Introduction
Excessive alcohol consumption has become one of the
major causes of diverse diseases (1), and alcohol abuse results in ~3 million
deaths per year and is accountable for ~5.1% of the global burden
of disease according to the report of the World Health Organization
for the year 2018(2). The toxic
effects of alcohol on the human body, particularly intestine and
liver tissues, are largely attributed to alcohol-induced metabolic
disorders and oxidative stress, and damage to energy homeostasis
(3). Of note, studies have revealed
that alcohol disturbs the gut microbiota imbalance and damages the
gut barrier to cause translocation of microbial products such as
lipopolysaccharide (LPS) (4-6).
Alcohol-associated endotoxemia also contributes to the development
and progression of liver inflammation and activation of the innate
immune response (7,8).
The mammalian gastrointestinal tract is colonized by
trillions of microorganisms that coevolved with their hosts
(9). It is established that the gut
microbiota is dynamic and it is pertinent to a wide variety of
diseases, including inflammatory bowel disease, obesity, diabetes,
Parkinson's disease and even cancer (10-14).
The gut microbiota dysbiosis induced by alcohol exposure has been
well-characterized, with increased abundance of Proteobacteria and
Actinobacteria and decreased abundance of Lachnospiraceae,
Ruminococcaceae and Bifidobacterium (4,5,15,16).
Of these, the overgrowth of Proteobacteria, particularly
Enterobacteriaceae, leads to disorders of the healthy gut
microbiota and promotes its pathogenic potential by producing
endotoxins, such as LPS. In addition, the damaged intestinal
barrier enables LPS translocation from the intestinal lumen into
the portal circulation (17), which
is subsequently recognized by the toll-like receptor 4 (TLR4)
complex and induces specific intracellular signalling pathways
affecting inflammation (18).
It is well recognized that temperance is the best
way to prevent the damage of alcohol to human body (19). However, the alcohol addiction and
drinking culture often allow easy exposure of humans to alcohol, at
least in the Chinese and Russian population (20,21).
Therefore, there is in urgent need for exploring novel approaches
to alleviate the alcoholic injury in humans. Yogurt containing
Lactobacillus or other probiotics has gradually become a
popular beverage in China (https://marketingtochina.com/yogurt-health-supplement-for-chinese/).
Probiotics have been demonstrated to be able to prevent the
occurrence and progression of ethanol-induced injury. Specifically,
supplementation with Lactobacillus species, particularly
Lactobacillus acidophilus (LA), is able to restore the gut
microbiota homeostasis and effectively attenuate alcohol-induced
liver injury via reducing the accumulation of plasma endotoxin
(22). Furthermore, administration
of Lactobacillus species is able to prevent harmful bacteria
from residing in the intestine due to their product, lactic acid
(23), and increase the abundance
of other healthy bacteria, such as Bifidobacteria (24), which likely contributes to the
maintenance of the immune balance of the intestine (25). On the other hand, a variety of
studies suggested that alcohol consumption leads to vitamin C (VC)
deficiency and VC supplementation was able to alleviate
ethanol-induced impairment (26,27).
While treatment with single LA or VC is capable of improving
alcohol-induced injury in mice to a certain extent, this level of
improvement may not be sufficient to support their further
application, particularly in the clinic.
Therefore, the aim of the present study was to
determine whether symbiotic supplementation of LA plus VC is able
to reduce ethanol-induced intestine and liver injury by modulating
gut microbiota dysbiosis and restoring intestinal barrier function
in mice.
Materials and methods
Bacterial strains and VC
preparation
Lactobacillus acidophilus (cat. no. AS1.3342;
Biobw Biotechnology Co., Ltd.) was used in the present study.
Separate colonies of LA were cultured in 5 ml MRS broth (cat. no.
M8540; Beijing Solarbio Science & Technology Co., Ltd.) at 37˚C
for 48 h with shaking (200 rpm) under aerobic conditions.
Subsequently, the cultures were centrifuged at 12,000 x g for 5 min
at 4˚C, diluted in sterile normal saline solution and mixed
thoroughly to obtain the appropriate bacterial density [LA:
5x108 colony-forming units (CFU)/ml]. VC (cat. no.
A8100; Solarbio Life Sciences) was dissolved in sterile water and
gavage-fed to mice daily at a dose of 100 mg/kg.
Construction of an ethanol-fed mouse
model
The animal experiments of the present study were
approved by the Ethics and Clinical Research Committee of Tianjin
Medical University (Tianjin, China). Forty male C57BL/6J mice (age,
7-8 weeks; weight, 21-23 g) were obtained from Huafukang Biological
Technology Co., Ltd. and maintained in a specific pathogen-free
environment at 23˚C and 40-60% humidity with a 12-h light/12-h dark
cycle. The ethanol feeding mouse model was constructed based on the
Lieber-DeCarli diet (cat. no. TP 4030C/TP 4030A; TROPHIC Animal
Feed High-Tech Co. Ltd.) (28). All
of the mice were randomly divided (10 mice per group) into 5
groups: Control group (Ctrl), ethanol-fed group (EH), ethanol-fed
and LA supplementation group (LA), ethanol-fed and VC
supplementation group (VC), ethanol-fed and LA plus VC
supplementation group (LA+VC). All of the mice were allowed to
adapt to the laboratory environment for 2 days and to the
ethanol-free liquid diet for another 3 days, and then, the diet
containing ethanol (5% vol/vol) was fed to these ethanol-fed mice
for 10 days, while the control mice received an isocaloric amount
of maltodextrin. LA (~1x108 CFU per mouse) in saline
solution, VC (100 mg/kg body weight) in saline solution or vehicle
alone (saline solution) were gavage-fed to mice daily after 3 days
of ethanol-free liquid diet acclimatization. On the last day of the
experiment, the groups of EH, LA, VC and LA+VC received a single
dose of ethanol via gavage (5 g/kg body weight), and the control
mice received a single dose of maltodextrin (9 g/kg body weight).
After 9 h, all mice were anaesthetised (4% isoflurane by
inhalation) until loss of paw reflex, exsanguinated and then
euthanized by cervical dislocation. Subsequently, distal colon
tissues (~1 cm) were collected for H&E staining and fixed in 4%
paraformaldehyde. The proximal colon tissues (~1 cm) were collected
for reverse transcription-quantitative (RT-q)PCR (stored at -80˚C),
and the remaining middle part of the colon was used for flow
cytometry.
FITC assays
To determine intestinal permeability, FITC-dextran
(cat. no. 68059; Sigma-Aldrich; Merck KGaA) was orally administered
to mice (600 mg/kg body weight) at 4 h prior to sacrifice. These
blood samples were collected from the isoflurane-anesthetised mice
and blood samples were centrifuged (2,000 x g, 4˚C) for 10 min to
obtain serum (200 µl). The fluorescence of these serum samples was
immediately recorded by a spectrophotometer (Tecan) at an
excitation wavelength of 485 nm and emission wavelength of 528
nm.
Isolation of lymphocytes and flow
cytometry
For all mice, the middle part of colon tissues was
collected and the colon lamina propria lymphocytes (LPMCs) were
prepared as described previously (29). In brief, after clearance of feces,
residual mesenteric fat tissue and Peyer's patches of the colon
were resected, cut into 1-cm pieces and washed in ice-cold PBS.
After digestion with predigestion solution (Hank's balanced salt
solution and 5 mM EDTA) and digestion solution [collagenase D (cat.
no. DH073-2; Beijing Dingguo Changsheng Biotechnology Co., Ltd.),
DNase I (cat. no. DH113-5; Beijing Dingguo Changsheng Biotechnology
Co., Ltd.) and dispase (cat. no. S10013; Shanghai Yuanye
Biotechnology Co., Ltd.)], the collected cells were further
purified by a Percoll gradient (40/80%) and the LPMCs were
collected and washed with PBS supplemented with 10% fetal bovine
serum (Zhejiang Tianhang Biotechnology Co., Ltd.) for flow
cytometric analysis. The cells were then stained with
anti-CD45-peridinin chlorophyll (1:500; cat. no. 103130; Biolegend)
and anti-CD4-FITC (1:500; cat. no. 100510; Biolegend) diluted in 1X
PBS at 4˚C in the dark for 45 min, and then fixed and permeabilized
with the forkhead box (Fox)P3/True-Nuclear™ Transcription factor
buffer set (cat. no. 424401; Biolegend) and stained with
anti-FoxP3-phycoerythrin (1:500; cat. no. 320007; Biolegend)
diluted in DMEM medium (cat. no. 31600; Beijing Solarbio Science
& Technology Co., Ltd.) at 4˚C in the dark for 45 min.
Biochemical analysis
Colon samples (100 mg) were homogenized with a
mini-bead beater (cat. no. KZ-II) and glass beads (cat. no.
G0101-200G; both from Wuhan Servicebio Technology Co., Ltd.) in 1
ml 1X PBS buffer and 700 µl supernatant was transferred to a new
centrifuge tube after centrifugation. The superoxide dismutase
(SOD) kit (cat. no. BC0170; Solarbio Life Sciences),
myeloperoxidase (MPO) kit (cat. no. ab105136; Abcam) and
glutathione peroxidase (GSH-PX) kit (cat. no. BC1190; Solarbio Life
Sciences) were used to determine the SOD activity, MPO activity and
GSH-PX activity according to the manufacturer's protocols. Serum
aspartate transaminase (AST) and alanine transaminase (ALT) were
determined by a blood biochemical analyser (Fujifilm DRI-CHEM
3500s; Fujifilm) according to the manufacturer's protocol. Mouse
ELISA Kits were used to determine the serum levels of LPS (cat. no.
