Introduction
Abdominal aortic aneurysm (AAA) is a complicated and
dangerous cardiovascular disease characterized by high morbidity
and mortality globally (1,2). In developing countries, the incidence
of AAA rises to 3-4% in people aged over 65, and rupture of AAA
causes ~4,500 deaths in the United States (1,2). Most
AAA-related deaths are attributed to AAA rupture (3). Currently, surgery is the only
treatment option for AAA; however, the benefits of surgery are
limited by the aortic diameter (4).
Moreover, AAA pathogenesis during formation and development is yet
to be fully elucidated. Apoptosis of vascular smooth muscle cells
(VSMCs) aggravates AAA progression at the histopathological level
(5). Thus, it is necessary to
explore new therapeutic approaches, such as inhibition of apoptosis
in VSMCs, in order to minimize AAA progression.
Findings of previous studies report that AAA and
atherosclerosis share similar histopathological characteristics
(6,7). Inhibitor of nuclear factor-κB kinase
subunit ε (IKKε) regulates a variety of pathophysiological
processes associated with the occurrence of atherosclerosis, such
as morphological changes and lipid accumulation in the aorta
(8). It is also an important signal
regulator that plays a crucial role in tumorigenesis, inflammation,
metabolic disorders and the reduction-oxidation process (9). AAA is characterized by inflammatory
cell infiltration and apoptosis of VSMCs (5). Therefore, it was inferred that IKKε
plays an important role in AAA development. A previous study
revealed that IKKε expression is significantly increased in
patients with AAA (10). The
results of a another study indicated that the lack of IKKε reduced
AAA formation by reducing apoptosis and inflammation in the
abdominal aorta of mice (11).
However, the mechanism underlying IKKε function remains
unclear.
Autophagy and apoptosis are two key forms of cell
death (12). In the cardiovascular
system, autophagy protects blood vessels from dysfunction (13). However, excessive autophagy leads to
cell death during the pathological state under ER stress (14). Myocardin regulates the apoptosis of
VSMCs by regulating autophagy to promote the occurrence of aortic
aneurysms (15). A previous study
has reported that the targeted knockdown of autophagy-related
protein (ATG)7 or the autophagy inhibitor 3-methyladenine inhibited
caspase activation and reduced apoptosis (16). Furthermore, a recent study
postulated that IKKε is an autophagy-activating gene in breast
cancer (17). Multiple
autophagy-related genes, such as ATG5 and ATG7 are markedly
upregulated in human aortic aneurysm disease (18). The present study investigated the
effects of IKKε on autophagy and apoptosis in a VSMC model of
AAA.
In the present study, loss of IKKε ameliorated
angiotensin II (AngII)-induced cellular AAA model by inhibiting
autophagy and apoptosis in VSMCs. This phenomenon was partially
dependent on activated ERK1/2 signaling.
Materials and methods
Cell line and culture
Mouse thoracic aortic vascular smooth muscle cell
(VSMCs; cat. no. CRL-2797) were obtained from the American Type
Culture Collection and cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% antibiotic-antimycotic solution (100 U/ml penicillin
and 100 µg/ml streptomycin). Cells were incubated in a humidified
incubator at 37˚C with 5% CO2 (Thermo Fisher Scientific,
Inc.). VSMCs were passaged 3-5 times and seeded in six-well plates
at a density of 1.0x104 cells/well. The following day,
cells were treated with AngII (1 µmol/l; Sigma Aldrich; Merck KGaA)
for 24 h.
Transfection
VSMCs at 60% confluence were cultured in six-well
plates and transfected with IKKε small interfering (si)RNA (20
µmol/l; Shanghai GenePharma Co., Ltd.) for 6 h using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
siRNA was used to infect cells for 6 h at 37˚C and cell medium was
replaced with complete culture medium. The following day, cells
were exposed to AngII for 24 h. The sequences were as follows: A
sense, 5'-GCAUACUGAUGACCUGCUATT-3' and antisense,
5'-UAGCAGGUCAUCAGUAUGCTT-3'; b sense, 5'-CCCACAACACGAUUGCCAUTT-3'
and antisense 5'-AUGGCAAUCGUGUUGUGGGTT-3'; c sense,
5'-GCAACCUAUGGCUCCUCAUTT-3' and antisense,
5'-AUGAGGAGCCAUAGGUUGCTT-3' and the control sense,
5'-UUCUCCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'.
Western blot analysis
Total proteins of VSMCs were extracted using RIPA
buffer (Beyotime Institute of Biotechnology) and their
concentrations were determined using the BCA protein assay kit
(cat. no. KGP902; Nanjing KeyGen Biotech Co., Ltd.). The proteins
(30 µg per lane) were then separated via 10 and 12% SDS-PAGE, and
the resultant bands were transferred onto PVDF membranes
(Sigma-Aldrich; Merck KGaA). The membranes were blocked with 5%
skimmed milk powder for 1 h at room temperature and subsequently
incubated with primary antibodies at 4˚C overnight. The antibodies
included anti-IKKε (1:1,000; cat. no. 3416S; Cell Signaling
Technology, Inc.), anti-LC3B (1:2,000; cat. no. ab51520; Abcam),
anti-P62 (1:1,000; cat. no. 23214S; Cell Signaling Technology,
Inc.), anti-ATG7 (1:1,000; cat. no. 8558S; Cell Signaling
Technology, Inc.), anti-Bax (1:1,000; cat. no. 2772S; Cell
Signaling Technology, Inc.), anti-cleaved (c)-caspase-3 (1:1,000;
cat. no. 9661S; Cell Signaling Technology, Inc.), anti-caspase-9
(1:1,000; cat. no. 9508T; Cell Signaling Technology, Inc.),
anti-p38 (1:1,000; cat. no. 9212S; Cell Signaling Technology,
Inc.), anti-phosphorylated (p)-p38 (1:1,000; cat. no. 4511S; Cell
Signaling Technology, Inc.), anti-MEK1/2 (1:1,000; cat. no. 9122S;
Cell Signaling Technology, Inc.), anti-p-MEK1/2 (1:1,000; cat. no.
