Introduction
Sepsis is a complicated disorder that develops as a
result of dysregulated host response to infection, may manifest as
acute organ dysfunction, and it is associated with a high mortality
rate (1). Sepsis is a
life-threatening syndrome with a global mortality rate of ~25%
(2). Sepsis impairs the function of
numerous vital organs, including the brain, kidneys and heart
(3-7).
Amongst the various sepsis-induced complications, which may result
in multiple organ failure, acute lung injury (ALI) is the most
likely to occur during the early phase of sepsis, due to the
purulent inflammatory involvement of the lungs (8).
Sepsis-induced ALI is characterized by an aggressive
inflammatory process that generates inflammatory cytokines and
chemokines. The oxidative stress in sepsis-induced ALI is
hypothesized to be initiated by the activation of products from
lung macrophages and infiltrating neutrophils, and these products
rapidly diffuse to the lung epithelial and endothelial cells
(9). Redox stress induces
production of redox-sensitive transcription factors (such as NF-κB
and activator protein-1), leading to increased secretion of
proinflammatory cytokines and chemokines, further exacerbating
inflammation and oxidative stress (10). Dysregulated lung cell apoptosis is
another pathophysiological mechanism implicated in ALI (11). However, there are currently no
suitable medications recommended as standard treatment for ALI.
According to a recent genome-wide expression
analysis, ~80% of genetic elements are reported to be abnormally
expressed in patients with sepsis, with non-coding RNAs, including
microRNAs (miRNAs/miRs), long non-coding RNAs (lncRNAs) and
circular RNAs, serving as key regulators of the pathogenesis of
sepsis (12). miRNAs are ~19-22
nucleotides in length and participate in a wide range of
physiological processes by regulating the expression of target
genes, and novel therapies that focus on miRNA interventions have
been attracting increasing attention (13-15).
miR-139-5p is a critical modulator of the
progression of several illnesses, including sepsis. Overexpression
of miR-139-5p reduces the levels of IL-1β, IL-6 and TNF-α by
inhibiting the activity of NF-κB (16). miR-139-5p has been shown to inhibit
the expression of transformation-dependent proteins, thereby
regulating hypoxia/ischemia-induced neuronal apoptosis in neonatal
rats (17). Additionally,
miR-139-5p has also been found to inhibit cell viability and
metastasis and induce apoptosis in non-small cell lung cancer
(18).
It was previously demonstrated that Rho-kinase 1
(ROCK1) plays a key role in sepsis-induced lung injury, and that
the mechanism may involve oxidative and/or nitrosative
stress-mediated caspase cleavage, resulting in apoptosis (19). By searching the miRDB database
(mirdb.org), ROCK1 was predicted to be a target gene of
miR-139-5p. However, the precise effect and regulatory mechanism of
miR-139-5p in ALI have yet to be fully elucidated.
In the present study, the regulatory role and
potential molecular mechanism of action of miR-139-5p in
sepsis-induced ALI were examined using a cecal ligation and
puncture (CLP) mouse model, as well as normal human bronchial
epithelial cells (NHBEs). The extent of lung tissue damage,
apoptosis and expression of inflammatory cytokines were
investigated. The results may highlight potential novel therapeutic
targets and provide a theoretical basis for the treatment of
sepsis-induced ALI.
Materials and methods
Mouse model of CLP-induced sepsis
A total of 20 adult male C57BL/6 mice were purchased
from Nanjing Biomedical Research Institute of Nanjing University
(Nanjing, China) and reared under normal conditions (temperature
23˚C; humidity 50%; 12/12 h light/dark cycle; lights on at 09:00
am) with free access to food and water. All animals were handled in
accordance with the guidelines approved by the Experimentation
Ethics Review Committee of Nanjing University. Following
acclimation for 1 week, the mice were randomly assigned to two
groups: Normal and CLP (n=10 per group). A total of 12 mice
underwent electrosurgery to establish a mouse model of
sepsis-induced ALI (20,21). Briefly, pentobarbital sodium (50
mg/kg) was injected intraperitoneally to anesthetize the mice,
which were then fixed in a supine position on the operating table.
A 0.4-cm longitudinal midline incision was performed in the abdomen
to expose the cecum. After ligating with a 3-0 silk thread 1 cm
from the tip, the cecum was punctured once with a 20-gauge needle,
0.5 cm distal to the ligature. After gently compressing the cecum
to squeeze out a small amount of feces, the intestine was
repositioned in the abdomen and then sutured. The mice were given a
subcutaneous injection of saline immediately after surgery. The
same procedure was performed for the normal group, omitting the
cecal ligation and puncture. After the mice were awakened following
CLP surgery, they were given free access to water and observed
under normal conditions for 2 days. At the end of that period, the
mice were anesthetized by intraperitoneal injection of sodium
pentobarbital (50 mg/kg), 0.2-0.3 ml blood was extracted from the
eye, placed at room temperature for 2 h, centrifuged at 1,000 x g
for 10 min at 4˚C, and the supernatant was separated and stored in
a refrigerator at -80˚C. Following blood collection, pentobarbital
sodium (100 mg/kg) was injected intraperitoneally to euthanize the
mice, the thorax was cut open to expose the trachea and
bronchoalveolar lavage fluid (BALF) was collected.
Hematoxylin and eosin (HE)
staining
The HE staining procedure was performed as described
previously (22). The lung tissues
were immersed in 4% paraformaldehyde solution overnight in 4˚C.
Subsequently, the lung tissues were embedded in paraffin, and cut
into 5-µm thick serial sections. The tissue sections were
dehydrated with an ascending ethanol gradient, cleared using xylene
and then stained with hematoxylin solution for 6 min at room
temperature, followed by soaking in 1% acidic ethanol and washing
with distilled water. Next, the sections were stained with eosin
solution for 5 min at room temperature. Finally, the sections were
dehydrated with a graded series of alcohol solutions and cleared
with xylene. Images were captured using a light microscope
(magnification, x400; Olympus Corporation).
Analysis of pulmonary edema
Lung tissue was obtained and weighed, dried at 80˚C,
and then weighed again after 48 h to calculate the wet-to-dry (W/D)
ratio.
ELISA
TNF-α, IL-6 and IL-1β ELISA kits (cat. nos.
130-101-688, 130-094-065, 130-094-053, respectively) were purchased
from Miltenyi Biotec, Inc. ELISA was performed in accordance with
the manufacturer's protocol, and absorbance was measured at 450
nm.
Lung tissues were homogenized and diluted to 10%,
and centrifuged at 3,000 x g for 10 min at 4˚C. The supernatant was
collected in preparation for further analysis. The levels of
reactive oxygen species (ROS), malondialdehyde (MDA), lactate
dehydrogenase (LDH) and glutathione peroxidase (GSH-px) in the
serum and BALF, as well as superoxide dismutase (SOD) levels in the
serum, were detected according to the protocol of the manufacturer
of the ROS kit (Jianglai Biotech, cat. no. JL20383), lipid
peroxidation MDA kit (Beyotime Institute of Biotechnology, cat. no.
S0131S), LDH cytotoxicity assay kit (Beyotime Institute of
Biotechnology, cat. no. C0016), GSH assay kit (Nanjing Jiancheng
Bioengineering Institute, cat. no. S0052) and total SOD assay kit
with WST-8 (Beyotime Institute of Biotechnology, cat. no. S0101S),
respectively.