JL20691-96T; Jiang Lai Biological), TNF-α (cat. no. ml002095;
Enzyme Link Biotechnology Co., Ltd.), IL-1β (cat. no. ml301814;
Enzyme Link Biotechnology Co., Ltd.) and IL-6 (cat. no. M*ml002301;
Enzyme Link Biotechnology Co., Ltd.), and spectrophotometric
methods were used to measure hepatic triglyceride (cat. no.
JL46662-96T; Jiang Lai Biological) and hepatic malonaldehyde (MDA;
cat. no. JL13329-96T; Jiang Lai Biological) via a spectrophotometer
(Infinite F50; Tecan Group, Ltd.). All the assays were performed in
triplicate and all the experiments were performed according to the
manufacturer's protocol.
Histopathological observation
For the histological analysis, the liver and colonic
tissues were stained with hematoxylin and eosin (H&E). In
brief, the tissues were fixed in 10% formalin for 48 h at room
temperature, and paraffin-embedded sections (5 µM) were stained
with H&E. For the evaluation of mucins (Muc), Alcian
Blue-Periodic acid-Schiff (AB-PAS) Stain Kit (cat. no. G1285;
Beijing Solarbio Science & Technology Co., Ltd.) was used to
stain the paraffin-embedded intestinal tissue sections according to
the manufacturer's protocol. H&E- and AB-PAS-stained colonic
sections were observed under an optical microscope (IX73; Olympus
Corporation) at x100 magnification.
RNA isolation and gene expression
analysis
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and the RNA
concentration was quantified using the NanoPhotometer®
N50 (Implen) and RT was performed with the PrimeScript RT reagent
kit with gDNA Eraser (cat. no. RR047Q; Takara Biotechnology Co.
Ltd.) according to the manufacturer's protocol. RT-qPCR was
performed on a LightCycler 96 System (Roche) using TB Green Premix
Ex Taq II (Tli RNaseH Plus; cat. no. RR820A; Takara Biotechnology
Co. Ltd.) and a cycling program of initial denaturation for 10 min
at 95˚C, then 40 cycles of 10 sec at 95˚C, 10 sec at 62˚C and 10
sec at 72˚C, followed by 95˚C for 60 sec and a dissociation curve
analysis. The primer sequences are listed in Table SI and the relative gene expression
was normalized to 18S and calculated by the 2-ΔΔCq
method (30).
DNA extraction and 16S ribosomal RNA
amplification sequencing
Faecal genomic DNA was collected from 150-200 mg of
fecal samples by the QIAamp PowerFecal DNA Kit (cat. no. 51804;
Qiagen GmbH). The hypervariable V3-V4 region (341F and 805R) was
amplified and purified. Sequencing was performed on the paired-end
Illumina MiSeq PE300 (2x300 bp) platform (Illumina, Inc.) at
Novogene Corp. according to the manufacturer's protocol. These raw
sequences were processed using the QIIME (v1.9.1) pipeline
(31) and the gut microbiota
diversity and composition of fecal samples were determined.
Statistical analysis
All experimental results were obtained from at least
three independent experiments. Values are expressed as the mean ±
standard deviation. Statistical comparisons were performed by
one-way ANOVA and Tukey's post-hoc test. GraphPad Prism 8.0
(GraphPad Software, Inc.) was used for statistical analysis and R
(version 3.6.3) was used for plotting the graphs. P<0.05 was
considered to indicate a statistically significant difference.
Results
LA plus VC treatment ameliorates
ethanol-induced injury in mice
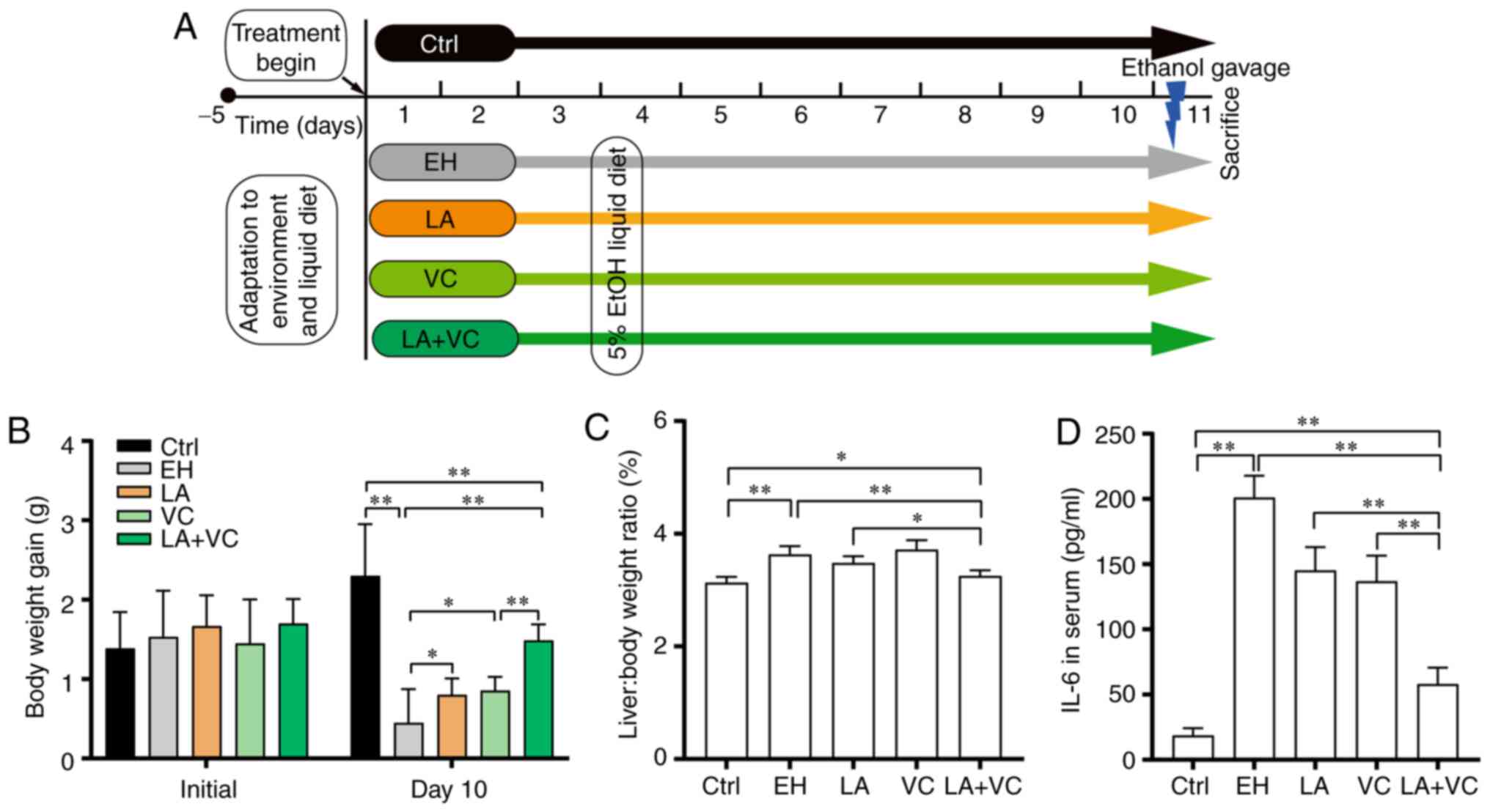
The effects of LA plus VC to reduce ethanol-induced
injury were explored using the NIAAA model (mouse model of chronic
and binge ethanol feeding) as described previously (28) (Fig.
1A). In the present study, ethanol treatment significantly
lowered the body weight gain of the mice (Ctrl: 2.30±0.97 g; EH:
0.45±0.52 g; P<0.01; Fig. 1B)
and obviously increased the liver/body weight ratio (P<0.01;
Fig. 1C). Supplementation with LA
or VC slightly alleviated the decline in body weight gain [LA:
0.80±0.25 g, P<0.05 (LA vs. EH); VC: 0.86±0.21 g, P<0.05 (VC
vs. EH); Fig. 1B] and
supplementation with LA partially reduced the liver/body weight
ratio (Fig. 1C). Of note, the
efficiency of alleviating the decline in body weight gain [LA+VC:
1.48±0.27 g; P<0.01 (LA+VC vs. EH); Fig. 1B] and reducing the liver/body weight
ratio [P<0.01 (LA+VC vs. EH); Fig.
1C] of LA+VC was greater than that of single administration of
LA or VC. Furthermore, the efficiency of attenuating the IL-6
levels by supplementation with LA+VC [P<0.01 (LA+VC vs. EH);
Fig. 1D] was greater than that of
the single treatments. In conclusion, treatment of mice with LA+VC
attenuated ethanol-induced injury more effectively than single LA
or VC supplementation.
