considered to be the result of a T-cell-mediated
autoimmune response to antigens that may be unmasked by contact
allergens such as dental restorative materials, drugs, mechanical
trauma or viral infections, leading to activation of auto-cytotoxic
CD8+ T cells that trigger apoptosis of the basal cells
of the oral epithelium (2).
Clinical manifestations of OLP include white
striations (Wickham striae), white papules, white plaques,
erythema, mucosal atrophy, erosions, or pigmented patches,
symmetrically distributed on the oral mucosal membranes. The
diagnosis of lichen planus is usually based on clinical aspects
correlated with histopathological examination of the lesions.
Currently, classical invasive diagnostic methods have been replaced
by modern non-invasive techniques (dermoscopy, reflectance confocal
microscopy, optical coherence tomography, ultrasound and diffuse
reflection spectrophotometry) for the diagnosis and therapeutic
monitoring of lichen planus (3,4). These
modern methods are useful for appreciating the risk of malignant
transformation and an earlier diagnosis of oral squamous carcinoma
(5,6).
A chronic relapsing clinical course of OLP has a
severe impact on the quality of life of patients, mainly in cases
of atrophic and erosive types. The risk of malignant transformation
is still debated, with a rate of 0.04-1.7% reported in the
literature, especially in erosive forms and in female patients, or
in association with HPV infection (7,8). High
levels of IL-6 are also associated with an increase in vascular
endothelial growth factor (VEGF) production. VEGF is one of the
most important angiogenesis stimulators and it has long been
demonstrated to facilitate tumor development and metastasis,
explaining the correlation between IL-6 and oral squamous cell
carcinoma (9).
Current therapeutic options are only partially
effective, leading to prolonged local chronic inflammation over the
course of several years in these patients (1,10).
Previous reports have focused on the association
between LP and alterations in lipid metabolism (11-14),
concluding that it may also be associated with a high risk of
cardiovascular morbidities (15).
Systemic inflammation leads to lipid metabolism disturbances
resulting in a redistribution of different nutrients to cells
involved in host defense in order to ensure detoxification and
tissue repair (16). Prolonged
inflammatory status causes prolonged dyslipidemia resulting in
atherosclerotic plaque formation and an increase in cardiovascular
risk (17,18).
IL-6 is a pleiotropic cytokine with a critical
defense role in infections and posttraumatic injuries, promoting
the production of IL-1 receptor antagonist, upregulating the
synthesis of acute phase proteins in hepatocytes (e.g., reactive C
protein), the terminal differentiation of B cells into
immunoglobulin producing cells and specific differentiation of T
CD4+ naïve cells into effector T cell subsets (including
antigen-specific Th17 cells) and inhibiting regulatory T cells
(19-23).
The persistence of proinflammatory IL-6 activity leads to the
conversion of acute defense inflammation into chronic damaging
inflammatory process (19). This
observational case-control study was designed to emphasize the
correlation between IL-6, a biomarker of systemic inflammation, and
lipid metabolism disturbances in patients with OLP, in order to
ascertain whether the disease, even localized oral lesions, may be
considered a marker of systemic inflammation and to support the
screening of these patients for these disturbances and treating
them with IL-6 antagonist aimed to control the cardiovascular
risk.
Patients and methods
We performed an observational case-control study on
a cohort of 36 patients diagnosed with several oral mucosal
disorders. The patients were divided into two groups: 18 patients
with OLP lesions diagnosed clinically and histologically in
accordance to the WHO criteria (24) (OLP group) and a control group of 18
patients with other oral manifestations (candidiasis, geographic
tongue, canker sore, oral dysesthesia, mucocele and erythema
multiforme). Patients over the age of 18 years, without previous
treatment with corticosteroids, retinoids or immunosuppressants for
6 months, were included. The study was conducted at the Dermatology
Clinic of the University Hospital of the Railways System, Iasi,
Romania from January 2015 to December 2019, and patients were
followed up for a period of 6 months. Ethical approval from The
Ethics Committee of the Railways University Hospital Iasi (Iasi,
Romania) and an informed written consent from all 36 enrolled
patients were obtained.
Venous blood samples were collected from all
patients for analyzing serum levels of IL-6, triglycerides (TG),
total cholesterol and high density lipoprotein cholesterol (HDL-C).
For this purpose, blood samples were obtained between 8:00 and 9:00
a.m., a jeun, with no alcohol intake during the previous 24 hours.
The lipid profile (serum triglycerides, total cholesterol,
HDL-cholesterol) and blood glucose were analyzed using DIASYS
(German Diagnostic System GmbH) commercial kits on an automated
CS-800 machine. Serum IL-6 levels were assessed on blood samples
drawn without anticoagulant, centrifuged and frozen at -80˚C on an
automated IMMULITE 2000 (Siemens) machine using a chemiluminescence
technique.
Statistical analysis
Statistical analysis was performed utilizing SPSS
version 18.0 software (SPSS, Inc.). ANOVA test was used to
demonstrate the relation between intergroup and intragroup
variables at 95% signification threshold and a linear regression
analysis was also performed.
Results
The 36 patients were aged 21-78 years (median 55.21
years) and 24 of them (66.0%) were females. In the OLP group, the
sex ratio F:M was 5:1, with 15 cases (83.3%) of female patients.
The most frequent clinical types of OLP were the atrophic-erosive
and erosive forms, diagnosed in 10 cases (55.5%). One case was in
accordance to Grinspan syndrome criteria (OLP, arterial
hypertension and type 2 diabetes mellitus) and one case fulfilled
the Hewitt-Pelisse syndrome criteria (coexistent lichen planus
erosive lesions of gums and genitalia in a 43-year-old woman). The
reticular form was present in 5 cases (27.7%) and OLP in plaques in
3 cases (16.6%). Erosive and atrophic-erosive types of OLP have the
most long lasting course, ranging between 8 and 30 months.
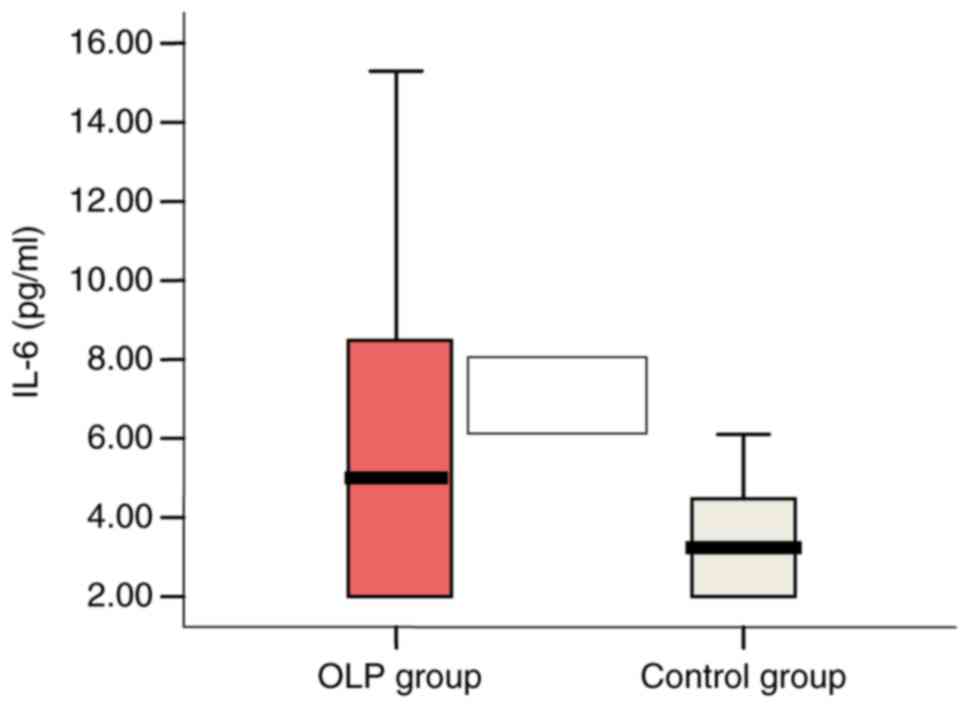
High IL-6 serum levels ranging between 8.29 pg/ml
(>5.9 pg/ml) and 15.30 pg/ml were found in 6 OLP patients
(33.3%) vs. 1 patient (5.5%) with a value of 6.1 pg/ml in the
control group. Mean values of IL-6 serum levels were significantly
higher in the OLP patient group: 5.66 vs. 3.40 pg/ml in the control
group (P=0.05) (Fig. 1).
