Pain is an important global health problem. Recent
data suggest that >30% of the population in China suffers from a
form of chronic pain, including joint pain, headaches, severe back
pain and cancer-related pain, with a low rate of treatment
(1). The aetiology of pain is a
complex, transdisciplinary process with multiple causes, including
cancer, rheumatoid arthritis, spinal problems, injuries and surgery
(2). Individuals who experience
chronic pain usually have a reduced quality of life resulting from
both the physical and mental toll of this condition. For instance,
numerous individuals who suffer from chronic pain experience
depression, anxiety and even suicidal thoughts (3). Opiates, which were discovered
thousands of years ago, are to date the most common and effective
analgesics for the treatment of pain and pain-associated disorders
(4). Important progress has been
made in the development of opioids derived from opiates in the last
century, but several of their side effects persist (5). An Italian study revealed that the
average duration of opioid treatment in patients with cancer is
~105 days (6). Long-term opioid
treatment results in decreased analgesic efficacy via increased
tolerance and an increased potential for addiction (7).
Tolerance is defined as the reduction in the effects
of a drug following its prolonged administration, leading to the
loss of drug potency and the increase in dosage to maintain its
analgesic effects (8). However,
this increase in dosage may accelerate tolerance and its side
effects, including respiratory depression, gastrointestinal
immobility and addiction (8,9). The
drug interactions with opioid receptors and the dose and frequency
of administration are the primary reasons for the development and
extension of tolerance (10).
Numerous mechanisms are involved in opioid tolerance, including the
receptor downregulation and signalling desensitisation,
upregulation of drug metabolism and initiation of
compensatory/opponent processes (11). Because of the loss of the analgesic
effect and the severity of the side effects, drug tolerance is one
of the most challenging issues in the clinical application of
opioids and can ultimately lead to poor patient compliance and
treatment discontinuation (12).
Thus, a better understanding of the molecular mechanisms underlying
the development of opioid receptor tolerance, regulation and signal
transduction may help in the identification of novel strategies to
tackle these clinical problems.
Although it has been widely established that opioid
drugs are critical to proper pain management and that their
receptor desensitisation is closely associated with opioid
tolerance, the role of inflammation and the associated molecular
mechanisms in this phenomenon have not been well studied until
relatively recently (13). A
growing body of literature suggests that activated microglia and
astrocytes (glia) are the primary targets in pain management,
because of their function in pain transmission and opioid analgesia
(14,15). Morphine, one of the most effective
analgesics, affects the glia through Toll like receptor 4 (TLR4),
inducing proinflammatory cytokine production and increasing
morphine tolerance (16). Due to
its strong anti-analgesic effects and critical role in morphine
tolerance, IL-1β stands out among the morphine-induced cytokines
(17). Morphine treatment
upregulates IL-1β production, and antagonisation of the IL-1
receptor reverses morphine tolerance (14). Thus, both the NOD-like receptor
family pyrin domain containing 3 (NLRP3) inflammasome, a major
signalling pathway involved in IL-1β release, and TLR4 signalling
are receiving increasing attention for the regulation of morphine
tolerance (18).
The present review summarized the current knowledge
on the interactions between opioid systems and the function of
inflammatory factors and their impact on the development of opioid
tolerance. The cellular and molecular mechanisms involved in opioid
tolerance and the inflammatory factors implicated in TLR4- and
NLRP3-inflammasome-mediated tolerance responses were discussed. The
aim of the current review was to reveal the full image of opioid
receptors and inflammatory factors and their impact on opioid
tolerance and aid in the identification of potential therapeutic
targets for the enhancement of analgesic efficacy and the reduction
of side effects in chronic pain management.
Opioid receptors are a class of
seven-transmembrane-spanning inhibitory G protein-coupled
receptors, with a high affinity for β-endorphin and enkephalins and
a low affinity for dynorphins (19). They are widely expressed in the
pain-modulating descending pathways of various tissues, including
the brain, spinal cord, peripheral neurons and digestive tract
(20). The activation of these
receptors is critical for the analgesic effects of these drugs and
is achieved via the direct inhibition of the spinal cord neurons,
preventing pain signalling in the spinal cord (7,21).
There are four different types of opioid receptors related to
analgesia: µ opioid receptors (MORs), δ opioid receptors (DORs), κ
opioid receptors (KORs) and opioid receptor like-1 (ORL-1), which
are all well characterised at both the molecular and
pharmacological levels (22)
(Table I).
MORs are the most common and well-studied opioid
receptors in pain management. MORs were the first opioid receptors
identified in 1973(23). In 2012,
the first X-ray crystal structure of the murine MOR was
characterised. It revealed significant detail about ligand and
receptor binding patterns, providing valuable insights for the
identification of novel bioactive molecules and the development of
better drugs for pain management (24). The phosphorylation of MORs is one of
the most significant methods of receptor internalisation and
desensitisation, eventually causing opioid tolerance (25). There are >15 serine, threonine
and tyrosine residues in MORs, which are accessible for
phosphorylation by various protein kinases (26). Ser375 at the C-terminus of the rat
MOR is phosphorylated to varying degrees upon treatment with both
morphine and [D-Ala2, N-MePhe4,
Gly-ol]-enkephalin (DAMGO; a strong synthetic agonist of MORs)
(27). Another study has indicated
that the phosphorylation of Tyr166 inhibited the G protein
activation mediated by DAMGO (28).
In addition, multi-phosphorylation in the specific region of the
C-terminal tail of MORs has also been demonstrated to be involved
in the endocytosis of MORs (29).
