Introduction
Osteoimmunology, a field that focuses on the
interaction between the skeletal and immune systems, proposes that
an imbalance between pro- and anti-inflammatory cytokines may serve
as the mechanism underlying osteogenesis (1). A wide repertoire of cytokines secreted
by T cells, some pre-osteogenic and some antiosteogenic, are
closely associated with bone metabolism (1). Interleukin (IL)-17 is a family of
cytokines released by CD4+ T cells (2). Numerous studies have demonstrated the
role of IL-17 in bone diseases, including spondyloarthropathies,
rheumatoid arthritis and ankylosing spondylitis (3-5).
IL-17 has also been reported to promote osteoblast differentiation,
bone regeneration and remodeling in mice (6). The IL-17 family comprises six members,
from IL-17A to IL-17F. IL-17A and IL-17F are dominant
proinflammatory cytokines, exhibiting the highest degree of
sequence homology (7,8). IL-17A enhances bone regeneration and
induces the osteogenic differentiation of human mesenchymal stem
cells (9,10). IL-17F family members have been
identified as important regulators of bone regeneration during the
early phase of fracture repair (11). IL-17F treatment promotes MC3T3-E1
cell differentiation and maturation (12,13),
and increases the expression of osteoblast bone markers (11). Therefore, the aforementioned studies
suggested a novel association between T-cells and osteoblast
biology, highlighting IL-17F as a key element. In the present
study, osteoblasts derived from newborn rats were employed to
examine the potential role of IL-17F in osteoblastic osteogenesis,
including proliferation, differentiation and mineralization
activity in vitro.
The activation of bone morphogenetic protein-2
(BMP-2) signaling is a critical regulator of osteogenesis (14). BMP-2 is one of the most potent
cytokines that promotes mesenchymal cell differentiation into
osteoblasts in vitro and induces bone formation in
vivo (15,16). Noggin, an extracellular BMP
antagonist, limits BMP-2 action and is induced by BMP-2(17). The imbalance between BMP-2 and
Noggin can cause abnormal bone metabolism (18). Runt-related transcription factor-2
(Runx2) and Osterix, essential transcription factors for
osteogenesis, are BMP-2-regulated targets (19). It was hypothesized that IL-17F
influences the aforementioned factors, thus affecting osteogenesis.
Therefore, in the present study, the expression levels of BMP-2,
Runx2, Osterix and Noggin were detected following IL-17F
treatment.
IL-17 receptor (IL-17R) is expressed in almost every
cell type, acting on diverse tissues throughout the body (20). IL-17R consists of five subunits,
from IL-17RA to IL-17RE (21,22).
IL-17A and IL-17F exist as homodimers or heterodimers to bind
IL-17RA and IL-17RC receptor complexes, activating downstream IL-17
receptor intracellular signaling (20). IL-17F signal transduction has been
observed in non-osteoblasts, such as immune cells, epithelial cell,
astrocytes and fibroblasts (20,23).
The ligand-receptor interaction mediates tumor necrosis factor
receptor-associated factor 6 ubiquitination, resulting in the
phosphorylation of downstream kinases, including ERK1/2, which is a
member of the MAPK family, ultimately inducing IL-17F target gene
expression (24). MAPK/ERK1/2
serves a crucial role in numerous cellular responses, including
cell proliferation, differentiation and survival (25,26).
The present study aimed to determine whether IL-17F affected
osteoblast osteogenesis via the MAPK/ERK1/2 signaling pathway and
to determine which type of IL-17R was involved in the process.
Materials and methods
Osteoblast isolation, culture and
identification
All animal experimental protocols were approved by
the Medical Ethics Committee of Jinan Central Hospital Affiliated
to Shandong University, Shandong, China (approval no.
GG2016-006-02). Calvarias were obtained from 8 Wistar rats (age,
<24 h; weight, 5-8 g; 4 male and 4 female) procured from the
Laboratory Animal Center of Shandong University, Shandong, China
following sacrifice by cervical dislocation. Osteoblasts were
isolated using trypsin (Jinan Fowler Biotechnology Co., Ltd.), type
II collagenase (Suzhou BioTOP Technical Service Co., Ltd.) and the
improved tissue-culture method (27). Osteoblasts were incubated in 5%
CO2 at 37˚C with 100% relative humidity. Osteoblasts
were purified by the differential adhesion method (28). Cell morphology and proliferation
were assessed using an inverted phase contrast microscope.
Osteoblasts were identified by alkaline phosphatase (ALP) staining
using a cALP stain kit (Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's protocol. Briefly,
cells were fixed for 3 min at 25˚C and rinsed with distilled water.
Following this, matrix liquid (provided in the cALP stain kit) was
added and cells were kept in the dark at 37˚C for 15 min. Cells
were then stained with staining reagent for 5 min at 25 ˚C, rinsed
with distilled water for 30 sec at 25˚C, re-stained 30 sec at 25˚C
and rinsed with distilled water for 30 sec at 25˚C, in accordance
with kit instructions. Images were captured at a magnification of
x200 under a light microscope. After staining, 100 osteoblasts and
all ALP-positive-osteoblasts was counted. The ALP-positive rate was
then calculated by assessing ALP-positive-osteoblasts/100
osteoblasts. Purified osteoblasts (4th generation) were used for
subsequent experiments.
Prior to IL-17F treatment, osteoblasts were seeded
into a 96-well plate at 4x104/well for the MTT analysis
and ALP activity assay, or into a 6-well plate at
2x105/well for RT-qPCR and western blotting at 37˚C for
24 h. Subsequently, cells were serum starved at 37˚C for 24 h in
serum-free DMEM (Jinan Fowler Biotechnology Co., Ltd.) for
synchronization. Osteoblasts were randomly divided into the control
and treatment groups. Groups were treated with 0, 1, 10, 20, 50 or
100 ng/ml of IL-17F (R&D Systems, Inc.) in low glucose (1 g/l)
DMEM supplemented with 10% FBS (Jinan Fowler Biotechnology Co.,
Ltd.).
