Introduction
Chronic heart failure (CHF) is a worldwide public
health problem that severely threatens human health. Its main
manifestation is abnormal cardiac structure, leading to impaired
cardiac filling or ejection function (1). The majority of cases of heart failure
(HF) have a poor prognosis. According to European data, acute HF
accounts for 17.4% of all-cause mortality per year, while CHF
accounts for 7.2% (2). In
high-income countries, the cost of HF treatment accounts for 2-3%
of the total health system expenditure, and is expected to more
than double in the next 20 years (3). At present, the clinical treatment of
HF drugs mainly includes diuretics, angiotensin converting enzyme
inhibitors, angiotensin receptor blockers and glucocorticoid
receptor antagonists (4). Although
there are diverse HF clinical treatments, the mortality and
disability rates due to HF remain high (5).
Traditional Chinese Medicine, including astragalus,
ginseng, ginsenoside and pepperweed seed, has a long history in the
treatment of HF, and has the advantages of multiple targets, low
cost and few side effects (6).
Astragalus has been widely used in the treatment of cardiovascular
diseases in China, and astragaloside IV (AS-IV) is one of its
important effective components. The main pharmacological effects of
AS-IV include enhancing immunity, anti-inflammatory, antioxidation
and anti-viral (7). In recent
years, it has been demonstrated that AS-Ⅳ exerts cardiac protection
by regulating intracellular calcium homeostasis and the antioxidant
response, improving myocardial energy metabolism, inhibiting
apoptosis and alleviating cytotoxicity. The results of a previous
study supported the effectiveness and safety of AS-IV in HF models
in vivo and in vitro (7). However, the mechanism of its action in
HF requires further study.
Ubiquitin is a small molecule protein that exists in
the majority of eukaryotic cells. The main function of protein
ubiquitination is to mark the proteins for degradation, followed by
removal by proteolytic enzyme hydrolysis. More than 80% of cellular
proteins are degraded by the ubiquitin proteasome system (8). Small ubiquitin-like modifier (SUMO) is
a post-translational modification protein with similar structure
but different functions to ubiquitin. SUMO binds to specific lysine
sites on the target protein, to stabilize the target protein
structure, mediate cytosol-nuclear translocation, regulate
downstream transcription factors, regulate protein interactions and
suppress ubiquitination (9). Like
the ubiquitination pathway, SUMO precursor synthesis, hydrolysis
and activation involve a series of enzymes, including E1 activating
enzyme, E2 binding enzyme, E3 ligase and SUMO proteases (SENPs). At
present, six SENP proteins (SENP1, SENP2, SENP3, SENP5, SENP6,
SENP7) participate in the SUMOylation process. Furthermore,
deSUMOylation serves an important role in determining the degree of
protein SUMOylation (10). It has
been demonstrated that SENP1, an important deSUMOylation enzyme,
serves a central role in inhibiting cardiac hypertrophy and cardiac
dysfunction (11). It has also been
demonstrated that SENP1 serves an important regulatory role in
oxidative stress and mitochondrial damage (12). However, its role in the AS-Ⅳ
protective effect has not been reported. Therefore, it was unclear
whether AS-Ⅳ inhibits the occurrence and development of HF by
regulating SENP1.
In the present study, a mouse HF model was
established by aortic coarctation, and the protective effects of
AS-IV on cardiac function and oxidative stress of myocardial cells
were examined in vivo. Furthermore, the expression of Senp1
in the HL-1 cell line was modulated to determine whether the
protective effects of AS-Ⅳ on HF myocardium are associated with
Senp1. The results of the present study provided a novel
theoretical basis and clinical reference for HF treatment based on
SUMOylation in the future.
Materials and methods
Animals and main reagents
Male C57BL/6J mice (8 weeks old) were purchased from
the Institute of Experimental Animals, Chinese Academy of Medical
Sciences. The mouse atrial muscle HL-1 cell line was purchased from
ATCC. A lentiviral plasmid containing HA-Senp1 was
constructed by Suzhou Jima gene Co., Ltd. AS-IV was purchased from
Sigma-Aldrich; Merck KGaA. Claycomb Medium (51800C),
penicillin/streptomycin (V900929) and glutamine (200 mM, G8540)
were from Sigma-Aldrich; Merck KGaA. Fetal bovine serum (10099141C)
and trypsin (25200072) were purchased from Gibco; Thermo Fisher
Scientific, Inc. Antibodies against cleaved-caspase-3 (cat. no.
9661, 1:1,000), caspase-3 (cat. no. 9662, 1:1,000), BCL2 (cat. no.
15071, 1:1,000), Bax (cat. no. 2774, 1:1,000), Senp1 (cat. no.
11929, 1:1,000) and β-actin (cat. no. 3700, 1:1,000) were purchased
from Cell Signaling Technology, Inc. Goat anti-mouse IgG (cat. no.
115-035-003) and goat anti-rabbit IgG (cat. no. 111-035-003) were
purchased from Jackson Laboratory. Amplex Red reagent (cat. no.
A22177) was purchased from Invitrogen; Thermo Fisher Scientific,
Inc. Mitochondrial membrane potential detection kit (kit. no.
C2006), lactate dehydrogenase (LDH) cytotoxicity test kit (kit. no.
C0016), and reactive oxygen species (ROS) detection kit (kit. no.
S0033M) were purchased from Shanghai Beyotime Biotechnology Co.,
Ltd. Terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) kit (kit. no. 11684817910) was purchased from Roche
Diagnostics GmbH.
Animal model
Animal experiments were performed according to the
regulations and guidelines approved by the Animal Ethics Committee
of The Fifth Central Hospital of Tianjin (approval. no.
TJWZX2019018). Animal studies adhered to the Guide for the Care and
Use of Laboratory Animals (8th edition, 2011, the Institute for
Laboratory Animal Research of the National Research Council in the
USA). A total of 24 male C57BL/6J mice (8-week-old; mean weight, 20
g) were purchased from the Animal Center of Nanjing University.
Animals were housed in the Experimental Animal Center of The Fifth
Central Hospital of Tianjin, and maintained under a controlled
temperature (22-24˚C), stable humidity (40-60%) and a 12
h-light/dark cycle with ad libitum access to food and water.
To establish the HF model, the mice were anesthetized with 2%
isoflurane from Zaozhuang Shuitailan Chemical Co. Ltd., and then
transferred to the operating table. Following endotracheal
intubation, they were connected to a ventilator, and further
anesthetized with 1.5% isoflurane. After the mouse chest was
disinfected, a longitudinal incision was made in the second rib to
open the chest and expose the thoracic segment of the aorta.
Finally, a 7-0 thread was inserted below the brachiocephalic trunk
of the aorta and the left common carotid artery. A 26 G fine needle
was used to ligate the aortic arch, and the needle was removed
following ligation. After 6 weeks, echocardiography was used to
determine whether the model was successfully established. The mice
were randomly divided into control group, HF group, HF+AS-IV group
and AS-IV group. The HF+AS-IV group and AS-IV group were
intraperitoneally injected with 40 mg/kg AS-IV every other day. The
control group and HF group were given the same dose of solvent
(dimethyl sulfoxide, 0.1%). After 4 weeks of drug intervention, the
mice underwent cardiac ultrasound examination. At the end of the
experiments, the mice were euthanized by CO2 exposure
(CO2 displacement rate equivalent to 20% of the chamber
volume/min, October 2019) and cervical dislocation.
