Introduction
Obesity is caused by excess fat accumulation, which
exerts a negative effect on human health (1). Being overweight or obese increases the
risks of various complications, particularly cardiovascular
diseases, diabetes and dyslipidemia (2). Therefore, methods for overcoming
obesity have become a current topic of considerable interest.
Increased energy expenditure might be an alternative option to
diet, exercise and gastric bypass surgery (3). Based on accumulating evidence from
recent studies, brown adipose tissue (BAT), a type of powerful
energy-consuming fat, ameliorates diet-induced and genetic obesity
(4,5). Thus, an increase in BAT mass or
activation may represent a novel approach to treating obesity and
its complications.
Fatty liver, also known as hepatic steatosis or
simple steatosis, is defined as abnormal triglyceride (TG)
accumulation in hepatocytes. Nonalcoholic fatty liver disease
(NAFLD) is the most common liver disorder occurring in developed
countries (6). Generally, >80%
of obese people develop fatty liver (7), and certain patients may progress to
fibrosis, cirrhosis, or even hepatocellular carcinoma (8). According to previous studies,
adipokines from visceral adipose tissue (VAT) modulate fatty liver
disease (9). For example, leptin
reverses NAFLD in patients with severe lipodystrophy (10). Lower adiponectin levels and
increased inflammation in patients with non-alcoholic
steatohepatitis (NASH) suggest that adiponectin deficiency is an
important risk factor for the development of steatohepatitis
(11). Thus, adipose tissue is
closely associated with the formation of fatty liver.
Body weight control via a combination of diet and
exercise has been regarded as the main intervention to rescue
hepatic fatty deposits; however, drug interventions, including
peroxisome proliferator-activated receptor (PPAR) agonists, bile
acid analogs, de novo lipogenesis inhibitors, antioxidants
and immune modulators, are required for liver dysfunction in some
patients with serious clinical cases (8,12).
Bile acids are common negative feedback medications used to
decrease hepatic lipogenesis and steatosis (13). In addition, bile acid analogs and
sequestrants may help modulate the bile acid concentration in the
enterohepatic circulation and decrease serum lipid levels and
hepatic fat accumulation (14,15).
Obeticholic acid (OCA), a bile acid analog, was
originally developed to treat NAFLD as a natural ligand of the
farnesoid X receptor (FXR). OCA indirectly inhibits cytochrome 7A1,
the rate-limiting enzyme in bile acid biosynthesis, to reduce fat
content (16). Although the
activation of FXR target genes by OCA has consistently been
demonstrated in fatty liver and OCA exerts a browning effect on
white adipose tissue (17), it
remains unclear whether OCA alters BAT function and/or improves
hepatic fatty deposits through the endocrine regulation of brown
fat.
The aim of the present study was to explore the
effect of OCA on brown fat. C3H10T1/2 cells and db/db mice were
used to investigate the potential effect of OCA on BAT function.
For the first time, the present study confirmed that OCA increased
BAT activity in vitro and in vivo, increased energy
expenditure, and ameliorated hepatic steatosis and obesity in db/db
mice. The current findings establish a previously unrecognized role
of OCA in activating BAT and reducing obesity, which may provide
insights into a potentially novel therapeutic approach to treat
metabolic disorders.
Materials and methods
Animals
In total, 40 7-week-old male mice (weight, 35-38 g)
of the genetic obesity model strain C57BLKS/J-Lepr-/Lepr-(db/db)
and their wild-type littermates were purchased from the Model
Animal Research Center of Nanjing University. For 8 weeks, all mice
were housed with a 12/12-h light/dark cycle, fed ad libitum
and provided free access to water. OCA (Shenzhen Botaier Scientific
Co., Ltd.) was administered at doses of 7.5, 15 and 30 mg/kg per
day, which were hereinafter referred to as the low, medium and high
groups, respectively. OCA was prepared as homogeneous solution in
2% (v/v) Tween-80 and orally administered once per day immediately
after preparation. The wild-type littermates and db/db control
group were treated with 2% (v/v) Tween-80 vehicle solution. The
dose conversion between cell and animal experiment were referred to
the peripheral blood volume in mice (accounting for 6% body weight)
to clarify the possible effective concentrations. For example, if
the experimental animal dose is 3 mg/kg, then the blood
concentration of this drug would be 50 µg/ml, which is 10X
LC50 for cells, making the LC50 in
vitro 5 µg/ml and vice versa. Therefore, in the present study,
25 µg/ml OCA in vitro would be equal to 15 mg/kg animal dose
in vivo.
All mice were euthanized using CO2 (with
2 l/min flow rate and in 20% of the chamber volume displacement per
min) until complete cardiac arrest and sacrificed to collect
organs. All animal studies were approved by the Institutional
Animal Care and Use Committee of the Institute of Zoology (Beijing,
China), and all experiments were performed under the oversight of
the Office of Laboratory Animal Welfare (Chinese Academy of
Sciences).
Cell culture
The mesenchymal stem cell line C3H10T1/2, which was
purchased from National Experimental Cell Resource Sharing Platform
(cat. no. 3111C0001CCC000665; https://cellresource.cn/fdetail.aspx?id=2778), was
cultured in basal medium (DMEM supplemented with 10% FBS; Gibco,
Thermo Fisher Scientific, Inc.) until the cells were 100%
confluent. Cells were incubated with basal medium supplemented with
1 µg/ml insulin, 1 nM 3,3',5-triiodo-L-thyronine (T3), 1 µM
dexamethasone, 0.5 mM isobutylmethylxanthine and 0.125 mM
indomethacin for the first 2 days (all from Sigma-Aldrich; Merck
KGaA). Then, the medium was replaced with differentiation medium
(DMEM supplemented with 10% FBS, including 1 µg/ml insulin and 1 nM
T3) for another 4 days until maturation, at which point the cells
were subjected to phenotypic and functional evaluations. C3H10T1/2
was treated by 25 µg/ml OCA during the processing of brown
adipogenesis differentiation. The constant culture condition is
37˚C and 5% CO2 for conventional culture or brown
adipogenesis differentiation.
