Introduction
With the wide application of intervention and
cardiac surgery in heart disease, myocardial ischemia-reperfusion
injury (MIRI) has increased, becoming an urgent problem in the
medical field. The main consequence of ischemia is bioenergetic
exhaustion caused by an insufficient transport of oxygen and
nutrients (1). MIRI is a phenomenon
in which the ischemic injury of tissues and organs is not
alleviated, but further aggravated after blood perfusion; it is
caused by an increase in oxygen free radical content, calcium
overload, inflammatory cell infiltration and other phenomena
associated with ischemia-reperfusion, leading to cardiomyocyte
necrosis and apoptosis (2).
However, timely reperfusion is effective to resuscitate myocardial
tissue and improve clinical prognosis, paradoxically, this type of
reperfusion therapy causes more severe myocardial injury than
simple ischemia (3). A previous
study has reported that certain interventions, such as primary
unloading, could limit infarct size after acute myocardial
infarction (4). In addition, a
number of anti-ischemia-reperfusion drugs have emerged, but their
effect in clinical application has not been exceptional (5). Therefore, the mechanism of reperfusion
injury needs to be further elucidated.
Heat shock proteins (HSPs) are highly conserved
proteins. The expression of HSPs is induced to protect body
functions during stress exposure (6). HSP70 is an endogenous protective
protein that plays a protective role in MIRI (7,8). The
PI3K/AKT signaling pathway has also been indicated to serve an
important role in the protection of cardiomyocytes in MIRI
(9-11).
The PI3K family is divided into class I, II and III forms, class I
being more commonly detected in MIRI (12). Previous studies have indicated that
apoptosis during reperfusion is an important cause of fatal
cardiomyocyte injury. The remedial kinase pathway against apoptosis
during reperfusion is called the reperfusion injury salvage kinase
(RISK) pathway (13). The PI3K/AKT
pathway is one of the RISK signaling pathways (14), which functions through the
regulation of cell morphology, cardiomyocyte survival, apoptosis,
protein synthesis and metabolic integration (15). Previous studies have identified a
close association between the PI3K/AKT pathway and HSP70, and this
mutual regulation has been demonstrated in brain and lung tissues
(16,17). However, the association between
PI3K/AKT and HSP70 in the myocardium requires further
elucidation.
DL-3-n-butylphthalide (NBP) is a natural product
extracted from celery seeds (18).
Previous studies have revealed that NBP promoted vasodilation and
improved cerebral microcirculation by promoting the production of
nitric oxide (19,20). Furthermore, NBP has been indicated
to stabilize the blood-brain barrier and mitochondrial membrane
structure by inhibiting inflammation and oxidative stress (21-24).
Interestingly, NBP has also been hypothesized to play an important
role in MIRI by reducing myocardial infarction and the incidence of
arrhythmia (25,26). Recent studies have indicated that
NBP significantly improved cardiac function by regulating the
PI3K/AKT pathway in MIRI, and that HSP70 exhibited protective
effects on MIRI (7,27). However, the role of HSP70 in the
cardioprotective effect of NBP remains unknown. Furthermore,
further research is required to explore the relationship between
HSP70 and the PI3K/AKT signaling pathway.
Therefore, the present study aimed to explore
whether NBP protected against MIRI by reducing inflammation and
oxidative stress through the PI3K/AKT signaling pathway and HSP70.
The association between NBP, PI3K/AKT and HSP70 was also
explored.
Materials and methods
Materials and reagents
NBP (cat. no. H20100041) was purchased from CSPC
Enbipu Pharmaceutical Co., Ltd. LY294002 (cat. no. A8250; PI3K
inhibitor) was purchased from APeXBIO Technology LLC. FBS (cat. no.
SFBS) was purchased from Bovogen Biologicals Pty Ltd. DMEM
(low-glucose, cat. no. 30021; high-glucose, cat. no. 30022) was
purchased from HyClone; Cytiva. ChamQ Universal SYBR qPCR Master
Mix (cat. no. Q711-02) and HiScript II Q RT SuperMix for qPCR +
gDNA wiper (cat. no. R223-01) were purchased from Vazyme Biotech
Co., Ltd. All antibodies and reagents were of analytical grade and
are commercially available.
Cell culture
H9c2 cells (cat. no. CL-0089) were obtained from
Procell Life Science & Technology Co., Ltd. H9c2 cells
(1x106 cells/ml) were seeded in 25-cm2
cell-culture flasks containing high-glucose DMEM (10% FBS; 1%
penicillin/streptomycin). The cells were cultured in a cell
incubator with 95% air and 5% CO2 at 37˚C. After H9c2
cells were cultured for 24 h, they were randomly assigned to four
groups: i) Control group (CON); ii) MIRI group; iii) NBP
pretreatment group (MIRI + NBP); and iv) PI3K inhibitor group (MIRI
+ NBP + LY294002). CON cells were cultured at 37˚C with 95% air.
MIRI cells were incubated at 37˚C with 5% CO2, 93%
N2 and 2% O2 for 6 h, then reoxygenated for 4
h. MIRI + NBP cells were pretreated with 100 µM NBP for 2 h before
being subjected to hypoxia for 6 h, followed by reoxygenation for 4
h. MIRI + NBP + LY294002 cells were pretreated with 10 µM
LY294002(28) for 1 h before
treatment with NBP for 2 h, followed by hypoxia for 6 h and
reoxygenation for 4 h.
Construction of the H9c2 MIRI
model
To simulate the in vivo model of
ischemia-reperfusion injury, the cell culture medium was replaced
with low-glucose DMEM without FBS, and the cells were incubated at
37˚C with 5% CO2, 93% N2 and 2%
O2. A total of 10 µM of LY294002 was added 1 h before
NBP treatment. NBP pretreatment lasted for 2 h before hypoxia.
After MIRI, the medium was replaced with high-glucose DMEM (10%
FBS; 1% penicillin/streptomycin) and the cells were cultured in an
incubator (37˚C; 5% CO2; 95% air) for 4 h.