Cell culture
HL-1 cardiomyocytes from mouse heart tissue were
purchased from ATCC. The cells were cultured in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum, 100 U
penicillin/streptomycin and 2 mM glutamine at 37˚C, 5%
CO2, 95% air and 95% humidity. Following vector or
HA-Senp1 plasmid transfection for 24 h, the HF group was
treated with isoprenaline (ISO, 20 µmol/l) for 24 h, and the
HF+AS-IV group was pretreated with 25, 50, 100 or 200 µmol/l AS-IV
for 30 min at 37˚C, and then with 20 µmol/l ISO for 24 h at 37˚C.
The mitochondrial membrane potential (Δψm) and ROS were examined
after 24 h of treatment.
Isolation of mouse left ventricular
mitochondria
The mitochondria and cytoplasm were separated by a
Tissue Mitochondrial Separation kit (Beyotime Institute of
Biotechnology) using differential centrifugation, according to the
manufacturer's protocols. Briefly, heart tissue blocks of the same
weight and from the same region were washed with precooled PBS
solution, cut into small pieces and transferred to a precooled
glass homogenizer. Mitochondrial isolation solution containing
protease inhibitors was added and the tissue was homogenized in an
ice bath ~10 times. The supernatant was extracted following
centrifugation at 4˚C for 5 min at 600 x g. Subsequently, the
supernatant was discarded following centrifugation at 4˚C for 10
min at 11,000 x g. The remaining precipitate comprised the isolated
mitochondria.
Measurement of mitochondrial membrane
potential
Isolated mitochondria or HL-1 cells were incubated
with JC-1 (10 µg/ml) for 20 min at 37˚C. Mitochondria were examined
by a fluorescence reader and HL-1 cells were examined by a laser
scanning confocal microscope (Olympus FV 1200; Olympus Corporation;
magnification x60). The excitation and emission wavelengths of the
green fluorescence of JC-1 monomer were 488 and 525 nm,
respectively, and of the red fluorescence of JC-1 aggregate were
543 and 590 nm, respectively. The change in fluorescence intensity
of each experimental group is expressed as polymer/monomer. The
temperature was maintained at 37˚C.
Measurement of ROS
Isolated myocardial mitochondria or HL-1 cells were
incubated with DCFH-DA (10 µM) for 20 min at 37˚C. Mitochondria
were examined by a fluorescence reader and HL-1 cells were examined
by a laser scanning confocal microscope (Olympus FV 1200; Olympus
Corporation; magnification x100). The excitation and emission
wavelengths of DCFH-DA were 488 and 525 nm, respectively. The
fluorescence intensity of DCFH-DA is expressed as a percentage of
the control group.
Measurements of mitochondrial hydrogen
peroxide (H2O2)
The Amplex Red
H2O2 detection kit was used
according to the manufacturer's protocols;
H2O2 in isolated mitochondria or HL-1 cells
was detected by a fluorescence plate reader. The excitation and
emission wavelengths of H2O2 were 543 and 590
nm, respectively. The change in fluorescence intensity in each
experimental group is expressed as a percentage of the control
group.
Western blotting
Total protein was extracted from cells or tissues
using RIPA buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) supplemented with 1 mM PMSF and 20 mM N-ethylmaleimide. The
protein supernatant was collected following centrifugation at
10,000 x g at 4˚C for 15 min. Protein concentration was measured
using a BCA protein assay (Beijing Solarbio Science &
Technology Co., Ltd.). Equal amounts of protein lysates (40 µg per
lane; 2 µg/µl) were separated on SDS-polyacrylamide gel using 10%
gels and were transferred onto PVDF membranes. The membranes were
then blocked with 5% skimmed milk for 1 h at room temperature.
Next, the membranes were incubated overnight with the
aforementioned primary antibodies at 4˚C overnight, and then with
secondary antibodies for 1 h at room temperature. Finally, the
protein bands were visualized by enhanced chemiluminescence (EMD
Millipore). Densitometric semi-quantification analysis of the
Western blot bands was performed using image analysis software
(ImageJv1.48; National Institutes of Health). Protein band
intensity was normalized to β-actin and expressed as a percentage
of the naive control.
TUNEL staining
To detect apoptosis, a TUNEL kit (Roche Diagnostics
GmbH) was used. Following treatment, HL-1 cells were fixed in 4%
paraformaldehyde for 40 min at 25˚C, blocked with 3%
H2O2 for 10 min at 25˚C, and permeated with
0.1% Triton X-100 for 3 min. Apoptotic cells were labeled with the
TUNEL reaction mixture and nuclei were stained with DAPI (1 µg/ml)
for 3 min at 25˚C. Cells were washed twice with PBS for 5 min at
room temperature and mounted with ProLong Gold Antifade reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Images were randomly
obtained using a fluorescence microscope (Olympus ix73; Olympus
Corporation; dp73 camera; magnification x10).
Echocardiography
Eight weeks after the operation, mice were
anesthetized with pentobarbital sodium (30 mg/kg) and placed on a
warm pad. Vevo 770® (Visualsonics Inc.) and a 716 probe
were used to dynamically evaluate the cardiac function of mice by
echocardiography. Left ventricular end-systolic diameter (LVESD)
and left ventricular end-diastolic diameter (LVEED) were obtained
by M-type tracing. Ejection fraction (EF) and shortening fraction
(FS) were automatically obtained by high-resolution
echocardiography (ECG).
Histological examination
Myocardial tissue was fixed with 4% paraformaldehyde
for 24 h at 4˚C, dehydrated, paraffin embedded and sectioned (4 µm
thick). Pathological sections were stained with hematoxylin for 5
min at 4˚C and eosin for 3 min at 4˚C (Nanjing SenBeiJia Biological
Technology Co., Ltd.). H&E staining was observed under a light
microscope (Olympus Medical Systems; magnification, x20). The
hypertrophy of cardiomyocytes was quantified by counting the
cross-sectional area of cardiomyocytes using Image-Pro Plus 6.0
(Media Cybernetics, Inc.).
HA-Senp1-overexpression plasmid and
cell transfection
Total RNA was extracted from the HL-1 cells using a
TRIzol® RNA extraction kit (Tiangen Biotech Co., Ltd.).
The RNA extracted from HL-1 cells was used to obtain the
full-length cDNA of mouse Senp1 by RT-PCR using a RT-PCR kit
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc.). The full-length Senp1 cDNA (Accessions;
NM_001379573.1; Insert size: 2019 bp) was subcloned into mammalian
Lentivirus Expression Vector pwpxl (Thermo Fisher Scientific, Inc.)
with a HA tag at the C-terminus (HA-SENP1), and the empty plasmid,
pwpxl, was used as the negative control group (vector). The empty
vector and the vectors encoding the SENP1 (HA-SENP1) were
transiently transfected into HL-1 cells using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols (DNA=4 µg, reagent=10 µl). In brief, cells
were seeded into a 6-well plate at 37˚C, 5% CO2 and 95%
humidity. A total of 18-24 h after seeding, the density of the HL-1
cells reached 40-50%, prior to being transfected with empty vector
or HA-SENP1 using Lipofectamine 2000 transfection reagent (DNA=4
µg, reagent=10 µl). Cells were then inoculated into fresh medium 24
h after the transfection. All subsequent experiments were performed
48 h after transfection.
