Introduction
Stroke is a heterogeneous disease and among the
leading causes of mortality worldwide, while cerebral ischemia is
considered as the most common cause of stroke (1). Cerebral ischemia/reperfusion (I/R)
injury may irreversibly impair brain function via partly
interrupting blood supply to the brain, thus resulting in
thrombosis, embolism or hypoperfusion, eventually leading to death
or long-term disability (2). To
date, there is no effective treatment available for cerebral
ischemic stroke. Therefore, the prevention and treatment of
cerebral I/R injury is becoming increasingly important in the
treatment of ischemic cerebrovascular diseases.
A growing body of literature has suggested that
energy metabolism disorders, oxidative stress, inflammation and
apoptosis are involved in the pathogenesis of cerebral I/R injury
(3,4). Neuroinflammation and oxidative stress
are two well-known factors promoting neuronal injury and apoptosis
during ischemic stroke (5).
Traditional Chinese medicine (TCM) has been widely used to treat
cerebral ischemia-related diseases, and has demonstrated notable
therapeutic efficacy in clinical practice. TCM comprises several
natural active substances (6).
Aloperine (ALO) is a novel quinolizidine alkaloid derived from the
leaves and seeds of the Sophora alopecuroides L.
(Leguminosae) (Fig. 1A) (7). Matrine, one of the major alkaloids of
the Sophora plant, has been demonstrated to be effective in
ameliorating the histological consequences of middle cerebral
artery occlusion (MCAO) in mice through its antioxidant activity
and by inhibiting apoptosis (8). As
a novel quinolizidine alkaloid derived from Sophora plant,
ALO has been recognized as an effective treatment for several
neurological diseases. For example, it was previously demonstrated
that ALO could attenuate the production of reactive oxygen species
(ROS) and cell apoptosis in a cell model of Alzheimer's disease
(9). A previous study reported that
ALO could protect mice against I/R-induced renal injury via
attenuating inflammatory infiltration and tubular cell apoptosis
(10). Of note, it has also been
reported that the protective mechanisms of ALO on cultured rat
hippocampal neurons injured by oxygen-glucose deprivation and
reperfusion were associated with its antioxidant properties
(11). In addition, ALO could
ameliorate oxidative damage during early brain injury following
subarachnoid hemorrhage via activating the nuclear factor
E2-related factor 2/antioxidant response element pathway (12). ALO can easily diffuse across
biological membranes and the blood-brain barrier in an
energy-deficient environment (11).
Therefore, it was hypothesized that ALO may serve a protective role
against cerebral I/R injury.
The present study aimed to investigate the
protective effects of ALO against cerebral I/R injury using a rat
model of reversible MCAO, in the hope that the results may uncover
a novel therapeutic option for the prevention of stroke.
Materials and methods
Animals
A total of 60 specific pathogen-free grade male
Sprague-Dawley rats (weight, 200-250 g) were provided by the
Shanghai Laboratory Animal Center (Shanghai, China). All rats were
housed under standard conditions with a 12-h light/dark cycle, and
were given ad libitum access to food and water. The
temperature and relative humidity were 22±2˚C and 55±10%,
respectively. All animal experimental procedures were approved by
the Affiliated Hospital of North Sichuan Medical College (approval
no. NSMC201901).
Grouping and drug treatment
Animals were randomly assigned into the following
six groups (n=10 per group): Sham, I/R, I/R + 2 mg/kg nimodipine
(I/R + NIM; positive control group), I/R + 2 mg/kg ALO, I/R + 25
mg/kg ALO and I/R + 50 mg/kg ALO. ALO (Santa Cruz Biotechnology,
Inc.) was dissolved in saline supplemented with 10% acetic acid
(2.5 mg/ml), as previously described (10). Rats in the ALO groups were treated
with the corresponding doses of ALO by intraperitoneal injection
for 7 consecutive days (4,8,13).
Animals in the positive control group received intraperitoneal
administration of 2 mg/kg NIM (Shandong Fangming Pharmaceutical
Group Co., Ltd.) instead of ALO, while those in the sham group
received the same volume of saline (containing 10% acetic acid).
The doses of ALO were determined based on previous studies
(14,15). NIM is a dihydropyridine calcium
antagonist, which can reduce nerve cell apoptosis through calcium
influx, increase the oxygen supply and blood supply to brain tissue
by improving the deformability of red blood cells, reducing the
permeability of the blood-brain barrier and improving blood
rheology, so as to protect cerebrovascular and nerve cells
(16,17). NIM can reduce brain inflammation and
promote neuronal recovery by inhibiting the expression of
inflammatory factors, such as TNF-α, IL-1β and IL-6, to relieve the
inflammatory response of the body (18,19).
NIM has been widely used as the positive control in the cerebral
I/R injury (20,21). The MCAO surgery was performed at 2 h
following the last drug administration.
Induction of cerebral I/R injury
Reversible MCAO surgery was performed using an
improved Longa-Zea method (22).
Following anesthetization with intraperitoneal injection of 40
mg/kg pentobarbital sodium, the rats were fixed in a supine
position. The left common carotid artery, the external carotid
artery and the internal carotid artery were carefully exposed and
dissected away from the adjacent nerves. A V-shaped oblique
incision was made at the bifurcation of the external and internal
carotid arteries with vascular scissors. Then, the artery clamp was
reopened, while a paraffin bolt was inserted through the external
carotid artery stump into the internal carotid artery until a
slight resistance was felt. The time was set as the beginning of
the embolism. The upper end of the common carotid artery was then
ligated, and the paraffin bolt was gently pulled back through the
incision of the external carotid artery 90 min after the embolism
to allow reperfusion. After 24 h, I/R injury was evaluated using
H&E and 2,3,5-triphenyltetrazolium chloride (TTC) staining.
Rats in the model group exhibited notable histopathological changes
and increased infarct volume, suggesting the success of I/R model.
In rats subjected to sham surgery, the arteries were isolated but
not ligated or occluded. During the surgical procedure, the body
temperature of the rats was maintained at 37±5˚C using a heating
pad.