Both point mutations and alanine substitution of the serine and
threonine residues from 375 to 379 (STANT sequence) has been
indicated to markedly decrease the agonist-induced recruitment of
β-arrestin and receptor internalisation. However, this did not
eliminate the induction of acute desensitisation (29-31),
suggesting that multiple amino acid residues are involved in the
regulation of MORs. These ligand-mediated differences also
indicated that receptor regulation and trafficking vary depending
on the agonist and require further elucidation.
Numerous studies have revealed that DORs and MORs
directly interact with each other to form heteromers, which have
been confirmed via co-immunoprecipitation (32,33).
Interestingly, DOR agonists not only activate DORs, but also induce
MOR internalisation and degradation, thereby inhibiting MOR agonist
activity and inducing morphine tolerance (34). It has been suggested that blocking
DOR function via gene knockout or DOR antagonists may reduce
morphine tolerance (33,35-38).
However, DOR knockout in mice presented conflicting results with
either no alteration (39) or in
certain cases, reduced morphine analgesic effects (40). This may be due to the high amino
acid sequence similarity (~60%) in murine DOR and MOR proteins.
High doses of their specific ligands can bind to both receptors and
enhance the analgesic effect (35).
Similar to MORs, phosphorylation of the C-terminal residues results
in DOR desensitisation and regulation. G protein-coupled receptor
kinase (GRK) 2 phosphorylates the Ser363 residue, which is a key
event in DOR regulation (41).
Thr353, at the COOH-terminal tail, is critical for the
downregulation of DORs in response to [D-Ala2,
D-Leu5]-enkephalin (42). The phosphorylation of Thr161 by
cyclin-dependent kinase 5 is required for DOR expression and the
production of MOR-DOR heterodimers (43), and serves an important role in the
development of morphine tolerance. Taken together, these studies
suggested that DOR phosphorylation may attenuate opioid tolerance
during pain management.
KORs represent another appealing therapeutic target
activated by endogenous dynorphin, a specific endogenous ligand of
KOR. KORs are phosphorylated, desensitised and internalised by
their agonists (44). Salvinorin A,
nalfurafine hydrochloride and type II thioesterase from the
rifamycin biosynthetic pathway, which are three structurally
distinct kappa ligands, induce KOR internalisation in a
dose-dependent manner with different potency rankings (45). The phosphorylation of Ser369
mediated by GRK and β-arrestin binding causes KOR desensitisation
and sustains analgesic tolerance. These results were confirmed both
in vitro in transfected cells and in vivo using mice
(46). In addition to its analgesic
benefit, KOR signalling also plays several other important roles
across the nervous system. It is involved in the mediation of
negative emotional states, such as drug reinstatement, depression
and aversion (47-50),
and its natural agonist, salvinorin A, functions as a psychoactive
drug (51,52).
ORL-1 is also known as the nociception opioid
peptide receptor. It is the most recently discovered opioid
receptor and is known to bind to its natural ligand nociceptin, a
17-amino acid neuropeptide (53).
Although ORL-1 shares high sequence identity with the classical
opioid receptors (µ, δ and κ), ORL-1 ligands possess low affinity
for these other opioid receptors (54,55).
Reciprocally, agonists of the classic opioid receptors, such as
opioid peptides or morphine-like compounds, possess low or no
affinity for ORL-1(56). Both
nociceptin and the ORL-1 agonist Ro646198 induce rapid
internalisation of ORL-1 in a concentration-dependent manner within
minutes of exposure, much faster than that of the other three
receptors (57), although they
share a similar internalisation mechanism. ORL-1 plays a critical
role in the regulation of several brain activities, including
instinctive and emotional behaviours (53). Studies on ORL-1 have indicated its
potential therapeutic use for non-addictive painkillers, depression
