|
1
|
Yongjun Z, Tingjie Z, Xiaoqiu Y, Zhiying
F, Feng Q, Guangke X, Jinfeng L, Fachuan N, Xiaohong J and Yanqing
L: A survey of chronic pain in China. Libyan J Med.
15(1730550)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mills SEE, Nicolson KP and Smith BH:
Chronic pain: A review of its epidemiology and associated factors
in population-based studies. Br J Anaesth. 123:e273–e283.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Strand EB, Mengshoel AM, Sandvik L,
Helland IB, Abraham S and Nes LS: Pain is associated with reduced
quality of life and functional status in patients with Myalgic
Encephalomyelitis/Chronic Fatigue Syndrome. Scand J Pain. 19:61–72.
2019.PubMed/NCBI View Article : Google Scholar
|
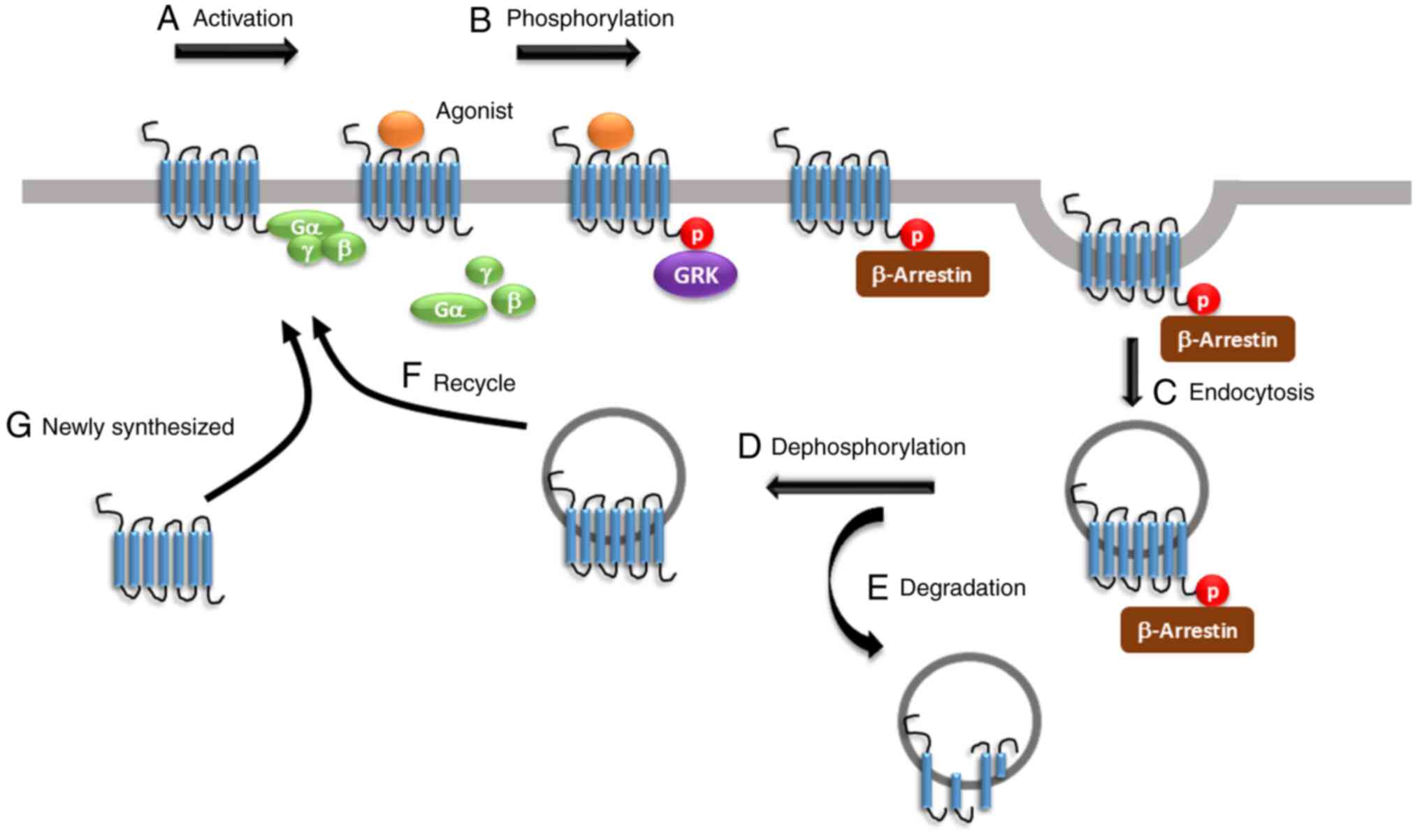
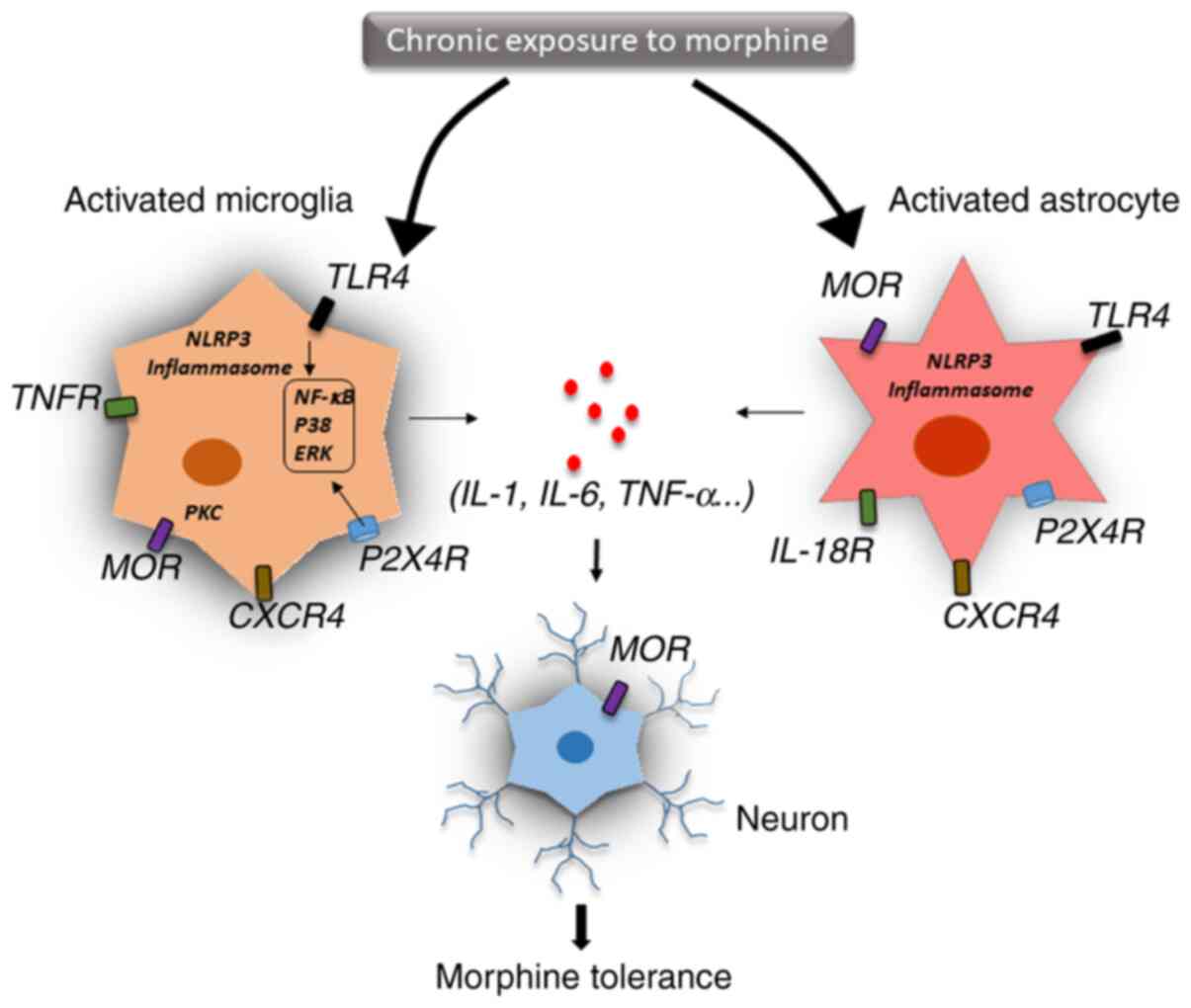
|
4
|
Chou R, Hartung D, Turner J, Blazina I,
Chan B, Levander X, McDonagh M, Selph S, Fu R and Pappas M: Opioid
Treatments for Chronic Pain. Agency for Healthcare Research and
Quality (US), Rockville, MD, 2020.
