Introduction
Breast cancer is the leading cause of
cancer-associated mortality in women worldwide (1). Triple-negative breast cancer (TNBC) is
a special subtype of breast cancer, accounting for ~10% of all
breast cancers (2). The clinical
manifestations of TNBC include high invasive capacity, tendency for
early metastasis and a lack of recognizable therapeutic targets,
which is directly associated with the poor clinical prognosis of
TNBC (3). Identification of
relevant biological markers could provide novel strategies for the
diagnosis and treatment of TNBC.
MicroRNAs (miRNAs/miRs) are a type of
single-stranded non-coding small RNA with a length of ~20
nucleotides (4). It has been
indicated that miRNAs serve important regulatory roles in
development, cell differentiation, proliferation, apoptosis and
tumor invasion, metastasis and the tumor microenvironment (5). miRNAs specifically bind to the
3'-untranslated region (3'-UTR) of their downstream target gene
mRNA to promote mRNA degradation or inhibit mRNA translation
(4). It has been indicated that the
abnormal expression of miRNAs is closely associated with the
occurrence and progression of several malignant tumors. For
instance, miR-183 has been revealed to target sirtuin 1 or the
PI3K/AKT/mTOR signaling pathway to regulate the apoptosis and
autophagy of gastric cancer cells (6). miR-488 has been demonstrated to
inhibit the apoptosis of CD8+ T cells in colon cancer
through the inhibition of indoleamine 2,3-dioxygenase 1, which may
be beneficial for the development of novel immunotherapy drugs for
colon cancer (7). Upregulation and
downregulation of multiple miRNAs has also been demonstrated to
exist in breast cancer. For example, the miR-10 family (8), miR-21(9) and the miR-17/92 cluster (10) are considered to serve a role in
promoting cancer, while the let-7(11) and miR-200 families (12) are considered as inhibitors in breast
cancer. Localized at 11p15.5, miR-483-5p is located in the second
intron of insulin-like growth factor 2(13). miR-483-5p has been revealed to be
dysregulated and has been associated with poorer survival in
adrenocortical carcinoma and lung adenocarcinoma (14,15).
However, the role of miR-483-5p in breast cancer and its associated
molecular mechanisms remain unclear.
The present study measured the expression of
miR-483-5p in TNBC to investigate its role and the underlying
molecular mechanism.
Materials and methods
Clinical samples
Tumor and adjacent tissues (>5 cm away from tumor
tissues) of 25 female patients with TNBC who underwent surgical
resection in Tangshan Maternal Child Health Care Hospital
(Tangshan, China) between October 2018 and October 2019 were
collected. The age range of the patients was 29-68 years, and the
mean age was 42±1.9 years. None of the patients received any
treatment before surgery. Immediately after the excision, the
tissues were immersed in liquid nitrogen and stored at -80˚C. The
present study was approved by Yantai Muping Hospital of Traditional
Chinese Medicine (Yantai, China), and written informed consent was
obtained from all the participants.
Cell culture
The non-malignant breast epithelial cell line
MCF-10a and TNBC cell lines (BT-20, MDA-MB-231, MDA-MB-468 and
BT-549) were obtained from American Type Culture Collection (ATCC)
and maintained in RPMI-1640 medium (HyClone; Cytiva) containing 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37˚C in a humidified atmosphere with 5% CO2.
293T cells were obtained from ATCC and cultured in high-glucose
DMEM (HyClone; Cytiva) containing 10% FBS, at 37˚C in a 5%
CO2 incubator.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from BT-549 cells using an
RNeasy Mini kit (Qiagen GmbH). cDNA was synthesized from 500 ng RNA
using PrimeScript RT Master Mix (cat. no. RR036Q; Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
Total RNA was reverse-transcribed into mature miRNAs using a Mir-X
miRNA First-Strand Synthesis kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. SOCS3 expression was
measured using SYBR-Green RT-qPCR kit (Qiagen GmbH). The reactions
were carried out in a Veriti Thermal Cycler (Applied Biosystems;
Thermo Fisher Scientific, Inc.) following the cycling: 50˚C For 30
min, 95˚C for 15 min, followed by 45 cycles of 94˚C for 30 sec,
58˚C for 30 sec, 72˚C for 30 sec and a final extension of 72˚C for
10 min. miR-483-5p expression was detected with a Hairpin-it™
miRNAs qPCR kit (Shanghai GenePharma Co., Ltd.). The reactions were
carried in Veriti Thermal Cycler following the cycling: 95˚C For 3
min followed by 40 cycles of 95˚C for 12 sec, 58˚C for 30 sec and a
final extension of 72˚C for 10 min. Quantitative analysis was
performed using the 2-ΔΔCq method (16). U6 and GAPDH were used as endogenous
normalization controls for miRNA and mRNA, respectively. The
primers used are listed as follows: miR-483-5p forward, 5'-AGT
TGGCTCACGGTTCTTTCAA-3' and reverse, 5'-ATCGCCA TGGCCCGCATGTCGG-3';
SOCS3 forward, 5'-AGAGCGG ATTCTACTGGAGCG-3' and reverse,
5'-CTGGATGCGTAGG TTCTTGGTC-3'; U6 forward, 5'-CTCGCTTCGGCAGC ACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'; GAPDH forward,
5'-ATGTTGCAACCGGGAAGGAA-3' and reverse,
5'-GCAAATTCGTGAAGCGTTCCATA-3'.
Cell transfection
miR-483-5p mimics, miR-483-5p inhibitor, small
interfering (si)RNA targeting SOCS3 (si-SOCS3; forward,
5'-UAGGAGACUCGCCUUAAAUTT-3'; reverse, 5'-AUUUA AGGCGAGUCUCCUATT-3')
and non-targeting sequence (si-Ctrl; forward,
5'-UUCUCCGAACGUGUCACGUTT-3' and reverse,
5'-ACGUGACACGUUCGGAGAATT-3') were purchased from Shanghai GeneChem
Co., Ltd. SOCS3 overexpression vector (SOCS3-OE; forward,
5'-AAAGCTAGCCC ATGGTCACCCACAGCAAGTT-3' and reverse, 5'-AAACTC
GAGTCCTTAAAGTGGAGCATCATAC-3') and empty vector (pcDNA3.1) were
obtained from Invitrogen (Thermo Fisher Scientific, Inc.). BT-549
cells were seeded at 1.5x105 cells/well in a six-well
plate and cultured for 24 h. The cells were transiently transfected
24 h post-seeding with mimics (50 pmol/ml), inhibitors (50
pmol/ml), si-SOCS3 (50 nM) or SOCS3-OE (2 µg) using Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions.