LA plus VC treatment restores the gut
microbiota homeostasis
Chronic ethanol consumption is a major cause of gut
microbiota dysbiosis, which may support the pathophysiology of
ethanol-related morbidity (5,32). In
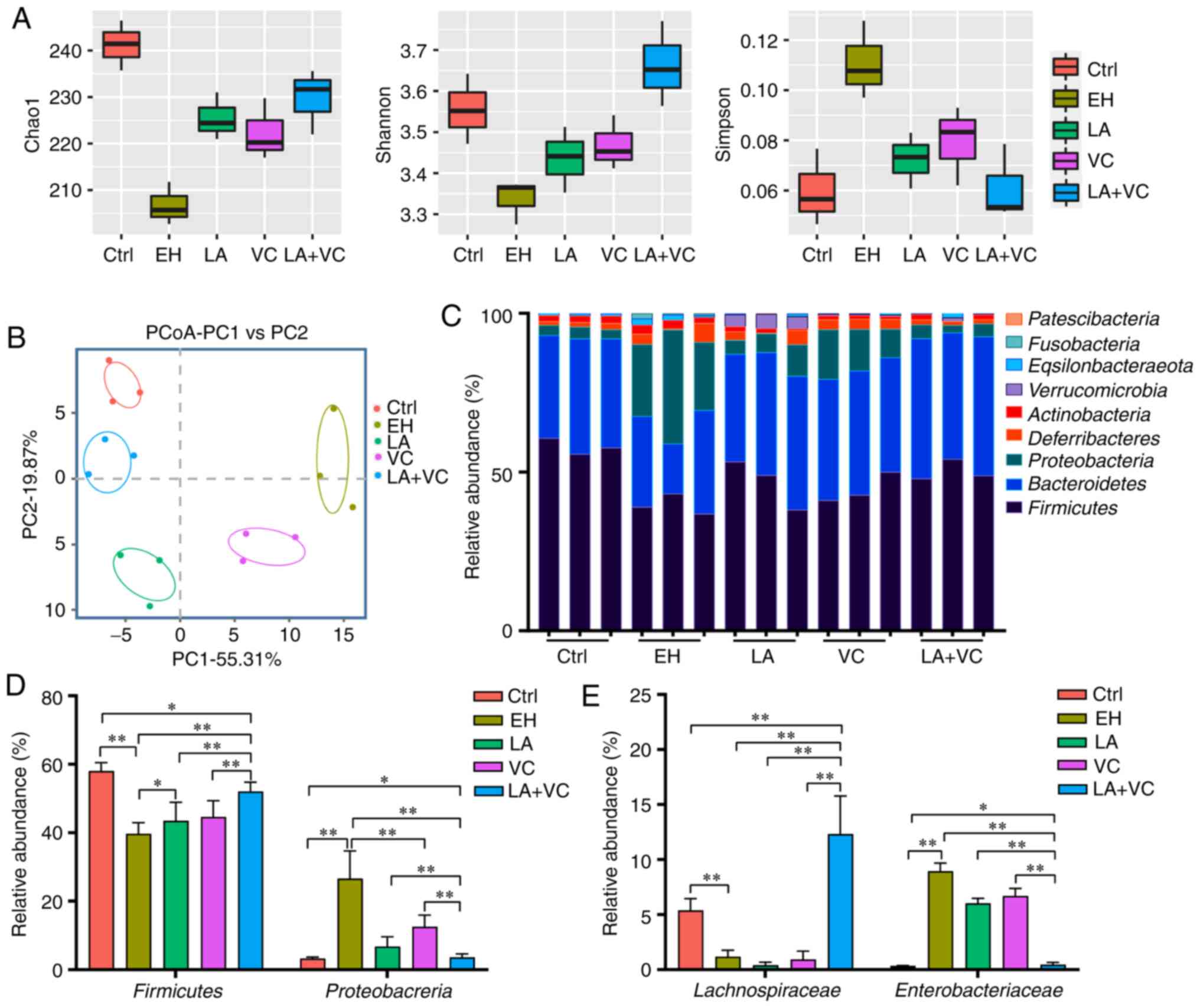
the present study, ethanol treatment markedly reduced the gut
microbiota abundance and diversity compared with that in the
control group (Fig. 2A-C). The LA,
VC and LA+VC treatments obviously increased the Chao1 and Shannon
index and reduced the Simpson index in comparison to the EH group
(Fig. 2A), which suggested that
these treatments markedly increased the community richness and
diversity of the gut microbiota. In addition, β-diversity analysis
based on the Bray-Curtis distance indicated that the gut microbiota
of different groups clustered separately and treatment with LA+VC
obviously restored the gut microbiota composition and diversity
(Fig. 2B).
Subsequently, the taxonomic changes in the bacterial
community were explored. At the phylum level, Firmicutes and
Bacteroidetes were dominant in the faecal microbiota of the control
group, whereas Firmicutes, Proteobacteria and Bacteroidetes were
dominant in the EH group (Fig. 2C).
Of note, LA+VC treatment significantly elevated the proportion of
Firmicutes and reduced the Proteobacteria abundance in
ethanol-treated mice (P<0.01; Fig.
2D). However, single LA or VC treatment exhibited this
effect to a lesser extent. After treatment with LA+VC, the family
of Lachnospiraceae (Firmicutes phyla), which is able to ferment
diverse polysaccharides to short-chain fatty acids (5), was significantly enriched compared
with the EH group (P<0.01; Fig.
2E). Furthermore, ethanol exposure markedly increased the
abundance of Enterbacteriaceae, which are closely associated with
intestinal diseases (33), and this
was significantly suppressed by the treatments of LA, VC and LA+VC
(P<0.01; Fig. 2E). Collectively,
the LA+VC treatment suppressed the potentially pathogenic
ethanol-associated changes of the gut microbiota and restored the
gut microbiota perturbances caused by ethanol treatment.
LA plus VC treatment attenuates
ethanol damage to the intestinal barrier
Ethanol and ethanol-associated gut microbiota
dysbiosis directly influence the physiological status of the
intestine (34). To determine
whether LA+VC has a beneficial effect on ethanol-induced intestinal
injury, changes in gut permeability were first explored. It was
observed that ethanol exposure markedly increased the serum FITC
concentration; however, the LA+VC treatment significantly reduced
the serum FITC levels compared with those in ethanol-fed mice
(P<0.01; Fig. 3A), suggesting
recovery of intestinal integrity. The beneficial effects were
further confirmed by the results of the serum LPS concentration
(P<0.01; Fig. 3B), which also
demonstrated that the LA+VC treatment reduced the translocation of
microbial products via restoring intestinal permeability. In
addition, the intestinal injury induced by ethanol was visualized
in the colon sections stained by HE (Fig. 3C), and LA+VC obviously attenuated
the ethanol-induced injury and restored the crypt structure and
length (P<0.01; Fig. 3C and
D). These beneficial effects were
further confirmed by the results of the relative expression of the
genes Claudin-2, zona occludens (ZO)-1 and occludin (P<0.01;
Fig. 3E), which suggested that
LA+VC reversed the ethanol-associated injury on the intercellular
tight junction of the intestine. On the other hand, ethanol
treatment significantly reduced the number of goblet cells in
colonic crypts that are responsible for the Muc excretion; however,
treatment with LA, VC and LA+VC markedly increased the number of
goblet cells (Fig. 3F and G; P<0.01). Furthermore, the relative
expression of mucus secretion-related genes, such as Muc2/3/4 and
Kruppel-like factor (Klf)4 was examined (Fig. 3H). The RT-qPCR results indicated
that the expression of Muc2/3/4 and Klf4 were markedly decreased in
the ethanol-treated mice and the decreased expression of these
genes was significantly improved by treatment of LA, VC and LA+VC
(P<0.01; Fig. 3H). Collectively,
treatment with LA+VC achieved better results in improving the
intestinal tight junction and restoring mucus secretion than single
treatment with LA or VC in the ethanol-challenged mice.
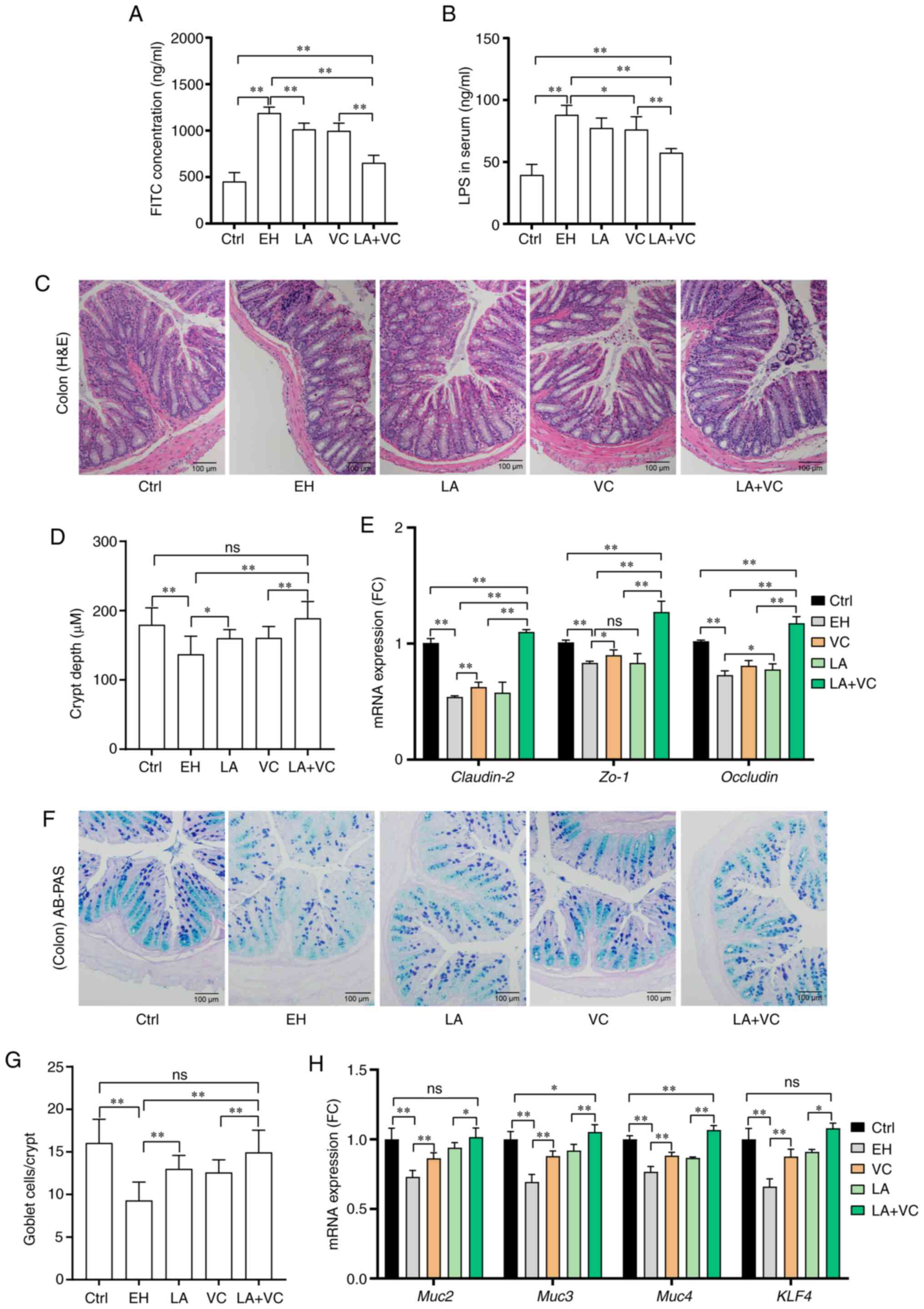 | Figure 3LA plus VC improves ethanol-induced
intestinal barrier dysfunction. (A) Serum concentration of
FITC-dextran. (B) LPS levels in serum. (C) Representative histology
images of colon sections (magnification, x100; scale bar, 100 mm;
H&E). (D) Quantified crypt depth in colon tissues. (E) Relative
mRNA expression of Muc2, ZO-1 and occludin in colon tissues. (F)
Representative colon sections with AB-PAS staining (magnification,
x100; scale bar, 100 mm). (G) Quantified goblet cells per crypt.