Cell viability assay
Cell viability was examined using a Cell Counting
Kit-8 (CCK-8; Beijing Solarbio Science & Technology Co., Ltd.)
assay. Hl-1 cells were cultured in 96-well plates (2x103
cells/well) and treated as described in ‘cell culture’.
Subsequently, 10 µl CCK-8 solution was added to each well and
incubated at 37˚C for 2 h. A microplate reader (VersaMax™
Microplate Reader; Molecular Devices, LLC) was used to analyze the
absorbance at 450 nm. To increase the reliability of measures, each
experiment was performed three times.
Statistical analysis
All HL-1 cell culture dishes and mice were randomly
assigned to different experimental groups. Data are presented as
the mean ± standard deviation. Statistical analysis was performed
using GraphPad Prism 6.0 software (GraphPad Software, Inc.). The
differences between the experimental and control groups were tested
using unpaired t-test. For comparing differences among more than
two experimental groups, the means were compared using one-way
analysis of variance (ANOVA), followed by Tukey's test, or two-way
ANOVA, followed by Bonferroni's test. Normal distribution was
assessed with the Shapiro-Wilk test. P<0.05 was considered to
indicate a statistically significant difference.
Results
AS-IV treatment improves contraction
function in transverse aortic constriction (TAC)-induced heart
failure
Compared with the control group, the heart weight
and the heart mass/body weight ratio were increased in the HF group
(P<0.05); however, AS-IV reversed this increase induced by
pressure overload-driven heart failure (P<0.05; Fig. 1). The ultrasound results
demonstrated that, compared with the control group, the EF, FS,
LVID;d and LVID;s were increased in the HF group (P<0.05;
Fig. 1A and B), suggesting that the heart function of
the HF group was impaired. However, AS-IV improved the heart
function.
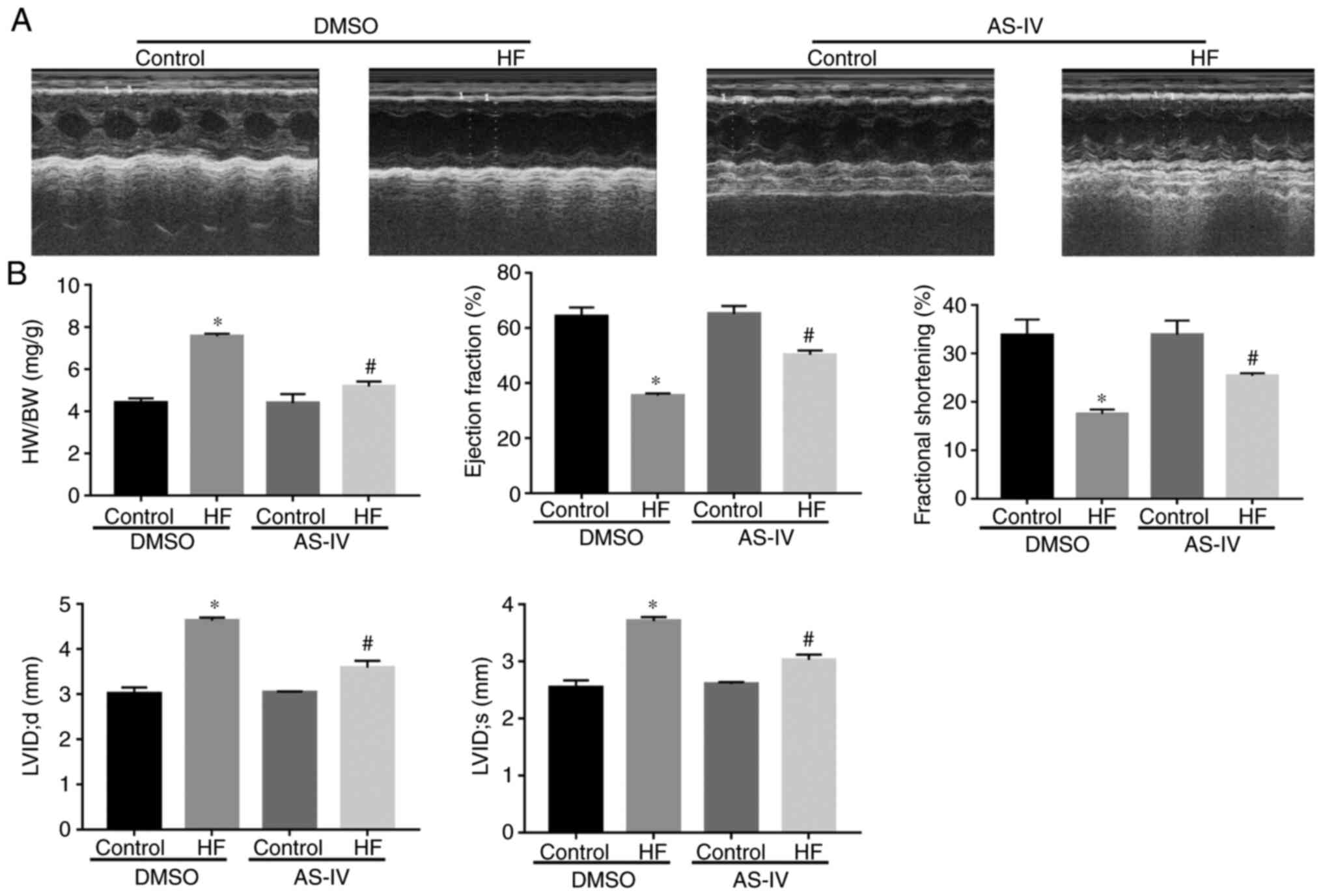 | Figure 1AS-IV treatment improved the
contraction function in transverse aortic constriction-induced HF.
(A) Representative M-mode echocardiography images. (B) Summarized
data of HW/BW, ejection fraction, fractional shortening; LVIDs,
LVIDd, (n=6). Data are presented as the mean ± standard deviation.
One-way analysis of variance, followed by Tukey's test.
*P<0.05 vs. control; #P<0.05 vs. HF.
