1. Introduction
Dental resorption is considered to be a challenge to
dentistry due to its complexity. Root resorption (RR) represents a
pathological process that causes the loss of cementum, dentine
and/or enamel, almost irreversibly, concerning vital and non-vital
teeth, as a result of odontoclastic function (1). Although root resorption is necessary
in temporary dentition enabling the eruption of the permanent
successors (physiological root resorption), root resorption of
permanent teeth is unfavorable as it may lead to perpetual injury,
which might require dental treatment or, sometimes, extraction
(2).
Generally, it can simply be classified as internal
or external resorption, depending on the location on the root
surface. External root resorption can be further subclassified into
surface resorption, external inflammatory resorption, external
replacement resorption, external cervical resorption, transient
apical resorption (3-6).
One of the least understood types of external resorption is
external cervical resorption.
External cervical resorption (ECR) is a clinical
term used to define a quite uncommon, insidious and often
aggressive form of external root resorption, which can appear in
any permanent tooth (5). ECR is a
dynamic and pathological process characterized by its cervical
position on the tooth, arising immediately below the epithelial
attachment and the coronal part of the bone, this zone being named
the zone of the connective tissue attachment (5). Its nature is an extremely aggressive
one, concerning periodontal, dental and in later phases pulpal
tissues, causing substantial damage to the tooth structure
(7).
Other various terms used to describe ECR are
invasive cervical resorption, odontoclastoma, idiopathic external
resorption, fibrous dysplasia of the teeth, burrowing resorption,
late cervical resorption, extra-canal invasive resorption,
peripheral cervical resorption, supra-osseous inflammatory root
resorption, subepithelial inflammatory resorption, and periodontal
infection resorption (6,8).
In the present article, the term used to describe
this entity will be external cervical resorption (ECR), as it
reveals exactly the starting point of the resorptive process on the
tooth surface.
Electronic literature databases (PubMed and Web of
Science) were searched to identify relevant studies related to ECR.
The inclusion criteria were reviews, case reports, case series,
clinical studies, clinical trial and histological studies up until
2020. Of the 121 studies identified from the electronic PubMed's
database search, 62 studies were included for this review. The
electronic Web of Science's search revealed 182 articles, from
which only 19 were reviews. The review of the literature
demonstrated that there are few original scientific articles on
this topic. Most of the articles were case reports, case series,
describing the possible etiology and the possible therapeutic
options regarding ECR.
On the other hand, few articles on histopathology
and diagnosis were found in the literature, leading to poor
diagnosis, under-reported and mismanagement of this entity
(2).
2. Pathogenesis and etiology
ECR is a dynamic and complex process. The process of
external resorption can be described by three main phases: The
initiation of resorption, expansion of resorption and the
reparative stage (2,7,9).
The resorption initiation phase is symbolized by
disruption of the periodontal ligament (PDL) and by damage of the
protective unmineralized cementum (10). This process leads to a localized
inflammatory response of the exposed dentine so macrophages can
migrate to the inflamed area, causing the formation of granulation
tissue, which can link to the exposed dentine. The exposed dentine
is susceptible to resorption from the adjacent bone or to immune
cells (2). Three circumstances are
required in order for cervical resorption to take place: Presence
of blood supply, recruitment of circulating cells and lack of
protective layer (5,11). Some studies suggest that hypoxia can
lead to activation of osteoclastic phenomena by disturbing the
metabolism and interfering with the recovery of fibroblasts
(2,12).
After starting from its location, the resorption
extends in a circumferential and apicocoronally direction around
the root canal, invading the tooth structure by resorbing cementum,
dentine and enamel (2,11,13).
The pulpal tissue remains vital, being protected by the thin
pre-dentine layers so the resorptive defect does not perforate into
the root canal (2,7,13).
Wedenberg and Lindskog suggest that an inhibitor of macrophage
propagation is included in the organic component of dentine, making
the pre-dentine and dentine resilient to resorption (14). These changes may be the result of
various stimulating factors such as: Infection (microorganisms),
continuous and discontinuous mechanical force on PDL (orthodontic
treatment, parafunction), which can lead to hypoxia (2,12,14).
Resorption and repair can take place in parallel in
different parts of the same lesion. Osteoblast-like cells can form
bone-like tissue that can penetrate into the resorptive defect.
This is thought to be a form of healing (2,7).
Regarding the involvement of bacteria, two
mechanisms can be described for the ECR lesions. The first one
proposes that microorganisms are not essential to the initiation of
ECR but may appear as secondary aggressors to maintain the
resorptive process. After the loss of protective unmineralized
tissue, clastic cells moving in the subepithelial connective tissue
area connect to the root. The bacteria from the periodontal pocket
that may invade the lesion can promote and maintain the
inflammatory resorptive process. According to the second mechanism,
microorganisms are vital to the beginning and propagation of ECR.
Loss of protective unmineralized tissue (pre-cementum) with
simultaneous inflammation produced by microorganisms in the
periodontal pocket originates the resorptive development at the
cervical area of the ECR tooth. Bacteria in the lesion participate
in the perpetuation of this resorptive process (7,8,15,16).
Recent histological findings seem to indicate that
bacteria are not essential for the initiation of the defect. It
appears that microorganisms colonize the resorptive process in
later stages of the resorption, based on the observation that
microorganisms are identified only at the outer layers of the
defects (7,15,16).
Research indicates a lack of clarity surrounding the
etiology of this condition. The etiology of ECR remains unclear,
although several potential predisposing factors have been
identified and associated with this condition (2,9).
Orthodontic treatment, previous history of traumatic injury
(luxation, avulsion), restorative and endodontic procedures,
internal bleaching using hydrogen peroxide 30% and dentoalveolar
surgery are the principle potential predisposing factors for ECR
(2,6,9,17-19).
Based on a study by Heithersay, in which 257 ECR
teeth were analyzed, orthodontic treatment (28.4%) was the most
common factor and previous traumatic injury was the second one
(17). Additional factors such as
internal bleaching, surgery and restorative treatment were
identified as predisposing factors (2,17).