(H) Relative mRNA expression of mucus secretion-associated genes
and Klf4 gene in colon tissues. The markedly decreased expression
of Muc2/3/4 and Klf4 in the EH mice was significantly improved by
the LA+VC treatment. Values are expressed as the mean ± standard
deviation of at least three independent experiments.
*P<0.05; **P<0.01. ns, no significance;
VC, vitamin C; LA, Lactobacillus acidophilus; Ctrl, control;
EH; ethanol; AB-PAS, Alcian blue-periodic acid-Schiff; ZO-1, zona
occludens-1; Muc, mucin; KLF, kruppel-like factor; LPS,
lipopolysaccharide. |
LA plus VC treatment alleviates
ethanol-induced intestinal inflammation
Since the immune imbalance and intestinal
inflammation are essential for the onset and progression of
ethanol-associated intestinal injury (35), it was investigated whether LA+VC
treatment has any influence on intestinal immunity and
inflammation. The results indicated that ethanol considerably
reduced the proportion of T-regulatory (Treg) cells
(CD4+CD45+Foxp3+) in colon lamina
propria and promoted the mRNA expression of pro-inflammatory genes
such as IL-1β, IL-6 and TNF-α, along with reducing the expression
of the anti-inflammation gene IL-10 in the colonic tissues of
ethanol-challenged mice (P<0.01; Fig. 4A-C). Of note, treatment with LA+VC
significantly increased the proportion of Treg cells from
14.30±2.58 to 22.60±1.06% (P<0.01; Fig. 4A and B), which indicated that the LA+VC
treatment was able to reinstate the immune balance of colonic Treg
cells. Consistently with this, LA+VC treatment significantly
inhibited the mRNA expression of IL-1β, IL-6 and TNF-α, and
promoted the mRNA expression of IL-10 (P<0.01; Fig. 4C). In addition, the ethanol-induced
alterations in the production of IL-10 and IL-17A were partially
abrogated by treatment with LA+VC (P<0.01; Fig. 4D and E). Furthermore, the LA+VC treatment
obviously decreased the activity of MPO (marker of inflammation)
induced by ethanol from 5.27±0.35 to 4.17±0.40 U/g protein
(P<0.05; Fig. 4F). Overall,
these results revealed that the LA+VC restored the Treg cells'
immune balance that was perturbed by ethanol and inhibited the
inflammatory responses induced by ethanol.
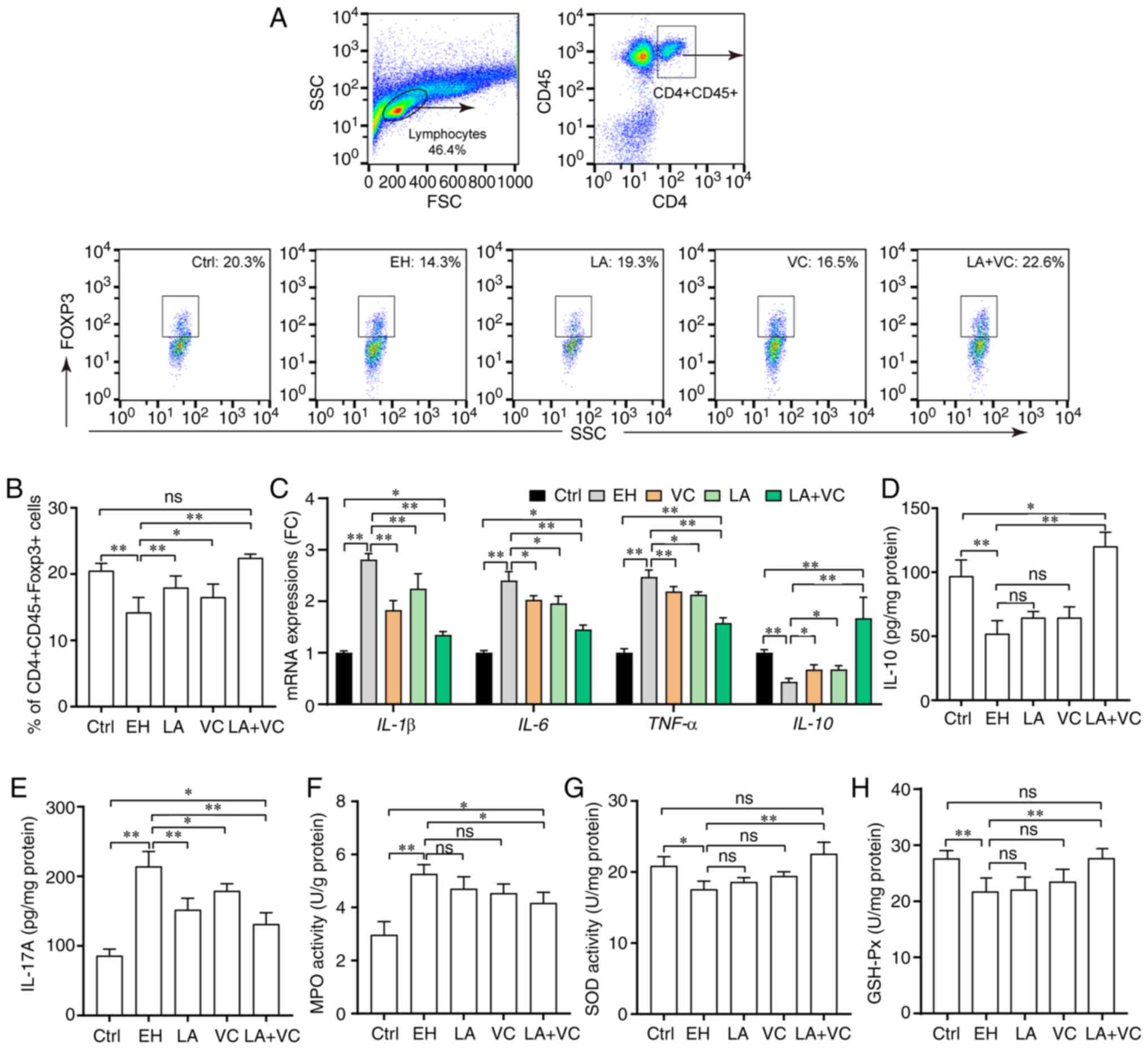 | Figure 4LA plus VC alleviates ethanol-induced
inflammatory responses. (A) Flow cytometry plots and quantification
of Treg cells (CD4+CD45+Foxp3+) in
the colon lamina propria. (B) Quantified percentage of Treg cells
in the colon lamina propria in the different groups. (C) Relative
mRNA expression of pro-inflammatory and anti-inflammatory genes.
(D) Liver IL-10 levels in colon tissues. (E) Liver IL-17A levels in
colon tissues. (F) MPO activity (U/g protein) in colon tissues. (G)
SOD activity (U/mg protein) in colon tissues. (H) GSH-Px activity
(U/mg protein) in colon tissues. Values are expressed as the mean ±
standard deviation of at least three independent experiments.
*P<0.05; **P<0.01. ns, no significance;
VC, vitamin C; LA, Lactobacillus acidophilus; Ctrl, control;
EH; ethanol; Treg, regulatory T; Foxp3, forkhead box p3; SOD,
superoxide dismutase; MPO, myeloperoxidase; GSH-Px, glutathione
peroxidase; FC, fold change; SSC, side scatter; FSC, forward
scatter. |
To evaluate the effects of LA plus VC on oxidative
stress, SOD activity and GSH-Px activity in the colon were
determined. The results indicated that ethanol exposure
significantly reduced the activity of SOD from 20.80±1.29 to
17.57±1.14 U/mg protein (P<0.05; Fig. 4G). Treatment with LA+VC led to a
significant increment of SOD activity (28.46%) compared with
ethanol treatment (P<0.01; Fig.