AS-IV, astragaloside; HF, heart failure; HW/BW, heart mass/body
weight; EF, ejection fraction; FS, fractional shortening; LVIDs,
left ventricular internal systolic diameter; LVIDd, left
ventricular internal diastolic diameter; DMSO, dimethyl
sulfoxide. |
AS-IV reduces TAC-induced
cardiomyocyte hypertrophy and mitochondrial damage
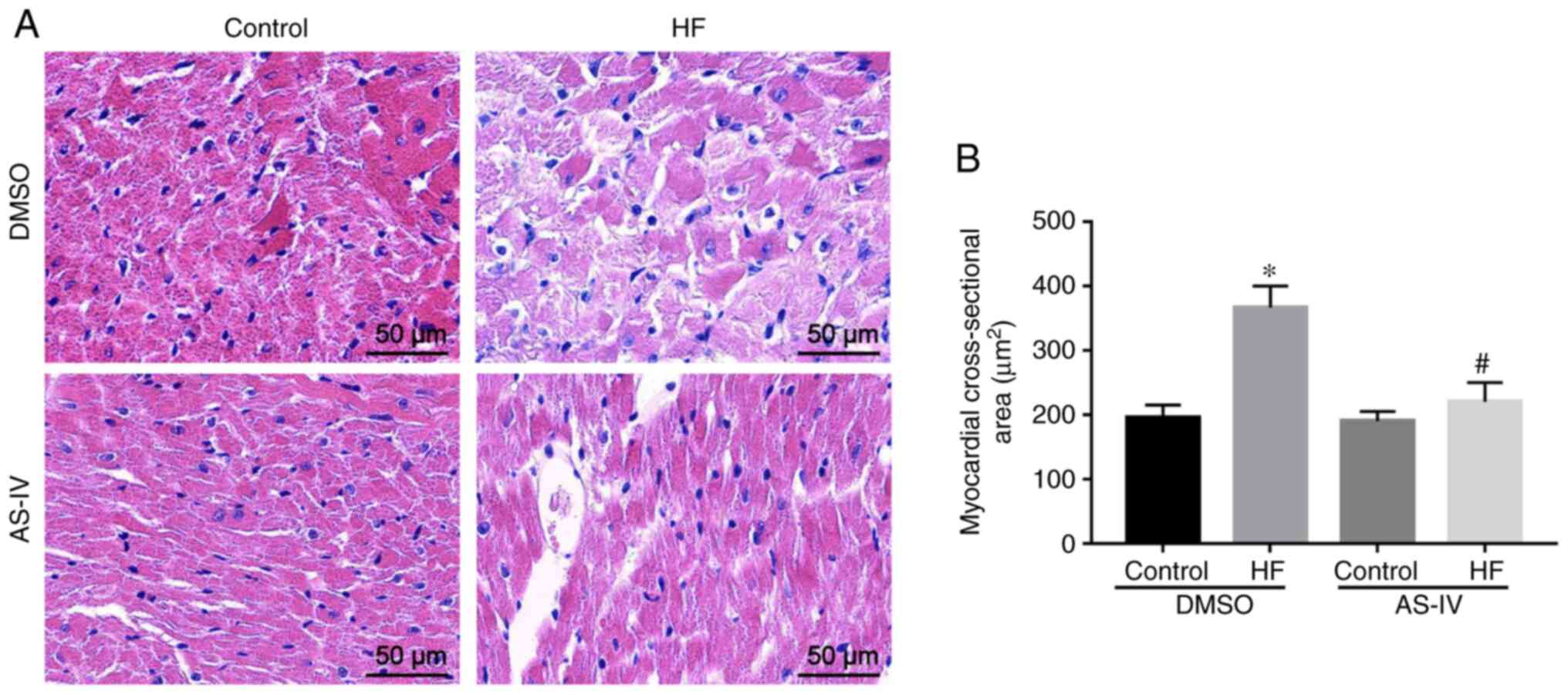
H&E staining demonstrated that, compared with
the control group, myocardial cells in the HF group exhibited
hypertrophy, disordered arrangement, increased intercellular stroma
and inflammatory infiltration. However, AS-IV reversed the
morphological changes of the myocardial tissue induced by TAC
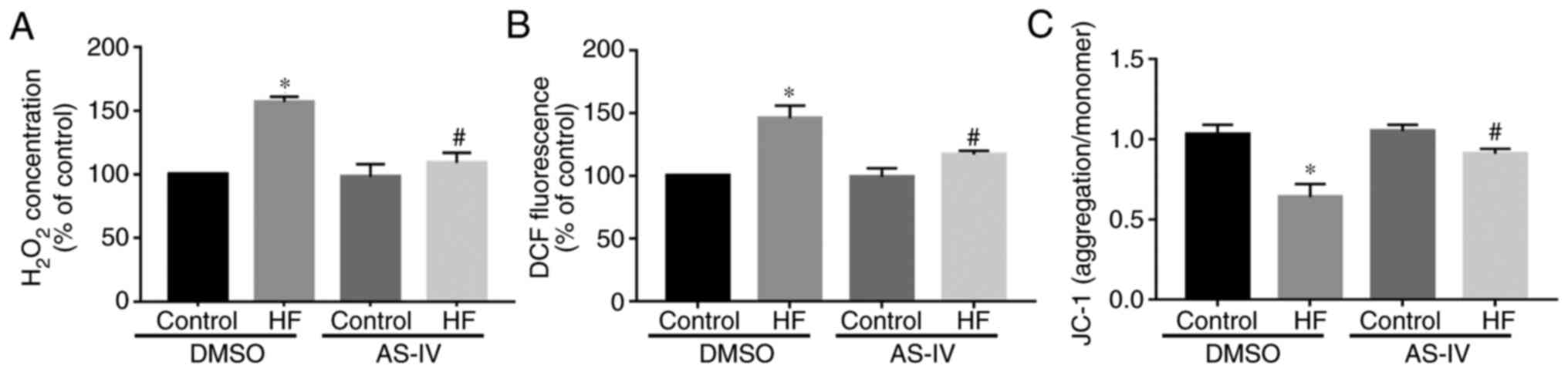
(Fig. 2). Furthermore, compared
with the control group, the ROS levels in myocardial mitochondria
of the HF group were increased, while AS-IV decreased the ROS
content in HF myocardial cells (Fig.
3A). The Amplex Red results demonstrated that, compared with
the control group, the H2O2 content in
myocardial mitochondria of the HF group was increased, which was
abrogated by AS-IV (Fig. 3B). The
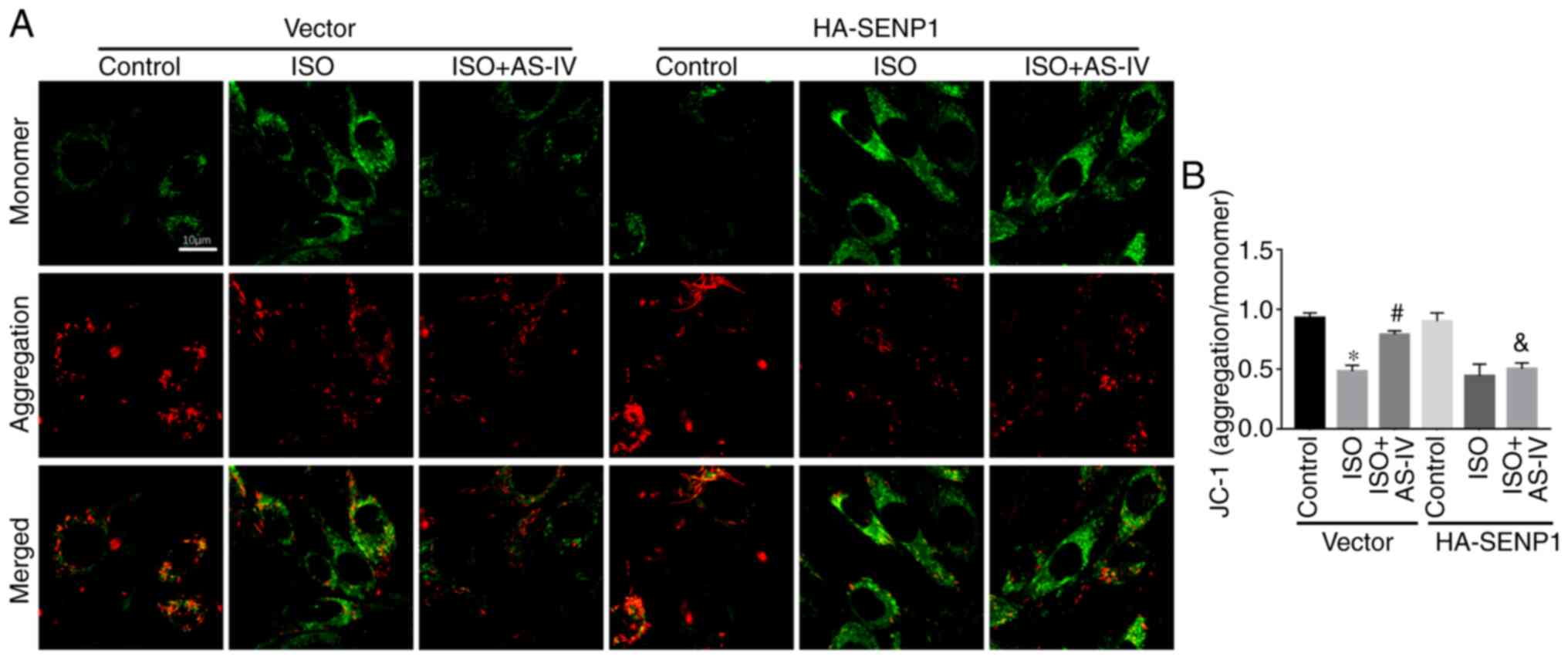
JC-1 results demonstrated that, compared with the control group,
the Δψm of the HF group was decreased, which was also reversed by
AS-IV (Fig. 3C).
