Introduction
Knee joint pain, a common morbidity in clinical
orthopedics, has various causes. Chronic knee pain is frequently
caused by osteoarthritis (OA) (1);
in elderly patients, it may be caused by rheumatoid arthritis
(2). The knee joint is a complex
and important joint (3) and knee
joint pain severely reduces the quality of life of patients. In the
clinic, treatments to alleviate pain and maintain joint mobility
are selected based on the situations of individual patients.
Treatment generally includes pain management, physical therapy and
replacement therapy, which are usually adopted in combination. In
the treatment of knee joint OA, glucosamine combined with
chondroitin sulfate have relevant roles in clinical analgesia
(4) and non-steroidal
anti-inflammatory drugs (NSAIDs) may alleviate pain, though they
are not suitable for certain patients due to their side effects,
such as injury to the intestinal mucous membrane following their
long-term oral use (5-7).
Proximal fibula osteotomy, a novel type of surgery, may be adopted
to relieve pain and improve joint function in patients, whereas
partial or total knee arthroplasty should only be considered when
all conservative treatment measures have been attempted (8).
Pulsed radiofrequency is widely adopted to relieve
pain in clinical practice (9-11).
In this process, a pulsed current emitted by a radiofrequency
generator applies a high voltage to the area near the nerve tissue.
Such energy transmission will neither destroy the anatomic basis of
pain impulse transmission, nor destroy motor nerve function; thus,
pulsed radiofrequency is a safe treatment with little risk. Erdem
and Sir (12) investigated the
effects of ultrasound-guided knee radiofrequency treatment on knee
joint pain in patients with severe knee OA or patients who
underwent knee arthroplasty and indicated that perceived pain and
disability in the knee medial nerves were relieved after the
treatment. Relevant studies have confirmed that pulsed
radiofrequency treatment for the knee joint, a novel technique for
relieving pain in OA, is able to reduce pain, relax the muscles and
improve knee function (13). Such
injuries stimulate large increases in the levels of catabolic
species, which contribute to progressive cartilage destruction in
the synovial fluid (14). Single
pulsed radiofrequency may alleviate pain, but its regulation does
not last for a long period of time. To date, only a small number of
clinical studies (15,16) have reported the use of repeated
intra-articular pulsed radiofrequency for the treatment of knee
joint pain.
Studies have indicated that knee OA is associated
with inflammatory mediators (17,18).
For instance, IL-6- and TNF-α-mediated diet and exercise affect the
pain associated with knee OA (19).
In addition, a recent study suggested that cytokine and
neuropeptide levels are associated with pain and pain relief in
patients with joint disease (20).
Several groups have focused their attention on the potential of
IL-10 as a therapeutic tool for OA therapy and prevention (21,22).
IL-10 may be a useful marker for systemic inflammatory diseases
(23). However, these studies may
underestimate the variety of biochemical mediators implicated in
long-term outcomes of OA (24). The
level of inflammatory mediators may be adopted for a comprehensive
diagnosis of patients with knee joint pain and provide an effective
reference for successful treatment strategies, such as the main
index for the degree and assessment of clinical efficacy.
The present study utilized repeated intra-articular
pulsed radiofrequency to treat patients with knee joint pain and
observed its clinical efficacy and safety in patients as well as
its effects on IL-6, IL-10 and TNF-α levels in the synovial
fluid.
Patients and methods
Subjects
A total of 64 patients with chronic knee joint pain
admitted to Caoxian People's Hospital (Caoxian, China) between
October 2016 and May 2018 were enrolled in our study and analyzed
prospectively. The 64 patients included 31 males and 33 females
between the ages of 50 and 60 years with a mean age of 52.23±11.57
years. This study was approved by the Ethics Committee of the
Caoxian People's Hospital (Caoxian, China) and all subjects signed
an informed consent form. Kellgren and Lawrence's radiological
diagnostic criteria (25) were
used, in which OA is classified into five levels: Grade 0, normal;
Grade Ⅰ, suspected narrowing of the joint space and possible
osteophytes; Grade Ⅱ, obvious osteophytes and the joint space is
suspiciously narrowed; Grade Ⅲ, moderate number of osteophytes, the
joint space is narrowed and there are sclerosing changes; Grade Ⅳ,
a large number of osteophytes, the joint space is notably narrowed
and there are severe sclerosing lesions and obvious
deformities.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients
between 50 and 60 years of age for whom complete clinical data were
available; diagnosed with knee pain lasting for 0.5-1 years with
limited joint activity; presence of a large amount of effusion
confirmed by physical examination and MRI; willingness to cooperate
with the medical staff at the hospital. The exclusion criteria were
as follows: Patients with a history of long-term analgesic drug
usage; cardio-cerebrovascular disease; severe organ failure;
combined injuryperipheral neuropathy; mental disease or
communication obstacles; and those transferred to another hospital
half-way through the study period. Patients from whom synovial
fluid collection was not successful were also excluded.
Methods
The study only included patients whose knee effusion
was confirmed by physical examination and MRI. Synovial fluid was
collected either directly or by small volume saline lavage when
direct aspiration failed. The patients were randomly divided into a
control group, who received treatment with a single intra-articular
pulsed radiofrequency through the knee joint (n=32), and the
experimental group, who received multiple intra-articular pulsed
radiofrequency treatments through the knee joint (n=32). The
subjects in the control group were treated once and those in the
experimental group were treated once every two weeks (for a total
of four times). The treatment period was two months.