Determination of optimal NBP
concentration and cell viability
H9c2 cells (1x104 cells/well) were seeded
in 96-well plates for 24-48 h, and then pretreated with different
doses of NBP (1, 50, 100, 200, 300 and 500 µM). After reperfusion,
the original medium was discarded and 100 µl medium containing 10
mg/ml Cell Counting Kit-8 (CCK-8; cat. no. CA1210-100; Beijing
Solarbio Science & Technology Co., Ltd.) were added to each
well. The absorbance at 450 nm was measured after culture for 1 h
without light. The final cell viability of each group was
calculated with the following formula: Cell viability
(%)=(experimental group-blank)/(MIRI only group-blank) x100.
Determination of oxidation index
After ischemia-reperfusion treatment, total proteins
were extracted using RIPA lysis buffer (cat. no. R0020; Beijing
Solarbio Science & Technology Co., Ltd.). BCA Protein Assay Kit
(cat. no. CW0014; CoWin Biosciences) was used to measure the
protein concentration. According to the instructions of the Lipid
Peroxidation malondialdehyde (MDA) Assay Kit (cat. no. S0131;
Beyotime Institute of Biotechnology), freshly prepared MDA
detection solution was added to blank, standard and sample tubes,
heated at 100˚C for 15 min, centrifuged at room temperature and
1,000 x g for 10 min, and 200 µl of each supernatant were placed in
a 96-well plate. The absorbance was determined at 530 nm using
microplate reader (Bio-Rad Laboratories, Inc.) and the absolute
value of MDA of each group was calculated. The MDA content per
weight of protein (nmol/mg) was calculated according to the protein
concentration of each group.
Determination of lactate dehydrogenase
(LDH) in the culture medium
According to the instructions of the LDH
Cytotoxicity Assay Kit (cat. no. C0017; Beyotime Institute of
Biotechnology), after the ischemia-reperfusion treatment, the
culture medium of each group was centrifuged for 5 min at room
temperature and 400 x g, and 120 µl supernatant of each sample were
placed in a 96-well plate. LDH detection solution (60 µl) was added
to each well for a 30-min incubation at room temperature. The
optical density was measured at 490 nm using a microplate reader,
and the amount of LDH (mU/ml) released by the cells in each group
was determined.
mRNA expression of IL-1β and TNF-α in
H9c2 cells
The mRNA expression of inflammatory factors in H9c2
cells was determined via reverse transcription-quantitative PCR
(RT-qPCR). Total RNA was extracted using TRIzol® reagent
(cat. no. 15596018; Thermo Fisher Scientific, Inc.), and the RNA
concentration was quantified in each group. Complementary DNA
(cDNA) was obtained from RNA using HiScript II Q RT SuperMix for
qPCR + gDNA wiper according to the manufacturer's instructions. A
PCR Master Mix of 10 µl (4.8 µl cDNA template; 5 µl qPCR Master
Mix; 0.2 µl gene primers) and a LightCycler® 480 system
Ⅱ [Roche Diagnostics (Shanghai) Co., Ltd.] were used to perform
qPCR. The following thermocycling conditions were used for qPCR:
Pre-denaturation at 95˚C for 5 min; 40 cycles of 95˚C for 10 sec
and 60˚C for 30 sec. The expression levels were normalized to
GAPDH. The primers used were as follows: TNF-α forward,
5'-TGATCGGTCCCAACAAGGA-3' and reverse, 5'-TGCTTGGTGGTTTGCTACGA-3';
IL-1β forward, 5'-GGGATGATGACGACCTGC-3' and reverse,
5'-CCACTTGTTGGCTTATGTT-3'; GAPDH forward,
5'-GTTACCAGGGCTGCCTTCTC-3' and reverse, 5'-ACCAGCTTCCCATTCTCAGC-3'.
The mRNA expression levels were calculated using the
2-ΔΔCq method (29).
Western blot analysis
After establishing the MIRI model, the original
medium was discarded and H9c2 cells were washed three times using
TBS with Tween-20 (TBST; cat. no. T1085; Beijing Solarbio Science
& Technology Co., Ltd.). A total of 120 µl RIPA lysis buffer
was added to each 25-cm2 culture flask. The lysates were
centrifuged at 4˚C and 12,000 x g for 5 min. BCA Protein Assay Kit
was used to measure protein concentration. The protein samples were
mixed with 5X loading buffer (cat. no. P1015; Beijing Solarbio
Science & Technology Co., Ltd.) at a 4:1 ratio, and
subsequently boiled at 100˚C for 5 min. A total of 30 µg
protein/sample were separated by 10% SDS-PAGE. The proteins were
transferred onto PVDF membranes (MilliporeSigma), which were then
blocked with 5% non-fat milk at room temperature for 2 h. The
membranes were incubated at 4˚C overnight with primary antibodies
as follows: Anti-PI3K (1:10,000; cat. no. 09-482; MilliporeSigma);
anti-AKT (1:2,000; cat. no. 05-591; MilliporeSigma);
anti-phosphorylated (p)-AKT (1:7,000; cat. no. ab81283; Abcam);
anti-HSP70 (1:1,000; cat. no. ab181606; Abcam) or anti-GAPDH
(1:10,000; cat. no. ab181602; Abcam). After washing with TBST three
times, the goat anti-rabbit immunoglobulin G (IgG; H+L) horseradish
peroxidase (HRP; 1:5,000; cat. no. GAR007; MultiSciences Biotech
Co., Ltd.) or goat anti-mouse IgG (H+L) HRP (1:5,000 dilution,
GAM007; MultiSciences Biotech Co., Ltd.) was incubated with the
membrane for 2 h at room temperature, and the membranes were
subsequently washed three times with TBST. Protein bands were
visualized using ECL detection reagents (cat. no. CW0049M; CWBIO).
The relative band intensity was measured by Image-Pro Plus 6.0
software (Media Cybernetics, Inc.).
Immunofluorescence staining
Cells were cultured for 24 to 48 h before the MIRI
model was established. Subsequently, the cells were washed three
times with PBS and fixed with 4% paraformaldehyde (cat. no. P1110;
Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 30 min, then blocked with 10% goat serum (cat. no.
