Introduction
Prostate cancer is a high-risk malignant tumor of
the urinary tract, typically diagnosed in middle-aged and elderly
men (1). The worldwide incidence
rate of prostate cancer accounted for 15% of the total number of
malignant tumors in males in 2012(2), displaying a significant upward trend
each year (3). Prostate cancer is
the third largest cause of cancer-associated death in males in the
United States (4) and the second
largest cause worldwide (2). At
present, the treatment of prostate cancer includes surgery,
radiotherapy, chemotherapy and endocrine therapy (5). However, for advanced prostate cancer,
surgery is ineffective and other treatment options are not
satisfactory, with the majority being associated with severe
adverse reactions (5). The main
therapeutic strategy for prostate cancer is endocrine therapy,
however, after a median of 18-20 months, the majority of patients
will eventually develop androgen resistance, with a median survival
of 12 months (6,7). Therefore, the treatment of
castration-resistant prostate cancer (CRPC) has become a focus in
urology research and the identification of novel treatment options
is required (8).
Bufalin is a steroidal terpene compound, and is one
of the active ingredients in traditional Chinese medicine (9). Bufalin has been reported to be useful
in the treatment of liver and pancreatic cancers, as well as other
tumors (9-12).
Previous studies have reported that bufalin can significantly
inhibit the proliferation of PC3 CRPC cells in vitro
(13,14). However, the therapeutic dose is
close to the toxic dose, which limits its clinical application
(15,16). Bufalin is a DNA topoisomerase
(TOP)II inhibitor (17), and its
mechanism of action is related to the inhibition of tumor
proliferation, metastasis and angiogenesis, as well as the reversal
of tumor resistance (18-21).
Cortés and Piñero (22) reported
that DNA TOPI inhibition in ovarian cells by the TOPI inhibitor
irinotecan resulted in increased levels of TOPII mRNA and protein
expression, allowing DNA metabolism to continue (23,24).
This phenomenon is called the side-channel sensitivity of TOP, and
is required to maintain the normal physiological state of the cells
(25,26). In oral cancer research, Ding et
al (27) combined the TOPI
inhibitor irinotecan and the TOPII inhibitor doxorubicin to improve
treatment efficacy.
Previous studies have reported that the DNA TOPI
inhibitor hydroxycamptothecin and the TOPII inhibitor bufalin can
inhibit the growth of the CRPC cell line DU 145 in vitro
(28,29). These studies also suggested that
simultaneous administration of the two inhibitors was not as
effective as individual drug administration, due to antagonism, but
sequential administration significantly improved the results
obtained (28,29).
The present study investigated whether bufalin in
combination with hydroxycamptotecin would exhibit the same effect
in vivo, as has been reported in previous in vitro
studies. Furthermore, the present study aimed to identify a dose of
low-toxicity bufalin combined with hydroxycamptothecin for further
clinical applications.
Materials and methods
Cell lines and cell culture
The human prostate cancer cell line DU 145,
purchased from Thermo Fisher Scientific, Inc., was cultured in RPMI
1640 complete medium (Sigma-Aldrich; Merck KGaA) containing 10%
fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA) and 1%
penicillin and streptomycin solution, at 37˚C with 5%
CO2 and fully saturated humidity. Cells were subcultured
each for 2-3 days.
Establishment of a CRPC xenograft
model in nude mice and treatment administration
A total of 41 male BALB/c nude mice (age, 22-25 g;
6-8 weeks), obtained from the Experimental Animal Center of
Shanghai University of Traditional Chinese Medicine were housed
under pathogen-free condition at 22±2˚C with 40-60% humidity, 12-h
light/dark cycles and free access to food and water. Cell
suspensions of DU 145 cells in the logarithmic growth phase were
prepared at a concentration of 1x107 cells/ml, using
physiological saline. To induce tumor formation, five mice were
subcutaneously injected in the abdomen with 0.2 ml cell suspension.
At three weeks post-inoculation, when the diameters of the primary
implanted tumors had grown to 1 cm3, tumors were removed
and abdominally implanted into the other 36 mice. After 8 days,
drugs were administered once every other day for 30 days. For drug
administration, the 36 tumor-bearing mice were randomly divided
into six groups, with six mice in each group. The groups were as
follows: Normal saline negative control group (SN),
hydroxycamptothecin (2 mg/kg; BioCrick) single drug positive
control group (H), bufalin (1 mg/kg, Sigma-Aldrich; Merck KGaA)
single drug positive control group (B) and hydroxycamptothecin (2
mg/kg) sequentially combined with 0.4 mg/kg bufalin treatment group
(H4B), 0.6 mg/kg bufalin treatment group (H6B) or 0.8 mg/kg bufalin
treatment group (H8B). No adverse reactions were reported in the
mice in the combined treatment groups. The sequential
co-administration method involved administration of the
corresponding dose of bufalin 8 h after the administration of
hydroxycamptothecin, as previously described (30). The present study was approved by the
Institutional Ethics Committee of Shanghai University of
Traditional Chinese Medicine.
Morphological and histological
observation of tumors
A total of one day post-treatment, the six mice in
each group were sacrificed and the tumors were completely removed.
The tumors from each group were compared by morphological analysis.
The long diameter (a) and short diameter (b) of the tumor were
measured. Tumor parameters were calculated using the following
formulae: Tumor volume (V; mm3)=(ab2)/2;
tumor-inhibition rate (%)=[1-(V administration group/V negative
control group)] x100; and tumor mass was determined using an
electronic balance. A section of the tumor mass was removed, fixed
in 10% formalin (pH 7.4) at room temperature for 24 h and embedded
in paraffin. The paraffin-embedded samples were sectioned (4 µm).
For pathological analysis, sections were stained at room
temperature using haemotoxylin for 3 min and eosin for 30 sec (HE).
Subsequently, tissue sections were examined using the Leica DM6 B
light microscope (Leica Microsystems Inc.; magnification, x4, x10
and x40).
TUNEL detection
The TUNEL Assay kit-FITC (cat. no. ab66108; Abcam)
was used to detect apoptosis in paraffin-embedded sections,
according to the manufacturer's protocol. The staining of tumor
cells in each group was observed under a fluorescence microscope
(Leica DMLB; Leica Microsystems Inc.; magnification, x400) and
necrotic areas were avoided. TUNEL-positive cells were observed in
five randomly-selected high-power fields. The integrated optical
density (IOD) values of the images were analyzed using Image-Pro
Plus software (version 6.0; Media Cybernetics, Inc.) to assess the
extent of apoptosis in tumor cells.
Detection of proliferating cell
nuclear antigen (PCNA) protein expression by
immunohistochemistry
Immunohistochemical staining was performed to detect
the expression of PCNA in the xenograft tumors. Paraffin sections
were rehydrated. The sections were subsequently treated as follows:
Microwave antigen retrieval (700 W for 8 min; twice in 10 mM sodium
citrate; pH 6.0) was followed by incubation with 3% hydrogen
peroxide to block endogenous peroxidase and 10% goat serum (cat.
no. 5560-0007; Seracare Life Sciences, Inc.) at 4˚C for 30 min to
block nonspecific binding. PCNA was detected with rabbit anti-PCNA
(1:100; cat. no. ab18197; Abcam) overnight at 4˚C and horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary
antibody (1:400; cat. no. ab205718; Abcam) for 1 h at room
temperature. After that, 3,3-diaminobenzidine tetrahydrochloride
(DAB; cat. no. 30015; Biotium, Inc.) was used for the chromomeric
reaction, and hematoxylin was used to stain the nucleus for 5 min
at room temperature. The staining of the tumor nuclei in each group
was observed using a Leica DFC300 FX light microscope (Leica
Microsystems Inc.; IL; magnification, x400). PCNA-positive cells,
displaying brown-yellow granules in the nuclei, were observed in
five randomly-selected high-power fields. The integrated optical
density (IOD) values of the images were analyzed using Image-Pro
Plus software (version 6.0; Media Cybernetics, Inc.). For the
negative control, the primary antibody was replaced by normal
rabbit IgG.