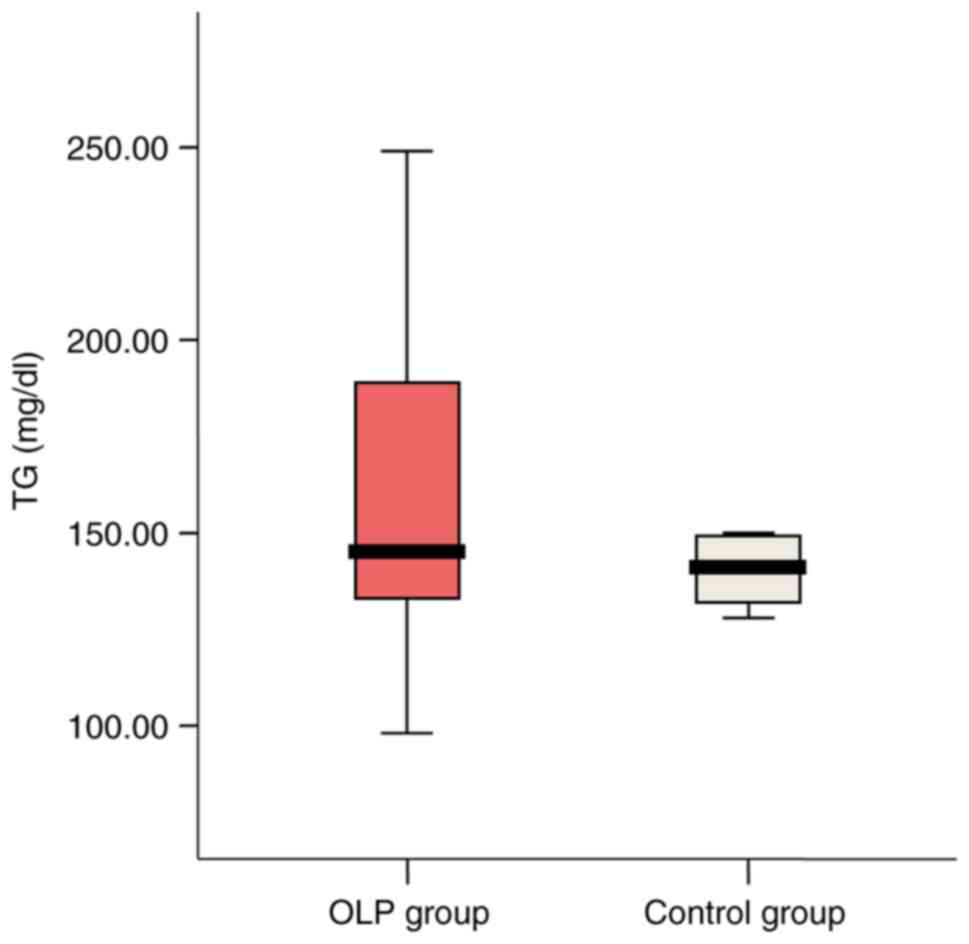
High TG serum levels (>150 mg/dl) were detected
in 33.3% (6 patients) of OLP group vs. 16.7% (3 cases) in the
control group. Mean value was slightly higher in the OLP group
(162.17l vs. 154.28 mg/dl; P=0.625) (Fig. 2).
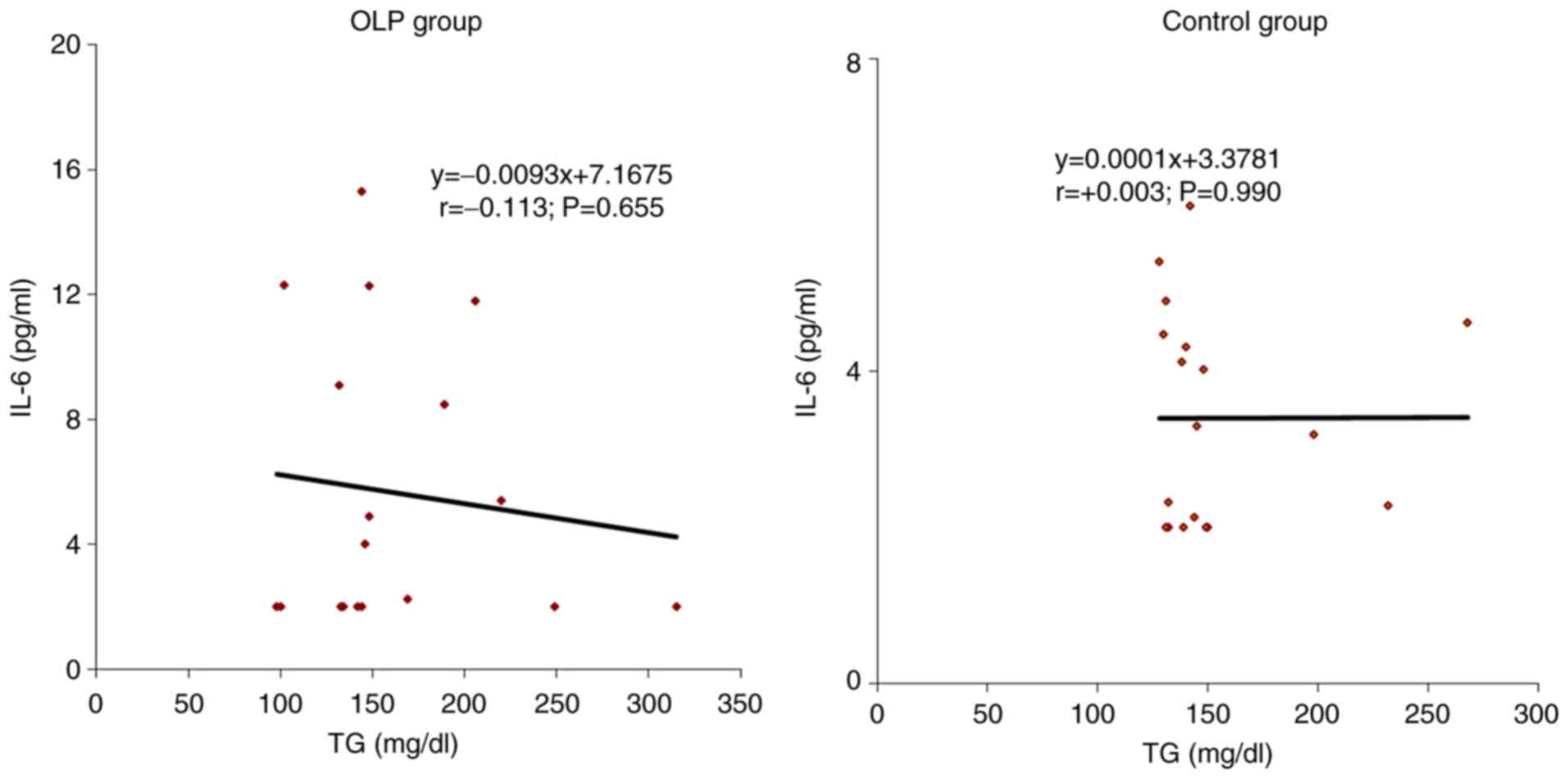
The correlations of IL-6 with individual values of
serum TG was indirect, of low intensity in patients with OLP
(r=-0.113; P=0.655) and these parameters were independent in those
from the control group (r=0.003; P=0.990) (Fig. 3).