4G). However, there was no significant difference among the LA,
VC and EH groups (Fig. 4G). In
addition, treatment with LA+VC slightly increased the GSH-Px
activity damaged by alcohol from 21.70±2.33 to 26.98±1.59 U/mg
protein (P<0.01; Fig. 4H), while
the results of the LA and VC groups were not significantly from
those in the EH group (P>0.05; Fig.
4H).
LA plus VC treatment attenuates
ethanol-induced liver injury
To determine whether LA+VC protects the liver
against ethanol-induced damage, HE staining of liver sections was
performed. The results indicated distinct pathological alterations
upon ethanol exposure, including neutrophil infiltration and
steatosis, whereas LA+VC led to improvement of these pathological
alterations (Fig. 5A). Next, it was
observed that ethanol exposure markedly increased the serum levels
of ALT and AST, and that LA+VC significantly improved the liver
function in ethanol-treated mice with reduced serum levels of ALT
and AST (P<0.01; Fig. 5B and
C). Additionally, ethanol exposure
dramatically increased the hepatic triglyceride and MDA levels,
which was significantly inhibited by LA+VC (P<0.01; Fig. 5D and E).
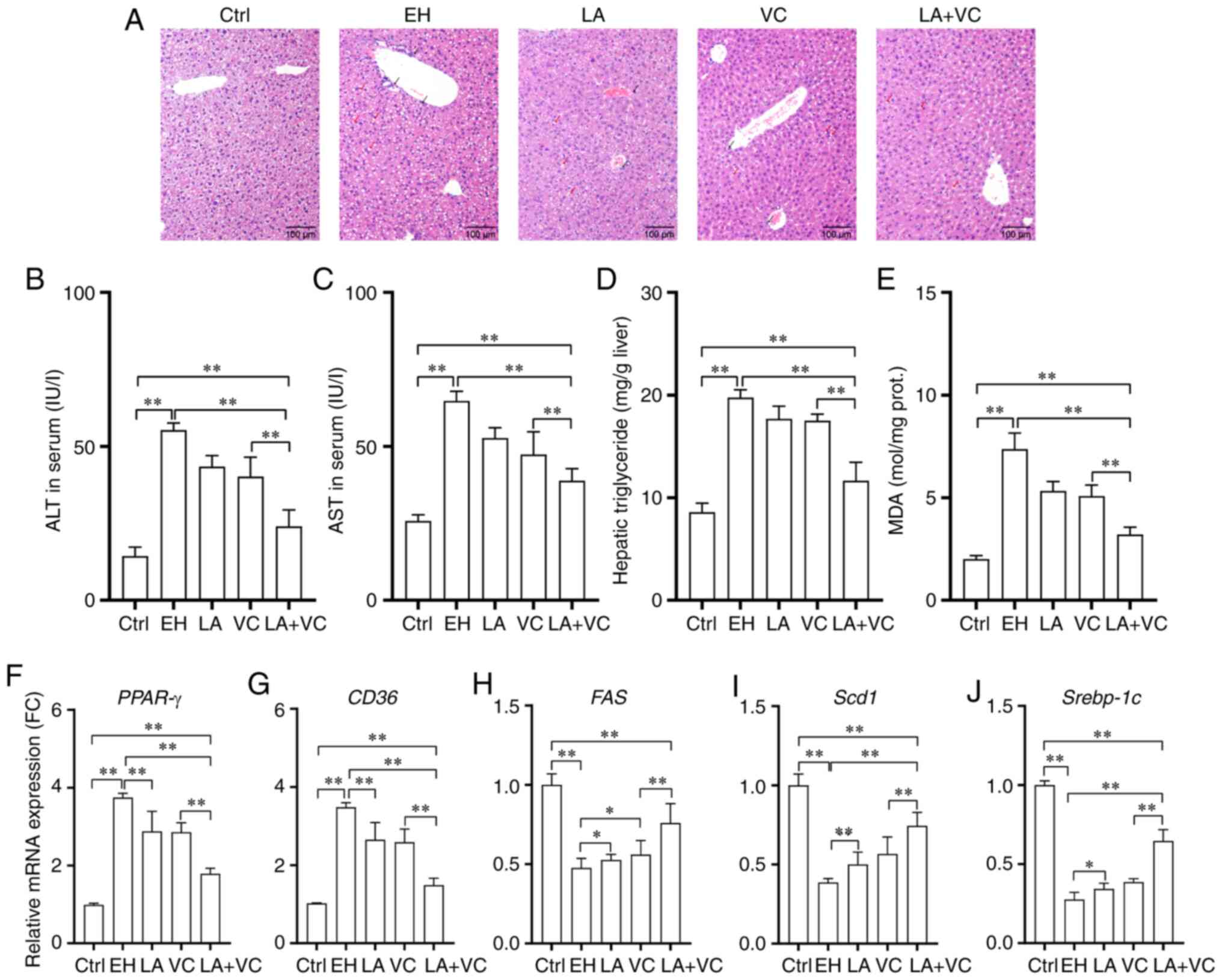 | Figure 5LA plus VC restores ethanol-induced
liver function disorders. (A) Representative histological sections
of the liver (H&E; magnification, x100; scale bar, 100 µm; red
arrows indicate areas of steatosis and the black arrows indicate
the sites of neutrophil infiltration in liver tissues). (B) Serum
ALT levels. (C) Serum AST levels. (D) Hepatic triglyceride levels.
(E) Hepatic MDA levels. Relative mRNA expression of (F) PPAR-γ, (G)
CD36, (H) Fas, (I) Scd1 and (J) Srebp-1c in liver tissues. Values
are expressed as the mean ± standard deviation of at least three
independent experiments. *P<0.05;
**P<0.01. VC, vitamin C; LA, Lactobacillus
acidophilus; Ctrl, control; EH; ethanol; PPAR-γ, peroxisome
proliferator activated receptor-γ; ALT, alanine aminotransferase;
AST, aspartate aminotransferase; MDA malondialdehyde; FC, fold
change; prot., protein; Scd1, stearoyl-CoA desaturase-1; Srebp-1c,
sterol regulatory element-binding transcription protein 1c. |
Furthermore, the mRNA expression of genes related to
steatosis was determined. The RT-qPCR results revealed that ethanol
exposure markedly increased the expression of genes encoding
peroxisome proliferator activated receptor-γ (PPAR-γ) and
transporter CD36 for fatty acids (P<0.01; Fig. 5F and G), which demonstrated that ethanol led to
disorders of the liver functions of triglyceride synthesis and
fatty acid uptake. However, LA+VC treatment significantly improved
the liver function of triglyceride synthesis and fatty acid uptake
with obviously reduced mRNA expression of PPAR-γ and CD36
(P<0.01; Fig. 5F and G). In addition, the decreased expression
of Fas, stearoyl-CoA desaturase-1 (Scd1) and sterol regulatory
element-binding transcription protein 1c (Srebp-1c) induced by
ethanol exposure were improved via LA+VC treatment, suggesting that
the LA+VC treatment likely accelerated fatty acid metabolism and
attenuated the impairment of hepatic function induced by ethanol
exposure. Treatment with LA and VC alone also alleviated
alcohol-induced liver injury, but the combined effects of LA+VC
were stronger.
LA plus VC treatment ameliorates
ethanol-induced liver inflammation
Ethanol exposure markedly increased the gut
permeability and caused LPS translocation into the bloodstream,
which contributed to ethanol-associated liver inflammation
(36). To evaluate the effects of
LA plus VC on liver inflammation states of ethanol-challenged mice,
the TNF-α and IL-1β concentration in liver tissues was examined.
The results revealed that ethanol exposure markedly stimulated the
inflammatory response with increased TNF-α and IL-1β levels in
liver tissues, which was significantly reduced by LA+VC (P<0.01;
Fig. 6A and B). Next, the mRNA expression of genes in
the LPS/TLR4-associated pathway was determined. The RT-qPCR results
revealed that ethanol exposure obviously activated the myeloid
differentiation primary response 88 (Myd88)-dependent TLR4
signaling pathway with increased mRNA expression levels of TLR4,
Myd88, IL-1 receptor associated kinase 4 (IRAK4) and TNF receptor
associated factor 6 (TRAF6) (P<0.01; Fig. 6C). Since the LA+VC treatment
obviously reduced the serum LPS accumulation in the
ethanol-challenged mice (P<0.01; Fig. 3B), these mice had reduced mRNA
expression of TLR4, Myd88, IRAK4 and TRAF6 (P<0.01; Fig. 6C). Furthermore, the mRNA expression
of pro-inflammatory markers was examined and the results indicated
that ethanol exposure significantly stimulated the mRNA expression
of TNF-α, NF-κB, IL-1β and monocyte chemoattractant protein (MCP-1)
(P<0.01; Fig. 6D). Of note, the
LA+VC treatment significantly reduced the mRNA levels of TNF-α,
NF-κB, IL-1β and MCP-1 in the ethanol-challenged mice (P<0.01;
Fig. 6D). Taken together, LA+VC
treatment alleviated the inflammatory response in the liver via the
MyD88-dependent TLR4 signalling pathway.