Western blotting
A homogenizer was used to prepare lysates from
xenograft tumor tissues. The xenograft tumor tissues were lysed in
radioimmunoprecipitation assay lysis buffer (Rockland
Immunochemicals, Inc.) according to the standard protocol. Total
protein was quantified using a bicinchoninic acid assay. Total
protein lysate (50 µg) was loaded into each lane and separated
using 12% SDS-PAGE gels. The separated proteins were then
transferred onto PVDF membranes (Merck KGaA) and 3% BSA (cat. no.
PRO-22; ProSpec-Tany TechnoGene, Ltd.) in TBS-Tween-20 (TBST) was
added to the membranes for 1 h at room temperature. The membranes
were washed three times with TBST and were subsequently incubated
with diluted primary antibodies overnight. Primary antibodies
including Bax (1:1,000; cat. no. 2772), Bcl-XL (1:1,000; cat. no.
2764), p53 (1:1,000; cat. no. 2527), PDCD4 (1:1,000; cat. no.
9535), GSK-3β (1:1,000; cat. no. 12456), p-AKT (1:2,000; cat. no.
4060), AKT (1:1,000; cat. no. 4685) and GAPDH (1:1,000; cat. no.
5174) were added and incubated overnight at 4˚C. Following the
primary incubation, membranes were washed with TBST three times (x5
min) and then incubated with the horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin G secondary antibody (1:1,000; cat.
no. 4030-05; SouthernBiotech) for 1 h at room temperature. After
washing the membranes three times with TBST, Immunoblot detection
and visualization were performed using enhanced chemiluminescence
western blotting detection reagents (SuperSignal™ West
Pico PLUS Chemiluminescent Substrate; cat. no. 34577; Thermo Fisher
Scientific, Inc.). Immunoblotting was performed with target
antibodies and protein bands were scanned and quantified using a
ChemiDoc image analysis system (Bio-Rad Laboratories, Inc.). ImageJ
software (version 1.46; Natioanl Institutes of Health) was used for
densitometry analysis. All Primary antibodies were purchased from
the Cell Signaling Technology, Inc.
Data analysis and statistical
processing
Statistical analysis was performed using SPSS
software (version 21.0; IBM Corp.). Data are presented as the mean
± standard deviation. All experiments were performed in triplicate.
Data were analyzed by one-way ANOVA followed by Bonferroni post-hoc
test. The Wilcoxon rank sum test was used to compare non-normally
distributed data sets in non-parametric tests. The Mann-Whitney U
method was used to test the significant differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of tumor size in different
groups
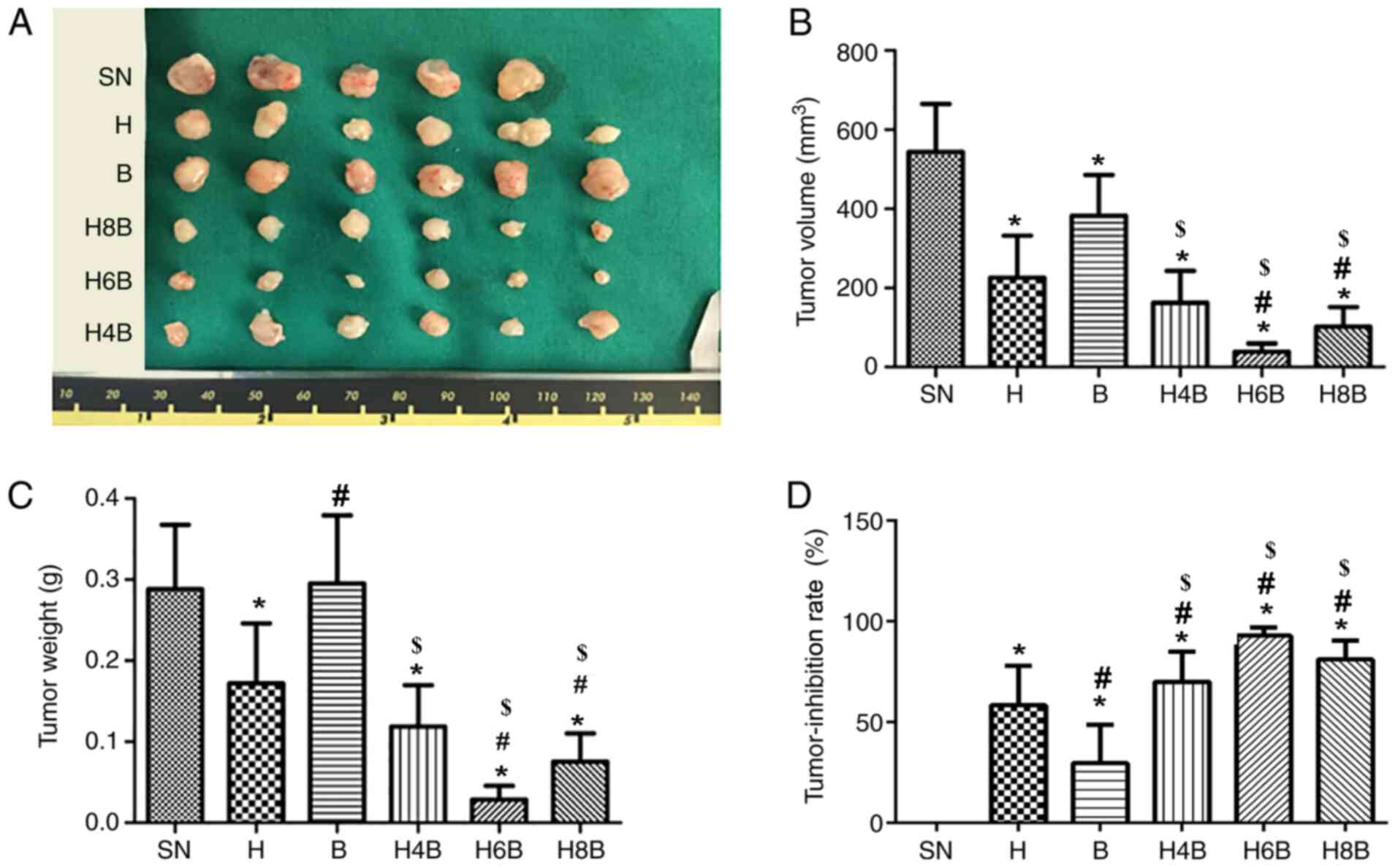
After successful establishment of CRPC xenografts in
mice, the mice were weighed and no statistically significant
difference was observed between treatment groups (Table I; P>0.05). The mice in the
treatment groups displayed no abnormal changes in body weight or
behavior. The mice were sacrificed one day post-treatment and
comparisons of tumor size of the xenograft tumors isolated from
different groups were performed (Fig.
