Introduction
Anti-neutrophil cytoplasmic antibody
(ANCA)-associated vasculitis (AAV) is characterized as necrotizing
inflammation of small-sized to medium-sized blood vessels. AAV is a
common cause of chronic kidney disease in China, which is a major
public health problem (1-4).
ANCAs usually have specificity for either myeloperoxidase (MPO) or
proteinase 3 (PR3). A total of four major phenotypes of AAV have
been described: Microscopic polyangiitis (MPA), granulomatosis with
polyangiitis (GPA), eosinophilic GPA (EGPA) and renal-limited
vasculitis. Although the pathogenesis of AAV remains elusive,
accumulating evidence suggests that chronic inflammation has an
essential role in AAV (5-7).
Numerous different markers of inflammation,
including C-reactive protein (CRP) and the erythrocyte
sedimentation rate (ESR), have been used to assess inflammatory
status in AAV (6). Hu et al
(8) developed a novel systemic
immune-inflammation index (SII) based on lymphocyte, neutrophil and
platelet counts and demonstrated that the SII is a useful
prognostic indicator of poor outcome in patients with
hepatocellular carcinoma. Since then, the SII has been reported to
be a potential prognostic indicator in patients with various
different types of tumor and to be associated with poor patient
outcomes (9). There is currently a
paucity of information regarding the clinical role of SII in
patients with AAV (8). Furthermore,
it has been demonstrated that there are numerous differences
between patients with PR3-AAV and those with MPO-AAV and there is
evidence that PR3-AAV and MPO-AAV may be two distinct diseases
(10-12).
The major phenotype of AAV and the major target antigen of ANCAs in
Chinese patients with AAV are quite different from those in Western
populations. There is a striking preponderance of MPA in Chinese
patients with AAV (13).
Accordingly, MPO is the major target antigen of ANCA in Chinese
patients with AAV (13).
Therefore, the present study aimed to analyze the
relationship between SII at diagnosis and inflammatory response and
disease activity among patients with MPO-AAV at a single center in
China. Furthermore, it was evaluated whether the SII was able to
predict the progression to end-stage renal disease (ESRD) and
mortality in patients with MPO-AAV in this study.
Patients and methods
Patients
The present single-center, retrospective
observational study included all patients with MPO-AAV who were
diagnosed between January 2009 and November 2018 at the Department
of Nephrology and the Department of Rheumatology and Immunology of
Xiangya Hospital (Changsha, China). All patients with AAV (n=190)
met the 2012 revised Chapel Hill Consensus Conference criteria for
AAV and were then reclassified according to the algorithm published
by the European Medicines Agency (14,15).
Patients with any of the following conditions were excluded: i)
EGPA or secondary vasculitis; ii) Comorbid kidney diseases, such as
anti-glomerular basement membrane nephritis, IgA nephropathy,
membranous nephropathy or diabetic nephropathy; and iii) Hepatitis
b virus, hepatitis C virus or HIV infection. The study protocol was
in accordance with the Declaration of Helsinki and was approved by
the Ethics Committee of Xiangya Hospital (Changsha, China).
Baseline demographic data and laboratory parameters
were extracted from the electronic medical record system of the
hospital. The estimated glomerular filtration rate (eGFR) was
calculated as described previously (16). Vasculitis activity was assessed by
determining the Birmingham vasculitis activity score (BVAS)
(17). The serum ANCA level was
detected by both indirect immunofluorescence assay (Euroimmun; cat.
no. FA 1201-1005) and antigen-specific ELISA (Inova Diagnostics;
cat. nos. 708700 and 704660) for PR3-ANCAs and MPO-ANCAs according
to the manufacturer's instructions in all patients.
Renal histology
The renal biopsy specimens were evaluated using
immunofluorescence, light microscopy and electron microscopy. As
proposed by Berden et al (18), the biopsy specimens were assigned to
4 categories. All the specimens met the requirement of a minimum of
10 whole glomeruli (18).
Tubulointerstitial lesions were graded semiquantitatively, as
previously reported (19).
Treatment
As described previously, all patients received
standard induction therapy, including oral prednisone combined with
cyclophosphamide (CTX) (20). Oral
prednisone was prescribed at an initial dosage of 1 mg/kg/day for
4-6 weeks, with tapering over time to 12.5-15 mg by 3 months. In
general, prednisone therapy should not last longer than 24 months.