According to Mavridou et al, who assessed 337 ECR teeth,
several additional predisposing factors were reported (19). These included: Malocclusion,
parafunctional habits, poor oral hygiene, extraction of an adjacent
tooth, impacted teeth, eruption disorders (pressure generated by
canine eruption on lateral incisors), viral etiology, individual
genetic propensity and playing wind instruments (19). Orthodontic treatment as a
predisposing factor increased from 28.4 to 45.7% (17,19).
Any surgical procedure that injures the cervical
region (cementoenamel junction) can be considered an etiological
factor for ECR (5). Extraction of
an adjacent tooth that led to damage of the cervical area of a
neighboring tooth, may result in ECR (20). Fuss et al postulated that
impacted teeth such as third mandibular molars could cause
resorptive lesions affecting mandibular second molars (21). Sometimes, spontaneous resorption of
impacted tooth can occur. Orthognathic surgery can be considered as
a predisposing factor for ECR; heat damage to bone, impairment of
blood supply are important predisposing factors associated with
root resorption (17,19). Lateral incisors can suffer cervical
resorption lesions due to the pressure exerted by canine eruption
(22,23).
Both Heithersay's and Mavridou's studies have shown
that internal bleaching can be a possible predisposing factor of
ECR (17,19). The 30% hydrogen peroxide
(H2O2) liberated during internal bleaching
presents permeability through dentinal tubules and moves out
through the dentine gaps in amelodentinal junction, which may
denature the organic components of dentine, cementum, provoking a
foreign body reaction near cementoenamel junction (CEJ). This
immunological response results in activation of phagocytes and
attachment of clastic cells, which can lead to the initiation of
the resorptive process (17,19).
An acidic environment caused by bleaching paste may enhance the
osteoclastic activity leading to ECR (24-26).
While Heithersay reported internal bleaching as a potential
predisposing factor in 13.6% of cases (17), Mavridou et al postulated that
only 2.7% of ECR cases appear due to internal bleaching (19). The lower percentage of appearance of
internal bleaching as a predisposing potential factor in Mavridou's
study may be due to softer bleaching products that are currently
used (19). However, patients
should be advised of the potential risk for ECR (2,19).
Recently, ECR has been associated with viral
etiology. ECR also occurs in domestic, captive and wild cats and
the pathology is known in the veterinary literature as feline
dental resorptive lesions, feline odontoclastic resorptive lesions
(FORL), tooth resorption (TR), or neck lesions (27,28).
Von Arx et al indicated that feline herpes viruses (FHV) can
be transmitted to humans and may have a relevant position in the
pathogenesis of human cervical resorptive lesions (29). Nevertheless, future studies are
necessary to confirm the likelihood of viral transmission from cats
to humans and the role of FORL/TR in cervical lesions.
Teeth presenting with previous traumatic injury
episode such as concussion or avulsion can lead to rupture/damage
of PDL, which may be a cause for resorptive lesions (10,17,19).
Mavridou et al and Gunst et al described cases of ECR
in individuals who play wind instruments (19,30).
Apart from these, systematic factors, poor oral health, nutrition,
and an individual's genetic propensity can be associated factors
for ECR (11,17,19).
A recent study of Irinakis et al based on a
10-year observation of endodontic patients showed a prevalence of
ECR of 2.3%, in which predisposing factors were identified in
approximately 78% patients with ECR defects (31).
Therefore, the exact etiology of ECR has not been
confirmed, because in the majority of cases, more than one
predisposing factor has been identified, showing that ECR is a
multifactorial entity and not idiopathic (17,19,31).
However, more research is needed to establish the cause-and-effect
relationship of all of these suggested etiological factors.
3. Histology
ECR is a complex and dynamic process. According to
Mavridou et al, ECR cases share several common
characteristics such as: Initiative resorption phase (portals of
entry), propagation resorption phase in which channels and external
interconnections are found (portals of exit), the presence of the
so-called pericanalar resorption-resistant sheet (PRRS), the
reparative phase characterized by replacement of the resorbed
tissue with bone-like tissue and the remodelling phase of the
repaired tissue (7).
The initiation point is characterized by the
so-called portal of entry, which is located at the level of the
cement, under the epithelial attachment. In order to occur, ECR
requires the presence of localized damage and/or removal of the
periodontal ligament from the entry orifices. This area is
described by the presence of connective tissue with lympho-plasma
infiltrates and an increase in bone-like tissue with the fusion of
the neighboring alveolar bone to dentine (2,7).
The resorptive area described by channels and
external interconnections, starts from the initiation point
(portals of entry) and extends three-dimensionally, being able to
encircle or progress towards the pulpal tissue, resulting in
destruction of dental tissues (dentin, enamel, cement). In this
way, certain channels and interconnections with the periodontal
ligament are created, the so-called portals of exit (2,7).
Mavridou et al and Gunst et al, based
on histological and micro-CT findings, suggested that PRRS, an
uneven layer located around the root canal, which is characterized
by dentine and, occasionally, by bone-like tissue, may function as
a protective film against perforation of the root canal against the
resorptive lesion (7,13,20).
PRRS resistance to resorption can be attributed to the low mineral
content that inhibits the attachment of clastic cells as well as
normal oxygen tension from blood supply in the pulp tissue that
decreases clastic action (7).
In vitro studies of Wedenberg and Lindskog
demonstrated that an inhibitor of macrophage spreading is present
in the organic components of dentin and that this inhibitor may be
responsible for the resistance of pre-dentine to resorption
(14). However, in later and
advanced stages, pulp damage can take place (7).
There are some differences between endodontically
treated and untreated teeth, these differences being related to the
presence of PRRS. The intensity of resorption was observed to be
higher in endodontically treated teeth, an aspect associated with
the removal of this protective layer during endodontic treatment,
which involves chemo-mechanical preparation (15). Moreover, the loss of pulpal vitality
can induce a hypoxic environment that can stimulate the
continuation of clastic activity. The apposition of bone-like
tissue into the resorptive defect is more common in vital teeth
(7,15). The hypoxic environment, generated by
the excision of the pulpal tissue can be a factor for the constant
growth of the granulomatous tissue, thus inhibiting osteoblast
formation and encouraging the osteoclastic process to occur
(32-34).