1A).
 | Table IComparison of the weight of nude
mice, as well as the volume, weight and inhibition rate of the
xenograft tumors. |
Table I
Comparison of the weight of nude
mice, as well as the volume, weight and inhibition rate of the
xenograft tumors.
| Group | n | Tumor volume
(mm3) | Tumor weight
(g) | Tumor-inhibition
rate (%) | Nude mice weight
(g) |
|---|
| SN | 6 | 543.55±121.51 | 0.29±0.08 | | 21.82±0.84 |
| H | 6 | 214.76±115.54 | 0.17±0.07 | 58.47±19.65 | 23.25±2.28 |
| B | 6 | 378.86±115.39 | 0.30±0.08 | 29.72±19.04 | 21.38±1.54 |
| H4B | 6 | 175.42±83.06 | 0.12±0.05 | 70.10±14.85 | 22.70±2.57 |
| H6B | 6 | 34.76±22.26 | 0.03±0.02 | 92.99±3.96 | 22.32±2.65 |
| H8B | 6 | 101.93±55.82 | 0.08±0.04 | 81.26±9.19 | 23.50±2.06 |
The volume and weight of the xenograft tumors were
measured. All drug treatments significantly reduced the tumor
volume compared with the SN group (P<0.05; Fig. 1B). Among the different drug
treatment groups, the H6B and H8B groups were more effective at
inhibiting increases in the tumor volume compared with the single
drug administration groups (H or B) and the H4B group (P<0.05;
Table I; Fig. 1B). However, the H6B group displayed
the lowest tumor volume out of all of the groups (Table I; Fig.
1B). Similarly, in terms of tumor weight, the H6B and H8B
groups were more effective at inhibiting tumor growth compared with
all other groups (P<0.05; Table
I; Fig. 1C). The tumor weight
of the H4B and H groups was significantly reduced compared with the
SN group (P<0.05; Table I;
Fig. 1C). However, the B group did
not display a significant difference in tumor weight compared with
the SN group (P>0.05; Table I;
Fig. 1C). Additionally, although
the H6B group appeared to limit tumor weight to a further extent
than the H8B group, the difference was not significant (P>0.05;
Table I; Fig. 1C).
The tumor-inhibition rate of each group was
calculated in each treatment group. The tumor-inhibition rate of
the H6B and H8B groups was >80%, which was significantly higher
than that of the single drug administration groups (P<0.05;
Fig. 1D). The tumor-inhibition rate
in the H6B group reached 92.99±3.96%, but there was no statistical
difference between the H6B and H8B groups (P>0.05; Table I; Fig.
1D). The tumor-inhibition rate in the H4B group was not
significantly different (P>0.05) from that in the H group, but
was significantly (P<0.05) improved compared with the B group
(Fig. 1D).
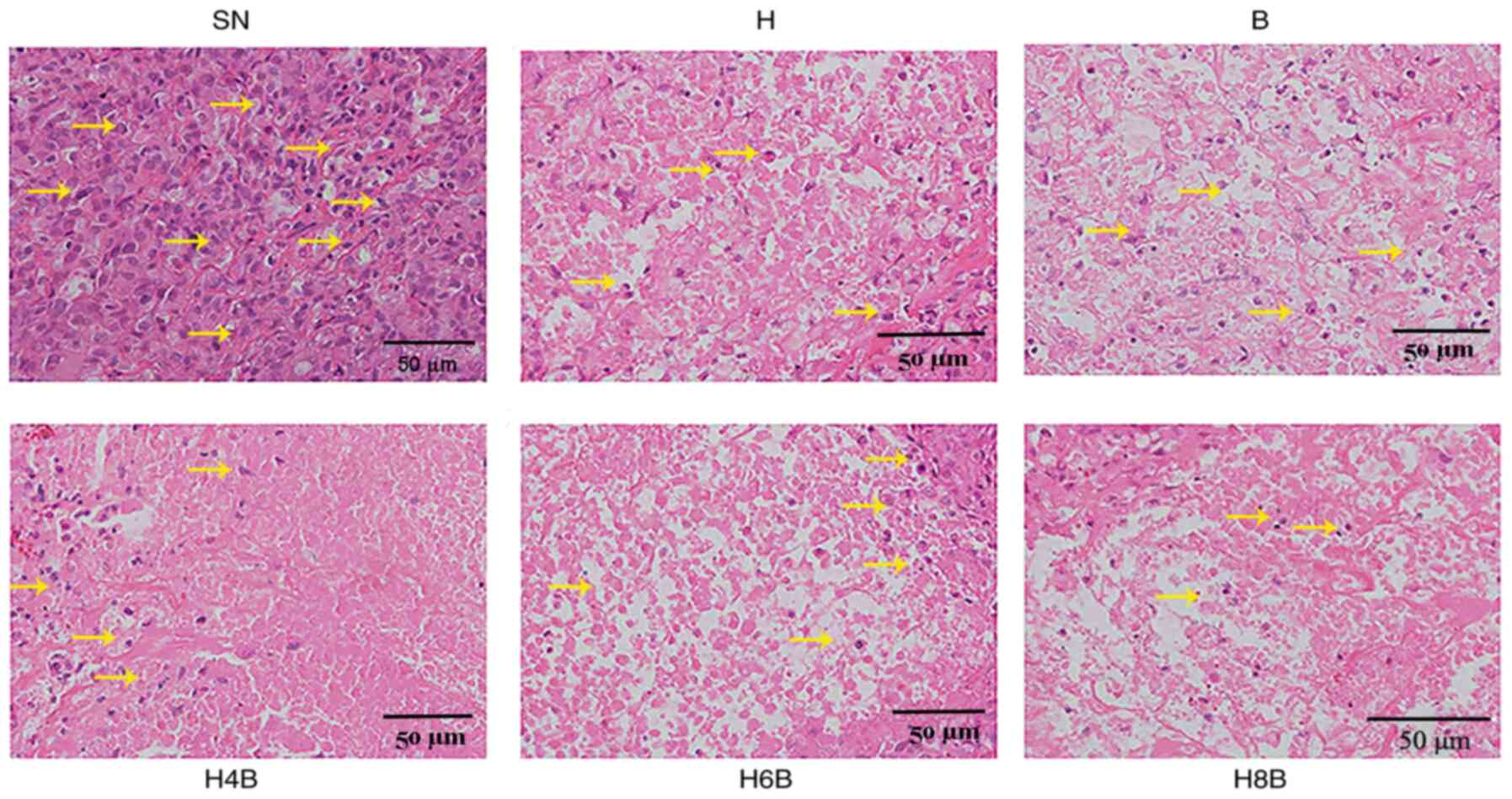
Pathological observation by HE
staining
HE staining was performed on the xenograft tumors
and suggested that the normal tissue structure was disorganized in
each group, as indicated by disorderly cell distribution and
different cell sizes. The nuclei morphology was irregular and
several nuclei were much larger in the treatment groups compared
with the SN group. In addition, the nucleus to cytoplasmic ratio
was increased in the treatment groups compared with the SN group.