CTX was prescribed intravenously at 0.5-0.75 g/m2 once a
month or a daily oral dose of 2 mg/kg/day. A 25% dose reduction of
CTX was prescribed for those who were older than 65 years or those
with GFR <20 ml/min/1.73 m2, and CTX was temporarily
discontinued for those who developed leukocytopenia (number of
leukocytes <4x109/l). Certain patients with rapidly
progressive glomerulonephritis or pulmonary hemorrhage received
methylprednisolone pulse therapy and/or plasma exchange prior to
standard induction therapy. Intravenous CTX every 3 months or daily
oral azathioprine or mycophenolate mofetil was given during
maintenance therapy.
Patients were evaluated at the time of diagnosis, at
1, 2 and 3 months, and then every 3 months until the end of the
study. The patients were followed up until their death, progression
to ESRD or the final follow-up date (May 31, 2019). ESRD was
defined by dialysis dependence for >3 months or kidney
transplantation. All follow-up data were collected from the
hospital's electronic medical records and by contacting the
individual patients directly.
Statistical analysis
The SII was calculated as follows: SII=PxN/L, where
P, N and L are the absolute peripheral cell counts of platelets,
neutrophils and lymphocytes, respectively. The laboratory data of
absolute peripheral cell counts were collected from the most recent
routine blood test prior to the commencement of immunosuppressive
induction therapy, usually within 3 days before the beginning of
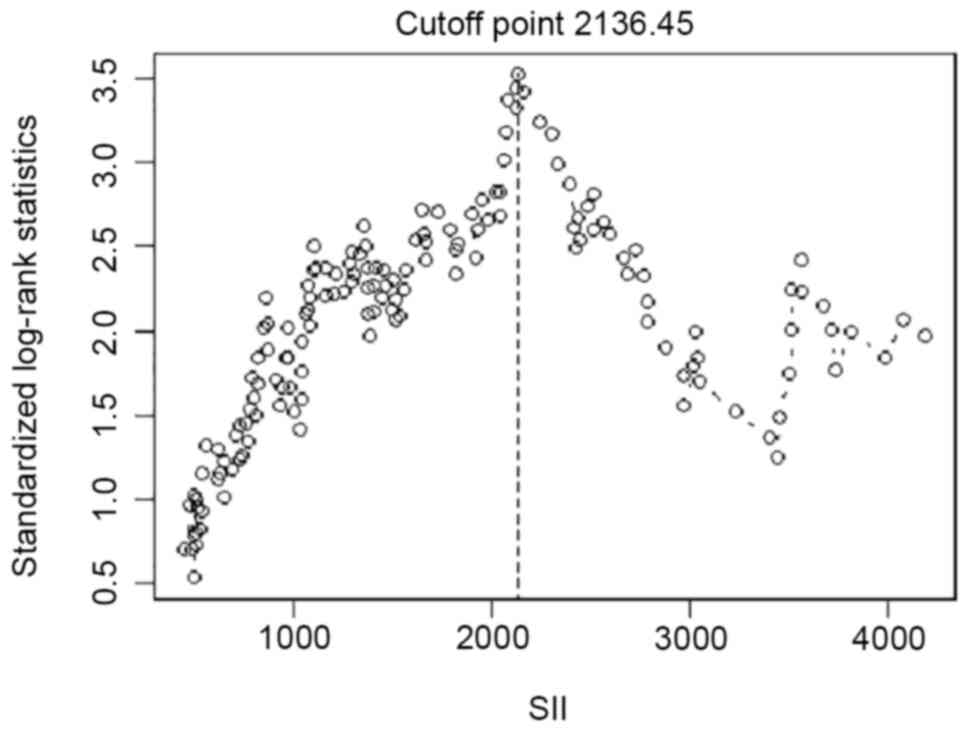
induction therapy. The cutoff point for the SII level was selected
by identifying the maximum log-rank statistic (21). Quantitative data were expressed as
the mean ± standard deviation, median (interquartile range) or n
(%). All analyses were performed using SPSS software (version 23.0;
IBM Corp.) and R for statistical computing (version 3.5.1). The
Kolmogorov-Smirnov test was used to check the normality of data
distribution. Differences between the two groups were evaluated by
one-way analysis of variance or the Kruskal-Wallis test for
continuous variables and the χ2 test or Fisher's test
for categorical variables. To examine the correlation between two
continuous variables, the Spearman correlation coefficient was
calculated. Kaplan-Meier curves and log-rank tests were used to
analyze patient and renal survival. P<0.05 was considered to
indicate statistical significance.