Dual-luciferase reporter assay
To determine if SOCS3 is a target of miR-483-5p, the
target gene prediction software miRTarBase (https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2019/php/index.php)
was used. Wild-type (Wt) and mutant (Mut) SOCS3 3'-UTRs were
amplified and cloned into the luciferase reporter vector pmirGLO
(Promega Corporation). BT-549 and 293T cells from the miR-negative
control (NC) and miR-483-5p groups were seeded in 24-well plates
(1x104 cells/well), and transfected with SOCS3-Wt or
SOCS3-Mut plasmids (0.5 µg), respectively, using Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), and cultured for
48 h at 37˚C. After 48 h of incubation, cells were collected and
firefly and Renilla luciferase activities were measured
using a dual-luciferase reporter assay (Promega Corporation)
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to Renilla luciferase activity.
Western blotting
A total of 100 µl RIPA cell lysis buffer (cat. no.
78425; Pierce; Thermo Fisher Scientific, Inc.) containing protease
inhibitors was added to BT-549 cells on ice for 20 min. Cell
lysates were centrifuged at 1,000 x g for 15 min in a high-speed
centrifuge at 4˚C, and supernatants were collected for protein
concentration determination using a BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Protein samples (40 µg/lane) were loaded on a 10%
SDS-PAGE gel, and then transferred to a PVDF membrane. The membrane
was blocked with 1X TBS-0.1% Tween-20 buffer (TBST) containing 5%
skimmed milk powder for 2 h at room temperature. Subsequently, the
membrane was incubated with the corresponding primary antibodies
against SOCS3 (cat. no. ab280884; 1:1,000; Abcam) and GAPDH (cat.
no. ab181602; 1:1,000; Abcam) at 4˚C overnight. After washing with
TBST (10X TBS; 20% Tween-20), the membrane was incubated with the
horseradish peroxidase-conjugated secondary goat anti-rabbit IgG
antibody (cat. no. ab6721; 1:2,000; Abcam) or goat anti-mouse IgG
antibody (cat. no. ab205719; 1:2,000; Abcam) for 1 h at room
temperature. Immunoreactive bands were visualized using enhanced
chemiluminescent substrate (Beyotime Institute of Biotechnology).
Optical density was measured and relative protein expression was
calculated by normalization to GAPDH using ImageJ v1.51 (National
Institutes of Health).
MTT assay
Following transfection for 24 h, BT-549 cells were
seeded in a 96-well plate at a density of 1x104/well and
cultured at 37˚C in a 5% CO2 incubator. At 24, 48 and 72
h, 20 µl of 5 mg/ml MTT solution (Beijing Solarbio Science &
Technology Co., Ltd.) were added to each well. The supernatant was
subsequently removed after centrifugation (1,000 x g; 10 min; room
temperature). A total of 200 µl DMSO was added to each well,
followed by incubation for 30 min at 37˚C. The plate was then
placed in a microplate reader and the optical density was measured
at 570 nm. All measurements were repeated in triplicate, and each
experiment was repeated at least three times.
Flow cytometry
BT-549 cells were transfected for 48 h and digested
with trypsin (at 4˚C for 1 h) without EDTA and collected. After
washing with PBS, the cells were centrifuged at 2,000 rpm for 5
min. The cells were resuspended in 500 µl of 1X PBS solution. 100
µl of cell suspension was transferred to a new tube, and 5 µl of
Annexin V-FITC solution (C1062S) and 5 µl of propidium iodide (PI)
(both from Beyotime Institute of Biotechnology) solution were added
to the cell suspension. Cells were incubated for 5 min in the dark
at room temperature. Detection was performed on a BD FACSCanto II
flow cytometer (BD Biosciences) and the data was analyzed using
FlowJo software v7.6.1 (FlowJo LLC).
Wound healing assay
BT-549 cells in the logarithmic growth phase (24 h
after transfection) were seeded in 24-well plates at
2x105 cells/well and incubated at 37˚C in a 5%
CO2 incubator to ~100% confluence. The surface of the
cell monolayer in the center of each well was scratched with a
200-µl sterile pipette tip. After rinsing with sterile PBS, cells
were cultured in serum-free RPMI-1640 medium. The width of the
wound was photographed at 0 and 48 h under a light microscope
(magnification, x100; Olympus Corporation).
Transwell assay
A total of 30 µl pre-diluted Matrigel (37˚C for 30
min; BD Biosciences) was placed on top of each Transwell with an
8-µm pore size. A total of 24 h after transfection, BT-549 cells
were resuspended in 200 µl serum-free RPMI-1640 medium at 5x
105/well and seeded into the upper chamber. In the lower
chamber, 700 µl complete RPMI-1640 medium containing 20% FBS was
added. The cells were then incubated at 37˚C and 5% CO2 for 48 h.
Subsequently, the cells in the top chamber were wiped with a cotton
swab. The cells invading the lower compartment of the chamber were
fixed at room temperature with 4% methanol for 15 min, and
subsequently stained with 0.1% crystal violet for 10 min at room
temperature. The number of invading cells were counted in five
random fields a light microscope at x100 magnification.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of IL-1β (cat. no. PI305), IL-6 (cat. no.
PI330), TNF-α (cat. no. PT518) and MCP-1 (cat. no. PC130) were
detected by ELISA (Beyotime Institute of Biotechnology) according
to the manufacturer's protocols. Briefly, total proteins were
extracted from the BT-549 cells using lysis buffer and the
supernatants were collected by centrifuging at 4˚C at 12,000 x g
for 15 min. Subsequently, 96-well microplates were coated with 100
µl biotinylated primary antibodies mixed with 100 µl EIA buffer
provided in the kit, plus 100 µl standard and sample aliquots.
Plates were incubated for 2 h at 30˚C, followed by aspiration of
the samples and subsequent washing of them three times with wash
buffer. Next, 100 µl solution of streptavidin-horseradish
peroxidase conjugate was added to each well and incubated for 30
min at 30˚C, prior to washed again. Thereafter, 100 µl substrate
solution provided in the kits was added to each well and the plates
were incubated for 30 min at 30˚C. The optical density values were
read at 450 nm using a Biotek Synergy 2 plate reader (BioTek
Instruments, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were carried out with SPSS 20.0 (IBM Corp.)