A non-toxic environment is preserved throughout the PRRS, allowing
the substitution of granulomatous tissue from the resorptive
defects with bone-like tissue during the repair and remodelling
stages (7). Repair of the resorbed
tissue takes place by apposition of reparative bone-like tissue
through portals of entry into the tooth. The histological findings
of Mavridou et al showed bone-like related cells
(osteoblast-like cells, osteocytes) and osteoid tissue (7).
Cyclic resorption and reforming of the bone-like
tissue containing clastic and blastic cells are the main activities
that describe the last phase called the remodelling phase. It is
likely that in various regions of the same tooth, resorption of
dentine, active repair and remodelling of the bone-like tissue can
occur together (7).
4. Classification
Heithersay developed the first classification of ECR
in 1999(17). This classification
is based on periapical radiographs, being a two-dimensional one.
Heithersay divides ECR lesions into 4 classes, depending on the
size and extent of the resorption process in the dentine: Class 1,
a small lesion in the cervical area of the tooth, with a slight
penetration into the dentine; Class 2, a deeper penetration of the
defect towards the pulp chamber, with little or no involvement of
the root dentin; Class 3, a deep cervical resorbable lesion,
affecting the root dentin of the coronal third part; Class 4 is
characterized by an extensive resorptive defect beyond the coronal
third of the root dentine (17).
Unfortunately, this classification, being a two-dimensional one,
based on the interpretation of periapical radiographs, does not
provide accurate information concerning the circumferential damage,
the depth of the defect, the surface on which the lesion is located
(buccal or palatal), the proximity of the lesion to the root canal
or the true phase in which the lesion is found (reparative or
resorptive) (35). This
bi-dimensional interpretation does not provide an accurate
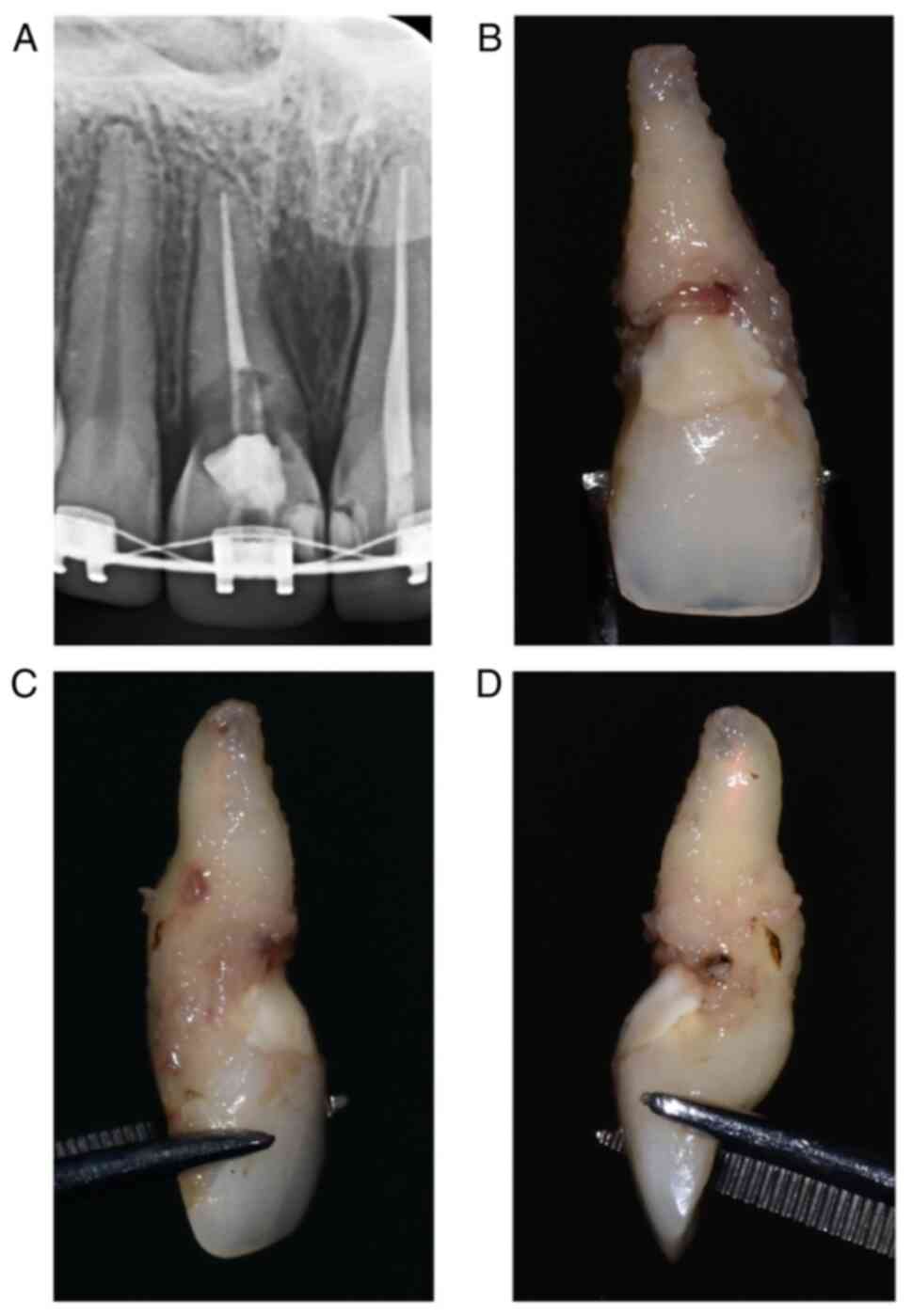
assessment of this entity (as observed in Fig. 1) and may lead to incorrect
establishment of treatment and management plans.
Recently, Patel et al described a new
three-dimensional classification of ECR based on CBCT
interpretation (18). This new
classification takes into account three parameters, namely: The
height of the lesions, the circumferential spread and the proximity
to the pulpal tissue. The score for the height of the lesions is
conducted as follows: Score ‘1’ is given for the supra-crestal
defects, ‘2’ for sub-crestal ones, ‘3’ for those extending in the
middle third of the root and ‘4’ for defects that extend to the
apical area of the root. Regarding the circumferential spread, the
lesions that do not exceed 90˚ are noted with ‘A’; ‘B’ are those
that are found between 90˚ and 180˚; ‘C’ indicates lesions larger
than 180˚ but smaller than 270˚ and ‘D’ indicates defects that
exceed 270˚. The third parameter, the proximity to the root canal
is noted with the small letter ‘d’, which shows that the lesion is
confined with dentine and with the letter ‘p’ if the lesion
perforates the root canal (18).