In the drug-administered groups, obvious cavities were identified
in the central part of the tumor cells and the cell size was
different compared with the SN group. Furthermore, the
drug-administered groups displayed no structural eosinophilic red
staining or nuclear debris. The number of tumor cells in the cavity
of drug-administered groups was reduced compared with the SN group,
and it was impossible to distinguish apoptosis and necrosis. In the
H, B and H4B groups, small changes to the structure and an
interstitial connection in the cavity were observed, while in the
H6B and H8B groups, severe cavity changes were observed and the
original tissue structures could not be seen under the microscope.
Therefore, it was speculated that the tumor suppressing effect of
the two drugs was stronger in the combination therapy groups
compared with the monotherapy groups (Fig. 2).
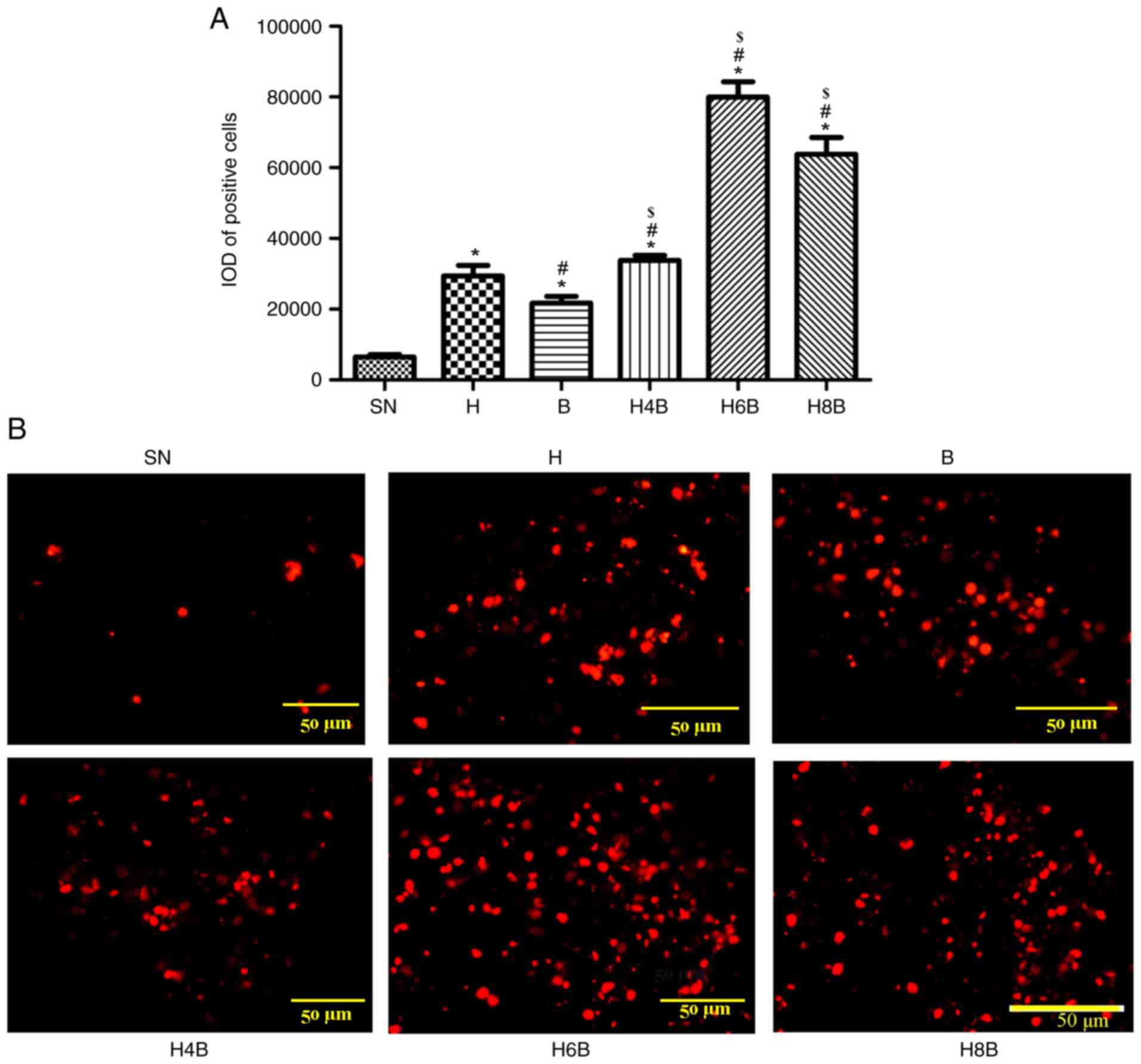
TUNEL assay for apoptosis
The TUNEL kit was used to detect apoptosis in the
xenograft tumors of each group. The drug-administered groups
displayed significantly higher levels of apoptosis compared with
the SN group (P<0.05; Fig. 3A
and B). Among the drug-administered
groups, the H6B group displayed the highest level of apoptosis. The
H8B group did not induce apoptosis to the same extent as the H6B
group, but still displayed significantly (P<0.05) higher levels
of apoptosis compared with the single drug administration groups (H
and B) and the H4B group. No statistically significant (P>0.05)
differences were found between the H4B group and the single drug
administration groups (Fig. 3A and
B).
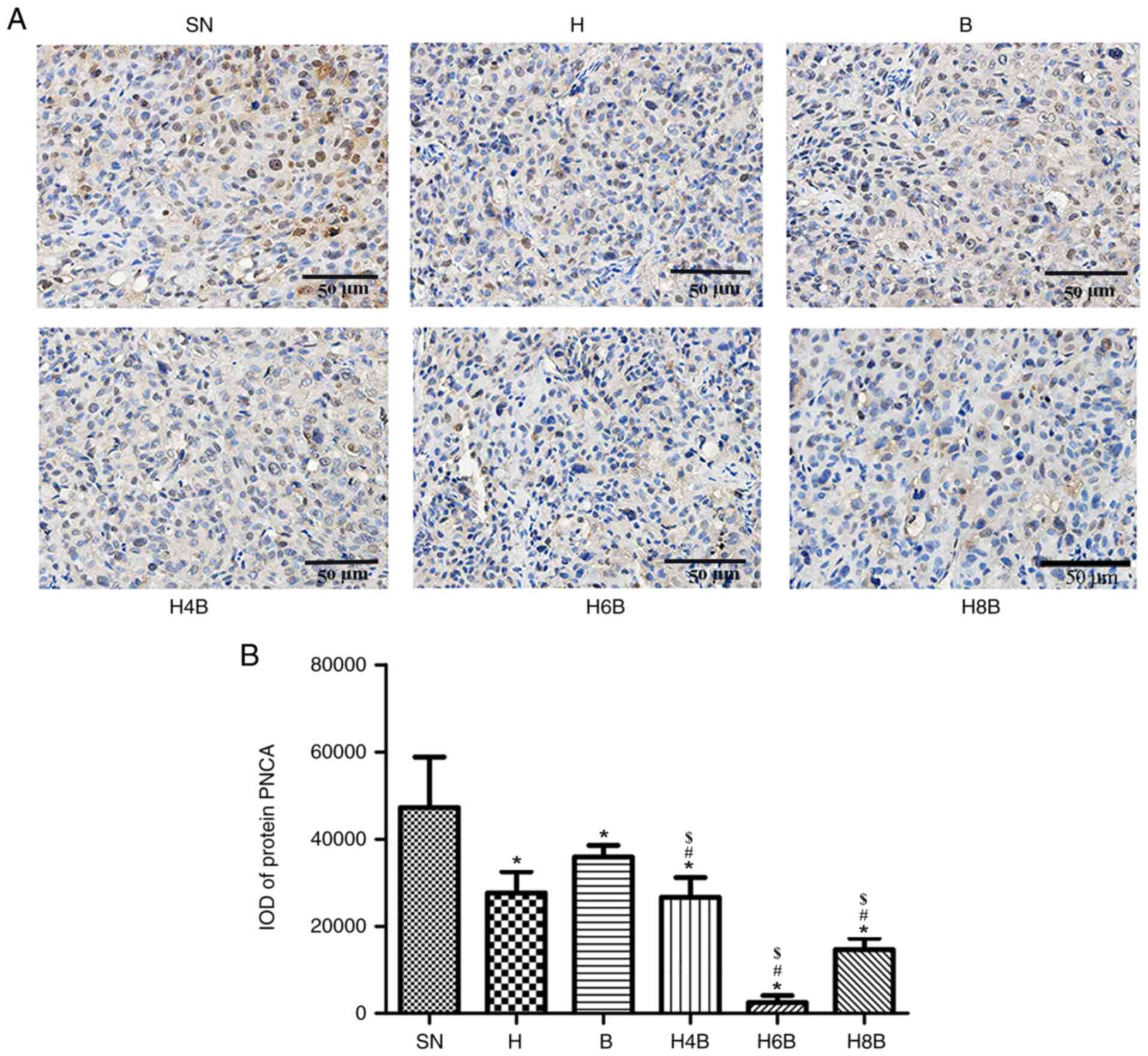
Differential expression of PCNA
protein
The PCNA protein is ubiquitously expressed in the
nucleus, and its nuclear content is consistent with the synthesis
of DNA, making PCNA an indicator of cell proliferation (31). The expression of PCNA in prostate
cancer xenograft tumors was analyzed by immunohistochemistry.