MTT analysis. Osteoblasts (4x104/well) in a
96-well plate were incubated with 0, 1, 10, 20, 50 or 100 ng/ml
IL-17F at 37˚C for 1, 3 or 5 days. Subsequently, 120 µl MTT working
fluid was added to each well at 37˚C for 4 h. DMSO was added to
dissolve the purple formazan. The optical density (OD) of each well
was measured at a wavelength of 570 nm using a microplate reader
and was calculated as follows:
OD=(ODtreated-ODzero)/(ODcontrol-ODzero).
ALP activity assay
Osteoblasts (4x104/well) in a 96-well
plate were treated with 0, 20, 50 or 100 ng/ml IL-17F at 37˚C for
1, 3 or 5 days. Subsequently, the cell supernatant was
centrifugated at 1,600 x g for 10 min at 25˚C and ALP activity was
detected using an ALP activity assay kit (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
protocol. The OD at a wavelength of 520 nm was determined utilizing
a microplate reader and ALP activity was calculated as follows: ALP
activity=(ODtest-ODcontrol)/(ODstandard
value-ODcontrol) x0.02 mg/ml x100 ml.
Alizarin red staining
Osteoblasts (5x105/ml) were inoculated at
37˚C for 24 h in culture dishes prior to treatment with 0 or 100
ng/ml IL-17F. The medium was changed every 3 days. After 10 days,
mineralized nodules were detected. Osteoblasts were rinsed twice
with cold PBS buffer, fixed with 95% ethanol at 25˚C for 10 min and
incubated with 0.1% Alizarin Red dye (Beyotime Biotechnology) at
37˚C for 30 min at pH 4.3. Subsequently, stained cells were
observed under light microscope and a magnification of x100.
Untreated cells were used as controls.
Reverse transcription-quantitative PCR
(RT-qPCR)
Osteoblasts (2x105/well) in a 6-well
plate were treated with 0, 1, 10, 20, 50 or 100 ng/ml IL-17F for 1,
3 or 5 days at 37˚C. Osteoblast RNA was extracted using the
TRIzol® Plus kit (Jinan Fowler Biotechnology Co., Ltd.),
according to the manufacturer's protocol. Total RNA was reverse
transcripted into cDNA using the cDNA Synthesis kit (Jinan Fowler
Biotechnology Co., Ltd.) for 15 min at 37˚C and 5 min at 98˚C.
Following this, the transcription levels were determined using a
RT-PCR with SYBR-Green PCR kit (Jinan Fowler Biotechnology Co.,
Ltd.) according to the manufacturer's protocol utilizing the
LightCycler 2.0 Real-Time PCR system (Roche Molecular Systems,
Inc.). The thermocycling conditions were as follows: 95˚C for 30
sec; followed by 40 cycles of 95˚C for 15 sec, 60˚C for 10 sec and
72˚C for 30 sec. The expression levels of the following genes were
measured via qPCR: IL-17RA, IL-17RC, BMP-2, Noggin, Runx2 and
Osterix. The sequences of the primers used for qPCR are listed in
Table I. Arithmetic formulae
(2-∆∆Cq method) was used to determine relative changes
in gene expression over the internal control, β-actin (29).
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| IL-17A | F:
TCATAAGCGGTGGCGGTTCTC |
| | R:
AGTCATCTTCATCTCCGTGTCCTC |
| IL-17C | F:
CCTAGTGTTGCCTCCACGAGAG |
| | R:
TCCCAGGTCATCATCATTCCACAG |
| BMP-2 | F:
GATGTCACCCCGGCTGTGATGCG |
| | R:
GGGATGTCCTTTACCGTCGTGGCC |
| Noggin | F:
TCGCCCTGGTGGTGGTCCTGG |
| | R:
GCAGCGAGCGCAGCAGCGTCT |
| Runx2 | F:
CATGGCCGGGAATGATGAG |
| | R:
TGTGAAGACCGTTATGGTCAAAGTG |
| Osterix | F:
GGATGGCGTCCTCTCTGCTT |
| | R:
TGTATGGCTTCTTTGTGCCTCCT |
| β-actin | F:
GTGGGCCGCTCTAGGCACCA |
| | R:
CGGTTGGCCTTAGGGTTCAGGGGG |
Western blotting
Osteoblasts were treated with 0, 1, 10, 20, 50 or
100 ng/ml IL-17F for 3 days at 37˚C. Osteoblasts were rinsed with
ice-cold PBS, collected, lysed with lysis buffer (Beyotime
Institute of Biotechnology) and centrifuged at 16,000 x g for 15
min at 4˚C. Total protein was quantified using a BCA kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Protein (20 µg) from each sample were then separated on
10% SDS-PAGE gel and transferred to a PVDF membrane. The membrane
was blocked with 5% BSA in Tris-buffered saline containing 0.1%
Tween-20 for 90 min at 37˚C. Following blocking, the membranes were
incubated with primary antibodies targeted against: IL-17RA (cat.
no. ab180904; 1:1,000; Abcam), ERK1/2 (cat. no. ab17942; 1:1,000;
Abcam), phosphorylated (p)-ERK1/2 (cat. no. ab17942; 1:1,000;
Abcam), BMP-2 (cat. no. ab14933; 1:1,000; Abcam), Runx2(cat. no.
ab23981; 1:500; Abcam), Osterix (cat. no. ab22552; 1:500; Abcam)
and Noggin (cat. no. ab16054; 1:1,000; Abcam) overnight at 4˚C.
Subsequently, the membranes were incubated with appropriate IRDye
800CW-conjugated secondary antibodies (cat. no. ab253031; 1:4,000;
Abcam) for 2 h at 37˚C. Protein bands were visualized using an ECL
kit (Gibco; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Antibody-specific binding intensity was
detected using an Odyssey luminescence apparatus (LI-COR
Biosciences). The obtained bands were quantified using Quantity One
software (version 25.0; Bio-Rad Laboratories, Inc.) and ratios of
the protein of interest and β-actin were calculated to determine
changes in protein levels.
Osteoblasts were cultured for 3 days at 37˚C with
the following treatments: i) No treatment; ii) 100 ng/ml IL-17F
treatment; or iii) 100 ng/ml IL-17F + 10 µM U0126 (Beyotime
Institute of Biotechnology) treatment (specific MAPK/ERK1/2
inhibitor). Subsequently, phosphorylated (p)-ERK1/2, Runx2 and
Osterix protein expression levels were assessed via western
blotting, according to the aforementioned protocol.
Statistical analysis
Experiments were performed in triplicate.