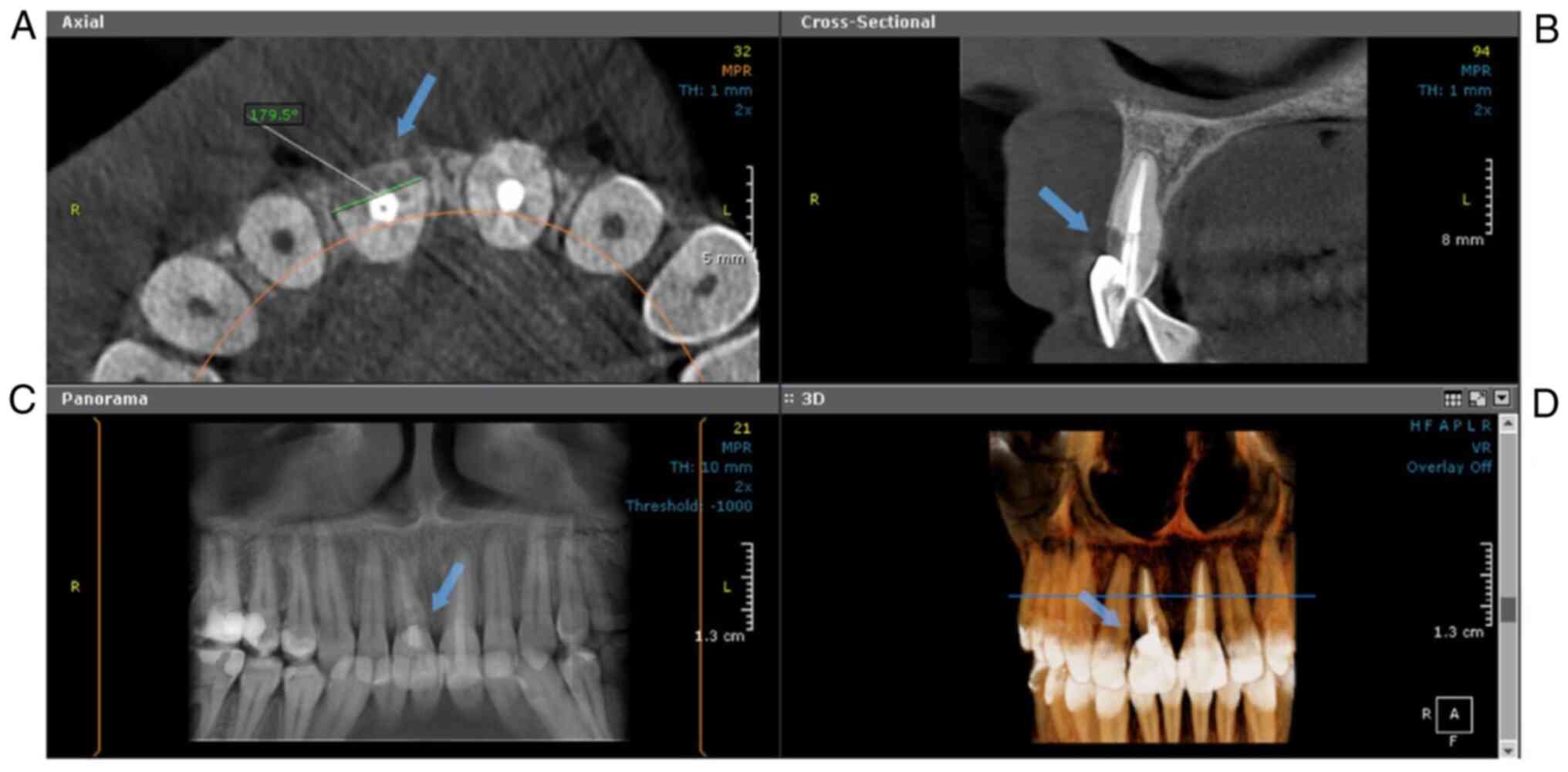
Fig. 2 shows an example of a Class
2Bp lesion diagnosed on CBCT.
The maximum height, circumferential spread and depth
of the lesion are noted after performing and interpreting
periapical radiographs and CBCT scans, thus resulting in a
three-dimensional gradation of the ECR lesion (18,36).
It is recommended that the ECR resorptive lesions be diagnosed and
assessed with the help of CBCT before establishing a treatment plan
as indicated by the American Association of Endodontists
(AAE)/American Academy of Oral and Maxillofacial Radiology Joint
Position Statement (AAOMR) and European Society of Endodontology
Position Statement (ESE) (36,37).
A three-dimensional classification of ECR is
necessary and relevant, because in vivo CBCT studies, ex
vivo micro-CT and nano-CT have demonstrated the complexity of
ECR invasion (portal of entry, small channels and their
interconnections with the periodontal ligament), an invasion that
can be different in all three dimensions (7,20,38).
This provides a three-dimensional insight into the
lesion and the capacity to correctly assess the entity and to
establish the most proper treatment plan and management.
5. Clinical and radiographic
presentation
Clinically, there is no classic presentation or
particular symptomatology for ECR. The lesion often begins
asymptomatically, insidiously, being accidentally discovered at a
clinical and/or radiological routine check-up (8). This is characteristic for the initial
lesions, discovered in early stages. In more advanced cases, when
the defect is in the proximity of the pulp chamber or even
perforates it, patients may report symptoms similar to reversible
or irreversible pulpitis (temperature sensitivity) and/or symptoms
similar to apical periodontitis (percussion pain, fistula)
(1,8). In initial stages, vitality tests are
within normal limits, as long as there is no involvement of the
pulp and the pulp has not become necrotic (1).
This is why the diagnosis of ECR is challenging, and
the differential diagnosis with cervical defects produced by caries
or cavities is difficult to achieve, but this aspect is of a
tremendous importance in establishing a correct treatment plan.
Resorptive defects of different etiology can be assessed or
diagnosed using periodontal probes or periodontal scalers. The
defects determined by caries lesions are soft and sticky on
probing, due to disintegration of the organic component of the
dentin (1,3,39).