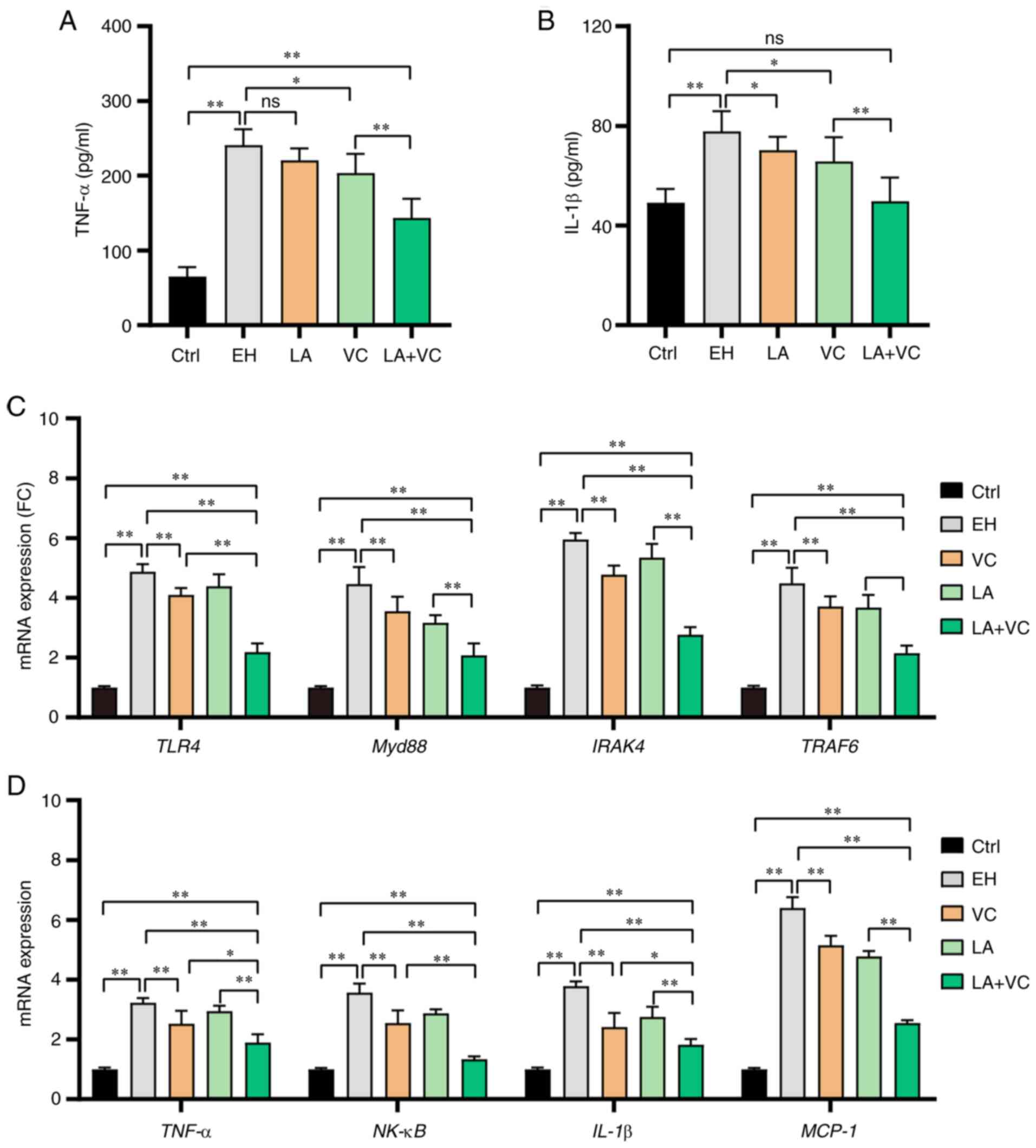 | Figure 6LA plus VC alleviates ethanol-induced
liver inflammation. (A) TNF-α levels in liver tissues. (B) IL-1β
levels in liver tissues. (C) Relative mRNA expression of TLR4,
Myd88, IRAK4 and TRAF6 in liver tissues. (D) Relative mRNA
expression of the proinflammatory cytokines TNF-α, NF-κB and IL-1β
and the chemokine MCP-1 in liver tissues. Values are expressed as
the mean ± standard deviation of at least three independent
experiments. *P<0.05; **P<0.01. ns, no
significance; MCP, monocyte chemoattractant protein; TLR4,
Toll-like receptor 4; IRAK4, IL-1 receptor associated kinase 4;
TRAF6, TNF receptor associated factor 6; Myd88, myeloid
differentiation primary response 88; VC, vitamin C; LA,
Lactobacillus acidophilus; Ctrl, control; EH; ethanol; FC,
fold change. |
Discussion
Mounting evidence revealed that gut microbiota
dysbiosis has a crucial role in ethanol-associated organ injury and
gut microbiota-targeted therapy is emerging as an important
adjuvant therapy for protecting the body against ethanol-induced
damage (34,37-39).
Previous studies have reported that single Lactobacillus
species or VC treatment had beneficial effects by protecting
against ethanol damage in murine models (40,41).
However, to the best of our knowledge, no previous study has
explored the efficiency and possibility of compatibility of
Lactobacillus species and VC in the reduction of ethanol
damage. The results of the present study indicated that LA plus VC
restored gut microbiota homeostasis and improved gut barrier
dysfunction via upregulating the tight junction proteins and mucus
secretion, which prevented the translocation of LPS into
circulatory systems and thus reduced the inflammatory responses
induced by TLR4 in liver tissues. In this context, LA plus VC
attenuated liver injury in ethanol-challenged mice.
Ethanol exposure leads to significant gut microbiota
dysbiosis and reduces the abundance of Lactobacillus species
(6,42). Of note, Lactobacillus species
as probiotics inhibit pathogens within the Enterobacteriaceae
family by producing bacteriocins and protect the intestine against
invasive bacteria via adhering to intestinal epithelial cells
(43,44). On the other hand, ethanol intake
generally leads to the deficiency of VC in the gut, which
contributes to the overgrowth and transcytosis of enteric bacteria
and causes accumulation of circulating LPS (45). The gut microbiota dysbiosis (reduced
diversity and overgrowth of enteric pathogenic bacteria)
exaggerates the intestinal inflammation and gut leakage induced by
ethanol, which likely poses a great threat to the liver. In
agreement with prior studies, single treatment with LA or VC
partially restored the gut microbiota dysbiosis and slightly
reduced the abundance of the enteric bacteria within the
Enterobacteriaceae family. Specifically, treatment with LA+VC
markedly increased the abundance of Firmicutes and Bacteroides and
suppressed the overgrowth of Enterobacteriaceae, which suggests
that LA+VC is able to relieve the ethanol damage to the gut
microbiota. A balanced gut microbiota and microbial metabolites
contribute to regulating the proportion of Foxp3+ Treg
cells in the intestine (46,47),
which are critical in maintaining immune tolerance and homeostasis
of the immune system (48). In
terms of cytokines, Foxp3+ Treg cells express the
immunosuppressive cytokine IL-10, which is important for the
control of the inflammatory response. As indicated in previous
studies, elevated levels of IL-10 may participate in suppressing
the secretion of Th17 cytokines (49); thus, the inflammatory responses in
alcohol-challenged mice were markedly attenuated by LA+VC.
Previous studies have revealed that alcohol
consumption results in depletion of GSH levels and declined
antioxidant activity (50,51). In the present study, single
treatment with LA or VC was not able to significantly restore the
SOD and GSH-Px enzyme activity in the intestine. However, combined
treatment of LA+VC significantly improved the SOD and GSH-Px enzyme
activity in ethanol-challenged mice, which markedly attenuated the
ethanol-induced oxidative stress in the intestine. In addition,
activation of neutrophil granulocytes facilitates secretion of MPO
and generation of oxidants (hypochlorous acid and tyrosyl radicals)
which have an important role in the body's inflammatory response
(52,53). In the present study, LA+VC treatment
reduced the MPO activity in the colon tissues of ethanol-challenged
mice, which contributed to alleviating ethanol-associated oxidative
stress and inflammatory response in tissues. The improved
intestinal oxidative stress and inflammatory response were
associated with the restoration of intestinal barrier function. The
reduced intestinal permeability in the mice treated with LA+VC was
further confirmed by the reduced FITC and LPS concentrations in
serum. In addition, ethanol exposure considerably reduced the
excretion of mucus as well as the mRNA expression of mucus
secretion-related proteins (Muc2/3/4 and Klf4) and tight
junction-related components (claudin-2, ZO-1 and occludin), which
was significantly restored by the treatment with LA+VC.
Collectively, LA+VC treatment attenuated alcohol-induced intestinal
injury, enhanced the intestinal barrier function and reduced the
translocation of LPS from the gut into the circulation.
Increased LPS in the circulatory system triggers the
innate immune response and leads to inflammatory response via the
TLR4 pathway (54). The activated
TLR4 receptor then stimulates the expression of inflammatory
cytokines such as TNF-α, as well as NF-κB (55), which maintains a constant low-grade
inflammatory state and has a negative influence on the liver
(56). LA+VC treatment markedly
reduced steatosis and neutrophil infiltration, as well as reducing
oxidative stress in the liver of ethanol-challenged mice. In
addition, LA+VC treatment improved triglyceride synthesis and fatty
acid uptake via regulating PPAR-γ and CD36, and accelerated fatty
acid metabolism through upregulating Fas, Scd1 and Srebp-1c in the
liver of ethanol-challenged mice. Excessive ethanol intake leads to
lipid metabolism disorders and perturbs fatty-acid transport and
oxidation. Ethanol exposure activates the PPAR-γ receptor and
inactivates the PPAR-α receptor in liver tissues (57-59),
which promotes dysbiosis of fatty-acid metabolism via the retinoid
X receptor (60). Ethanol exposure
also decreases the mitochondrial membrane potential and causes
mitochondrial dysfunction in liver tissues, and mitochondrial
dysfunction and lipid metabolism disorders may lead to steatosis in
the liver (61,62). Furthermore, inflammatory responses
induced by ethanol exposure are frequently associated with an
abnormal redox state in liver tissues, which promotes the
phosphorylation of the p65 subunit of NF-κB and its nuclear
translocation (63), and this
inflammatory pathway may be interrupted by antioxidants (64,65).