and Parkinson's disease in the future (58).
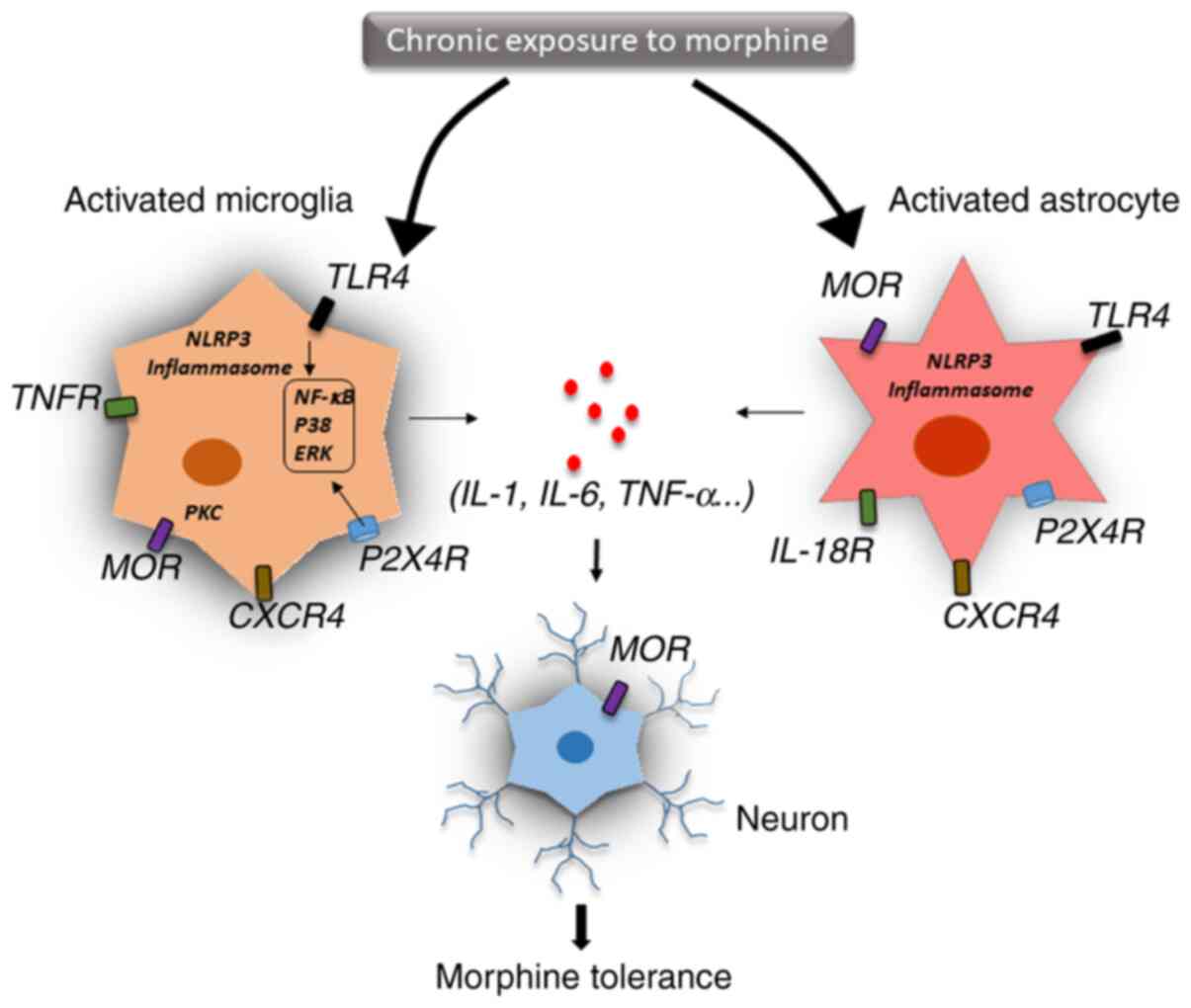
The two possible mechanisms involved in drug
tolerance are within-system and between-system adaptation (67). Within-system drug tolerance occurs
when opposite reactions are elicited within the same system. A
recent study has indicated that chronic morphine use induced a
shift in MOR signalling from the predominantly inhibitory Gi/Go
adenylyl cyclase to the stimulatory Gs adenylyl cyclase through the
upregulation of different MOR receptor variants (68). Specifically, chronic morphine use
induced the phosphorylation of the carboxyl terminal sites on
MOR-1B2 and MOR-1C1, enhancing Gs protein association (68). In between-system adaptation,
different drug-sensitive systems are linked to the drugs' primary
action system. Neuroinflammation and the release of proinflammatory
cytokines, as well as innate immune signalling, such as TLR4- and
NLRP3-mediated inflammasomes, are the key molecular mechanisms for
between-system adaptation (Fig. 2)
(69).
Glial cells are activated by cytokines in the
central nervous system and release other mediators that trigger
neuroinflammation (70). IL-1β, a
key proinflammatory cytokine, plays an important role in host
defence and inflammation and is a critical mediator of inflammatory
pain and opioid analgesia (71). In
a mouse study, the administration of IL-1β abolished morphine
analgesia, and genetic or pharmacological inhibition of IL-1
signalling prevented the development of morphine tolerance
(72). Prolonged morphine treatment
was indicated to induce glial TLR4 activation, which promoted
neurotoxicity and amplified nociceptive signalling in the spinal
cord (73). Moreover, chronic
morphine use activated TLR4 signalling in the brain, especially in
the periaqueductal grey region, resulting in changes in
inflammatory cytokine expression, thereby inducing glutamatergic
signalling and eventually leading to opioid tolerance (74). Expression of IL-1β could also be
induced by morphine via activated microglia, which disrupted
glutamate homeostasis by downregulating glutamate transporter 1 and
increasing glutamate, thereby triggering the release of ATP from
the glia. These events may contribute to excitotoxicity and chronic
inflammation, which leads to continued morphine discontinuation
(75). Interestingly, IL-1β
treatment significantly upregulated MOR mRNA expression in various
cell types, including primary astrocytes, neurons and microvascular
endothelial cells, which further supports the interaction between
IL-1β and the opioid system (76,77).
In addition, other inflammatory cytokines, such as TNF-α, IFN-α,
IL-4 and IL-6 are also associated with MOR expression in neural and
immune cells (78). IL-1β mediates
its function through IL-1 receptor type 1 protein (78). Previous studies have indicated that
IL-1β stimulation activated downstream signalling, including the
janus kinase-STAT, MAPK and NF-κB pathways, which altered MOR
transcriptional level (79,80). Chronic morphine treatment has been
indicated to upregulate purinergic P2X7 receptor (P2X7R) and
increase the expression of IL-18 in the microglia, IL-18 receptor
in astrocytes and protein kinase Cγ (PKCγ) in neurons of the spinal
dorsal horn. Thus, targeting the P2X7R/IL-18/PKCγ cascade may
present a novel therapeutic target for reducing and understanding
morphine tolerance in the clinical management of chronic pain
(81).
Since IL-1β and IL-18 are both classical cytokines
released during inflammasome activation, TLR4 and NLRP3 signalling
may be critical for opioid tolerance and should be investigated
more thoroughly (18). TLR4, a
member of the toll-like receptor family, can recognise specific
danger-associated molecular patterns and initiate an immune
response (82,83). A previous study reported that
morphine resulted in microglial activation via TLR4/myeloid
differentiation factor 2 binding (84). Blocking TLR4 signalling inhibited
microglial activation, attenuated morphine tolerance and
facilitated pain management (85).