Moreover, gingival recession may occur (40). On the other hand, the cavities
resulting from ECR are hard and scratchy on probing, giving a
feeling of mineralized tissue. In addition, the edges are sharp,
like a knife (1,39).
A pink spot can be observed in the cervical third
area of the ECR teeth, being a pathognomonic sign of this entity
(3,8). This is due to the high vascularity of
the granulomatous tissue, which can be visible through the thin
enamel in the cervical area. The presence of granulomatous tissue
is also the reason why bleeding is observed during probing
(1,39). However, the appearance of pink spots
is quite rare. Teeth with ECR may also have dyschromia due to
mortification of the pulpal tissue. In this case their color will
be grey (2).
From a radiological point of view, the lesions can
be symmetrical or asymmetrical. Their margins can vary from
well-defined and smooth, to poorly defined and rough, or with no
clear limits between the lesion and the healthy dental tissues
(41). ECR may appear radiolucent
(if the lesion is identified in its active resorption phase),
radiopaque (if the lesion is detected in the repair phase as a
result of ossification of the granulomatous tissue) or there may be
combinations of both phases (the lesion appearing radiopaque and
radiolucent) (7,20).
In addition, ECR lesions can be misdiagnosed as
internal resorption (IR). In ECR without perforation, the walls of
the root canal should be intact, and the entire contour of the root
canal must be observed, with the lesion located on the lateral
surface of the root. In IR, the contour of the root canal is
enlarged, but no defect is observed on the outer surface of the
root, if the lesion did not complicate with a perforation or root
fracture (42).
In order to avoid establishing an erroneous
diagnosis of IR, multiple periapical radiographs can be performed
from various angles. In the case of ECR, the lesion will look as if
it is moving with the change of horizontal angle of the X-ray tube.
The lesions located buccally move in the opposite direction, while
those located lingually move in the same direction with the
parallax shift. This can also help to determine the location of the
lesion when clinically it is not possible (41).
However, periapical radiographs (PRs) have some
disadvantages: The true extent of the lesion can be distorted which
can result in underestimating or overestimating the size of the
lesion. Being a two-dimensional image, only the height and width of
the lesion can be analysed; details concerning depth and
circumferential damage are minimal (35,43).
All of these disadvantages can lead to misdiagnosis and wrong
management of the lesion.
Rodriguez et al established that the use of
CBCT could lead to a more accurate diagnosis, assessment and/or
management of difficult endodontic cases (44,45).
The use of CBCT has exceeded the limits of
bi-dimensional radiographs; the true nature of the lesion, size,
circumferential extension and proximity to the root canal can be
identified (13).
The European Society of Endodontology (ESE) also
underlined the relevance and importance of using CBCT for the
management of potential ECR lesions in 2014, as well as the
AAE/AAOMR in 2015 (36,37).
The efficiency in the detection and classification
of ECR was studied by Vaz de Souza et al in an ex
vivo study, using and comparing two different CBCT scanners,
Kodak and Morita with periapical radiographs (PRs) (43). Examiners were asked to locate and
identify the tooth surface area/areas on which simulated lesions of
ECR were located, by analyzing PRs and CBCTs. They correctly
identified the lesion's location on the tooth surface in 87.8% with
the KODAK CBCT scanner, in 89.1% with MORITA CBCT, and in only
49.4% on periapical radiographs. In addition, examiners were asked
to classify the identified ECR lesions according to Heithersay's
classification. Only on 32% PRs, lesions were correctly classified,
compared to 70% for the MORITA CBCT, and 71.4% for KODAK CBCT
(43).
Thus, the improved precision of CBCT has not only
the advantage of a more accurate recognition, evaluation and
classification of ECR, but helps also in the selection of the most
suitable management plan.
6. Treatment
The final aim of the management strategy is to
ensure that the tooth diagnosed with ECR is maintained in a healthy
and functional status on the dental arch, avoiding tooth
extraction, and to improve aesthetics when indicated.
In order to maintain the affected tooth on the
dental arch, in a healthy and functional status, it is necessary to
proper restore the tooth, by excavating the resorptive tissue,
closing of the subsequent defect and portals of entry in order to
arrest the resorptive process (9).
Performing a preoperative CBCT helps identify the
true nature of the resorption, providing valuable information that
can help establish a correct treatment plan (9,18,35,46).
Depending on the extent of the damage, the location of the lesion
and its nature, two main therapeutic strategies can be proposed:
Internal repair or/and external repair. In some of the cases, when
the lesion has perforated the root canal walls or this may happen
during the excavation of the resorptive process, endodontic
treatment might be required as well (16,46,47).
External approach can be combined with surgical
treatment, such as flap surgery, when the lesions are extended far
apically than the gingival margin. In cases where the resorptive
lesions are located above/at the gingival margin level and are
accessible to excavation, non-surgical repair and treatment can be
the optimal solutions (16).
Small lesions, with inaccessible entry points which
may extend apicocoronally and circumferentially around the root
canal space, can be treated only using an internal approach,
defined by the endodontic treatment and the restoration of the
lesion (7,46).
External approach
External repair of smaller resorptive processes,
such as Heithersay class 1 and 2 or 3D Patel classes 1Ad, 2Ad, 2Bd,
is thought to have a positive outcome; in these cases, the root
canal is typically intact and the pulpal tissue is healthy, without
any symptoms of reversible or irreversible pulpitis (18,47).
The external non-surgical approach can be performed
on ECR teeth on which the lesions are located above the coronal
third of the root, whereas the ECR lesions located below the
gingival margin can benefit from surgical repair, including an
intra-crevicular incision or muco-periosteal flap surgery (47,48).
The first external repair technique of ECR lesions was described by
Heithersay and was characterized by topical application of 90%
aqueous trichloroacetic acid solution (TCA) to the resorptive
cavities, excavation of the infected tissue, endodontic treatment
if the root canal was perforated and restoration with glass ionomer
(GI) (8,47).
The application of TCA is used in order to stimulate
coagulation necrosis of the lesion and tissue located in an
accessible channel of ECR (49).
The TCA further penetrates the more inaccessible resorptive
channels, which may not be visible and opened for excavation by
mechanical instruments (38,47).