|
|
5
|
Daoust R, Paquet J, Cournoyer A, Piette E,
Morris J, Lessard J, Castonguay V, Williamson D and Chauny JM: Side
effects from opioids used for acute pain after emergency department
discharge. Am J Emerg Med. 38:695–701. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roggeri D, Saramin C, Terrazzani G, Zusso
M, Giusti P and Chinellato A: Resource consumption and costs of
treating pain in patients affected by cancer in a district of
northeast Italy. Pharmacol Res. 56:329–334. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahlbeck K: Opioids: A two-faced Janus.
Curr Med Res Opin. 27:439–448. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eidson LN and Murphy AZ: Inflammatory
mediators of opioid tolerance: Implications for dependency and
addiction. Peptides. 115:51–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gulur P, Williams L, Chaudhary S, Koury K
and Jaff M: Opioid tolerance-a predictor of increased length of
stay and higher readmission rates. Pain Physician. 17:E503–E507.
2014.PubMed/NCBI
|
|
10
|
Martyn JAJ, Mao J and Bittner EA: Opioid
tolerance in critical illness. N Engl J Med. 380:365–378.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Allouche S, Noble F and Marie N: Opioid
receptor desensitization: Mechanisms and its link to tolerance.
Front Pharmacol. 5(280)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rosenblum A, Marsch LA, Joseph H and
Portenoy RK: Opioids and the treatment of chronic pain:
Controversies, current status, and future directions. Exp Clin
Psychopharmacol. 16:405–416. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Uniyal A, Gadepalli A and Akhilesh Tiwari
V: Underpinning the neurobiological intricacies associated with
opioid tolerance. ACS Chem Neurosci. 11:830–839. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liang Y, Chu H, Jiang Y and Yuan L:
Morphine enhances IL-1β release through toll-like receptor
4-mediated endocytic pathway in microglia. Purinergic Signal.
12:637–645. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sanna MD, Borgonetti V and Galeotti N: µ
Opioid receptor-triggered notch-1 activation contributes to
morphine tolerance: Role of neuron-glia communication. Mol
Neurobiol. 57:331–345. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang X, Loram LC, Ramos K, de Jesus AJ,
Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR
and Yin H: Morphine activates neuroinflammation in a manner
parallel to endotoxin. Proc Natl Acad Sci USA. 109:6325–6330.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Johnston IN, Milligan ED, Wieseler-Frank
J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P,
Fleshner M, et al: A role for proinflammatory cytokines and
fractalkine in analgesia, tolerance, and subsequent pain
facilitation induced by chronic intrathecal morphine. The Journal
of neuroscience: J Neurosci. 24:7353–7365. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang H, Zhang Y, Ma X, Wang W, Xu X, Huang
M, Xu L, Shi H, Yuan T, Jiang W, et al: Spinal TLR4/P2X7
receptor-dependent NLRP3 inflammasome activation contributes to the
development of tolerance to morphine-induced antinociception. J
Inflamm Res. 13:571–582. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pasternak GW and Pan YX: Mu opioids and
their receptors: Evolution of a concept. Pharmacol Rev.