Reverse transcription-quantitative PCR
(RT-qPCR)
The differentiated C3H10T1/2 was washed with icy PBS
(Gibco) for three times. After draining the last PBS, TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) was added into every
culture well containing differentiated C3H10T1/2 cells and cell
lysis was obtained. RNA concentration was then measured using a
Nanodrop 2000 machine (Thermo Fisher Scientific, Inc.). A total of
2 µg total RNA was reverse transcribed with M-MLV Reverse
Transcriptase (cat. no. M1705; Promega Corporation). The reaction
system contained 1.0 µg Oligo (dT) primer (Takara Bio, Inc.) and 2
µg RNA sample in a total volume up to 17.75 µl for the annealing
solution. This annealing solution was heated to 70˚C for 5 min and
cooled immediately on ice before the following components were
added: 5 µl M-MLV 5X Reaction Buffer, 10 mM dNTP (Takara Bio,
Inc.), 10 mM 1.25 µl Recombinant RNasin® Ribonuclease
Inhibitor 25 units (Promega Corporation) and M-MLV Reverse
Transcriptase. This reaction underwent 42˚C incubation for 1 h to
finish the first chain synthesis reaction. qPCR was performed with
a GoTaq® qPCR Master Mix (cat. no. A6001; Promega
Corporation) using an ABI Prism VIIA7 real-time PCR cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermal cycling
conditions: Polymerase activation for 1 cycle: 95˚C for 2 min;
followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min.
Cyclophilin A was used as an internal reference gene. Relative fold
changes in mRNA expression were calculated using the formula
2-ΔΔCq (18). The primer
sequences are listed in Table
I.
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| PPARγ2 |
TCGCTGATGCACTGCCTATG |
GAGAGGTCCACAGAGCTGATT |
| Ucp1 |
GAGGTCGTGAAGGTCAGAATG |
AAGCTTTCTGTGGTGGCTATAA |
| Elovl3 |
ATGCAACCCTATGACTTCGAG |
ACGATGAGCAACAGATAGACG |
| PRDM16 |
CAGCACGGTGAAGCCATTC |
GCGTGCATCCGCTTGTG |
| CyclophillinA |
TCCAAAGACAGCAGAAAACTTTCG |
TCTTCTTGCTGGTCTTGCCATTCC |
Western blotting
The cells were washed three times with PBS and lysed
in RIPA buffer [50 mM Tris-HCl with 150 mM NaCl, 1% NP40, 0.25%
sodium deoxycholate and inhibitors cocktail mixture (Roche
Diagnostics; cat. nos. 4906837001 and 4693124001)] following the
manufacturer's protocols to make a working solution. Brown fat
tissue was isolated and rapidly dipped in RIPA buffer and ground
with TissueLyser (Qiagen GmbH). After centrifugation at 13,000 x g
and 4˚C, the supernatant of extracted fluid from cells and brown
fat was carefully separated, and the total protein concentration
was quantified using a bicinchoninic acid protein assay (Pierce;
Thermo Fisher Scientific, Inc.). A total of 20 µg protein from each
sample was separated by 10% SDS/PAGE and transferred to PVDF
membranes (EMD Millipore). Then the membranes were incubated with
5% fat-free milk blocking buffer for 1 h at room temperature, and
blotted with primary antibodies overnight at 4˚C. After washing
with TBST (including 0.1% Tween 20) for three times, the membranes
were incubated with HRP-conjugated secondary antibodies for 1 h at
room temperature. The imaging signals were detected with a
SuperSignal West Pico chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc.). Primary antibodies against Ucp1 (1:1,000;
Abcam; cat no. ab10983), oxidative phosphorylation-related proteins
[dilution 1:250; Abcam; cat. no. ab110413; a mixture of antibodies
against ATP synthase F1 subunit α (ATP5α), ubiquinol-cytochrome c
reductase core protein 2 (UQCRC2), mitochondrially encoded
cytochrome c oxidase I (MTCO1), succinate dehydrogenase complex
iron sulfur subunit B (SDHB) and NADH: Ubiquinone oxidoreductase
subunit B5 (NDUFB5)], PPARγ (1:1,000; Cell Signaling Technology,
Inc.; cat. no. 2443) and β-tubulin (dilution 1:1,000; Santa Cruz
Biotechnology, Inc.; cat. no. sc-9104) were used. The secondary
antibodies included the horseradish peroxidase (HRP)-conjugated
goat anti-rabbit antibody (dilution 1:5,000; ZSGB-BIO; cat. no.
ZDR-5306) and the HRP-conjugated goat anti-mouse antibody
(ZSGB-BIO; cat. no. ZDR-5307). Densitometry analyses were performed
using ImageJ software 1.48v (National Institutes of Health) from
3-4 samples per group.
Metabolic rate and physical
activity
The metabolic rate was evaluated using a TSE
Labmaster system (19). Each mouse
was placed in a metabolic cage alone, and the volumes of
O2 consumption and CO2 release were monitored
in real-time over 24 h. The physical activity of each mouse was
measured with an optical beam technique (Opto-M3 animal activity
meter; Columbus Instruments) and quantified by summing all motion
points over 24 h (19).
Histology analysis
Brown adipose tissues were fixed with 4%
paraformaldehyde at room temperature for 24 h and then embedded in
paraffin. Sections with a thickness of 5 µm were stained with
hematoxylin and eosin (H&E). De-paraffinization was performed
using xylene. H&E staining step, the sections were stained with
hematoxylin for 10 min at room temperature until the nucleus turned
blue before being transferred into an eosin solution for 1-2 sec at
room temperature and sealing with Neutral balsam. The stained
sections would be kept in room temperature.
Fresh livers were fixed at room temperature for 24 h
and dehydrated in a 30% (v/v) sucrose solution overnight twice at
room temperature. Properly sized tissues were embedded in OCT
compound, frozen at -80˚C and prepared for cryosectioning (Sakura
Finetek USA, Inc.), and the thickness of the sections was
maintained at 12-15 µm. Oil Red O staining was performed using a
previously described method (20).