Treatment methods
The patients were placed in a supine position on an
operating table with a thin pillow under the knee. The patients
were locally anesthetized by subcutaneous injection on both sides
of the knee joint. Two radiofrequency trocars (10 cm long and 10 mm
wide at the working end) were inserted into the knee articular
cavity from the knee eyes to the center of the knee joint with
assisted positioning using ultrasound-guided radiofrequency
(STARmed) manipulation of the sensory nerve around the knee. A
radiofrequency therapy device was connected and regulated to have a
voltage of <45V and a temperature of <45˚C, for 15 min. The
puncture points were covered with sterile drug film (Kanglidi
aseptic dressing; China Yangzhou Guo Tai Co., Ltd.) after
treatment. All operations were performed by the same group of
physicians and the patients' adverse reactions were closely
monitored during the treatment. If infection occurred after the
operation, adequate drainage was required, bacterial culture and
drug sensitivity tests were carried out on the pus and relevant
antibiotics were then used for anti-infection treatment according
to the drug sensitivity test.
Evaluation standards of clinical
efficacy
The degree of pain in the patients was assessed by
determining the visual analog scale score (VAS) prior to treatment
and at a minimum of 2 years following surgery (26). The pain was scored as follows: No
pain, 0; tolerable mild pain, 0-4; pain that affects sleep, 4-7;
and intolerable, severe pain, 7-10.
Treatment efficacy was evaluated as follows: ‘Cure’
was defined as complete disappearance of knee joint disease and the
patient's activity returning to normal; a ‘marked effect’ was
defined as relieved knee joint pain and flexion and improved
motion; and ‘no effect’ was defined as no relief or improvement in
symptoms and knee function, and possibly worsening of the condition
(27). The following formula was
used for evaluating the efficacy of treatment: Total effective rate
= (number of patients cured + number of patients with marked
effect)/total number of cases.
Knee function was evaluated based on the Lysholm
Knee Score Scale (LKSS) score (28)
determined prior to and after treatment. Higher scores indicated
better knee function.
Detection of inflammatory
cytokines
Inflammatory cytokines were determined as described
previously (29). The patients'
knee synovial fluid was sampled prior to and after treatment; the
synovial fluid samples were centrifuged at 299 x g for 15 min at
4˚C and then stored at -80˚C. Inflammatory indexes, including IL-6
(cat. no. KIT10395; Sino Biological Inc.), IL-10 (cat. no. BE45601;
IBL International) and TNF-α (cat. no. XF16189Q; Shanghai Xinfan
Biotechnology Co., Ltd.) were detected through ELISA in strict
accordance with the instructions of the kit. The optical density of
each well was measured at a wavelength of 450 nm using a
BIOBASE2000 ELISA automatic analyzer (Jinan Biobase Biotechnology
Co., Ltd.). From these values, the concentrations of IL-6, IL-10
and TNF-α were then calculated.
Statistical analysis
All statistical analyses were performed using SPSS
24.0 statistical software (IBM Corp.). All graphs were generated
using GraphPad 8 (GraphPad Software, Inc.). Enumeration data were
expressed as n (%) and comparisons between groups were performed
using the χ2 test or Fisher's exact test. The
Jarque-Bera test was used to test the normality of distribution of
the data. Continuous variables were expressed as the mean ±
standard deviation or median (interquartile range). Comparisons of
continuous variables were assessed using the paired t-test (before
vs. after treatment) or an unpaired t-test (control vs.
experimental group). For ordinal variables, Mann-Whitney U tests
were used for unpaired comparisons (control vs. experimental group)
and Wilcoxon signed-rank tests for paired comparisons (before vs.
after treatment). Bonferroni correction was applied for multiple
comparisons. P<0.05 was considered to indicate a significant
difference.
Results
Comparison of general characteristics
and clinical parameters
No significant differences in terms of age, sex,
body mass index, course of disease, marital status, VAS at
baseline, area of residence, smoking, drinking and exercise status
between the experimental group and the control group were present
(P>0.05), which indicated that the two groups were comparable.
The basic data of the two groups are presented in Table I.
 | Table IComparison of clinical data between
the groups. |
Table I
Comparison of clinical data between
the groups.
| Item | Experimental group
(n=32) | Control group
(n=32) | χ2 or
t | P-value |
|---|
| Age, years | 52.30±5.44 | 53.10±5.74 | 0.572 | 0.569 |
| Sex | | | 0.063 | 0.802 |
|
Male | 15 (46.87) | 16 (50.00) | | |
|
Female | 17 (52.13) | 16 (50.00) | | |
| BMI,
kg/m2 | 24.45±2.64 | 23.81±2.45 | 1.005 | 0.318 |
| Course of disease,
months | 1.84±1.24 | 1.82±1.36 | 0.061 | 0.951 |
| Marital status | | 0.075 | 0.784 | |
|
Married | 22 (68.75) | 23 (71.87) | | |
|
Unmarried | 10 (31.25) | 9 (28.13) | | |
| VAS | | | 0.169 | 0.918 |
|
0-4 | 3 (9.37) | 4 (12.5) | | |
|
4-7 | 19 (59.38) | 18 (56.25) | | |
|
7-10 | 10 (31.25) | 10 (31.25) | | |
| Area of
residence | | 0.063 | 0.802 | |
|
Town | 17 (52.13) | 16 (50.00) | | |
|
Rural | 15 (46.87) | 16 (50.00) | | |
| Smoking | | | 0.064 | 0.800 |
|
Yes | 14 (43.75) | 13 (40.62) | | |
|
No | 18 (56.25) | 19 (59.38) | | |
| Drinking | | | 0.063 | 0.801 |
|
Yes | 14 (43.75) | 15 (46.87) | | |
|
No | 18 (56.25) | 17 (52.13) | | |
VAS at different time-points
After treatment, both the control and experimental
group exhibited a lower VAS and experienced pain relief
(P<0.001). The control group after treatment had a higher VAS
than the experimental group (4.38±1.48 and 2.48±1.25, respectively)
and experienced less pain relief than the experimental group
(P<0.001; Table II).