9154S; Cell Signaling Technology, Inc.), anti-ERK1/2 (1:1,000; cat.
no. 4695S; Cell Signaling Technology, Inc.), anti-p-ERK1/2
(1:1,000; cat. no. 4370S; Cell Signaling Technology, Inc.) and
anti-GAPDH antibody (1:5,000; cat. no. 8884S; Cell Signaling
Technology, Inc.). The PVDF membranes were then incubated with
secondary antibodies, including HRP-anti-mouse IgG (1:5,000; cat.
no. HRP-60004; ProteinTech Group, Inc.) and HRP-anti-Rabbit IgG
(1:5,000; cat. no. 7074P2; Cell Signaling Technology, Inc.) for 1 h
at room temperature. The protein bands were subsequently visualized
using chemiluminescent HRP Substrate (cat. no. P90720;
MilliporeSigma) and captured on a Hyperfilm (Amersham; Cytiva), and
the results were analyzed using ImageJ software (version 1.8.0;
National Institutes of Health) for semi-quantitation of the mean
gray value of each blot.
Flow cytometry analysis
Apoptosis was measured using the Annexin-V/PI double
staining method. VSMCs were seeded in six-well plates at a density
of 1.0x104 cells/well and treated with AngII for 24 h,
washed with PBS, and subsequently trypsinized to obtain a
single-cell suspension. The Annexin V-FITC Apoptosis Detection Kit
(Nanjing KeyGen Biotech Co., Ltd.) was used to stain the cells
according to the manufacturer's protocol. Apoptotic cells were
subsequently evaluated using a flow cytometer (BD FACSCalibur™; BD
Biosciences). The results were analyzed using ModFit LT version 5.0
(Verity Software House, Inc.).
Transmission electron microscopy
VSMCs were centrifuged to form clusters at 67 x g
and room temperature for 5 min, and subsequently fixed with 2.5%
glutaraldehyde at 4˚C overnight. Cells were further fixed in 1%
buffered osmium tetroxide and dehydrated in graded ethanol. Cluster
sections (60-70 nm) were double stained with 2% uranyl acetate for
2 h at room temperature and lead citrate for 5 min at room
temperature. The samples were examined under a JEOL JEM-1010
transmission electron microscope (JEOL, Ltd.). The autophagosomes
were manually counted.
Mitochondrial membrane potential
analysis
The JC-1 probe (Beyotime Institute of Biotechnology)
was employed to measure mitochondrial depolarization in VSMCs. The
cells were first cultured with AngII in six-well plates at a
density of 1.0x104 cells/well for 24 h and subsequently
incubated with an equal volume of JC-1 staining solution (5 pg/ml)
at 37˚C for 20 min. Cells were then rinsed twice with PBS, followed
by monitoring of the mitochondrial membrane potentials by
determining the relative amounts of dual emissions from
mitochondrial JC-1 monomers and aggregates. Mitochondrial membrane
potentials were monitored using an Olympus fluorescent microscope
(Olympus Corporation) under Argon-ion 488 nm laser excitation. An
increase in the ratio of green/red (488/594 nm) fluorescence
intensity indicated mitochondrial depolarization. The results were
analyzed using ImageJ software (version 1.8.0; National Institutes
of Health).
Autophagic flux analysis
VSMCs were first treated with bafilomycin A1
(Baf-A1; 400 nM; Selleck Chemicals) for 4 h prior to AngII
treatment as previously described, to block autophagosome-lysosome
fusion. Autophagic flux was measured via western blot analysis, as
previously described.
Statistical analysis
Data are presented as the mean ± standard error.
Differences between groups were determined using ANOVA followed by
Tukey's post hoc test. Comparisons between two groups were
performed using a paired Student's t-test. All statistical analyses
were performed using GraphPad Prism 6.0 software (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
IKKε knockdown attenuates
AngII-induced apoptosis in VSMCs
IKKε deficiency has been indicated to attenuate AAA
formation in mice by inhibiting apoptosis (11). In the present study, VSMCs were
transfected with siRNA to knock down IKKε, and were subsequently
exposed to AngII for 24 h to elucidate the role of IKKε in
apoptosis in vitro. The sequences of IKKε siRNA with the
highest knockdown efficiency were selected (Fig. S1A and B). IKKε knockdown efficiency in VSMCs was
40-60% compared with the control group. (Fig. 1A and B). Western blot analysis revealed that
IKKε knockdown reduced the expression of caspase-9, c-caspase-3 and
Bax in VSMCs after induction with AngII (Fig. 1A and B). The analysis of the apoptosis of VSMCs
detected by flow cytometry revealed that IKKε knockdown reduced the
apoptosis of VSMCs following AngII treatment (Fig. 1C and D). Furthermore, JC-1 staining revealed
that IKKε deficiency reduced mitochondrial damage after AngII
treatment, which occurs at an early stage of apoptosis (19) (Fig.