Expression of cardiomyocyte
apoptosis-related proteins and Senp1 protein
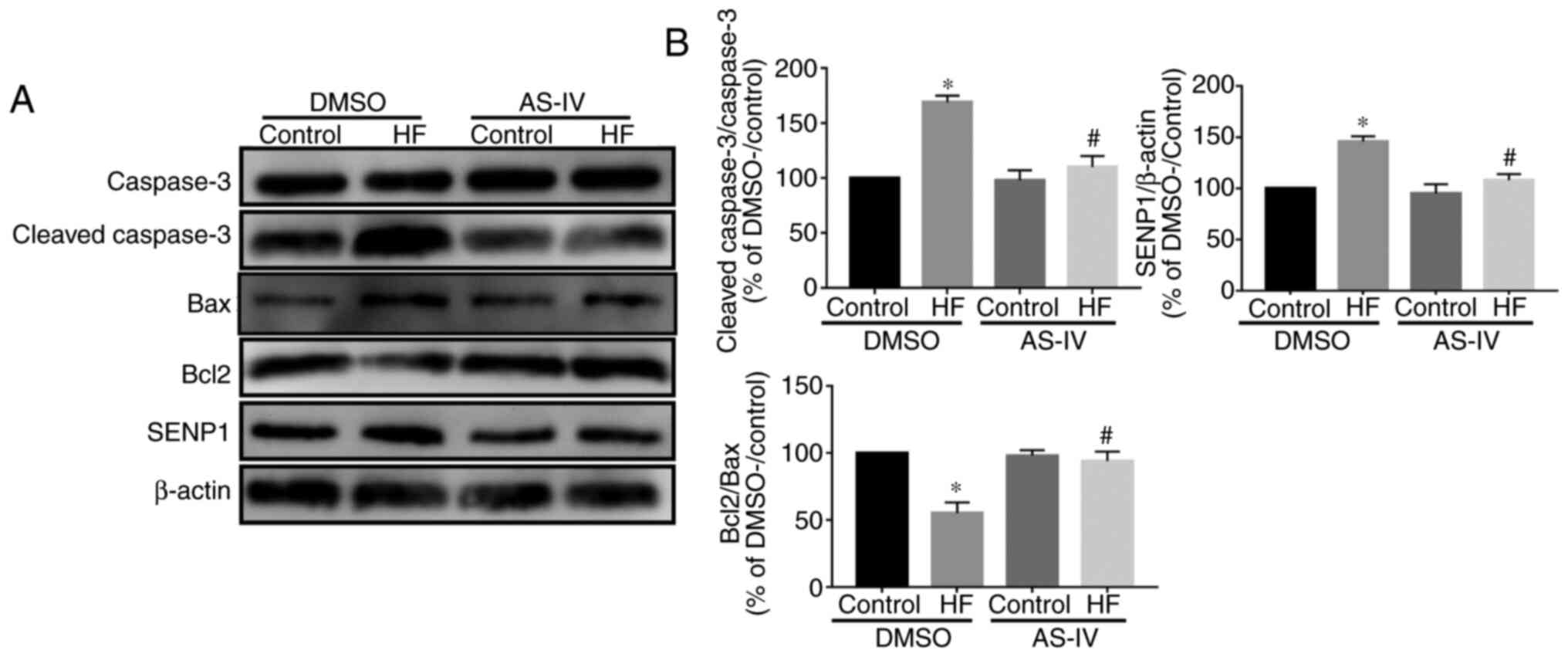
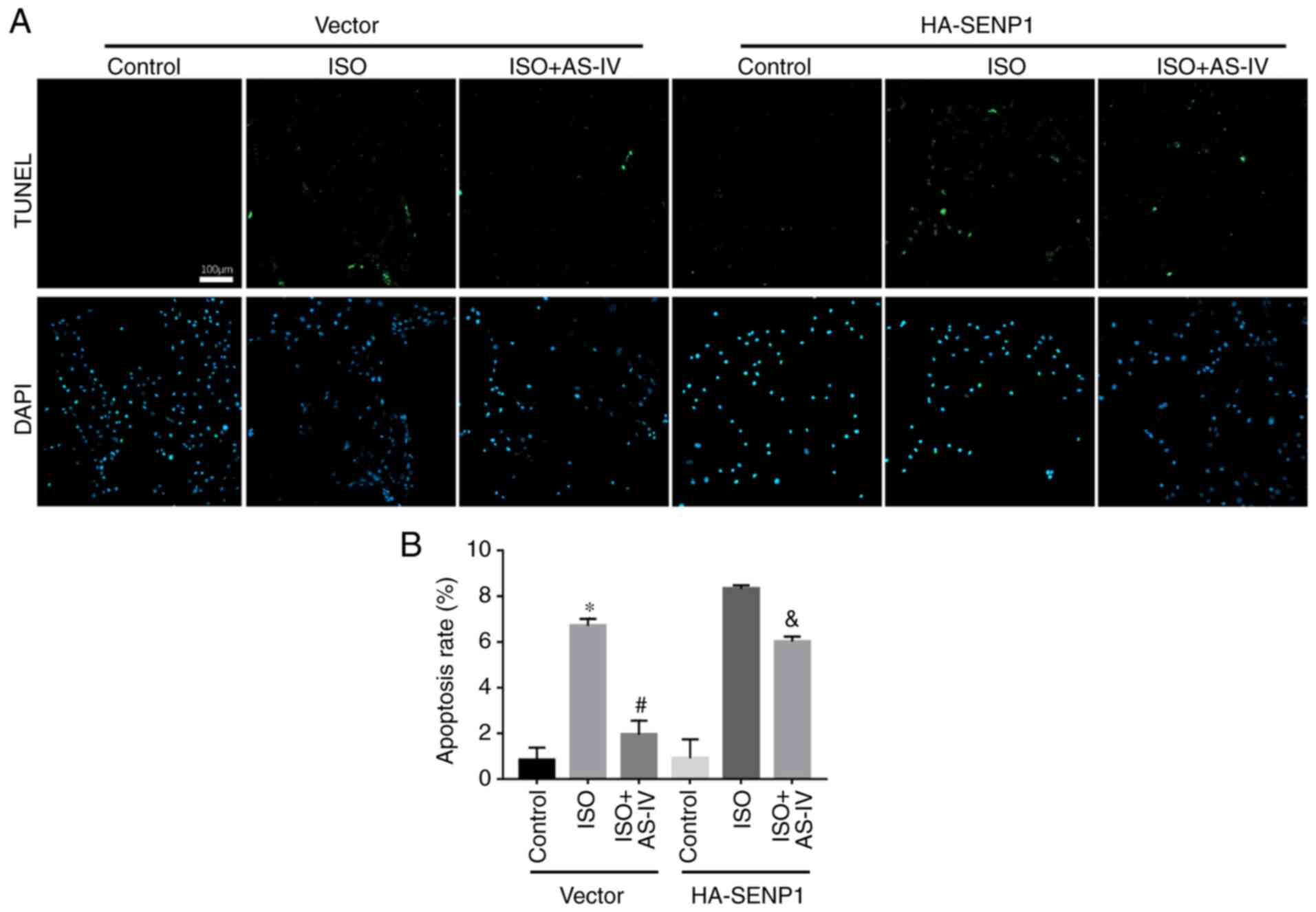
Western blotting demonstrated that, compared with
the control group, the cleaved-caspase-3/caspase-3 ratio was
increased, but the Bcl2/Bax ratio was decreased in the HF group,
indicating that apoptosis of cardiomyocytes in the HF group was
increased. This effect was abolished by AS-IV (Fig. 4A and B). Additionally, compared with the control
group, the expression of Senp1 was increased in the HF model, while
AS-IV decreased this expression (Fig.