 | Table IIVisual analog scale score of the two
groups at different time-points. |
Table II
Visual analog scale score of the two
groups at different time-points.
| Group | Before
treatment | After
treatment | t |
P-valuea |
|---|
| Control (n=32) | 6(3) | 4(5) | 5.610 | <0.001 |
| Experimental group
(n=32) | 7(6) | 2(3) | 12.002 | <0.001 |
|
P-valueb | 6(3) | 4(5) | | |
Comparison of the efficacy (%) of
treatment between the groups
Treatment in the control group had a total
effectiveness rate of 78.13%, with 13 patients cured (40.63%), 12
patients reporting a marked effect (37.5%) and no effect reported
by 7 patients (21.87%). The experimental group had a total
effectiveness rate of 90.63%, with 18 patients cured (56.25%), 11
patients reporting a marked effect (34.37%) and no effect reported
by 3 patients (9.38%; Table
III).
 | Table IIIComparison of the clinical efficacy
between the two groups. |
Table III
Comparison of the clinical efficacy
between the two groups.
| Group | Cure | Marked effect | No effect | Total effective
rate |
|---|
| Control (n=32) | 13 (40.63) | 12 (37.5) | 7 (21.87) | 25 (78.13) |
| Experimental group
(n=32) | 18 (56.25) | 11 (34.37) | 3 (9.38) | 29 (90.63) |
Comparison of adverse reactions (%)
between the groups
In the experimental group, two patients suffered an
infection (6.25%) and two patients had sciatica (6.25%). In the
control group, one patient suffered an infection (3.13%). There was
no evidence of deep venous thrombosis in either group. The
experimental group had a higher incidence of adverse reactions than
the control group (P<0.05; Table
IV).
 | Table IVComparison of adverse reactions
between the two groups. |
Table IV