PCNA-positive cells were stained brown or yellow, indicating that
the cells were proliferating and dividing (Fig. 4A).
The xenograft tumors of the drug-administered groups
displayed significantly reduced PCNA levels compared with the SN
group (P<0.05; Fig. 4A and
B). The H6B and H8B groups
displayed the lowest levels of PCNA expression compared with all
the other treatment groups (P<0.05; Fig. 4A and B). There was no significant difference
(P>0.05) between the PCNA expression in xenograft tumors from
the H4B group and the single drug administration groups (H and B).
The H6B group exhibited the most significant effect on PCNA
expression, and there was a statistical difference compared with
the single drug administration groups (P<0.05; Fig. 4B).
Expression of apoptosis-related
proteins Bax, p53, programmed cell death 4 (PDCD4) and glycogen
synthase kinase (GSK)-3β
To further explore the mechanisms of drug inhibition
on tumor growth, the expression of cytoplasmic proteins in
xenograft tumors was determined by western blotting. All drug
treatments increased the protein expression levels of the tumor
suppressor genes p53 and PDCD4, the mitochondrial apoptosis-related
protein Bax and the PI3K/AKT/GSK-3β apoptosis signaling
pathway-related protein GSK-3β, compared with the SN group
(Fig. 5). Furthermore, all drug
treatments decreased the protein expression levels of the
mitochondrial apoptosis-related protein Bcl-XL and the
PI3K/AKT/GSK-3β apoptosis signaling pathway-related protein p-AKT,
compared with the SN group (Fig.
5). Additionally, the H4B, H6B and H8B groups significantly
increased the protein expression levels of Bax, p53, PDCD4 and
GSK-3β, and decreased the protein expression levels of Bcl-XL and
p-AKT compared with the single drug administration groups (H and B;
P<0.05; Fig. 5). Among the
combination treatment groups, the changes were the most prominent
in the H6B group, followed by the H8B and H4B treatment groups,
respectively, although there was no significant difference among
the three groups.
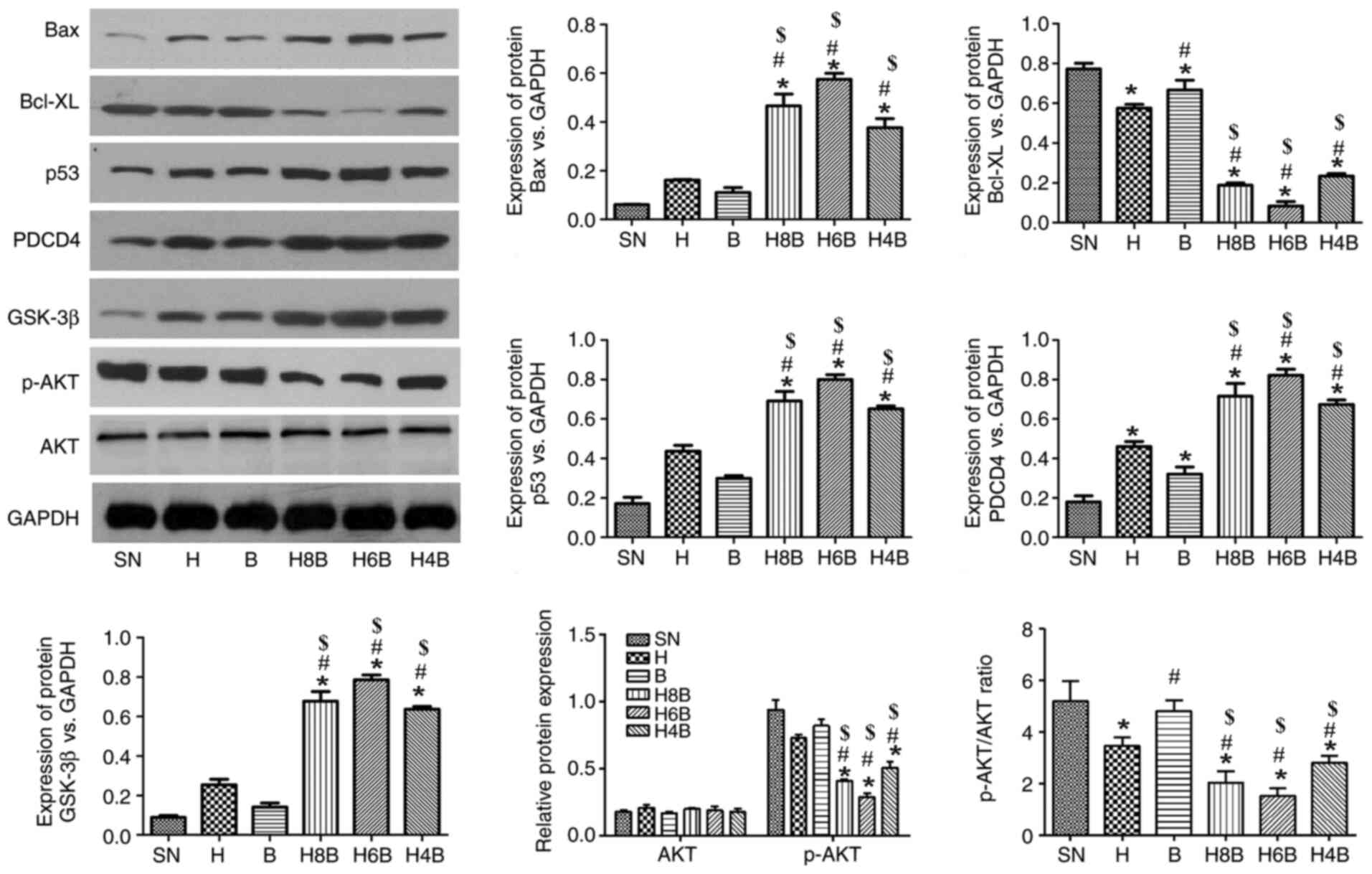 | Figure 5Expression of apoptosis-related
proteins. The expression of apoptosis-related proteins, Bax, p53,
PDCD4, Bcl-XL, p-AKT and GSK-3β in prostate cancer xenograft tumors
of nude mice in each group. Data are presented as the mean ± SD
(n=6 per group). *P<0.05 vs. the SN group;
$P<0.05 vs. the H group and #P<0.05 vs.