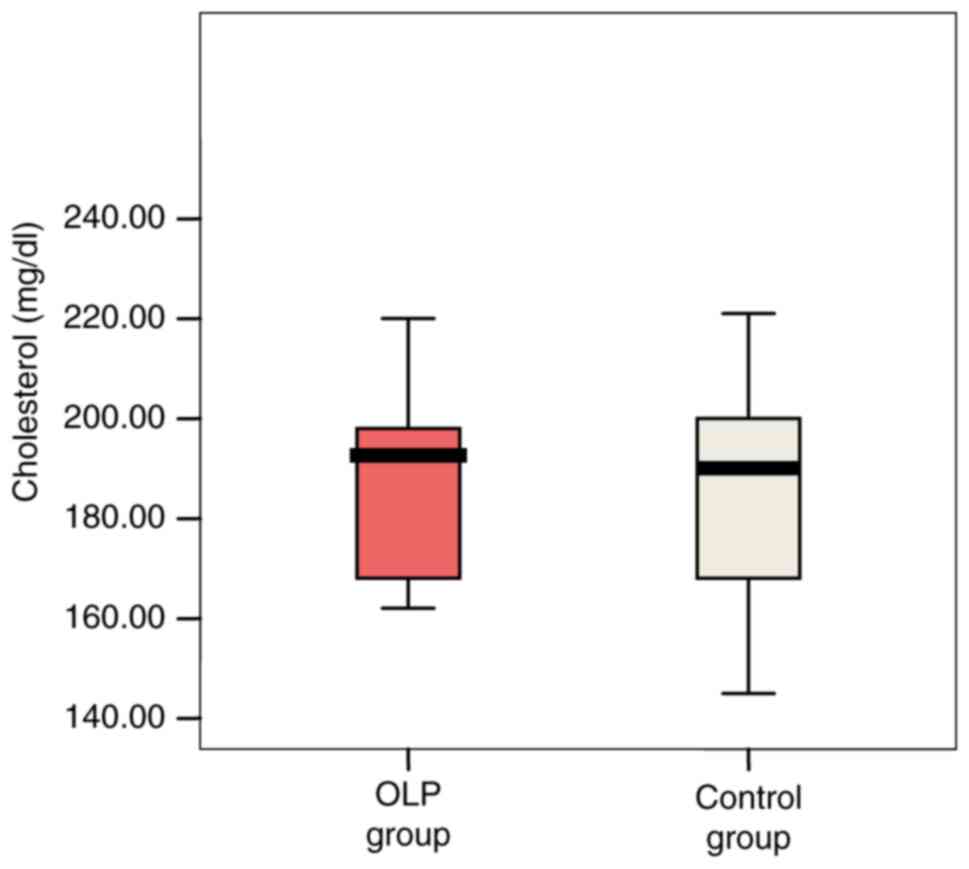
Cholesterol serum levels ranged between 145-298
mg/dl, extreme values being recorded in the OLP patient group. Mean
values were slightly higher in patients with OLP compared with
controls (194.78 vs. 194.72 mg/dl; P=0.921) (Fig. 4).
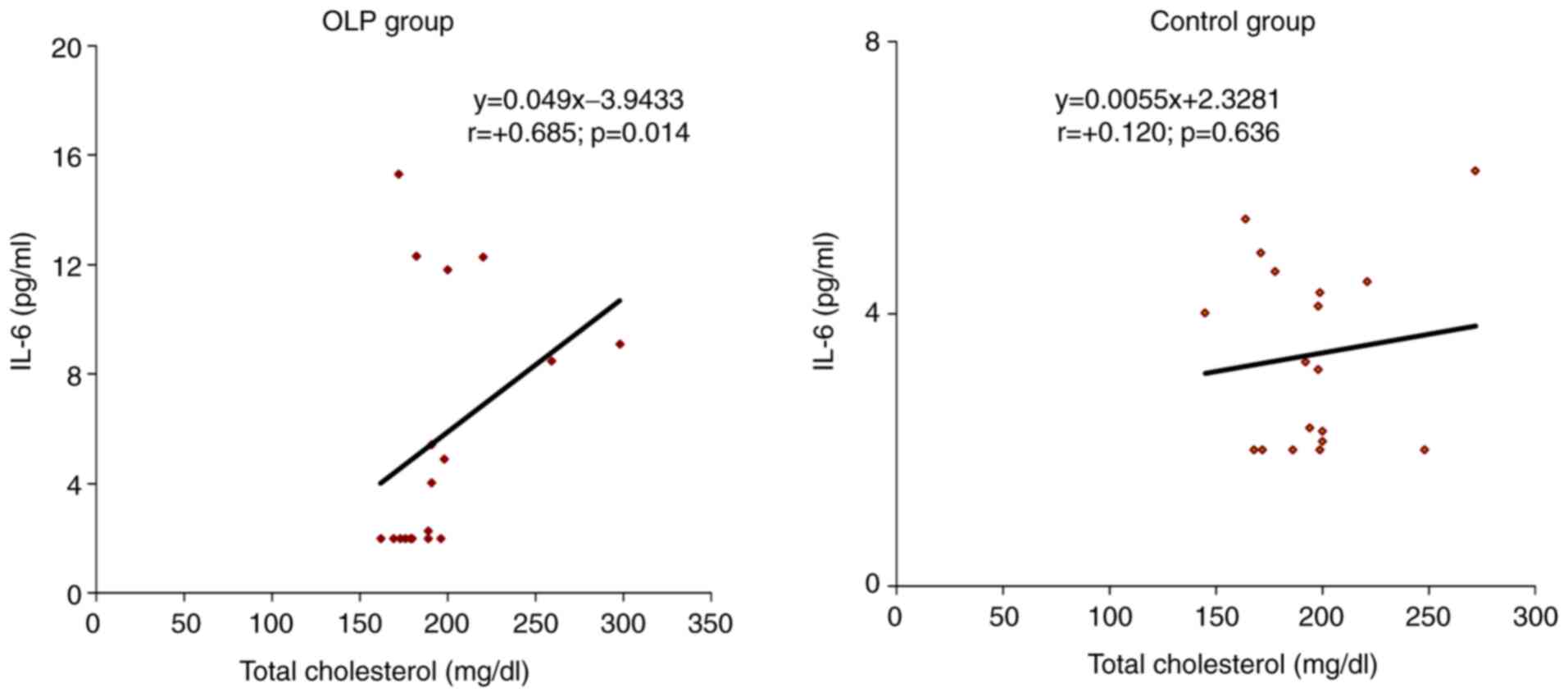
In the OLP group, the correlation of IL-6 with
individual values of serum total cholesterol was direct, of
moderate intensity (r=0.685; P=0.014), a result that can be
extrapolated to the general population. In the control group, the
correlation of IL-6 with total cholesterol serum values was also
direct but of low intensity (r=0.120; P=0.636) (Fig. 5).