Comparison of adverse reactions
between the two groups.
| Group | Infection | Sciatica | Deep venous
embolism | Total |
|---|
| Experimental group
(n=32) | 2 (6.25) | 2 (6.25) | 0 (0) | 4 (12.5) |
| Control (n=32) | 1 (3.13) | 0 (0)a | 0 (0) | 1
(3.13)a |
Evaluation of knee function at
different time-points
After treatment, the control group and the
experimental group both exhibited higher LKSS values and improved
knee joint function (P<0.001). The control group after treatment
had a lower LKSS value than the experimental group (73.31±9.17 and
84.24±13.52, respectively; P<0.001; Table V).
 | Table VEvaluation of knee joint function in
the two groups at different time-points. |
Table V
Evaluation of knee joint function in
the two groups at different time-points.
| Group | Before
treatment | After
treatment | ta | P-value |
|---|
| Control (n=32) | 41.34±7.24 | 73.31±9.17 | 21.286 | <0.001 |
| Experimental group
(n=32) | 42.19±7.18 | 84.24±13.52 | 18.751 | <0.001 |
| tb | 0.472 | 3.785 | | |
| P-value | 0.638 | <0.001 | | |
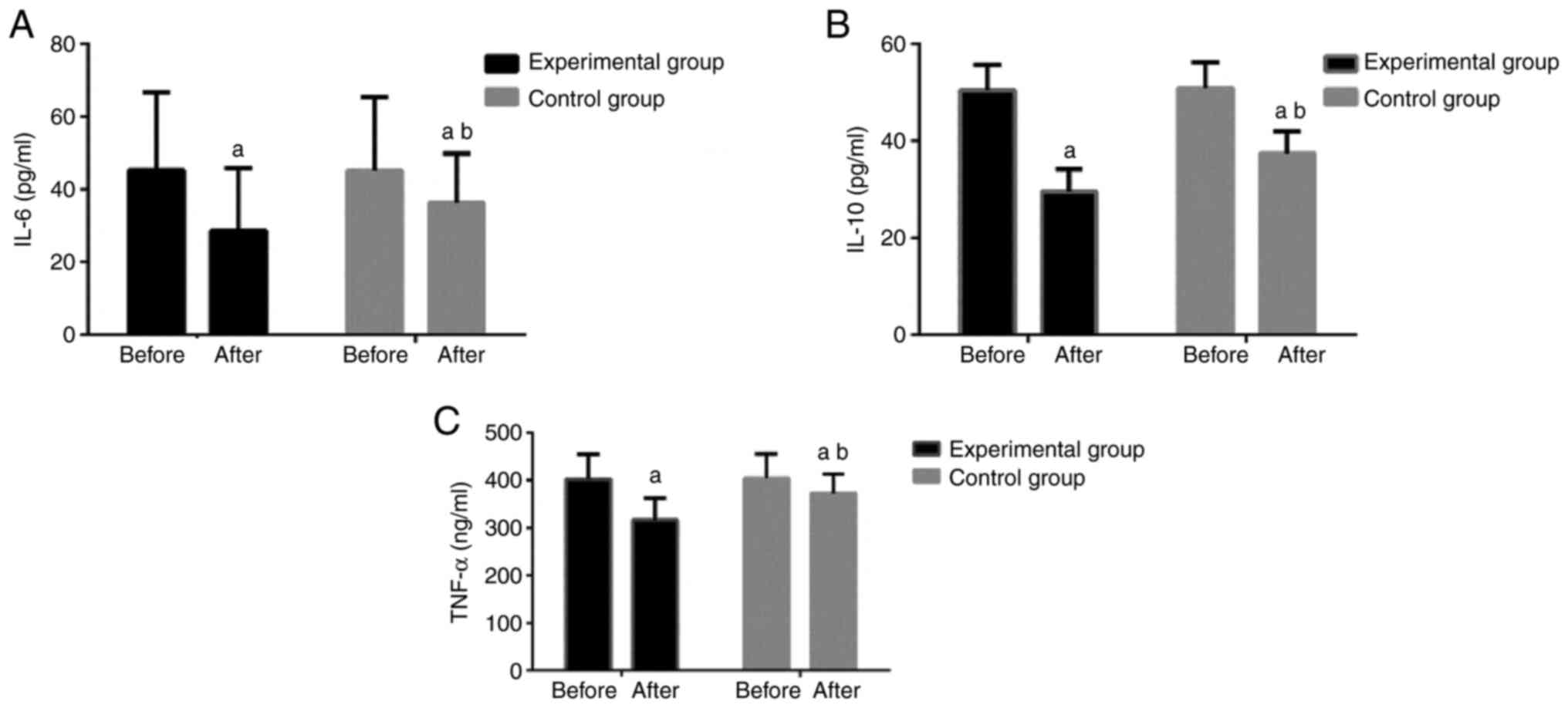
Comparison of IL-6, IL-10 and TNF-α
levels
After treatment, both groups exhibited decreased
concentrations of IL-6, IL-10 and TNF-α in the knee joint synovial
fluid (P<0.05). The experimental group had lower concentrations
of IL-6, IL-10 and TNF-α than the control group (P<0.05; and
Fig. 1).
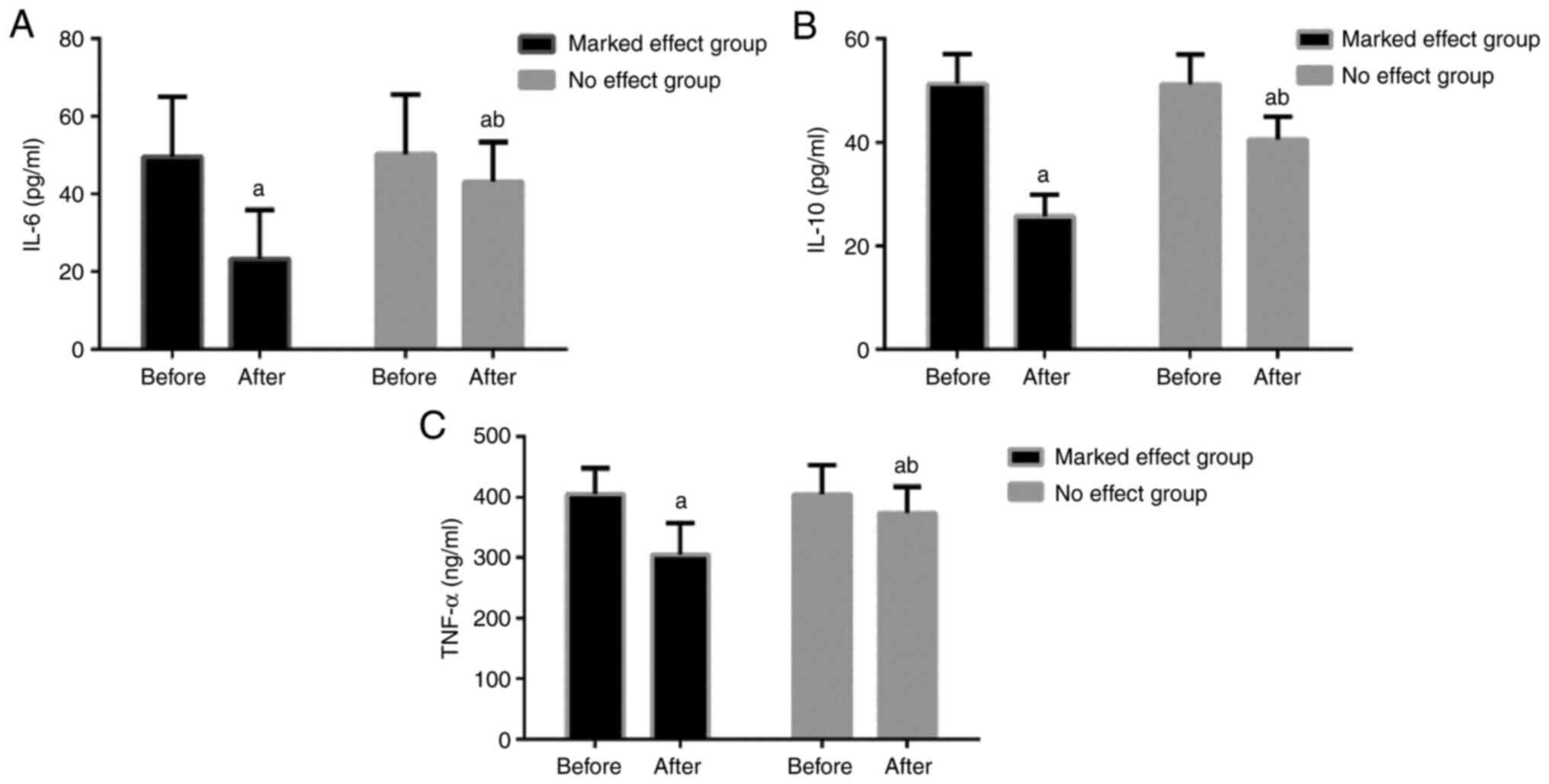
Comparison between patients with
marked effects and those with no effects within the experimental
group
The experimental group was divided into a marked
effect group, containing 29 patients, and a no effect group,
containing 3 patients, based on the clinical efficacy
evaluation.