H&E staining
The rats were euthanized with intraperitoneal
injection of 200 mg/kg pentobarbital sodium at 24 h post-I/R, and
their whole brains were removed and washed with saline. Then, the
whole brain cortical tissue sample from each animal (n=5 for each
group) was soaked in 4% paraformaldehyde overnight at room
temperature. Following dehydration through an ethanol gradient, the
tissues were transparentized with xylene, and the brain blocks were
embedded in paraffin. The paraffin-embedded tissue samples were
then cut into 5-µm coronal sections using a microtome (Leica
Microsystems GmbH), deparaffinized in xylene and rehydrated through
a descending ethanol series. Following dehydration via a graded
ethanol and xylene series, the histological structure of the brain
tract was observed under a light microscope (magnification, x400;
Olympus Corporation). Finally, three tissue sections from each
animal were stained with H&E using standard techniques
according to a previous study (23)
and the stained slides were observed under a light microscope
(magnification, x400; Olympus Corporation).
Measurement of cerebral infarct
volume
To evaluate the cerebral infarct size, TTC staining
was performed. Briefly, the rats were euthanatized with 200 mg/kg
pentobarbital sodium at 24 h post-I/R and thoroughly perfused with
saline. The brains (n=5 for each group) were removed quickly, and
the olfactory bulb, cerebellum and brainstem were excised and cut
into five 2-mm coronal sections. Subsequently, the brain sections
were first immersed in 1% TTC solution at 37˚C for 20 min, followed
by fixation in 4% paraformaldehyde for 6 h at 4˚C. Images of the
TTC-stained sections were captured under a light microscope
(magnification, x50). TTC stains the non-infarcted region with a
deep red pigment, while the infarcted brain area appears white.
Infarct size was calculated using ImageJ software (1.52r; National
Institutes of Health) and the result was expressed as a percentage
of infarct area to total brain area. The infarct volume percentage
in the ischemic cerebral hemisphere was calculated using the
following equation: [(Contralateral hemisphere volume-volume of
non-ischemic ipsilateral hemisphere)/contralateral hemisphere
volume] x100, as previously reported (24).
Evaluation of oxidative stress-related
markers
The brain tissues (n=5 for each group) were obtained
at the end of reperfusion and the ischemic hemispheres were then
cut into small pieces. These pieces were homogenized with RIPA
lysis buffer (Beyotime Institute of Biotechnology), centrifuged at
12,000 x g for 10 min at 4˚C, and the supernatant was collected.
The levels of ROS (cat. no. E004-1-1), malondialdehyde (MDA; cat.
no. A003-1-2) and reduced glutathione (GSH; cat. no. A006-1-1), as
well as the activities of superoxide dismutase (SOD; cat. no. cat.
no. A001-1-2) and catalase (CAT; cat. no. A007-2-1), were measured
in tissue homogenate supernatant with corresponding commercially
available kits (Nanjing Jiancheng Bioengineering Institute) using
chemical colorimetry, according to the manufacturer's
recommendations.
Measurement of inflammatory
factors
To determine the levels of inflammation-related
factors, including TNF-α (cat. no. F16960), IL-1β (cat. no. F15810)
and IL-6 (cat. no. F15870), the brain tissue homogenate supernatant
was collected as described above and ELISA was carried out using
corresponding kits (Shanghai XiTang Biotechnology Co., Ltd.)
according to the manufacturer's instructions. The optical density
values were measured at 450 nm and were read using a plate reader
(BioTek Instruments, Inc.).
TUNEL staining
The sections were fixed with 4% paraformaldehyde for
20 min at room temperature, deparaffinized, rehydrated and then
permeated with proteinase K for 10 min at room temperature to
increase cell membrane permeability. To evaluate cell apoptosis in
the hippocampus post-I/R, TUNEL assay was performed using a
commercially available kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. The sections were washed
by PBS and then incubated with TUNEL solution for 1 h at 37˚C in a
dark chamber, followed by the counterstaining with 4',
6-diamidino-2-phenylindole (DAPI) for the nuclei for 20 min at room
temperature. A total of three sections from each animal (n=5 for
each group) were analyzed by two investigators, blinded to the
origin of the sections. For each section, TUNEL-positive cells were
counted in five non-overlapping high-power fields under a light
microscope (magnification, x200). The nuclei of healthy cells were
stained blue, whereas apoptotic cells with nuclei presented
brown/yellow staining were identified as TUNEL-positive cells.
Western blot analysis
For immunoblotting, the brain tissues were lysed on
ice and centrifuged at 4˚C and at 12,000 x g for 10 min. The
supernatant was extracted using RIPA buffer (Beyotime Institute of
Biotechnology) containing protease inhibitor cocktail (Beyotime
Institute of Biotechnology) and the protein concentration was
examined by means of a bicinchoninic acid protein quantification
kit (Beyotime Institute of Biotechnology). For each sample, equal
amounts of protein (40 µg/lane) were separated by 10% SDS-PAGE and
transferred to a nitrocellulose blotting membrane (Cytiva).
Possible non-specific binding was blocked by 5% skimmed milk for
1.5 h at room temperature and the membranes were then incubated
overnight at 4˚C with specific primary antibodies. On the following
day, horseradish peroxidase-conjugated secondary antibody (cat. no.
7074S; 1:3,000; Cell Signaling Technology, Inc.) were added to the
membranes and incubated for 1 h at room temperature. Protein bands
was scanned and visualized using an enhanced chemiluminescence
detection system (Thermo Fisher Scientific, Inc.). The relative
intensity of each band was quantified using ImageJ software (1.52r;
National Institutes of Health). The protein expression was
normalized to GAPDH levels. Anti-Bax (cat. no. 14796S; 1:1,000),
anti-phosphorylated (p-)PI3K (cat. no. 17366S; 1:1,000), anti-PI3K
(cat. no. 4249S; 1:1,000), anti-p-AKT (cat. no. 4060T; 1:1,000),
anti-AKT (cat. no. 4691T; 1:1,000) and anti-GAPDH (cat. no. 5174T;
1:1,000) antibodies were obtained from Cell Signaling Technology,
Inc. Anti-Bcl-2 (cat. no. sc-7382; 1:1,000) antibody was provided
by Santa Cruz Biotechnology, Inc.
Statistical analysis
All experiments were repeated independently in
triplicate. Data are presented as the mean ± standard deviation.
One-way analysis of variance was used to assess multiple
differences, followed by a Tukey's post hoc test with GraphPad
Prism 6.0 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate statistically significant differences.