Furthermore, we hypothesized that nuclear factor erythroid 2 like
2, as the master regulator of the intracellular adaptive
antioxidant response to oxidative stress, probably participated in
the protective effect of LA+VC treatment against ethanol exposure
via regulating the antioxidant response and impacting on ethanol
metabolism (66-68).
In the present study, the improved liver function was likely
attributed to the alleviation of the inflammatory response,
amelioration of the redox state and reduced mitochondrial
dysfunction in liver tissues. Further studies should be performed
to confirm the potential role of LA+VC in ethanol-challenged
mice.
The primary mechanisms by which LA+VC significantly
attenuated alcohol-induced intestinal injury involved restoring the
gut microbiota, reinstating the immune balance, inhibiting
pro-inflammatory cytokines, reducing oxidative stress and
maintaining gut barrier function. Based on all of these results, it
may be concluded that LA+VC attenuated intestinal inflammatory
responses and oxidative stress, and restored the intestinal tight
junction and mucus excretion, which markedly alleviated the
translocation of gut-derived LPS. In addition, the decrease of LPS
in serum contributed to the relief of inflammatory cytokine
expression in the Myd88-dependent TLR4 pathway, which was
responsible for the amelioration of liver function in
ethanol-challenged mice. These results provide mechanisms by which
LA+VC attenuated ethanol-induced intestinal and liver injury, and
hence, guide the further exploration of synbiotics based on
Lactobacillus species and VC. However, there were some
limitations in the present study. The specific modulation of LA+VC
treatment on gut microbiota and intestine of ethanol-treated mice
were not clearly identified. In the examination of gene expression
in intestine and liver tissues, three samples from each group were
randomly selected, which may result in different error bar
values.
Supplementary Material
Primers for PCR.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Major Science and
Technology Special Project for Chronic Disease Prevention and
Treatment in Tianjin (grant no. 17ZXMFSY00170).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request. Raw sequencing reads of 16S rRNA sequencing have been
deposited in the NCBI Sequence Read Archive (accession nos.
PRJNA732292 and SRX10973300; https://www.ncbi.nlm.nih.gov/bioproject/PRJNA732292,
https://www.ncbi.nlm.nih.gov/sra/?term=SRX10973300).
Authors' contributions
FW designed the experiments; XL performed
experimental experiments and data analysis under the supervision of
FW. XL and FW wrote the manuscript, and confirmed the authenticity
of the raw data. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments of the present study were
approved by the Ethics and Clinical Research Committee of Tianjin
Medical University (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parry CD, Patra J and Rehm J: Alcohol
consumption and non-communicable diseases: Epidemiology and policy
implications. Addiction. 106:1718–1724. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
World Health Organization: Global status
report on alcohol and health. Geneva, Switzerland, WHO Press,
2018.
|
|
3
|
Rusyn I and Bataller R: Alcohol and
toxicity. J Hepatol. 59:387–388. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bajaj JS: Alcohol, liver disease and the
gut microbiota. Nat Rev Gastroenterol Hepatol. 16:235–246.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dubinkina VB, Tyakht AV, Odintsova VY,
Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS,
Alexeev DG, Taraskina AY, et al: Links of gut microbiota
composition with alcohol dependence syndrome and alcoholic liver
disease. Microbiome. 5(141)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Leclercq S, Matamoros S, Cani PD, Neyrinck
AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke
K, et al: Intestinal permeability, gut-bacterial dysbiosis, and
behavioral markers of alcohol-dependence severity. Proc Natl Acad
Sci USA. 111:E4485–E4493. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cho YE, Yu LR, Abdelmegeed MA, Yoo SH and
Song BJ: Apoptosis of enterocytes and nitration of junctional
complex proteins promote alcohol-induced gut leakiness and liver
injury. J Hepatol. 69:142–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kirpich IA, McClain CJ, Vatsalya V,
Schwandt M, Phillips M, Falkner KC, Zhang L, Harwell C, George DT
and Umhau JC: Liver injury and endotoxemia in male and female
alcohol-dependent individuals admitted to an alcohol treatment
program. Alcohol Clin Exp Res. 41:747–757. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pickard JM, Zeng MY, Caruso R and Núñez G:
Gut microbiota: Role in pathogen colonization, immune responses,
and inflammatory disease. Immunol Rev. 279:70–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Caruso R, Lo BC and Nunez G:
Host-microbiota interactions in inflammatory bowel disease. Nat Rev
Immunol. 20:411–426. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sampson TR, Debelius JW, Thron T, Janssen
S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S,
Gradinaru V, et al: Gut microbiota regulate motor deficits and
neuroinflammation in a model of Parkinson's disease. Cell.
167:1469–1480.e12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fattorusso A, Di Genova L, Dell'Isola GB,
Mencaroni E and Esposito S: Autism spectrum disorders and the gut
microbiota. Nutrients. 11(521)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Torres-Fuentes C, Schellekens H, Dinan TG
and Cryan JF: The microbiota-gut-brain axis in obesity. Lancet
Gastroenterol Hepatol. 2:747–756. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bordalo Tonucci L, Dos Santos KM, De Luces
Fortes Ferreira CL, Ribeiro SM, De Oliveira LL and Martino HS: Gut
microbiota and probiotics: Focus on diabetes mellitus. Crit Rev
Food Sci Nutr. 57:2296–2309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bajaj JS, Kakiyama G, Zhao D, Takei H,
Fagan A, Hylemon P, Zhou H, Pandak WM, Nittono H, Fiehn O, et al:
Continued alcohol misuse in human cirrhosis is associated with an
impaired gut-liver axis. Alcohol Clin Exp Res. 41:1857–1865.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bajaj JS, Heuman DM, Hylemon PB, Sanyal
AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR,
et al: Altered profile of human gut microbiome is associated with
cirrhosis and its complications. J Hepatol. 60:940–947.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rao R: Endotoxemia and gut barrier
dysfunction in alcoholic liver disease. Hepatology. 50:638–644.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Szabo G and Bala S: Alcoholic liver
disease and the gut-liver axis. World J Gastroenterol.
16:1321–1329. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mehta G, Macdonald S, Cronberg A, Rosselli
M, Khera-Butler T, Sumpter C, Al-Khatib S, Jain A, Maurice J,
Charalambous C, et al: Short-term abstinence from alcohol and
changes in cardiovascular risk factors, liver function tests and
cancer-related growth factors: A prospective observational study.
BMJ Open. 8(e020673)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu W, Xu J, Taylor AW, Bewick BM, Fu Z, Wu
N, Qian L and Yin P: Analysis of the alcohol drinking behavior and
influencing factors among emerging adults and young adults: A
cross-sectional study in Wuhan, China. BMC Public Health.
19(458)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Saburova L, Keenan K, Bobrova N, Leon DA
and Elbourne D: Alcohol and fatal life trajectories in Russia:
Understanding narrative accounts of premature male death in the
family. BMC Public Health. 11(481)2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chiu WC, Huang YL, Chen YL, Peng HC, Liao
WH, Chuang HL, Chen JR and Yang SC: Synbiotics reduce
ethanol-induced hepatic steatosis and inflammation by improving
intestinal permeability and microbiota in rats. Food Funct.
6:1692–1700. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sivieri K, Morales ML, Adorno MA, Sakamoto
IK, Saad SM and Rossi EA: Lactobacillus acidophilus CRL 1014
improved ‘gut health’ in the SHIME reactor. BMC Gastroenterol.
13(100)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ahrne S and Hagslatt ML: Effect of
lactobacilli on paracellular permeability in the gut. Nutrients.
3:104–117. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
van Baarlen P, Troost F, van der Meer C,
Hooiveld G, Boekschoten M, Brummer RJ and Kleerebezem M: Human
mucosal in vivo transcriptome responses to three lactobacilli
indicate how probiotics may modulate human cellular pathways. Proc
Natl Acad Sci USA. 108 (Suppl 1):S4562–S4569. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Susick RL Jr and Zannoni VG: Effect of
ascorbic acid on the consequences of acute alcohol consumption in
humans. Clin Pharmacol Ther. 41:502–509. 1987.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xiaoqiang G, Wenjie L, Qiliang X, Hui D,
Caiyun Z, Yanzhong C and Xianglin D: Vitamin C protective role for
alcoholic liver disease in mice through regulating iron metabolism.
Toxicol Ind Health. 27:341–348. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bertola A, Mathews S, Ki SH, Wang H and
Gao B: Mouse model of chronic and binge ethanol feeding (the NIAAA
model). Nat Protoc. 8:627–637. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Weigmann B, Tubbe I, Seidel D, Nicolaev A,
Becker C and Neurath MF: Isolation and subsequent analysis of
murine lamina propria mononuclear cells from colonic tissue. Nat
Protoc. 2:2307–2311. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Caporaso JG, Kuczynski J, Stombaugh J,
Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich
JK, Gordon JI, et al: QIIME allows analysis of high-throughput
community sequencing data. Nature Methods. 7:335–336.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bjørkhaug ST, Aanes H, Neupane SP,
Bramness JG, Malvik S, Henriksen C, Skar V, Medhus AW and Valeur J:
Characterization of gut microbiota composition and functions in
patients with chronic alcohol overconsumption. Gut Microbes.