However, despite its advantages, TCA is very caustic, and attention
must be paid when utilizing it because it can cause inflammation
and possible irritation in neighboring tissues such as: Periodontal
tissue, oral mucosa and/or skin. In order to protect the
neighboring tissues, the rubber dam isolation system can be applied
to the causal tooth. The TCA manipulation technique involves
soaking a cotton pellet or micro-brush in the solution, then
removing the excess and applying it in direct contact with the
granulomatous tissue of the resorptive process for about 3-4 min,
until coagulation necrosis occurs (47,49).
The technique is very sensitive, and it necessitates
the use of magnification, such as surgical microscope or loups, for
the excavation of the granulomatous tissue with the help of hand
instruments (excavators); the use of burs should be reduced to the
maximum, in order to protect the intact remaining tooth structure
(46). Small internal resorptive
lesions can be accessed by using ultrasonic instruments under
magnification without any risk of removing unnecessary sound
dentine and can securely debride the lesions without damaging the
periodontal tissues (16). The use
of magnification helps to differentiate between healthy dentine and
reparative hard tissues, and to remove as much as possible from the
resorptive tissue. Being unable to completely remove the resorptive
tissue, the lesions can recur (1).
If during the excavation of the ECR lesion the root
canal is close to/or it is perforated, an indirect or direct pulp
caping may be indicated if the pulp is healthy, with no
inflammation. Bioactive materials such as Biodentine (Biodentine™
Septodont, Saint-Maurs-des-fosses, France) or MTA (ProRoot MTA,
Dentsply Sirona Endodontics, Tulsa Dental, USA) have several
advantages including bond strength, short setting time,
antibacterial effect and biocompatibility (50-54).
Moreover, they help with the formation of reparative dentine,
cement and encourage the differentiation of odontoblasts (55,56).
Bioactive materials, such as Biodentine (Septodont),
Endosequence Root Repair Material (Brasseler) can replace the use
of GI or resin-modified glass ionomer (RMGI) in order to restore
the ECR sub-gingival cavity. Although GI and RMGI have certain
advantages such as biocompatibility, fluoride release and chemical
adhesion to dentine, they do not ensure bone and cement
regeneration (57-60).
These bioactive restorative materials have the ability to
regenerate the cement, the PDL and the bone around them (50,61).
If the ECR tooth that is being restored is not located in an area
of aesthetic interest, the entire cavity can be restored with
bioactive materials; in cases where the aesthetic prevails, 3-4 mm
can be removed from the placed material and the remaining cavity
can be restored with GI and/or adhesive composite resin (46).
As stated above, endodontic treatment may be needed
if the ECR tooth presents with symptoms of irreversible pulpitis,
pulp necrosis and/or apical periodontitis and/or the ECR lesion has
perforated the root canal walls. As a technique, it is recommended
to initially identify, negotiate and shape the root canal, and then
to block the canal space using a suitable gutta-percha cone in
order to maintain it patent during the restoration of the ECR
lesion. After complete restoration of the defect (as described
above), endodontic treatment may be completed. In this way, the
risk of blocking the root canal is limited (62).
As an alternative to surgical approach, orthodontic
extrusion can be performed in order to access the ECR lesions
located far below the gingival margin (38).
Internal approach
When the ECR lesion has perforated or is close to
perforate the root canal, and an external repair is not a viable
solution, as there is no optimal access, the treatment option is an
internal approach. The internal repair technique requires
endodontic treatment and subsequent restoration of the ECR cavity
(46).
When small entry points are located apically from
the epithelial attachment, an internal repair is recommended. An
internal repair, meaning endodontic treatment as a first step, can
assess teeth with minor communications between the root canal space
and the resorptive lesion. After the endodontic treatment is
complete, longneck burs and/or ultrasonic tips can be used under
the surgical microscope, in order to extend the access cavity so
that the ECR defect is included (46,63).
In cases with larger ECR resorptive defects, the
endodontic access cavity should be realized with longneck burs
and/or ultrasonic instruments in order that both the root canal and
the defect are accessed (46).
In order to decontaminate the endodontic system,
sodium hypochlorite can be used as the main irrigant. Moreover,
sodium hypochlorite can dissolve the granulomatous tissue and can
achieve hemostasis. Then, a calcium hydroxide paste, which has an
alkaline pH, may be placed in the root canal to stimulate the
coagulation necrosis and to inhibit the osteoclastic action,
whereas the osteoblastic one occurs (64,65).
For the protection of the PDL, a polytetrafluoroethylene tape may
be compacted between the tooth and the adjacent tissues. Endodontic
treatment may then be finalized.
For restoration of the resulting cavity, materials
such GI and/or composite resins may be used. Salzano and Tirone
effectively treated four cases of ECR using MTA or Biodentine
(66). After the removal of
granulomatous tissue through the access cavity and the root canals,
the defects were repaired with MTA or Biodentine (66). Applying bioactive materials on the
ECR cavities will result in an alkaline pH that may impede the
osteoclastic function of any remaining ECR tissues, reducing the
risk of recurrence (67).
7. Conclusions
External cervical resorption (ECR) still remains a
relatively uncommon pathology which leads to the loss of hard
dental tissues due to osteoclastic activity. Its multifactorial
etiology being poorly understood, more research is needed to
establish the cause-and-effect relationship of all the etiological
factors. The improved radiographic detection using the cone-beam
computed tomography (CBCT) allows a precise diagnosis, a more
accurate classification of the lesion and leads to a more
predictable treatment plan for the benefit of the patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The authors confirm that all the information
provided in this review is documented by relevant references.
Authors' contributions
RMTN, LMN and MP were strongly involved in the
literature review and in writing the manuscript. STN performed the
radiological analysis and prepared the images. LCR carefully
revised and reviewed the paper in light of the literature data. All
authors read and approved the final version of the manuscript for
publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patel S, Kanagasingam S and Pitt Ford T:
External cervical resorption: A review. J Endod. 35:616–625.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Patel S, Mavridou AM, Lambrechts P and
Saberi N: External cervical resorption-part 1: Histopathology,
distribution and presentation. Int Endod J. 51:1205–1223.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Patel S and Ford TP: Is the resorption
external or internal? Dent Update. 34:218–20, 222, 224-6, 229.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Patel S and Saberi N: The ins and outs of
root resorption. Br Dent J. 224:691–699. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heithersay GS: Clinical, radiologic, and
histopathologic features of invasive cervical resorption.