Results
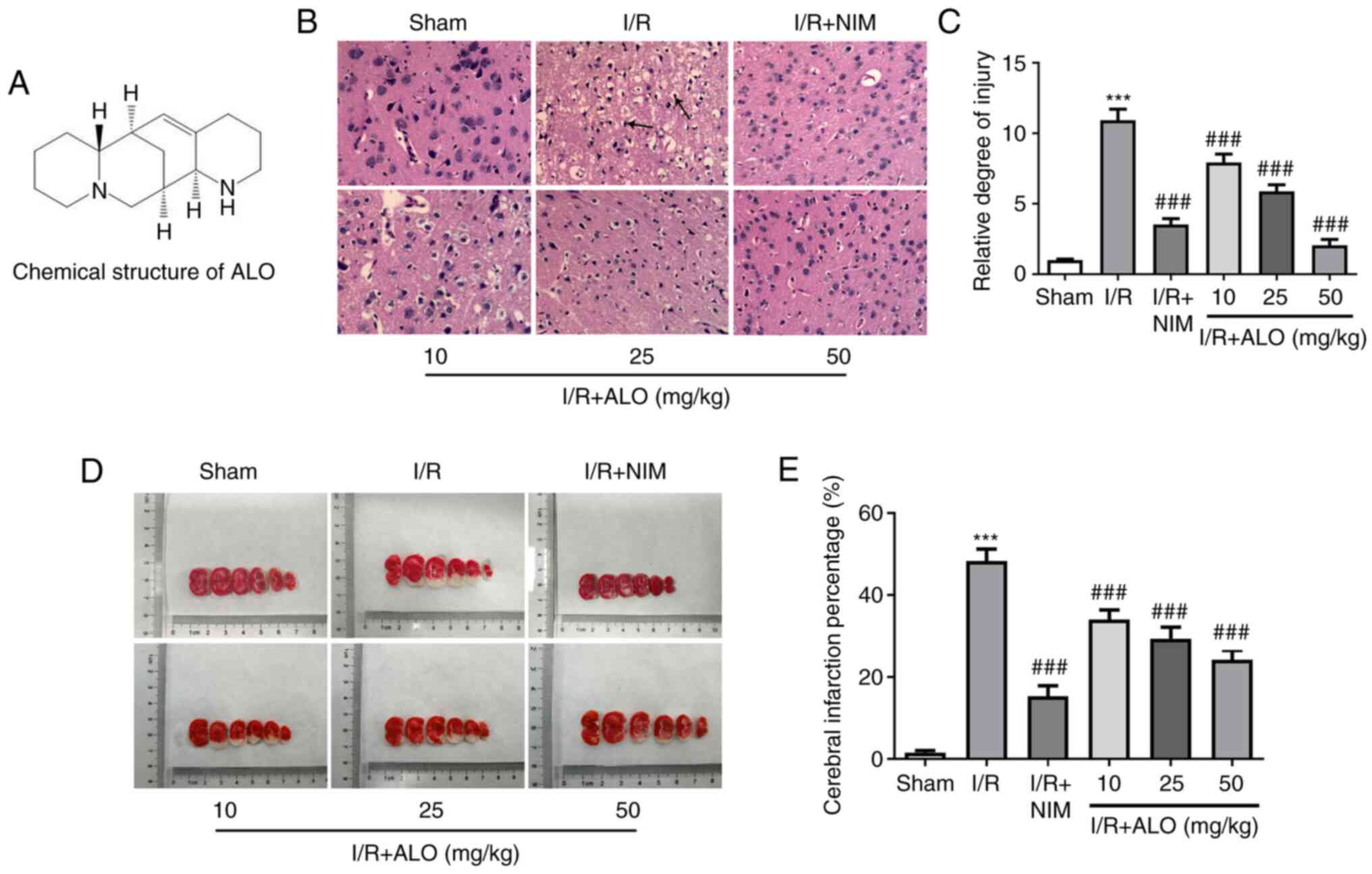
ALO treatment significantly alleviates
brain injury and reduces the cerebral ischemic area in MCAO
rats
To investigate the potential neuroprotective effect
of ALO in cerebral ischemic stroke, the histopathological changes
in the brain tissues were evaluated using H&E staining
following treatment of MACO rats with ALO. In the sham group, the
outline of the cortex neurons was clear and their structure was
compact, with abundant cytoplasm. In addition, I/R injury was
associated with unequivocal signs of pyknotic and shrunken nuclei,
nuclear loss and numerous vacuolated spaces, which were markedly
restored following ALO preconditioning in a dose-dependent manner
(Fig. 1B and C; P<0.001). Additionally, treatment
with high doses of ALO had the same effects as NIM treatment.
Consistently, as shown in Fig. 1D
and E, I/R challenge notably
increased the infarct volume compared with the sham group
(P<0.001), while significantly decreased infarct volumes were
observed in the ALO and NIM intervention groups compared with the
I/R group (P<0.001). These findings suggested that ALO treatment
significantly improved brain injury and the cerebral ischemic area
in MCAO rats.
ALO preconditioning ameliorates
MCAO/reperfusion-induced oxidative stress and inflammation
Subsequently, the levels of oxidative stress- and
inflammation-related markers in rat brain tissue homogenates were
assessed using commercially available kits. As shown in Fig. 2A-E, I/R injury markedly increased
the contents of ROS and MDA (P<0.001), while it reduced SOD, CAT
and GSH compared with the sham group (P<0.001). Furthermore,
compared with the I/R group, pretreatment with ALO dose-dependently
decreased ROS and MDA and increased SOD, CAT and GSH (P<0.01,
P<0.001). Simultaneously, the levels of inflammatory factors,
including TNF-α, IL-1β and IL-6, were notably enhanced following
I/R exposure compared with the sham group (P<0.001), whereas ALO
or NIM preconditioning markedly reduced the levels of the
aforementioned inflammatory factors (P<0.001). These findings
indicated that ALO treatment markedly attenuated oxidative stress
and neuroinflammation in rats post-MCAO/reperfusion.