10:663–675. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kuprys PV, Cannon AR, Shieh J, Iftekhar N,
Park SK, Eberhardt JM, Ding X and Choudhry MA: Alcohol decreases
intestinal ratio of Lactobacillus to Enterobacteriaceae and induces
hepatic immune tolerance in a murine model of DSS-colitis. Gut
Microbes. 12:1–16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sarin SK, Pande A and Schnabl B:
Microbiome as a therapeutic target in alcohol-related liver
disease. J Hepatol. 70:260–272. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vacca M, Celano G, Calabrese FM,
Portincasa P, Gobbetti M and De Angelis M: The controversial role
of human gut lachnospiraceae. Microorganisms. 8(573)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bode C and Bode JC: Effect of alcohol
consumption on the gut. Best Pract Res Clin Gastroenterol.
17:575–592. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Szabo G: Gut-liver axis in alcoholic liver
disease. Gastroenterology. 148:30–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Seo B, Jeon K, Moon S, Lee K, Kim WK,
Jeong H, Cha KH, Lim MY, Kang W, Kweon MN, et al: Roseburia Spp.
Abundance associates with alcohol consumption in humans and its
administration ameliorates alcoholic fatty liver in mice. Cell Host
Microbe. 27:25–40.e6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Roychowdhury S, Glueck B, Han Y, Mohammad
MA and Cresci GAM: A designer synbiotic attenuates chronic-binge
ethanol-induced gut-liver injury in mice. Nutrients.
11(97)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sönmez MF, Narin F, Akkuş D and Türkmen
AB: Melatonin and vitamin C ameliorate alcohol-induced oxidative
stress and eNOS expression in rat kidney. Ren Fail. 34:480–486.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Forsyth CB, Farhadi A, Jakate SM, Tang Y,
Shaikh M and Keshavarzian A: Lactobacillus GG treatment ameliorates
alcohol-induced intestinal oxidative stress, gut leakiness, and
liver injury in a rat model of alcoholic steatohepatitis. Alcohol.
43:163–172. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yan AW, Fouts DE, Brandl J, Stärkel P,
Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA and
Schnabl B: Enteric dysbiosis associated with a mouse model of
alcoholic liver disease. Hepatology. 53:96–105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Turroni F, Ventura M, Buttó LF, Duranti S,
O'Toole PW, Motherway MO and van Sinderen D: Molecular dialogue
between the human gut microbiota and the host: A Lactobacillus and
Bifidobacterium perspective. Cell Mol Life Sci. 71:183–203.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bernet MF, Brassart D, Neeser JR and
Servin AL: Lactobacillus acidophilus LA 1 binds to cultured
human intestinal cell lines and inhibits cell attachment and cell
invasion by enterovirulent bacteria. Gut. 35:483–489.
1994.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Levine M, Conry-Cantilena C, Wang Y, Welch
RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King
J and Cantilena LR: Vitamin C pharmacokinetics in healthy
volunteers: Evidence for a recommended dietary allowance. Proc Natl
Acad Sci USA. 93:3704–3709. 1996.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Round JL and Mazmanian SK: Inducible
Foxp3+ regulatory T-cell development by a commensal
bacterium of the intestinal microbiota. Proc Natl Acad Sci USA.
107:12204–12209. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Smith PM, Howitt MR, Panikov N, Michaud M,
Gallini CA, Bohlooly-Y M, Glickman JN and Garrett WS: The microbial
metabolites, short-chain fatty acids, regulate colonic Treg cell
homeostasis. Science. 341:569–573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li Z, Li D, Tsun A and Li B:
FOXP3+ regulatory T cells and their functional
regulation. Cell Mol Immunol. 12:558–565. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chaudhry A, Samstein RM, Treuting P, Liang
Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller
W and Rudensky AY: Interleukin-10 signaling in regulatory T cells
is required for suppression of Th17 cell-mediated inflammation.
Immunity. 34:566–578. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cho YE and Song BJ: Pomegranate prevents
binge alcohol-induced gut leakiness and hepatic inflammation by
suppressing oxidative and nitrative stress. Redox Biol. 18:266–278.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lei P, Zhao W, Pang B, Yang X, Li BL, Ren
M and Shan YJ: Broccoli sprout extract alleviates alcohol-induced
oxidative stress and endoplasmic reticulum stress in C57BL/6 mice.
J Agric Food Chem. 66:5574–5580. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Faith M, Sukumaran A, Pulimood AB and
Jacob M: How reliable an indicator of inflammation is
myeloperoxidase activity? Clin Chim Acta. 396:23–25.
2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Olza J, Aguilera CM, Gil-Campos M, Leis R,
Bueno G, Martínez-Jiménez MD, Valle M, Cañete R, Tojo R, Moreno LA
and Gil A: Myeloperoxidase is an early biomarker of inflammation
and cardiovascular risk in prepubertal obese children. Diabetes
Care. 35:2373–2376. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li
P, Hu L and Shao F: Inflammatory caspases are innate immune
receptors for intracellular LPS. Nature. 514:187–192.
2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang H, Song X, Li M, Wang X, Tao Y, Xiya
X, Liu H, Zhao Y, Chang D and Sha Q: The role of TLR4/NF-κB
signaling pathway in activated microglia of rats with chronic high
intraocular pressure and vitro scratch injury-induced microglia.
Int Immunopharmacol. 83(106395)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Furman D, Campisi J, Verdin E,
Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW,
Fasano A, Miller GW, et al: Chronic inflammation in the etiology of
disease across the life span. Nat Med. 25:1822–1832.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Valenzuela R and Videla LA: Impact of the
co-administration of N-3 fatty acids and olive oil components in
preclinical nonalcoholic fatty liver disease models: A mechanistic
view. Nutrients. 12(499)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang W, Xu MJ, Cai Y, Zhou Z, Cao H,
Mukhopadhyay P, Pacher P, Zheng S, Gonzalez FJ and Gao B:
Inflammation is independent of steatosis in a murine model of
steatohepatitis. Hepatology. 66:108–123. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Echeverría F, Valenzuela R, Bustamante A,
Álvarez D, Ortiz M, Espinosa A, Illesca P, Gonzalez-Mañan D and
Videla LA: High-fat diet induces mouse liver steatosis with a
concomitant decline in energy metabolism: Attenuation by
eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation
and the additive effects upon EPA and HT co-administration. Food
Funct. 10:6170–6183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xie Z, Gao G, Wang H, Li E, Yuan Y, Xu J,
Zhang Z, Wang P, Fu Y, Zeng H, et al: Dehydroabietic acid
alleviates high fat diet-induced insulin resistance and hepatic
steatosis through dual activation of PPAR-γ and PPAR-α. Biomed
Pharmacother. 127(110155)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gyamfi D, Everitt HE, Tewfik I, Clemens DL
and Patel VB: Hepatic mitochondrial dysfunction induced by fatty
acids and ethanol. Free Radic Biol Med. 53:2131–2145.
2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Begriche K, Massart J, Robin MA,
Borgne-Sanchez A and Fromenty B: Drug-induced toxicity on
mitochondria and lipid metabolism: Mechanistic diversity and
deleterious consequences for the liver. J Hepatol. 54:773–794.
2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Küper C, Beck FX and Neuhofer W: Toll-like
receptor 4 activates NF-κB and MAP kinase pathways to regulate
expression of proinflammatory COX-2 in renal medullary collecting
duct cells. Am J Physiol Renal Physiol. 302:F38–F46.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mardones M, Valenzuela R, Romanque P,
Covarrubias N, Anghileri F, Fernández V, Videla LA and Tapia G:
Prevention of liver ischemia reperfusion injury by a combined
thyroid hormone and fish oil protocol. J Nutr Biochem.
23:1113–1120. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Valenzuela R, Illesca P, Echeverría F,
Espinosa A, Rincón-Cervera MÁ, Ortiz M, Hernandez-Rodas MC,
Valenzuela A and Videla LA: Molecular adaptations underlying the
beneficial effects of hydroxytyrosol in the pathogenic alterations
induced by a high-fat diet in mouse liver: PPAR-α and Nrf2
activation, and NF-κB down-regulation. Food Funct. 8:1526–1537.
2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Sun J, Fu J, Zhong Y, Li L, Chen C, Wang
X, Wang L, Hou Y, Wang H, Zhao R, et al: NRF2 mitigates acute
alcohol-induced hepatic and pancreatic injury in mice. Food Chem
Toxicol. 121:495–503. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Echeverría F, Valenzuela R, Bustamante A,
Álvarez D, Ortiz M, Soto-Alarcon SA, Muñoz P, Corbari A and Videla
LA: Attenuation of high-fat diet-induced rat liver oxidative stress
and steatosis by combined hydroxytyrosol-(HT-) eicosapentaenoic
acid supplementation mainly relies on HT. Oxid Med Cell Longev.
2018(5109503)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Barrera C, Valenzuela R, Rincón M,
Espinosa A, Echeverria F, Romero N, Gonzalez-Mañan D and Videla LA:
Molecular mechanisms related to the hepatoprotective effects of
antioxidant-rich extra virgin olive oil supplementation in rats
subjected to short-term iron administration. Free Radic Biol Med.
126:313–321. 2018.PubMed/NCBI View Article : Google Scholar
|