The stained sections would be kept in 4˚C. All photos are captured
by light microscopy (ECLIPSE 80i; Nikon Corporation) and the
magnification is x200.
Glucose tolerance test
Mice were fasted for 12 h (21:00-9:00) with free
access to water. D-Glucose (Sigma-Aldrich; Merck KGaA) was
intraperitoneally (i.p.) injected at a concentration of 0.75 g/kg
in saline, and blood glucose levels were measured with an Accu-Chek
glucose monitor (Roche Diagnostics) at the indicated timepoints.
The specific calculation method was described in a previous study
(19).
ELISA
The following ELISA kits were used to detect blood
lipid content in plasma samples according to the manufacturer's
instructions: High-density lipoprotein cholesterol (HDL-C) assay
kit (cat. no. E1017; Applygen Technologies Inc.), low-density
lipoprotein cholesterol/very low-density lipoprotein cholesterol
(LDL-C/VLDL-C) assay kit (cat. no. E1018; Applygen Technologies
Inc.), serum triglyceride assay kit (cat. no. E1002; Applygen
Technologies Inc.) and serum total cholesterol assay kit (cat. no.
E1005; Applygen Technologies Inc.).
Statistical analysis
All data are presented as means ± SEM. Data from
multiple groups were analyzed using one-way ANOVA and Tukey's post
hoc test, using GraphPad Prism version 6.04 (GraphPad Software,
Inc.). Data from two groups were analyzed using the unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
OCA enhances brown adipogenesis in
C3H10T1/2 cells
C3H10T1/2 cells were treated with OCA to investigate
the potential effect of OCA on brown adipogenesis. OCA
significantly increased the mRNA expression levels of Ucp1, a
BAT-specific gene, in C3H10T1/2 cells when administered at a
concentration of 25 µg/ml for 24 h (Fig. 1A). Based on this result, the dose of
25 µg/ml was used in further experiments to evaluate the effect of
OCA on brown adipogenesis. OCA treatment enhanced brown
adipogenesis, as evidenced by the increase in Oil Red cell staining
(Fig. 1B) and by the upregulation
of the late adipogenic marker PPARγ2 (Fig. 1C). In addition, OCA treatment
upregulated the mRNA expression levels of the BAT-specific genes
Ucp1, ELOVL fatty acid elongase 3 and PRDM16 (Fig. 1C). These results were further
confirmed by the increased protein expression levels of UCP1 and
PPARγ2 following OCA treatment in C3H10T1/2 cells (Fig. 1D). Notably, OCA administration did
not alter the levels of proteins involved in the oxidative
phosphorylation pathway, such as ATP5α, UQCRC2, SDHB and NDUFB5
(Fig. 1D). Thus, OCA enhanced the
brown adipocyte differentiation of C3H10T1/2 cells in vitro
and specifically increased Ucp1 expression.
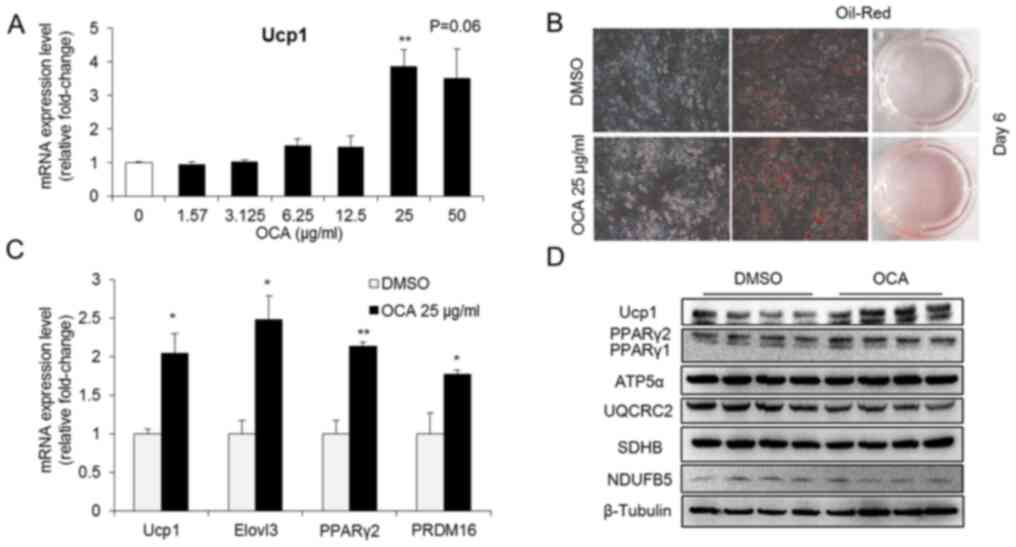 | Figure 1OCA enhances brown adipogenesis in
C3H10T1/2 cells. (A) Effects of different concentrations of OCA on
Ucp1 expression in undifferentiated C3H10T1/2 cells after treatment
for 24 h (n=5). **P<0.05 vs. 0. (B) Representative
photographs of Oil Red O staining in OCA-treated and control cells
on day 6 of brown adipogenesis (Original magnification, x200). (C)
The mRNA expression levels of brown adipocyte-specific genes and
differentiation-related genes were detected in differentiated
C3H10T1/2 cells from the control and OCA-treated groups (n=6).
*P<0.05, **P<0.01 compared with DMSO
group. (D) The levels of fatty acid oxidation-related proteins were
detected in differentiated C3H10T1/2 cells using western blotting
(n=4). All data are presented as means ± SEM. OCA, obeticholic
acid; Ucp1, uncoupling protein 1; Elovl3, ELOVL fatty acid elongase
3; PPARγ2, peroxisome proliferator-activated receptor γ2; ATP5α,
ATP synthase F1 subunit α; UQCRC2, ubiquinol-cytochrome c reductase
core protein 2; SDHB, succinate dehydrogenase complex iron sulfur
subunit B; NDUFB5, NADH: Ubiquinone oxidoreductase subunit B5. |
OCA delays body weight gain in db/db
mice and increases whole-body O2 consumption
An increase in brown adipogenesis and Ucp1
expression is linked to energy metabolism (21,22).