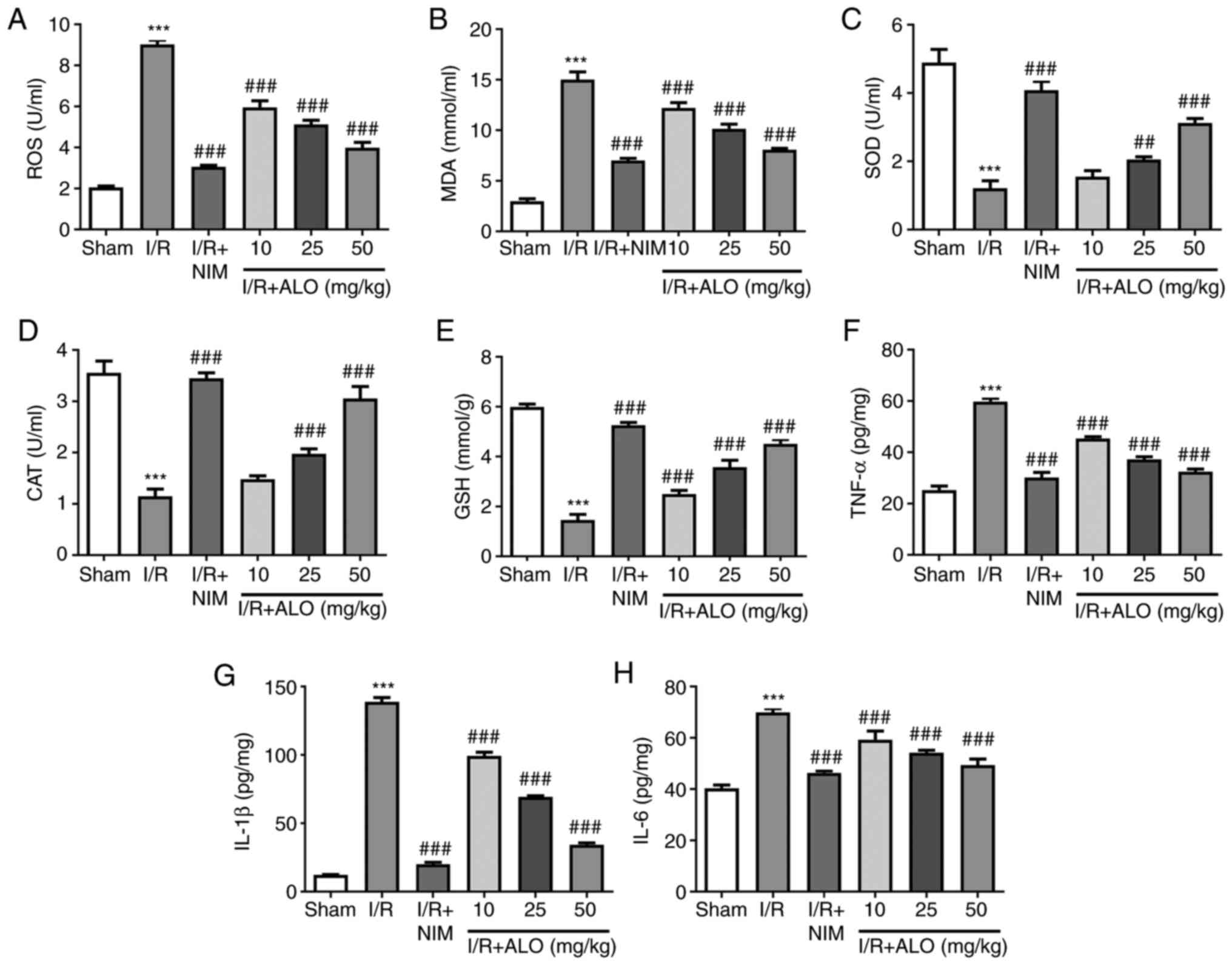 | Figure 2ALO preconditioning alleviates the
MCAO/reperfusion-induced oxidative stress and inflammation. The
levels of (A) ROS, (B) MDA, (C) SOD, (D) CAT and (E) GSH were
measured using commercially available kits. The levels of (F)
TNF-α, (G) IL-1β and (H) IL-6 were assessed by ELISA. n=5 per
group. ***P<0.001 vs. sham group;
##P<0.01 and ###P<0.001 vs. I/R group.
ALO, aloperine; ROS, reactive oxygen species; MDA, malondialdehyde;
SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; I/R,
ischemia/reperfusion; NIM, nimodipine. |
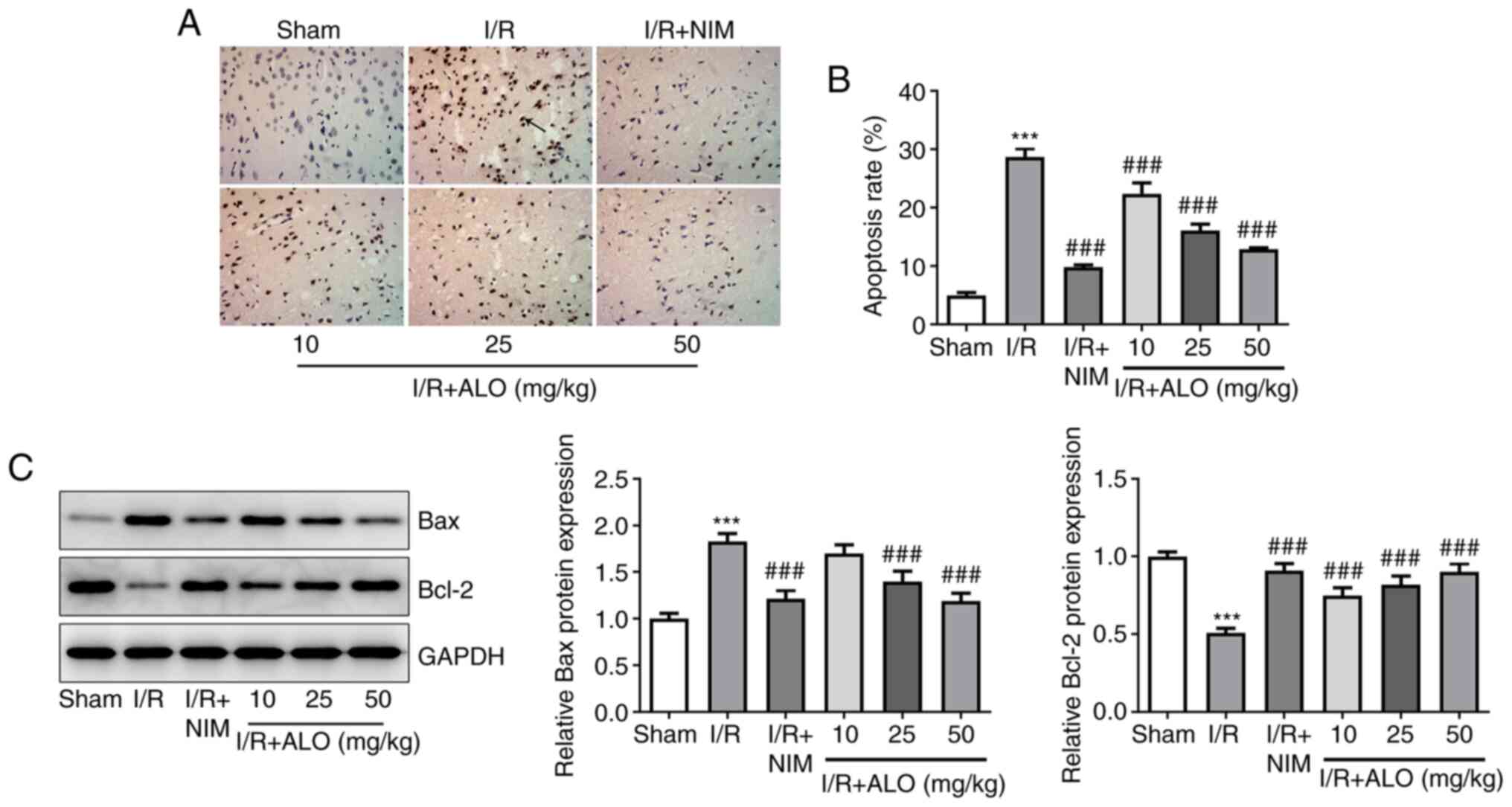
ALO intervention inhibits cell
apoptosis in the brain tissues of rats following MCAO/reperfusion
injury
To evaluate the effect of ALO on cell apoptosis
post-MCAO/reperfusion, TUNEL staining was performed on rat brain
sections. A significant increase in the number of apoptotic cells
was observed in the I/R group compared with the sham group
(Fig. 3A and B; P<0.001). Of note, treatment with ALO
dose-dependently reduced the number of apoptotic cells in the brain
tissues of rats with MCAO/reperfusion injury (P<0.001).
Concurrently, compared with the sham group, the expression levels
of the pro-apoptotic protein Bax was notably upregulated in the I/R
group, accompanied by decreased expression of the anti-apoptotic
protein Bcl-2 (Fig. 3C;
P<0.001). Importantly, the effect of MCAO/reperfusion on the
expression of apoptosis-related proteins was restored by ALO
preconditioning in a dose-dependent manner (P<0.001). These
findings indicated that ALO could attenuate neuronal apoptosis in
response to cerebral I/R injury in rats.
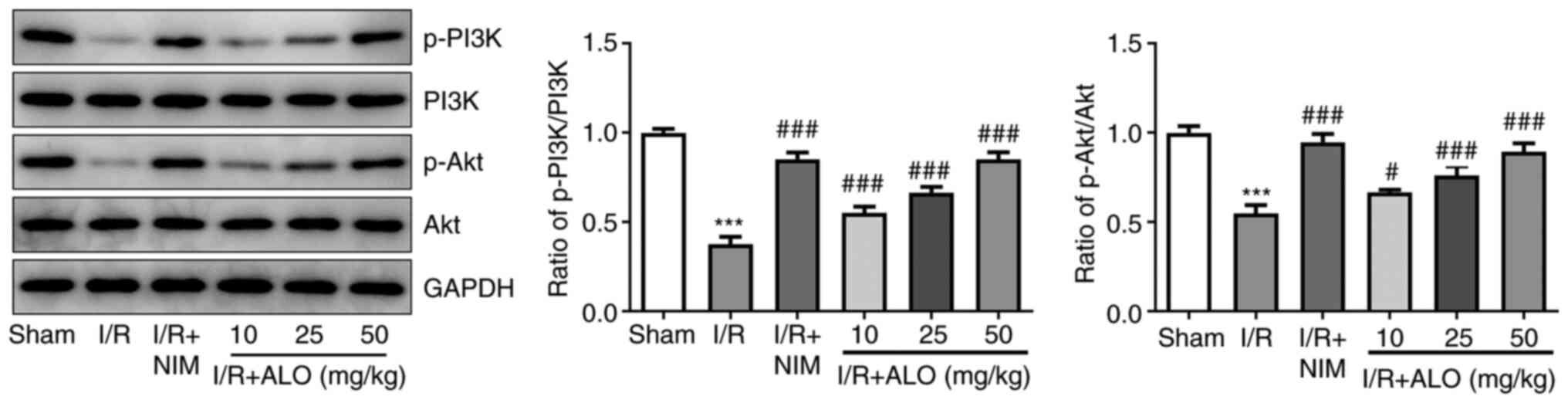