65:1257–1317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yam MF, Loh YC, Tan CS, Khadijah Adam S,
Abdul Manan N and Basir R: General pathways of pain sensation and
the major neurotransmitters involved in pain regulation. Int J Mol
Sci. 19(2164)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McNicol E, Horowicz-Mehler N, Fisk RA,
Bennett K, Gialeli-Goudas M, Chew PW, Lau J and Carr D: Americal
Pain Society. Management of opioid side effects in cancer-related
and chronic noncancer pain: A systematic review. J Pain. 4:231–256.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dougall IG: A critical review of the
classification of opioid receptors. Biotechnol Appl Biochem.
10:488–499. 1988.PubMed/NCBI
|
|
23
|
Pert CB and Snyder SH: Opiate receptor:
Demonstration in nervous tissue. Science. 179:1011–1014.
1973.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Manglik A, Kruse AC, Kobilka TS, Thian FS,
Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK and Granier
S: Crystal structure of the micro-opioid receptor bound to a
morphinan antagonist. Nature. 485:321–326. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Williams JT, Ingram SL, Henderson G,
Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ and Christie
MJ: Regulation of µ-opioid receptors: Desensitization,
phosphorylation, internalization, and tolerance. Pharmacol Rev.
65:223–254. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chavkin C, McLaughlin JP and Celver JP:
Regulation of opioid receptor function by chronic agonist exposure:
Constitutive activity and desensitization. Mol Pharmacol. 60:20–25.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schulz S, Mayer D, Pfeiffer M, Stumm R,
Koch T and Hollt V: Morphine induces terminal micro-opioid receptor
desensitization by sustained phosphorylation of serine-375. EMBO J.
23:3282–3289. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Clayton CC, Bruchas MR, Lee ML and Chavkin
C: Phosphorylation of the mu-opioid receptor at tyrosine 166
(Tyr3.51) in the DRY motif reduces agonist efficacy. Mol Pharmacol.
77:339–347. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lau EK, Trester-Zedlitz M, Trinidad JC,
Kotowski SJ, Krutchinsky AN, Burlingame AL and von Zastrow M:
Quantitative encoding of the effect of a partial agonist on
individual opioid receptors by multisite phosphorylation and
threshold detection. Sci Signal. 4(ra52)2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arttamangkul S, Heinz DA, Bunzow JR, Song
X and Williams JT: Cellular tolerance at the micro-opioid receptor
is phosphorylation dependent. Elife. 7(e34989)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Birdsong WT, Arttamangkul S, Bunzow JR and
Williams JT: Agonist binding and desensitization of the µ-opioid
receptor is modulated by phosphorylation of the C-terminal tail
domain. Mol Pharmacol. 88:816–824. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fan T, Varghese G, Nguyen T, Tse R, O'Dowd
BF and George SR: A role for the distal carboxyl tails in
generating the novel pharmacology and G protein activation profile
of mu and delta opioid receptor hetero-oligomers. J Biol Chem.
280:38478–38488. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gomes I, Gupta A, Filipovska J, Szeto HH,
Pintar JE and Devi LA: A role for heterodimerization of mu and
delta opiate receptors in enhancing morphine analgesia. Proc Natl
Acad Sci USA. 101:5135–5139. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B,
Wang HB, Li Q, Yang H, Luo J, Li ZY, et al: Facilitation of
µ-opioid receptor activity by preventing δ-opioid receptor-mediated
codegradation. Neuron. 69:120–131. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang D, Tawfik VL, Corder G, Low SA,
Francois A, Basbaum AI and Scherrer G: functional divergence of
delta and mu opioid receptor organization in CNS pain circuits.
Neuron. 98:90–108, e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chefer VI and Shippenberg TS: Augmentation
of morphine-induced sensitization but reduction in morphine
tolerance and reward in delta-opioid receptor knockout mice.