1E and F). Collectively, the
results of the present study demonstrated that IKKε knockdown
reduced apoptosis of VSMCs at an early stage with AngII
treatment.
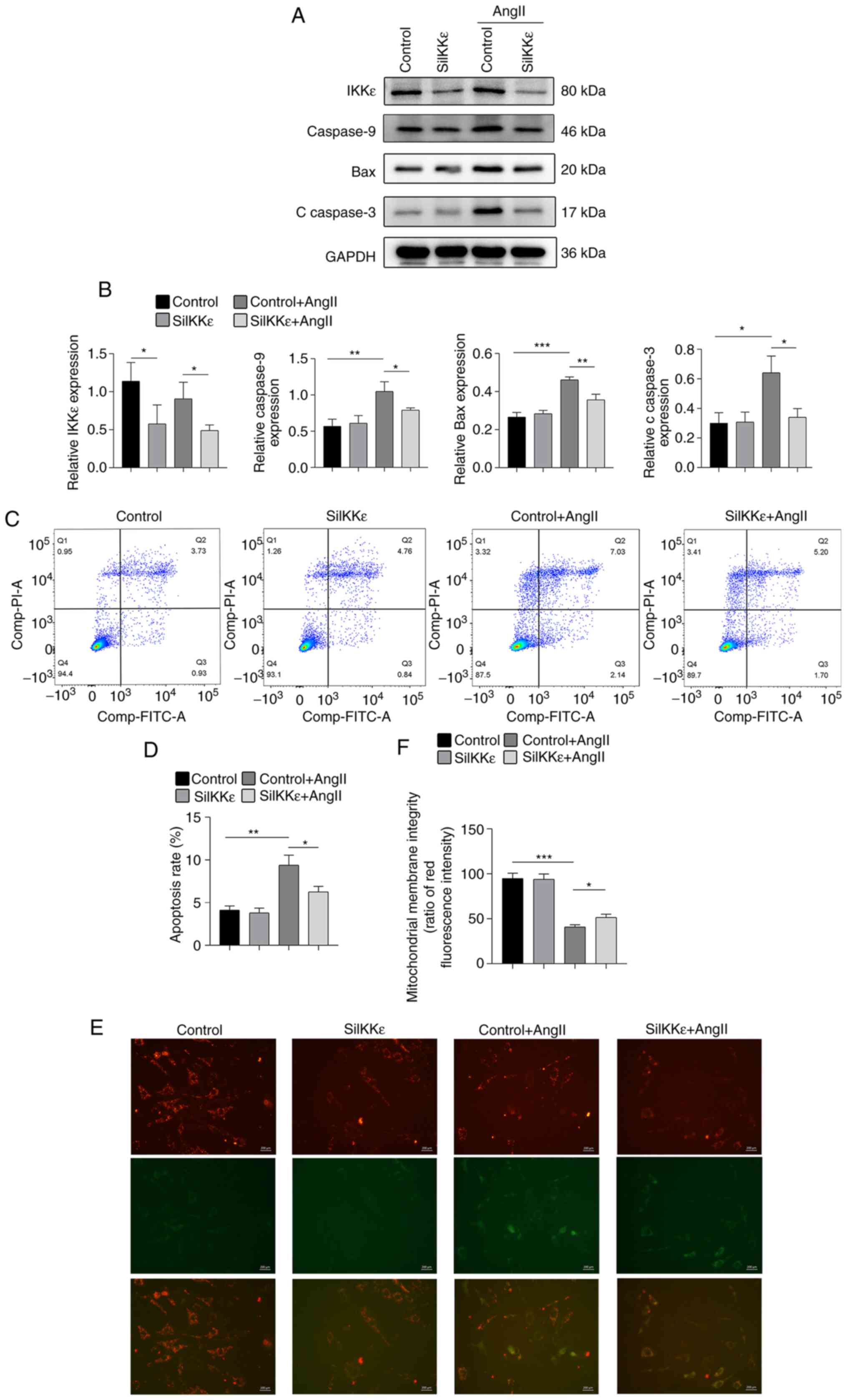 | Figure 1Knockdown of IKKε attenuates
AngII-induced apoptosis in VSMCs. (A) Bax, caspase-9, c-caspase-3
and IKKε expression levels were detected via western blot analysis
in AngII-induced VSMCs following siRNA transfection for 24 h (n=4-5
per experimental group). (B) Quantitative results of expression
levels of Bax, caspase-9 and c-caspase-3 (n=4-5 per experimental
group). (C and D) Quantitative flow cytometry results of the
apoptosis rate of VCMCs exposed to AngII for 24 h (n=4-5 per
experimental group). (E) Representative images and (F) quantitative
results of JC-1 staining in VSMCs exposed to AngII for 24 h (n=6
per experimental group; magnification, x400). Scale bar, 200 µm.