4A and B), indicating that
Senp1 may participate in the myocardial protection of AS-IV.
Effect of Senp1-overexpression on
oxidative stress
To further investigate the effect of
Senp1-overexpression on oxidative stress in HL-1 cells, the present
study examined the effect of different concentrations of AS-IV on
the survival of HL-1 cells subjected to ISO, which induces
myocardial injury. The CCK-8 results demonstrated that HL-1 cells
treated with ISO had significantly decreased cell viability. AS-IV
at 50 and 100 mmol/l significantly improved the cell viability, but
the protective effect of 25 and 200 mmol/l was not notable
(Fig. 5A). Next, the HA-Senp1
plasmid was transfected into HL-1 cells. In the subsequent
experiments, 50 mmol/l AS-IV was used as the intervention
concentration. Western blotting demonstrated that, compared with
the vector group, the expression of Senp1 was increased in the
HA-Senp1 group (Fig. 5B). Compared
with the HF group, AS-IV decreased the increase in intracellular
H2O2, while the Senp1-overexpression in HL-1
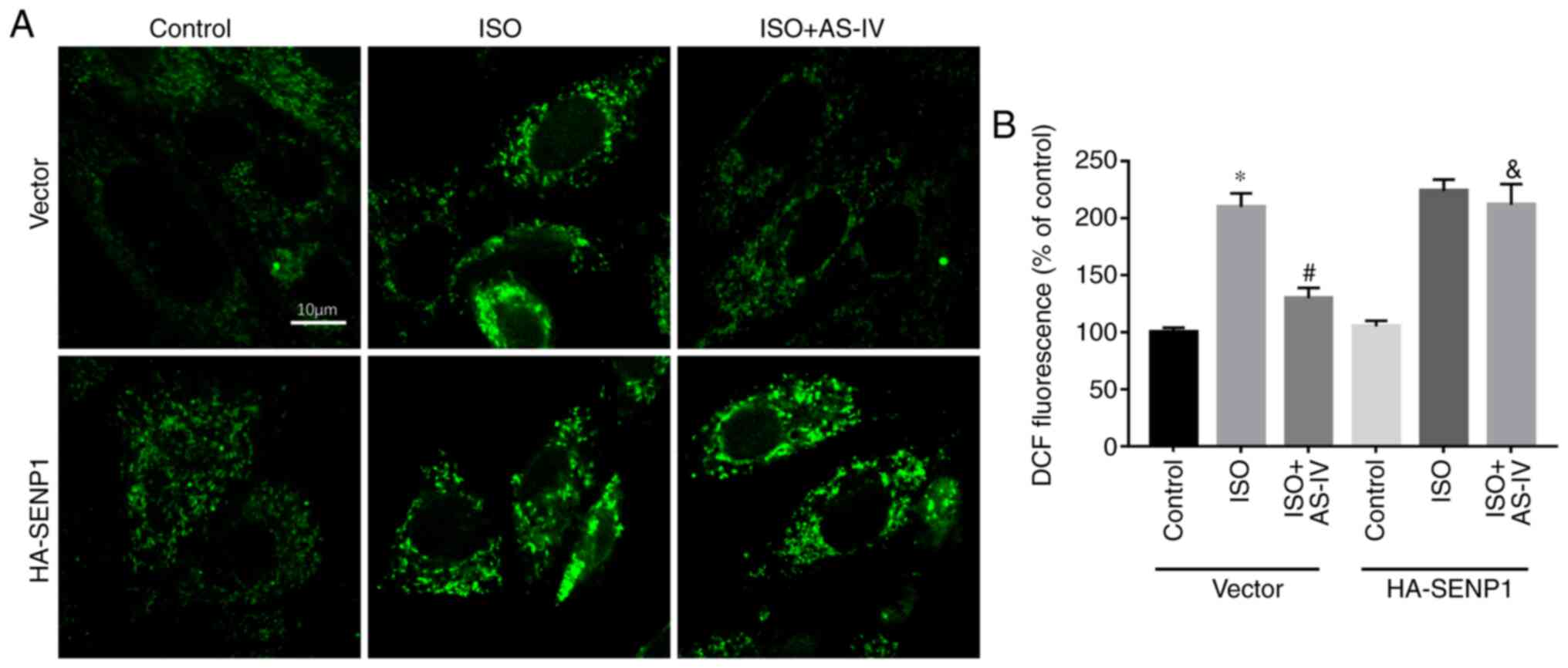
cells inhibited the effect of AS-IV (Fig. 5C). Furthermore, confocal detection
of DCF demonstrated that AS-IV decreased the increase in ROS
compared with the HF group, while Senp1-overexpression in HL-1
cells abolished the effect of AS-IV (Fig. 6).