SL038; Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 30 min. The cells were incubated with the primary
antibody overnight at 4˚C using PI3K (1:5,000; cat. no. 09-482;
MilliporeSigma), phosphorylated (p)-Akt (1:200; cat. no. ab81283;
Abcam), and HSP70 (1:50; cat. no. ab181606; Abcam). The cells were
then washed and incubated with goat anti-mouse IgG-FITC (1:500;
cat. no. SA0015; Beijing Solarbio Science & Technology Co.,
Ltd.) or goat anti-rabbit IgG-FITC (1:500; cat. no. SA0025; Beijing
Solarbio Science & Technology Co., Ltd.) for 30 min without
light at 37˚C. Nuclei were stained with a DAPI solution (cat. no.
C0065; Beijing Solarbio Science & Technology Co., Ltd.) at 37˚C
for 5 min. The cells were mounted with an anti-fluorescence
attenuator and the staining was observed under a fluorescence
microscope.
Statistical analysis
SPSS v20.0 statistical software (IBM Corp.) was used
to analyze the data. Results are presented as the mean ± SEM of
three experimental repeats. Significant differences were determined
using one-way ANOVA followed by Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
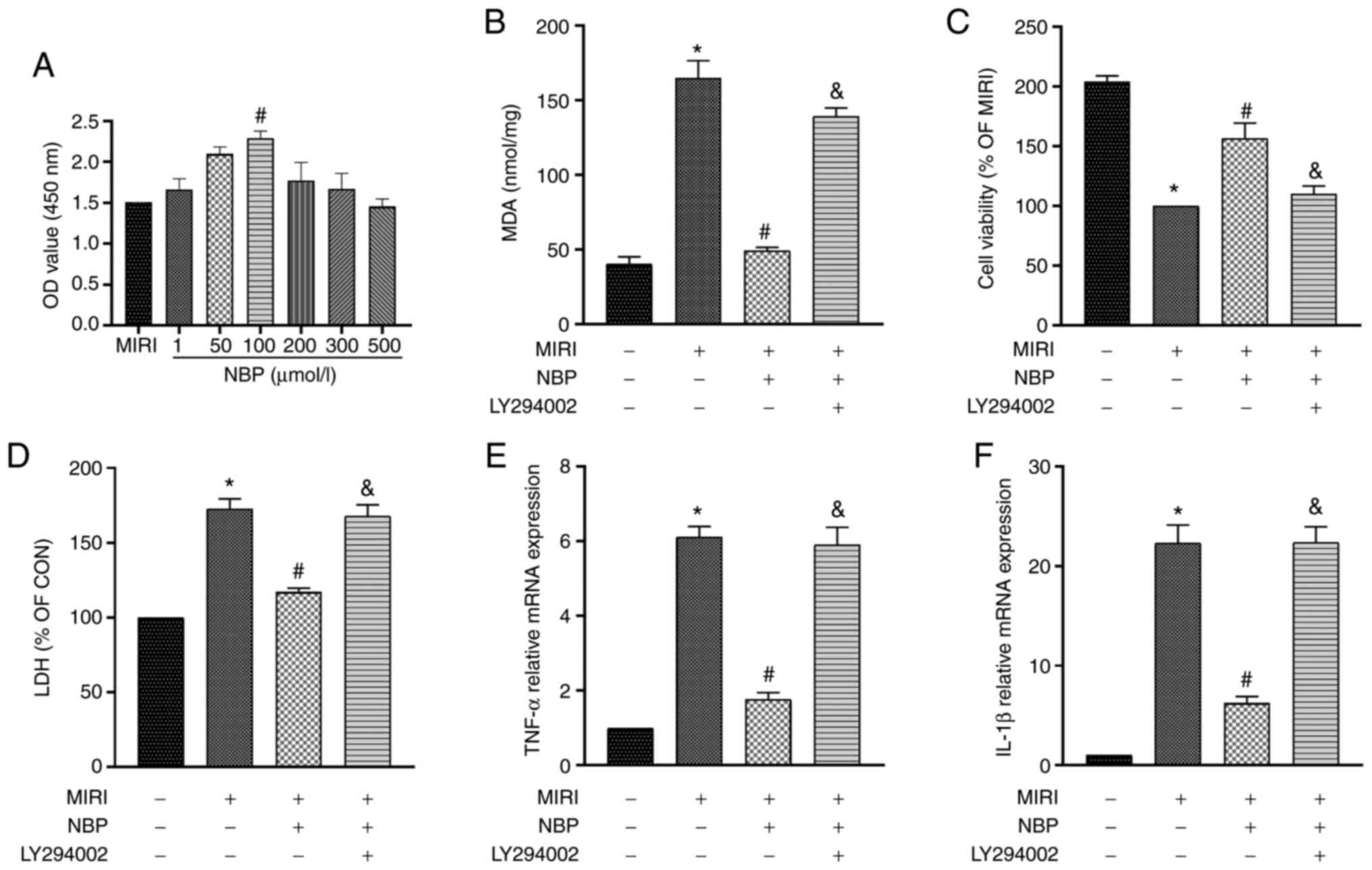
Effect of different concentrations of
NBP on the viability of H9c2 cells
The viability of H9c2 cells treated with different
NBP doses was examined via the CCK-8 colorimetric assay. Compared
with the MIRI group, 100 µM NBP increased cell viability
(P<0.05; Fig. 1A). However, NBP
concentrations ≥200 µM slightly decreased cell viability (Fig. 1A). The present results indicated
that 100 µM NBP was the optimal concentration for protecting cells
from MIRI.
NBP inhibits the oxidative stress
response during MIRI
When ischemia-reperfusion injury occurs in
cardiomyocytes, oxidative stress also plays an important role
(30). An MDA kit was used to
explore the effect of NBP on the oxidative stress index after MIRI
and the role of PI3K in this process. The oxidation level in the
MIRI group was significantly higher compared with the CON group
(P<0.05). However, NBP pretreatment significantly decreased
oxidative stress (P<0.05). In contrast, LY294002 eliminated the
antioxidant effect of NBP (P<0.05; Fig. 1B). Namely, NBP significantly
decreased the oxidative stress response of H9c2 cells during MIRI,
but this antioxidant protective effect was eliminated by blocking
PI3K.