TUNEL assay
The 5-µm section of lung tissue embedded in paraffin
were dewaxed in the oven at 60˚C for 2 h, washed with xylene at
room temperature, rehydrated in a descending alcohol series (100,
90, 80 and 75%) and washed three times with PBS at room
temperature. A TUNEL kit (Roche Applied Science) was to detect
apoptotic cells according to the manufacturer's protocol. Cell
permeability solution was added for 8 min and 500 µl TUNEL reaction
mixture was added (50 µl TdT and 450 µl fluorescein-labeled dUTP)
at 37˚C for 1 h in the dark box. After DAPI staining (10 µg/ml; at
room temperature for 5 min), the sample was mounted under glass
coverslip with a mixture of PBS and glycerol (1:2). Using
fluorescence microscopy (magnification, x400; Olympus Corporation),
five high-power microscope fields were randomly selected from each
glass slide to count the number of TUNEL-positive cells.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR analysis
Total RNA from mouse lungs was extracted using the
RNA Isolation kit (cat. no. 4992456; Tiangen Biotech, Co., Ltd.)
according to the manufacturer's protocol and samples were stored at
-80˚C until subsequent use. The PrimeScript™ RT reagent kit (cat.
no. DRR037A; Takara Bio, Inc.) was used for the RT of RNA into cDNA
using the following conditions: 37˚C for 5 min, 42˚C for 60 min and
followed by 70˚C for 10 min. qPCR under the conditions of
pre-denaturation at 95˚C for 3 min, 40 cycles of denaturation 95˚C
for of 15 sec, annealing at 58˚C for 1 min, and extension at 72˚C
for 30 sec was performed using SYBR® Premix Ex Taq™
(Takara Bio, Inc. cat. no. DRR041A). The sequences of the PCR
primers used were as follows: MiR-139-5p forward,
5'-TCTACAGTGCACGTGTC-3' and reverse, 5'-GAATACCTCGGACCCTGC-3';
ROCK1 forward, 5'-TGGAAAGACATGCTTGCTCAT-3' and reverse,
5'-CGGTTAGAACAAGAGGTAAAT-3'; mucin (MUC)1 forward,
5'-TGCCGCCGAAAGAACTACG-3' and reverse, 5'-TGGGGTACTCGCTCATAGGAT-3';
MUC5AC forward, 5'-TGCGTCCCACGACATCTG-3' and reverse,
5'-CAGGTGAATGGGCACATGTG-3 and GAPDH forward,
5'-TTCAACGGCACAGTCAAGG-3' and reverse, 5'-CTCAGCACCAGCATCACC-3' and
U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'. U6 was used as an endogenous control
for miR-139-5p and GAPDH was used as a control for other genes.
Relative gene expression levels were quantified using the
2-ΔΔCq method (23) and
normalized to GAPDH.
Western blotting
Total protein was extracted from lung tissues using
the RIPA lysis buffer (Beyotime Institute of Biotechnology). The
protein concentration was measured with a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology) and equal
quantities (30 µg) of protein extract were loaded on a 10% SDS gel.
Proteins were resolved using SDS-PAGE and transferred to a PVDF
membrane. The membranes were first blocked using fat-free milk (5%)
in Tris-buffered saline (TBS) for 2 h at room temperature and then
incubated overnight at 4˚C with rabbit monoclonal anti-ROCK1 (cat.
no. 4035; 1:1,000; Cell Signaling Technology, Inc.), rabbit
monoclonal anti-NLR family pyrin domain containing 3 (anti-NLRP3;
cat. no. 15101; 1:1,000; Cell Signaling Technology, Inc.), rabbit
monoclonal anti-apoptosis-associated speck-like protein containing
a CARD (anti-ASC; cat. no. 67824; 1:1,000; Cell Signaling
Technology, Inc.), rabbit monoclonal anti-caspase-1 (cat. no.
24232; 1:1,000; Cell Signaling Technology, Inc.), rabbit monoclonal
anti-caspase-3 (cat. no. 9662; 1:1,000; Cell Signaling Technology,
Inc.) and anti-cleaved caspase-3 (cat. no. 9661; 1:1,000; Cell
Signaling Technology, Inc.), rabbit monoclonal anti-Bcl-2 (cat. no.
4223; 1:1,000; Cell Signaling Technology, Inc.), rabbit monoclonal
anti-Bax (cat. no. 14796; 1:1,000; Cell Signaling Technology, Inc.)
or rabbit polyclonal anti-GAPDH (cat. no. sc-32233; 1:2,000; Santa
Cruz Biotechnology, Inc.), and subsequently with horseradish
peroxidase-conjugated goat anti-rabbit IgG antibody (cat. no.
98164; 1:2,000; Cell Signaling Technology, Inc.) for 2 h at room
temperature. The optical density values of all bands were
standardized to the respective GAPDH band and visualized using
luminescent reagents (Santa Cruz Biotechnology, Inc.) and analyzed
via ImageJ software 1.4 (National Institutes of Health).
Dual-luciferase reporter assay
The ROCK1 3'-untranslated region (3'-UTR) fragment
with a putative binding site for miR-139-5p obtained from ensemble
database (version 38; https://asia.ensembl.org;) was entered into the
Primer3Plus website (https://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi)
to acquire the primer sequences, which was cloned into the
luciferase gene in a pmirGlo vector (Shanghai GenePharma, Co.,
Ltd.) by Shanghai GenePharma, Co., Ltd. Mutant (MUT) ROCK1 3'-UTR
was used to construct the ROCK1-MUT vector. 293 cells obtained from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences (5x104 cells/well) were plated in 12-well
plates (Thermo Fisher Scientific, Inc.) with 10% FBS and 1%
penicillin/streptomycin in a humidified incubator at 37˚C with 5%
CO2 and co-transfected with miR-139-5p mimics (50 nM,
5'-UCUACAGUGCACGUGUCUCCAGU-3') or negative control (NC) mimics (50
nM, 5'-UUUGUACUACACAAAAGUACUG-3') and ROCK1-wild type (WT, 100 ng)
or ROCK1-MUT (100 ng) when they reached 70-80% confluence.
Luciferase activity was assessed using a Dual-Luciferase Reporter
assay system (Promega Corporation) 2 days post-transfection and
normalized to that of Renilla luciferase activity.
Cell culture
NHBE cells were purchased from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences. NHBE cells
were cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
with 10% FBS and 1% penicillin/streptomycin in a humidified
incubator at 37˚C with 5% CO2. The six cell groups were
as follows: i) Control, ii) LPS, iii) LPS plus miR-NC mimics, iv)
LPS plus miR-139-5p mimics, v) LPS plus miR-139-5p mimics and empty
vector and vi) LPS plus miR-139-5p mimics and overexpression
(Ov)-ROCK1. miR-139-5p mimics (5'-UCUACAGUGCACGUGUCUCCAGU-3'; 50
nM) and control mimics (5'-UUUGUACUACACAAAAGUACUG-3'; 50 nM) were
prepared by Shanghai GenePharma Co., Ltd., and transfected into
NHBE cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). At 24 h post-transfection, cells were
treated with 1 mg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck
KGaA). miR-139-5p, IL-1b, IL-6 and ROCK1 levels were assessed 6 h
after LPS treatment. The ROCK1 overexpression plasmid (OV) was
established into the pcDNA3.1 vector (Invitrogen; Thermo Fisher
Scientific, Inc.), whereas an empty vector served as the Vector.
NHBEs were co-transfected with 1 µg Ov-ROCK1 (or Vector) and 50 nM
miR-139-5p mimics 24 h prior to treatment with LPS using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). NHBEs co-transfected with empty vector and
control mimics were used as the respective controls.
Cell apoptosis assay
Cells were seeded (1x106 cells/well) in
six-well plates and placed in an incubator for 12 h, followed by
another 48 h of incubation after transfection, as described above.
Next, cells were digested, washed twice with PBS and incubated in
1X binding buffer (provided in the Annexin V-FITC/PI apoptosis kit;
cat. no. C1062M; Beyotime Institute of Biotechnology), adjusting
the cell concentration to 1x106/ml. Subsequently, 10 µl
Annexin V-FITC and 5 µl propidium iodide (PI) were added to the
cells (200 µl) and cells were incubated for 15 min at room
temperature in the dark. BD FACSCalibur™ flow cytometer (BD
Biosciences) was used to quantify stained cells and data were
analyzed by FlowJo software (version 7.6.1; FlowJo LLC).