C57BLKS/J-Lepr-/Lepr-(db/db) male mice were used as a model of
obesity and diabetes to further investigate the function of OCA
in vivo. According to the dose conversion from the in
vitro experiment, mice were treated with three different doses
(7.5, 15 and 30 mg/kg per day, which were defined as low, medium
and high groups, respectively) of OCA beginning at 7 weeks of age
(Fig. 2A). OCA significantly
inhibited body weight gain in the three OCA treatment groups
compared with the control group (mean body weight at the end of
experiment: Control group, 52.8 g; low group, 49.7 g; medium group,
50.4 g; and high group, 48.5 g; decrease in body weight compared
with the control group, 5.8, 4.5 and 8.1%, respectively; Fig. 2A). Of note, OCA treatment
significantly increased energy metabolism, as evidenced by
increased whole-body O2 consumption (Fig. 2B), without significantly altering
food intake or physical activity (Fig.
2C). Furthermore, the results of the glucose tolerance test
demonstrated that OCA treatment dramatically improved glucose
tolerance (Fig. 2D). The area under
the curve revealed a positive correlation between the improved
glucose tolerance and the OCA dosage (Fig. 2D).
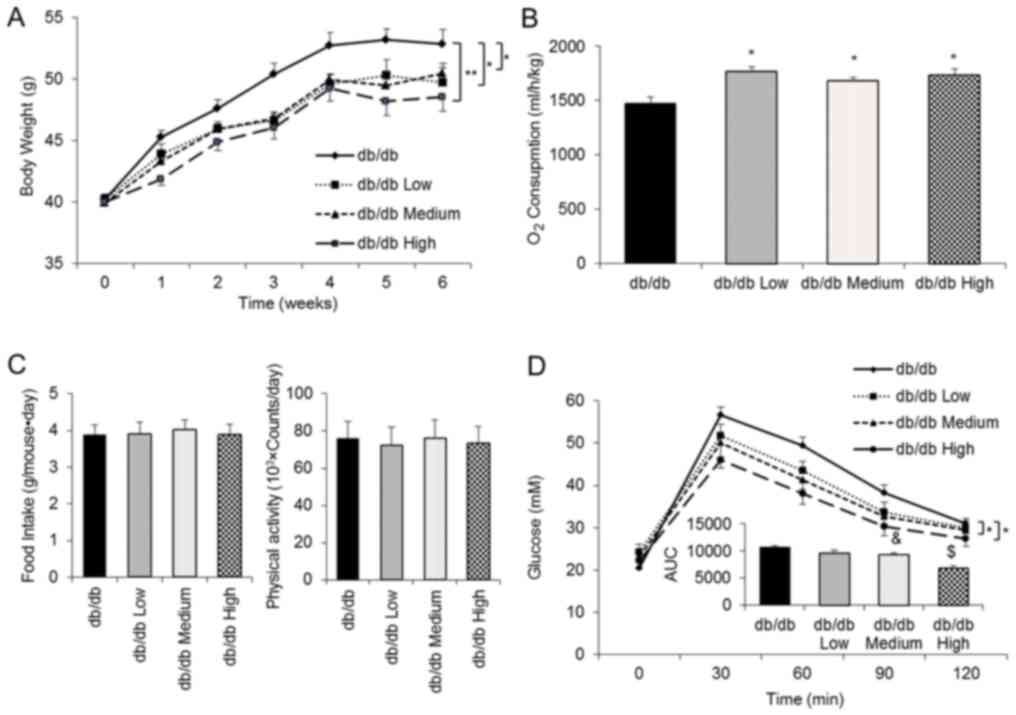 | Figure 2OCA inhibits body weight gain in
db/db mice and increases whole-body O2 consumption. (A)
Body weight was recorded weekly for 6 consecutive weeks (n=10 mice
per group). (B) The whole-body metabolic rate was determined by
measuring O2 consumption in a single day after 4 week
oral treatment (db/db control, n=6; db/db Low, n=8; db/db Medium,
n=6; and db/db High, n=8). (C) Food intake and physical activity
were recorded for a single day after 4 week oral treatment (db/db
control, n=7; db/db Low, n=9; db/db Medium, n=8; and db/db High,
n=9). (D) The glucose tolerance test was conducted in the 5th week
of treatment (db/db control, n=10; db/db Low, n=10; db/db Medium,
n=9; and db/db High, n=10). All data are presented as means ± SEM.
*P<0.05 and **P<0.01 compared with the
db/db control group; &P<0.05 compared with the
db/db Low group; $P<0.05 compared with the db/db
Medium group. OCA, obeticholic acid; AUC, area under the curve. |
OCA activates endogenous BAT to
ameliorate hepatic steatosis
Firstly, BAT morphology was investigated to obtain
additional insights into the potential mechanism of OCA action. OCA
treatment substantially reduced the size of BAT cells compared with
the control treatment group and activated endogenous BAT (Fig. 3A). Accordingly, the expression
levels of the UCP1 protein were significantly increased in medium
group, but the levels of oxidative phosphorylation-related proteins
were not altered (Fig. 3B and
C). Considering the therapeutic
potential of OCA as a treatment for hepatic steatosis, the present
study investigated the effect of OCA on hepatic steatosis. OCA
treatment significantly reversed hepatic steatosis, as evidenced by
the Oil Red O staining of frozen liver sections (Fig. 4A). In addition, OCA administration
significantly decreased serum total cholesterol, TG and HDL-C
levels, but not the levels of LDL-C (Fig. 4B-E).