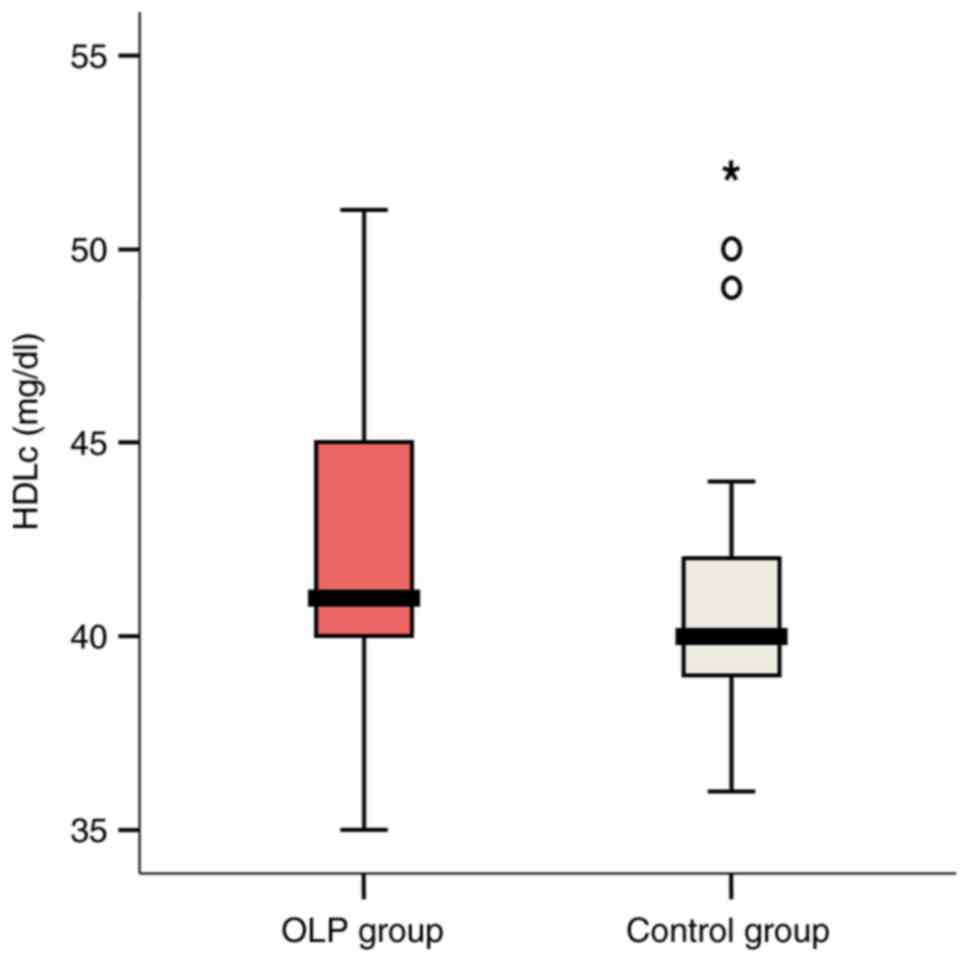
HDL-C serum values ranged between 35 and 54 mg/dl in
the OLP group. Pathological individual values were recorded in
27.8% of these patients vs. 72.2% of controls. Mean values were
slightly higher in OLP cases compared with controls (43.11 vs.
41.28 mg/dl; P=0.342) (Fig. 6).
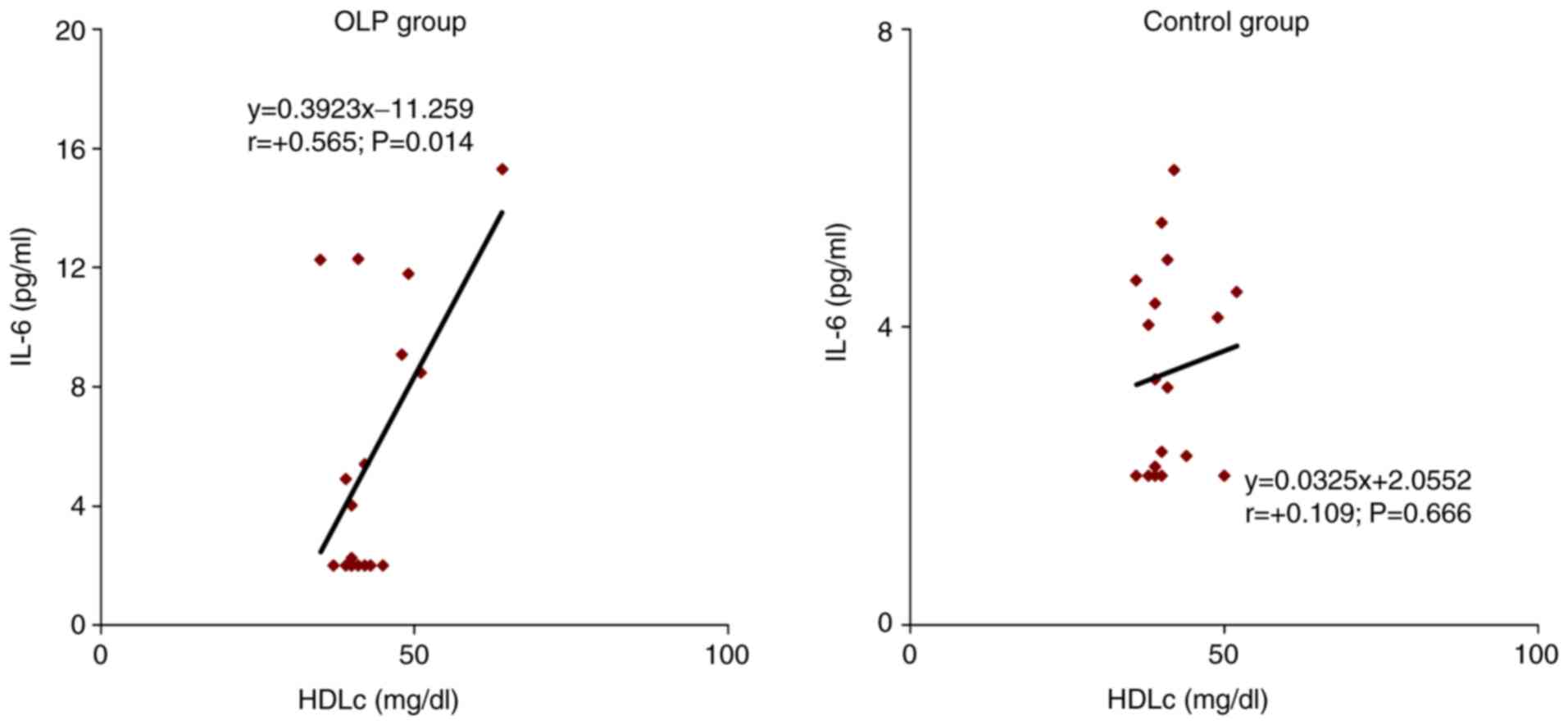
The correlation of IL-6 with individual values of
HDL-C in OLP patients was direct, of moderate intensity (r =0.565;
P=0.014). In 56.5% of cases, lower levels of HDL-C and high levels
of IL-6 were recorded. In the control group, the correlation of
IL-6 with HDL-C serum levels was also direct but of low intensity
(r=0.109; P=0.666) (Fig. 7).