Statistical analysis
All experiments were repeated three times, and the
data are presented as the mean ± standard deviation. Comparisons
between two groups were performed using a unpaired Student's
t-test. Comparisons between multiple groups were performed using
one-way ANOVA followed by Tukey's post hoc test. All data were
analyzed using GraphPad Prism version 7.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Lung histological analysis
To investigate the effects of miR-139-5p in the
regulation of sepsis-induced ALI, a mouse model of sepsis was
established by CLP surgery. The results of lung tissue histological
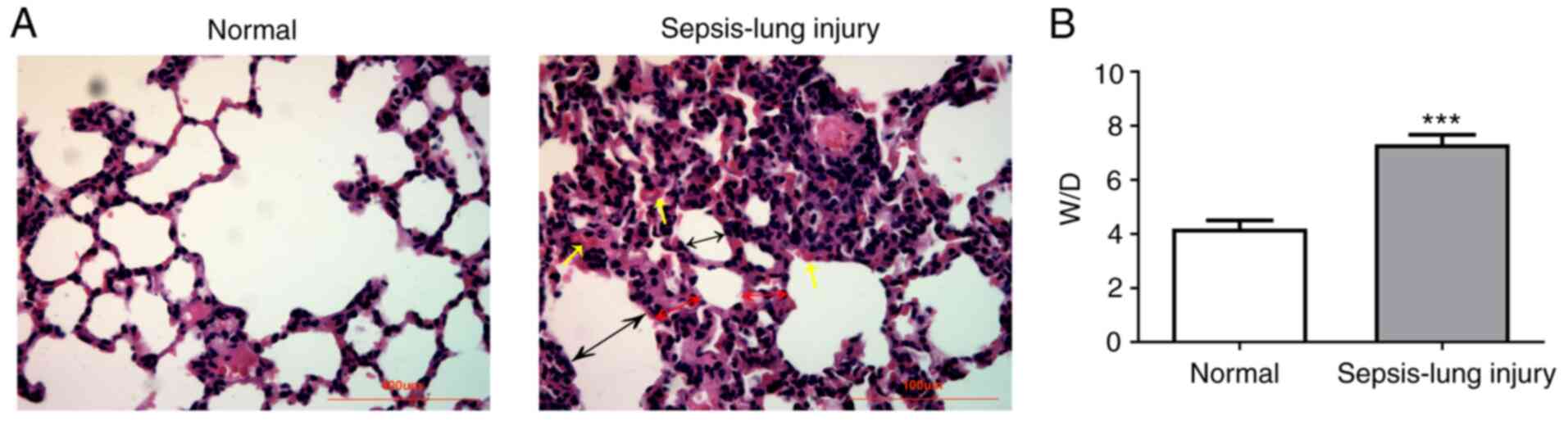
examination are shown in Fig. 1. No
pathological changes were observed in the normal group (Fig. 1A). However, serious injury was
present in the sepsis group compared with the normal control group,
where the normal alveolar structure of the lung injury group was
partially destroyed, inflammatory cells infiltrated, alveolar
septum was significantly thickened, alveolar cavity was narrowed,
and some alveoli were atrophied. In addition, the sepsis group had
a significantly higher W/D ratio compared with the normal group
(Fig. 1B). These results indicated
that the ALI mouse model was successfully established.
Inflammatory cytokines in the serum
and BALF, and oxidative stress
In the normal group, TNF-α, IL-6 and IL-1β were
maintained at low levels in the serum and BALF (Fig. 2A and B). However, the expression levels of
TNF-α, IL-6 and IL-1β were significantly increased following CLP,
suggesting a notable increase in the release of inflammatory
factors.
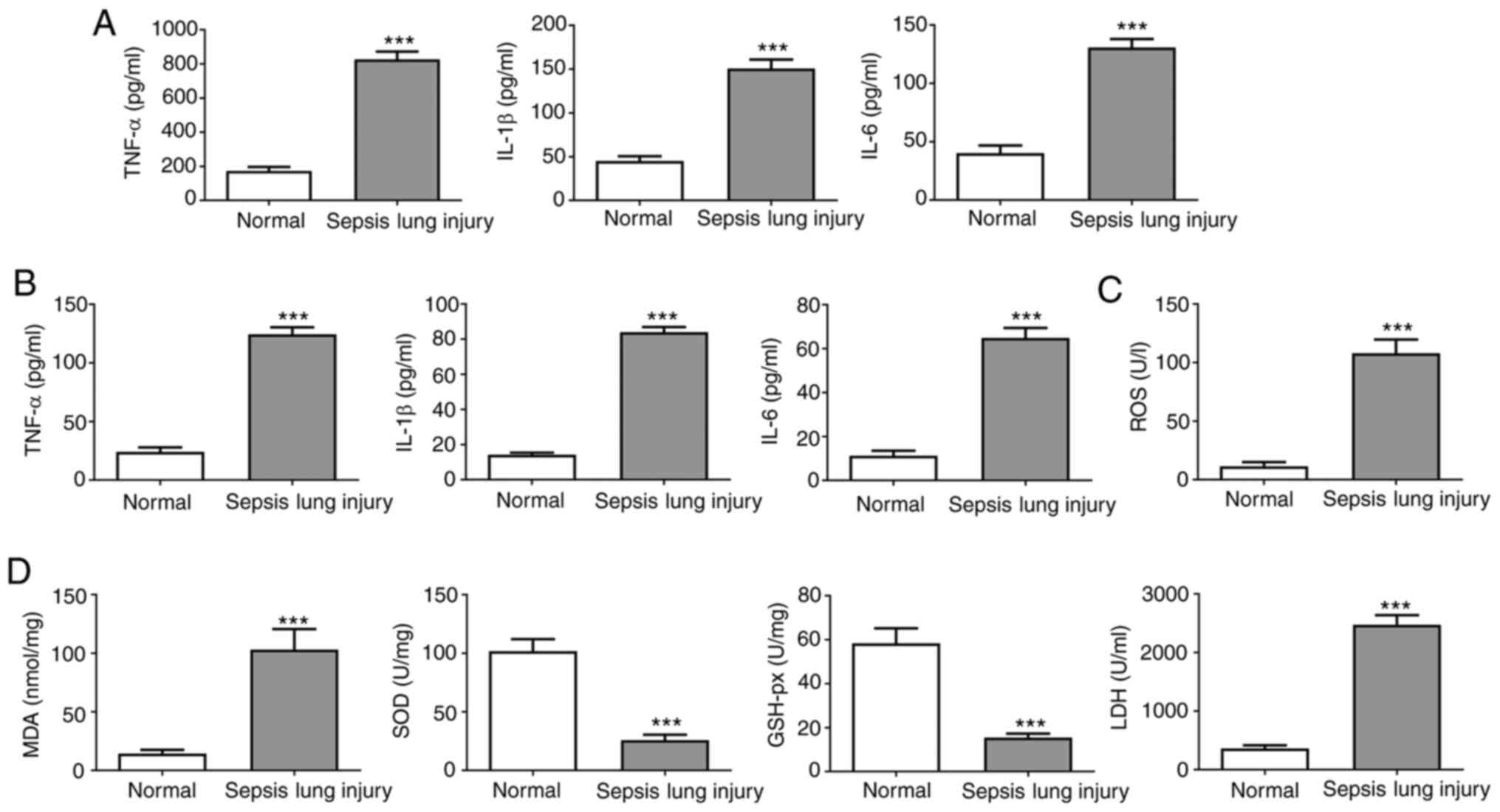 | Figure 2Effect of induction of ALI on the
expression of inflammatory factors. (A) IL-1β, TNF-α and IL-6 serum
levels were assessed by ELISA. (B) IL-1β, TNF-α and IL-6 BALF
levels were evaluated by ELISA. (C) ROS levels in mouse lung
tissues were measured using commercial ROS assay kits. (D) MDA,
SOD, LDH and GSH-px activity in mouse lung tissues was evaluated
using commercial assay kits. Data are presented as the mean ±
standard deviation. ***P<0.001 vs. normal group. ALI,
acute lung injury; SOD, superoxide dismutase; MDA, malondialdehyde;
LDH, lactate dehydrogenase; GSH-px, glutathione peroxidase; BALF,
bronchoalveolar lavage fluid; ROS, reactive oxygen species. |
Oxidative stress levels were analyzed by measuring
the ROS levels in the lung tissues (Fig. 2C) and measuring SOD, GSH-px, MDA and
LDH activity (Fig. 2D). ROS levels
were increased in septic mice compared with the normal group.
Furthermore, the SOD and GSH-px activities were notably reduced in
septic mice compared with the controls. By contrast, MDA and LDH
activities were higher in the sepsis group compared with the normal
group. These results suggested that the levels of inflammatory
factors and oxidative stress in the serum and BALF were upregulated
in mice following sepsis-induced lung injury.