Statistical analyses were conducted using SPSS software (version
19.0; IBM Corp.). Data are presented as the mean ± standard
deviation. Comparisons among multiple groups were analyzed using
two-way (Figs. 2, 3 and 4A-D)
or one-way (Figs. 4F and 5) ANOVA followed by Tukey's (equal
variance assumed) or Dunnett's T3 (equal variance not assumed) post
hoc tests. P<0.05 was considered to indicate a statistically
significant difference.
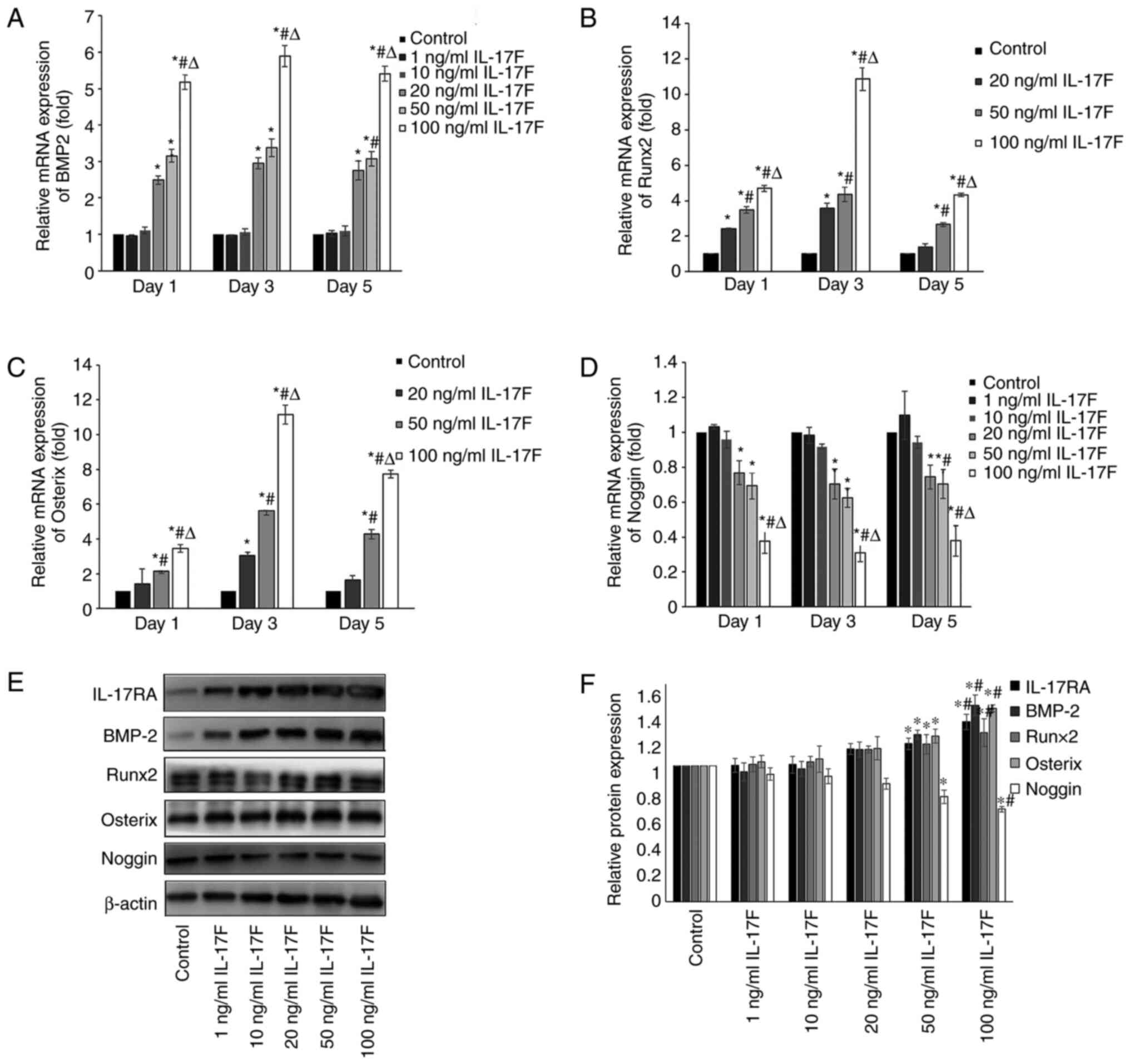 | Figure 4IL-17F upregulates BMP-2, Runx2 and
Osterix expression levels and downregulates Noggin expression
levels. (A) BMP-2, (B) Runx2, (C) Osterix and (D) Noggin mRNA
expression levels in IL-17F-treated osteoblasts. Protein expression
levels of IL-17RA, BMP-2, Runx2, Osterix and Noggin were determined
by (E) western blotting and (F) semi-quantified.