ALO protects against cerebral I/R
injury via activating the PI3K/AKT signaling pathway
To uncover the potential mechanisms underlying the
role of ALO in cerebral ischemic stroke, the expression levels of
PI3K/AKT pathway-related proteins were determined by western blot
analysis. As shown Fig. 4, I/R
injury significantly reduced the protein expression levels of
p-PI3K and p-AKT compared with the sham group (P<0.001), whereas
this inhibitory effect was reversed following treatment with ALO or
NIM (P<0.05 and P<0.001, respectively). Overall, the
aforementioned data suggested that ALO treatment could protect
against cerebral I/R injury via activating the PI3K/AKT signaling
pathway.
Discussion
Brain tissue is highly sensitive to injury and
neuronal apoptosis due to I/R (25). TCM has become increasingly important
in the treatment of cerebral I/R injury. It has been reported that
an alkaloid-free ethyl acetate extract from the root of Sophora
flavescens Ait. exerted neuroprotective effects on focal
cerebral ischemia in rats (26). In
addition, oxymatrine and matrine, as the main alkaloids extracted
from the traditional Chinese herb, Sophora flavescens Ait.,
have been shown to be effective in improving hypoxic-ischemic brain
injury in neonatal rats and MCAO mice by antioxidant and
anti-apoptotic activities (8,27). As
a novel quinolizidine alkaloid derived from Sophora
alopecuroides L., ALO has important beneficial effects in the
treatment of neurological diseases. ALO was shown to alleviate
oxygen-glucose deprivation and reperfusion-induced cultured rat
hippocampal neuron injury and ameliorate early brain injury
following subarachnoid hemorrhage (11,12).