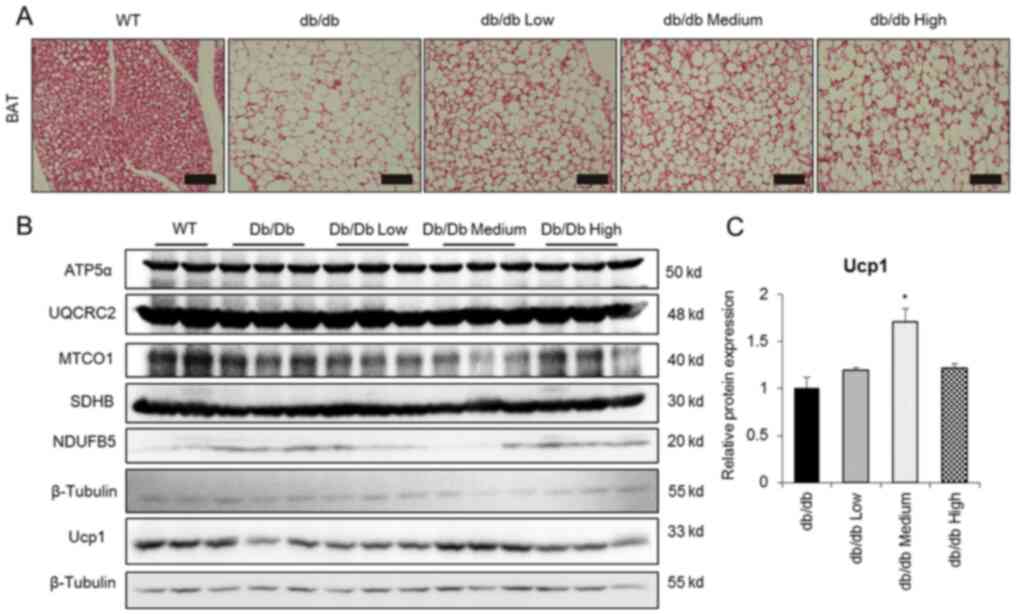 | Figure 3OCA induces Ucp1 expression in
endogenous BAT. (A) Representative images of hematoxylin and eosin
staining of endogenous BAT. Scale bar, 100 µm. (B) Representative
blot images and (C) quantification of western blot analysis showing
the levels of fatty acid oxidation-related proteins and Ucp1 in BAT
from db/db mice (n=3). β-Tubulin was used to normalize Ucp1 levels.
All data are presented as means ± SEM. *P<0.05
compared with the db/db control group. OCA, obeticholic acid; Ucp1,
uncoupling protein 1; BAT, brown adipose tissue; WT, wild-type;
ATP5α, ATP synthase F1 subunit α; UQCRC2, ubiquinol-cytochrome c
reductase core protein 2; MTCO1, mitochondrially encoded cytochrome
c oxidase I; SDHB, succinate dehydrogenase complex iron sulfur
subunit B; NDUFB5, NADH: Ubiquinone oxidoreductase subunit B5. |
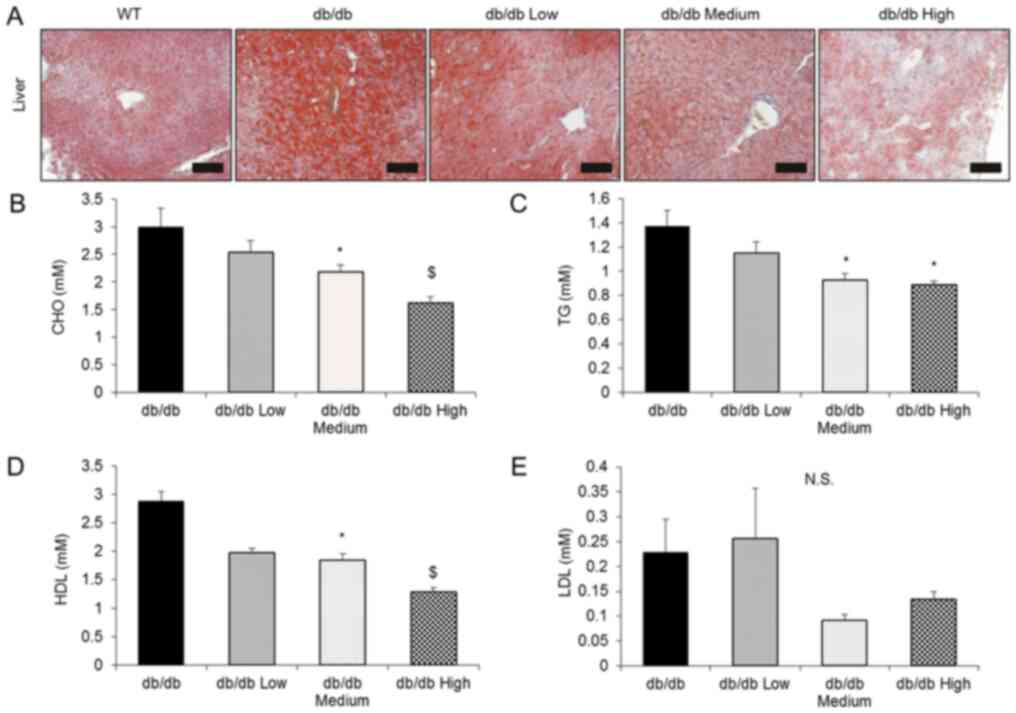 | Figure 4OCA reverses hepatic steatosis and
hyperlipidemia. (A) Comparison of the liver morphology in each
group using Oil Red O staining. Scale bar, 100 µm. (B) Serum CHO,
(C) TG, (D) HDL-C and (E) LDL-C levels were detected using ELISA
kits (db/db control, n=10; db/db Low, n=10; db/db Medium, n=9; and
db/db High, n=10). All data are presented as means ± SEM.
