Introduction
Schizophrenia is one of the most common psychiatric
disorders, with an annual incidence rate of approximately 0.05%
(1). Although studied for over a
century, the neuropathology of schizophrenia is still unknown
(2). Schizophrenia is thought to be
caused by a complex interplay of genetic and environmental risk
factors that affect early brain growth and biological adaptation to
life experiences (3,4). With the development of computed
tomography (CT) and magnetic resonance imaging (MRI) technologies,
the number of imaging studies performed in patients with
schizophrenia has increased. Numerous neuroimaging studies have
identified structural and functional abnormalities in patients with
schizophrenia, but no diagnostic value can be attributed to any
change. Thus, studies on brain volume did not show statistically
significant differences in MRI investigations between patients with
schizophrenia and normal control subjects. Other cerebral anomalies
reported in some studies on the imaging aspects of patients with
schizophrenia were enlargement of the lateral ventricles or only
their temporal horn, enlargement of ventricle three or four, and a
reduction in the volume of the temporal or frontal or parietal or
occipital brain lobes (5-7).
In some studies changes in the volume of the corpus callosum have
also been reported (8-10).
A total of 92% of imaging studies in patients with schizophrenia
reported doubling of the septum pellucidum (11,12).
The presence of enlarged Virchow spaces was reported in a
significant proportion in patients with schizophrenia compared to
the control group (30.3%) (13).
Najjar and Pearlman described, in a review of 15
imaging studies that included 792 patients with schizophrenia, the
presence of a significant proportion of white matter abnormalities,
which would suggest the existence of structural and functional
dysconnectivity, even in the early stages of psychoses (14). Changes in the structure of the white
matter may influence perception, thought and behavior (15).
Imaging studies did not show significant differences
between patients in the first psychotic episode and those with
chronic forms of schizophrenia except for an expansion of the
volume of the lateral ventricles (16-19).
Schizophrenia in pediatric patients described as
early onset schizophrenia (EOS), ages 13-18, and very early onset
schizophrenia (VEOS), ages <13, is observed less frequently than
adult-onset schizophrenia, but is generally more severe, from both
a clinical and neurobiological perspective (20). Findings from the structural MRI
studies of patients with EOS and VEOS have shown smaller total
cerebral volume in VEOS patients compared to controls (21,22),
increased lateral ventricular volume (21), reduced thalamic volume (21,23),
and enlarged cavum septum pellucidum in VEOS vs. controls (24). Information provided by longitudinal
studies has shown that in EOS and VEOS patients decrements in grey
matter volume in frontal, temporal and parietal cortices were
identified over time (25). It is
difficult to determine how medication affects the process of grey
matter loss in schizophrenia (26).
Medication-naive childhood-onset schizophrenia (COS) subjects are
difficult to identify due to the severity of the disorder (27). Grey matter loss and ventricular
enlargement have been identified in many adult schizophrenia trials
using brain MRI, which are progressive (19,28-31).
In the literature, it is speculated that since puberty is a period
of massive brain reorganization, the grey matter changes in COS
would be more pronounced than in the schizophrenic adult
population, which may be the trigger or the effect of the more
serious COS phenotype (32). Since
there are currently no methods for reliably identifying and
studying individuals until the onset of schizophrenia, determining
when the structural brain abnormalities occurred is difficult
(31). Differences in brain volumes
between schizophrenic patients and healthy adults are also evident
around the time patients receive treatment (11,33).
Additionally, it has been found that in schizophrenic individuals,
brain volume alterations tend to be most pronounced in the first
years of disease (31,34,35).
In this study, neuroimaging studies in adult and
pediatric patients with schizophrenia were assessed. The main MRI
abnormalities identified in patients with schizophrenia included in
the study were: enlarged Virchow spaces, sinusitis, white matter
abnormalities, hemosiderotic spots, but also cortical atrophy,
cerebral palsy or venous malformations.
Patients and methods
Patient data
Adult and pediatric patients diagnosed with
schizophrenia admitted to the ‘Prof. Dr. Alexandru Obregia’
Clinical Psychiatry Hospital between September 2019 and December
2020 were included in the study. For the inclusion in the study of
patients with schizophrenia, a study protocol was used, which
included anamnesis data, clinical evaluation with general clinical
examination, neurological examination and psychiatric examination
in which the scales specific to schizophrenia were applied [PANSS
(36), Calgary Scale (37), Sheehan Disability Scale (38), Addenbrooke's Cognitive Examination
(39)] and psychological
examination. The diagnosis of schizophrenia was established
according to DSM-IV-TR and ICD 10 criteria (40,41).
The inclusion of patients in the study was achieved
after the patients or legal guardian signed the informed consent.