It is well understood that TLR4 functions as a prime signal that
triggers downstream signalling pathways, enhancing the
transcription of NLRP3 and pro-IL-1β (86). Subsequently, a second signal
triggers several NLRP3 subunits into forming a protein complex
known as the inflammasome, which then recruits caspase-1, and
eventually leads to the maturation and secretion of IL-1β and
IL-18(87). Numerous studies have
suggested that the NLRP3 inflammasome plays an important role in
pain conditions, such as post-herpetic neuralgia, postoperative
pain and neuropathic pain through secreted proinflammatory
cytokines (88,89). It has been demonstrated that
inhibition of the NLRP3 inflammasome attenuated morphine tolerance
(90). A previous study reported
that morphine activated the potassium ATP channel, and blocking
this channel alleviated morphine tolerance by inhibiting the heat
shock protein 70/TLR4/NLRP3 cascade-mediated neuroinflammation
(91). Taken together, these
results suggested that TLR4/NLRP3 inflammasome-mediated
neuroinflammation is critical for morphine tolerance and pain
management.
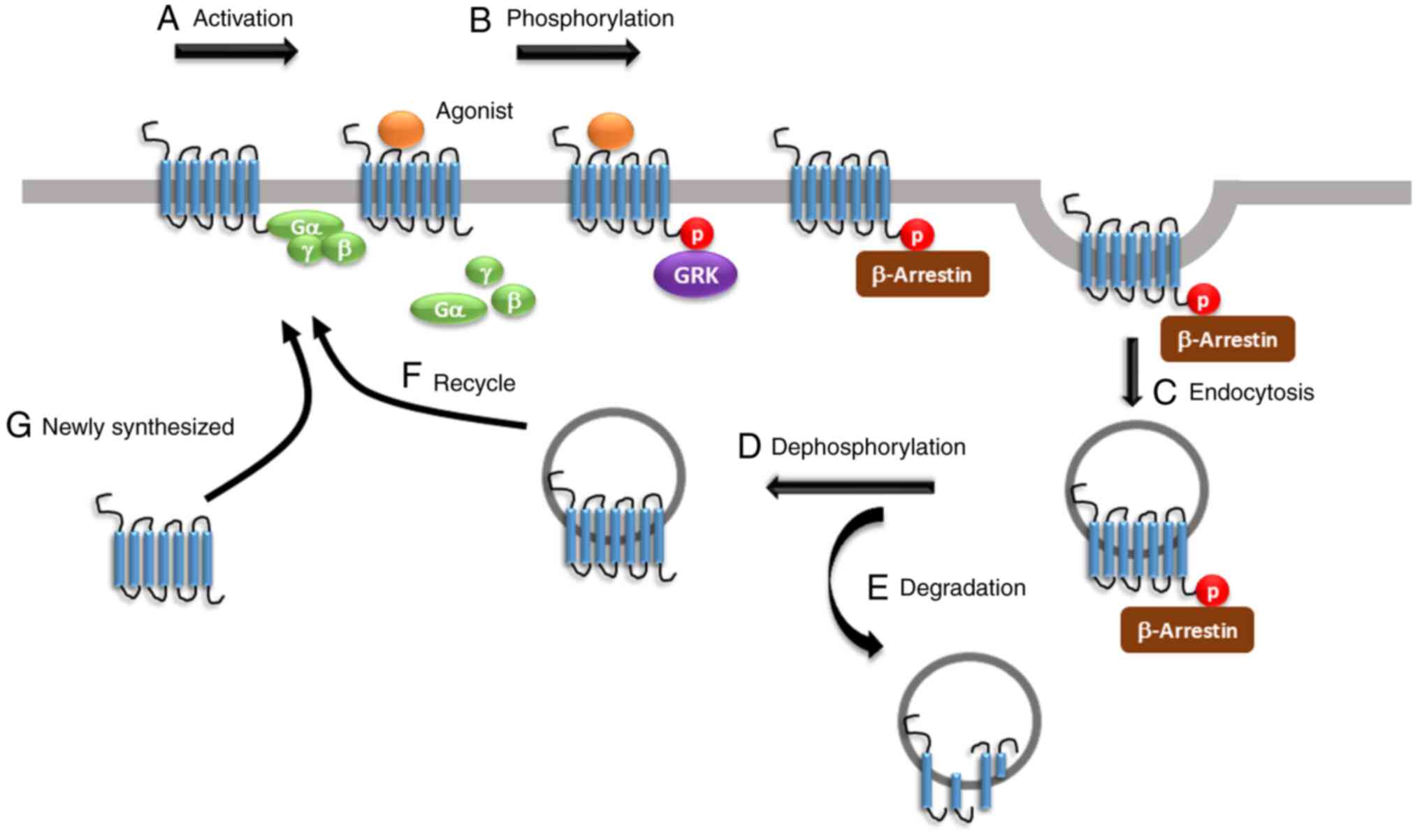
The present review summarized the underlying
molecular mechanisms of opioid tolerance facilitated by both
within-system and between-system regulation. Agonist activation
results in the phosphorylation of the opioid receptors by various
kinases, including GRK and PKC, leading to G protein uncoupling,
β-arrestin binding, receptor desensitisation and endocytosis,
followed by degradation or recycling. Drugs targeting opioid
receptors and inhibiting G protein dissociation or β-arrestin
binding are still in preclinical or early clinical studies designed
to evaluate their impact on opioid tolerance (25). Interestingly, prolonged opioid
treatment accelerates the production and secretion of
proinflammatory cytokines, such as IL-1β and IL-18, which suppress
morphine analgesia and lead to opioid tolerance (14). Inflammatory immune responses are
considered a critical contributor to the regulation of the opioid
receptors. Numerous studies have suggested that suppression of the
TLR4/NLRP3-mediated inflammasome activation and the inflammatory
response in microglia lead to an important attenuation of morphine
tolerance (18). Taken together,
these results could reveal novel therapeutic targets of anti-opioid
tolerance, from opioid receptors to inflammatory factors. Several
studies have indicated that inflammatory factors promote morphine
tolerance; however, how inflammatory factors act on opioid
receptors and how they affect opioid analgetic tolerance is still
unknown (8,78,92). A
better understanding of the association between inflammatory
factors and opioid receptors will help understand the mechanism
underlying opioid resistance and assist in the identification of
potential therapeutic targets, the development of anti-opioid drugs
and the treatment of its side effects. There is abundant basic
information describing the mechanism and treatment of morphine
tolerance, but there are few clinical trials completed to draw
definitive conclusions (93),
indicating that there is still a lack of effective measures and
methods to inhibit morphine tolerance. Multicentre randomised
controlled clinical trials are needed in the future. With the
development of biomarkers and genetic diagnostic tests, opioid
treatment could be personalized, which may aid in addressing opioid
tolerance and improving therapeutic outcomes for individual
patients. With a deepened understanding of the opioid lifecycle and
the underlying molecular mechanisms of morphine tolerance, more
effective drugs with fewer side effects may be produced in the
future.