Each experiment was repeated three times. *P<0.05;
**P<0.01; ***P<0.001. IKKε, inhibitor
of nuclear factor-κB kinase subunit ε; AngII, angiotensin II;
VSMCs, vascular smooth muscle cells; si, small interfering; c,
cleaved. |
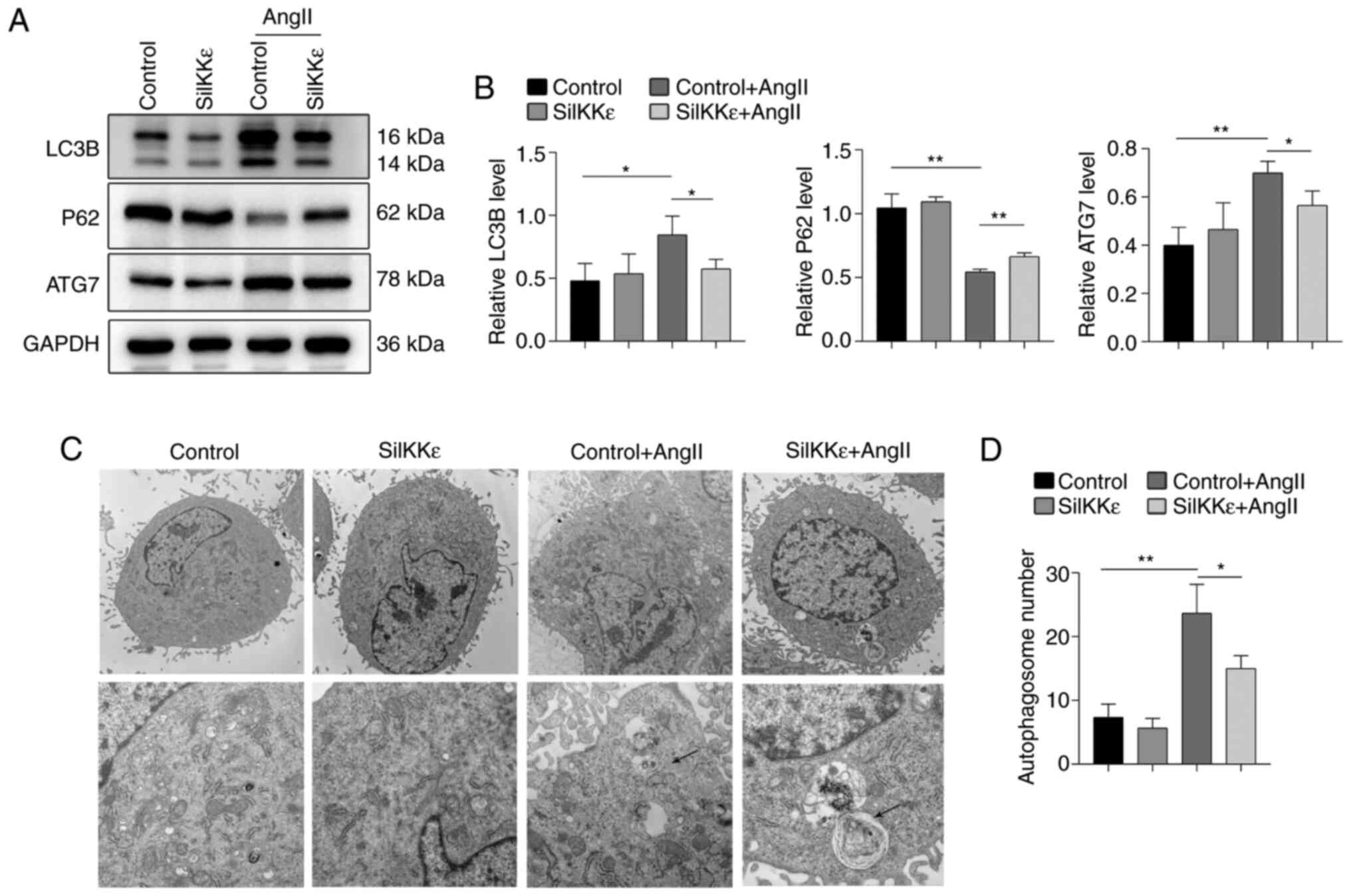
IKKε knockdown attenuates
AngII-induced excessive autophagy in VSMCs
Autophagy in VSMCs was further investigated to
verify the role of IKKε on apoptosis in VSMCs. Western blot
analysis of LC3B, ATG7 and P62 revealed that IKKε knockdown reduced
autophagy in AngII-induced VSMCs (Fig.
2A and B). Transmission
electron microscopy further revealed that autophagic vacuoles were
significantly reduced in AngII-induced VSMCs in the SiIKKε group
compared with the control group (Fig.
2C and D).