NBP protects H9c2 cells from MIRI
In vitro, the destruction of the cell
membrane structure caused by apoptosis or necrosis leads to LDH
release into the culture medium (31). Therefore, LDH activity can
indirectly indicate the degree of cell damage. To explore the
effect of NBP on MIRI and the involvement of PI3K in MIRI, H9c2
cell viability was determined via CCK-8 colorimetric assay, while
an LDH kit was used to determine the LDH content in the culture
medium. Compared with the CON group, the cell viability of the MIRI
group was significantly lower (P<0.05). However, the cell
viability of the MIRI + NBP group was higher than that of the MIRI
group (P<0.05). By contrast, addition of the PI3K inhibitor
LY294002 decreased cell viability (P<0.05; Fig. 1C). Furthermore, the LDH content of
the MIRI group was significantly higher than that of the CON group
(P<0.05). Pretreatment with NBP decreased the LDH release
(P<0.05), but addition of LY294002 reversed this effect
(P<0.05; Fig. 1D). Thus, NBP
significantly increased the viability of H9c2 cells after
ischemia-reperfusion injury and reduced H9c2 cell injury. However,
the PI3K inhibitor LY294002 reversed the protective effect of NBP
on H9c2 cells.
NBP inhibits inflammation by
decreasing the TNF-α and IL-1β mRNA expression
Activation of inflammatory cytokines during MIRI
aggravates the injury of cardiomyocytes (32). Therefore, to determine the
association between the protective effect of NBP during MIRI and
inflammatory factors, the mRNA expression of TNF-α and IL-1β was
examined in H9c2 cells via RT-qPCR. Compared with the CON group,
the expression of TNF-α and IL-1β in the MIRI group significantly
increased (P<0.05). However, the expression of these genes
decreased in the MIRI + NBP compared with the MIRI group
(P<0.05). By contrast, addition of LY294002 increased the
expression of TNF-α and IL-1β compared with the MIRI + NBP group
(P<0.05; Fig. 1E and F). Therefore, it was revealed that NBP
effectively reduced the expression of inflammatory factors during
MIRI, while the application of the PI3K inhibitor LY294002 reversed
this anti-inflammatory effect.
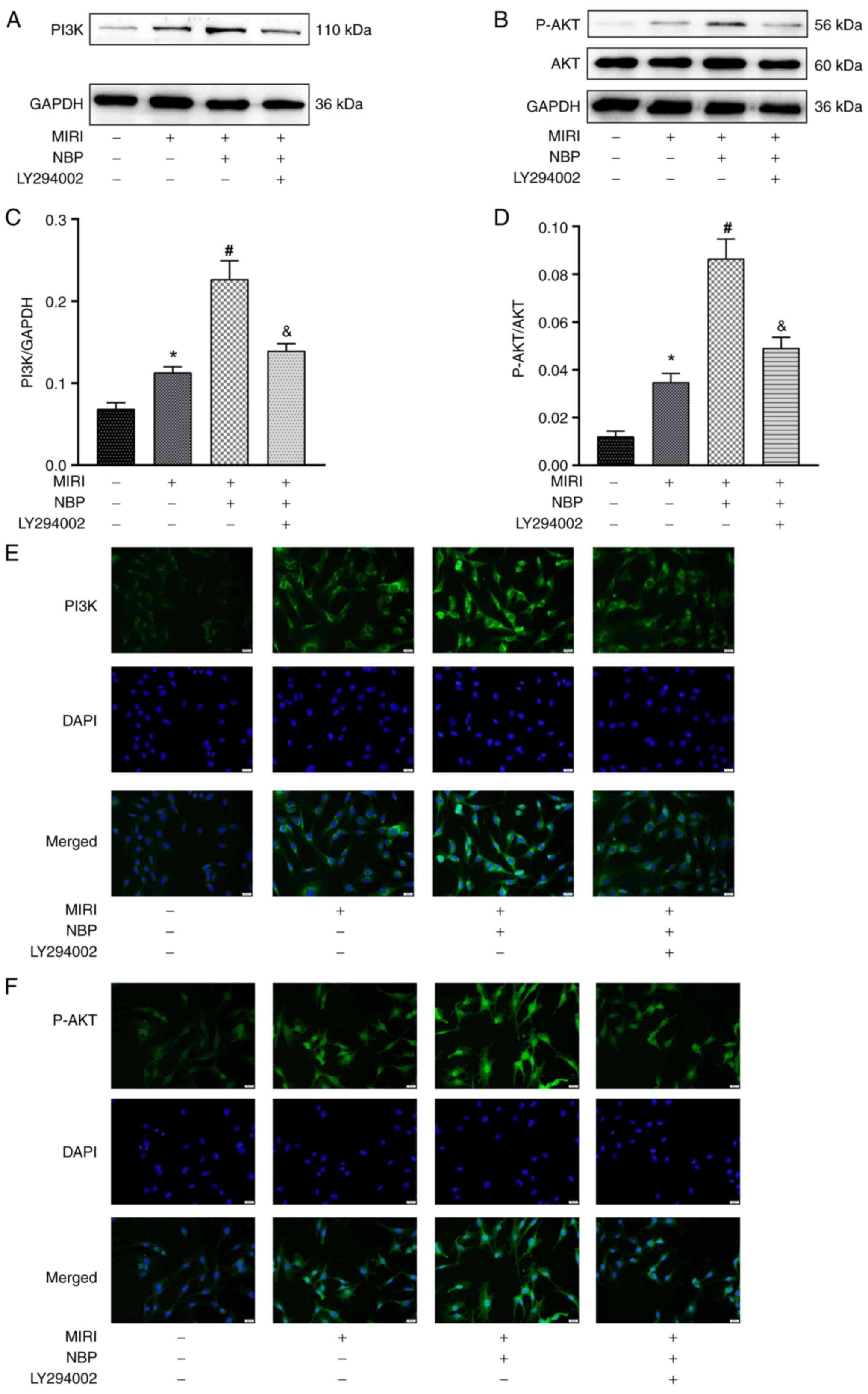
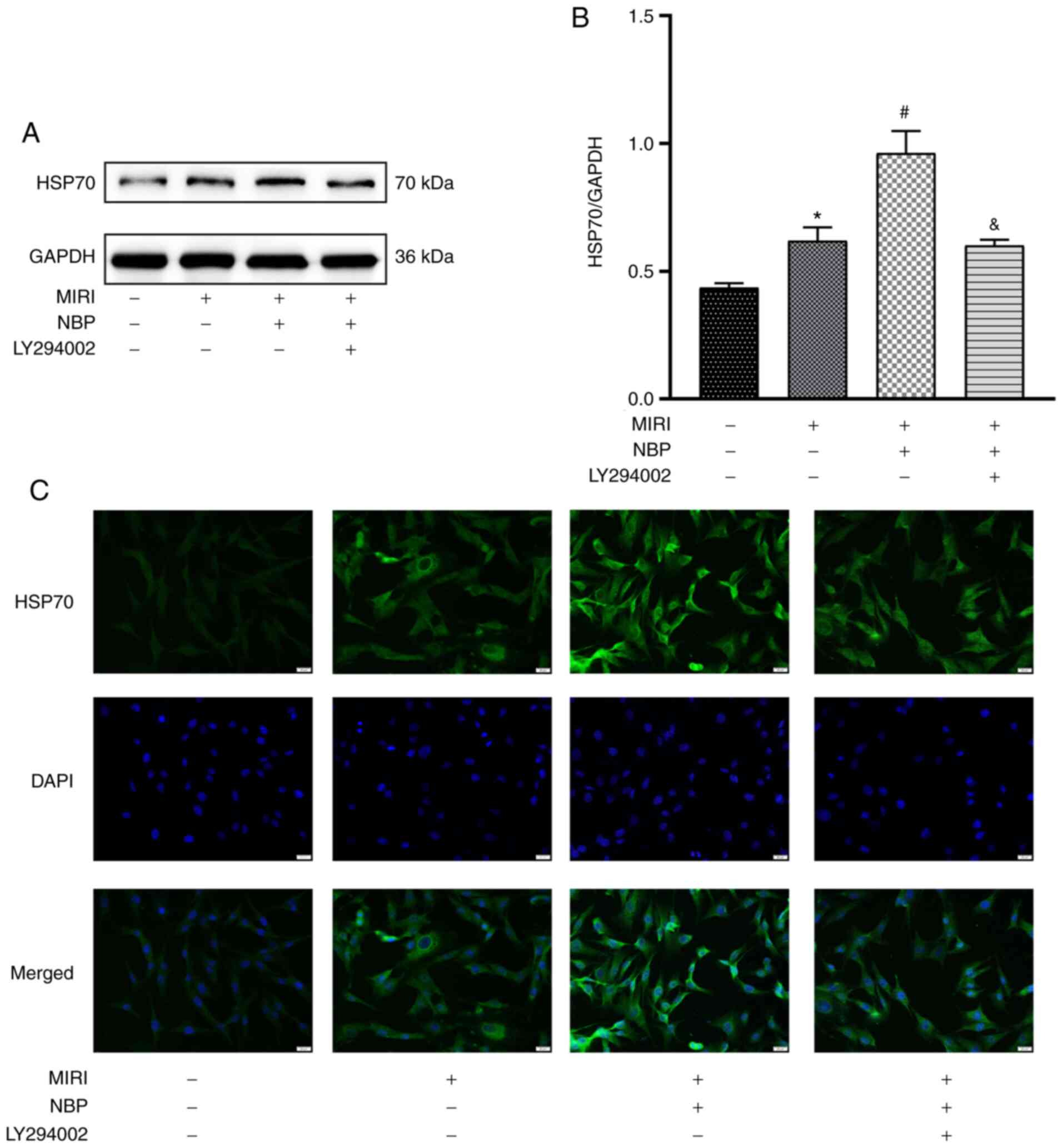
NBP activates the HSP70 and PI3K/AKT
signaling pathway
During MIRI, the expression of the stress-protective
proteins HSP70 and PI3K/AKT was upregulated. Western blotting and