The levels of IL-6 in the marked effect group prior
to and after treatment were 49.64±15.37 and 23.36±12.54 pg/ml,
respectively, and those of the no effect group were 50.29±15.35 and
43.18±10.24 pg/ml, respectively. The two groups had higher IL-6
levels before treatment (P<0.05) and the marked effect group had
lower IL-6 levels than the no effect group after treatment
(P<0.05 Table VI).
 | Table VIComparison of IL-6, IL-10 and TNF-α
levels between the two groups before and after treatment. |
Table VI
Comparison of IL-6, IL-10 and TNF-α
levels between the two groups before and after treatment.
| | Experimental
group | | Control group | |
|---|
| Index | Before | After | t | P-value | Before | After | t | P-value |
|---|
| IL-6, pg/ml | 45.31±21.38 |
28.60±17.27a | 2.467 | 0.020 | 45.21±20.23 |
36.39±13.43a,b | 2.452 | 0.0262 |
| IL-10, pg/ml | 50.48±5.23 |
29.54±4.64a | 11.56 | <0.001 | 50.86±5.39 |
37.43±4.59a,b | 7.982 | <0.001 |
| TNF-α, ng/ml | 402.14±53.14 |
316.35±46.53a | 9.864 | <0.001 | 403.78±52.29 |
372.71±40.26a,b | 3.271 | 0.003 |
The levels of IL-10 in the marked effect group
before and after treatment were 51.29±5.72 and 25.71±4.18 pg/ml,
respectively, and those of the no effect group were 51.23±5.73 and
40.53±4.39 pg/ml, respectively. The two groups had higher IL-10
levels prior to treatment (P<0.05) and the marked effect group
had lower IL-10 levels than the no effect group after treatment
(P<0.05).
The levels of TNF-α in the marked effect group prior
to and after treatment were 405.34±42.38 and 304.72±52.47 ng/ml,
respectively, and those of the no effect group were 404.85±48.34
and 373.84±43.10 ng/ml, respectively. The two groups had higher
TNF-α levels prior to treatment (P<0.05) and the marked effect
group had lower TNF-α levels than the no effect group after
treatment (P<0.05). These results are presented in Fig. 2.
Discussion
Knee OA is a universally disabling joint disease
that is frequently accompanied by severe joint pain, swelling,
stiffness and loss of movement (30). It is the major cause of knee joint
pain and is usually treated with conservative methods (31-34).
The European Society for Clinical and Economic Aspects of
Osteoporosis and Osteoarthritis recommends NSAIDs as the first
choice for the treatment of knee pain, particularly for OA patients
who are >75 years of age and patients with complications or
increased risks of cardiovascular, gastrointestinal or
kidney-related side effects (35).
However, for certain patients with renal insufficiency, NSAIDs
induce high nephrotoxicity (36),
indicating the need for novel therapeutic regimens.
Radiofrequency has been adopted for numerous years
to treat diseases associated with neuropathic pain (37). Pulsed radiofrequency is a
non-pharmacological treatment that has been indicated to reduce
severe chronic joint pain; this safe and minimally invasive
treatment may be performed in outpatient settings (38,39).
Recent studies have investigated the effectiveness of pulsed
radiofrequency in patients with chronic pain who were difficult to
treat with conservative methods (40) and found pulsed radiofrequency to be
an effective and reliable technique for the palliative treatment of
chronic pain in patients with gonarthritis. However, only a small
number of clinical studies (41,42) on
repeated intra-articular pulsed radiofrequency for the treatment of
knee joint pain have been performed. It has been reported that the
expression levels of cytokines, such as IL-1 receptor α, IL-6,
IL-8, IL-10, IL-15 and monocyte chemo-attractant protein-1 are
increased in the synovial fluid of patients with traumatic
anklebone arthritis, due to inflammatory injury (43). However, only a small number of
studies (16,44) on the changes in the levels of
inflammatory cytokines in the synovial fluid of patients with knee
joint pain undergoing repeated treatment with intra-articular
pulsed radiofrequency have been performed, which is worth
investigating.
The results of the present study revealed that after
treatment with intra-articular pulsed radiofrequency, the subjects
in the experimental group had a lower VAS and higher total
effectiveness rate than those in the control group, while
experiencing a higher degree of pain relief and improved knee joint
function. This indicated that the efficacy of the treatment in the
experimental group was better than that in the control group. Nagar
et al (45) compared pulsed
radiofrequency therapy and continuous radiofrequency therapy in the
treatment of patients with facet joint lower back pain and
demonstrated that continuous radiofrequency therapy was more
effective, which is similar to the results of the present study. In
the present study, the subjects in the experimental group had a
higher incidence of adverse reactions than those in the control
group. It may be hypothesized that the increase in the number of
treatments in the experimental group led to an increase in the
number of adverse reactions, which suggests that close attention
must be paid to whether patients are affected by other diseases
during treatment.