*P<0.05 compared with the db/db control group;
$P<0.05 compared with the db/db Medium group. OCA,
obeticholic acid; CHO, cholesterol; TG, triglyceride; HDL-C,
high-density lipoprotein cholesterol; LDL-C, low-density
lipoprotein cholesterol; N.S., not significant. |
Discussion
The db/db mouse strain is a leptin
receptor-deficient mouse model that exhibits excess obesity,
diabetes, polyuria, dyslipidemia and even liver damage during
growth and development (23). These
mice exhibit low levels of BAT activity and severe hepatic
steatosis; therefore, the present study used db/db mice as a model
to evaluate the effects of OCA on obesity and fatty liver disease.
The results demonstrated that OCA treatment increased brown
adipogenesis, increased the expression of the BAT-specific gene
Ucp1, enhanced whole-body energy consumption, improved glucose
tolerance, and ultimately significantly reversed hepatic
steatosis.
As an important heat production organ, BAT was
initially shown to primarily maintain the body temperature of
rodents and human infants in cold environments (24). However, based on accumulating
evidence, activated BAT may serve critical roles in energy
metabolism in rodents and adults (4). Unfortunately, several reports have
detected only a small amount of BAT in adult humans. Therefore,
affecting energy metabolism may be achieved by increasing the
activity of BAT or by increasing beige fat cell formation (25). Although cold exposure activates
endogenous BAT (26), it is not
suitable for people with cardiovascular disease, in whom cold
stimulation might increase the risk of death (27). Thus, other alternative methods of
activation, such as orally available drugs, may be more
suitable.
Bile acid participates in the intestinal absorption
of fat and is recycled in the liver (28). Previous studies confirmed that bile
acid, and its analogs, increase BAT mass and activity and
ultimately decrease diet-induced obesity (29,30).
In addition, OCA modulates the adipose tissue phenotype by inducing
the mRNA expression levels of fatty acid binding protein 4,
CCAAT/enhancer-binding protein (C/EBP) α and PPARγ2 in 3T3-L1 cells
and white adipocyte browning in atherogenic diet-fed wild-type mice
(17,31). In the present study, OCA treatment
substantially increased PPARγ2 expression, but not C/EBPα and
C/EBPβ expression (data not shown). Notably, for technical reasons,
the expression levels of other BAT-related genes were not detected
in the present study (data not shown), except for the upregulating
PR/SET domain 16 gene (PRDM16). The expression of Zic family member
1 (Zic1), myelin protein zero like 2 (Eva1) or LIM homeobox protein
8 (Lhx8) could not be detected by RT-qPCR in the present study.
Therefore, an analysis of the changes between control and treatment
groups was not technically possible for these genes in the present
study, although previous studies have shown expression of Zic1 in
adipogenesis differentiation of C3H10T1/2 cells (32,33).
In the future, primary brown adipocyte cells will be used as a
model for adipogenesis differentiation in vitro. As a
typical example of drug repurposing, in the present study, OCA
increased Ucp1 expression and activated endogenous BAT without
altering fatty acid oxidation. Additionally, OCA increased
whole-body energy consumption similar to bile acid in a high-fat
diet mouse model (34). Evidence
from another study similarly demonstrated that OCA treatment
increased insulin sensitivity and improved glucose homeostasis in a
phase II trial (35).
In the present study, the side effects of higher
serum cholesterol and LDL-C concentrations but lower HDL-C observed
in the OCA treatment group may be ascribed to the disrupted
conversion of cholesterol to bile acids reported in clinical trials
with humans (36,37). Of note, serological indicators, such
as a decrease in TG and cholesterol levels, in mouse and rat models
are not identical to those in human samples (38,39).
This difference might be derived from the suppression of hepatic
bile acid synthesis, alteration of the bile acid composition and
subsequent inhibition of cholesterol absorption in the intestine
(38). On the one hand, OCA may
increase serum cholesterol concentrations by blocking bile acid
biosynthesis from cholesterol and reducing bile acid deposition in
humans (40). By contrast, the
alteration of the bile acid composition and inhibition of
cholesterol absorption in the intestine contribute to the serum
cholesterol clearance in rodent models (41,42).
In the present study, the serum cholesterol levels were decreased,
including total cholesterol, TG and HDL-C, but not LDL-C, while
liver steatosis was improved. A previous study indicated that
‘indirect factors’ may exist in adipose tissue or other organs to
reverse fatty liver disease while failing to activate FXR in
subjects treated with OCA (17).
In previous studies, strategies designed to increase
BAT activity were proposed as a promising approach to improve fatty
liver and decrease blood lipid levels (43,44).
BAT activation improves corticosterone-induced hyperlipidemia by
reducing de novo lipogenesis in the liver and TG secretion
(45). In addition, higher BAT
activity in individual adults has a negative correlation with
NAFLD-related morbidity (46).
Furthermore, brown adipocytes significantly alter hepatic lipid
homeostasis and NAFLD in patients with diabetes (47). Given the spatial inaccessibility
between BAT and the liver, BAT-derived secretory factors might have
a critical role in organ crosstalk. Moreover, the transplantation
of fetal brown adipocytes into type 1 diabetic mice decreases
hepatic glucose production by inducing the production of
insulin-like growth factor 1 through a direct and/or indirect
effect on liver metabolism (48).
Another batokine, neuregulin 4 (NRG4), selectively binds to
hepatocytes and reduces de novo lipogenesis through an LXR
and sterol regulatory element binding transcription factor
1-dependent mechanism (43).
Similarly, the fat content in the human liver may be regulated by
NRG4 through the same pathway. Taken together, several BAT-derived
endocrine factors may indirectly influence hepatic lipid
metabolism. In the present study, it was indicated that OCA may
reverse steatohepatitis via the BAT endocrine network. Future
studies will aim to screen serum samples in detail to identify the
direct factors and investigate their functions.
In summary, to the best of our knowledge, the
present study is the first to demonstrate that OCA treatment
ameliorated hepatic steatosis by increasing BAT activity. OCA
stimulated brown adipogenesis and Ucp1 expression in BAT in
vitro. In addition, OCA treatment increased whole-body energy
metabolism and glucose homeostasis by increasing BAT activity in
vivo. In our next study, a mouse model of diet-induced obesity
will be used to confirm the function of OCA. Furthermore, although
OCA shows therapeutic potential in animal models, its benefits and
safety profile must be confirmed in humans in clinical trials in
the future. In summary, the present results describe a novel
approach to activate BAT as a potential treatment for obesity and
other metabolic disorders.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Key
Research and Development Program of China (grant no.