Results
Patient characteristics. The cut-off point
for the SII for ESRD was determined as 2,136.45 (Fig. 1). The baseline characteristics of
patients with MPO-AAV based on a dichotomy of baseline SII are
presented in Table I. A higher
baseline SII was associated with older age, higher serum levels of
alanine transaminase, aspartate transaminase, total bilirubin,
direct bilirubin, CRP, ESR and eGFR and lower serum levels of
albumin, IgM and serum creatinine.
 | Table IBaseline demographic characteristics
of patients with myeloperoxidase-anti-neutrophil cytoplasmic
antibody-associated vasculitis according to SII. |
Table I
Baseline demographic characteristics
of patients with myeloperoxidase-anti-neutrophil cytoplasmic
antibody-associated vasculitis according to SII.
| Variable | SII<2,136.45 | SII≥2,136.45 | P-value |
|---|
| Age (years) | 57.41±15.56 | 61.60±11.69 | 0.040 |
| Males/females | 67/61 | 38/24 | 0.245 |
| MPA/GPA/RLV | 121/2/5 | 54/5/3 | 0.078 |
| Median follow-up
(months) | 16.5
(7.75,29.25) | 17 (6,34) | 0.601 |
| White blood cells
(109/l) | 8.23±3.31 | 13.60±5.26 | <0.0001 |
| Hemoglobin (g/l) | 81.46±20.20 | 82.53±20.90 | 0.735 |
| Platelet
(109/l) | 222.14±101.82 | 310.69±107.32 | <0.0001 |
| Neutrophil
(109/l) | 6.18±2.86 | 12.00±5.69 | <0.0001 |
| Lymphocyte
(109/l) | 1.50±1.07 | 0.93±0.47 | <0.0001 |
| Serum albumin
(g/l) | 36.05
(28.38,59.55) | 30 (25.05,57.45) | 0.049 |
| Serum globulin
(g/l) | 30.1
(26.18,35.5) | 28.6
(25.65,36.85) | 0.323 |
| Alanine transaminase
(U/l) | 11.6 (7.7,18.43) | 22.8
(12.75,35.95) | <0.0001 |
| Aspartate
transaminase (U/l) | 17.7
(14.03,26.2) | 25.4
(17.75,35.45) | <0.0001 |
| Total bilirubin
(µmol/l) | 5.65
(4.08,7.0) | 6.2 (4.65,8.9) | 0.007 |
| Direct bilirubin
(µmol/l) | 2.5 (1.8,3.2) | 2.8 (2,4.15) | 0.007 |
| Proteinuria
(g/d) | 1.065
(0.50,1.86) | 0.99
(0.555,1.5) | 0.880 |
| Serum creatinine
(µmol/l) | 409.75
(195.95,642.75) | 281
(103.15,484.7) | 0.002 |
| eGFR (ml/min/1.73
m2) | 12.115
(8.18,31.15) | 23.09
(10.65,61.17) | 0.005 |
| ESR (mm/h) | 64
(37.75,95.25) | 81 (51.5,120) | 0.030 |
| CRP (mg/l) | 13.5
(4.7,42.9225) | 75.4
(22.05,116) | <0.0001 |
| C3 (mg/l) | 799.09±243.29 | 792.68±301.69 | 0.883 |
| C4 (mg/l) | 229.66±88.67 | 281.43±239.37 | 0.132 |
| IgA (mg/l) |
2,566.85±1,212.17 |
2,581.89±1,356.20 | 0.943 |
| IgG (g/l) | 13.95±4.58 | 15.03±5.11 | 0.171 |
| IgM (mg/l) |
1,224.26±841.65 | 966.80±542.51 | 0.043 |
| Organ
involvement | | | |
|
Kidney | 122 (95.31) | 59 (95.16) | 0.963 |
|
Pulmonary | 71 (55.47) | 36 (58.06) | 0.735 |
|
Cardiovascular | 21 (16.41) | 10 (16.13) | 0.961 |
|
Nervous
system | 30 (23.44) | 19 (31.15) | 0.873 |
| BVAS | 16 (11.75,20) | 15 (11.5,17) | 0.258 |
| EUVAS
classification | | | |
|
Focal | 3 | 1 | 0.801 |
|
Mixed | 19 | 4 | |
|
Crescentic | 16 | 4 | |
|
Sclerotic | 11 | 1 | |
| Tubulointerstitial
injury score | | | |
|
0 | 0 | 0 | 0.319 |
|
1 | 27 | 5 | |
|
2 | 15 | 5 | |
|
3 | 7 | 0 | |
Correlations of SII with clinical
disease activity and clinical characteristics of patients with
MPO-AAV
As presented in Table
II, SII was positively correlated with CRP (r=0.274,
P<0.0001) and ESR (r=0.481, P<0.0001). The SII had no
significant correlation with the BVAS (r=-0.024, P=0.737).