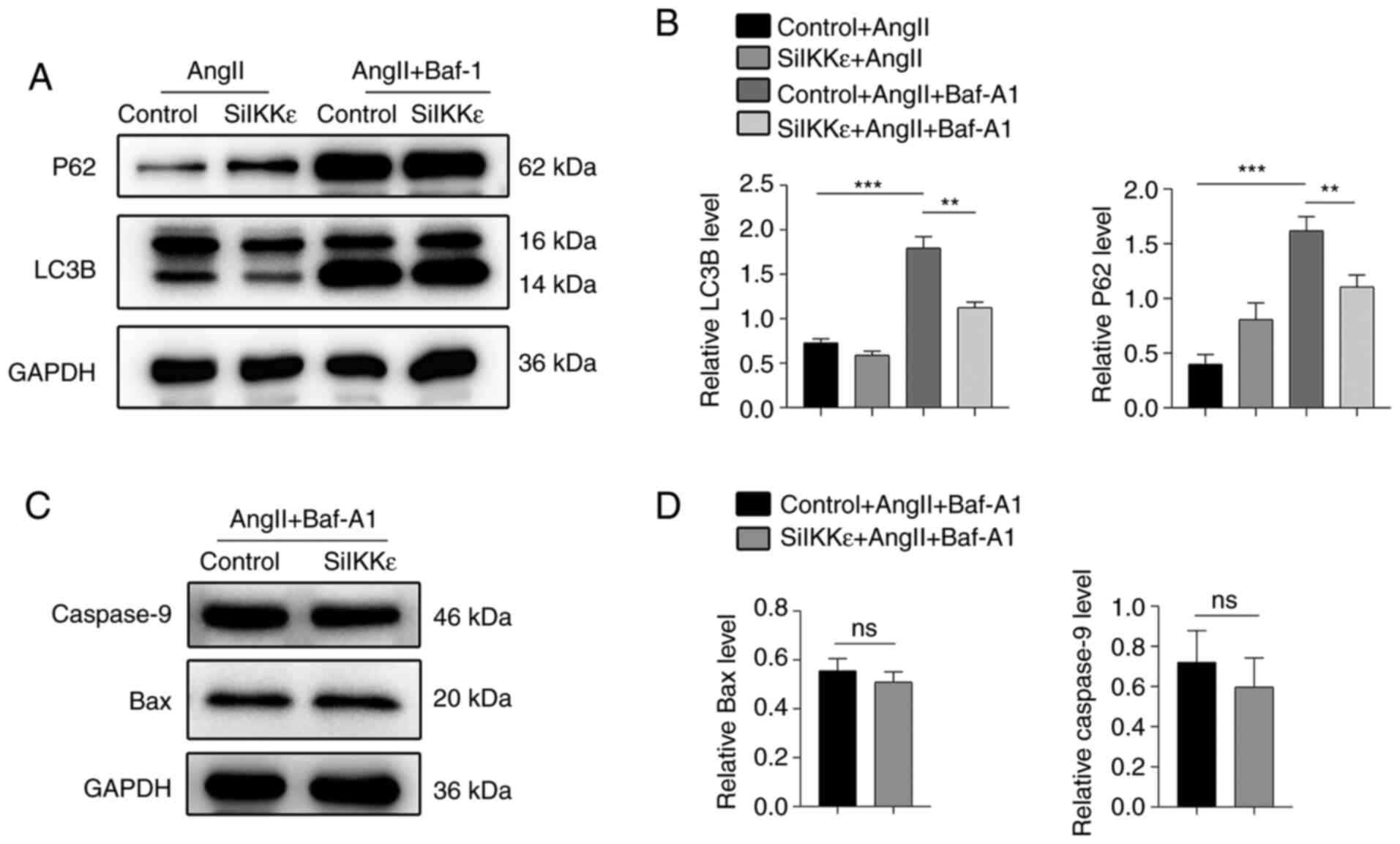
IKKε plays an important role in the
association of autophagy and apoptosis
To detect the autophagy flow in vitro, Baf-A1
was used to block the autophagy flow in AngII-induced VSMCs. The
expression levels of LC3B and P62 in the VSMCs were significantly
increased following autophagy blocking with Baf-A1 and AngII
compared with the AngII group, indicating that the autophagic
process was active in the AngII-induced VSMC autophagosomes
(20) (Fig. 3A and B). A smaller decrease in the expression of
LC3B and P62 was demonstrated in the IKKε deficiency with Baf-A1 +
AngII group compared with the control + AngII + Baf-A1 group,
indicating that IKKε potentially played a role in autophagy
(Fig. 3A and B). Notably, there was no significant
difference in the expression levels of caspase-9 and Bax in the
SiIKKε + AngII + Baf-A1 group compared with the control + AngII +
Baf-A1 group (Fig. 3C and D). Thus, it was hypothesized that IKKε
increased apoptosis by promoting autophagy in VSMCs.
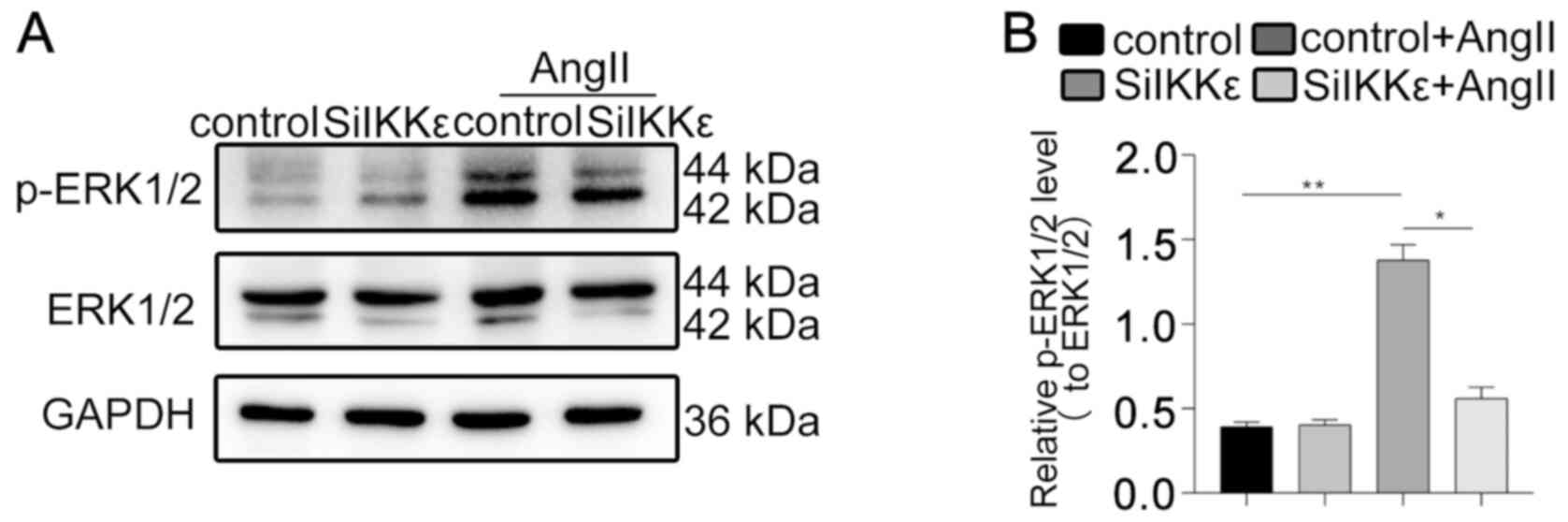
IKKε regulates autophagy and apoptosis
via the ERK1/2 signaling pathway
The activation of the ERK1/2 pathway in VSMCs was
assessed to further investigate the mechanism underlying IKKε in
autophagy regulation in vitro. The level of p-ERK1/2 was
decreased in SiIKKε cells exposed to AngII compared with control
cells exposed to AngII (Fig. 4A and
B). The phosphorylation levels of
MEK1/2 and p38 were investigated, and no significant difference was
demonstrated in the phosphorylation of MEK1/2 and p38 in VSMCs with
AngII treatment (Fig. S2A and
B). These results indicated that
IKKε knockdown reduced autophagy and apoptosis by inhibiting the
ERK1/2 signaling pathway.