2017YFC1001003) and grants from the National Natural Science
Foundation of China (grant nos. 81770577, 81770834 and 81370951),
the Strategic Priority Research Program from the Chinese Academy of
Sciences (grant no. XDB13030000), the National Natural Science
Foundation of China (grant no. Y21JA71234), the Open and
Cooperation in Science and Technology Project of Henan Province
(grant no. 182106000047) and the Innovative Talents in Universities
of Henan Province (grant no. 19HASTIT015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and MD conceived the project and wrote the
manuscript. HZ performed the experiments. MD and XL analyzed the
results. All authors were involved in editing the paper and
approved the final manuscript. MD and XL confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal studies were approved by the
Institutional Animal Care and Use Committee of the Institute of
Zoology (Beijing, China), and all experiments were performed under
the oversight of the Office of Laboratory Animal Welfare (Chinese
Academy of Sciences).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Srivastava G and Apovian CM: Current
pharmacotherapy for obesity. Nat Rev Endocrinol. 14:12–24.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cypess AM, Haft CR, Laughlin MR and Hu
HCH: Brown fat in humans: Consensus points and experimental
guidelines. Cell Metab. 20:408–415. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen
Y, Chi Q, Wang D, Zhang Z, Li C, et al: Brown adipose tissue
transplantation improves whole-body energy metabolism. Cell Res.
23:851–854. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu X, Wang S, You Y, Meng M, Zheng Z,
Dong M, Lin J, Zhao Q, Zhang C, Yuan X, et al: Brown adipose tissue
transplantation reverses obesity in Ob/Ob mice. Endocrinology.
156:2461–2469. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rinella ME: Nonalcoholic fatty liver
disease: A systematic review. JAMA. 313:2263–2273. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Younossi Z, Anstee QM, Marietti M, Hardy
T, Henry L, Eslam M, George J and Bugianesi E: Global burden of
NAFLD and NASH: Trends, predictions, risk factors and prevention.
Nat Rev Gastroenterol Hepatol. 15:11–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American association for the study of
liver diseases. Hepatology. 67:328–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Antuna-Puente B, Feve B, Fellahi S and
Bastard JP: Adipokines: The missing link between insulin resistance
and obesity. Diabetes Metab. 34:2–11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chong AY, Lupsa BC, Cochran EK and Gorden
P: Efficacy of leptin therapy in the different forms of human
lipodystrophy. Diabetologia. 53:27–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Polyzos SA, Toulis KA, Goulis DG, Zavos C
and Kountouras J: Serum total adiponectin in nonalcoholic fatty
liver disease: A systematic review and meta-analysis. Metabolism.
60:313–326. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rotman Y and Sanyal AJ: Current and
upcoming pharmacotherapy for non-alcoholic fatty liver disease.
Gut. 66:180–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Watanabe M, Houten SM, Wang L, Moschetta
A, Mangelsdorf DJ, Heyman RA, Moore DD and Auwerx J: Bile acids
lower triglyceride levels via a pathway involving FXR, SHP, and
SREBP-1c. J Clin Invest. 113:1408–1418. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Luo YH, Wang XX, Orlicky DJ and Levi M:
Bile acid sequestrant prevents NAFLD and NASH in western diet fed
mice independent of FXR. Hepatology. 62:280A–282A. 2015.
|
|
15
|
Yuan L and Bambha K: Bile acid receptors
and nonalcoholic fatty liver disease. World J Hepatol. 7:2811–2818.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Massafra V, Milona A, Vos HR, Ramos RJJ,
Gerrits J, Willemsen ECL, Ramos Pittol JM, Ijssennagger N,
Houweling M, Prinsen HCMT, et al: Farnesoid X receptor activation
promotes hepatic amino acid catabolism and ammonium clearance in
mice. Gastroenterology. 152:1462–1476.e10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Haczeyni F, Poekes L, Wang H, Mridha AR,
Barn V, Geoffrey Haigh W, Ioannou GN, Yeh MM, Leclercq IA, Teoh NC
and Farrell GC: Obeticholic acid improves adipose morphometry and
inflammation and reduces steatosis in dietary but not metabolic
obesity in mice. Obesity (Silver Spring). 25:155–165.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan X, Wei G, You Y, Huang Y, Lee HJ,
Dong M, Lin J, Hu T, Zhang H, Zhang C, et al: Rutin ameliorates
obesity through brown fat activation. FASEB J. 31:333–345.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang C, Wang J, Zhang H, Liu S, Lee HJ,
Jin W and Cheng J: Hepatitis C virus core protein induces hepatic
steatosis via Sirt1-dependent pathway. Liver Int. 38:803–812.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trayhurn P, Thurlby PL and James WP:
Thermogenic defect in pre-obese ob/ob mice. Nature. 266:60–62.
1977.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Kozak LP and Anunciado-Koza R: UCP1: Its
involvement and utility in obesity. Int J Obes (Lond). 32 (Suppl
7):S32–S38. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Masuo K, Straznicky NE, Lambert GW,
Katsuya T, Sugimoto K, Rakugi H, Socratous F, Hastings J, Lambert
EA, Ogihara T and Esler MD: Leptin-receptor polymorphisms relate to
obesity through blunted leptin-mediated sympathetic nerve
activation in a Caucasian male population. Hypertens Res.
31:1093–1100. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cannon B and Nedergaard J: Brown adipose
tissue: Function and physiological significance. Physiol Rev.
84:277–359. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Carey AL and Kingwell BA: Brown adipose
tissue in humans: Therapeutic potential to combat obesity.