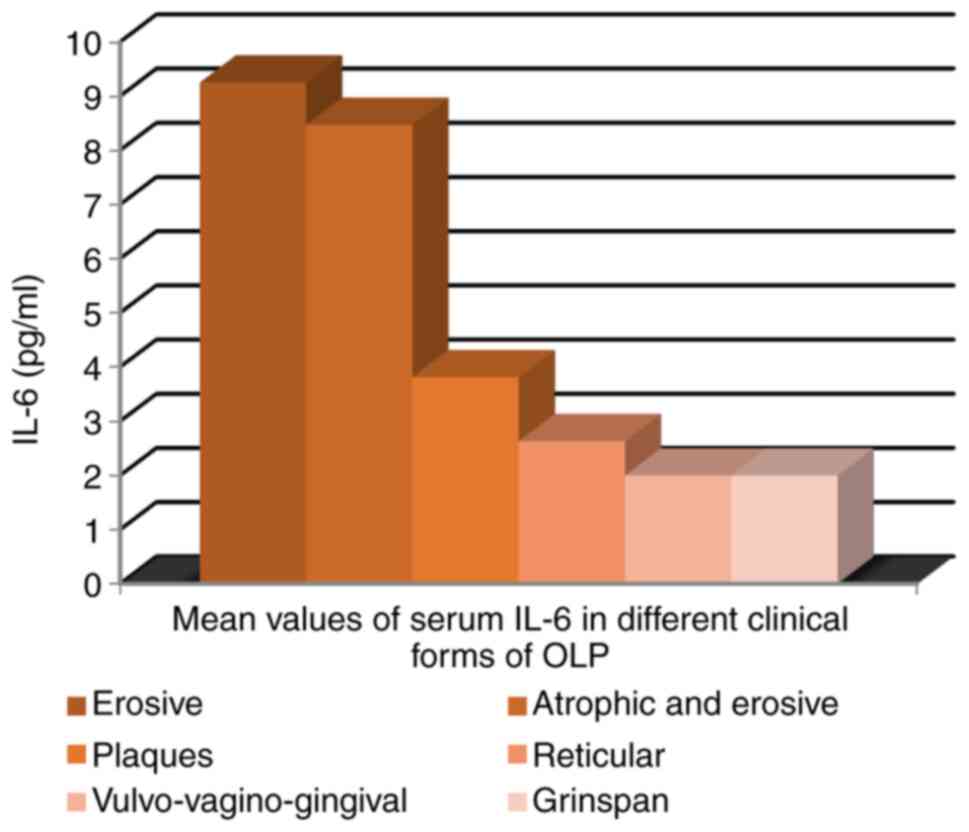
The highest IL-6 mean serum value was recorded in
patients with erosive lesions of OLP (9.25 pg/ml) and
atrophic-erosive clinical form (8.47 pg/ml) (Fig. 8).
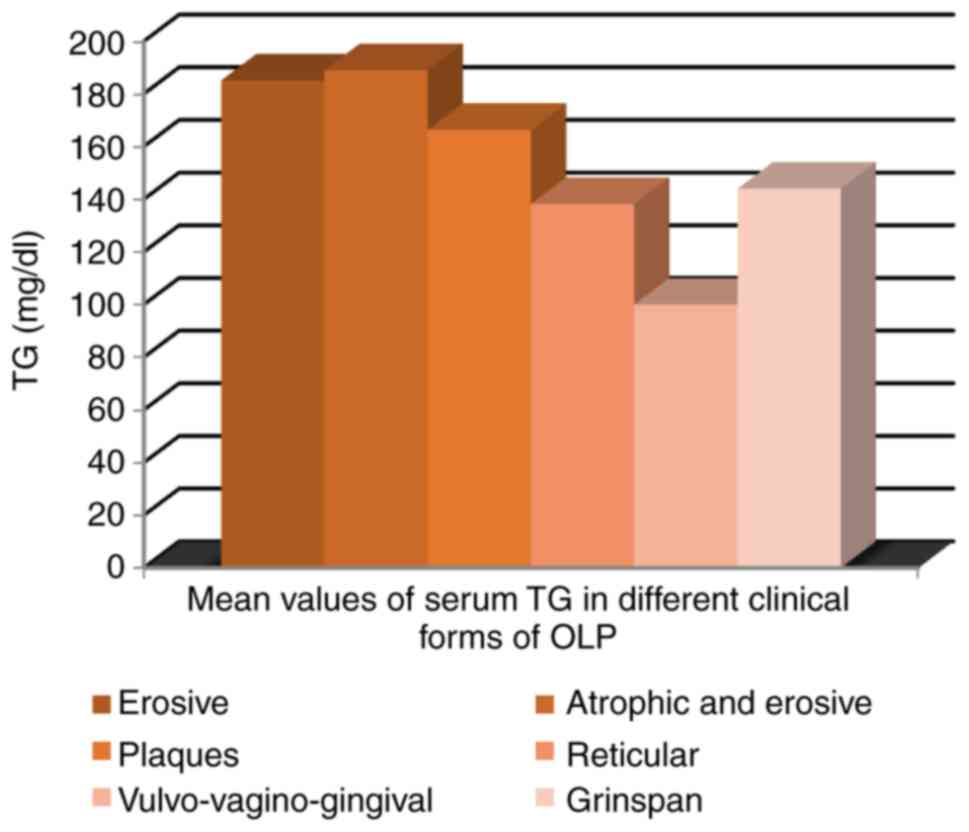
The mean value of serum TG was significantly
different depending on the clinical form of OLP; mean value was
higher in OLP with erosive and atrophic lesions, ranging from 189
mg/ml in atrophic-erosive form to 100 mg/ml in
vulvo-vagino-gingival syndrome (P=0.05) (Fig. 9).