Apoptosis and inflammation in lung
tissues
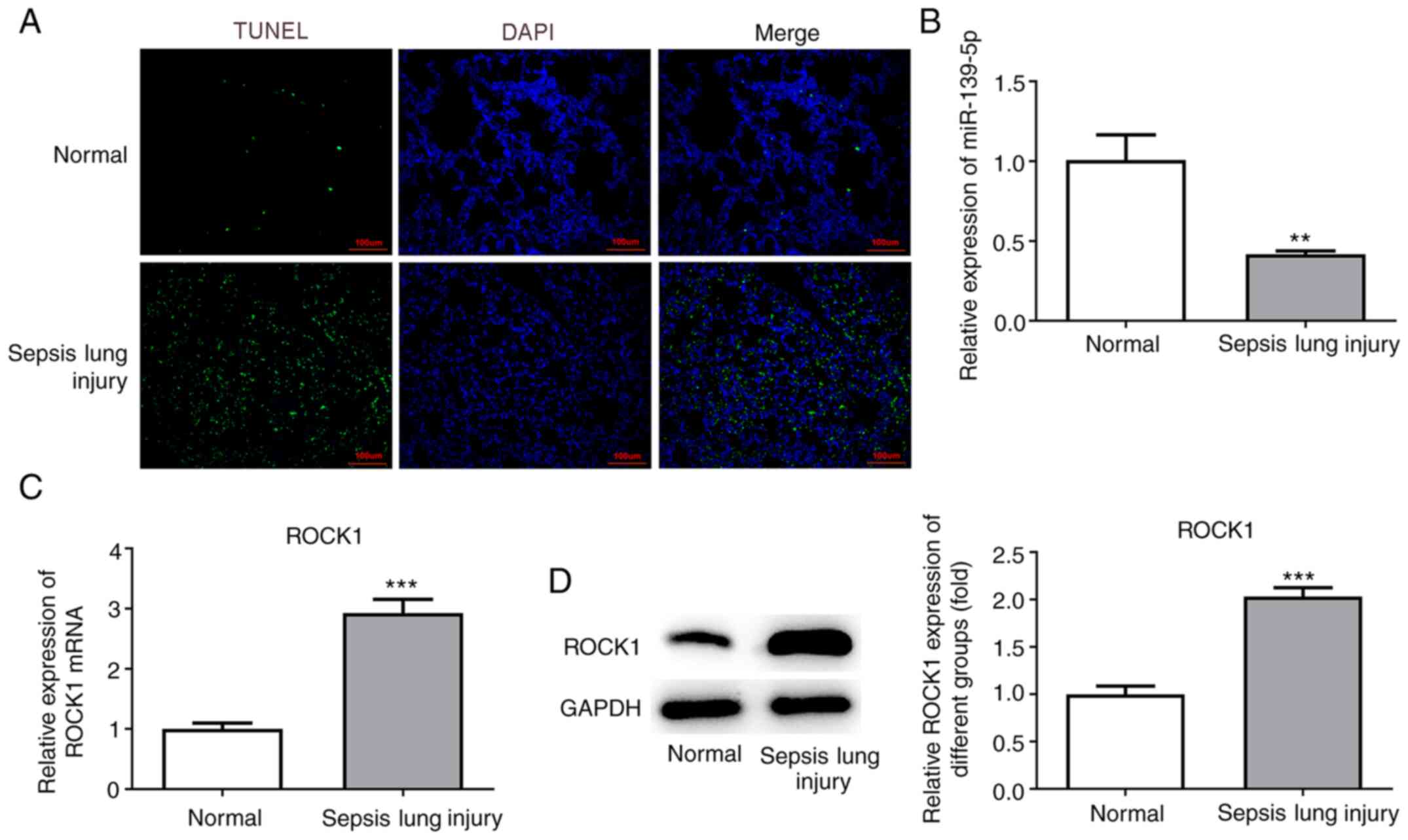
Lung tissue sections were stained using TUNEL
reagent to examine apoptotic cell death. An increased number of
TUNEL-positive (apoptotic) cells were detected in mice with
sepsis-induced lung injury compared with the normal group (Fig. 3A). The results suggested that
sepsis-induced ALI promoted cell apoptosis.
Expression of miR-139-5p and ROCK1 in
lung tissues
Next, the levels of miR-139-5p were analyzed by
RT-qPCR, and the results revealed that miR-139-5p levels were
decreased in the sepsis-induced lung injury group compared with the
normal group (Fig. 3B). ROCK1
expression was detected by RT-qPCR and western blot analyses and
was found to be significantly upregulated in the sepsis-induced
lung injury group compared with the normal group (Fig. 3C and D).
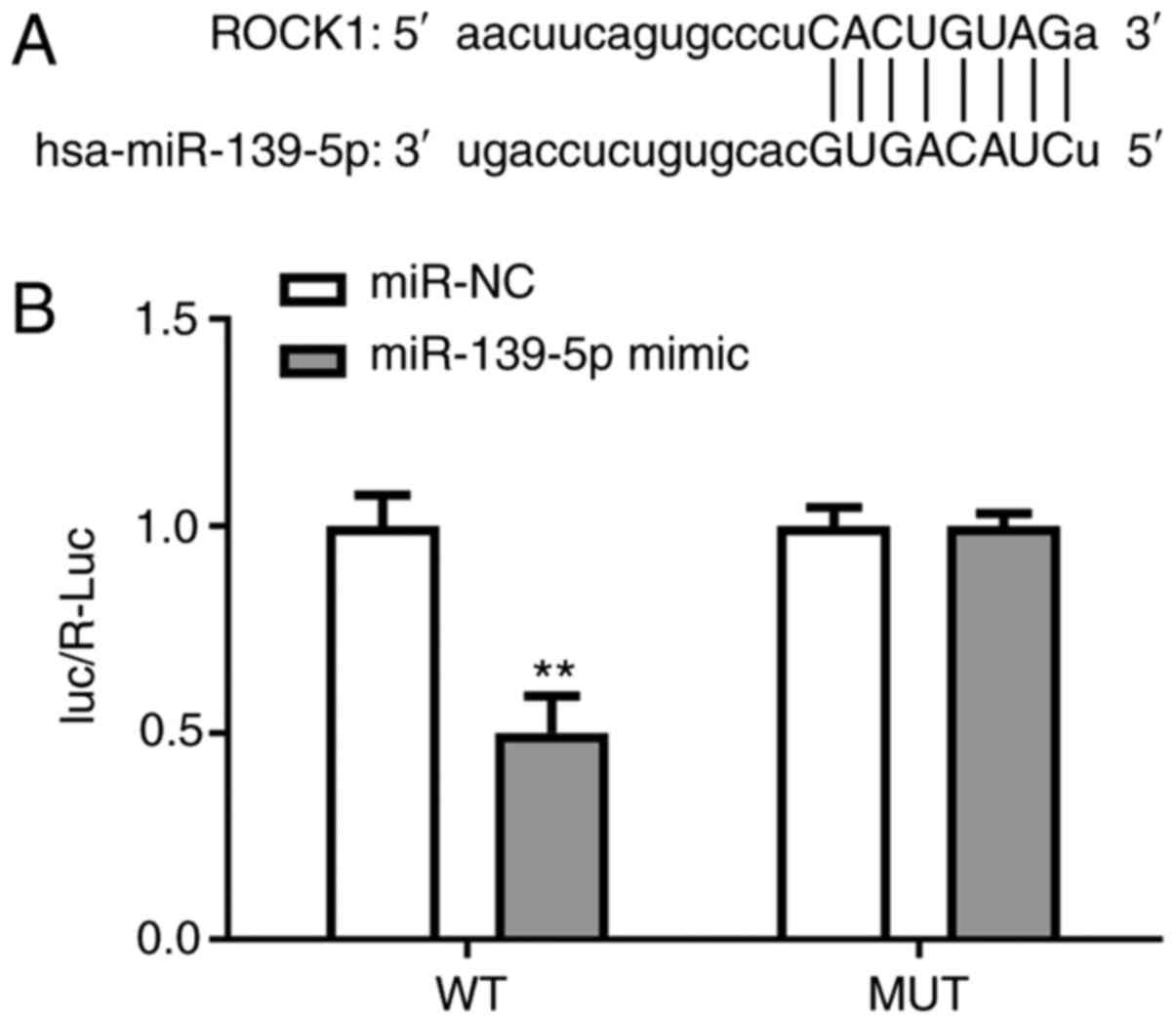
miR-139-5p targets ROCK1
The predicted binding site between ROCK1 3'-UTR and
miR-139-5p is shown in Fig. 4A. WT
and MUT luciferase reporter gene plasmids containing the 3'-UTR
region of ROCK1 were created and co-transfected with miR-139-5p
mimics and mir-NC. Dual-luciferase reporter experiments
demonstrated that the luciferase activity of ROCK1-WT was
significantly reduced in cells transfected with miR-139-5p compared
with the control mock cells. These findings indicated that ROCK1
regulates the expression of its downstream target, miR-139-5p
(Fig. 4).
miR-139-5p overexpression inhibits
LPS-induced inflammation and oxidative stress via targeting ROCK1
in NHBEs
In order to further determine the beneficial effects
of miR-139-5p overexpression on sepsis-induced lung injury, an
in vitro NHBE cell model was developed to mimics in
vivo sepsis-induced epithelial cell stimulation using LPS.