To the best of our knowledge, the present study was the first to
investigate the effects of ALO on cerebral I/R injury in rats
subjected to MCAO and its potential regulatory mechanisms. The
results of the present study demonstrated the beneficial effect of
ALO against neuronal injury in a rat model of MCAO/reperfusion, and
further suggested that these protective effects may be mediated by
the PI3K/AKT pathway.
The pathophysiological mechanisms underlying
neuronal injury in cerebral I/R are complex and multifactorial, and
a considerable body of evidence has demonstrated that oxidative
stress plays a dominant role in the occurrence and development of
this condition (28,29). It has been reported that increased
production of ROS and lipid peroxidation can be detected at the
early stages of cerebral I/R (13,30).
MDA, the end product of lipid peroxidation, is a marker of
oxidative stress. SOD and CAT are crucial enzymes of the
antioxidant defense system produced under oxidative stress, which
together protect neuronal cells against ROS-induced cell death
(31,32). GSH, a tripeptide with a free
sulfhydryl group, reduces oxidative stress and helps to maintain
the normal reduced redox state in cells via directly interacting
with ROS (33). Emerging evidence
has suggested that the excessive accumulation of ROS accompanied by
an exhausted antioxidant defense system are involved in the
pathogenesis of cerebral I/R injury (34). ALO is a quinolizidine alkaloid
derived from the leaves and seeds of the Sophora plant,
which has several pharmacological functions in human diseases
through its antioxidant activity (7,35).
Compelling evidence has indicated that ALO exerts protective
effects against hydrogen peroxide-induced oxidative stress and
apoptosis in human retinal pigment epithelial cells (36). In addition, a study revealed that
ALO could inhibit ROS formation and cell apoptosis in an
Alzheimer's disease cellular model (9). Additionally, the significant
neuroprotective effects of ALO on oxygen-glucose
deprivation/reperfusion-injured neonatal rat primary cultured
hippocampal neurons were attributed to its antioxidant activity
(11). In the present study,
pretreatment of rats post-MCAO/reperfusion with ALO markedly
reduced the levels of oxidative stress, supporting the potential
protective effects of ALO against cerebral I/R injury.
Accumulating evidence has shown that the
inflammatory response is triggered immediately after ischemia, thus
resulting in the deterioration of delayed cerebral injury and poor
functional recovery of neurons (37). Oxidative stress increases the levels
of inflammatory cytokines, including TNF-α, IL-6 and IL-1β, and is
closely associated with the injury caused by the excessive
secretion of these mediators (38).
A cascade of physiological events during the inflammatory responses
after cerebral I/R may result in neuronal cell death and
neurological dysfunction, eventually leading to brain injury
(39,40). Apoptosis is considered as an
important type of neuronal cell death that occurs after cerebral
I/R injury (41,42). A study revealed that ALO could
attenuate human pulmonary vascular smooth muscle cell proliferation
via suppressing inflammatory response (43). Mao et al (44) demonstrated that ALO could restrain
cardiomyocyte apoptosis and alleviate coronary
microembolization-induced rat myocardial injury. A previous study
demonstrated that ALO could protect mice against I/R-induced renal
injury via suppressing inflammatory infiltration and tubular cell
apoptosis (10). Consistent with
the aforementioned findings, the present study revealed that
cerebral MCAO/reperfusion could markedly enhance the secretion of
inflammatory cytokines (TNF-α, IL-6 and IL-1β) and promote cell
apoptosis in brain tissues. These effects were effectively
inhibited following treatment with ALO, indicating that the
protective effect of ALO on cerebral I/R injury could be associated
with the inhibition of inflammation and apoptosis.
The PI3K/AKT signaling pathway is a known regulator
of a wide range of cellular functions, such as proliferation,
oxidative stress, inflammation and apoptosis (45). Mounting evidence indicates that the
activation of PI3K/AKT signaling plays an important role in
cerebral I/R injury via promoting the repair and survival of
ischemic nerve cells (46,47). In the nervous system, PI3K is
involved in the survival and differentiation of neuronal cells, and
PI3K activation can modulate the expression of downstream target
genes via activating AKT expression (48). A previous study demonstrated that
AKT could exert a critical protective effect on cerebral I/R injury
in rats, since its overexpression was found to reduce the volume of
the infarcted brain tissue (49).
Therefore, the development of effective PI3K/AKT activators may be
a focus of future research on ischemic cerebrovascular diseases.
ALO was found to attenuate coronary microembolization-induced
myocardial injury in rats via activating the PI3K/AKT signaling
pathway (44). Additionally, ALO
was shown to regulate inflammatory responses in colitis via
suppressing the PI3K/AKT pathway in a PP2A-dependent manner
(50). The present study revealed
that ALO treatment markedly upregulated the expression of p-PI3K
and p-Akt in rats post-MCAO/reperfusion, suggesting that ALO may
protect against cerebral I/R injury via activating the PI3K/AKT
signaling pathway.
Taken together, the findings of the present study
demonstrated that ALO exerted anti-neuroinflammatory, antioxidant
and anti-apoptotic effects during cerebral I/R injury via
activating the PI3K/AKT signaling pathway. These data support the
therapeutic potential of ALO in cerebral ischemic stroke. However,
the lack of behavioral measures and neurological deficit scores
constitute limitations of the present study, and will be addressed
in future studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, XC and LX searched the literature, designed the
experiments and conducted the experiments. XC and RZ analyzed and
interpreted the data. ZL wrote the manuscript. RZ revised the
manuscript. ZL and XC confirmed the authenticity of all the raw
data. All the authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Animal Care and Use Committee of the Affiliated Hospital of
North Sichuan Medical College (approval no. NSMC201901).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang Z, Zhou W, Dong H, Ma X and He Z:
Dexmedetomidine pretreatment inhibits cerebral
ischemia/reperfusioninduced neuroinflammation via activation of
AMPK. Mol Med Rep. 18:3957–3964. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
GBD 2017 Causes of Death Collaborators.
Global, regional, and national age-sex-specific mortality for 282
causes of death in 195 countries and territories, 1980-2017: A
systematic analysis for the Global Burden of Disease Study 2017.