and GraphPad Prism v8.0 (GraphPad Software, Inc.). An unpaired
two-tailed Student's t-test was used to assess the statistical
significance for comparisons between two groups. Comparisons
between multiple groups were performed using one-way ANOVA followed
by Tukey's post hoc test. In vitro experiments were repeated
three times in triplicate. The correlation between miR-483-5p and
SOCS3 protein in TNBC tissues was analyzed using Pearson's
correlation coefficient analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
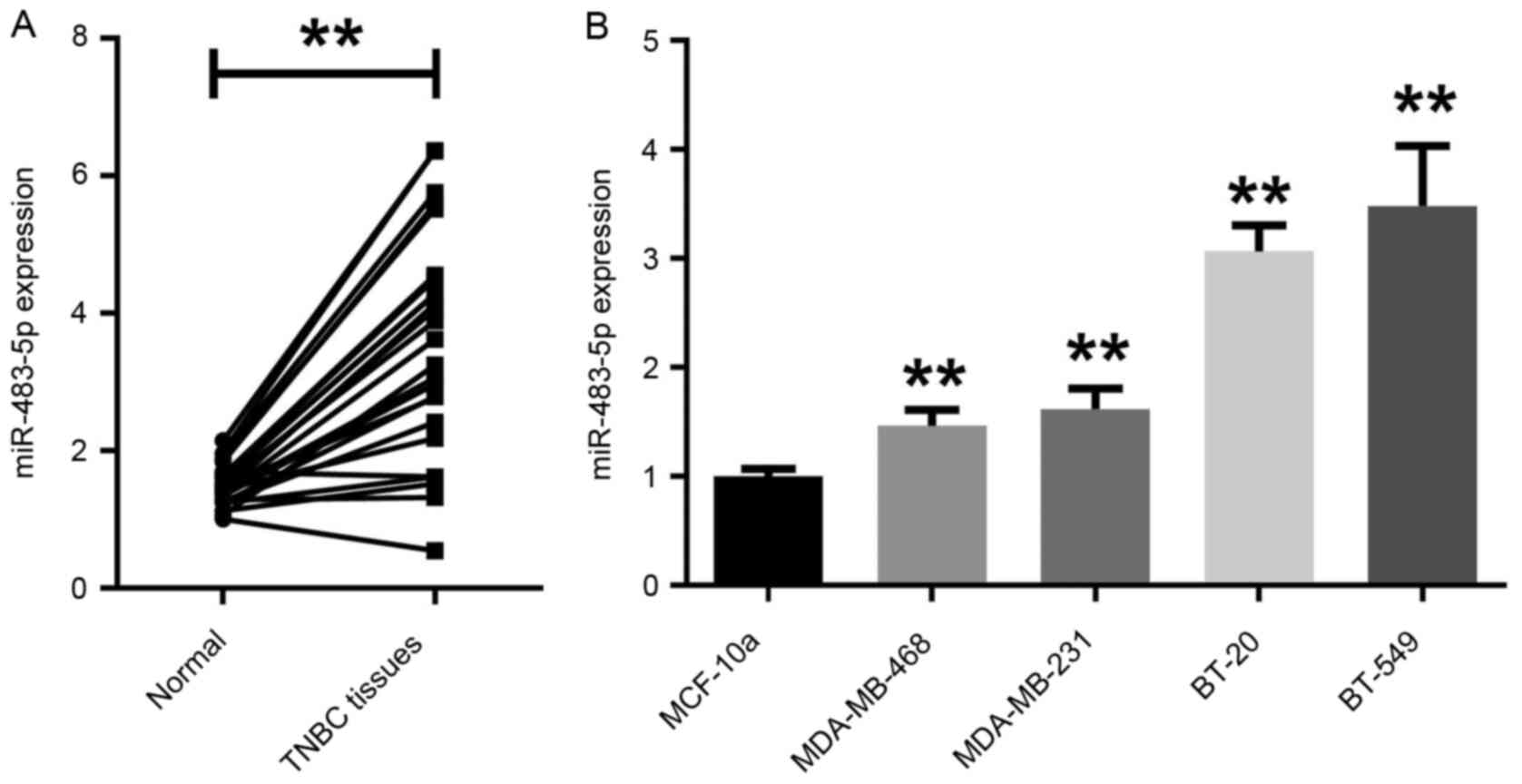
miR-483-5p is upregulated in TNBC
tissues and cell lines
The expression level of miR-483-5p in TNBC tissues
and adjacent normal tissues was first assessed by RT-qPCR.
miR-483-5p expression levels were significantly higher in TNBC
tissues compared with adjacent normal tissues (Fig. 1A). Additionally, in the TNBC cell
lines BT-20, MDA-MB-231, MDA-MB-468 and BT-549, miR-483-5p
expression was also significantly higher compared with in the
non-malignant epithelial cell line MCF-10a (Fig. 1B). As the expression level of
miR-483-5p was higher in BT-549 cells compared with the other TNBC
cell lines, BT-549 was selected for subsequent experiments.
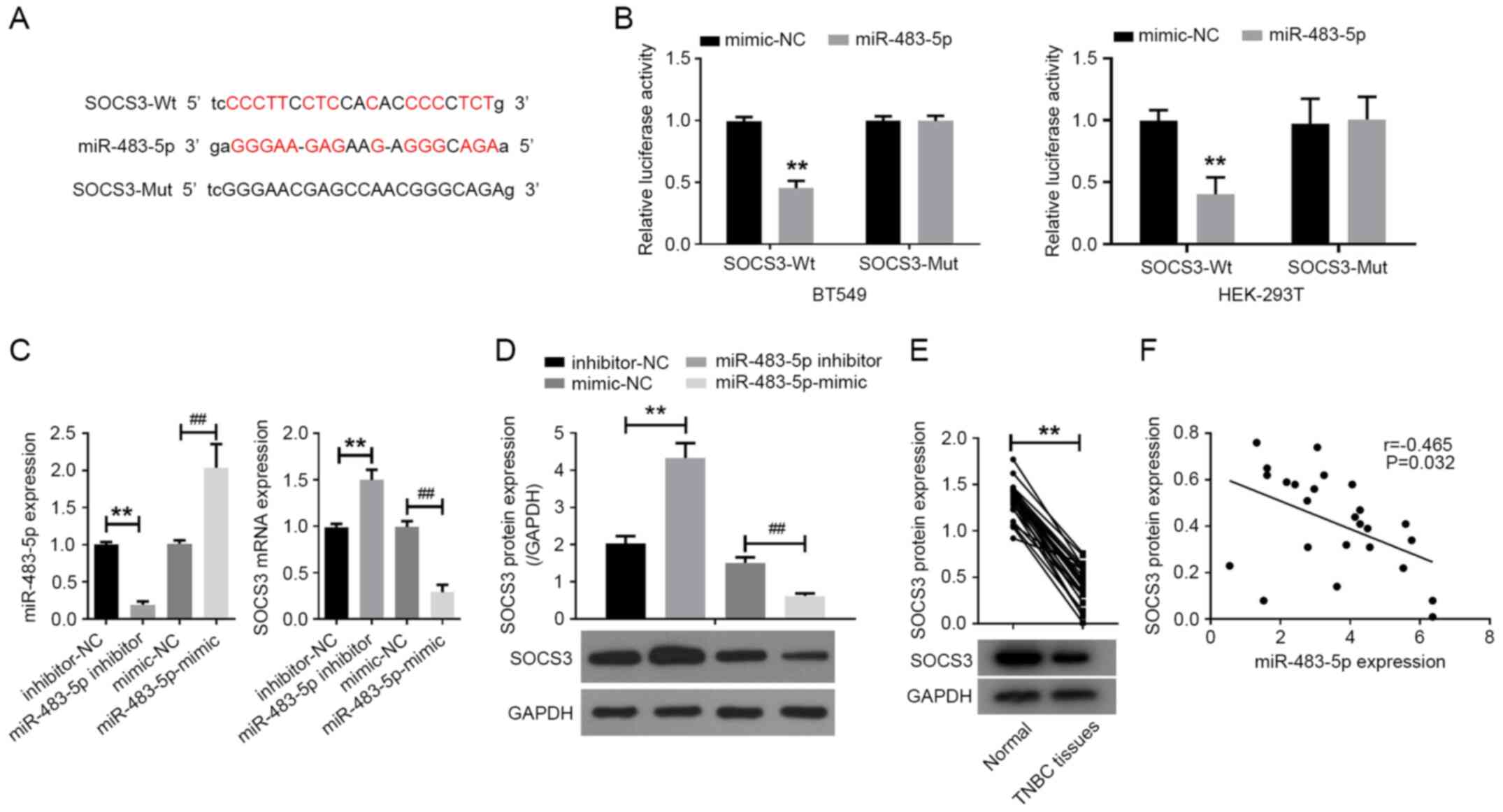
SOCS3 is a target of miR-483-5p
The online software miRTarBase was used to predict
the mRNAs putatively targeted by miR-483-5p. A binding site of
miR-483-5p was detected in the 3'-UTR of SOCS3 (Fig. 2A). Subsequently, luciferase reporter
vectors containing either SOCS3-WT or SOCS3-MUT 3'-UTRs were
constructed and co-transfected with miR-483-5p mimics or mimic-NC
into BT-549 and 293T cells to perform luciferase reporter assays.