*P<0.05 vs. control; #P<0.05 vs. 20
ng/ml IL-17F; ∆P<0.05 vs. 50 ng/ml IL-17F. IL-17,
interleukin-17; BMP-2, bone morphogenetic protein-2; Runx2,
Runt-related transcription factor-2; IL-17R, interleukin-17
receptor. |
Results
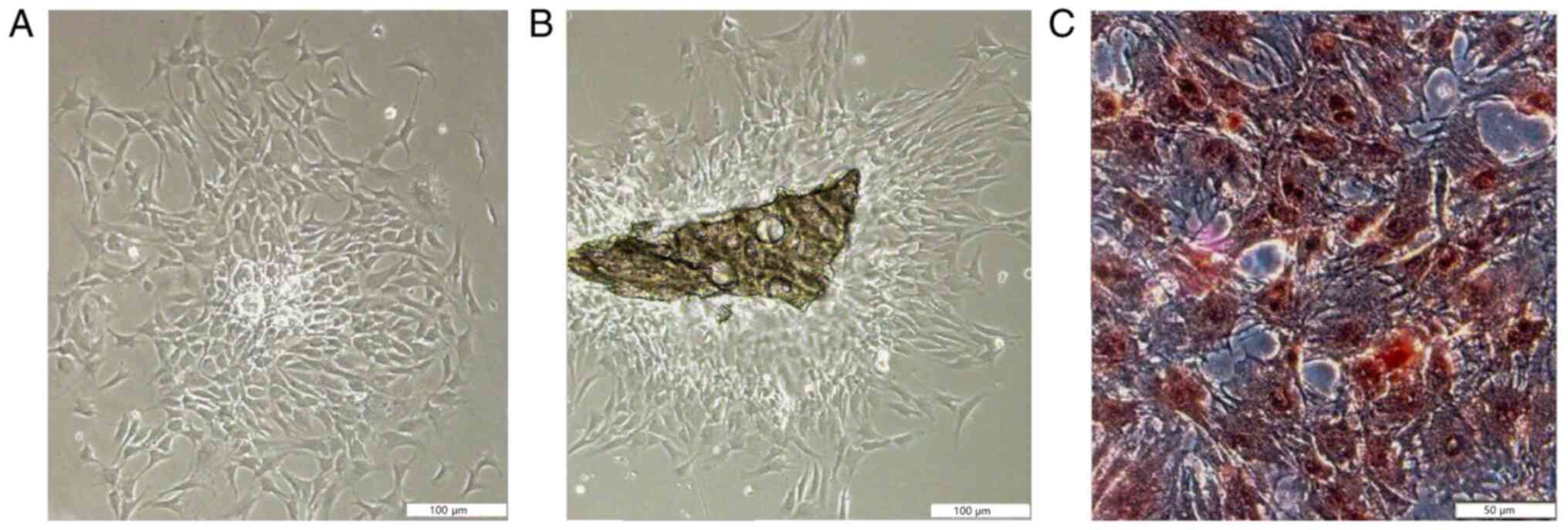
Calvarial osteoblast cell isolation,
culture and identification
The characteristics and proliferation of the
adherent cells was in accordance with the features of osteoblasts
(Fig. 1). The ALP staining results
demonstrated that the positive cells, which were osteoblasts,
exhibited numerous black dye precipitates. ALP staining was not
quantified. The ALP-positive rate of osteoblasts derived from the
trypsin and type II collagenase method and the improved
tissue-culture method was >95%.
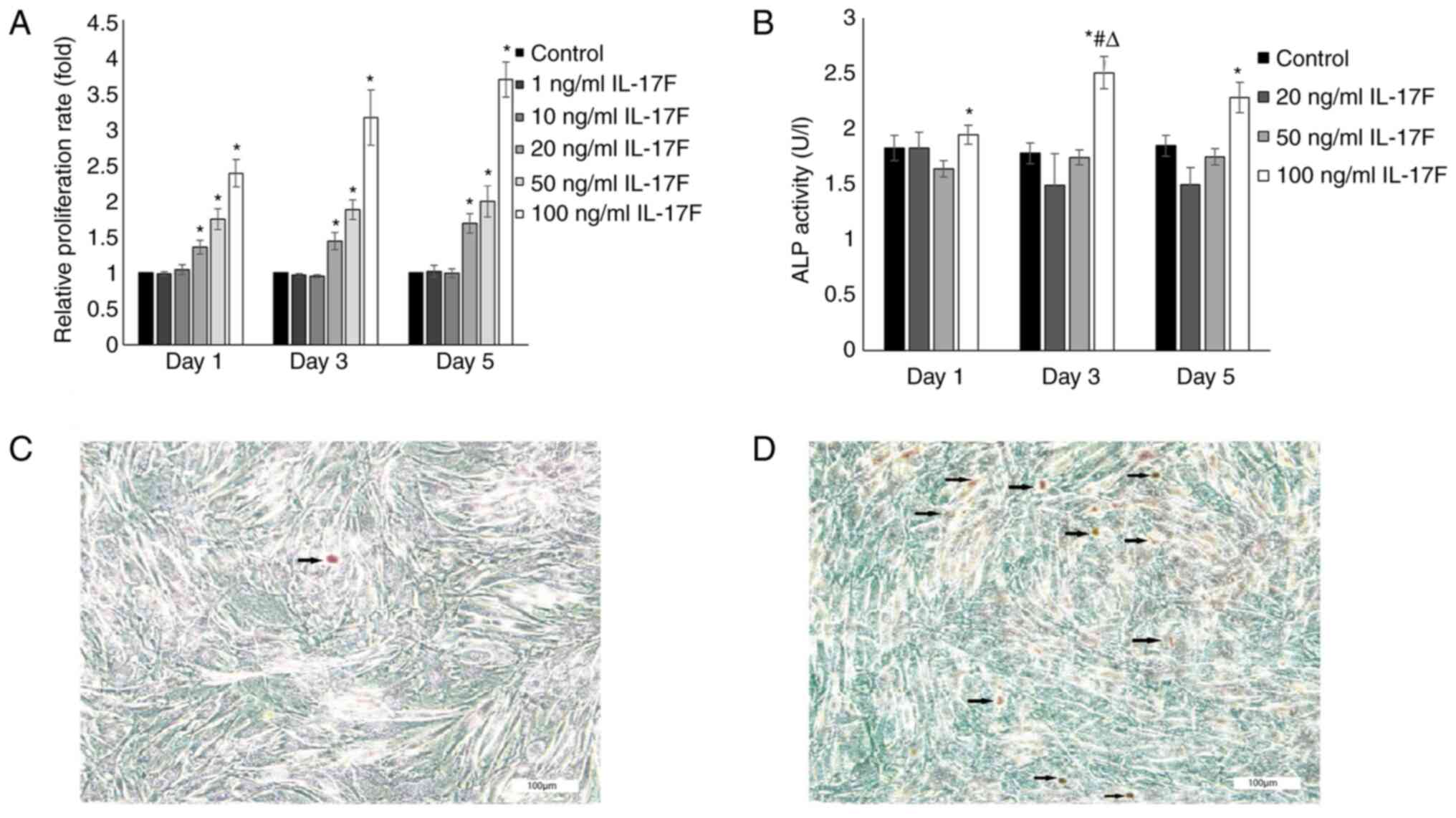
IL-17F promotes osteoblast
proliferation
To assess the direct effects of IL-17F on osteoblast
proliferation, rat osteoblasts were treated with various
concentrations of IL-17F (1, 10, 20, 50 or 100 ng/ml) for 1, 3 or 5
days (Fig. 2A). The MTT assay
results demonstrated that osteoblast proliferation was increased by
20, 50 and 100 ng/ml IL-17F in a dose- and time-dependent manner
compared with the control group. Furthermore, the maximal positive
effect of IL-17F on cell proliferation was observed with a
concentration of 100 ng/ml on day 5. By contrast, treatment with 1
and 10 ng/ml IL-17F had no significant effect on osteoblast
proliferation compared with the control group.