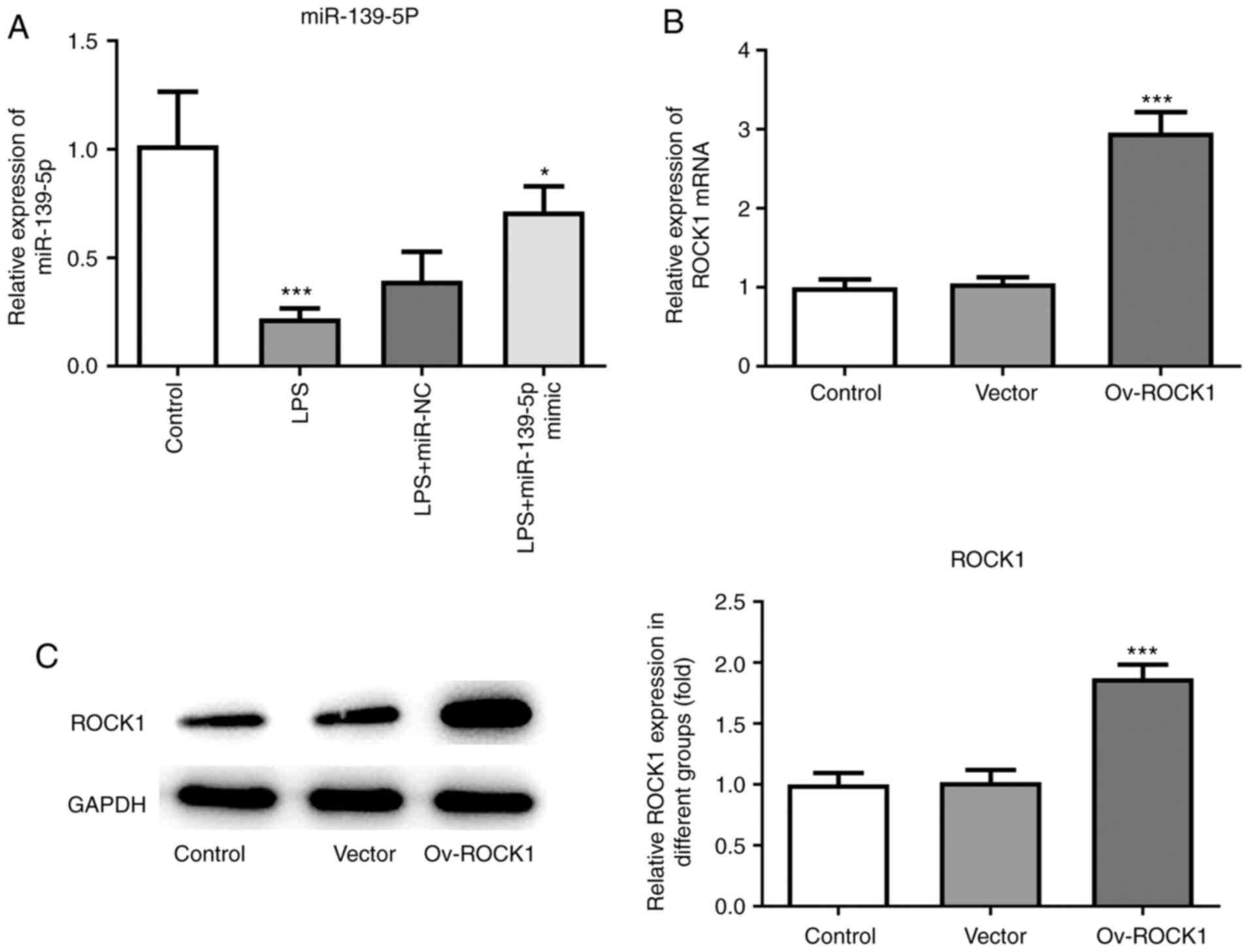
miR-139-5p mimics were transfected into NHBE cells, and RT-qPCR was
used to confirm overexpression. Compared with the control group,
the expression of miR-139-5p was significantly decreased in the
LPS-treated group, whereas transfection of miR-139-5p mimics prior
to LPS treatment significantly increased the levels of miR-139-5p
in NHBEs (Fig. 5A). Next, ROCK1
overexpression plasmid was transfected in NHBE cells, and
overexpression was confirmed using both RT-qPCR and western
blotting. Compared with the control group, the expression of ROCK1
was significantly increased in the Ov-ROCK1 group and the Vector
group (Fig. 5B and C).
MUC5AC and MUC1 are markers of lung mucosal
epithelial cell damage (24). To
further explore whether miR-139-5p modulates inflammation and
oxidative stress by specifically targeting ROCK1, NHBEs were
co-transfected with miR-139-5p mimics and vectors expressing ROCK1
(or empty vector). LPS-treated cells exhibited significantly higher
levels of MUC5AC and MUC1 compared with the untreated cells. The
NHBEs transfected with control mimics exhibited similar levels of
MUC5AC and MUC1, whereas transfection of miR-139-5p mimics
significantly decreased the expression of MUC5AC and MUC1. Compared
with the miR-139-5p mimics group, there were no differences between
miR-139-5p and empty vector co-transfected NHBEs, whereas
miR-139-5p and Ov-ROCK1 co-transfected NHBEs exhibited
significantly increased expression of MUC5AC and MUC1 (Fig. 6A). The levels of inflammatory
factors in the cell supernatants were assessed using ELISA.
Compared with the control group, the expression of TNF-α, IL-1β and
IL-6 were significantly increased by LPS treatment. Overexpression
of miR-139-5p largely suppressed the production of pro-inflammatory
cytokines in NHBEs, whereas ROCK1 overexpression resulted in
significant upregulation of TNF-α, IL-1β and IL-6 at the protein
level (Fig. 6B).