Co-transfection with miR-483-5p mimics was observed to
significantly reduce the luciferase activity of the SOCS3-WT
vector, while the luciferase activity of the reporter containing
SOCS3-MUT was unaffected in cells following transfection with
miR-483-5p mimics compared with mimic-NC (Fig. 2B).
Additionally, miR-483-5p inhibitor or miR-483-5p
mimics were transfected into BT-549 cells. The expression levels of
miR-483-5p, SOCS3 mRNA and SOCS3 protein were quantified by RT-qPCR
and western blot assay. RT-qPCR analysis indicated that miR-483-5p
inhibitor decreased miR-483-5p expression in BT-549 cells, while
miR-483-5p mimics exhibited the opposite effect compared with the
respective NC (Fig. 2C). Moreover,
compared with inhibitor-NC, miR-483-5p inhibitor markedly increased
the expression level of SOCS3 mRNA and protein in BT-549 cells. By
contrast, compared with mimic-NC, miR-483-5p mimics significantly
reduced SOCS3 mRNA and protein expression levels in BT-549 cells
(Fig. 2C and D).
Detection of SOCS3 protein in TNBC tissues via
western blot analysis indicated that the expression level of SOCS3
protein in TNBC tissues was significantly downregulated compared
with in adjacent normal tissues (Fig.
2E). Correlation analysis revealed that miR-483-5p expression
was negatively correlated with SOCS3 protein expression in TNBC
tissues (Fig. 2F). Taken together,
these results indicated that SOCS3 was one of the targets of
miR-483-5p, and that miR-483-5p could regulate the expression of
SOCS3 in TNBC cells.
Knockdown of miR-483-5p inhibits the
proliferation and promotes apoptosis of the TNBC cell line BT-549
cells by regulating SOCS3
To investigate whether miR-483-5p could participate
in TNBC progression by regulating SOCS3, miR-483-5p inhibitor and
si-SOCS3 were co-transfected into BT-549 cells. As illustrated in
Fig. 3A, transfection with si-SOCS3
successfully decreased the expression level of SOCS3 in BT-549
cells compared with the control siRNA. Western blot assay results
indicated that miR-483-5p inhibitor promoted the expression of
SOCS3, which could be reversed by si-SOCS3 (Fig. 3B). The effects of miR-483-5p on the
proliferation and apoptosis of BT-549 cells were further evaluated
by MTT assays and flow cytometry. As depicted in Fig. 3C, the decrease of miR-483-5p
inhibited the proliferation of BT-549 cells, which was markedly
attenuated by si-SOCS3 transfection. Additionally, flow cytometry
revealed that the inhibition of miR-483-5p significantly increased
apoptosis in BT-549 cells, which was reversed by si-SOCS3 (Fig. 3D).
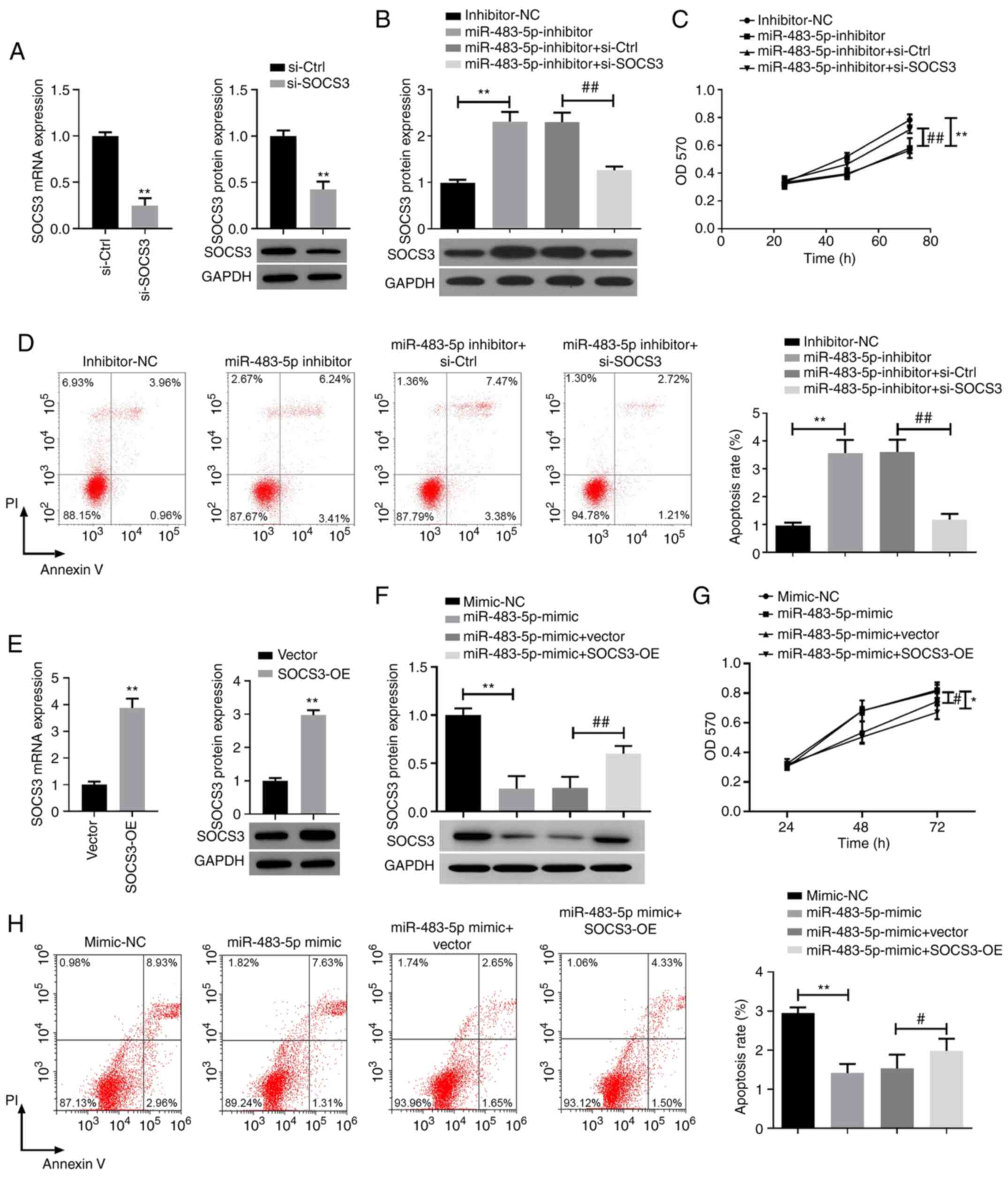 | Figure 3miR-483-5p knockdown inhibits the
proliferation and promotes apoptosis of BT-549 cells by regulating
SOCS3. (A) Expression of SOCS3 mRNA and protein in BT-549 cells
transfected with si-Ctrl or si-SOCS3 was analyzed using RT-qPCR or
western blot assay. **P<0.01 vs. si-Ctrl. (B) SOCS3
protein expression was analyzed via western blotting, (C) cell
proliferation was detected using MTT assay and (D) flow cytometry
was used to determine apoptosis in BT-549 cells co-transfected with
miR-483-5p inhibitor and si-SOCS3. **P<0.01;
##P<0.01. (E) Expression of SOCS3 mRNA and protein in
BT-549 cells transfected with vector or SOCS3-OE was analyzed using
RT-qPCR or western blot assays. **P<0.01 vs. Vector.