IL-17F promotes osteoblast ALP
activity
ALP activity is a key marker in the early stage of
osteoblast differentiation (30).
To investigate whether IL-17F promoted osteoblast ALP activity,
cells were treated with 20, 50 or 100 ng/ml IL-17F for 1, 3 or 5
days (Fig. 2B). ALP activity was
only significantly increased in the 100 ng/ml IL-17F group compared
with the control group at each time point. However, treatment with
20 and 50 ng/ml IL-17F had no significant effect on ALP activity
compared with the control group.
IL-17F promotes osteoblast
mineralization activity
The effects of IL-17F on mineralization activity
were examined by measuring mineral nodule formation. The results
demonstrated that the number of mineralized nodules was markedly
increased following treatment with 100 ng/ml IL-17F compared with
the control group, as evidenced by orange patches/blocks (Fig. 2C and D).
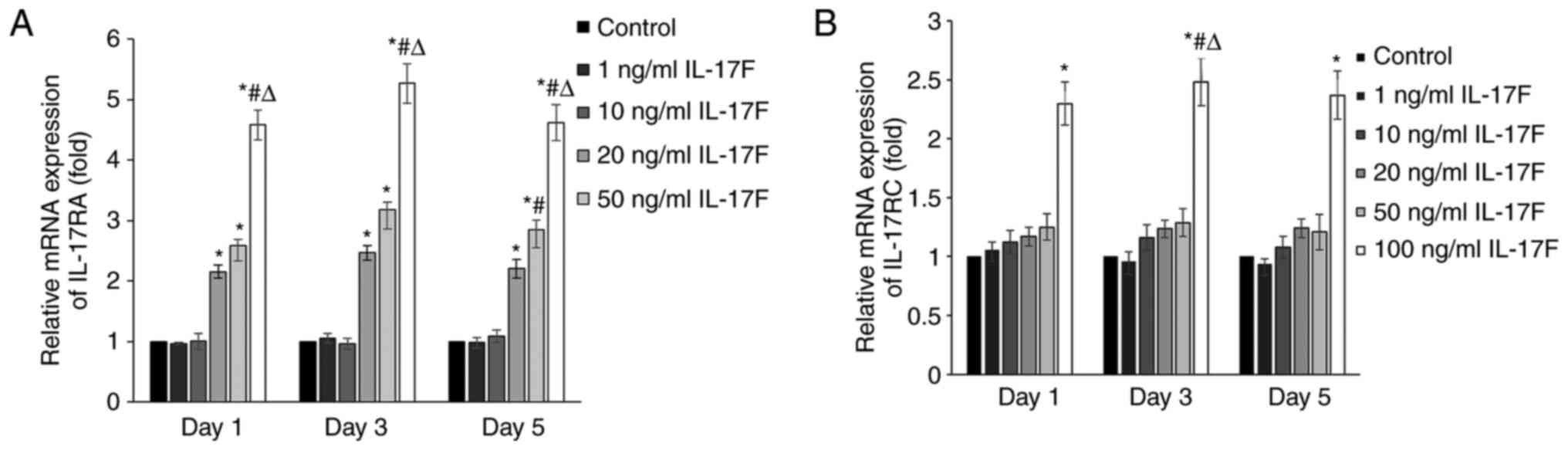
IL-17F treatment increases IL-17RA and
IL-17RC mRNA expression levels
To determine whether IL-17F receptors participated
in IL-17F-mediated promotion of osteogenesis, the mRNA expression
levels of IL-17RA and IL-17RC were determined via RT-qPCR (Fig. 3). At each time point, IL-17RA mRNA
expression levels were significantly increased in the 20, 50 and
100 ng/ml IL-17F groups compared with the control group. However,
IL-17RC mRNA expression levels were only significantly increased in
the 100 ng/ml IL-17F group compared with the control group.
Therefore, the results suggested that IL-17RA was associated with
the IL-17F-mediated promotion of osteogenesis with low
concentrations of IL-17F (20 and 50 ng/ml); however, at a high
concentration (100 ng/ml), IL-17F interacted with both IL-17RA and
IL-17RC.
IL-17F upregulates BMP-2/Runx2/Osterix
mRNA expression and downregulates Noggin mRNA expression
The mRNA expression levels of BMP-2, Runx2, Osterix
and Noggin were examined to determine the effects of IL-17F on
osteoblasts. Because osteoblast proliferation and differentiation
demonstrated no significant increase or decrease in 1 and 10 ng/ml
IL-17F groups, Runx 2 and Osterix mRNA expression levels were not
detected in these two groups. IL-17F (20, 50 and 100 ng/ml)
significantly increased BMP-2 mRNA expression levels on days 1, 3
and 5 compared with the control group, particularly in the 100
ng/ml IL-17F group (Fig. 4A). As an
antagonist to BMP-2, Noggin mRNA expression levels displayed an
opposite trend to BMP-2 in response to IL-17F treatment (Fig. 4D). The results indicated that
compared with the control group, the mRNA expression levels of
Runx2 and Osterix were significantly increased in a dose-dependent
manner in the 20, 50 and 100 ng/ml IL-17F groups, with peak
expression levels observed on day 3 (Fig. 4B and C).
IL-17F promotes BMP-2/Runx2/Osterix
protein expression and downregulates Noggin protein expression
To further determine the effects of IL-17F on BMP-2,
Runx2, Osterix and Noggin, western blotting analysis was conducted
on day 3 to measure protein expression levels (Fig. 4E and F). Following treatment with 50 or 100
ng/ml IL-17F, the protein expression levels of BMP-2, Runx2 and
Osterix were significantly increased, whereas Noggin expression
levels were significantly decreased compared with the control
group. IL-17F-induced alterations in protein expression were
consistent with IL-17F-induced alterations in mRNA expression. By
contrast, treatment with 1, 10 or 20 ng/ml IL-17F had no
significant effect on BMP-2, Runx2, Osterix and Noggin protein
expression levels compared with the control group.