immunofluorescence staining were used to determine the protein
expression of HSP70 and PI3K/AKT after NBP treatment. The
expression levels of p-AKT and PI3K in the MIRI group were
significantly increased compared with the CON group (P<0.05). In
the MIRI + NBP group, the expression of p-AKT and PI3K was
increased compared with the MIRI group (P<0.05). By contrast, in
the MIRI + NBP + LY294002 group, the expression of p-AKT and PI3K
decreased compared with the MIRI + NBP group (P<0.05; Fig. 2A-D). This tendency was also observed
in the immunofluorescence assay (Fig.
2E and F). Additionally, the
expression level of HSP70 in the MIRI group increased compared with
the CON group (P<0.05). NBP pretreatment further increased the
expression of HSP70 (P<0.05), whereas LY294002 decreased it
compared with the MIRI + NBP group (P<0.05; Fig. 3A and B). This tendency was also observed in the
immunofluorescence assay (Fig. 3C).
The present results indicated that NBP pretreatment could increase
the expression level of PI3K, p-AKT and HSP70 following MIRI, and
that the inhibition of the PI3K pathway also altered HSP70
expression.
Discussion
MIRI mechanism involves several processes, including
energy metabolism disorder, oxidative stress, calcium overload,
inflammation, apoptosis and autophagy (33). In the clinic, the best treatment for
patients with myocardial infarction is immediate reperfusion,
causing a paradoxical situation because of its adverse effects
(34). Therefore, an in-depth
understanding of the mechanism of MIRI has become a priority.
As a drug used for the treatment of stroke, NBP has
been widely studied in the nervous system. For instance, a previous
study has indicated that NBP could reduce nerve cell death during
cerebral ischemia by inhibiting inflammation, oxidative stress,
autophagy and apoptosis (35). NBP
has a wide range of functions, as it can protect the nervous system
and delay the onset and progression of diabetic cataract (25,36).
Furthermore, a previous study has revealed that NBP exhibited a
protective effect against MIRI, and its mechanism may be associated
with the regulation of the mitochondrial apoptosis and the
AKT/Nuclear factor erythroid 2-related factor 2 signaling pathways
(37). However, the specific
mechanism underlying the NBP effect on MIRI needs to be further
explored.