Not applicable.
Funding: The present study was supported by Natural Science
Foundation of Zhejiang Province (grant nos. LQ21H270008 and
LQ18H270001), Zhejiang Province Top Discipline of Chinese Medicine
(grant no. ZTK2017A02) and Innovation Fund for Youth of Zhejiang
Chinese Medical University (grant no. KC201947).
Not applicable.
JZ led the present review. YJ, YL and JiF
contributed to the conception of the review. JZ and YL developed
the research methods, according to the comments and feedback from
YL and RM. JZ, JuF and XS performed the literature search and
applied the selection criteria. JZ and RM synthesised the data and
wrote the first draft of the manuscript. RM, JD and JiF contributed
to the modification of the first draft of the manuscript. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Yongjun Z, Tingjie Z, Xiaoqiu Y, Zhiying
F, Feng Q, Guangke X, Jinfeng L, Fachuan N, Xiaohong J and Yanqing
L: A survey of chronic pain in China. Libyan J Med.
15(1730550)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mills SEE, Nicolson KP and Smith BH:
Chronic pain: A review of its epidemiology and associated factors
in population-based studies. Br J Anaesth. 123:e273–e283.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Strand EB, Mengshoel AM, Sandvik L,
Helland IB, Abraham S and Nes LS: Pain is associated with reduced
quality of life and functional status in patients with Myalgic
Encephalomyelitis/Chronic Fatigue Syndrome. Scand J Pain. 19:61–72.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chou R, Hartung D, Turner J, Blazina I,
Chan B, Levander X, McDonagh M, Selph S, Fu R and Pappas M: Opioid
Treatments for Chronic Pain. Agency for Healthcare Research and
Quality (US), Rockville, MD, 2020.
|
|
5
|
Daoust R, Paquet J, Cournoyer A, Piette E,
Morris J, Lessard J, Castonguay V, Williamson D and Chauny JM: Side
effects from opioids used for acute pain after emergency department
discharge. Am J Emerg Med. 38:695–701. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roggeri D, Saramin C, Terrazzani G, Zusso
M, Giusti P and Chinellato A: Resource consumption and costs of
treating pain in patients affected by cancer in a district of
northeast Italy. Pharmacol Res. 56:329–334. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahlbeck K: Opioids: A two-faced Janus.
Curr Med Res Opin. 27:439–448. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eidson LN and Murphy AZ: Inflammatory
mediators of opioid tolerance: Implications for dependency and
addiction. Peptides. 115:51–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gulur P, Williams L, Chaudhary S, Koury K
and Jaff M: Opioid tolerance-a predictor of increased length of
stay and higher readmission rates. Pain Physician. 17:E503–E507.
2014.PubMed/NCBI
|
|
10
|
Martyn JAJ, Mao J and Bittner EA: Opioid
tolerance in critical illness. N Engl J Med. 380:365–378.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Allouche S, Noble F and Marie N: Opioid
receptor desensitization: Mechanisms and its link to tolerance.
Front Pharmacol. 5(280)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rosenblum A, Marsch LA, Joseph H and
Portenoy RK: Opioids and the treatment of chronic pain:
Controversies, current status, and future directions. Exp Clin
Psychopharmacol. 16:405–416. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Uniyal A, Gadepalli A and Akhilesh Tiwari
V: Underpinning the neurobiological intricacies associated with
opioid tolerance. ACS Chem Neurosci. 11:830–839. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liang Y, Chu H, Jiang Y and Yuan L:
Morphine enhances IL-1β release through toll-like receptor
4-mediated endocytic pathway in microglia. Purinergic Signal.
12:637–645. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sanna MD, Borgonetti V and Galeotti N: µ
Opioid receptor-triggered notch-1 activation contributes to
morphine tolerance: Role of neuron-glia communication. Mol
Neurobiol. 57:331–345. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang X, Loram LC, Ramos K, de Jesus AJ,
Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR
and Yin H: Morphine activates neuroinflammation in a manner
parallel to endotoxin. Proc Natl Acad Sci USA. 109:6325–6330.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Johnston IN, Milligan ED, Wieseler-Frank
J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P,
Fleshner M, et al: A role for proinflammatory cytokines and
fractalkine in analgesia, tolerance, and subsequent pain
facilitation induced by chronic intrathecal morphine. The Journal
of neuroscience: J Neurosci. 24:7353–7365. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang H, Zhang Y, Ma X, Wang W, Xu X, Huang
M, Xu L, Shi H, Yuan T, Jiang W, et al: Spinal TLR4/P2X7
receptor-dependent NLRP3 inflammasome activation contributes to the
development of tolerance to morphine-induced antinociception. J
Inflamm Res. 13:571–582. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pasternak GW and Pan YX: Mu opioids and
their receptors: Evolution of a concept. Pharmacol Rev.