 | Table IICorrelations of the systemic
immune-inflammation index with laboratory findings in patients with
myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated
vasculitis. |
Table II
Correlations of the systemic
immune-inflammation index with laboratory findings in patients with
myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated
vasculitis.
| Parameter | r | P-value |
|---|
| White blood
cells | 0.634 | <0.0001 |
| HB | -0.024 | 0.743 |
| PLT | 0.499 | <0.0001 |
| N | 0.735 | <0.0001 |
| Lymphocytes | -0.414 | <0.0001 |
| ESR | 0.274 | <0.0001 |
| CRP | 0.481 | <0.0001 |
| C3 | 0.001 | 0.993 |
| C4 | 0.006 | 0.936 |
| BVAS | -0.024 | 0.737 |
Incidence rates of ESRD are associated
with SII
It was evaluated whether the SII at diagnosis is
able to predict all-cause mortality and ESRD in patients with
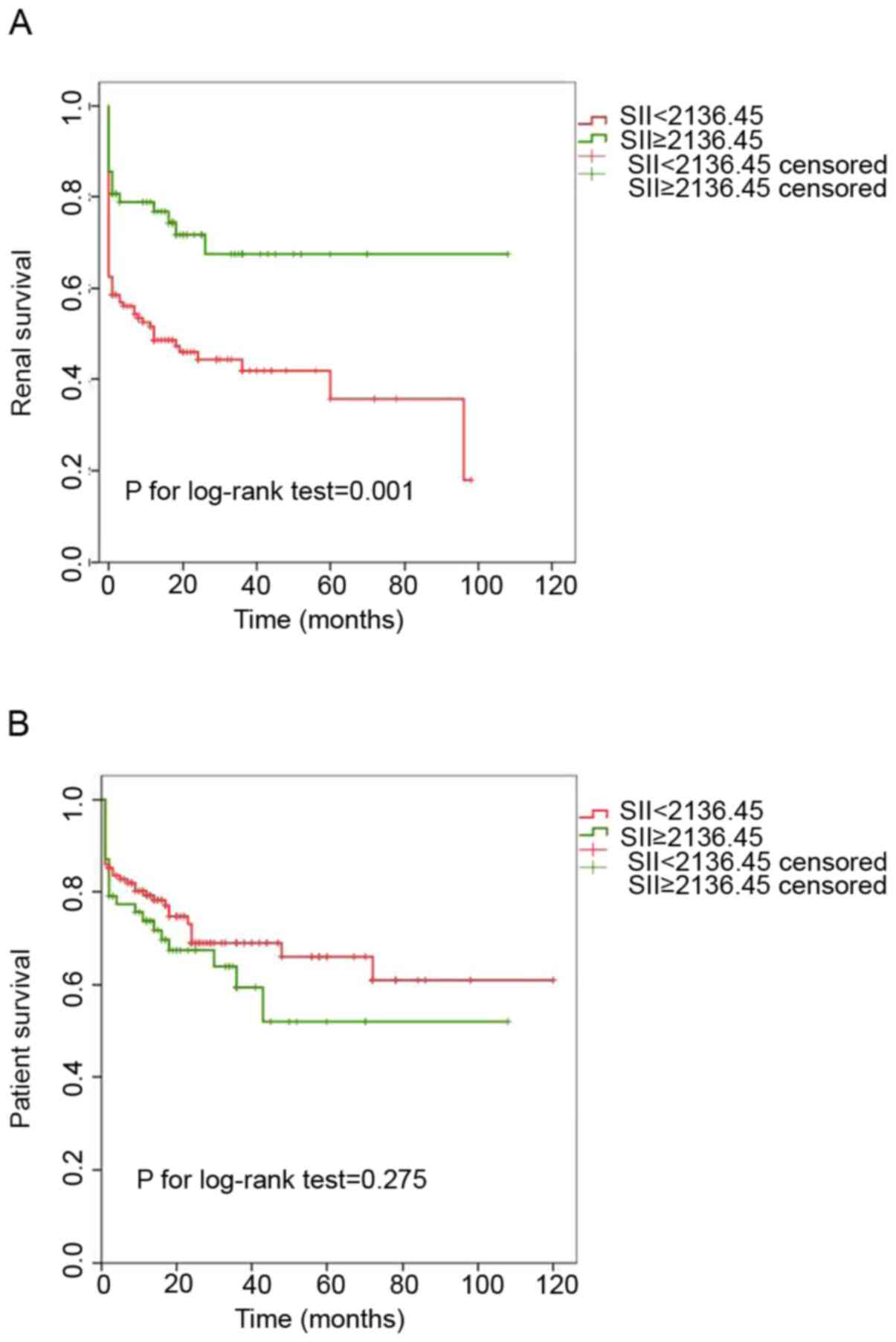
MPO-AAV during follow-up. Kaplan-Meier analysis of cumulative renal
survival and patient survival rates are provided in Fig. 2. Patients with SII≥2,136.45
exhibited better cumulative renal survival rates than those with
SII<2,136.45 (P=0.001). However, no significant difference in
the cumulative patient survival rates between patients with
SII≥2,136.45 and those with SII<2,136.45 at diagnosis was
obtained (P=0.275).
Discussion
The key finding of the present study was that a
higher SII in patients with MPO-AAV was able to predict a decreased
risk of ESRD. Another important result was that the SII was
positively correlated with CRP levels and the ESR in patients with
MPO-AAV. These results indicated that the SII may reflect the
inflammatory response and estimate prognosis during the follow-up
of patients with MPO-AAV.
With accumulating evidence suggesting the
detrimental role of inflammation in the pathogenesis of AAV,
researchers have focused on evaluating the value of inflammatory
markers in this disease. Two typical noninvasive markers of
inflammation, CRP and ESR, are widely used. The SII is a relatively
novel inflammatory index in routine blood tests. The calculation of
SII depends on absolute cell numbers of neutrophils, lymphocytes
and platelets. Neutrophils have been proven to be the key effector
cells in the pathogenesis of MPO-AAV in animal models (22). In vitro experiments using
human neutrophils and clinical evidence also suggested that