the B group. PDCD4, programmed cell death 4; p, phosphorylated;
GSK-3β, glycogen synthase kinase-3β; SN, saline negative control
group; H, hydroxycamptothecin positive control group; B, bufalin
positive control group; H4B, combination of hydroxycampothecin and
0.4 mg/kg bufalin; H6B, combination of hydroxycampothecin and 0.6
mg/kg bufalin; H8B, combination of hydroxycampothecin and 0.8 mg/kg
bufalin. |
Discussion
The use of bufalin for the inhibition of tumor cell
growth has been researched extensively in precious years (32-37).
Low-dose bufalin displays an inhibitory effect on the growth of
prostate cancer DU 145 cells in a time and dose-dependent manner
(38). Administration of a
combination of a specific dose of hydroxycamptothecin with a single
agent was found to be effective against cancer cells (28). Similar synergy was also reported for
the combination of hydroxycamptothecin and etoposide in human colon
carcinoma HT-29 cells (39).
The present study suggested that in a nude mouse
CRPC xenograft model, low-dose bufalin inhibited increases in tumor
volume and weight, but bufalin (0.6 and 0.8 mg/kg) combined with
hydroxycamptothecin had an improved effect on tumor volume, weight
and inhibition rate compared with the administration of either drug
alone. Histopathological analysis of sections of the xenograft
tumors indicated increased cell death with the combined
administration of bufalin and hydroxycamptothecin compared with the
other groups. The TUNEL assay suggested that the H6B and H8B groups
promoted higher levels of tumor cell apoptosis compared with the
single drug administration groups. Immunohistochemical staining
indicated that the H6B and H8B groups were more effective at
inhibiting the proliferation of prostate cancer cells than all
other treatment groups. The proapoptotic and growth-inhibiting
effects of bufalin, hydroxycamptothecin or their combination may be
related to the mitochondrial, p53-related and PI3K/AKT/GSK-3β
apoptotic signaling pathways.
In recent years, bufalin has been reported to
exhibit proapoptotic effects in a number of tumors (40-44,41),
but the effective dose (≥1.5 mg/kg) utilized in previous studies is
close to the toxic dose. According to the Dictionary of Traditional
Chinese Medicine, the median lethal dose (LD50) of
bufalin in nude mice is 2.2 mg/kg (45), and a number of previous studies have
reported that 1.5 mg/kg bufalin significantly promoted the
apoptosis of transplanted tumor cells and exhibited antitumor
effects in nude mice (37,46,16,37).
In the present study, the dose of bufalin used in the combination
treatment groups (0.4, 0.6 and 0.8 mg/kg) was much lower than the
LD50 value. There was no significant difference in the
body weight of mice in the treatment groups compared with the SN
group. The effects of the three combined treatment groups were no
less than those of the bufalin (1.0 mg/kg) alone positive control
group. Therefore, it can be suggested that the use of low-dose
bufalin combined with hydroxycamptothecin may have a significant
therapeutic effect, and may not be associated with toxicity,
providing rationale for the clinical use of low-dose bufalin.
However, the administration of a bufalin and hydroxycamptothecin
combination would need to follow a specific protocol. The present
study further suggested that the simultaneous administration of
bufalin and hydroxycamptothecin was effective in the treatment of
CRPC. The use of bufalin and hydroxycamptothecin simultaneously
leads to drug antagonism, but sequential administration may lead to
a synergistic effect (47).
Therefore, administration of hydroxycamptothecin for a certain
period of time prior to the administration of bufalin may be more
effective (47). The present study
suggested that the sequential administration of bufalin (0.6 and
0.8 mg/kg) 8 h after the administration of hydroxycamptothecin (2
mg/kg) was more beneficial. However, the role of
hydroxycamptothecin in CRPC requires further investigation.
Bcl-XL and Bax belong to the Bcl-2 protein family
(48). By controlling the
permeability of the mitochondrial inner membrane structure, Bcl-XL
and Bax affect proapoptotic factors in the cytoplasm, including
cytochrome C, and transmit apoptotic signals to regulate cell death
(49). Bax is an important
component of mitochondrial membrane ion channels (50). After receiving the apoptotic signal,
Bax expression is increased, proapoptotic factors in the
mitochondria, such as cytochrome C, enter the cytoplasm and the
caspase protein family is activated to induce apoptosis (51-53).
Bcl-XL is primarily located in the cytoplasm and can be
translocated to the mitochondrial outer membrane to bind Bax and
form Bcl-XL/Bax heterodimers, under the action of apoptotic signals
(20,21). Subsequently, the Bcl-XL/Bax
heterodimers maintain the integrity of the mitochondrial outer
membrane and interfere with apoptosis induction (54,55).
The sequential administration of bufalin 8 h after the
administration of hydroxycamptothecin enhanced the expression of
Bax and inhibited the expression of Bcl-XL, potentially promoting
apoptosis. The present study suggested that the combination of
hydroxycamptothecin and bufalin, at the dose of 0.6 mg/kg, was the
most beneficial treatment option.
Both p53 and PDCD4 are tumor suppressor genes, which
play roles in cell apoptosis and DNA damage repair (56,57).
Under physiological conditions, p53 levels are low in the cell
(58). When DNA damage occurs in
cells, p53 accumulates in the cells and promotes the apoptosis of
abnormal cells via the p53/Bax apoptosis regulatory signaling
pathway to prevent excessive proliferation of abnormal cells
(59,60). The present study suggested that the
apoptotic effect of bufalin on tumor cells is related to the
activation of p53 and an increase in PDCD4 expression, which could
potentially prevent the excessive proliferation of prostate cancer
cells.
The PI3K/AKT signaling pathway is important for cell
membrane receptor signaling (61).
AKT regulates the proliferation of downstream proteins, including
caspase 9, Bad, NF-κB and GSK-23, by phosphorylation, thereby
regulating cell proliferation, differentiation, apoptosis and
migration (62). GSK-3β can inhibit
the expression of transcription factors, including β-catenin, Nrf2
and NFAT and activate the caspase pathway to induce apoptosis
(63). The combination of bufalin
and hydroxycamptothecin promoted the expression of GSK-3β and
inhibited the expression of p-AKT, potentially inhibiting the
growth of tumor cells. Furthermore, the combination of
hydroxycamptothecin and bufalin at a dose of 0.6 mg/kg was the most
effective at promoting GSK-3β expression and inhibiting p-AKT
expression.