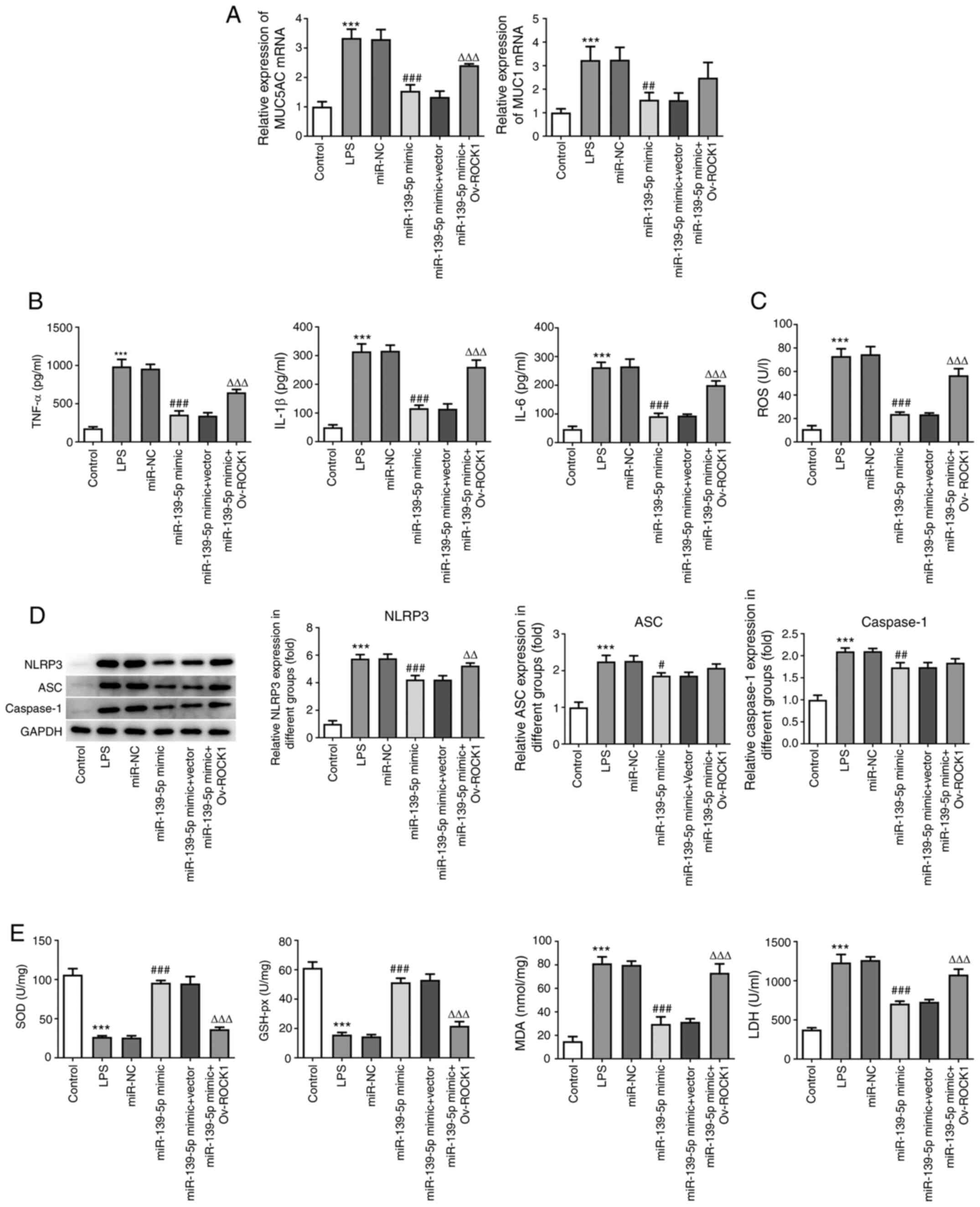 | Figure 6miR-139-5p overexpression inhibits
LPS-induced inflammation and oxidative stress via targeting ROCK1
in NHBEs. NHBEs were divided into six groups: Control, LPS, LPS
plus miR-NC mimics, LPS plus miR-139-5p mimics, LPS plus miR-139-5p
mimics and empty vector, and LPS plus miR-139-5p mimics and
Ov-ROCK1. (A) Relative mRNA expressions of MUC5AC and MUC1 were
measured using RT-qPCR. (B) Proinflammatory cytokine levels in the
treated NHBEs were examined using ELISA. (C) ROS levels in
different groups. (D) Expression levels of NLRP3, ASC and caspase-1
in the treated NHBEs were examined using western blotting. (E) SOD,
GSH-px and LDH activities and MDA levels were determined using
commercial assay kits. Data are presented as the mean ± standard
deviation. ***P<0.001 vs. control;
#P<0.01, ##P<0.005 and
###P<0.001 vs. miR-NC; ΔΔP<0.005 and
ΔΔΔP<0.001 vs. miR-139-5p mimics + Vector. ROCK1,
Rho-associated kinase 1; miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; NHBEs, normal human bronchial
epithelial cells; Ov, overexpression; SOD, superoxide dismutase;
MDA, malondialdehyde; LDH, lactate dehydrogenase; GSH-px,
glutathione peroxidase; LPS, lipopolysaccharide; NC, negative
control; MUC, mucin; NLRP3, NLR family pyrin domain containing 3;
ASC, apoptosis-associated speck-like protein containing a CARD. |
Next, the protein expression levels of NLRP3, ASC
and caspase-1 were examined and found to be significantly increased
following LPS treatment compared with the control group
(P<0.05). miR-139-5p mimics transfection alone or
co-transfection with the empty vector control resulted in
significant decreases in NLRP3, ASC and caspase-1. However, cells
co-transfected with miR-139-5p mimics and Ov-ROCK1 exhibited
significant increases in the expression of NLRP3, ASC and caspase-1
(Fig. 6C). These data suggest that
miR-139-5p inhibits the expression of inflammatory factors in NHBEs
cells by targeting ROCK1.
To determine the effects of miR-139-5p and ROCK1 on
oxidative stress, the levels of indicators of ROS were detected.
Compared with the control group, LPS treatment significantly
increased the levels of ROS; miR-139-5p mimics and miR-139-5p
mimics co-transfection with empty vector control resulted in
significant decreases in the levels of ROS. However, miR-139-5p
mimics co-transfection with Ov-ROCK1 resulted in a significant
increase in the levels of ROS (Fig.
6D). Additionally, the levels of SOD, GSH-px, MDA and LDH were
determined. The changes in expression of MDA and LDH were the same
as those of ROS, whereas SOD and GSH-px levels exhibited the
opposite trend. Compared with the control group, LPS treatment
significantly decreased the levels of SOD and GSH-px; miR-139-5p
mimics and miR-139-5p mimics co-transfected with the empty vector
control significantly increased, whereas miR-139-5p mimics
co-transfected with Ov-ROCK1 significantly decreased the levels of
SOD and GSH-px (Fig. 6E). Taken
together, these findings indicated that miR-139-5p overexpression
may inhibit LPS-induced inflammation and oxidative stress via
targeting ROCK1 in NHBEs.
miR-139-5p reduces apoptosis via
suppression of ROCK1 expression in NHBEs
To further examine whether miR-139-5p regulated cell
apoptosis by targeting ROCK1, NHBEs were co-transfected with
miR-139-5p mimics and vector expressing ROCK1 (or empty vector
control). The LPS-treated cells exhibited a markedly higher rate of
apoptosis compared with the control cells. The NHBEs transfected
with miR-NC exhibited a similar rate of apoptosis as the LPS group,
whereas transfection with miR-139-5p mimics significantly decreased
the proportion of apoptotic cells. NHBEs transfected with
miR-139-5p mimics and empty vector control had a similar proportion
of apoptotic cells as that of the miR-139-5p mimics group; however,
miR-139-5p mimics and Ov-ROCK1 co-transfection significantly
increased the proportion of apoptotic NHBEs (Fig. 7A).