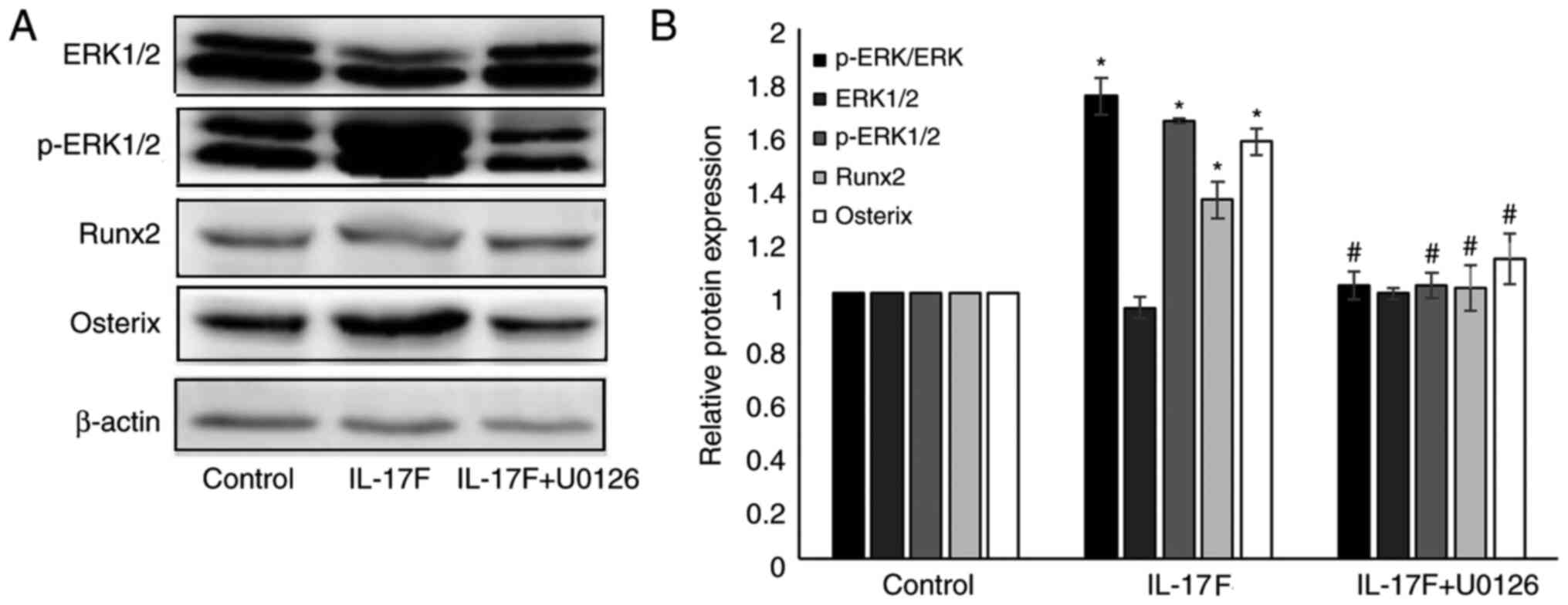
IL-17F-induced p-ERK1/2, Runx2 and
Osterix protein expression is inhibited by U0126
To further verify whether p-ERK1/2 served a role in
IL-17F-mediated osteoblastogenesis, a specific MAPK/ERK1/2
inhibitor (U0126) was used (Fig.
5). Following treatment with 100 ng/ml IL-17F for 3 days,
p-ERK, p-ERK1/2, Runx2 and Osterix expression levels were
significantly increased compared with the control group.
Co-treatment with U0126 significantly reversed IL-17F-induced
protein expression. However, no significant differences were
observed in the expression of p-ERK, Runx2 and Osterix between the
control group and IL-17F+U0126 groups.
Discussion
The IL-17F cytokine was reported for the first time
in 2001 and is expressed in Th17, natural killer, monocytes and
T-cells (31,32). IL-17F is involved in numerous
inflammatory and autoimmune settings, such as rheumatoid arthritis,
asthma, psoriasis, systemic lupus erythematosus and cancer
(33-37).
IL-17F has attracted increasing attention in bone disease research
(38). The results of the present
study suggested that IL-17F exerted a pro-osteogenic effect on
calvaria-derived rat osteoblasts. Compared with the control group,
osteoblast proliferation was significantly increased in a dose- and
time-dependent manner following treatment with 20, 50 or 100 ng/ml
IL-17F. Treatment with 100 ng/ml IL-17F significantly promoted
osteoblast ALP activity and mineralized nodule expression compared
with the control group, which represented the capacity of
differentiation and mineralization.
BMP-2 is one the most widely studied BMPs with the
most potent bone inductive activity and has been reported to induce
osteogenic differentiation in vitro (39,40)
and bone formation in vivo (41). The BMP-2 signaling pathway is a
vital positive modulator of bone homeostasis (42,43).
BMP-2 binding to its receptors regulates target genes, such as
Runx2 and Osterix (44). In the
present study, compared with the control group, BMP-2, Runx2 and
Osterix mRNA expression levels were increased by IL-17F treatment
in rat osteoblasts, with expression levels peaking at a
concentration of 100 ng/ml on day 3, which was consistent with ALP
activity. The promoting effect of IL-17F was also observed at the
protein level. Therefore, the results indicated that promoting
effects of IL-17F on osteoblasts occurred via stimulation of BMP-2,
Runx2 and Osterix signaling pathway expression.
Noggin, an extracellular BMP antagonist,
specifically blocks BMP/BMP receptor interaction, inhibits the
phosphorylation of downstream targets and suppresses the activity
of osteoblasts (45,46). Yunan et al (47) demonstrated that there may be a
negative feedback regulation of Noggin in the BMP signaling pathway
in vitro. In the present study, IL-17F treatment upregulated
BMP-2 expression and downregulated Noggin expression in osteoblasts
compared with the control group.