As a protective protein under stress, HSP70 has been
indicated to play a protective role in cardiomyocytes during MIRI
(38). However, it is unclear
whether HSP70 is involved in the NBP protective effect on
cardiomyocytes. The present results indicated that NBP regulated
the expression of HSP70 via the PI3K/AKT signaling pathway, thereby
protecting H9c2 cells from MIRI. The present study provided a basis
for the application of NBP in the clinical treatment of
cardiovascular diseases, and elucidated the protective mechanism of
NBP to a certain extent.
The current study determined the optimal
concentration of NBP pretreatment against MIRI by establishing a
concentration gradient of NBP, indicating that 100 µM NBP exhibited
the strongest protective effect compared with the other
concentrations. NBP pretreatment improved the decrease in cell
viability induced by MIRI and significantly reduced LDH release,
thus protecting H9c2 cells from MIRI. LY294002 treatment reversed
the protective effect of NBP, decreased cell viability and
increased LDH release. The present results suggested that the
protective effect of NBP on H9c2 cells depended on the activation
of the PI3K signaling pathway.
During myocardial ischemia, a large number of oxygen
free radicals are produced, eventually leading to the destruction
of the mitochondrial structure, mitochondrial swelling, cell
membrane structure damage and the disturbance of cell energy
metabolism (39). MDA is the final
product of reactive oxygen species oxidation of arachidonic acid,
representing a biomarker of lipid peroxidation produced by
oxidative stress (40). The present
results indicated that the MDA content in H9c2 cells significantly
increased after MIRI; however, the oxidation index indicated by the
MDA content significantly decreased after NBP pretreatment. By
contrast, the oxidation index increased again after LY294002
intervention. NBP inhibited oxidative stress in a process of
myocardial protection, and this antioxidant mechanism was blocked
by inhibiting the PI3K pathway.
A number of inflammatory cytokines are activated
during MIRI, which can cause systemic inflammation (41,42).
TNF-α causes local inflammation and apoptosis, eventually
contributing to cardiac insufficiency and even cardiac infarction
(43). IL-1 plays a central role in
human autoinflammatory diseases and its target genes are numerous,
such as IL-1β, IL-6 and IL-8(44).
Among those, IL-1β expression was quantified in the present study.
IL-1β is a typical pro-inflammatory factor that mediates the
infiltration of neutrophils and macrophages in local tissues during
ischemia-reperfusion, which causes myocardial fibrosis and
structural remodeling (45). To
elucidate the effect of NBP on H9c2 cells, RT-qPCR was performed to
determine the expression level of inflammatory cytokines. The
present results indicated that the mRNA expression of IL-1β and
TNF-α in the MIRI group significantly increased compared with the
CON group. Pretreatment with NBP significantly inhibited the
inflammatory response; however, this anti-inflammatory effect was
reversed by blocking the PI3K signaling pathway. Namely, blocking
the PI3K pathway eliminated the inflammatory inhibitory effect of
NBP.
The protective role of the PI3K/AKT signaling
pathway against MIRI is well-known. For instance, a previous study
has demonstrated that activation of the insulin-induced PI3K/AKT
pathway could inhibit cardiomyocyte apoptosis and protect or
improve local and global cardiac function (46). Upon PI3K-induced activation, AKT
activates GSK-3β through phosphorylation, this junction of several
pathways plays a notable role in myocardial protection (47). Relevant experiments have confirmed
that the increase of heat shock factor 1 (HSF1) results from GSK-3β
phosphorylation (48,49). HSP70 is a stress-protective protein
that protects cardiomyocytes during MIRI (50). HSP70 induction is mediated by the
interaction with HSF1; therefore, PI3K/AKT and HSP70 may be
associated through GSK-3β and HSF1 (51,52).
It was previously demonstrated that there is a mutual regulation
between the PI3K/AKT pathway and HSP70 in brain and lung tissues
(16,17). To further elucidate the association
between the PI3K/AKT pathway and HSP70 in H9c2 cells, the
expression of HSP70 was examined upon application of the PI3K
inhibitor LY294002. Western blotting and immunofluorescence
staining indicated that MIRI increased the expression levels of
HSP70, PI3K and p-AKT, and that NBP pretreatment further increased
this expression. By contrast, LY294002 suppressed the expression
levels of PI3K, p-AKT and HSP70. Therefore, the protective effect
of NBP against MIRI was indicated to be associated with the
PI3K/AKT signaling pathway and HSP70.
Certain main limitations can be taken into account
in the present work. Firstly, the present regulation pathway needs
to be further investigated in an animal model. Secondly, the
protective effect of NBP on MIRI has not been reflected in clinical
treatment, and further studies are required to explore the drug
administration route and dosages. Thirdly, H9c2 cells are rat
myoblasts and not cardiomyocytes; therefore, they present
differences in their characteristics and protein expression
compared with adult cardiomyocytes. Future studies will investigate
the present hypotheses in primary cardiomyocytes.
In summary, NBP upregulated HSP70 through the
PI3K/AKT pathway and reduced the inflammatory response, oxidative
stress and injury of H9c2 cells, thereby attenuating MIRI. These
findings may provide a novel therapeutic target for the clinical
treatment of MIRI.
Acknowledgements
Not applicable.
Funding
Funding: The present work was supported by National Natural
Science Foundation of China (grant no. 81870593) and Natural
Science Foundation of Shandong Province of China (grant nos.
ZR2018MH008 and ZR2019PH037).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH, XDS and XTS designed the research and revised
the manuscript. YY, YZ and YL conducted experiments. MW and BD
analyzed the data and participated in technical editing of the
manuscript. YY and XTS wrote the draft manuscript. YY and WH
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang BF and Yoshioka J: The emerging role
of thioredoxin-interacting protein in myocardial
ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther.
22:219–229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li X, Liu M, Sun R, Zeng Y, Chen S and
Zhang P: Protective approaches against myocardial ischemia
reperfusion injury. Exp Ther Med. 12:3823–3829. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chin KY, Qin C, May L, Ritchie RH and
Woodman OL: New pharmacological approaches to the prevention of
myocardial ischemia-reperfusion injury. Curr Drug Targets.