Neuropsychopharmacology. 34:887–898. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fujita W, Gomes I and Devi LA: Heteromers
of µ-δ opioid receptors: new pharmacology and novel therapeutic
possibilities. Br J Pharmacol. 172:375–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schiller PW: Opioid peptide-derived
analgesics. AAPS J. 7:E560–E565. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Scherrer G, Imamachi N, Cao YQ, Contet C,
Mennicken F, O'Donnell D, Kieffer BL and Basbaum AI: Dissociation
of the opioid receptor mechanisms that control mechanical and heat
pain. Cell. 137:1148–1159. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhu Y, King MA, Schuller AG, Nitsche JF,
Reidl M, Elde RP, Unterwald E, Pasternak GW and Pintar JE:
Retention of supraspinal delta-like analgesia and loss of morphine
tolerance in delta opioid receptor knockout mice. Neuron.
24:243–252. 1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei
G and Ma L: Identification of G protein-coupled receptor kinase 2
phosphorylation sites responsible for agonist-stimulated
delta-opioid receptor phosphorylation. Mol Pharmacol. 58:1050–1056.
2000.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Al-Hasani R and Bruchas MR: Molecular
mechanisms of opioid receptor-dependent signaling and behavior.
Anesthesiology. 115:1363–1381. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xie WY, He Y, Yang YR, Li YF, Kang K, Xing
BM and Wang Y: Disruption of Cdk5-associated phosphorylation of
residue threonine-161 of the delta-opioid receptor: Impaired
receptor function and attenuated morphine antinociceptive
tolerance. J Neurosci. 29:3551–3564. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jean-Charles PY, Kaur S and Shenoy SK: G
Protein-coupled receptor signaling through β-arrestin-dependent
mechanisms. J Cardiovasc Pharmacol. 70:142–158. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Y, Tang K, Inan S, Siebert D,
Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A and Liu-Chen LY:
Comparison of pharmacological activities of three distinct kappa
ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors
in vitro and their antipruritic and antinociceptive activities in
vivo. J Pharmacol Exp Ther. 312:220–230. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
McLaughlin JP, Myers LC, Zarek PE, Caron
MG, Lefkowitz RJ, Czyzyk TA, Pintar JE and Chavkin C: Prolonged
kappa opioid receptor phosphorylation mediated by G-protein
receptor kinase underlies sustained analgesic tolerance. J Biol
Chem. 279:1810–1818. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lalanne L, Ayranci G, Kieffer BL and Lutz
PE: The kappa opioid receptor: From addiction to depression, and
back. Front Psychiatry. 5(170)2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lutz PE and Kieffer BL: Opioid receptors:
Distinct roles in mood disorders. Trends Neurosci. 36:195–206.
2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nygard SK, Hourguettes NJ, Sobczak GG,
Carlezon WA and Bruchas MR: Stress-induced reinstatement of
nicotine preference requires dynorphin/kappa opioid activity in the
basolateral amygdala. J Neurosci. 36:9937–9948. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Taylor GT and Manzella F: Kappa opioids,
salvinorin a and major depressive disorder. Curr Neuropharmacol.
14:165–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sheffler DJ and Roth BL: Salvinorin A: The
‘magic mint’ hallucinogen finds a molecular target in the kappa
opioid receptor. Trends Pharmacol Sci. 24:107–109. 2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chavkin C, Sud S, Jin W, Stewart J,
Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ and Roth BL:
Salvinorin A, an active component of the hallucinogenic sage salvia
divinorum is a highly efficacious kappa-opioid receptor agonist:
Structural and functional considerations. J Pharmacol Exp Ther.
308:1197–1203. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mollereau C and Mouledous L: Tissue
distribution of the opioid receptor-like (ORL1) receptor. Peptides.
21:907–917. 2000.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Thompson AA, Liu W, Chun E, Katritch V, Wu
H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, et al:
Structure of the nociceptin/orphanin FQ receptor in complex with a
peptide mimetic. Nature. 485:395–399. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen Y, Fan Y, Liu J, Mestek A, Tian M,
Kozak CA and Yu L: Molecular cloning, tissue distribution and
chromosomal localization of a novel member of the opioid receptor
gene family. FEBS Lett. 347:279–283. 1994.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mogil JS and Pasternak GW: The molecular
and behavioral pharmacology of the orphanin FQ/nociceptin peptide
and receptor family. Pharmacol Rev. 53:381–415. 2001.PubMed/NCBI
|
|
57
|
Spampinato S, Baiula M and Calienni M:
Agonist-regulated internalization and desensitization of the human
nociceptin receptor expressed in CHO cells. Curr Drug Targets.