(F) SOCS3 protein expression was analyzed via western blotting in
BT-549 cells co-transfected with miR-483-5p mimic and SOCS3-OE. (G)
Proliferation and (H) apoptosis of BT-549 cells co-transfected with
miR-483-5p mimic and SOCS3-OE was analyzed using MTT assays and
flow cytometry, respectively. Data are presented as the mean ± SD
of three independent experiments. *P<0.05,
**P<0.01; #P<0.05,
##P<0.01. RT-qPCR, reverse transcription-quantitative
PCR; miR, microRNA; NC, negative control; SOCS3, suppressor of
cytokine signaling 3; Ctrl, control; si, small interfering; OE,
overexpression; OD, optical density; PI, propidium iodide. |
miR-483-5p mimics and SOCS3-OE were then
co-transfected into BT-549 cells. The expression level of SOCS3 was
increased in BT-549 cells transfected with SOCS3-OE compared with
the empty vector (Fig. 3E).
SOCS3-OE attenuated the inhibitory effect of miR-483-5p on SOCS3
expression in BT-549 cells (Fig.
3F). SOCS3-OE also partially suppressed the promoting effect of
miR-483-5p on the proliferation of BT-549 cells (Fig. 3G). Consistently, SOCS3
overexpression attenuated the miR-483-5p-mediated inhibition of
apoptosis (Fig. 3H). These data
suggested that knockdown of miR-483-5p could inhibit the
proliferation of BT-549 cells and induce apoptosis by targeting
SOCS3.
Knockdown of miR-483-5p inhibits the
migration and invasion of the TNBC cell line BT-549 cells by
regulating SOCS3
Metastasis is the leading cause of death in clinical
patients with breast cancer (17).
The effects of miR-483-5p on BT-549 migration and invasion were
investigated. Wound healing assay results indicated that the
decrease of miR-483-5p inhibited BT-549 cell migration, which was
markedly reversed by SOCS3 siRNA transfection (Fig. 4A). Consistent with this observation,
Transwell assays revealed that SOCS3 siRNA reversed the inhibitory
effect of miR-483-5p inhibitor on BT-549 cell invasion (Fig. 4B). Additionally, the effect of
miR-483-5p mimics on the migration (Fig. 4C) and invasion (Fig. 4D) of BT-549 cells was reversed by
SOCS3-OE transfection.
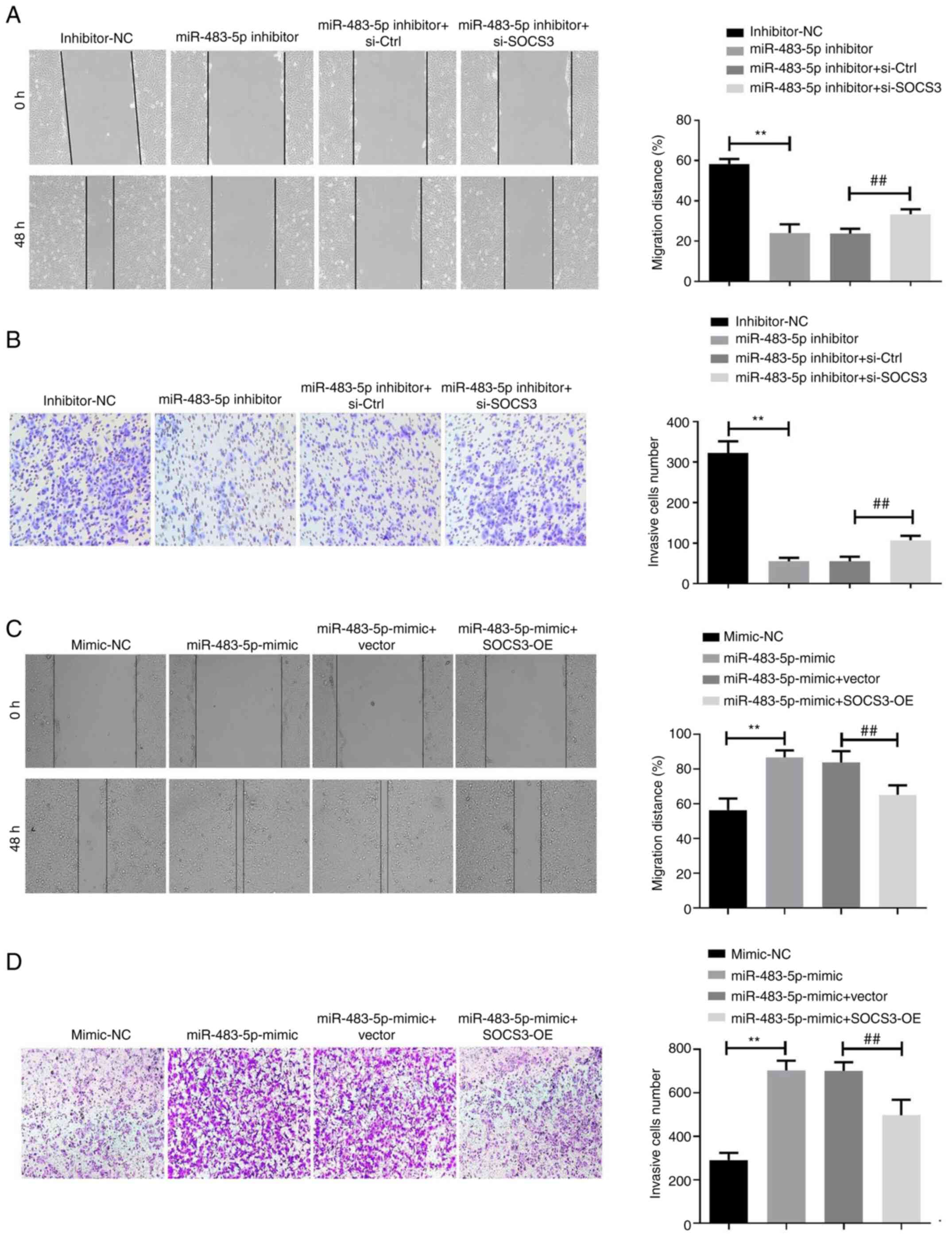 | Figure 4miR-483-5p knockdown inhibits
migration and invasion of the triple-negative breast cancer cell
line BT-549 by regulating SOCS3. (A) Wound healing assay was used
to analyze the migration of BT-549 cells co-transfected with
miR-483-5p inhibitor and si-SOCS3. Scale bar, 200 µm. (B) Transwell
assay was used to analyze the invasion of BT-549 cells