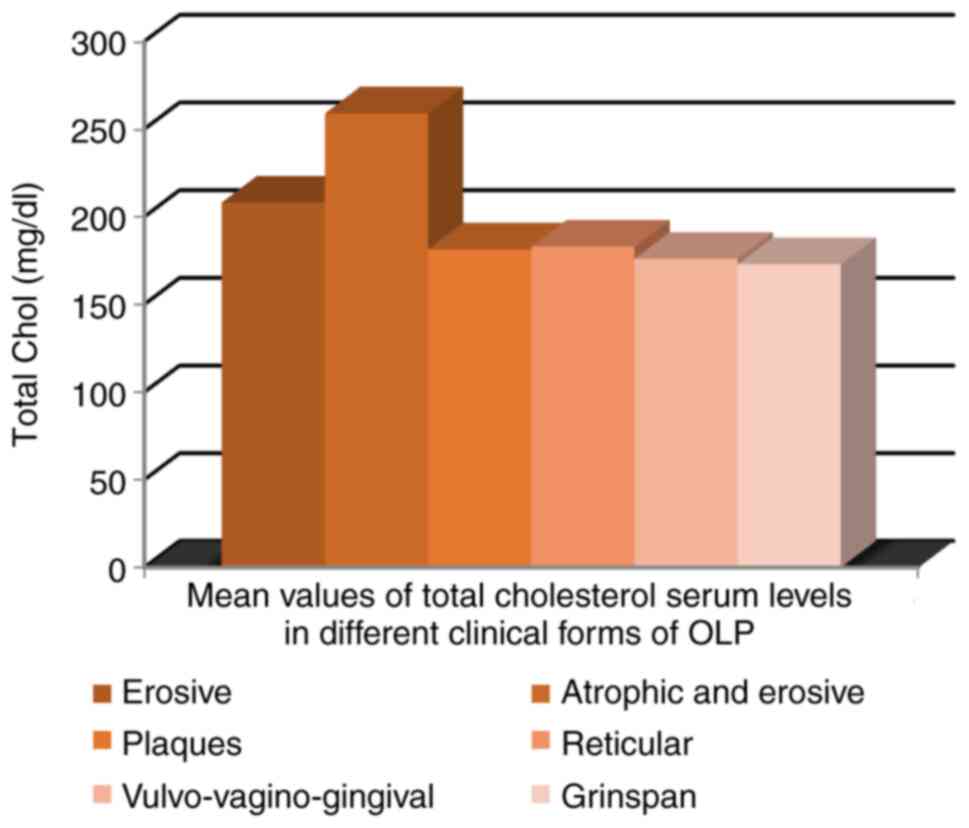
The highest mean value of serum total cholesterol
was recorded in patients with atrophic-erosive clinical forms (259
mg/dl). However, mean serum cholesterol values were not dependent
on the clinical aspect of OLP (Fig.
10).
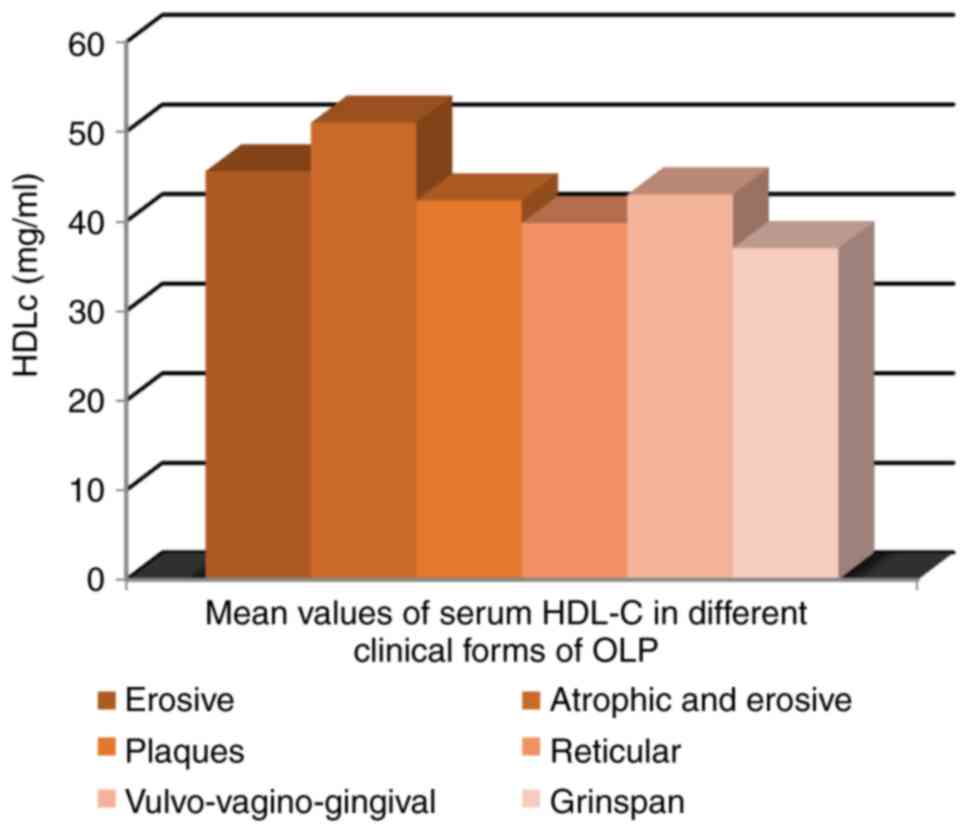
No significant differences were observed between
clinical forms of OLP with respect to mean serum HDL-C values
(Fig. 11).
There was an association between erosive clinical
forms of OLP and the pathological serum values of IL-6 and TG
respectively. Thus, these two parameters would be valuable
predictive factors of the clinical form of OLP. Serum levels of all
the studied parameters in relation to the clinical forms of OLP are
presented in Table I.
 | Table ISerum levels of IL-6 (pg/ml),
triglycerides (mg/dl), total cholesterol (mg/dl) and HDL
cholesterol (mg/dl) in relation with the clinical form of OLP. |
Table I
Serum levels of IL-6 (pg/ml),
triglycerides (mg/dl), total cholesterol (mg/dl) and HDL
cholesterol (mg/dl) in relation with the clinical form of OLP.
| | Serum IL-6 levels
(pg/ml) P=0.007 | Serum triglycerides
(mg/dl) P =0.05 | Serum total
cholesterol (mg/dl) P =0.259 | Serum HDL-C (mg/dl)
P =0.530 |
|---|
| Clinical form of
OLP | No. of
patients | Mean | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. |
|---|
| Erosive | 7 | 9.25 | <2.00 | 15.30 | 185.14 | 102.00 | 315.00 | 208.14 | 172.00 | 298.00 | 45.57 | 35.00 | 64.00 |
| Atrophic and
erosive | 1 | 8.47 | 8.47 | 8.47 | 189.00 | 189.00 | 189.00 | 259.00 | 259.00 | 259.00 | 51.00 | 51.00 | 51.00 |
| Plaques | 3 | 3.81 | <2.00 | 5.40 | 166.33 | 133.00 | 220.00 | 181.33 | 162.00 | 191.00 | 42.33 | 40.00 | 45.00 |
| Reticular | 5 | 2.63 | <2.00 | 4.90 | 138.20 | 98.00 | 169.00 | 183.00 | 169.00 | 198.00 | 39.80 | 39.00 | 41.00 |
|
Vulvo-vagino-gingival syndrome | 1 | 2.00 | 2.00 | 2.00 | 100.00 | 100.00 | 100.00 | 176.00 | 176.00 | 176.00 | 43.00 | 43.00 | 43.00 |
| Grinspan's
syndrome | 1 | 2.00 | 2.00 | 2.00 | 144.00 | 144.00 | 144.00 | 173.00 | 173.00 | 173.00 | 37.00 | 37.00 | 37.00 |
Discussion
OLP is the most frequent mucosal involvement of a
chronic inflammatory muco-cutaneous disease. Recent studies
emphasize the association between LP, dyslipidemia, systemic
inflammation and cardiovascular risk (11,13,14,25-28).
Alterations in serum plasma lipid profile occur normally during
inflammatory processes in order to annihilate the toxicity of
causative agents and promote tissue repair (16,17).
Although LP etiology and pathogenesis are still not completely
understood, current scientific data support the hypothesis of T
cell-mediated disease in which proinflammatory cytokines [e.g.,
tumor necrosis factor (TNF)α, interleukin (IL)-2, IL-4, IL-6,
IL-10] secreted by various cells including activated keratinocytes
and cytotoxic CD8+ T cells, participate in perpetuating
and persistence of inflammation (11). Recent research regarding
cardiovascular risk in psoriasis suggests that chronic inflammation
may be considered a component of the metabolic syndrome in which
activation of type 1 T helper cells is present. The upregulation of
type 1 T helper cells was observed in metabolic syndrome in
correlation with several cytokines such as IL-6 and TNFα (29-31).