Quintessence Int. 30:27–37. 1999.PubMed/NCBI
|
|
6
|
Tronstad L: Root resorption-etiology,
terminology and clinical manifestations. Endod Dent Traumatol.
4:241–252. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mavridou AM, Hauben E, Wevers M, Schepers
E, Bergmans L and Lambrechts P: Understanding external cervical
resorption in vital teeth. J Endod. 42:1737–1751. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Heithersay GS: Invasive cervical
resorption. Endod Topics. 7:73–92. 2004.
|
|
9
|
European Society of Endodontology (ESE)
developed by. Patel S, Lambrechts P, Shemesh H and Mavridou A:
European society of endodontology position statement: External
cervical resorption. Int Endod J. 51:1323–1326. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Andreasen JO and Andreasen FM: Textbook
and Color Atlas of Traumatic Injuries to the Teeth. 4th edition.
Blackwell Munksgaard, Copenhagen, pp1358-1381, 2007.
|
|
11
|
Kandalgaonkar SD, Gharat LA, Tupsakhare SD
and Gabhane MH: Invasive cervical resorption: A review. J Int Oral
Health. 5:124–130. 2013.PubMed/NCBI
|
|
12
|
Knowles HJ and Athanasou NA: Canonical and
non-canonical pathways of osteoclast formation. Histol and
Histopathol. 24:337–346. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mavridou AM, Pyka G, Kerckhofs G, Wevers
M, Bergmans L, Gunst V, Huybrechts B, Schepers E, Hauben E and
Lambrechts P: A novel multimodular methodology to investigate
external cervical tooth resorption. Int Endod J. 49:287–300.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wedenberg C and Lindskog S: Evidence for a
resorption inhibitor in dentin. Scand J Dent Res. 95:205–211.
1987.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mavridou AM, Hauben E, Wevers M, Schepers
E, Bergmans L and Lambrechts P: Understanding external cervical
tooth resorption patterns in endodontically treated teeth. Int
Endod J. 50:1116–1133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rotondi O, Waldon P and Kim SG: The
disease process, diagnosis and treatment of invasive cervical
resorption: A review. Dent J (Basel). 8(64)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Heithersay GS: Invasive cervical
resorption: An analysis of potential predisposing factors.
Quintessence Int. 30:83–95. 1999.PubMed/NCBI
|
|
18
|
Patel S, Foschi F, Mannocci F and Patel K:
External cervical resorption: A three-dimensional classification.
Int Endod J. 51:206–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mavridou AM, Bergmans L, Barendregt D and
Lambrechts P: Descriptive analysis of factors associated with
external cervical resorption. J Endod. 43:1602–1610.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gunst V, Mavridou A, Huybrechts B, Van
Gorp G, Bergmans L and Lambrechts P: External cervical resorption:
An analysis using cone beam and microfocus computed tomography and
scanning electron microscopy. Int Endod J. 46:877–887.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fuss Z, Tsesis I and Lin S: Root
resorption-diagnosis, classification and treatment choices based on
stimulation factors. Dent Traumatol. 19:175–182. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alqerban A, Jacobs R, Lambrechts P, Loozen
G and Willems G: Root resorption of the maxillary lateral incisor
caused by impacted canine: A literature review. Clin Oral Investig.
13:247–255. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hadler-Olsen S, Pirttiniemi P, Kerosuo H,
Bolstad Limchaichana N, Pesonen P, Kallio-Pulkkinen S and
Lähdesmäki R: Root resorptions related to ectopic and normal
eruption of maxillary canine teeth-A 3D study. Acta Odontol Scand.
73:609–615. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Friedman S, Rotstein I, Libfield H,
Stabholz A and Heling I: Incidence of external root resorption and
esthetic results in 58 bleached pulpless teeth. Endod Dent
Traumatol. 4:23–26. 1988.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Attin T, Paque F, Ajam F and Lennon AM:
Review of the current status of tooth whitening with the walking
bleach technique. Int Endod J. 36:313–329. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Heithersay GS, Dahlstrom SW and Marin PD:
Incidence of invasive cervical resorption in bleached root-filled
teeth. Aust Dent J. 39:82–87. 1994.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reiter AM, Lewis JR and Okuda A: Update on
the etiology of tooth resorption in domestic cats. Vet Clin North
Am Small Anim Pract. 35:913–942. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Reiter AM and Mendoza KA: Feline
odontoclastic resorptive lesions an unsolved enigma in veterinary
dentistry. Vet Clin North Am Small Anim Pract. 32:791–837.
2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Von Arx T, Schawalder P, Ackermann M and
Bosshardt DD: Human and feline invasive cervical resorptions: The
missing link?-Presentation of four cases. J Endod. 35:904–913.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gunst V, Huybrechts B, De Almeida Neves A,
Bergmans L, Van Meerbeek B and Lambrechts P: Playing wind
instruments as a potential aetiologic cofactor in external cervical
resorption: Two case reports. Int Endod J. 44:268–282.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Irinakis E, Aleksejuniene J, Shen Y and
Haapasalo M: External cervical resorption: A retrospective
case-control study. J Endod. 46:1420–1427. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Utting JC, Robins SP, Brandao-Burch A,
Orriss IR, Behar J and Arnett TR: Hypoxia inhibits the growth,
differentiation and bone-forming capacity of rat osteoblasts. Exp
Cell Res. 312:1693–1702. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arnett TR: Acidosis, hypoxia and bone.
Arch Biochem Biophys. 503:103–109. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Arnett TR, Gibbons DC, Utting JC, Orriss
IR, Hoebertz A, Rosendaal M and Meghji S: Hypoxia is a major
stimulator of osteoclast formation and bone resorption. J Cell
Physiol. 196:2–8. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Patel K, Mannocci F and Patel S: The
assessment and management of external cervical resorption with
periapical radiographs and cone-beam computed tomography: A
clinical study. J Endod. 42:1435–1440. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
AAE and AAOMR joint position statement:
Use of cone beam computed tomography in endodontics update. J
Endod. 41:1393–1396. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Patel S, Durack C, Abella F, Roig M,
Shemesh H, Lambrechts P and Lemberg K: European Society of
Endodontology. European society of endodontology position
statement: The use of CBCT in endodontics. Int Endod J. 47:502–504.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schwartz RS, Robbins JW and Rindler E:
Management of invasive cervical resorption: Observations from three
private practices and a report of three cases. J Endod.