co-transfected with miR-483-5p inhibitor and si-SOCS3. Scale bar,
100 µm. **P<0.01; ##P<0.01. (C) Wound
healing assay was used to analyze the migration of BT-549 cells
co-transfected with miR-483-5p mimic and SOCS3-OE. Scale bar, 200
µm. (D) Transwell assay was used to analyze the invasion of BT-549
cells co-transfected with miR-483-5p mimic and SOCS3-OE. Scale bar,
100 µm. Data are presented as the mean ± SD of three independent
experiments. **P<0.01; ##P<0.01. miR,
microRNA; NC, negative control; SOCS3, suppressor of cytokine
signaling 3; Ctrl, control; si, small interfering; OE,
overexpression. |
Knockdown of miR-483-5p reduces the
secretion of inflammatory factors in TNBC cells by regulating
SOCS3
In addition to metastasis, the occurrence of
inflammation is an important factor in the worsening of breast
cancer. It has been indicated that inflammatory factors, such as
TNF-α, IL-6, IL-1β and monocyte chemoattractant protein-1 (MCP-1),
are closely associated with the recurrence and metastasis of breast
cancer (18). Therefore, TNF-α,
IL-6, IL-1β and MCP-1 were quantified in BT-549 cell supernatant
via ELISA. Compared with the inhibitor-NC group, miR-483-5p
inhibitor notably reduced the secretion of IL-1β, IL-6, TNF-α and
MCP-1 in BT-549 cells, while si-SOCS3 partially reversed the effect
of miR-483-5p inhibitor on the inflammatory response (Fig. 5A-D). On the contrary, increases in
inflammatory factor secretion induced by miR-483-5p mimics in
BT-549 cells was reversed after transfection with SOCS3-OE
(Fig. 5E-H).
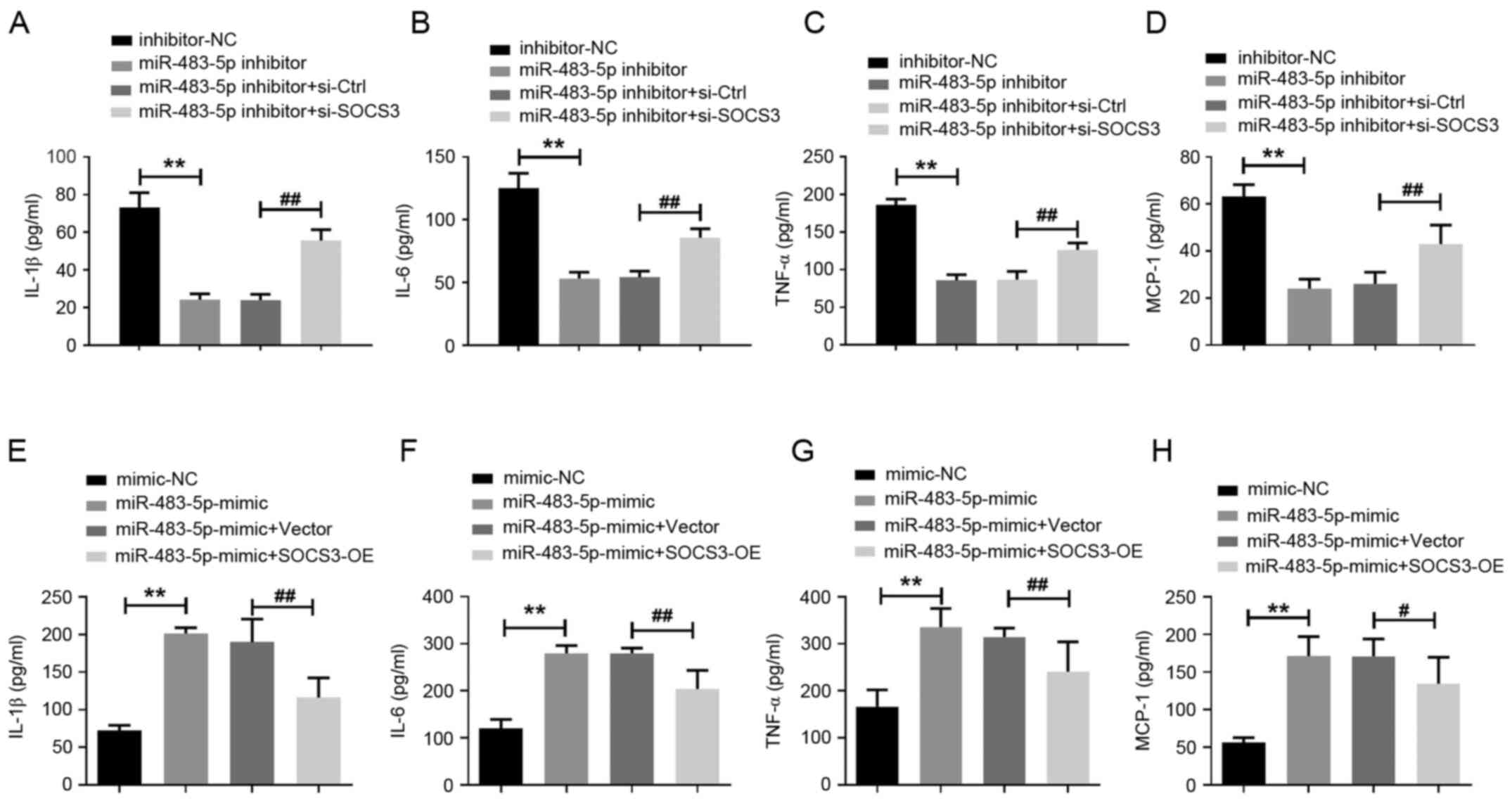 | Figure 5Knockdown of miR-483-5p reduces the
secretion of inflammatory factors in triple-negative breast cancer
cells by regulating SOCS3. ELISA was used to analyze the quantity
of (A) IL-1β, (B) IL-6, (C) TNF-α and (D) MCP-1 in the supernatant
of BT-549 cells co-transfected with miR-483-5p inhibitor and
si-SOCS3. **P<0.01; ##P<0.01. ELISA was
used to analyze the quantity of (E) IL-1β, (F) IL-6, (G) TNF-α and
(H) MCP-1 in the supernatant of BT-549 cells co-transfected with
miR-483-5p mimic and SOCS3-OE. **P<0.01;
#P<0.05, ##P<0.01. Data are presented
as the mean ± SD of three independent experiments. MCP-1, monocyte
chemoattractant protein-1; miR, microRNA; NC, negative control;
SOCS3, suppressor of cytokine signaling 3; Ctrl, control; si,small
interfering; OE, overexpression. |
Discussion
miRNAs have been indicated to play roles similar to
oncogenes or tumor suppressors (4).