Discussion
AAA is a disease associated with serious
complications such as the rupture of aneurysm and death, and is
more severe than common heart disease (21). However, the specific pathogenesis of
AAA is yet to be elucidated. The results of the present study
demonstrated the important role of IKKε in autophagy, apoptosis and
AAA development. Knockdown of IKKε attenuated AngII-induced
apoptosis and excessive autophagy in VSMCs. IKKε also increased
apoptosis by promoting the autophagy of VSMCs. Moreover, IKKε
knockdown reduced autophagy and apoptosis by inhibiting the ERK1/2
signaling pathway.
Apoptosis of VSMCs is a key biomarker of AAA
formation (5). The results of a
previous study demonstrated that IKKε played an important role in
regulating apoptosis of VSMCs in mice (11). In the present study, the in
vitro results were consistent with previous findings (8). It has been previously revealed that
when the membrane potential of mitochondria decreases, the
permeability of the mitochondrial membrane increases and the
proapoptotic factors are released into the cytoplasm (22). In the present study, IKKε deficiency
reduced mitochondrial damage and apoptosis in vitro,
indicating that IKKε regulated the apoptosis of VSMCs via the
mitochondrial apoptosis pathway.
Activated autophagy is generally considered a cell
protection mechanism due to the promotion of cell survival
(23). However, excessive
activation of autophagy has been indicated to result in autophagic
cell death (24). Functional
autophagy is essential for cardiac homeostasis (25); however uncontrolled autophagy
induction in ischemia-reperfusion injury response resulted in
excessive cardiomyocyte apoptosis, thereby aggravating the injury
(26). VSMCs may exhibit excessive
autophagy leading to cell death when exposed to severe stimuli
(27). This mechanism serves a role
in the occurrence of a number of vascular diseases, such as
atherosclerosis. A previous study postulated that suppressing
autophagy inhibited AAA development (28). Although previous findings have
suggested that the IKK family plays an important role in autophagy
(29), few studies have reported
the role of IKKε in autophagy. A previous study demonstrated that
IKKε played a protective role against cardiovascular diseases
(30). Thus, inhibition of the
autophagy of VSMCs may provide a novel treatment against AAA.
The autophagic flow plays an important role in
autophagy (31). In the present
study, autophagy was blocked with Baf-A1 to prevent the downstream
events of autophagy by inhibiting lysosomal degradation (32). IKKε knockdown suppressed the
synthesis of autophagy initiation-related autophagy vesicles and
the accumulation of autophagosomes; therefore, it was hypothesized
that IKKε played an important role in the early stages of
autophagic flow in VSMCs.
Apoptosis and autophagy are important biological
activities that contribute to the stability of the internal
environment, thus promoting the organism's survival (33). Maintaining a balance between
autophagy and apoptosis is critical for cell development (33). Abnormal induction of the autophagic
flux has been indicated to promote apoptotic neuronal cell death
(34). Autophagy has been
demonstrated to play a protective role in attenuating AngII-induced
oxidative stress and inflammation (35). However, autophagy has been indicated
to contribute to apoptosis in cardiac microvascular endothelial
cells (36). In the present study,
treatment with Baf-A1 inhibited the expression levels of
AngII-induced Bax and Caspase-9 in VSMCs. Therefore, it was
hypothesized that IKKε deficiency reduced the apoptosis of VSMCs by
decreasing autophagy to alleviate AAA formation.
The results of a previous study indicated that
activation of ERK signaling is detected in a number of tumor cells,
such as melanomas and gastric carcinoma (37). Furthermore, it has been reported
that the phosphorylation of ERK induced during tumor development is
dependent on IKKε (38). Continuous
activation of the MEK/ERK signaling pathway directly induced
autophagy (39) and induced
apoptosis in tumor cells (40,41).
The findings of the present study revealed that the ERK1/2 pathway
was activated in parallel with AngII-induced apoptosis in VSMCs,
consistent with the findings generated from a previous study
(11). The ERK1/2 pathway was
possibly associated with AngII-induced autophagy. Furthermore,
there was no significant difference in the phosphorylation of
MEK1/2 and p38 in VSMCs after AngII treatment. Thus, the present
study revealed that IKKε knockdown inhibited the phosphorylation of
ERK1/2 in VSMCs.