36:1721–1730. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bergmans L, Van Cleynenbreugel J, Verbeken
E, Wevers M, Van Meerbeek B and Lambrechts P: Cervical external
root resorption in vital teeth, X-ray microfocus-tomographical and
histopathological case study. J Clin Periodontol. 29:580–585.
2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liang H, Burkes EJ and Frederiksen NL:
Multiple idiopathic cervical root resorption: Systematic review and
report of four cases. Dentomaxillofac Radiol. 32:150–155.
2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Patel S, Durack C, Abella F, Shemesh H,
Roig M and Lemberg K: Cone beam computed tomography in
endodontics-a review. Int Endod J. 48:3–15. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Patel S, Dawood A, Wilson R, Horner K and
Mannocci F: The detection and management of root resorption lesions
using intraoral radiography and cone beam computed tomography-an in
vivo investigation. Int Endod J. 42:831–838. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vaz de Souza D, Schirru E, Mannocci F,
Foschi F and Patel S: External cervical resorption: A comparison of
the diagnostic efficacy using 2 different cone-beam computed
tomographic units and periapical radiographs. J Endod. 43:121–125.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rodriguez G, Abella F, Duran-Sindreu F,
Patel S and Roig M: Influence of cone-beam computed tomography in
clinical decision making among specialists. J Endod. 43:194–199.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rodriguez G, Patel S, Duran-Sindreu F,
Roig M and Abella F: Influence of cone-beam computed tomography on
endodontic retreatment strategies among general dental
practitioners and endodontists. J Endod. 43:1433–1437.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Patel S, Foschi F, Condon R, Pimentel T
and Bhuva B: External cervical resorption: Part 2-management. Int
Endod J. 51:1224–1238. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Heithersay GS: Treatment of invasive
cervical resorption: An analysis of results using topical
application of trichloroacetic acid, curettage, and restoration.
Quintessence Int. 30:96–110. 1999.PubMed/NCBI
|
|
48
|
Frank AL and Torabinejad M: Diagnosis and
treatment of extra-canal invasive resorption. J Endod. 24:500–504.
1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Heithersay GS and Wilson DF: Tissue
responses in the rat to trichloroacetic acid-an agent used in the
treatment of invasive cervical resorption. Aust Dent J. 33:451–461.
1988.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Prati C and Gandolfi MG: Calcium silicate
bioactive cements: Biological perspectives and clinical
applications. Dent Mater. 31:351–370. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Grech L, Mallia B and Camilleri J:
Invesitigation of the physical properties of tricalcium silicate
cement-based root-end filling materials. Dent Mater. 29:e20–e28.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guo YJ, Du TF, Li HB, Shen Y, Mobuchon C,
Hieawy A, Wang ZJ, Yang Y, Ma J and Haapasalo M: Physical
properties and hydration behavior of a fast-setting bioceramic
endodontic material. BMC Oral Health. 16(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Torabinejad M and Parirokh M: Mineral
trioxide aggregate: A comprehensive literature review-part II:
Leakage and biocompatibility investigations. J Endod. 36:190–202.
2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Parirokh M and Torabinejad M: Mineral
trioxide aggregate: A comprehensive literature review-part I:
Chemical, physical, and antibacterial properties. J Endod.
36:16–27. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Torabinejad M, Parirokh M and Dummer PMH:
Mineral trioxide aggregate and other bioactive endodontic cements:
An updated overview-part II: Other clinical applications and
complications. Int Endod J. 51:284–317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Parirokh M, Torabinejad M and Dummer PMH:
Mineral trioxide aggregate and other bioactive endodontic cements:
An updated overview-part I: Vital pulp therapy. Int Endod J.
51:177–205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rajasekharan S, Martens LC, Cauwels RG and
Verbeeck RM: Biodentine material characteristics and clinical
applications: A review of the literature. Eur Arch Paediatr Dent.
15:147–158. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hasan AMHR, Sidhu SK and Nicholson JW:
Fluoride release and uptake in enhanced bioactivity glass ionomer
cememt (‘glass carbomer™’) compared with conventional and
resin-modified glass ionomer cements. J Appl Oral Sci.
27(e20180230)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sidhu SK and Nicholson JW: A review of
glass-ionomer cements for clinical dentistry. J Funct Biomater.
7(16)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Santamaria MP, Ambrosano GM, Casati MZ,
Nociti Júnior FH, Sallum AW and Sallum EA: Connective tissue graft
plus resin-modified glass ionomer restoration for the treatment of
gingival recession associated with non-carious cervical lesion: A
randomized-controlled clinical trial. J Clin Periodontol.
36:791–798. 2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Parirokh M and Torabinejad M: Mineral
trioxide aggregate: A comprehensive literature review-Part III:
Clinical applications, drawbacks, and mechanism of action. J Endod.
36:400–413. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Patel S, Durack C and Ricucci D: Root
resorption. In: Pathways of the pulp. 11th edition. Hargreaves KM
and Berman LH (eds). Elsevier, St. Louis, pp660-683, 2016.
|
|
63
|
Frank AL: External-internal progressive
resorption and its nonsurgical correction. J Endod. 7:473–476.
1981.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Aeinehchi M, Eslami B, Ghanbariha M and
Saffar AS: Mineral trioxide aggregate (MTA) and calcium hydroxide
as pulp-capping agents in human teeth: A preliminary report. Int
Endod J. 36:225–231. 2003.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Narita H, Itoh S, Imazato S, Yoshitake F
and Ebisu S: An explanation of the mineralization mechanism in
osteoblasts induced by calcium hydroxide. Acta Biomater. 6:586–590.
2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Salzano S and Tirone F: Conservative
nonsurgical treatment of class 4 invasive cervical resorption: A
case series. J Endod. 41:1907–1912. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Arnett TR: Extracellular pH regulates bone
cell function. J Nutr. 138:415S–418S. 2008.PubMed/NCBI View Article : Google Scholar
|