In the present study, the expression level of miR-483-5p was
observed to be increased in TNBC tissues and cell lines compared
with normal adjacent tissues and cells, and the inhibition of
miR-483-5p could inhibit the proliferation, invasion and
inflammatory response of TNBC cells. More importantly, SOCS3 was
one of the targets of miR-483-5p, and SOCS3 silencing reversed the
anti-oncogenic effect of miR-483-5p knockdown on TNBC cells.
In the present study, miR-483-5p was identified as a
novel therapeutic target for TNBC. Upregulation of miR-483-5p
expression has previously been reported in adrenal cortical cancer
and esophageal squamous cell carcinoma (14,19).
Consistent with these reports, the present study provided new
evidence for the abnormal expression of miR-483-5p in TNBC.
Interestingly, Wang et al (20) reported that miR-483-5p is decreased
in human glioma. Considering that gene expression is tumor-specific
and that the reason for the differential expression of miRNAs
depends to a large extent on the condition of the tumor (21,22),
these results are not contradictory. miR-483-5p was inhibited in
the TNBC cell line BT-549 to analyze cell proliferation and
invasion. The results indicated that inhibition of miR-483-5p
expression inhibited the proliferative and invasive capabilities of
BT-549 cells. In addition, the anti-proliferative effect of
miR-483-5p silencing may be related to its positive effect on
apoptosis. As the interaction between the tumor microenvironment
and tumor cells has received increasing attention, tumor-associated
inflammation is also considered to be an important feature of
cancers. For instance, inflammation has been revealed to be closely
associated with the growth, angiogenesis and distant metastasis of
breast tumor cells (23). Moreover,
excessive inflammation can disrupt the acquired immune response and
reduce the sensitivity of cells to chemotherapeutics (24). Furthermore, blocking
pro-inflammatory cytokines can be used as a therapeutic strategy
for targeting TNBC (25). In the
present study, inhibition of miR-483-5p reduced the secretion of
inflammatory cytokines in breast cancer cells. These results
indicated that miR-483-5p may be a potential oncogenic factor for
the progression of TNBC.
It is well known that miRNAs can perform biological
functions by regulating the expression of multiple target genes
(4). In the current study,
miRTarBase analysis revealed that SOCS3 was one of the direct
targets of miR-483-5p. Luciferase reporter assays and expression
level analysis confirmed that miR-483-5p was able to negatively
regulate SOCS3 expression in BT-549 cells. SOCS3 is a cytokine
signaling inhibitor protein, and its abnormal expression has been
associated with the occurrence and development of numerous
diseases, including tumors (26).
It has been previously demonstrated that SOCS3 can regulate the
proliferation, metastasis and invasion of several types of tumor
cells (27-29),
including breast cancer (30).
Furthermore, it has been indicated that the expression of the SOCS3
gene is directly associated with the expression of several
inflammatory genes, especially NF-κB, primary inflammatory
cytokines (such as TNF-α, IL-6, IL-1β) and the STAT3 cascade
(31), which have been considered
as the main molecular participants involved in cancer-related
inflammation (32,33). It has also been demonstrated in TNBC
that enhanced expression of SOCS3 can inhibit tumor growth and
metastasis by interfering with the IL-6/STAT3/NF-κB pathway
(21). In the present study,
miR-483-5p inhibitor and si-SOCS3 were co-transfected into BT-549
cells, and the results indicated that SOCS3 silencing reversed the
effects of miR-483-5p inhibition on cell proliferation, apoptosis,
migration, invasion and inflammatory factor secretion.
Additionally, co-transfection of miR-483-5p and SOCS3-OE also
abolished the effects of miR-483-5p alone on the malignant
phenotype of TNBC cells. Based on these results, it could be
speculated that miR-483-5p may exert a carcinogenic role in TNBC by
directly inhibiting the expression of SOCS3.
However, there were certain limitations in the
present study. Firstly, the sample size of patients with TNBC was
limited. Moreover, it was only confirmed that SOCS3 was one of the
targets of miR-483-5p. It is well known that miRNAs can target and
regulate a variety of genes (34).
Therefore, future studies may involve an extensive screening of
more targets through microarrays to further clarify the potential
mechanism of miR-483-5p in the progression of TNBC.
In conclusion, the expression level of miR-483-5p
was revealed to be increased in TNBC tissues and cell lines, and
inhibition of miR-483-5p inhibited the proliferation, migration,
invasion and inflammatory response, while promoting apoptosis of
TNBC cells by negatively regulating SOCS3. Therefore, miR-483-5p
may be a potential target for TNBC therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JR and GX wrote the manuscript and conducted the
majority of the experiments. HS and TL analyzed data and conducted
the experiments. SX acquired data and performed statistical
analysis. YZ designed the research, analyzed the data and approved
the study. JR and YZ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Yantai Muping
Hospital of Traditional Chinese Medicine (Yantai, China), and
written informed consent was obtained from all the
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Domínguez F, Maycotte P, Acosta-Casique A,
Rodríguez-Rodríguez S, Moreno DA, Ferreres F, Flores-Alonso JC,
Delgado-López MG, Pérez-Santos M and Anaya-Ruiz Μ: Bursera
copallifera Extracts have cytotoxic and migration-inhibitory
effects in breast cancer cell lines. Integr Cancer Ther.