IL-6 is a pleiotropic cytokine with a critical defense role in
acute inflammatory response during infections and posttraumatic
tissue injuries, promoting the synthesis of acute phase proteins
including reactive C protein, amyloid A and fibrinogen. This acute
phase response is associated with increased blood viscosity and
increased number of activated platelets. High plasma levels of
fibrinogen contributes to the decrease in serum levels of
HDL-cholesterol. Deposition of fibrinogen in the vascular
endothelium is stimulated by IL-6 activation and represents a risk
factor for cardiovascular morbidity (32). Recent research suggests IL-6 as an
important mediator of atherosclerosis, as a high serum level of
IL-6 is associated with the onset of acute coronary disease and
other ischemic conditions (33).
IL-6 is also involved in the upregulation of
terminal differentiation of B cells into immunoglobulin-producing
plasma cells and differentiation of naïve TCD4+ cells
into antigen-specific Th17 effector cells (17). It also downregulates regulatory T
cells leading to immune tolerance suppression and subsequent
development of autoimmune and inflammatory reaction (19). Disturbances in IL-6 secretion
resulting in high serum plasma levels and persistence of its
proinflammatory activity leads to the conversion of acute
inflammatory response to chronic inflammatory process (33). High serum levels of IL-6 have been
recorded in rheumatoid arthritis, systemic juvenile idiopathic
arthritis, systemic lupus erythematosus, psoriasis, Crohn disease
and ankylosing spondylitis (22,34).
Tocilizumab, a monoclonal humanized IgG1 class antibody which
inhibits IL-6 binding to its soluble and transmembrane receptors
was approved in over 100 countries for rheumatoid arthritis
treatment (35). The involvement of
IL-6 in general metabolic control was also demonstrated. Studies
suggest that in obese persons, IL-6 secretion in adipocytes
correlates with the size of these cells (18). Experimental research showed that
IL6-deficient mice developed glucose intolerance, systemic insulin
resistance, hepatic inflammation and mature-onset obesity (36,37,38).
Given the important pathogenic role of IL-6 in a multitude of
inflammatory, autoimmune and even proliferative conditions (e.g.,
multiple myeloma) we found it useful to study its involvement in
lipid metabolic disturbances in patients with OLP.
The current observational study demonstrated that in
patients with OLP, lipid serum profile changes are present and
there is a correlation between dyslipidemia and IL-6 as an
important marker of systemic inflammation. Thus, high IL-6 serum
levels were recorded in 33.3% cases of OLP vs. 5.6% in the control
group and mean IL-6 values were significantly higher in patients
with OLP (5.66 vs. 3.40 pg/ml; P=0.05). Our findings support other
studies that showed similar results, with IL-6 levels of serum and
saliva significantly higher in patients with OLP (13).
High serum TG levels were found in a higher
proportion OLP cases vs. controls, with TG mean values slightly
higher in OLP patients, but without statistical significance
(162.17 vs. 154.28 mg/dl; P=0.625). Total cholesterol levels were
higher in an equal proportion in the two groups (16.7%) but with
slightly higher mean values in the OLP group. Pathologic HDL-C
serum levels were found in 27.8% of OLP cases. Our findings
regarding dyslipidemia in OLP is in accordance with recent studies
suggesting that patients with OLP display more impaired lipid
metabolism alteration than patients with classic cutaneous lichen
planus (39).
We found that IL-6 serum levels in patients with OLP
correlated with all the studied parameters of lipid metabolism.
There was a significant direct and moderate correlation between
IL-6 and individual mean cholesterol values (r=0.685; P=0.014) in
the OLP group. The correlation of IL-6 with individual HDL-C values
was moderate and direct (r=0.565; P=0.014), result that can be
extrapolated to the general population. The correlation between
serum levels of IL-6 and TG in OLP cases was indirect (r=-0.113;
P=0.655) vs. the control group, where these parameters were
independent.
We must emphasize that the highest IL-6 mean serum
values were recorded in erosive and atrophic-erosive OLP lesions,
which can be explained by the long-lasting course of these clinical
forms (8-30 months) meaning a prolonged inflammatory status. TG
serum levels were also different in regards to the clinical forms
of OLP, the highest mean value (189 mg/ml) being recorded in
patients with atrophic-erosive lesions. Thus, we may consider IL-6
and triglycerides good predictive factors of the OLP clinical
form.
The present study has several limitations. The study
included a small number of patients and the period of survey was
short. We did not take into account other risk factors for
dyslipidemia (such as tobacco and alcohol consumption, diabetes
mellitus, sedentary lifestyle) and the presence of cardiovascular
and hepatic comorbidities in our patients.
In conclusion, the current observational
case-control study was designed and carried out to demonstrate the
correlation between OLP and systemic inflammation via the enhanced
production of IL-6, a biomarker of systemic inflammation and
disturbance of serum lipid profile. Our results suggest that this
chronic inflammatory condition may be considered a potential marker
of systemic inflammation and a cardiovascular risk factor. The
findings should be completed and interpreted in conjunction with
results of larger future studies aimed at other biomarkers of
systemic inflammation. However, OLP patients should be monitored
for anomalies of lipid metabolism and cardiovascular
comorbidities.
Acknowledgements
Not applicable.
Funding
Funding: The study was partly funded by a research grant from
the Romanian Society of Dermatology.
Availability of data and materials
The data that support the findings of this study are
available from the archives of the Railways University Hospital
Iasi, (Iasi, Romania), but restrictions apply to the availability
of these data which are not publicly available. Data are, however,
available from the authors upon reasonable request and with
permission from the Railways University Hospital Iasi.
Authors' contributions
MPT, TT and MMC conceived and supervised the study.
MPT, VVC and DO were responsible for the collection and analysis of
the experimental data. MM and ST performed the statistical
analysis, created the figures and drafted the manuscript. All
authors contributed equally to acquisition, analysis and
systematization of data, manuscript writing and critical revision
of the manuscript for important intellectual content. All the
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Railways University
Hospital Iasi (Iasi, Romania) approved the current study. Informed
written consent from all 36 enrolled patients was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Rossi SS and Ciarrocca K: Oral lichen
planus and lichenoid mucositis. Dent Clin North Am. 58:299–313.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Popovska M, Radojkova-Nikolovska V,
Minovska A, Agop Forna D, Muratovska I and Forna NC: Etiopathogenic
biochemical mechanism involved in oral lichen planus. Rev Chim.