In the current study, IL-17RA and IL-17RC served
differential roles relative to low and high concentrations of
IL-17F. Following treatment with 20 or 50 ng/ml IL-17F, IL-17RA was
increased compared with the control group. However, when
osteoblasts were treated with 100 ng/ml IL-17F, both IL-17RA and
IL-17RC expression levels were increased compared with the control
group. The peak mRNA expression levels of IL-17RA and IL-17RC mRNA
were observed on day 3 following IL-17F treatment, which was not
consistent with the proliferation assay results, but was in
accordance with BMP-2/Runx2/Osterix mRNA expression and ALP
activity. The expression of IL-17RC displayed a similar trend to
ALP activity regarding time and dose. Therefore, the results
suggested that IL-17F bound to different receptors at different
time periods. The different requirements of IL-17RA and IL-17RC in
combination with IL-17F observed in the present study corroborated
the significance of precise ligand-receptor interaction in
osteoblasts.
MAPK/ERK1/2 has been reported to regulate the cell
proliferation, differentiation and apoptosis (26,27).
The significance of the MAPK/ERK1/2 signaling pathway in IL-17F was
verified by performing western blotting. The expression levels of
Runx2 and Osterix were higher in osteoblasts in 100 ng/ml
IL-17F-treated osteoblasts on day 3 compared with the control
group. When the MAPK/ERK1/2 inhibitor, U0126, was employed,
IL-17F-induced upregulation of p-ERK1/2, Runx2 and Osterix was
reversed. However, U0126 had no significant effect on Runx2 and
Osterix expression compared with the control group. The results
indicated that activation of the MAPK/ERK1/2 signaling pathway was
essential for the effects of IL-17F on osteoblasts.
In conclusion, the results of the present study
demonstrated that IL-17F promoted osteogenesis, including
proliferation, differentiation and mineralization activity. The
pro-osteogenic effects were associated with the upregulation of
BMP-2/Runx2/Osterix expression and the downregulation of Noggin
expression. The IL-17 receptors, IL-17RA and IL-17RC, were involved
in the process. IL-17F promoted osteoblastic osteogenesis via the
MAPK/ERK1/2-mediated signaling pathway. Therefore, IL-17F may serve
as a therapeutic target for metabolic bone diseases.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Shandong Province (grant no. ZR2014HM055).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY and NZ majorly contributed to drafting the
manuscript. LY designed the current study and interpreted results.
NZ performed the rat osteoblast isolation, culture and
identification. XL, XZ and LC performed RT-qPCR and western
blotting. MC and YJ performed the proliferation, differentiation
and mineralization ability detection assays, as well as part of the
western blotting experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical
Ethical Committee of Jinan Central Hospital, Jinan, China (approval
no. GG2016-006-02).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weitzmann MN and Pacifici R: Estrogen
deficiency and bone loss: An inflammatory tale. J Clin Invest.
116:1186–1194. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage distinct
from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Chyuan IT and Chen JY: Role of
interleukin-(IL-) 17 in the pathogenesis and targeted therapies in
spondyloarthropathies. Mediators Inflamm.
2018(2403935)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu S, Meng Z and Zhang Y: Correlation
between rheumatoid arthritis and immunological changes in a
rheumatoid arthritis rat model. J Biol Regul Homeost Agents.
32:1461–1466. 2018.PubMed/NCBI
|
|
5
|
Koo BS, Jo S, Kwon E, Shin JH, Hur JW and
Kim TH: Effect of biologics in the level of cytokines in the
synovial fluid of patients with ankylosing spondylitis. Korean J
Intern Med. 35:465–473. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim HJ, Seo SJ, Kim JY, Kim JY, Kim YG and
Lee Y: IL-17 promotes osteoblast differentiation, bone
regeneration, and remodeling in mice. Biochem Biophys Res Commun.
524:1044–1050. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Patel DD and Kuchroo VK: Th17 cell pathway
in human immunity: Lessons from genetics and therapeutic
interventions. Immunity. 43:1040–1051. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ono T, Okamoto K, Nakashima T, Nitta T,
Hori S, Iwakura Y and Takayanaqi H: IL-17 produing γδ T cells
enhance bone regeneration. Nat Commun. 7(10928)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Osta B, Lavocat F, Eljaafari A and Miossec
P: Effects of interleukin-17A on osteogenic differentiation of
isolated human mesenchymal stem cells. Front Immunol.
5(425)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nam D, Mau E, Wang Y, Wright D, Silkstone
D, Whetstone H, Whyne C and Alman B: T-Lymphocytes enable
osteoblast maturation via IL-17F during the early phase of fracture
repair. PLoS One. 7(e40044)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Y, Kim J, Chan A, Whyne C and Nam D:
A two phase regulation of bone regeneration: IL-17F mediates
osteoblastogenesis via C/EBP-β in vitro. Bone. 116:47–57.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vachhani K, Pagotto A, Wang Y, Whyne C and
Nam D: Design of experiments confirms optimization of lithium
administration parameters for enhanced fracture healing. J Biomech.
66:153–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang W, Guo D, Harris MA, Cui Y,
Gluhak-Heinrich J, Wu J, Chen XD, Skinner C, Nyman JS, Edwards JR,
et al: Bmp2 in osteoblasts of periosteum and trabecular bone links
bone formation to vascularization and mesenchymal stem cells. J
Cell Sci. 126:4085–4098. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Salazar VS, Gamer LW and Rosen V: BMP
signaling in skeletal development, disease and repair. Nat Rev
Endocrinol. 12:203–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cohen MM: Bone morphogenetic proteins with
some comments on fibrodysplasia ossificans progressive and NOGGIN.
Am J Med Genet. 109:87–92. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Canalis E, Economides AN and Gazzerro E:
Bone morphogenetic proteins, their antagonists, and the skeleton.
Endocrine Rev. 24:218–235. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moffett SP, Dillon KA, Yerges LM, Goodrich
LJ, Nestlerode C, Bunker CH, Wheeler VW, Patrick AL and Zmuda JM:
Identification and association analysis of single nucleotide
polymorphisms in the human noggin (NOG) gene and osteoporosis
phenotypes. Bone. 44:999–1002. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Komori T: Regulation of osteoblast
differentiation by transcription factors. J Cell Biochem.
99:1233–1239. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Amatya N, Garg AV and Gaffen SL: IL-17
signaling: The Yin and the Yang. Trends Immunol. 38:310–322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ely LK, Fischer S and Garcia KC:
Structural basis of receptor sharing by interleukin 17 cytokines.