In conclusion, the present study demonstrated that
IKKε induced excessive autophagy in VSMCs, leading to increased
apoptosis and potentially AAA formation. Therefore, inhibition of
IKKε has the potential to act as a therapeutic target, to inhibit
autophagy and apoptosis to reduce AAA occurrence.
Nonetheless, the present study was limited by
several factors. The VSMC cell line used differs from primary
cells, and the levels of IKKε were not increased by AngII treatment
in the present study. The findings of the present study should be
extended by analyzing both upstream and downstream mechanisms of
IKKε and the ERK1/2 signaling pathway. Future studies should
therefore focus on these shortcomings to provide more comprehensive
results.
Supplementary Material
Transfection efficiency of three
different IKKε siRNA sequences. (A) Representative western blot and
(B) quantitative results of IKKε expression in vascular smooth
muscle cells (n=4-5 per group). Each experiment was repeated three
times. *P<0.05. IKKε, inhibitor of nuclear factor-κB
kinase subunit ε; si, small interfering; ns, not significant.
Knockdown of IKKε does not alter the
phosphorylation levels of MEK1/2 and p38. (A) Representative
western blots and (B) quantitative results of phosphorylated and
total protein levels of MEK1/2 and p38 in vascular smooth muscle
cells in the control, SiIKKε, control + AngII and SiIKKε + AngII
group (n=4-5 per group). Each experiment was repeated three times.
IKKε, inhibitor of nuclear factor-κB kinase subunit ε; si, small
interfering; AngII, angiotensin II; p, phosphorylated; ns, not
significant.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant. No. 81870193), the Project of
Invigorating Health Care through Science, Technology and Education,
Jiangsu Provincial Key Medical Discipline (grant. no. ZDXKA2016021)
and the Young Program of National Natural Science Foundation of
China (grant. no. 81700415).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GC, YC, YX and YY performed the experiments. GC, YL
and HC conducted data analysis. YX, WC and XC designed the
experiments and aided in data analyses. WC and XC confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brangsch J, Reimann C, Collettini F,
Buchert R, Botnar RM and Makowski MR: Molecular imaging of
abdominal aortic aneurysms. Trends Mol Med. 23:150–164.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Aggarwal S, Qamar A, Sharma V and Sharma
A: Abdominal aortic aneurysm: A comprehensive review. Exp Clin
Cardiol. 16:11–15. 2011.PubMed/NCBI
|
|
3
|
Laine MT, Laukontaus SJ, Sund R, Aho PS,
Kantonen I, Albäck A and Venermo M: A population-based study of
abdominal aortic aneurysm treatment in finland 2000 to 2014.
Circulation. 136:1726–1734. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Keisler B and Carter C: Abdominal aortic
aneurysm. Am Fam Physician. 91:538–543. 2015.PubMed/NCBI
|
|
5
|
Riches K, Angelini TG, Mudhar GS, Kaye J,
Clark E, Bailey MA, Sohrabi S, Korossis S, Walker PG, Scott DJ and
Porter KE: Exploring smooth muscle phenotype and function in a
bioreactor model of abdominal aortic aneurysm. J Transl Med.
11(208)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Peshkova IO, Schaefer G and Koltsova EK:
Atherosclerosis and aortic aneurysm-is inflammation a common
denominator? FEBS J. 283:1636–1652. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stegbauer J, Thatcher SE, Yang G,
Bottermann K, Rump LC, Daugherty A and Cassis LA: Mas receptor
deficiency augments angiotensin II-induced atherosclerosis and
aortic aneurysm ruptures in hypercholesterolemic male mice. J Vasc
Surg. 70:1658–1668.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cao C, Zhu Y, Chen W, Li L, Qi Y, Wang X,
Zhao Y, Wan X and Chen X: IIKKε knockout prevents high fat diet
induced arterial atherosclerosis and NF-κB signaling in mice. PLoS
One. 8(e64930)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang J, Tian M, Xia Z and Feng P: Roles
of IκB kinase ε in the innate immune defense and beyond. Virol Sin.
31:457–465. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang L, Wang L, Chen W, Xu Y, Wang L,
Iskandar R, Wang Y and Chen X: The expression of inhibitor of
nuclear factor kappa-B kinase epsilon (IKKe) in human aortic
aneurysm. Folia Morphol (Warsz). 76:372–378. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chai H, Tao Z, Qi Y, Qi H, Chen W, Xu Y,
Zhang L, Chen H and Chen X: IKK epsilon deficiency attenuates
angiotensin II-induced abdominal aortic aneurysm formation in mice
by inhibiting inflammation, oxidative stress, and apoptosis. Oxid
Med Cell Longev. 2020(3602824)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Simon HU: Autophagy in myocardial
differentiation and cardiac development. Circ Res. 110:524–525.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song S, Tan J, Miao Y, Li M and Zhang Q:
Crosstalk of autophagy and apoptosis: Involvement of the dual role
of autophagy under ER stress. J Cell Physiol. 232:2977–2984.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang J, Wang T, Wright AC, Yang J, Zhou
S, Li L, Yang J, Small A and Parmacek MS: Myocardin is required for
maintenance of vascular and visceral smooth muscle homeostasis
during postnatal development. Proc Natl Acad Sci USA.
112:4447–4452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yu L, Alva A, Su H, Dutt P, Freundt E,
Welsh S, Baehrecke EH and Lenardo MJ: Regulation of an ATG7-beclin
1 program of autophagic cell death by caspase-8. Science.