65:1257–1317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yam MF, Loh YC, Tan CS, Khadijah Adam S,
Abdul Manan N and Basir R: General pathways of pain sensation and
the major neurotransmitters involved in pain regulation. Int J Mol
Sci. 19(2164)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McNicol E, Horowicz-Mehler N, Fisk RA,
Bennett K, Gialeli-Goudas M, Chew PW, Lau J and Carr D: Americal
Pain Society. Management of opioid side effects in cancer-related
and chronic noncancer pain: A systematic review. J Pain. 4:231–256.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dougall IG: A critical review of the
classification of opioid receptors. Biotechnol Appl Biochem.
10:488–499. 1988.PubMed/NCBI
|
|
23
|
Pert CB and Snyder SH: Opiate receptor:
Demonstration in nervous tissue. Science. 179:1011–1014.
1973.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Manglik A, Kruse AC, Kobilka TS, Thian FS,
Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK and Granier
S: Crystal structure of the micro-opioid receptor bound to a
morphinan antagonist. Nature. 485:321–326. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Williams JT, Ingram SL, Henderson G,
Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ and Christie
MJ: Regulation of µ-opioid receptors: Desensitization,
phosphorylation, internalization, and tolerance. Pharmacol Rev.
65:223–254. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chavkin C, McLaughlin JP and Celver JP:
Regulation of opioid receptor function by chronic agonist exposure:
Constitutive activity and desensitization. Mol Pharmacol. 60:20–25.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schulz S, Mayer D, Pfeiffer M, Stumm R,
Koch T and Hollt V: Morphine induces terminal micro-opioid receptor
desensitization by sustained phosphorylation of serine-375. EMBO J.
23:3282–3289. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Clayton CC, Bruchas MR, Lee ML and Chavkin
C: Phosphorylation of the mu-opioid receptor at tyrosine 166
(Tyr3.51) in the DRY motif reduces agonist efficacy. Mol Pharmacol.
77:339–347. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lau EK, Trester-Zedlitz M, Trinidad JC,
Kotowski SJ, Krutchinsky AN, Burlingame AL and von Zastrow M:
Quantitative encoding of the effect of a partial agonist on
individual opioid receptors by multisite phosphorylation and
threshold detection. Sci Signal. 4(ra52)2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arttamangkul S, Heinz DA, Bunzow JR, Song
X and Williams JT: Cellular tolerance at the micro-opioid receptor
is phosphorylation dependent. Elife. 7(e34989)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Birdsong WT, Arttamangkul S, Bunzow JR and
Williams JT: Agonist binding and desensitization of the µ-opioid
receptor is modulated by phosphorylation of the C-terminal tail
domain. Mol Pharmacol. 88:816–824. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fan T, Varghese G, Nguyen T, Tse R, O'Dowd
BF and George SR: A role for the distal carboxyl tails in
generating the novel pharmacology and G protein activation profile
of mu and delta opioid receptor hetero-oligomers. J Biol Chem.
280:38478–38488. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gomes I, Gupta A, Filipovska J, Szeto HH,
Pintar JE and Devi LA: A role for heterodimerization of mu and
delta opiate receptors in enhancing morphine analgesia. Proc Natl
Acad Sci USA. 101:5135–5139. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B,
Wang HB, Li Q, Yang H, Luo J, Li ZY, et al: Facilitation of
µ-opioid receptor activity by preventing δ-opioid receptor-mediated
codegradation. Neuron. 69:120–131. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang D, Tawfik VL, Corder G, Low SA,
Francois A, Basbaum AI and Scherrer G: functional divergence of
delta and mu opioid receptor organization in CNS pain circuits.
Neuron. 98:90–108, e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chefer VI and Shippenberg TS: Augmentation
of morphine-induced sensitization but reduction in morphine
tolerance and reward in delta-opioid receptor knockout mice.
Neuropsychopharmacology. 34:887–898. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fujita W, Gomes I and Devi LA: Heteromers
of µ-δ opioid receptors: new pharmacology and novel therapeutic
possibilities. Br J Pharmacol. 172:375–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schiller PW: Opioid peptide-derived
analgesics. AAPS J. 7:E560–E565. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Scherrer G, Imamachi N, Cao YQ, Contet C,
Mennicken F, O'Donnell D, Kieffer BL and Basbaum AI: Dissociation
of the opioid receptor mechanisms that control mechanical and heat
pain. Cell. 137:1148–1159. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhu Y, King MA, Schuller AG, Nitsche JF,
Reidl M, Elde RP, Unterwald E, Pasternak GW and Pintar JE:
Retention of supraspinal delta-like analgesia and loss of morphine
tolerance in delta opioid receptor knockout mice. Neuron.
24:243–252. 1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei
G and Ma L: Identification of G protein-coupled receptor kinase 2
phosphorylation sites responsible for agonist-stimulated
delta-opioid receptor phosphorylation. Mol Pharmacol. 58:1050–1056.
2000.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Al-Hasani R and Bruchas MR: Molecular
mechanisms of opioid receptor-dependent signaling and behavior.