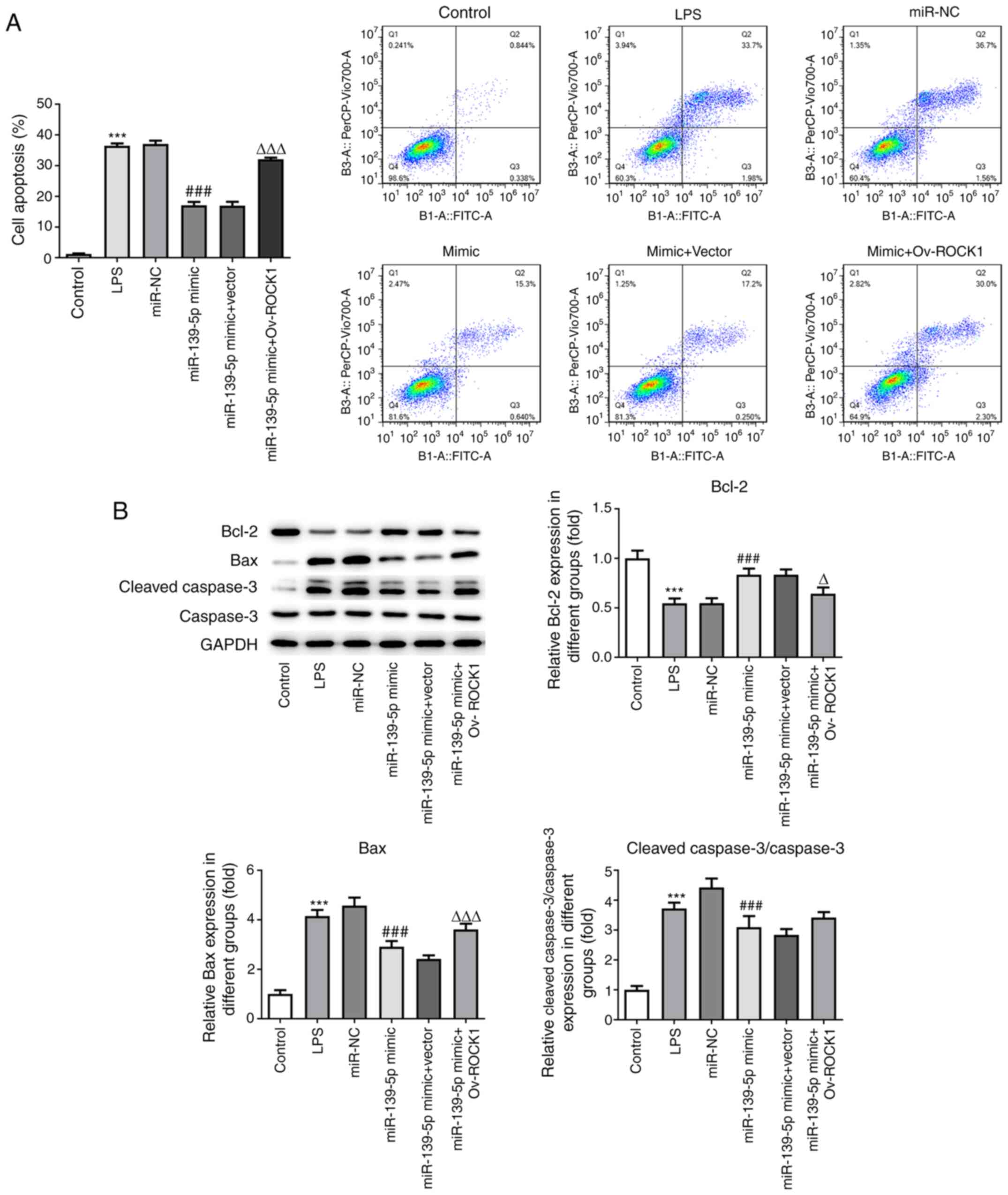 | Figure 7miR-139-5p overexpression reduces
LPS-induced apoptosis by targeting ROCK1 in NHBEs. (A) Proportion
of apoptotic cells among transfected NHBEs was determined using
flow cytometry. (B) Expression levels of Bax, Bcl-2, cleaved
caspase-3 and caspase-3 in the treated NHBEs were determined using
western blotting (left, representative blots; right, densitometry
analysis). Data are presented as the mean ± standard deviation.
***P<0.001 vs. control; ###P<0.001 vs.
miR-NC; ΔP<0.01 and ΔΔΔP<0.001 vs.
miR-139-5p mimics + Vector. NHBEs, normal human bronchial
epithelial cells; ROCK1, Rho-associated kinase 1; miR, microRNA;
LPS, lipopolysaccharide; NC, negative control; Ov,
overexpression. |
Furthermore, the levels of key proteins associated
with the apoptotic pathway were measured. The levels of Bax and
cleaved caspase-3 were higher in LPS-treated NHBEs as well as in
LPS-treated NHBEs transfected with miR-NC compared with NHBEs with
miR-139-5p mimics and empty vector co-transfection. Compared with
the miR-139-5p mimics and empty vector co-transfection groups, the
expression of Bax and cleaved caspase-3 were increased in the
miR-139-5p mimics plus Ov-ROCK1 group. However, the Bcl-2 protein
levels in the LPS group were significantly decreased compared with
those in the control group, and the miR-139-5p mimics and vector
co-transfection group exhibited significant decreases in Bcl-2
protein levels compared with the miR-139-5p mimics, as well as the
miR-139-5p mimics and empty vector co-transfection group (Fig. 7B). These results suggested that
miR-139-5p may inhibit cell apoptosis via suppression of ROCK1
expression in NHBEs.
Discussion
Sepsis-induced ALI is a clinical syndrome
characterized by injury of alveolar epithelial and endothelial
cells (25). ALI is a condition
that develops from excessive inflammation, and involves a variety
of potential therapeutic targets and signaling pathways (26). The pathophysiology of human sepsis
is similar to that caused by cecal perforation in the mouse CLP
model, and it has been widely used to study organ dysfunction
caused by sepsis (27). In the
present study, a mouse model of sepsis-induced ALI was successfully
established, and the mice exhibited alveolar wall thickening and
pulmonary edema.
miR-139-5p has been identified as a tumor
suppressor, and acts by reducing cell migration, invasion and
metastasis in breast, colorectal, prostate and esophageal squamous
cell carcinomas (28-31).
In addition, miR-139-5p is involved in the regulation of multiple
DNA repair and ROS defense pathways, and is a potent regulator of
the breast cancer response to radiotherapy (32). Furthermore, deficiency of miR-139-5p
was shown to result in the initiation of the MAPK, NF-κB and STAT3
signaling pathways, thereby contributing to the development of
intestinal inflammation and colorectal cancer (33). Therefore, in the present study, the
effects of miR-139-5p on lung injury, oxidative stress,
inflammation and autophagy were assessed, and the molecular
mechanisms in a mouse model of CLP-induced ALI were determined. In
the in vivo model, sepsis-induced ALI resulted in a
significant increase in the levels of inflammatory cytokines,
oxidative stress and apoptosis compared with the normal mice.
Additionally, miR-139-5p levels were decreased in mice with septic
lung injury.
ROCK1 has been reported to interact with miRNAs and
lncRNAs during cancer pathogenesis to regulate cell migration,
invasion and metastasis. For example, miR-202-5p was found to
inhibit tumor cell migration and invasion in osteosarcoma, whereas
upregulation of ROCK1 abrogated these effects (34). In the present study, ROCK1 was
predicted and confirmed to be a direct downstream target of
miR-139-5p. miR-139-5p expression was significantly downregulated
and positively correlated with ROCK1 expression during Ewing
sarcoma progression (35).
Moreover, the lncRNA X-inactive specific transcript was found to
sponge miR-139-5p, thereby reducing the levels of ROCK1(36). In the present study, miR-139-5p
expression was reduced in the mice with sepsis-induced lung injury,
whereas ROCK1 expression was significantly increased.
Further investigations on the role of miR-139-5p and
targeting of ROCK1 in sepsis-induced lung injury were performed
using LPS-treated NHBEs in vitro. Treatment with LPS
resulted in a significant increase in the levels of inflammatory
cytokines, oxidative stress and apoptosis in NHBEs compared with
the untreated cells. Overexpression of miR-139-5p reversed these
trends by decreasing the expression of ROCK1 in NHBEs.
In conclusion, miR-139-5p alleviated sepsis-induced
proinflammatory cytokine production, oxidative stress and apoptosis
induced by ALI via downregulation of ROCK1 expression levels.
Therefore, the findings of the present study highlight miR-139-5p
as a potential therapeutic target for the management of
sepsis-induced ALI.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Social
Development Projects of Jiangsu Province (grant no. BE2017720).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SN primarily designed the protocol; XZ wrote the
manuscript for this study and analyzed the data; MW and ZS
performed the animal experiments; SZ, WZ and ZY performed the in
vitro experiments; ZY and XH were involved in the coordination
of experiments and data analysis. All authors read and approved the
final manuscript. SN and XZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animals were handled in accordance with
guidelines approved by the Experimentation Ethics Review Committee
of Nanjing University (approval no. L2019320).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cecconi M, Evans L, Levy M and Rhodes A:
Sepsis and septic shock. Lancet. 392:75–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vincent JL, Marshall JC, Namendys-Silva
SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli
M, Pickkers P, Njimi H, et al: Assessment of the worldwide burden
of critical illness: The intensive care over nations (ICON) audit.
Lancet Respir Med. 2:380–386. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Singer BH, Dickson RP, Denstaedt SJ,
Newstead MW, Kim K, Falkowski NR, Erb-Downward JR, Schmidt TM,
Huffnagle GB and Standiford TJ: Bacterial dissemination to the
brain in Sepsis. Am J Respir Crit Care Med. 197:747–756.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Merx MW and Weber C: Sepsis and the heart.
Circulation. 116:793–802. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Meneses G, Cardenas G, Espinosa A, Rassy
D, Pérez-Osorio IN, Bárcena B, Fleury A, Besedovsky H, Fragoso G
and Sciutto E: Sepsis: Developing new alternatives to reduce
neuroinflammation and attenuate brain injury. Ann N Y Acad Sci.