66:1786–1790. 2015.
|
|
3
|
Ianosi SL, Forsea AM, Lupu M, Ilie MA,
Zurac S, Boda D, Ianosi G, Neagoe D, Tutunaru C, Popa CM and
Caruntu C: Role of modern imaging techniques for the in vivo
diagnosis of lichen planus. Exp Ther Med. 17:1052–1060.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lupu M, Caruntu A, Caruntu C, Boda D,
Moraru L, Voiculescu V and Bastian A: Non-invasive imaging of
actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin
Oncol. 8:640–646. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lupu M, Căruntu A, Moraru L, Voiculescu
VM, Boda D, Tanase C and Căruntu C: Non-invasive imaging techniques
for early diagnosis of radiation-induced squamous cell carcinoma of
the lip. Rom J Morphol Embryol. 59:949–953. 2018.PubMed/NCBI
|
|
6
|
Calenic B, Greabu M, Caruntu C, Nicolescu
MI, Moraru L, Surdu-Bob CC, Badulescu M, Anghel A, Logofatu C and
Boda D: Oral keratinocyte stem cells behavior on diamond like
carbon films. Rom Biotechnol Lett. 21:11914–11922. 2016.
|
|
7
|
Boda D, Docea AO, Calina D, Ilie MA,
Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE,
Voiculescu V, et al: Human papilloma virus: Apprehending the link
with carcinogenesis and unveiling new research avenues. Int J
Oncol. 52:637–655. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boda D, Neagu M, Constantin C, Voinescu
RN, Caruntu C, Zurac S, Spandidos DA, Drakoulis N, Tsoukalas D and
Tsatsakis AM: HPV strain distribution in patients with genital
warts in a female population sample. Oncol Lett. 12:1779–1782.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Solomon I, Voiculescu VM, Caruntu C, Lupu
M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine factors and head and neck squamous cell
carcinoma: An affair to remember. Dis Markers.
2018(9787831)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Giuliani M, Troiano G, Cordaro M,
Corsalini M, Gioco G, Lo Muzio L, Pignatelli P and Lajolo C: Rate
of malignant transformation of oral lichen planus: A systematic
review. Oral Dis. 25:693–709. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aniyan KY, Guledgud MV and Patil K:
Alterations of serum lipid profile patterns in oral lichen planus
patients: A case-control study. Contemp Clin Dent. 9 (Suppl
1):S112–S121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sezer E, Ozugurlu F, Ozyurt H, Sahin S and
Etikan I: Lipid peroxidation and antioxidant status in lichen
planus. Clin Exp Dermatol. 32:430–434. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lai YC, Yew YW and Schwartz RA: Lichen
planus and dyslipidemia: A systematic review and meta-analysis of
observational studies. Int J Dermatol. 55:e295–e304.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ozbagcivan O, Akarsu S, Semiz F and Fetil
E: Comparison of serum lipid parameters between patients with
classic cutaneous lichen planus and oral lichen planus. Clin Oral
Investig. 24:719–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arias-Santiago S, Eisman AB, Fernandez JA,
Girón-Prieto MS, Gutiérrez-Salmerón MT, García Mellado V and
Naranjo-Sintes R: Cardiovascular risk factors in patients with
lichen planus. Am J Med. 124:543–548. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Esteve E, Ricart W and Fernández-Real JM:
Dyslipidemia and inflammation: An evolutionary conserved mechanism.
Clin Nutr. 24:16–31. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krishnamoorthy B, Suma GN, Mamatha NS,
Sowbhagya MB and Komali Garlapati: Lipid profile and metabolic
syndrome status in patients with oral lichen planus, oral lichenoid
reactions and healthy individuals attending a dental college in
Northern India - a descriptive study. J Clin Diagn Res.
8:ZC92–ZC95. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hotamisigil GS: Inflammation and metabolic
disorders. Nature. 444:860–867. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Scheller J, Chalaris A, Schmidt-Aras D and
Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Act. 1813:878–888.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xing Z, Gauldie J, Cox G, Baumann H,
Jordana M, Lei XF and Achong MK: IL6 is an antiinflammatory
cytokine required for controlling local or systemic acute
inflammatory responses. J Clin Invest. 101:311–320. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Paquet P and Piérard GE: Interleukin-6 and
the skin. Int Arch Allergy Immunol. 109:308–317. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lipsky PE: Interleukin-6 and rheumatic
diseases. Arthritis Res Ther. 8 (Suppl 2)(S4)2006.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Khishimoto T: Interleukin-6: Discovery of
a pleiotropic cytokine. Arthritis Res Ther. 8 (Suppl
2)(S2)2006.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Patil S, Roopa SR, Sanketh DS, Sarode SC
and Sarode GS: A universal diagnostic criteria for oral lichen
planus: An exigency! Int J Contemp Dental Medical Rev. 1–4.
2014.
|
|
25
|
Baykal L, Arıca DA, Yaylı S, Örem A,
Bahadır S, Altun E and Yaman H: Prevalence of metabolic syndrome in
patients with lichen planus. A case-control study. Am J Clin
Dermatol. 16:439–445. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Romero MA, Seoane J, Varela-Centelles P,
Diz-Dios P and Garcia-Pola MJ: Prevalence of diabetes mellitus
amongst oral lichen planus patients. Clinical and pathological
characteristics. Med Oral. 7:121–129. 2002.PubMed/NCBI(In English, Spanish).
|
|
27
|
Eisen D: The clinical features, malignant
potential and systemic associations of oral lichen planus: A study
of 723 patients. J Am Acad Dermatol. 46:207–214. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lopez-Jornet P, Alonso CF and
Rodríguez-Martines MA: Alterations in serum lipid profile patterns
in oral lichen planus. A cross-sectional study. Am J Clin Dermatol.
13:399–404. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Neimann AL, Shin DB, Wang X, Margolis DJ,
Troxel AB and Gelfand JM: Prevalence of cardiovascular risk factors
in patients with psoriasis. J Am Acad Dermatol. 55:829–835.
2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sommer DM, Jenish S, Suchan M,
Christophers E and Weichenthal M: Increased prevalence of the
metabolic syndrome in patients with moderate to severe psoriasis.
Arch Dermatol Res. 298:321–328. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Grechin C, Solovăstru LG, Vâță D, Pătrașcu
AI, Grăjdeanu AI and Porumb-Andrese E: Inflammatory marker
alteration in response to systemic therapies in psoriasis. Exp Ther
Med. 20:42–46. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Boda D and Dehelean C:
Immuno-dermatological processes involved in chronic skin diseases:
Highlights of the Second Conference of the Romanian Society for
Immuno-Dermatology, Bucharest. Exp Ther Med. 18:873–874.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Narazaki M and Kishimoto T: The two-faced
cytokine IL-6 in host defenseand diseases. Int J Mol Sci.
19(3528)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pauli N, Puchałowicz K, Kuligowska A,
Krzystolik A, Dziedziejko V, Safranow K, Rać M, Chlubek D and Rać
ME: Associations between IL-6 and echo-parameters in patients with
early onset coronary artery disease. Diagnostics (Basel).
9(189)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gabay C: Interleukin-6 and chronic
inflammation. Arthritis Res Ther. 8 (Suppl 2)(S3)2006.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Tanaka T, Narazaki M, Masuda K and
Kishimoto T: Interleukin-6: Pathogenesis and treatment of
autoimmune inflammatory diseases. Inflam Regeneratin. 33:54–65.
2013.
|
|
37
|
Tanaka T, Narazaki M and Kishimoto T:
Therapeutic targeting of interleukin-6 receptor. Ann Rev Pharmacol
Toxicol. 52:199–219. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wallenius V, Wallenius K, Ahrén B, Rudling
M, Carlsten H, Dickson SL, Ohlsson C and Jansson JO: Interleukin-6
deficient mice develop mature-onset obesity. Nat Med. 8:75–79.
2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matthews VB, Allen TL, Risis S, Chan MH,
Henstridge DC, Watson N, Zaffino LA, Babb JR, Boon J, Meikle PJ, et
al: Interleukin-6-deficient mice develop hepatic inflammation and
systemic insulin resistance. Diabetologia. 53:2431–2441.
2010.PubMed/NCBI View Article : Google Scholar
|