Anesthesiology. 115:1363–1381. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xie WY, He Y, Yang YR, Li YF, Kang K, Xing
BM and Wang Y: Disruption of Cdk5-associated phosphorylation of
residue threonine-161 of the delta-opioid receptor: Impaired
receptor function and attenuated morphine antinociceptive
tolerance. J Neurosci. 29:3551–3564. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jean-Charles PY, Kaur S and Shenoy SK: G
Protein-coupled receptor signaling through β-arrestin-dependent
mechanisms. J Cardiovasc Pharmacol. 70:142–158. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Y, Tang K, Inan S, Siebert D,
Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A and Liu-Chen LY:
Comparison of pharmacological activities of three distinct kappa
ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors
in vitro and their antipruritic and antinociceptive activities in
vivo. J Pharmacol Exp Ther. 312:220–230. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
McLaughlin JP, Myers LC, Zarek PE, Caron
MG, Lefkowitz RJ, Czyzyk TA, Pintar JE and Chavkin C: Prolonged
kappa opioid receptor phosphorylation mediated by G-protein
receptor kinase underlies sustained analgesic tolerance. J Biol
Chem. 279:1810–1818. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lalanne L, Ayranci G, Kieffer BL and Lutz
PE: The kappa opioid receptor: From addiction to depression, and
back. Front Psychiatry. 5(170)2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lutz PE and Kieffer BL: Opioid receptors:
Distinct roles in mood disorders. Trends Neurosci. 36:195–206.
2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nygard SK, Hourguettes NJ, Sobczak GG,
Carlezon WA and Bruchas MR: Stress-induced reinstatement of
nicotine preference requires dynorphin/kappa opioid activity in the
basolateral amygdala. J Neurosci. 36:9937–9948. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Taylor GT and Manzella F: Kappa opioids,
salvinorin a and major depressive disorder. Curr Neuropharmacol.
14:165–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sheffler DJ and Roth BL: Salvinorin A: The
‘magic mint’ hallucinogen finds a molecular target in the kappa
opioid receptor. Trends Pharmacol Sci. 24:107–109. 2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chavkin C, Sud S, Jin W, Stewart J,
Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ and Roth BL:
Salvinorin A, an active component of the hallucinogenic sage salvia
divinorum is a highly efficacious kappa-opioid receptor agonist:
Structural and functional considerations. J Pharmacol Exp Ther.
308:1197–1203. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mollereau C and Mouledous L: Tissue
distribution of the opioid receptor-like (ORL1) receptor. Peptides.
21:907–917. 2000.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Thompson AA, Liu W, Chun E, Katritch V, Wu
H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, et al:
Structure of the nociceptin/orphanin FQ receptor in complex with a
peptide mimetic. Nature. 485:395–399. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen Y, Fan Y, Liu J, Mestek A, Tian M,
Kozak CA and Yu L: Molecular cloning, tissue distribution and
chromosomal localization of a novel member of the opioid receptor
gene family. FEBS Lett. 347:279–283. 1994.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mogil JS and Pasternak GW: The molecular
and behavioral pharmacology of the orphanin FQ/nociceptin peptide
and receptor family. Pharmacol Rev. 53:381–415. 2001.PubMed/NCBI
|
|
57
|
Spampinato S, Baiula M and Calienni M:
Agonist-regulated internalization and desensitization of the human
nociceptin receptor expressed in CHO cells. Curr Drug Targets.
8:137–146. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zaveri NT: Nociceptin opioid receptor
(NOP) as a therapeutic target: Progress in translation from
preclinical research to clinical utility. J Med Chem. 59:7011–7028.
2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zamponi GW and Snutch TP: Modulating
modulation: Crosstalk between regulatory pathways of presynaptic
calcium channels. Mol Interv. 2:476–478. 2002.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zamponi GW and Snutch TP: Modulation of
voltage-dependent calcium channels by G proteins. Curr Opin
Neurobiol. 8:351–356. 1998.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Torrecilla M, Quillinan N, Williams JT and
Wickman K: Pre- and postsynaptic regulation of locus coeruleus
neurons after chronic morphine treatment: A study of GIRK-knockout
mice. Eur J Neurosci. 28:618–624. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Diaz A, Florez J, Pazos A and Hurle MA:
Opioid tolerance and supersensitivity induce regional changes in
the autoradiographic density of dihydropyridine-sensitive calcium
channels in the rat central nervous system. Pain. 86:227–235.
2000.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bohn LM, Lefkowitz RJ and Caron MG:
Differential mechanisms of morphine antinociceptive tolerance
revealed in (beta)arrestin-2 knock-out mice. J Neurosci.
22:10494–10500. 2002.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bernstein MA and Welch SP: mu-Opioid
receptor down-regulation and cAMP-dependent protein kinase
phosphorylation in a mouse model of chronic morphine tolerance.
Brain Res Mol Brain Res. 55:237–242. 1998.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Stafford K, Gomes AB, Shen J and Yoburn
BC: mu-Opioid receptor downregulation contributes to opioid
tolerance in vivo. Pharmacol Biochem Behav. 69:233–237.
2001.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Fábián G, Bozó B, Szikszay M, Horváth G,
Coscia CJ and Szücs M: Chronic morphine-induced changes in
mu-opioid receptors and G proteins of different subcellular loci in
rat brain. J Pharmacol Exp Ther. 302:774–780. 2002.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gupta S and Kulhara P: Cellular and
molecular mechanisms of drug dependence: An overview and update.
Indian J Psychiatry. 49:85–90. 2007.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chakrabarti S, Liu NJ and Gintzler AR:
Phosphorylation of unique C-terminal sites of the mu-opioid
receptor variants 1B2 and 1C1 influences their Gs association
following chronic morphine. J Neurochem. 152:449–467.