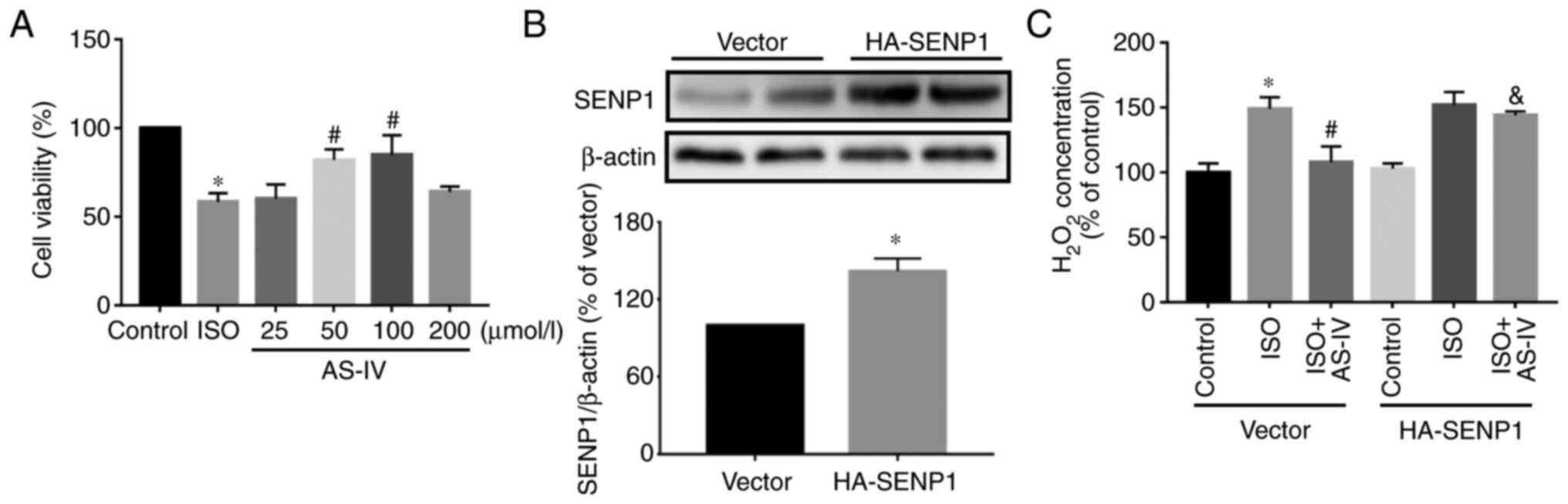 | Figure 5Effect of Senp1-overexpression on
H2O2 in HL-1 cells. (A) The effects of
different concentrations of AS-IV (25, 50, 100 or 200 µmol/l) on
the survival of HL-1 cells subjected to ISO. One-way ANOVA,
followed by Tukey's test. (B) The expression of Senp1 protein was
examined by Western blotting following HA-Senp1 plasmid
transfection. T-test. (C) Compared with the control (dimethyl
sulfoxide, 0.1%), H2O2 was increased in the
20 µmol/l ISO-induced HL-1 cells. AS-IV (50 µmol/l, n=6) prevented
the ISO-induced increase in H2O2, which was
inhibited by Senp1-overexpression (n=7). Data are presented as the
mean ± standard deviation. Two-way ANOVA followed by Bonferroni's
test. *P<0.05 vs. control; #P<0.05 vs.
HF; &P<0.05 vs. HF+AS-IV.
H2O2, hydrogen peroxide; AS-IV,
astragaloside; ANOVA, analysis of variance; ISO, isoprenaline;
Senp1, small ubiquitin-like modifier-specific protease 1. |
Effects of Senp1-overexpression on Δψm
and apoptosis of HL-1 cells
Mitochondria are associated with apoptosis, and the
decline in Δψm is an important indicator of early apoptosis.
Confocal detection of JC-1 demonstrated that, compared with the HF
group, AS-IV inhibited the decrease in Δψm, while
Senp1-overexpression in HL-1 cells abrogated the effect of AS-IV
(Fig. 7). The TUNEL assay also
demonstrated that, compared with the HF group, AS-IV decreased the
apoptotic rate, while Senp1-overexpression inhibited the protective
effect of AS-IV (Fig. 8).
Discussion
CHF is the end-stage of numerous cardiovascular
diseases, which severely affect the life span and quality of life
of patients and is a major public health problem. According to
epidemiological data, there are more than 5.8 million patients with
CHF in the United States and 23 million worldwide (13). It is estimated that the 5-year and
10-year survival rates of CHF patients are 50 and 10%,
respectively, and these are even lower in developing countries
(14). Ventricular remodeling is an
important pathological feature of CHF, including ventricular wall
thickening, ventricular dilatation and collagen fiber hyperplasia,
leading to ventricular dysfunction, increased oxygen consumption
and even cardiac death (15).
Inhibition of ventricular remodeling is the primary therapeutic
target for patients with CHF. The results of the present study
demonstrated that AS-IV prevents ventricular remodeling by
attenuating mitochondrial dysfunction, including the burst of ROS,
the loss of ΔΨm and the release of apoptotic factors via SENP1.
The effect of astragalus in the treatment of heart
failure has been verified in a variety of animal models. Its
protective effects involve improving myocardial contraction,
protecting myocardial cells, regulating the neuroendocrine system
and inhibiting left ventricular remodeling (16). In acute HF induced by pentobarbital
sodium, AS-IV increased systolic and diastolic functions without
increasing myocardial oxygen consumption (17). In CHF rats induced by ligation of
the left coronary artery (LAD), AS-IV significantly improved
cardiovascular parameters and cardiac function (18). AS-IV treatment reversed FS, the peak
values of left ventricular pressure and left ventricular systolic
pressure (LVSP), and the increase in diastolic left ventricular
diameter (LVIDd) and systolic left ventricular diameter (LVIDs)
(18). The present study also
revealed that AS-IV inhibited the increase in heart weight and
heart mass/body weight ratio, the decrease in EF and FS, and the
increase in LVIDd and LVIDs in mice subjected to pressure
overload-driven heart failure. AS-IV reversed the morphological
changes of the myocardial tissue induced by TAC. Similarly, AS-IV
(50 and 100 µmol/l) alleviated the HL-1 cell damage induced by ISO.
However, 25 and 200 µmol/l AS-IV did not demonstrate any protective
effect on ISO-induced cardiomyocyte injury, suggesting that the
treatment dose requires consideration, and the mechanism of AS-IV
may vary between high and low doses of the drug.
Basic and clinical studies have demonstrated that
ROS (superoxide, hydrogen peroxide, hydroxyl radical) produced by
HF myocardium are increased. Excessive ROS accumulation will not
only cause non-specific oxidative damage to DNA, protein and
lipids, but also regulate redox-related signaling cascades, leading
to further myocardial damage (19).
It has been demonstrated that in ApoE-/- mice,
AS-IV treatment decreased oxidative stress, inhibited cardiac
remodeling and improved ventricular function by decreasing
nicotinamide adenine dinucleotide phosphate oxidase (NOX)
expression and/or increasing superoxide dismutase (SOD) expression
(20). Yang et al (21) have reported that AS-IV significantly
enhanced cell viability, increased glutathione peroxidase and SOD
activity, and decreased ROS production, thereby serving a
protective role in cardiomyocytes against hypoxia/reoxygenation.
Similarly, AS-IV decreased the levels of ROS and
H2O2 and inhibited the decrease in Δψm in HF
mice (17). It has been reported
that SENP1 abnormality serves an important role in oxidative stress
injury (22). Therefore, the
present study further investigated whether AS-Ⅳ inhibits myocardial
oxidative stress in HF mice by modulating Senp1 expression. In the
present study, the expression of Senp1 in myocardial tissue samples
from the different treatment groups was examined. The results
showed that, compared with the control group, the expression of
Senp1 protein in the HF group was increased, and this upregulation
was inhibited by AS-IV treatment. Therefore, inhibition of Senp1
expression may serve an important role in the improvement of
ventricular function by AS-IV. To verify this hypothesis, an
Senp1-overexpression plasmid was constructed and the changes in
ROS, H2O2 and Δψm following
Senp1-overexpression in HL-1 cells were investigated.