Lancet. 392:1736–1788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jung JE, Kim GS, Chen H, Maier CM,
Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, et
al: Reperfusion and neurovascular dysfunction in stroke: From basic
mechanisms to potential strategies for neuroprotection. Mol
Neurobiol. 41:172–179. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao Y and Xu J: Sanggenon C Ameliorates
cerebral Ischemia-Reperfusion injury by inhibiting inflammation and
oxidative stress through regulating RhoA-ROCK signaling.
Inflammation. 43:1476–1487. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Esenwa CC and Elkind MS: Inflammatory risk
factors, biomarkers and associated therapy in ischaemic stroke. Nat
Rev Neurol. 12:594–604. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yin F, Zhou H, Fang Y, Li C, He Y, Yu L,
Wan H and Yang J: Astragaloside IV alleviates ischemia
reperfusion-induced apoptosis by inhibiting the activation of key
factors in death receptor pathway and mitochondrial pathway. J
Ethnopharmacol. 248(112319)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brosius AD, Ziller JW and Zhang Q:
Relative and absolute configuration of aloperine. Acta Crystallogr
C. 53 (Pt 10):1510–1512. 1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhao P, Zhou R, Zhu XY, Hao YJ, Li N, Wang
J, Niu Y, Sun T, Li YX and Yu JQ: Matrine attenuates focal cerebral
ischemic injury by improving antioxidant activity and inhibiting
apoptosis in mice. Int J Mol Med. 36:633–644. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao J, Zhang G, Li M, Luo Q, Leng Y and
Liu X: Neuro-protective effects of aloperine in an Alzheimer's
disease cellular model. Biomed Pharmacother. 108:137–143.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hu S, Zhang Y, Zhang M, Guo Y, Yang P,
Zhang S, Simsekyilmaz S, Xu JF, Li J, Xiang X, et al: Aloperine
protects mice against Ischemia-Reperfusion (IR)-induced renal
injury by regulating PI3K/AKT/mTOR signaling and AP-1 activity. Mol
Med. 21:912–923. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma NT, Zhou R, Chang RY, Hao YJ, Ma L, Jin
SJ, Du J, Zheng J, Zhao CJ, Niu Y, et al: Protective effects of
aloperine on neonatal rat primary cultured hippocampal neurons
injured by oxygen-glucose deprivation and reperfusion. J Nat Med.
69:575–583. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Song S, Chen Y, Han F, Dong M, Xiang X,
Sui J, Li Y, Yang H and Liu J: Aloperine activates the Nrf2-ARE
pathway when ameliorating early brain injury in a subarachnoid
hemorrhage model. Exp Ther Med. 15:3847–3855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang M, Chen Z, Yang L and Ding L:
Sappanone A protects against inflammation, oxidative stress and
apoptosis in cerebral Ischemia-Reperfusion injury by alleviating
endoplasmic reticulum stress. Inflammation. 44:934–945.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu F, Yao W, Yang J, Zhang M, Xu Y, Hao Y,
Yan L, Niu Y, Sun T, Yu J and Zhou R: Protective effects of
aloperin on monocroline-induced pulmonary hypertension via
regulation of Rho A/Rho kinsase pathway in rats. Biomed
Pharmacother. 95:1161–1168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song G, Huang Y, Xiong M, Yang Z, Liu Q,
Shen J, Zhao P and Yang X: Aloperine relieves Type 2 diabetes
mellitus via enhancing GLUT4 expression and translocation. Front
Pharmacol. 11(561956)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bork K, Wurm F, Haller H, Strauss C,
Scheller C, Gnanapragassam VS and Horstkorte R: Neuroprotective and
neuroregenerative effects of nimodipine in a model system of
neuronal differentiation and neurite outgrowth. Molecules.
20:1003–1013. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moran JM and Pedrera-Zamorano JD: Comments
on ‘Efficacy and safety assessment of acupuncture and nimodipine to
treat mild cognitive impairment after cerebral infarction: A
randomized controlled trial’. BMC Complement Altern Med.
17(119)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen X, Yao Z, Peng X, Wu L, Wu H, Ou Y
and Lai J: Eupafolin alleviates cerebral ischemia/reperfusion
injury in rats via blocking the TLR4/NF-κB signaling pathway. Mol
Med Rep. 22:5135–5144. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sanz JM, Chiozzi P, Colaianna M, Zotti M,
Ferrari D, Trabace L, Zuliani G and Di Virgilio F: Nimodipine
inhibits IL-1beta release stimulated by amyloid beta from
microglia. Br J Pharmacol. 167:1702–1711. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Han K, Rong W, Wang Q, Qu J, Li Q, Bi K
and Liu R: Time-dependent metabolomics study of cerebral
ischemia-reperfusion and its treatment: Focus on the combination of
traditional Chinese medicine and Western medicine. Anal Bioanal
Chem. 412:7195–7209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zou S, Zhang M, Feng L, Zhou Y, Li L and
Ban L: Protective effects of notoginsenoside R1 on cerebral
ischemia-reperfusion injury in rats. Exp Ther Med. 14:6012–6016.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Trotman-Lucas M, Kelly ME, Janus J and
Gibson CL: Middle cerebral artery occlusion allowing reperfusion
via common carotid artery repair in mice. J Vis Exp.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Shi Y, Peng XH, Li X, Luo GP and Wu MF:
Neuroprotective role of dexmedetomidine pretreatment in cerebral
ischemia injury via ADRA2A-mediated phosphorylation of ERK1/2 in
adult rats. Exp Ther Med. 16:5201–5209. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao
J, Lei J, Yang L, Wang K, Chen L, et al: Therapeutic application of
gene silencing MMP-9 in a middle cerebral artery occlusion-induced
focal ischemia rat model. Exp Neurol. 216:35–46. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hao MQ, Xie LJ, Leng W and Xue RW: Trim47
is a critical regulator of cerebral ischemia-reperfusion injury
through regulating apoptosis and inflammation. Biochem Biophys Res
Commun. 515:651–657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Park SJ, Nam KW, Lee HJ, Cho EY, Koo U and
Mar W: Neuroprotective effects of an alkaloid-free ethyl acetate
extract from the root of Sophora flavescens Ait. against focal
cerebral ischemia in rats. Phytomedicine. 16:1042–1051.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao P, Zhou R, Li HN, Yao WX, Qiao HQ,
Wang SJ, Niu Y, Sun T, Li YX and Yu JQ: Oxymatrine attenuated
hypoxic-ischemic brain damage in neonatal rats via improving
antioxidant enzyme activities and inhibiting cell death. Neurochem
Int. 89:17–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Peters O, Back T, Lindauer U, Busch C,
Megow D, Dreier J and Dirnagl U: Increased formation of reactive
oxygen species after permanent and reversible middle cerebral
artery occlusion in the rat. J Cereb Blood Flow Metab. 18:196–205.