304:1500–1502. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leonardi M, Perna E, Tronnolone S,
Colecchia D and Chiariello M: Activated kinase screening identifies
the IKBKE oncogene as a positive regulator of autophagy. Autophagy.
15:312–326. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramadan A, Al-Omran M and Verma S: The
putative role of autophagy in the pathogenesis of abdominal aortic
aneurysms. Atherosclerosis. 257:288–296. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Petit PX, Lecoeur H, Zorn E, Dauguet C,
Mignotte B and Gougeon ML: Alterations in mitochondrial structure
and function are early events of dexamethasone-induced thymocyte
apoptosis. J Cell Biol. 130:157–167. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Daye D and Walker TG: Complications of
endovascular aneurysm repair of the thoracic and abdominal aorta:
Evaluation and management. Cardiovasc Diagn Ther. 8 (Suppl
1):S138–S156. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gottlieb RA: Mitochondrial signaling in
apoptosis: Mitochondrial daggers to the breaking heart. Basic Res
Cardiol. 98:242–249. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lopez de Figueroa P, Lotz MK, Blanco FJ
and Carames B: Autophagy activation and protection from
mitochondrial dysfunction in human chondrocytes. Arthritis
Rheumatol. 67:966–976. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kosacka J, Nowicki M, Paeschke S, Baum P,
Bluher M and Kloting N: Up-regulated autophagy: As a protective
factor in adipose tissue of WOKW rats with metabolic syndrome.
Diabetol Metab Syndr. 10(13)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xing H, Peng M, Li Z, Chen J, Zhang H and
Teng X: Ammonia inhalation-mediated mir-202-5p leads to cardiac
autophagy through PTEN/AKT/mTOR pathway. Chemosphere. 235:858–866.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang W, Duan Q, Zhu X, Tao K and Dong A:
Follistatin-Like 1 attenuates ischemia/reperfusion injury in
cardiomyocytes via regulation of autophagy. Biomed Res Int.
2019(9537382)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang YY, Shi YN, Zhu N, Wang W, Deng CF,
Xie XJ, Liao DF and Qin L: Autophagy: A killer or guardian of
vascular smooth muscle cells. J Drug Target. 28:449–455.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Z, Guo J, Han X, Xue M, Wang W, Mi L,
Sheng Y, Ma C, Wu J and Wu X: Metformin represses the
pathophysiology of AAA by suppressing the activation of
PI3K/AKT/mTOR/autophagy pathway in ApoE-/-
mice. Cell Biosci. 9(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Criollo A, Senovilla L, Authier H, Maiuri
MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S,
et al: The IKK complex contributes to the induction of autophagy.
EMBO J. 29:619–631. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cao C, Zhu Y, Chen W, Li L, Qi Y, Wang X,
Zhao Y, Wan X and Chen X: IKKε knockout prevents high fat diet
induced arterial atherosclerosis and NF-κB signaling in Mice. PLoS
One. 8(e64930)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu L, Duan Q, Gao D, Wang Y, Xue S, Li W
and Lei M: Zearalenone blocks autophagy flow and induces cell
apoptosis during embryo implantation in gilts. Toxicol Sci.
175:126–139. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheng Z, Zhang M, Hu J, Lin J, Feng X,
Wang S, Wang T, Gao E, Wang H and Sun D: Mst1 knockout enhances
cardiomyocyte autophagic flux to alleviate angiotensin II-induced
cardiac injury independent of angiotensin II receptors. J Mol Cell
Cardiol. 125:117–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu J, Liu W and Yang H: Balancing
apoptosis and autophagy for Parkinson's disease therapy: Targeting
BCL-2. ACS Chem Neurosci. 10:792–802. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chung Y, Lee J, Jung S, Lee Y, Cho JW and
Oh YJ: Dysregulated autophagy contributes to caspase-dependent
neuronal apoptosis. Cell Death Dis. 9(1189)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lu Y, Li S, Wu H, Bian Z, Xu J, Gu C, Chen
X and Yang D: Beneficial effects of astragaloside IV against
angiotensin II-induced mitochondrial dysfunction in rat vascular
smooth muscle cells. Int J Mol Med. 36:1223–1232. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang R, Yang Q, Wang X, Wang W, Li J, Zhu
J, Liu X, Liu J and Du J: FoxO3α-mediated autophagy contributes to
apoptosis in cardiac microvascular endothelial cells under hypoxia.
Microvasc Res. 104:23–31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu YL, Lai F, Wilmott JS, Yan XG, Liu XY,
Luan Q, Guo ST, Jiang CC, Tseng HY, Scolyer RA, et al: Noxa
upregulation by oncogenic activation of MEK/ERK through CREB
promotes autophagy in human melanoma cells. Oncotarget.
5:11237–11251. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Goktuna SI, Shostak K, Chau TL, Heukamp
LC, Hennuy B, Duong HQ, Ladang A, Close P, Klevernic I, Olivier F,
et al: The prosurvival IKK-related kinase IKKε integrates LPS and
IL17A signaling cascades to promote wnt-dependent tumor development
in the intestine. Cancer Res. 76:2587–2599. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zucchini-Pascal N, de Sousa G and Rahmani
R: Lindane and cell death: At the crossroads between apoptosis,
necrosis and autophagy. Toxicology. 256:32–41. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cagnol S, Van Obberghen-Schilling EB and
Chambard JC: Prolonged activation of ERK1,2 induces
FADD-independent caspase 8 activation and cell death. Apoptosis.
11:337–346. 2006.PubMed/NCBI View Article : Google Scholar
|