17:654–664. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Geenen JJJ, Linn SC, Beijnen JH and
Schellens JH: PARP inhibitors in the treatment of triple-negative
breast cancer. Clin Pharmacokinet. 57:427–437. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu J, Shi Z, Bai Y, Liu L and Cheng K:
Prognostic significance of systemic immune-inflammation index in
triple-negative breast cancer. Cancer Manag Res. 11:4471–4480.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Paul S, Reyes PR, Garza BS and Sharma A:
MicroRNAs and child neuropsychiatric disorders: A Brief Review.
Neurochem Res. 45:232–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dai W, Lu H, Yang F, Dong H and Zhang X:
Accurate detection of intracellular microRNAs using functional Mo2C
quantum dots nanoprobe. Chem Commun (Camb). 55:10615–10618.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li H, He C, Wang X, Wang H, Nan G and Fang
L: MicroRNA-183 affects the development of gastric cancer by
regulating autophagy via MALAT1-miR-183-SIRT1 axis and
PI3K/AKT/mTOR signals. Artif Cells Nanomed Biotechnol.
47:3163–3171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lou Q, Liu R, Yang X, Li W, Huang L, Wei
L, Tan H, Xiang N, Chan K, Chen J, et al: miR-448 targets IDO1 and
regulates CD8+ T cell response in human colon cancer. J
Immunother Cancer. 7(210)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sahraei M, Chaube B, Liu Y, Sun J, Kaplan
A, Price NL, Ding W, Oyaghire S, García-Milian R, Mehta S, et al:
Suppressing miR-21 activity in tumor-associated macrophages
promotes an antitumor immune response. J Clin Invest.
129:5518–5536. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li H, Bian C, Liao L, Li J and Zhao RC:
miR-17-5p promotes human breast cancer cell migration and invasion
through suppression of HBP1. Breast Cancer Res Treat. 126:565–575.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Gao Y, Zhang W, Liu C and Li G: miR-200
affects tamoxifen resistance in breast cancer cells through
regulation of MYB. Sci Rep. 9(18844)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Girardot M, Cavaillé J and Feil R: Small
regulatory RNAs controlled by genomic imprinting and their
contribution to human disease. Epigenetics. 7:1341–1348.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Soon PS, Tacon LJ, Gill AJ, Bambach CP,
Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson
BG, et al: miR-195 and miR-483-5p identified as predictors of poor
prognosis in adrenocortical cancer. Clin Cancer Res. 15:7684–7692.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen
J, Zhang Y, Lai P, Fan X, Zhou X, et al: miR-483-5p promotes
invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1
and ALCAM. Cancer Res. 74:3031–3042. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meirson T and Gil-Henn H: Targeting
invadopodia for blocking breast cancer metastasis. Drug Resist
Updat. 39:1–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
De Sanctis V, Agolli L, Visco V, Monaco F,
Muni R, Spagnoli A, Campanella B, Valeriani M, Minniti G, Osti MF,
et al: Cytokines, fatigue, and cutaneous erythema in early stage
breast cancer patients receiving adjuvant radiation therapy. Biomed
Res Int. 2014(523568)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang H, Shi X, Chang W, Li Y and Wang L
and Wang L: Epigenetic alterations of the Igf2 promoter and the
effect of miR 483 5p on its target gene expression in esophageal
squamous cell carcinoma. Mol Med Rep. 17:2251–2256. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang L, Shi M, Hou S, Ding B, Liu L, Ji X,
Zhang J and Deng Y: MiR-483-5p suppresses the proliferation of
glioma cells via directly targeting ERK1. FEBS Lett. 586:1312–1317.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lemberger M, Loewenstein S, Lubezky N,
Nizri E, Pasmanik-Chor M, Barazovsky E, Klausner JM and Lahat G:
MicroRNA profiling of pancreatic ductal adenocarcinoma (PDAC)
reveals signature expression related to lymph node metastasis.
Oncotarget. 10:2644–2656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blenkiron C, Goldstein LD, Thorne NP,
Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE,
Green AR, Ellis IO, et al: MicroRNA expression profiling of human
breast cancer identifies new markers of tumor subtype. Genome Biol.
8(R214)2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Conteduca V, Caffo O, Galli L, Maugeri A,
Scarpi E, Maines F, Chiuri VE, Cristian Lolli C, Kinspergher S,
Schepisi G, et al: Association among metabolic syndrome,
inflammation, and survival in prostate cancer. Urol Oncol.
36:240.e1–.e11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Garbers C and Rose-John S: Dissecting
interleukin-6 classic-and trans-signaling in inflammation and
cancer. Methods Mol Biol. 1725:127–140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim G, Ouzounova M, Quraishi AA, Davis A,
Tawakkol N, Clouthier SG, Malik F, Paulson AK, D'Angelo RC, Korkaya
S, et al: SOCS3-mediated regulation of inflammatory cytokines in
PTEN and p53 inactivated triple negative breast cancer model.
Oncogene. 34:671–680. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Klepsch O, Namer LS, Köhler N, Kaempfer R,
Dittrich A and Schaper F: Intragenic regulation of SOCS3 isoforms.
Cell Commun Signal. 17(70)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li H, Zhang B, Ding M, Lu S, Zhou H, Sun
D, Wu G and Gan X: C1QTNF1-AS1 regulates the occurrence and
development of hepatocellular carcinoma by regulating
miR-221-3p/SOCS3. Hepatol Int. 13:277–292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Singh S, Chouhan S, Mohammad N and Bhat
MK: Resistin causes G1 arrest in colon cancer cells through
upregulation of SOCS3. FEBS Lett. 591:1371–1382. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li MZ, Lai DH, Zhao HB, Chen Z, Huang QX
and Situ J: SOCS3 overexpression enhances ADM resistance in bladder
cancer T24 cells. Eur Rev Med Pharmacol Sci. 21:3005–3011.
2017.PubMed/NCBI
|
|
30
|
Muhammad N, Bhattacharya S, Steele R and
Ray RB: Anti-miR-203 suppresses ER-positive breast cancer growth
and stemness by targeting SOCS3. Oncotarget. 7:58595–58605.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Christopher AF, Gupta M and Bansal P:
Micronome revealed miR-19a/b as key regulator of SOCS3 during
cancer related inflammation of oral squamous cell carcinoma. Gene.
594:30–40. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zakaria N, Mohd Yusoff N, Zakaria Z,
Widera D and Yahaya BH: Inhibition of NF-κB signaling reduces the
stemness characteristics of lung ccancer stem cells. Front Oncol.
8(166)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang X, Wan Q and Xu TS: Effect of
Grain-moxibustion on IL-6 and STAT 3 in inflammatory
microenvironment of Lewis lung cancer mice. Zhen Ci Yan Jiu.
42:235–239. 2017.PubMed/NCBI(In Chinese).
|
|
34
|
Chen L, Heikkinen L, Wang C, Yang Y, Sun H
and Wong G: Trends in the development of miRNA bioinformatics
tools. Brief Bioinform. 20:1836–1852. 2019.PubMed/NCBI View Article : Google Scholar
|