neutrophils have key roles in the pathogenesis of human MPO-AAV
(23). A decrease in the absolute
lymphocyte number has been reported in various autoimmune diseases
and an increase in the platelet count has been reported in patients
with active AAV (24,25). Therefore, it may be assumed that the
SII is correlated with an inflammatory response in patients with
AAV. The results of the present study, which suggested that the SII
was positively correlated with CRP and ESR in patients with
MPO-AAV, support this hypothesis. The present results suggested
that the SII may be a potential index reflecting inflammatory
response in MPO-AAV. However, no significant relationship between
the SII and BVAS was observed in the present study, which suggested
that the value of the SII in predicting and evaluating disease
activity in patients with MPO-AAV remains elusive.
The maximally selected log-rank statistical analysis
rather than the receiver operating characteristic curve analysis
was applied to the continuous variable in order to estimate the
most appropriate cut-off values for splitting patients into groups
with different renal survival probabilities (26). The decreased likelihood of ESRD in
patients with a higher SII in comparison with that in patients with
a lower SII is in accordance with our clinical experience. However,
this result is in contrast with data from a Korean cohort that
demonstrated that patients with higher levels of SII exhibited
significantly lower renal survival than those with lower levels of
SII at diagnosis (27). The most
plausible explanation for this difference is that a higher baseline
SII was associated with lower levels of serum creatinine in the
present study. The severity of renal dysfunction at presentation
has been demonstrated to be a key negative prognostic factor for
renal survival (28). Another
explanation may be that the SII is a potential index for reflecting
the inflammatory response in MPO-AAV, as mentioned above. Thus, the
phenomenon resembles what is observed in malignant disease, where
highly active proliferating cells are more sensitive to initial
chemotherapy (29), and findings in
patients with lupus nephritis, where patients having the highest
activity index in kidney biopsies are most likely to enter
remission (30).