To conclude, sequential administration of bufalin
and hydroxycamptothecin inhibited the growth of CRPC xenograft
tumors. The dosage used for co-administration influenced the degree
of drug inhibition. The administration of hydroxycamptothecin (2
mg/kg) followed by the administration of bufalin (0.6 mg/kg) 8 h
later was the most effective treatment method assessed in the
present study. The proapoptotic effect of bufalin and
hydroxycamptothecin may occur via signaling pathways associated
with mitochondrial apoptosis, PI3K/AKT/GSK-3β apoptotic signaling
and p53-dependent apoptosis regulation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Shanghai Putuo
Hospital, Shanghai University of Traditional Chinese Medicine
(grant no. 2016233A).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ performed the experimental work and collected and
interpreted the data. RG designed the study, performed the analysis
of the data, interpreted the data and drafted the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of Shanghai University of Traditional Chinese
Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perez-Cornago A, Key TJ, Allen NE, Fensom
GK, Bradbury KE, Martin RM and Travis RC: Prospective investigation
of risk factors for prostate cancer in the UK Biobank cohort study.
Br J Cancer. 117:1562–1571. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Salinas CA, Tsodikov A, Ishak-Howard M and
Cooney KA: Prostate cancer in young men: An important clinical
entity. Nat Rev Urol. 11:317–323. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Litwin MS and Tan HJ: The diagnosis and
treatment of prostate cancer: A review. JAMA. 317:2532–2542.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sountoulides P and Rountos T: Adverse
effects of androgen deprivation therapy for prostate cancer:
Prevention and management. ISRN Urol. 2013(240108)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Klarskov LL, Klarskov P, Mommsen S and
Svolgaard N: Effect of endocrine treatment on voiding and prostate
size in men with prostate cancer: A long-term prospective study.
Scand J Urol Nephrol. 46:37–43. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hotte SJ and Saad F: Current management of
castrate-resistant prostate cancer. Curr Oncol. 17 (Suppl
2):S72–S79. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cheng CS, Wang J, Chen J, Kuo KT, Tang J,
Gao H, Chen L, Chen Z and Meng Z: New therapeutic aspects of
steroidal cardiac glycosides: The anticancer properties of
Huachansu and its main active constituent Bufalin. Cancer Cell Int.
19(92)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qiu DZ, Zhang ZJ, Wu WZ and Yang YK:
Bufalin, a component in Chansu, inhibits proliferation and invasion
of hepatocellular carcinoma cells. BMC Complement Altern Med.
13(185)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen Y, Guo Q, Zhang B, Kang M, Xie Q and
Wu Y: Bufalin enhances the antitumor effect of gemcitabine in
pancreatic cancer. Oncol Lett. 4:792–798. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu T and Huang Q: Biodegradable
brush-type copolymer modified with targeting peptide as a
nanoscopic platform for targeting drug delivery to treat
castration-resistant prostate cancer. Int J Pharm. 511:1002–1011.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pastor N and Cortés F: Bufalin influences
the repair of X-ray-induced DNA breaks in Chinese hamster cells.
DNA Repair (Amst). 2:1353–1360. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han KQ, Huang G, Gu W, Su YH, Huang XQ and
Ling CQ: Anti-tumor activities and apoptosis-regulated mechanisms
of bufalin on the orthotopic transplantation tumor model of human
hepatocellular carcinoma in nude mice. World J Gastroenterol.
13:3374–3379. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hashimoto S, Jing Y, Kawazoe N, Masuda Y,
Nakajo S, Yoshida T, Kuroiwa Y and Nakaya K: Bufalin reduces the
level of topoisomerase II in human leukemia cells and affects the
cytotoxicity of anticancer drugs. Leuk Res. 21:875–883.
1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chang Y, Zhao Y, Zhan H, Wei X, Liu T and
Zheng B: Bufalin inhibits the differentiation and proliferation of
human osteosarcoma cell line hMG63-derived cancer stem cells.
Tumour Biol. 35:1075–1082. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang Y, Zhang Y, Luan J, Duan H, Zhang F,
Yagasaki K and Zhang G: Effects of bufalin on the proliferation of
human lung cancer cells and its molecular mechanisms of action.
Cytotechnology. 62:573–583. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chueh FS, Chen YY, Huang AC, Ho HC, Liao
CL, Yang JS, Kuo CL and Chung JG: Bufalin-inhibited migration and
invasion in human osteosarcoma U-2 OS cells is carried out by
suppression of the matrix metalloproteinase-2, ERK, and JNK
signaling pathways. Environ Toxicol. 29:21–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hong SH, Kim GY, Chang YC, Moon SK, Kim WJ
and Choi YH: Bufalin prevents the migration and invasion of T24
bladder carcinoma cells through the inactivation of matrix
metalloproteinases and modulation of tight junctions. Int J Oncol.
42:277–286. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cortés F and Piñero J: Synergistic effect
of inhibitors of topoisomerase I and II on chromosome damage and
cell killing in cultured Chinese hamster ovary cells. Cancer
Chemother Pharmacol. 34:411–415. 1994.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wethington SL, Wright JD and Herzog TJ:
Key role of topoisomerase I inhibitors in the treatment of
recurrent and refractory epithelial ovarian carcinoma. Expert Rev
Anticancer Ther. 8:819–831. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee YC, Lee CH, Tsai HP, An HW, Lee CM, Wu
JC, Chen CS, Huang SH, Hwang J, Cheng KT, et al: Targeting of
topoisomerase I for prognoses and therapeutics of
camptothecin-resistant ovarian cancer. PloS One.
10(e0132579)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cuya SM, Bjornsti MA and van Waardenburg
RCAM: DNA topoisomerase-targeting chemotherapeutics: What's new?
Cancer Chemother Pharmacol. 80:1–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Deweese JE, Osheroff MA and Osheroff N:
DNA topology and topoisomerases: Teaching a ‘Knotty’ subject.
Biochem Mol Biol Educ. 37:2–10. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ding X, Matsuo K, Xu L, Yang J and Zheng
L: Optimized combinations of bortezomib, camptothecin, and
doxorubicin show increased efficacy and reduced toxicity in
treating oral cancer. Anticancer Drugs. 26:547–554. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cho YS and Cho-Chung YS: Antisense protein
kinase A RIalpha acts synergistically with hydroxycamptothecin to
inhibit growth and induce apoptosis in human cancer cells:
Molecular basis for combinatorial therapy. Clin Cancer Res.
9:1171–1178. 2003.PubMed/NCBI
|
|
29
|
Griffith TS and Kemp TJ: The topoisomerase
I inhibitor topotecan increases the sensitivity of prostate tumor
cells to TRAIL/Apo-2L-induced apoptosis. Cancer Chemother
Pharmacol. 52:175–184. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wei W, Yu Y, Wang X, Yang L, Zhang H, Ji
H, Li Z, Hou J, Wu W and Guo D: Simultaneous determination of
bufalin and its nine metabolites in rat plasma for characterization
of metabolic profiles and pharmacokinetic study by
LC-MS/MS. Molecules. 24(E1662)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Keshav R and Narayanappa U: Expression of
proliferating cell nuclear antigen (PCNA) in oral submucous
fibrosis: An immunohistochemical study. J Clin Diagn Res.