8:137–146. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zaveri NT: Nociceptin opioid receptor
(NOP) as a therapeutic target: Progress in translation from
preclinical research to clinical utility. J Med Chem. 59:7011–7028.
2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zamponi GW and Snutch TP: Modulating
modulation: Crosstalk between regulatory pathways of presynaptic
calcium channels. Mol Interv. 2:476–478. 2002.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zamponi GW and Snutch TP: Modulation of
voltage-dependent calcium channels by G proteins. Curr Opin
Neurobiol. 8:351–356. 1998.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Torrecilla M, Quillinan N, Williams JT and
Wickman K: Pre- and postsynaptic regulation of locus coeruleus
neurons after chronic morphine treatment: A study of GIRK-knockout
mice. Eur J Neurosci. 28:618–624. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Diaz A, Florez J, Pazos A and Hurle MA:
Opioid tolerance and supersensitivity induce regional changes in
the autoradiographic density of dihydropyridine-sensitive calcium
channels in the rat central nervous system. Pain. 86:227–235.
2000.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bohn LM, Lefkowitz RJ and Caron MG:
Differential mechanisms of morphine antinociceptive tolerance
revealed in (beta)arrestin-2 knock-out mice. J Neurosci.
22:10494–10500. 2002.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bernstein MA and Welch SP: mu-Opioid
receptor down-regulation and cAMP-dependent protein kinase
phosphorylation in a mouse model of chronic morphine tolerance.
Brain Res Mol Brain Res. 55:237–242. 1998.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Stafford K, Gomes AB, Shen J and Yoburn
BC: mu-Opioid receptor downregulation contributes to opioid
tolerance in vivo. Pharmacol Biochem Behav. 69:233–237.
2001.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Fábián G, Bozó B, Szikszay M, Horváth G,
Coscia CJ and Szücs M: Chronic morphine-induced changes in
mu-opioid receptors and G proteins of different subcellular loci in
rat brain. J Pharmacol Exp Ther. 302:774–780. 2002.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gupta S and Kulhara P: Cellular and
molecular mechanisms of drug dependence: An overview and update.
Indian J Psychiatry. 49:85–90. 2007.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chakrabarti S, Liu NJ and Gintzler AR:
Phosphorylation of unique C-terminal sites of the mu-opioid
receptor variants 1B2 and 1C1 influences their Gs association
following chronic morphine. J Neurochem. 152:449–467.
2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20(3328)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Jha MK, Jeon S and Suk K: Glia as a link
between neuroinflammation and neuropathic pain. Immune Netw.
12:41–47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Pinho-Ribeiro FA, Verri WA Jr and Chiu IM:
Nociceptor sensory neuron-immune interactions in pain and
inflammation. Trends Immunol. 38:5–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shavit Y, Wolf G, Goshen I, Livshits D and
Yirmiya R: Interleukin-1 antagonizes morphine analgesia and
underlies morphine tolerance. Pain. 115:50–59. 2005.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lacagnina MJ, Watkins LR and Grace PM:
Toll-like receptors and their role in persistent pain. Pharmacol
Ther. 184:145–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Eidson LN, Inoue K, Young LJ, Tansey MG
and Murphy AZ: Toll-like receptor 4 mediates morphine-induced
neuroinflammation and tolerance via soluble tumor necrosis factor
signaling. Neuropsychopharmacology. 42:661–670. 2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Illes P, Rubini P, Ulrich H, Zhao Y and
Tang Y: Regulation of microglial functions by purinergic mechanisms
in the healthy and diseased CNS. Cells. 9(1108)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Mohan S, Davis RL, DeSilva U and Stevens
CW: Dual regulation of mu opioid receptors in SK-N-SH neuroblastoma
cells by morphine and interleukin-1beta: Evidence for opioid-immune
crosstalk. J Neuroimmunol. 227:26–34. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ruzicka BB and Akil H: The
interleukin-1beta-mediated regulation of proenkephalin and opioid
receptor messenger RNA in primary astrocyte-enriched cultures.