After treatment, both groups had decreased
concentrations of IL-6, IL-10 and TNF-α in the synovial fluid of
the knee joint. The experimental group performed better than the
control group with regard to these indexes; the marked effect group
had lower concentrations of IL-6, IL-10 and TNF-α than the control
group, which was consistent with the results of the study by Li
et al (46) on the
correlation of changes in the serum inflammatory cytokines with
knee joint pain symptoms. Their study revealed that the degree of
pain was closely related to TNF-α levels. It may be hypothesized
that repeated intra-articular pulsed radiofrequency may reduce the
inflammatory response and lower the degree of knee pain in patients
by inhibiting the expression of IL-6, IL-10 and TNF-α in the
synovial fluid. By assessing the duration of pulsed radiofrequency
in alleviating neuropathic pain, Ramzy et al (47) determined that a prolonged duration
of pulsed radiofrequency had a better analgesic effect and that an
increase in duration was associated with a significant decrease in
IL-6 and TNF-α levels; these results support the present hypothesis
that pulsed radiofrequency reduces the production of
pro-inflammatory cytokines. Moffett et al (48) studied the regulatory mechanism of
pulsed radiofrequency energy on peripheral pain and determined that
the levels of primary transcription products produced by structural
gene pre-mRNA, an endogenous opiate-like substance, and
corresponding opioid peptide levels were increased, which further
supports the present hypothesis.
The present study confirmed that repeated
intra-articular pulsed radiofrequency is a feasible treatment
method for patients with knee joint pain based on the comparison of
single and repeated treatments. However, there are certain
limitations to the present study. For instance, the number of
research subjects included in the study was low and all patients
undergoing repeated treatment were treated at the same time. The
treatment time of the patients was not determined based on their
individual conditions; thus, the patients' pain relief was
inconsistent. The patients' age was associated with certain
problems, e.g. inflammatory mediator levels in a 47-year-old
patient may not be comparable with those of a 60-year-old; however,
the median age was similar among groups. Of note, there was a lack
of homogeneity during patient selection. In future studies, it will
be endeavored to improve the study design and screen patients
according to strict inclusion and exclusion criteria in order to
obtain more consistent results in the future.
In conclusion, repeated intra-articular pulsed
radiofrequency is an effective method for the treatment of knee
joint pain with a good analgesic effect and it may be used in
clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
All authors conceived and designed the study and
interpreted the results of the experiments. JZ and ZW performed
experiments and analyzed data. HX prepared figures and drafted the
manuscript. ZY edited and revised the manuscript, designed the
current study and analyzed the data. All authors read and approved
the final version of the manuscript. JZ and HX confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Caoxian People's Hospital (Caoxian, China) and all research
subjects signed an informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teixeira JM, Bobinski F, Parada CA, Sluka
KA and Tambeli CH: P2X3 and P2X2/3 receptors play a crucial role in
articular hyperalgesia development through inflammatory mechanisms
in the knee joint experimental synovitis. Mol Neurobiol.
54:6174–6186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
McWilliams DF, Ferguson E, Young A, Kiely
PD and Walsh DA: Discordant inflammation and pain in early and
established rheumatoid arthritis: Latent Class Analysis of Early
Rheumatoid Arthritis Network and British Society for Rheumatology
Biologics Register data. Arthritis Res Ther. 18(295)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hirschmann MT and Müller W: Complex
function of the knee joint: The current understanding of the knee.
Knee Surg Sports Traumatol Arthrosc. 23:2780–2788. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Provenza JR, Shinjo SK, Silva JM, Peron CR
and Rocha FA: Combined glucosamine and chondroitin sulfate, once or
three times daily, provides clinically relevant analgesia in knee
osteoarthritis. Clin Rheumatol. 34:1455–1462. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu J and Wang F: Preoperative celecoxib
analgesia is more efficient and equally tolerated compared to
postoperative celecoxib analgesia in knee osteoarthritis patients
undergoing total knee arthroplasty: A randomized, controlled study.
Medicine (Baltimore). 97(e13663)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tellegen AR, Rudnik-Jansen I, Pouran B, de
Visser HM, Weinans HH, Thomas RE, Kik MJL, Grinwis GCM, Thies JC,
Woike N, et al: Controlled release of celecoxib inhibits
inflammation, bone cysts and osteophyte formation in a preclinical
model of osteoarthritis. Drug Deliv. 25:1438–1447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Inoue T, Iijima H, Arimitsu J, Hagihara K,
Kawai S, Shiraishi E, Hiyama S, Mukai A, Shinzaki S, Nishida T, et
al: Amelioration of small bowel injury by switching from
nonselective nonsteroidal anti-inflammatory drugs to celecoxib in
rheumatoid arthritis patients: A pilot study. Digestion.
89:124–132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang X, Wei L, Lv Z, Zhao B, Duan Z, Wu W,
Zhang B and Wei X: Proximal fibular osteotomy: A new surgery for
pain relief and improvement of joint function in patients with knee
osteoarthritis. J Int Med Res. 45:282–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lindquist J and Bäckryd E: Pulsed
radiofrequency in clinical practice - A retrospective analysis of
238 patients with chronic non-cancer pain treated at an academic
tertiary pain centre. Scand J Pain. 12:68–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang YH, Hou SY, Cheng JK, Wu CH and Lin
CR: Pulsed radiofrequency attenuates diabetic neuropathic pain and
suppresses formalin-evoked spinal glutamate release in rats. Int J
Med Sci. 13:984–991. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Albayrak I, Apiliogullari S, Onal O,
Gungor C, Saltali A and Levendoglu F: Pulsed radiofrequency applied
to the dorsal root ganglia for treatment of post-stroke complex
regional pain syndrome: A case series. J Clin Anesth. 33:192–197.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Erdem Y and Sir E: The efficacy of
ultrasound-guided pulsed radiofrequency of genicular nerves in the
treatment of chronic knee pain due to severe degenerative disease
or previous total knee arthroplasty. Med Sci Monit. 25:1857–1863.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vas L, Pai R, Khandagale N and Pattnaik M:
Pulsed radiofrequency of the composite nerve supply to the knee
joint as a new technique for relieving osteoarthritic pain: A
preliminary report. Pain Physician. 17:493–506. 2014.PubMed/NCBI
|
|
14
|
Bigoni M, Turati M, Zatti G, Gandolla M,
Sacerdote P, Piatti M, Castelnuovo A, Rigamonti L, Munegato D,
Franchi S, et al: Intra-articular cytokine levels in adolescent
patients after anterior cruciate ligament tear. Mediators Inflamm.