2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20(3328)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Jha MK, Jeon S and Suk K: Glia as a link
between neuroinflammation and neuropathic pain. Immune Netw.
12:41–47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Pinho-Ribeiro FA, Verri WA Jr and Chiu IM:
Nociceptor sensory neuron-immune interactions in pain and
inflammation. Trends Immunol. 38:5–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shavit Y, Wolf G, Goshen I, Livshits D and
Yirmiya R: Interleukin-1 antagonizes morphine analgesia and
underlies morphine tolerance. Pain. 115:50–59. 2005.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lacagnina MJ, Watkins LR and Grace PM:
Toll-like receptors and their role in persistent pain. Pharmacol
Ther. 184:145–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Eidson LN, Inoue K, Young LJ, Tansey MG
and Murphy AZ: Toll-like receptor 4 mediates morphine-induced
neuroinflammation and tolerance via soluble tumor necrosis factor
signaling. Neuropsychopharmacology. 42:661–670. 2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Illes P, Rubini P, Ulrich H, Zhao Y and
Tang Y: Regulation of microglial functions by purinergic mechanisms
in the healthy and diseased CNS. Cells. 9(1108)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Mohan S, Davis RL, DeSilva U and Stevens
CW: Dual regulation of mu opioid receptors in SK-N-SH neuroblastoma
cells by morphine and interleukin-1beta: Evidence for opioid-immune
crosstalk. J Neuroimmunol. 227:26–34. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ruzicka BB and Akil H: The
interleukin-1beta-mediated regulation of proenkephalin and opioid
receptor messenger RNA in primary astrocyte-enriched cultures.
Neuroscience. 79:517–524. 1997.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Liu DQ, Zhou YQ and Gao F: targeting
cytokines for morphine tolerance: A narrative review. Curr
Neuropharmacol. 17:366–376. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Allan SM, Tyrrell PJ and Rothwell NJ:
Interleukin-1 and neuronal injury. Nat Rev Immunol. 5:629–640.
2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Park JI, Strock CJ, Ball DW and Nelkin BD:
Interleukin-1beta can mediate growth arrest and differentiation via
the leukemia inhibitory factor/JAK/STAT pathway in medullary
thyroid carcinoma cells. Cytokine. 29:125–134. 2005.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chen ML, Cao H, Chu YX, Cheng LZ, Liang
LL, Zhang YQ and Zhao ZQ: Role of P2X7 receptor-mediated
IL-18/IL-18R signaling in morphine tolerance: Multiple
glial-neuronal dialogues in the rat spinal cord. J Pain.
13:945–958. 2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Janeway CA Jr and Medzhitov R: Innate
immune recognition. Annu Rev Immunol. 20:197–216. 2002.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801.
2006.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Hutchinson MR, Zhang Y, Shridhar M, Evans
JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler
J, et al: Evidence that opioids may have toll-like receptor 4 and
MD-2 effects. Brain Behav Immun. 24:83–95. 2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Eidson LN and Murphy AZ: Blockade of
Toll-like receptor 4 attenuates morphine tolerance and facilitates
the pain relieving properties of morphine. J Neurosci.
33:15952–15963. 2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Liu Y, Dai Y, Li Q, Chen C, Chen H, Song
Y, Hua F and Zhang Z: Beta-amyloid activates NLRP3 inflammasome via
TLR4 in mouse microglia. Neurosci Lett. 736(135279)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
de Rivero Vaccari JP, Dietrich WD and
Keane RW: Activation and regulation of cellular inflammasomes: Gaps
in our knowledge for central nervous system injury. J Cereb Blood
Flow Metab. 34:369–375. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kleibeuker W, Gabay E, Kavelaars A,
Zijlstra J, Wolf G, Ziv N, Yirmiya R, Shavit Y, Tal M and Heijnen
CJ: IL-1 beta signaling is required for mechanical allodynia
induced by nerve injury and for the ensuing reduction in spinal
cord neuronal GRK2. Brain Behav Immun. 22:200–208. 2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Grace PM, Loram LC, Christianson JP,
Strand KA, Flyer-Adams JG, Penzkover KR, Forsayeth JR, van Dam AM,
Mahoney MJ, Maier SF, et al: Behavioral assessment of neuropathic
pain, fatigue, and anxiety in experimental autoimmune
encephalomyelitis (EAE) and attenuation by interleukin-10 gene
therapy. Brain Behav Immun. 59:49–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Grace PM, Strand KA, Galer EL, Urban DJ,
Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI,
et al: Morphine paradoxically prolongs neuropathic pain in rats by
amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci
USA. 113:E3441–E3450. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Cunha TM, Roman-Campos D, Lotufo CM,
Duarte HL, Souza GR, Verri WA Jr, Funez MI, Dias QM, Schivo IR,
Domingues AC, et al: Morphine peripheral analgesia depends on
activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway.
Proc Natl Acad Sci USA. 107:4442–4447. 2010.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Roeckel LA, Le Coz GM, Gaveriaux-Ruff C
and Simonin F: Opioid-induced hyperalgesia: Cellular and molecular
mechanisms. Neuroscience. 338:160–182. 2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Jeffery MM, Chaisson CE, Hane C, Rumanes
L, Tucker J, Hang L, McCoy R, Chen CL, Bicket MC, Hooten WM, et al:
Assessment of potentially inappropriate prescribing of opioid
analgesics requiring prior opioid tolerance. JAMA Netw Open.
3(e202875)2020.PubMed/NCBI View Article : Google Scholar
|