Neuroscience. 79:517–524. 1997.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Liu DQ, Zhou YQ and Gao F: targeting
cytokines for morphine tolerance: A narrative review. Curr
Neuropharmacol. 17:366–376. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Allan SM, Tyrrell PJ and Rothwell NJ:
Interleukin-1 and neuronal injury. Nat Rev Immunol. 5:629–640.
2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Park JI, Strock CJ, Ball DW and Nelkin BD:
Interleukin-1beta can mediate growth arrest and differentiation via
the leukemia inhibitory factor/JAK/STAT pathway in medullary
thyroid carcinoma cells. Cytokine. 29:125–134. 2005.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chen ML, Cao H, Chu YX, Cheng LZ, Liang
LL, Zhang YQ and Zhao ZQ: Role of P2X7 receptor-mediated
IL-18/IL-18R signaling in morphine tolerance: Multiple
glial-neuronal dialogues in the rat spinal cord. J Pain.
13:945–958. 2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Janeway CA Jr and Medzhitov R: Innate
immune recognition. Annu Rev Immunol. 20:197–216. 2002.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801.
2006.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Hutchinson MR, Zhang Y, Shridhar M, Evans
JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler
J, et al: Evidence that opioids may have toll-like receptor 4 and
MD-2 effects. Brain Behav Immun. 24:83–95. 2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Eidson LN and Murphy AZ: Blockade of
Toll-like receptor 4 attenuates morphine tolerance and facilitates
the pain relieving properties of morphine. J Neurosci.
33:15952–15963. 2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Liu Y, Dai Y, Li Q, Chen C, Chen H, Song
Y, Hua F and Zhang Z: Beta-amyloid activates NLRP3 inflammasome via
TLR4 in mouse microglia. Neurosci Lett. 736(135279)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
de Rivero Vaccari JP, Dietrich WD and
Keane RW: Activation and regulation of cellular inflammasomes: Gaps
in our knowledge for central nervous system injury. J Cereb Blood
Flow Metab. 34:369–375. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kleibeuker W, Gabay E, Kavelaars A,
Zijlstra J, Wolf G, Ziv N, Yirmiya R, Shavit Y, Tal M and Heijnen
CJ: IL-1 beta signaling is required for mechanical allodynia
induced by nerve injury and for the ensuing reduction in spinal
cord neuronal GRK2. Brain Behav Immun. 22:200–208. 2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Grace PM, Loram LC, Christianson JP,
Strand KA, Flyer-Adams JG, Penzkover KR, Forsayeth JR, van Dam AM,
Mahoney MJ, Maier SF, et al: Behavioral assessment of neuropathic
pain, fatigue, and anxiety in experimental autoimmune
encephalomyelitis (EAE) and attenuation by interleukin-10 gene
therapy. Brain Behav Immun. 59:49–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Grace PM, Strand KA, Galer EL, Urban DJ,
Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI,
et al: Morphine paradoxically prolongs neuropathic pain in rats by
amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci
USA. 113:E3441–E3450. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Cunha TM, Roman-Campos D, Lotufo CM,
Duarte HL, Souza GR, Verri WA Jr, Funez MI, Dias QM, Schivo IR,
Domingues AC, et al: Morphine peripheral analgesia depends on
activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway.
Proc Natl Acad Sci USA. 107:4442–4447. 2010.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Roeckel LA, Le Coz GM, Gaveriaux-Ruff C
and Simonin F: Opioid-induced hyperalgesia: Cellular and molecular
mechanisms. Neuroscience. 338:160–182. 2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Jeffery MM, Chaisson CE, Hane C, Rumanes
L, Tucker J, Hang L, McCoy R, Chen CL, Bicket MC, Hooten WM, et al:
Assessment of potentially inappropriate prescribing of opioid
analgesics requiring prior opioid tolerance. JAMA Netw Open.
3(e202875)2020.PubMed/NCBI View Article : Google Scholar
|