2018(4210593)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shin SM, Kwak SG, Lee DG and Chang MC:
Clinical effectiveness of intra-articular pulsed radiofrequency
compared to intra-articular corticosteroid injection for management
of Atlanto-occipital joint pain: A prospective randomized
controlled pilot study. Spine. 43:741–746. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Filippiadis D, Charalampopoulos G, Mazioti
A, Alexopoulou E, Vrachliotis T, Brountzos E, Kelekis N and Kelekis
A: Interventional radiology techniques for pain reduction and
mobility improvement in patients with knee osteoarthritis. Diagn
Interv Imaging. 100:391–400. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ogura T, Suzuki M, Sakuma Y, Yamauchi K,
Orita S, Miyagi M, Ishikawa T, Kamoda H, Oikawa Y, Kanisawa I, et
al: Differences in levels of inflammatory mediators in meniscal and
synovial tissue of patients with meniscal lesions. J Exp Orthop.
3(7)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li S, Wan J, Anderson W, Sun H, Zhang H,
Peng X, Yu Z, Wang T, Yan X and Smith W: Downregulation of IL-10
secretion by Treg cells in osteoarthritis is associated with a
reduction in Tim-3 expression. Biomed Pharmacother. 79:159–165.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Runhaar J, Beavers DP, Miller GD, Nicklas
BJ, Loeser RF, Bierma-Zeinstra S and Messier SP: Inflammatory
cytokines mediate the effects of diet and exercise on pain and
function in knee osteoarthritis independent of BMI. Osteoarthritis
Cartilage. 27:1118–1123. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Singh JA, Noorbaloochi S and Knutson KL:
Cytokine and neuropeptide levels are associated with pain relief in
patients with chronically painful total knee arthroplasty: A pilot
study. BMC Musculoskelet Disord. 18(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bigoni M, Zanchi N, Omeljaniuk RJ, Zatti
G, Locatelli V, Torsello A and Turati M: Role of interleukin-10 in
the synovial fluid of the anterior cruciate ligament injured knee.
Eur Rev Med Pharmacol Sci. 23:932–940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Siqueira MB, Frangiamore S, Klika AK,
Gajewski N, Barsoum WK and Higuera CA: Comparison of synovial fluid
cytokine levels between traumatic knee injury and end-stage
osteoarthritis. J Knee Surg. 30:128–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wassilew GI, Lehnigk U, Duda GN, Taylor
WR, Matziolis G and Dynybil C: The expression of proinflammatory
cytokines and matrix metalloproteinases in the synovial membranes
of patients with osteoarthritis compared with traumatic knee
disorders. Arthroscopy. 26:1096–1104. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cuellar VG, Cuellar JM, Golish SR, Yeomans
DC and Scuderi GJ: Cytokine profiling in acute anterior cruciate
ligament injury. Arthroscopy. 26:1296–1301. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brandt KD, Fife RS, Braunstein EM and Katz
B: Radiographic grading of the severity of knee osteoarthritis:
Relation of the Kellgren and Lawrence grade to a grade based on
joint space narrowing, and correlation with arthroscopic evidence
of articular cartilage degeneration. Arthritis Rheum. 34:1381–1386.
1991.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tashjian RZ, Hung M, Keener JD, Bowen RC,
McAllister J, Chen W, Ebersole G, Granger EK and Chamberlain AM:
Determining the minimal clinically important difference for the
American Shoulder and Elbow Surgeons score, Simple Shoulder Test,
and visual analog scale (VAS) measuring pain after shoulder
arthroplasty. J Shoulder Elbow Surg. 26:144–148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Manimunda SP, Vijayachari P, Uppoor R,
Sugunan AP, Singh SS, Rai SK, Sudeep AB, Muruganandam N, Chaitanya
IK and Guruprasad DR: Clinical progression of chikungunya fever
during acute and chronic arthritic stages and the changes in joint
morphology as revealed by imaging. Trans R Soc Trop Med Hyg.