1437:43–56. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hato T, Maier B, Syed F, Myslinski J,
Zollman A, Plotkin Z, Eadon MT and Dagher PC: Bacterial sepsis
triggers an antiviral response that causes translation shutdown. J
Clin Invest. 129:296–309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fowler AA III, Truwit JD, Hite RD, Morris
PE, DeWilde C, Priday A, Fisher B, Thacker LR II, Natarajan R,
Brophy DF, et al: Effect of vitamin C infusion on organ failure and
biomarkers of inflammation and vascular injury in patients with
sepsis and severe acute respiratory failure: The CITRIS-ALI
randomized clinical trial. JAMA. 322:1261–1270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fernandez FG, Kosinski AS, Furnary AP,
Onaitis M, Kim S, Habib RH, Tong BC, Cowper P, Boffa D, Jacobs JP,
et al: Differential effects of operative complications on survival
after surgery for primary lung cancer. J Thorac Cardiovasc Surg.
155:1254–1264.e1. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vazquez-Medina JP, Tao JQ, Patel P,
Bannitz-Fernandes R, Dodia C, Sorokina EM, Feinstein SI, Chatterjee
S and Fisher AB: Genetic inactivation of the phospholipase A2
activity of peroxiredoxin 6 in mice protects against LPS-induced
acute lung injury. Am J Physiol Lung Cell Mol Physiol.
316:L656–L668. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo RF and Ward PA: Role of oxidants in
lung injury during sepsis. Antioxid Redox Signal. 9:1991–2002.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yi L, Chang M, Zhao Q, Zhou Z, Huang X,
Guo F and Huan J: Genistein-3'-sodium sulphonate protects against
lipopolysaccharide-induced lung vascular endothelial cell apoptosis
and acute lung injury via BCL-2 signalling. J Cell Mol Med.
24:1022–1035. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Swisher EM, Lin KK, Oza AM, Scott CL,
Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O'Malley DM,
et al: Rucaparib in relapsed, platinum-sensitive high-grade ovarian
carcinoma (ARIEL2 Part 1): An international, multicentre,
open-label, phase 2 trial. Lancet Oncol. 18:75–87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hennessy EJ, Parker AE and O'Neill LA:
Targeting Toll-like receptors: Emerging therapeutics? Nat Rev Drug
Discov. 9:293–307. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Seeley JJ, Baker RG, Mohamed G, Bruns T,
Hayden MS, Deshmukh SD, Freedberg DE and Ghosh S: Induction of
innate immune memory via microRNA targeting of chromatin
remodelling factors. Nature. 559:114–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gotts JE and Matthay MA: Sepsis:
Pathophysiology and clinical management. BMJ.
353(i1585)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu M, Zhang W, Ma J, Dai Y, Zhang Q, Liu
Q, Yang B and Li G: MicroRNA-139-5p regulates chronic inflammation
by suppressing nuclear factor-κB activity to inhibit cell
proliferation and invasion in colorectal cancer. Exp Ther Med.
18:4049–4057. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qu Y, Wu J, Chen D, Zhao F, Liu J, Yang C,
Wei D, Ferriero DM and Mu D: MiR-139-5p inhibits HGTD-P and
regulates neuronal apoptosis induced by hypoxia-ischemia in
neonatal rats. Neurobiol Dis. 63:184–193. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang N, Guo W, Ren K, Li W, Jiang Y, Sun
J, Dai W and Zhao W: LncRNA AFAP1-AS1 Supresses miR-139-5p and
promotes cell proliferation and chemotherapy resistance of
non-small cell lung cancer by competitively upregulating RRM2.
Front Oncol. 9(1103)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y, Wang X, Liu W and Zhang L: Role of
the Rho/ROCK signaling pathway in the protective effects of fasudil
against acute lung injury in septic rats. Mol Med Rep.
18:4486–4498. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ruiz S, Vardon-Bounes F, Merlet-Dupuy V,
Conil JM, Buléon M, Fourcade O, Tack I and Minville V: Sepsis
modeling in mice: Ligation length is a major severity factor in
cecal ligation and puncture. Intensive Care Med Exp.
4(22)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhuo Y, Li D, Cui L, Li C, Zhang S, Zhang
Q, Zhang L, Wang X and Yang L: Treatment with
3,4-dihydroxyphenylethyl alcohol glycoside ameliorates
sepsis-induced ALI in mice by reducing inflammation and regulating
M1 polarization. Biomed Pharmacother. 116(109012)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Matute-Bello G, Downey G, Moore BB,
Groshong SD, Matthay MA, Slutsky AS and Kuebler WM: An official
American Thoracic Society workshop report: Features and
measurements of experimental acute lung injury in animals. Am J
Respir Cell Mol Biol. 44:725–738. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shang G, Jin Y, Zheng Q, Shen X, Yang M,
Li Y and Zhang L: Histology and oncogenic driver alterations of
lung adenocarcinoma in Chinese. Am J Cancer Res. 9:1212–1223.
2019.PubMed/NCBI
|
|
25
|
Englert JA, Bobba C and Baron RM:
Integrating molecular pathogenesis and clinical translation in
sepsis-induced acute respiratory distress syndrome. JCI Insight.
4(e124061)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Konduri K, Gallant JN, Chae YK, Giles FJ,
Gitlitz BJ, Gowen K, Ichihara E, Owonikoko TK, Peddareddigari V,
Ramalingam SS, et al: EGFR fusions as novel therapeutic targets in
lung cancer. Cancer Discov. 6:601–611. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dejager L, Pinheiro I, Dejonckheere E and
Libert C: Cecal ligation and puncture: The gold standard model for
polymicrobial sepsis? Trends Microbiol. 19:198–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hong HC, Chuang CH, Huang WC, Weng SL,
Chen CH, Chang KH, Liao KW and Huang HD: A panel of eight microRNAs
is a good predictive parameter for triple-negative breast cancer
relapse. Theranostics. 10:8771–8789. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Du F, Cao T, Xie H, Li T, Sun L, Liu H,
Guo H, Wang X, Liu Q, Kim T, et al: KRAS Mutation-Responsive
miR-139-5p inhibits colorectal cancer progression and is repressed
by Wnt Signaling. Theranostics. 10:7335–7350. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xiu D, Liu L, Cheng M, Sun X and Ma X:
Knockdown of lncRNA TUG1 enhances radiosensitivity of prostate
cancer via the TUG1/miR-139-5p/SMC1A Axis. Onco Targets Ther.
13:2319–2331. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wen J, Wang G, Xie X, Lin G, Yang H, Luo
K, Liu Q, Ling Y, Xie X, Lin P, et al: Prognostic value of a
Four-miRNA signature in patients with lymph node positive
locoregional esophageal squamous cell carcinoma undergoing complete
surgical resection. Ann Surg. 273:523–531. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pajic M, Froio D, Daly S, Doculara L,
Millar E, Graham PH, Drury A, Steinmann A, de Bock CE,
Boulghourjian A, et al: MiR-139-5p modulates radiotherapy
resistance in breast cancer by repressing multiple gene networks of
DNA repair and ROS defense. Cancer Res. 78:501–515. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zou F, Mao R, Yang L, Lin S, Lei K, Zheng
Y, Ding Y, Zhang P, Cai G, Liang X and Liu J: Targeted deletion of
miR-139-5p activates MAPK, NF-κB and STAT3 signaling and promotes
intestinal inflammation and colorectal cancer. FEBS J.
283:1438–1452. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li C, Ma D, Yang J, Lin X and Chen B:
MiR-202-5p inhibits the migration and invasion of osteosarcoma
cells by targeting ROCK1. Oncol Lett. 16:829–834. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Roberto GM, Delsin LEA, Vieira GM, Silva
MO, Hakime RG, Gava NF, Engel EE, Scrideli CA, Tone LG and
Brassesco MS: ROCK1-PredictedmicroRNAs dysregulation contributes to
tumor progression in ewing sarcoma. Pathol Oncol Res. 26:133–139.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tian K, Sun D, Chen M, Yang Y, Wang F, Guo
T and Shi Z: Long Noncoding RNA X-Inactive specific transcript
facilitates cellular functions in melanoma via miR-139-5p/ROCK1
Pathway. Onco Targets Ther. 13:1277–1287. 2020.PubMed/NCBI View Article : Google Scholar
|