1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang Z, Weian C, Susu H and Hanmin W:
Protective effects of mangiferin on cerebral ischemia-reperfusion
injury and its mechanisms. Eur J Pharmacol. 771:145–151.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Q, Sun AY, Simonyi A, Jensen MD,
Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE,
Weisman GA and Sun GY: Neuroprotective mechanisms of curcumin
against cerebral ischemia-induced neuronal apoptosis and behavioral
deficits. J Neurosci Res. 82:138–148. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang TF, Lei Z, Li YX, Wang YS, Wang J,
Wang SJ, Hao YJ, Zhou R, Jin SJ, Du J, et al: Oxysophoridine
protects against focal cerebral ischemic injury by inhibiting
oxidative stress and apoptosis in mice. Neurochem Res.
38:2408–2417. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang B, Zhong Q and Chen X, Wu X, Sha R,
Song G, Zhang C and Chen X: Neuroprotective effects of celastrol on
transient global cerebral ischemia rats via regulating HMGB1/NF-ĸB
signaling pathway. Front Neurosci. 14(847)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ahmad A, Khan MM, Raza SS, Javed H,
Ashafaq M and Islam F, Safhi MM and Islam F: Ocimum sanctum
attenuates oxidative damage and neurological deficits following
focal cerebral ischemia/reperfusion injury in rats. Neurol Sci.
33:1239–1247. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wei W, Lan XB, Liu N, Yang JM, Du J, Ma L,
Zhang WJ, Niu JG, Sun T and Yu JQ: Echinacoside alleviates
hypoxic-ischemic brain injury in neonatal rat by enhancing
antioxidant capacity and inhibiting apoptosis. Neurochem Res.
44:1582–1592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu F, Hao Y, Yang J, Yao W, Xu Y, Yan L,
Niu Y, Sun T, Yu J and Zhou R: Protective effects of aloperine on
monocrotaline-induced pulmonary hypertension in rats. Biomed
Pharmacother. 89:632–641. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang J, Zhou H, Chen J, Lv X and Liu H:
Aloperine protects human retinal pigment epithelial cells against
hydrogen peroxide-induced oxidative stress and apoptosis through
activation of Nrf2/HO-1 pathway. J Recept Signal Transduct Res: Nov
30, 2020 (Epub ahead of print).
|
|
37
|
Han B, Lu Y, Zhao H, Wang Y, Li L and Wang
T: Electroacupuncture modulated the inflammatory reaction in MCAO
rats via inhibiting the TLR4/NF-κB signaling pathway in microglia.
Int J Clin Exp Pathol. 8:11199–11205. 2015.PubMed/NCBI
|
|
38
|
Hou SZ, Li Y, Zhu XL, Wang ZY, Wang X and
Xu Y: Ameliorative effects of diammonium glycyrrhizinate on
inflammation in focal cerebral ischemic-reperfusion injury. Brain
Res. 1447:20–27. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shichita T, Sakaguchi R, Suzuki M and
Yoshimura A: Post-ischemic inflammation in the brain. Front
Immunol. 3(132)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu Q and Zhang Y: PRDX1 enhances cerebral
ischemia-reperfusion injury through activation of TLR4-regulated
inflammation and apoptosis. Biochem Biophys Res Commun.
519:453–461. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kao TK, Ou YC, Liao SL, Chen WY, Wang CC,
Chen SY, Chiang AN and Chen CJ: Opioids modulate post-ischemic
progression in a rat model of stroke. Neurochem Int. 52:1256–1265.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao J, Li L and Fang G: Salvianolic acid
A attenuates cerebral ischemia/reperfusion injury induced rat brain
damage, inflammation and apoptosis by regulating miR-499a/DDK1. Am
J Transl Res. 12:3288–3301. 2020.PubMed/NCBI
|
|
43
|
Chang Z, Zhang P, Zhang M, Jun F, Hu Z,
Yang J, Wu Y and Zhou R: Aloperine suppresses human pulmonary
vascular smooth muscle cell proliferation via inhibiting
inflammatory response. Chin J Physiol. 62:157–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mao Q, Guo F, Liang X, Wu Y and Lu Y:
Aloperine activates the PI3K/Akt Pathway and protects against
coronary microembolisation-induced myocardial injury in rats.
Pharmacology. 104:90–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Feng C, Wan H, Zhang Y, Yu L, Shao C, He
Y, Wan H and Jin W: Neuroprotective effect of Danhong injection on
cerebral Ischemia-Reperfusion injury in rats by activation of the
PI3K-Akt pathway. Front Pharmacol. 11(298)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yu Y, Jia XJ, Zong QF, Zhang GJ, Ye HW, Hu
J, Gao Q and Guan SD: Remote ischemic postconditioning protects the
heart by upregulating ALDH2 expression levels through the PI3K/Akt
signaling pathway. Mol Med Rep. 10:536–542. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang Z, Han Y, Tian S, Bao J, Wang Y and
Jiao J: Lupeol alleviates cerebral Ischemia-Reperfusion injury in
correlation with modulation of PI3K/Akt pathway. Neuropsychiatr Dis
Treat. 16:1381–1390. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pignataro G, Meller R, Inoue K, Ordonez
AN, Ashley MD, Xiong Z, Gala R and Simon RP: In vivo and in vitro
characterization of a novel neuroprotective strategy for stroke:
Ischemic postconditioning. J Cereb Blood Flow Metab. 28:232–241.
2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fu X, Sun F, Wang F, Zhang J, Zheng B,
Zhong J, Yue T, Zheng X, Xu JF and Wang CY: Aloperine protects mice
against DSS-induced colitis by PP2A-mediated PI3K/Akt/mTOR
signaling suppression. Mediators Inflamm.
2017(5706152)2017.PubMed/NCBI View Article : Google Scholar
|