104:392–399. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Collins NJ, Misra D, Felson DT, Crossley
KM and Roos EM: Measures of knee function: International Knee
Documentation Committee (IKDC) Subjective Knee Evaluation Form,
Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury
and Osteoarthritis Outcome Score Physical Function Short Form
(KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale
(KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS),
Western Ontario and McMaster Universities Osteoarthritis Index
(WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score
(TAS). Arthritis Care Res (Hoboken). 63 (Suppl 11):S208–S228.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhai KF, Duan H, Khan GJ, Xu H, Han FK,
Cao WG, Gao GZ, Shan LL and Wei ZJ: Salicin from Alangium
chinense ameliorates rheumatoid arthritis by modulating the
Nrf2-HO-1-ROS pathways. J Agric Food Chem. 66:6073–6082.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wallace IJ, Worthington S, Felson DT,
Jurmain RD, Wren KT, Maijanen H, Woods RJ and Lieberman DE: Knee
osteoarthritis has doubled in prevalence since the mid-20th
century. Proc Natl Acad Sci USA. 114:9332–9336. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aciksoz S, Akyuz A and Tunay S: The effect
of self-administered superficial local hot and cold application
methods on pain, functional status and quality of life in primary
knee osteoarthritis patients. J Clin Nurs. 26:5179–5190.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Conaghan PG, Hunter DJ, Cohen SB, Kraus
VB, Berenbaum F, Lieberman JR, Jones DG, Spitzer AI, Jevsevar DS,
Katz NP, et al: FX006-2014-008 participating investigators: Effects
of a single intra-articular injection of a microsphere formulation
of triamcinolone acetonide on knee osteoarthritis pain: a
double-blinded, randomized, placebo-controlled, multinational
study. J Bone Joint Surg Am. 100:666–677. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yilmaz M, Sahin M and Algun ZC: Comparison
of effectiveness of the home exercise program and the home exercise
program taught by physiotherapist in knee osteoarthritis. J Back
Musculoskeletal Rehabil. 32:161–169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Özkuk K, Gürdal H, Karagülle M, Barut Y,
Eröksüz R and Karagülle MZ: Balneological outpatient treatment for
patients with knee osteoarthritis; an effective non-drug therapy
option in daily routine? Int J Biometeorol. 61:719–728.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rannou F, Pelletier JP and
Martel-Pelletier J: Efficacy and safety of topical NSAIDs in the
management of osteoarthritis: Evidence from real-life setting
trials and surveys. Semin Arthritis Rheum. 45 (Suppl 4):S18–S21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Strawson J: Nonsteroidal anti-inflammatory
drugs and cancer pain. Curr Opin Support Palliat Care. 12:102–107.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang RY, Liao CC, Tsai SY, Yen CT, Lin
CW, Chen TC, Lin WT, Chang CH and Wen YR: Rapid and delayed effects
of pulsed radiofrequency on neuropathic pain: Electrophysiological,
molecular, and behavioral evidence supporting long-term depression.
Pain Physician. 20:E269–E283. 2017.PubMed/NCBI
|
|
38
|
Gulec E, Ozbek H, Pektas S and Isik G:
Bipolar versus unipolar intraarticular pulsed radiofrequency
thermocoagulation in chronic knee pain treatment: A prospective
randomized trial. Pain Physician. 20:197–206. 2017.PubMed/NCBI
|
|
39
|
Eyigor C, Eyigor S, Akdeniz S and Uyar M:
Effects of intra-articular application of pulsed radiofrequency on
pain, functioning and quality of life in patients with advanced
knee osteoarthritis. J Back Musculoskeletal Rehabil. 28:129–134.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Masala S, Fiori R, Raguso M, Morini M,
Calabria E and Simonetti G: Pulse-dose radiofrequency for knee
osteoartrithis. Cardiovasc Intervent Radiol. 37:482–487.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schianchi PM, Sluijter ME and Balogh SE:
The treatment of joint pain with intra-articular pulsed
radiofrequency. Anesth Pain Med. 3:250–255. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rahimzadeh P, Imani F, Faiz SH, Entezary
SR, Nasiri AA and Ziaeefard M: Investigation the efficacy of
intra-articular prolotherapy with erythropoietin and dextrose and
intra-articular pulsed radiofrequency on pain level reduction and
range of motion improvement in primary osteoarthritis of knee. J
Res Med Sci. 19:696–702. 2014.PubMed/NCBI
|
|
43
|
Adams SB Jr, Nettles DL, Jones LC, Miller
SD, Guyton GP and Schon LC: Inflammatory cytokines and cellular
metabolites as synovial fluid biomarkers of posttraumatic ankle
arthritis. Foot Ankle Int. 35:1241–1249. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Filippiadis D, Velonakis G, Mazioti A,
Konstantos C, Brountzos E, Kelekis N and Kelekis A: Intra-articular
application of pulsed radiofrequency combined with
viscosupplementation for improvement of knee osteoarthritis
symptoms: A single centre prospective study. Int J Hyperthermia.
34:1265–1269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nagar VR, Birthi P, Grider JS and Asopa A:
Systematic review of radiofrequency ablation and pulsed
radiofrequency for management of cervicogenic headache. Pain
Physician. 18:109–130. 2015.PubMed/NCBI
|
|
46
|
Li S, An R, Wang Z, Kuang L, Tan W and
Fang C: Correlation analysis of bone marrow edema degree and serum
inflammatory factors change with knee joint pain symptoms in
patients with bone contusion around the knee joint. Zhongguo Xiu Fu
Chong Jian Wai Ke Za Zhi. 28:615–619. 2014.PubMed/NCBI(In Chinese).
|
|
47
|
Ramzy EA, Khalil KI, Nour EM, Hamed MF and
Taha MA: Evaluation of the effect of duration on the efficacy of
pulsed radiofrequency in an animal model of neuropathic pain. Pain
Physician. 21:191–198. 2018.PubMed/NCBI
|
|
48
|
Moffett J, Fray LM and Kubat NJ:
Activation of endogenous opioid gene expression in human
keratinocytes and fibroblasts by pulsed radiofrequency energy
fields. J Pain Res. 5:347–357. 2012.PubMed/NCBI View Article : Google Scholar
|