18:1689–1711. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Esposito ML, Zhang Y, Qiao X, Reyelt L,
Paruchuri V, Schnitzler GR, Morine KJ, Annamalai SK, Bogins C,
Natov PS, et al: Left ventricular unloading before reperfusion
promotes functional recovery after acute myocardial infarction. J
Am Coll Cardiol. 72:501–514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang
L, Chen L, Feng W, Shi H, Yu X, et al: bFGF attenuates endoplasmic
reticulum stress and mitochondrial injury on myocardial
ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J
Cell Mol Med. 19:595–607. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Radons J: The human HSP70 family of
chaperones: Where do we stand? Cell Stress Chaperones. 21:379–404.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guo S, Gao C, Xiao W, Zhang J, Qu Y, Li J
and Ye F: Matrine protects cardiomyocytes from ischemia/reperfusion
injury by regulating HSP70 expression via activation of the
JAK2/STAT3 pathway. Shock. 50:664–670. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wright MA, Aprile FA, Bellaiche MM,
Michaels TC, Muller T, Arosio P, Vendruscolo M, Dobson CM and
Knowles TP: Cooperative assembly of Hsp70 subdomain clusters.
Biochemistry. 57:3641–3649. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang L, Mo Y, Li Y, Zhong Y, He S, Zhang
Y, Tang Y, Fu S, Wang X and Chen A: Urolithin A alleviates
myocardial ischemia/reperfusion injury via PI3K/Akt pathway.
Biochem Biophys Res Commun. 486:774–780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang BF, Jiang H, Chen J, Guo X, Li Y, Hu
Q and Yang S: Nobiletin ameliorates myocardial ischemia and
reperfusion injury by attenuating endoplasmic reticulum
stress-associated apoptosis through regulation of the PI3K/AKT
signal pathway. Int Immunopharmacol. 73:98–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thokala S, Inapurapu S, Bodiga VL, Vemuri
PK and Bodiga S: Loss of ErbB2-PI3K/Akt signaling prevents zinc
pyrithione-induced cardioprotection during ischemia/reperfusion.
Biomed Pharmacother. 88:309–324. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vanhaesebroeck B, Guillermet-Guibert J,
Graupera M and Bilanges B: The emerging mechanisms of
isoform-specific PI3K signalling. Nat Rev Mol Cell Biol.
11:329–341. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Sulaiman D, Li J, Devarajan A, Cunningham
CM, Li M, Fishbein GA, Fogelman AM, Eghbali M and Reddy ST:
Paraoxonase 2 protects against acute myocardial
ischemia-reperfusion injury by modulating mitochondrial function
and oxidative stress via the PI3K/Akt/GSK-3β RISK pathway. J Mol
Cell Cardiol. 129:154–164. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hausenloy DJ and Yellon DM: New directions
for protecting the heart against ischaemia-reperfusion injury:
Targeting the reperfusion injury salvage kinase (RISK)-pathway.
Cardiovasc Res. 61:448–460. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Damilano F, Perino A and Hirsch E: PI3K
kinase and scaffold functions in heart. Ann N Y Acad Sci.
1188:39–45. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhai C, Lv J, Wang K, Li Q and Qu Y: HSP70
silencing aggravates apoptosis induced by hypoxia/reoxygenation
in vitro. Exp Ther Med. 18:1013–1020. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ji K, Xue L, Cheng J and Bai Y:
Preconditioning of H2S inhalation protects against cerebral
ischemia/reperfusion injury by induction of HSP70 through
PI3K/Akt/Nrf2 pathway. Brain Res Bull. 121:68–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abdoulaye IA and Guo YJ: A review of
recent advances in neuroprotective potential of 3-N-Butylphthalide
and its derivatives. Biomed Res Int. 2016(5012341)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao Y, Lee JH, Chen D, Gu X, Caslin A, Li
J, Yu SP and Wei L: DL-3-n-butylphthalide induced neuroprotection,
regenerative repair, functional recovery and psychological benefits
following traumatic brain injury in mice. Neurochem Int. 111:82–92.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qin C, Zhou P, Wang L, Mamtilahun M, Li W,
Zhang Z, Yang GY and Wang Y: Dl-3-N-butylphthalide attenuates
ischemic reperfusion injury by improving the function of cerebral
artery and circulation. J Cereb Blood Flow Metab. 39:2011–2021.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kawasaki K, Yano K, Sasaki K, Tawara S,
Ikegaki I, Satoh S, Ohtsuka Y, Yoshino Y, Kuriyama H, Asano T and
Seto M: Correspondence between neurological deficit, cerebral
infarct size, and rho-kinase activity in a rat cerebral thrombosis
model. J Mol Neurosci. 39:59–68. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao Q, Zhang C, Wang X, Chen L, Ji H and
Zhang Y: (S)-ZJM-289, a nitric oxide-releasing derivative of
3-n-butylphthalide, protects against ischemic neuronal injury by
attenuating mitochondrial dysfunction and associated cell death.
Neurochem Int. 60:134–144. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang P, Guo ZF, Xu YM, Li YS and Song JG:
N-Butylphthalide (NBP) ameliorated cerebral ischemia
reperfusion-induced brain injury via HGF-regulated TLR4/NF-κB
signaling pathway. Biomed Pharmacother. 83:658–666. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liao D, Xiang D, Dang R, Xu P, Wang J, Han
W, Fu Y, Yao D, Cao L and Jiang P: Neuroprotective effects of
dl-3-n-Butylphthalide against doxorubicin-induced
neuroinflammation, oxidative stress, endoplasmic reticulum stress,
and behavioral changes. Oxid Med Cell Longev.