Senp1-overexpression effectively inhibited the protective effect of
AS-IV against oxidative stress in the HF model. The findings of the
present study preliminarily confirm the connection between SENP1 in
AS-IV-induced cardio protection. However, the specific target of
Senp1 and the free radical types regulated by Senp1 following AS-IV
treatment remain unknown and will be the focus in future
studies.
The present study demonstrated that AS-IV decreased
oxidative stress and mitochondrial damage, inhibited ventricular
remodeling and improved cardiac function by decreasing
Senp1-upregulation in HF mice. The discovery of this mechanism
provides a novel theoretical basis and clinical reference for the
treatment of HF. However, the present study only investigated the
role of Senp1 in AS-IV-treated cardiomyocytes; therefore, the
specific target of Senp1 and the free radical types regulated by
Senp1 remain to be identified in order to provide a more
comprehensive theoretical basis for the protective effect of AS-IV
against cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from Tianjin
Natural Science Foundation of China (grant. no. 19JCZDJC35200) and
Tianjin Special Project of New Generation Artificial Intelligence
Technology (grant. no. 18ZXZNSY00260).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and XL designed the experiments. YL, XB and NX
performed the experiments, and collected and analyzed the data. SD
and JY drafted the manuscript. JY analyzed the data. SD interpreted
data and wrote the manuscript. All authors agreed to be accountable
for all aspects of the work in ensuring that questions related to
the accuracy and integrity of any part of the work are
appropriately investigated and resolved. XL and SD confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics statement and consent to
participate
The present study was performed in accordance with
the Guidelines of the Ethics Committee of Tianjin Medical
University Cancer Institute and Hospital (Tianjin, China). Animal
experiments were approved by the Animal Ethics Committee of Tianjin
Fifth Central Hospital (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McMurray JJ and Pfeffer MA: Heart failure.
Lancet. 365:1877–1889. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure: The task force for
the diagnosis and treatment of acute and chronic heart failure of
the European society of cardiology (ESC)Developed with the special
contribution of the heart Failure Association (HFA) of the ESC. Eur
Heart J. 37:2129–2200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ishihara S, Kawakami R, Nogi M, Hirai k,
Hashimoto Y, Nakada Y, Nakagawa H, Ueda T, Nishida T, Onoue K, et
al: Incidence and clinical significance of 30-day and 90-day
rehospitalization for heart failure among patients with acute
decompensated heart failure in Japan-From the NARA-HF Study. Circ
J. 84:194–202. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aimo A, Januzzi JL Jr, Bayes-Genis A,
Vergaro G, Sciarrone P, Passino C and Emdin M: Clinical and
prognostic significance of sST2 in heart failure: JACC review topic
of the week. J Am Coll Cardiol. 74:2193–2203. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feusette P, Gierlotka M, Tukiendorf A,
Płonka J, Bugajski J, Łabuz-Roszak B and Bryk R: Heart failure in
opole voivodeship-epidemiology and future perspectives. Wiad Lek.
72:112–119. 2019.PubMed/NCBI(In Polish).
|
|
6
|
Jia Q, Wang L, Zhang X, Ding Y, Li H, Yang
Y, Zhang A, Li Y, Lv S and Zhang J: Prevention and treatment of
chronic heart failure through traditional Chinese medicine: Role of
the gut microbiota. Pharmacol Res. 151(104552)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A research
review on the pharmacological effects. Adv Pharmacol. 87:89–112.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Swatek KN and Komander D: Ubiquitin
modifications. Cell Res. 26:399–422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Han ZJ, Feng YH, Gu BH, Li YM and Chen H:
The post-translational modification, SUMOylation, and cancer
(Review). Int J Oncol. 52:1081–1094. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kunz K, Piller T and Müller S:
SUMO-specific proteases and isopeptidases of the SENP family at a
glance. J Cell Sci. 131(jcs211904)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cai R, Gu J, Sun H, Liu X, Mei W, Qi Y,
Xue S, Ren S, Rabinowitz JE, Wang Y, et al: Induction of SENP1 in
myocardium contributes to abnormities of mitochondria and
cardiomyopathy. J Mol Cell Cardiol. 79:115–122. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chhunchha B, Kubo E, Singh P and Singh DP:
Sumoylation-deficient Prdx6 repairs aberrant Sumoylation-mediated
Sp1 dysregulation-dependent Prdx6 repression and cell injury in
aging and oxidative stress. Aging (Albany NY). 10:2284–2315.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bozkurt B: What is new in heart failure
management in 2017? Update on ACC/AHA heart failure guidelines.
Curr Cardiol Rep. 20(39)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kerpen K, Koutrolou-Sotiropoulou P, Zhu C,
Yang J, Lyon JA, Lima FV and Stergiopoulos K: Disparities in death
rates in women with peripartum cardiomyopathy between advanced and
developing countries: A systematic review and meta-analysis. Arch
Cardiovasc Dis. 112:187–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Frangogiannis NG: The extracellular matrix
in ischemic and nonischemic heart failure. Circul Res. 125:117–146.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Wu J, Guo S, Lin W, Zhang B, Chen
X, Mo H and Zhan T: The clinical efficacy and safety of the Chinese
herbal medicine Astragalus (Huangqi) preparation for the treatment
of acute myocardial infarction: A systematic review of randomized
controlled trials. Medicine. 98(e15256)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dai H, Jia G, Lu M, Liang C, Wang Y and
Wang H: Astragaloside IV inhibits isoprenaline-induced cardiac
fibrosis by targeting the reactive oxygen species/mitogen-activated
protein kinase signaling axis. Mol Med Rep. 15:1765–1770.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan LB, Hua CY, Gao S, Yin YL, Dai M,
Meng HY, Li PP, Yang ZX and Hu QH: Astragalus Polysaccharides
attenuate monocrotaline-induced pulmonary arterial hypertension in
rats. Am J Chin Med. 45:773–789. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
van der Pol A, van Gilst WH, Voors AA and
van der Meer P: Treating oxidative stress in heart failure: Past,
present and future. Eur J Heart Failure. 21:425–435.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song Z, Wei D, Chen Y, Chen L, Bian Y,
Shen Y, Chen J and Pan Y: Association of astragaloside IV-inhibited
autophagy and mineralization in vascular smooth muscle cells with
lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol Appl
Pharmacol. 364:45–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang P, Zhou Y, Xia Q, Yao L and Chang X:
Astragaloside IV regulates the PI3K/Akt/HO-1 signaling pathway and
inhibits H9c2 cardiomyocyte injury induced by
hypoxia-reoxygenation. Biol Pharm Bull. 42:721–727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Du FL, Dong WB, Zhang C, Li QP, Kang L,
Lei XP, Guo L and Zhai XS: Budesonide and poractant alfa prevent
bronchopulmonary dysplasia via triggering SIRT1 signaling pathway.
Eur Rev Med Pharmacol Sci. 23:11032–11042. 2019.PubMed/NCBI View Article : Google Scholar
|