The present study has several limitations. First,
the duration of the follow-up was relatively short. Furthermore,
with the retrospective nature of data collection, the maintenance
immunosuppressive regimen was not the same in all patients. In
addition, the number of patients with MPO-AAV in the present study
was relatively small. Therefore, a large cohort of patients is
required to validate the present results in future studies.
In conclusion, the SII was positively correlated
with CRP levels and the ESR in Chinese patients with MPO-AAV.
Furthermore, the SII may independently predict a reduced risk of
progression to ESRD.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Research and
Development Program of Hunan province (grant nos. 2020WK2008 and
2018WK2060), the National Natural Science Foundation of China
(grant no. 81800641), the Natural Science Foundation of Hunan
Province (grant nos. 2018JJ3853 and 2018JJ3818), the Project of
Health Commission of Hunan province (grant no. C2019184), the
Chinese Society of Nephrology (grant no. 18020010780).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JBC, RT, LH, YZ, XCX, QLZ and PX designed the study,
analyzed the data and wrote the manuscript. JBC, RT, YOZ, XXZ, HL,
TW, YQY, TM, ZX, WL, XA, QLZ and PX contributed to patient
enrollment and follow-up. JBC, RT and YZ analyzed the data. JBC and
YZ checked and confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics review
committee of Xiangya Hospital Central South University (Changsha,
China; reference. no. 20101006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu T, Peng J, Meng T, Liu Q, Ao X, Lin W,
Yin H, Chen J, Pu J, Peng Z, et al: Clinicopathological features
and prognostic analysis of 49 cases with crescentic
glomerulonephritis. Exp Ther Med. 18:3984–3990. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang L, Zhong Y, Ooi JD, Zhou YO, Zuo X,
Luo H, Chen JB, Wu T, Yang Y, Meng T, et al: The effect of pulse
methylprednisolone induction therapy in Chinese patients with
dialysis-dependent MPO-ANCA associated vasculitis. Int
Immunopharmacol. 76(105883)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhong Y, Pu J, Ao X, Peng W, Peng Z, Li X,
Xiao X, Zhou Q and Xiao P: Investigation of status for vascular
access in hemodialysis patients at Xiangya hospital of Central
South university. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
42:1270–1274. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
4
|
Peng Z, Wang J, Yuan Q, Xiao X, Xu H, Xie
Y, Wang W, Huang L, Zhong Y, Ao X, et al: Clinical features and
CKD-related quality of life in patients with CKD G3a and CKD G3b in
China: Results from the Chinese cohort study of chronic kidney
disease (C-STRIDE). BMC Nephrol. 18(311)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goodnow CC: Multistep pathogenesis of
autoimmune disease. Cell. 130:25–35. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hutton HL, Alikhan MA and Kitching AR:
Inflammasomes in the kidney. Exp Suppl. 108:177–210.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hutton HL, Holdsworth SR and Kitching AR:
ANCA-associated vasculitis: Pathogenesis, models, and preclinical
testing. Semin Nephrol. 37:418–435. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W,
Zhang X, Wang WM, Qiu SJ, Zhou J and Fan J: Systemic
immune-inflammation index predicts prognosis of patients after
curative resection for hepatocellular carcinoma. Clin Cancer Res.
20:6212–6222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang R, Chang Q, Meng X, Gao N and Wang W:
Prognostic value of systemic immune-inflammation index in cancer: A
meta-analysis. J Cancer. 9:3295–3302. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de Joode AA, Sanders JS and Stegeman CA:
Renal survival in proteinase 3 and myeloperoxidase ANCA-associated
systemic vasculitis. Clin J Am Soc Nephrol. 8:1709–1717.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hilhorst M, van Paassen P and Tervaert JW:
Limburg Renal Registry. Proteinase 3-ANCA vasculitis versus
myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol. 26:2314–2327.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lyons PA, Rayner TF, Trivedi S, Holle JU,
Watts RA, Jayne DRW, Baslund B, Brenchley P, Bruchfeld A, Chaudhry
AN, et al: Genetically distinct subsets within ANCA-associated
vasculitis. N Engl J Med. 367:214–223. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li ZY, Ma TT, Chen M and Zhao MH: The
prevalence and management of anti-neutrophil cytoplasmic
antibody-associated vasculitis in China. Kidney Dis (Basel).