Nat Immunol. 10:1245–1251. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Toy D, Kugler D, Wolfson M, Vanden Bos T,
Gurgel J, Derry J, Tocker J and Peschon J: Cutting edge:
Interleukin 17 signals through a heteromeric receptor complex. J
Immunol. 177:36–39. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gu C, Wu L and Li X: IL-17 family:
Cytokines, receptors and signaling. Cytokine. 64:477–485.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schwandner R, Yamaguchi K and Cao Z:
Requirement of tumor necrosis factor receptor-associated factor
(TRAF) 6 in interleukin 17 signal transduction. J Exp Med.
191:1233–1240. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nguyen TT, Lian S, Ung TT, Xia Y, Han JY
and Jung YD: Lithocholic acid stimulates IL-8 expression in human
colorectal cancer cells via activation of Erk1/2 MAPK and
Suppression of STAT3 Activity. J Cell Biochem. 118:2958–2967.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen T, Huang H, Zhou Y, Geng L, Shen T,
Yin S, Zhou L and Zheng S: HJURP promotes hepatocellular carcinoma
proliferation by destabilizing p21 via the MAPK/ERK1/2 and
AKT/GSK3β signaling pathways. J Exp Clin Cancer Res.
37(193)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Q, Xia T and Ye ZW: New SD Rat
osteoblasts by modified explant culture in vitro and
identification. Acta Universitatis Medicinalis Anhui. 47:1124–1127.
2006.
|
|
28
|
Sun L and Hou JM: Primary culture and
identification of rats cranial cover osteocytes. Med Innovation
China. 8:17–19. 2011.
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Orimo H: The mechanism of mineralization
and the role of alkaline phosphatase in health and disease. J
Nippon Med Sch. 77:4–12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Starnes T, Robertson MJ, Sledge G, Kelich
S, Nakshatri H, Broxmeyer HE and Hromas R: Cutting edge: IL-17F, a
novel cytokine selectively express in activated T cells and
monocytes, regulates angiogenesis and endothelial cell cytokine
production. J Immunol. 167:4137–4140. 2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kimura A and Kishimoto T: IL-6: Regulator
of Treg/Th17 balance. Eur J Immunol. 40:1830–1835. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Paradowska-Gorycka A, Sowinska A,
Stypinska B, Grobelna MK, Walczyk M, Olesinska M, Piotrowski P and
Jagodzinski PP: Impact of the IL-17F, IL-23 and IL-23R on
susceptibility and phenotype of systemic lupus erythematosus.
Autoimmunity. 49:373–382. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Soderstrom C, Berstein G, Zhang W, Valdez
H, Fitz L, Kuhn M and Fraser S: Ultra-sensitive measurement of
IL-17A and IL-17F in psoriasis patient serum and skin. AAPS J.
19:1218–1222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hatta M, Surachmanto EE, Islam AA and
Wahid S: Expression of mRNA IL-17F and sIL-17F in atopic asthma
patients. BMC Res Notes. 10(202)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gomes da Silva IIF, Angelo HD, Rushansky
E, Mariano MH, Diniz Maia MM and Eleuterio de Souza PRE:
Interleukin (IL)-23 Receptor, IL-17A and IL-17F gene polymorphisms
in brazilian patients with rheumatoid arthritis. Arch Immunol Ther
Exp (Warsz). 65:537–543. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang H, Zhang Y, Liu Z, Zhang Y, Zhao H
and Du S: The IL-17A G-197A and IL-17F 7488T/C polymorphisms are
associated with increased risk of cancer in Asians: A
meta-analysis. Drug Des Devel Ther. 9:5159–5168. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Erkolİnal E, Görükmez O, Eroğlu S, Özemri
SŞ, Solak Ö, Görükmez Ö and Yakut T: Associations between
polymorphisms of IL-17F and IL-17A genes with disease activity and
clinical outcome of Ankylosing Spondylitis. Acta Reumatol Port.
41:232–239. 2016.PubMed/NCBI
|
|
39
|
Luu HH, Song WX, Luo X, Manning D, Lou J,
Deng ZL, Sharff KA, Montag AG, Haydon RC and He TC: Distinct roles
of bone morphogenetic proteins in osteogenic differentiation of
mesenchymal stem cells. J Orthop Res. 2:665–677. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Taşli PN, Aydin S, Yalvaç ME and Sahin F:
Bmp 2 and bmp 7 induce odonto- and osteogenesis of human tooth germ
stem cells. Appl Biochem Biotechnol. 172:3016–3025. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jimi E, Hirata S, Shin M, Yamazaki M and
Fukushima H: Molecular mechanisms of BMP-induced bone formation:
Cross-talk between BMP and NF-κB signaling pathways in
osteoblastogenesis. Jpn Dent Sci Rev. 46:33–42. 2010.
|
|
42
|
Moon SH, Kim I and Kim SH: Mollugin
enhances the osteogenic action of BMP-2 via the p38-Smad signaling
pathway. Arch Pharm Res. 40:1328–1335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kim EC, Yoon SJ, Noh K and Lee DW: Dual
effect of curcumin/BMP-2 loaded in HA/PLL hydrogels on osteogenesis
in vitro and in vivo. J Nanosci Nanotechnol. 17:143–152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun WL, Wang N and Xu Y: Impact of
miR-302b on calcium-phosphorus metabolism and vascular
calcification of rats with chronic renal failure by regulating
BMP-2/Runx2/Osterix signaling pathway. Arch Med Res. 49:164–171.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yu X, Kawakami H, Tahara N, Olmer M,
Hayashi S, Akiyama R, Bagchi A, Lotz M and Kawakami Y: Expression
of Noggin and Gremlin1 and its implications in fine tuning BMP
activities in mouse cartilage tissues. J Orthop Res. 35:1671–1682.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
AlShaibi HF, Ahmed F, Buckle C, Fowles
ACM, Awlia J, Cecchini MG and Eaton C: The BMP antagonist Noggin is
produced by osteoblasts in response to the presence of prostate
cancer cells. Biotechnol Appl Biochem. 65:407–418. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yunan MA, Ying Y, Huanhuan S, Zhaozeng S,
Lin Z and Yunzhi FA: Effect of Noggin silencing on the BMP and Wnt
signaling pathways. Acta Laboratorium Animalis Scientia Sinica.
24:475–480. 2016.
|