9:ZC20–ZC23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu X, Xiao XY, Shou QY, Yan JF, Chen L,
Fu HY and Wang JC: Bufalin inhibits pancreatic cancer by inducing
cell cycle arrest via the c-Myc/NF-κB pathway. J Ethnopharmacol.
193:538–545. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Y, Tian X, Liu X and Gong P: Bufalin
inhibits human breast cancer tumorigenesis by inducing cell death
through the ROS-mediated RIP1/RIP3/PARP-1 pathways. Carcinogenesis.
39:700–707. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li H, Hu S, Pang Y, Li M, Chen L, Liu F,
Liu M, Wang Z and Cheng X: Bufalin inhibits glycolysis-induced cell
growth and proliferation through the suppression of Integrin β2/FAK
signaling pathway in ovarian cancer. Am J Cancer Res. 8:1288–1296.
2018.PubMed/NCBI
|
|
35
|
Pan Z, Xie Y, Bai J, Lin Q, Cui X and
Zhang N: Bufalin suppresses colorectal cancer cell growth through
promoting autophagy in vivo and in vitro. RSC Advances.
8:38910–38918. 2018.
|
|
36
|
Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill
TG, Wang J, Qi R, Matzuk AJ, Song X, Madoux F, et al: Bufalin is a
potent small-molecule inhibitor of the steroid receptor
coactivators SRC-3 and SRC-1. Cancer Res. 74:1506–1517.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang ZJ, Yang YK and Wu WZ: Bufalin
attenuates the stage and metastatic potential of hepatocellular
carcinoma in nude mice. J Transl Med. 12(57)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Choo GS, Lee HN, Shin SA, Kim HJ and Jung
JY: Anticancer effect of fucoidan on DU-145 prostate cancer cells
through inhibition of PI3K/Akt and MAPK pathway expression. Mar
Drugs. 14(E126)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bertrand R, O'Connor PM, Kerrigan D and
Pommier Y: Sequential administration of camptothecin and etoposide
circumvents the antagonistic cytotoxicity of simultaneous drug
administration in slowly growing human colon carcinoma HT-29 cells.
Eur J Cancer. 28A:743–748. 1992.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li M, Yu X, Guo H, Sun L, Wang A, Liu Q,
Wang X and Li J: Bufalin exerts antitumor effects by inducing cell
cycle arrest and triggering apoptosis in pancreatic cancer cells.
Tumour Biol. 35:2461–2471. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao H, Li Q, Pang J, Jin H, Li H and Yang
X: Blocking autophagy enhances the pro-apoptotic effect of bufalin
on human gastric cancer cells through endoplasmic reticulum stress.
Biol Open. 6:1416–1422. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wu SH, Bau DT, Hsiao YT, Lu KW, Hsia TC,
Lien JC, Ko YC, Hsu WH, Yang ST, Huang YP and Chung JG: Bufalin
induces apoptosis in vitro and has Antitumor activity against human
lung cancer xenografts in vivo. Environ Toxicol. 32:1305–1317.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of PI3K/Akt pathway. Int J Mol Sci. 13:2025–2035.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Miao Q, Bi LL, Li X, Miao S, Zhang J,
Zhang S, Yang Q, Xie YH, Zhang J and Wang SW: Anticancer effects of
bufalin on human hepatocellular carcinoma HepG2 cells: Roles of
apoptosis and autophagy. Int J Mol Sci. 14:1370–1382.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM,
Wu YX and Jiang SH: Induction of apoptosis by arsenic trioxide and
hydroxycamptothecin in gastric cancer cells in vitro. World J
Gastroenterol. 6:532–539. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu Y, Wang X, Jia Y and Liu Y: Effects of
bufalin on the mTOR/p70S6K pathway and apoptosis in esophageal
squamous cell carcinoma in nude mice. Int J Mol Med. 40:357–366.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Guo F, Fan Z, Yang J, Li Y, Wang Y, Zhao
H, Xie L and Hou Z: A comparative evaluation of hydroxycamptothecin
drug nanorods with and without methotrexate prodrug
functionalization for drug delivery. Nanoscale Res Lett.
11(384)2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lee EF, Harris TJ, Tran S, Evangelista M,
Arulananda S, John T, Ramnac C, Hobbs C, Zhu H, Gunasingh G, et al:
BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell
survival. Cell Death Dis. 10(342)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hu X, Bardhan K, Paschall AV, Yang D,
Waller JL, Park MA, Nayak-Kapoor A, Samuel TA, Abrams SI and Liu K:
Deregulation of apoptotic factors Bcl-xL and Bax confers apoptotic
resistance to myeloid-derived suppressor cells and contributes to
their persistence in cancer. J Biol Chem. 288:19103–19115.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Szabó I, Bock J, Grassmé H, Soddemann M,
Wilker B, Lang F, Zoratti M and Gulbins E: Mitochondrial potassium
channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc
Natl Acad Sci USA. 105:14861–14866. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis*. Annu Rev Genet. 43:95–118.
2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Er E, Oliver L, Cartron PF, Juin P, Manon
S and Vallette FM: Mitochondria as the target of the pro-apoptotic
protein Bax. Biochim Biophys Acta. 1757:1301–1311. 2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang L, Chen D, Chen Z and Moeckel GW:
Hypertonicity-induced mitochondrial membrane permeability in renal
medullary interstitial cells: Protective role of osmolytes. Cell
Physiol Biochem. 25:753–760. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Voutsadakis IA: Apoptosis and the
pathogenesis of lymphoma. Acta Oncol. 39:151–156. 2000.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schon EA and Giovanni M: Neuronal
degeneration and mitochondrial dysfunction. J Clin Invest.
111:303–312. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Horn HF and Vousden KH: Coping with
stress: Multiple ways to activate p53. Oncogene. 26:1306–1316.
2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lankat-Buttgereit B and Göke R: The tumour
suppressor Pdcd4: Recent advances in the elucidation of function
and regulation. Biol Cell. 101:309–317. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Brady CA and Attardi LD: p53 at a glance.
J Cell Sci. 123:2527–2532. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Holley AK and St Clair DK: Watching the
watcher: Regulation of p53 by mitochondria. Future Oncol.
5:117–130. 2009.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kuribayashi K, Krigsfeld G, Wang W, Xu J,
Mayes PA, Dicker DT, Wu GS and El-Deiry WS: TNFSF10 (TRAIL), a p53
target gene that mediates p53-dependent cell death. Cancer Biol
Ther. 7:2034–2038. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Li X, Li Z, Qiu L, Zhao C and Hu Z: Nerve
growth factor modulate proliferation of cultured rabbit corneal
endothelial cells and epithelial cells. J Huazhong Univ Sci
Technolog Med Sci. 25:575–577. 2005.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bassili M, Birman E, Schor NF and Saragovi
HU: Differential roles of Trk and p75 neurotrophin receptors in
tumorigenesis and chemoresistance ex vivo and in vivo. Cancer
Chemother Pharmacol. 65:1047–1056. 2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chen S, Guttridge DC, You Z, Zhang Z,
Fribley A, Mayo MW, Kitajewski J and Wang CY: Wnt-1 signaling
inhibits apoptosis by activating beta-catenin/T cell
factor-mediated transcription. J Cell Biol. 152:87–96.
2001.PubMed/NCBI View Article : Google Scholar
|