1:216–223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jennette JC, Falk RJ, Bacon PA, Basu N,
Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen
EC, et al: 2012 revised international chapel hill consensus
conference nomenclature of vasculitides. Arthritis Rheum. 65:1–11.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Watts R, Lane S, Hanslik T, Hauser T,
Hellmich B, Koldingsnes W, Mahr A, Segelmark M, Cohen-Tervaert JW
and Scott D: Development and validation of a consensus methodology
for the classification of the ANCA-associated vasculitides and
polyarteritis nodosa for epidemiological studies. Ann Rheum Dis.
66:222–227. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y,
Xu JS, Huang SM, Wang LN, Huang W, et al: Modified glomerular
filtration rate estimating equation for Chinese patients with
chronic kidney disease. J Am Soc Nephrol. 17:2937–2944.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mukhtyar C, Lee R, Brown D, Carruthers D,
Dasgupta B, Dubey S, Flossmann O, Hall C, Hollywood J, Jayne D, et
al: Modification and validation of the birmingham vasculitis
activity score (version 3). Ann Rheum Dis. 68:1827–1832.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Berden AE, Ferrario F, Hagen EC, Jayne DR,
Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, et
al: Histopathologic classification of ANCA-associated
glomerulonephritis. J Am Soc Nephrol. 21:1628–1636. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen YX, Xu J, Pan XX, Shen PY, Li X, Ren
H, Chen XN, Ni LY, Zhang W and Chen N: Histopathological
classification and renal outcome in patients with antineutrophil
cytoplasmic antibodies-associated renal vasculitis: A study of 186
patients and metaanalysis. J Rheumatol. 44:304–313. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang DY, Li ZY, Chen M and Zhao MH:
Myeloperoxidase-ANCA-positive granulomatosis with polyangiitis is a
distinct subset of ANCA-associated vasculitis: A retrospective
analysis of 455 patients from a single center in China. Semin
Arthritis Rheum. 48:701–706. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yuan Q, Wang J, Peng Z, Zhou Q, Xiao X,
Xie Y, Wang W, Huang L, Tang W, Sun D, et al:
Neutrophil-to-lymphocyte ratio and incident end-stage renal disease
in Chinese patients with chronic kidney disease: results from the
Chinese cohort study of chronic kidney disease (C-STRIDE). J Transl
Med. 17(86)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiao H, Heeringa P, Liu Z, Huugen D, Hu P,
Maeda N, Falk RJ and Jennette JC: The role of neutrophils in the
induction of glomerulonephritis by anti-myeloperoxidase antibodies.
Am J Pathol. 167:39–45. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiao H, Hu P, Falk RJ and Jennette JC:
Overview of the pathogenesis of ANCA-associated vasculitis. Kidney
Dis (Basel). 1:205–215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma TT, Huang YM, Wang C, Zhao MH and Chen
M: Coagulation and fibrinolysis index profile in patients with
ANCA-associated vasculitis. PLoS One. 9(e97843)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Willeke P, Kumpers P, Schlüter B, Limani
A, Becker H and Schotte H: Platelet counts as a biomarker in
ANCA-associated vasculitis. Scand J Rheumatol. 44:302–308.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff finder: A
comprehensive and straightforward web application enabling rapid
biomarker cutoff optimization. PLoS One. 7(e51862)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim Y, Choi H, Jung SM, Song JJ, Park YB
and Lee SW: Systemic immune-inflammation index could estimate the
cross-sectional high activity and the poor outcomes in
immunosuppressive drug-naive patients with antineutrophil
cytoplasmic antibody-associated vasculitis. Nephrology (Carlton).
24:711–717. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Basu N, Karabayas M and Pusey C: Prognosis
and future developments in vasculitis. Best Pract Res Clin
Rheumatol. 32:148–165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Collecchi P, Baldini E, Giannessi P,
Naccarato AG, Passoni A, Gardin G, Roncella M, Evangelista G,
Bevilacqua G and Conte PF: Primary chemotherapy in locally advanced
breast cancer (LABC): Effects on tumour proliferative activity,
bcl-2 expression and the relationship between tumour regression and
biological markers. Eur J Cancer. 34:1701–1704. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Najafi CC, Korbet SM, Lewis EJ, Schwartz
MM, Reichlin M and Evans J: Lupus Nephritis Collaborative Study
Group. Significance of histologic patterns of glomerular injury
upon long-term prognosis in severe lupus glomerulonephritis. Kidney
Int. 59:2156–2163. 2001.PubMed/NCBI View Article : Google Scholar
|