Pharmacol Ther. 140:26–33. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yoneshiro T, Aita S, Matsushita M,
Kayahara T, Kameya T, Kawai Y, Iwanaga T and Saito M: Recruited
brown adipose tissue as an antiobesity agent in humans. J Clin
Invest. 123:3404–3408. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Tian L, Qiu H, Sun S and Lin H: Emergency
cardiovascular hospitalization risk attributable to cold
temperatures in Hong Kong. Circ Cardiovasc Qual Outcomes.
9:135–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marin JJ, Macias RI, Briz O, Banales JM
and Monte MJ: Bile acids in physiology, pathology and pharmacology.
Curr Drug Metab. 17:4–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Broeders EP, Nascimento EB, Havekes B,
Brans B, Roumans KH, Tailleux A, Schaart G, Kouach M, Charton J,
Deprez B, et al: The bile acid chenodeoxycholic acid increases
human brown adipose tissue activity. Cell Metab. 22:418–426.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen X, Yan L, Guo Z, Chen Y, Li M, Huang
C, Chen Z and Meng X: Chenodeoxycholic acid attenuates high-fat
diet-induced obesity and hyperglycemia via the G protein-coupled
bile acid receptor 1 and proliferator-activated receptor γ pathway.
Exp Ther Med. 14:5305–5312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rizzo G, Disante M, Mencarelli A, Renga B,
Gioiello A, Pellicciari R and Fiorucci S: The farnesoid X receptor
promotes adipocyte differentiation and regulates adipose cell
function in vivo. Mol Pharmacol. 70:1164–1173. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rahman MS, Imran KM, Hossain M, Lee TJ and
Kim YS: Biochanin A induces a brown-fat phenotype via improvement
of mitochondrial biogenesis and activation of AMPK signaling in
murine C3H10T1/2 mesenchymal stem cells. Phytother Res. 35:920–931.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Zhang HL, Huang YY, Lee HJ and Jin WZ:
Zic1 negatively regulates brown adipogenesis in C3H10T1/2 cells.
Sci Bull. 60:1033–1035. 2015.
|
|
34
|
Watanabe M, Houten SM, Mataki C,
Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O,
Kodama T, et al: Bile acids induce energy expenditure by promoting
intracellular thyroid hormone activation. Nature. 439:484–489.
2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mudaliar S, Henry RR, Sanyal AJ, Morrow L,
Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe
E, et al: Efficacy and safety of the farnesoid X receptor agonist
obeticholic acid in patients with type 2 diabetes and nonalcoholic
fatty liver disease. Gastroenterology. 145:574–582.e1.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Papazyan R, Liu X, Liu J, Dong B, Plummer
EM, Lewis RD II, Roth JD and Young MA: FXR activation by
obeticholic acid or nonsteroidal agonists induces a human-like
lipoprotein cholesterol change in mice with humanized chimeric
liver. J Lipid Res. 59:982–993. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pencek R, Marmon T, Roth JD, Liberman A,
Hooshmand-Rad R and Young MA: Effects of obeticholic acid on
lipoprotein metabolism in healthy volunteers. Diabetes Obes Metab.
18:936–940. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xu Y, Li F, Zalzala M, Xu J, Gonzalez FJ,
Adorini L, Lee YK, Yin L and Zhang Y: Farnesoid X receptor
activation increases reverse cholesterol transport by modulating
bile acid composition and cholesterol absorption in mice.
Hepatology. 64:1072–1085. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cipriani S, Mencarelli A, Palladino G and
Fiorucci S: FXR activation reverses insulin resistance and lipid
abnormalities and protects against liver steatosis in Zucker
(fa/fa) obese rats. J Lipid Res. 51:771–784. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Owsley E and Chiang JY: Guggulsterone
antagonizes farnesoid X receptor induction of bile salt export pump
but activates pregnane X receptor to inhibit cholesterol 7
alpha-hydroxylase gene. Biochem Biophys Res Commun. 304:191–195.
2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lorbek G, Lewinska M and Rozman D:
Cytochrome P450s in the synthesis of cholesterol and bile
acids-from mouse models to human diseases. FEBS J. 279:1516–1533.
2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Modica S, Petruzzelli M, Bellafante E,
Murzilli S, Salvatore L, Celli N, Di Tullio G, Palasciano G,
Moustafa T, Halilbasic E, et al: Selective activation of nuclear
bile acid receptor FXR in the intestine protects mice against
cholestasis. Gastroenterology. 142:355–365.e1-e4. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang GX, Zhao XY, Meng ZX, Kern M,
Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al: The
brown fat-enriched secreted factor Nrg4 preserves metabolic
homeostasis through attenuation of hepatic lipogenesis. Nat Med.
20:1436–1443. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lee P, Linderman JD, Smith S, Brychta RJ,
Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et
al: Irisin and FGF21 are cold-induced endocrine activators of brown
fat function in humans. Cell Metab. 19:302–309. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
van den Beukel JC, Boon MR, Steenbergen J,
Rensen PC, Meijer OC, Themmen AP and Grefhorst A: Cold exposure
partially corrects disturbances in lipid metabolism in a male mouse
model of glucocorticoid excess. Endocrinology. 156:4115–4128.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yilmaz Y, Ones T, Purnak T, Ozguven S,
Kurt R, Atug O, Turoglu HT and Imeryuz N: Association between the
presence of brown adipose tissue and non-alcoholic fatty liver
disease in adult humans. Aliment Pharmacol Ther. 34:318–323.
2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Blondin DP, Labbé SM, Noll C, Kunach M,
Phoenix S, Guérin B, Turcotte ÉE, Haman F, Richard D and Carpentier
AC: Selective impairment of glucose but not fatty acid or oxidative
metabolism in brown adipose tissue of subjects with type 2
diabetes. Diabetes. 64:2388–2397. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Gunawardana SC and Piston DW:
Insulin-independent reversal of type 1 diabetes in nonobese
diabetic mice with brown adipose tissue transplant. Am J Physiol
Endocrinol Metab. 308:E1043–E1055. 2015.PubMed/NCBI View Article : Google Scholar
|


