2018(9125601)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang YG, Li Y, Wang CY, Ai JW, Dong XY,
Huang HY, Feng ZY, Pan YM, Lin Y, Wang BX and Yao LL:
L-3-n-Butylphthalide protects rats' cardiomyocytes from
ischaemia/reperfusion-induced apoptosis by affecting the
mitochondrial apoptosis pathway. Acta Physiol (Oxf). 210:524–533.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qiu H, Wu H, Ma J, Cao H, Huang L, Qiu W,
Peng Y and Ding C: DL-3-n-Butylphthalide reduces atrial
fibrillation susceptibility by inhibiting atrial structural
remodeling in rats with heart failure. Naunyn Schmiedebergs Arch
Pharmacol. 391:323–334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Qiu H, Ma J, Wu H and Ding C:
DL-3-n-butylphthalide improves ventricular function, and prevents
ventricular remodeling and arrhythmias in post-MI rats. Naunyn
Schmiedebergs Arch Pharmacol. 391:627–637. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yu P, Ma S, Dai X and Cao F: Elabela
alleviates myocardial ischemia reperfusion-induced apoptosis,
fibrosis and mitochondrial dysfunction through PI3K/AKT signaling.
Am J Transl Res. 12:4467–4477. 2020.PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao D, Yang J and Yang L: Insights for
oxidative stress and mTOR signaling in myocardial
ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev.
2017(6437467)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Amani M, Jeddi S, Ahmadiasl N, Usefzade N
and Zaman J: Effect of HEMADO on level of CK-MB and LDH enzymes
after ischemia/reperfusion injury in isolated rat heart.
Bioimpacts. 3:101–104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu Z, Wang D, Zhou Z, Chen Q, Zhang D,
Chen S, Jiang H, Jia C and Liu X: Dexmedetomidine attenuates renal
and myocardial ischemia/reperfusion injury in a dose-dependent
manner by inhibiting inflammatory response. Ann Clin Lab Sci.
49:31–35. 2019.PubMed/NCBI
|
|
33
|
Zhang C, He M, Ni L, He K, Su K, Deng Y,
Li Y and Xia H: The role of arachidonic acid metabolism in
myocardial ischemia-reperfusion injury. Cell Biochem Biophys.
78:255–265. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu H, Yang L, Wu HJ, Chen KH, Lin F, Li
G, Sun HY, Xiao GS, Wang Y and Li GR: Water-soluble acacetin
prodrug confers significant cardioprotection against
ischemia/reperfusion injury. Sci Rep. 6(36435)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang S, Ma F, Huang L, Zhang Y and Peng Y,
Xing C, Feng Y, Wang X and Peng Y: Dl-3-n-Butylphthalide (NBP): A
promising therapeutic agent for ischemic stroke. CNS Neurol Disord
Drug Targets. 17:338–347. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang F, Ma J, Han F, Guo X, Meng L, Sun Y,
Jin C, Duan H, Li H and Peng Y: DL-3-n-butylphthalide delays the
onset and progression of diabetic cataract by inhibiting oxidative
stress in rat diabetic model. Sci Rep. 6(19396)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bai M, Pan CL, Jiang GX, Zhang YM and
Zhang Z: Effects of butylphthalide on oxidative stress and
inflammatory response in rats with myocardial infarction through
Akt/Nrf2 signaling pathway. Eur Rev Med Pharmacol Sci.
23:9642–9650. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu X, Zhang C, Zhang C, Li J, Guo W, Yan
D, Yang C, Zhao J, Xia T, Wang Y, et al: Heat shock protein 70
inhibits cardiomyocyte necroptosis through repressing autophagy in
myocardial ischemia/reperfusion injury. In Vitro Cell Dev Biol
Anim. 52:690–698. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yuan X, Juan Z, Zhang R, Sun X, Yan R, Yue
F, Huang Y, Yu J and Xia X: Clemastine fumarate protects against
myocardial ischemia reperfusion injury by activating the
TLR4/PI3K/Akt signaling pathway. Front Pharmacol.
11(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ma C, Xu Z and Lv H: Low n-6/n-3 PUFA
ratio improves inflammation and myocardial ischemic reperfusion
injury. Biochem Cell Biol. 97:621–629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiong J, Yuan YJ, Xue FS, Wang Q, Cheng Y,
Li RP, Liao X and Liu JH: Postconditioning with α7nAChR agonist
attenuates systemic inflammatory response to myocardial
ischemia-reperfusion injury in rats. Inflammation. 35:1357–1364.
2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sun M, Dawood F, Wen WH, Chen M, Dixon I,
Kirshenbaum LA and Liu PP: Excessive tumor necrosis factor
activation after infarction contributes to susceptibility of
myocardial rupture and left ventricular dysfunction. Circulation.
110:3221–3228. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Weber A, Wasiliew P and Kracht M:
Interleukin-1 (IL-1) pathway. Sci Signal. 3(cm1)2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yao H and Han X and Han X: The
cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling
pathway. Am J Cardiovasc Drugs. 14:433–442. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wei D, Xu H, Gai X and Jiang Y:
Astragaloside IV alleviates myocardial ischemia-reperfusion injury
in rats through regulating PI3K/AKT/GSK-3β signaling pathways. Acta
Cir Bras. 34(e201900708)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Juhaszova M, Zorov DB, Yaniv Y, Nuss HB,
Wang S and Sollott SJ: Role of glycogen synthase kinase-3beta in
cardioprotection. Circ Res. 104:1240–1252. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Son TW, Yun SP, Yong MS, Seo BN, Ryu JM,
Youn HY, Oh YM and Han HJ: Netrin-1 protects hypoxia-induced
mitochondrial apoptosis through HSP27 expression via DCC- and
integrin a6b4-dependent Akt, GSK-3β, and HSF-1 in mesenchymal stem
cells. Cell Death Dis. 4(e563)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nair SP and Sharma RK: Heat shock proteins
and their expression in primary murine cardiac cell populations
during ischemia and reperfusion. Mol Cell Biochem. 464:21–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xu NW, Chen Y, Liu W, Chen YJ, Fan ZM, Liu
M and Li LJ: Inhibition of JAK2/STAT3 signaling pathway suppresses
proliferation of burkitt's lymphoma raji cells via cell cycle
progression, apoptosis, and oxidative stress by modulating HSP70.
Med Sci Monit. 24:6255–6263. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Staib JL, Quindry JC, French JP, Criswell
DS and Powers SK: Increased temperature, not cardiac load,
activates heat shock transcription factor 1 and heat shock protein
72 expression in the heart. Am J Physiol Regul Integr Comp Physiol.
292:R432–R439. 2007.PubMed/NCBI View Article : Google Scholar
|