The study was run in accordance with the World Medical Association
Declaration of Helsinki and was approved by the Institutional
Ethical Board of the institution Clinical Hospital of Psychiatry
‘Prof. Dr. Alexandru Obregia’.
Methods
Magnetic resonance imaging 3 Tesla was performed
according to the standard protocol, including T1, T2, FLAIR, DWI,
ADC, and SWI sequences.
Patients with contraindications for performing MRI,
such as cardiac pacemaker, implanted cardiac defibrillator,
internal pacing wires, clips such as cerebral, carotid or aortic
aneurysm, cochlear implants, any implant held in by magnet,
Swan-Ganz catheter, with claustrophobia or a possibly pregnancy,
were excluded. After exclusion criteria, 45 patients [21 male and
24 female; age range 13-61 years with a mean age of 26.71 years and
a standard deviation (SD)=14.39] with schizophrenia were qualified
to be introduced in the study at this stage.
Statistical analysis
For statistical analysis, the data obtained were
entered into Excel documents, then transferred to the IBM SPSS 22
(SPSS, Inc.) statistical analysis program. Statistical analysis was
performed using JASP 0.14.1.0 software. The χ² test and Bayesian
contingency table test were performed. These statistical tests were
applied to verify the existence of relationships between variables,
personal characteristics of patients and brain abnormalities
encountered in schizophrenia. P<0.05 was considered to indicate
statistical significance.
Results
Patient data
The study included 45 patients (21 male and 24
female), with an age range of 13-61 years (mean age, 26.71 years
and SD of 14.39).
MRI anomalies
The MRI studies showed variable anomalies, alone or
in different combinations: enlarged Virchow spaces in 44 patients,
sinusitis in 27 cases, white matter abnormalities in 16 subjects,
hemosiderotic spots in 2 patients, cortical atrophy in 4 cases,
lacuna and doubling septum pellucidum in 3 patients, respectively,
mega cisterna magna and calcification of the falx cerebri in 2
subjects, respectively, and 1 patient had a venous malformation
(Table I). A total of 26 of the
patients had both enlarged Virchow spaces and sinusitis, and 8 of
the patients had both sinusitis and white substance
abnormalities.
 | Table IContingency table concerning the
association between sex and brain abnormalities in the patients
with schizophrenia (N=45). |
Table I
Contingency table concerning the
association between sex and brain abnormalities in the patients
with schizophrenia (N=45).
| | Sex |
|---|
| Variables | M | F | Total |
|---|
| Enlarged Virchow
spaces | | | |
|
Absent | 1 | 0 | 1 |
|
Present | 20 | 24 | 44 |
|
Total | 21 | 24 | 45 |
| White substance
abnormalities | | | |
|
Absent | 15 | 14 | 29 |
|
Present | 6 | 10 | 16 |
|
Total | 21 | 24 | 45 |
| Sinusitis | | | |
|
Absent | 6 | 12 | 18 |
|
Present | 15 | 12 | 27 |
|
Total | 21 | 24 | 45 |
| Mega cisterna
magna | | | |
|
Absent | 19 | 24 | 43 |
|
Present | 2 | 0 | 2 |
|
Total | 21 | 24 | 45 |
| Doubling septum
pellucidum | | | |
|
Absent | 19 | 23 | 42 |
|
Present | 2 | 1 | 3 |
|
Total | 21 | 24 | 45 |
| Cortical
atrophy | | | |
|
Absent | 20 | 21 | 41 |
|
Present | 1 | 3 | 4 |
|
Total | 21 | 24 | 45 |
| Hemosiderotic
spots | | | |
|
Absent | 19 | 24 | 43 |
|
Present | 2 | 0 | 2 |
|
Total | 21 | 24 | 45 |
| Lacuna | | | |
|
Absent | 21 | 21 | 42 |
|
Present | 0 | 3 | 3 |
|
Total | 21 | 24 | 45 |
| Venous
malformation | | | |
|
Absent | 21 | 23 | 44 |
|
Present | 0 | 1 | 1 |
|
Total | 21 | 24 | 45 |
| Calcification of
the falx cerebri | | | |
|
Absent | 19 | 24 | 43 |
|
Present | 2 | 0 | 2 |
|
Total | 21 | 24 | 45 |
Considering sex of patients, 24 women and 20 men had
enlarged Virchow spaces, 6 men and 10 women had white matter
abnormalities, but without any statistical significance (P>0.05)
(Tables I and II).
 | Table IIThe χ² tests concerning the
association between sex and brain abnormalities in the patients
with schizophrenia (N=45). |
Table II
The χ² tests concerning the
association between sex and brain abnormalities in the patients
with schizophrenia (N=45).
| Variables | Value | df | P-value |
|---|
| Enlarged Virchow
spaces | 1.169 | 1 | 0.280 |
| White substance
abnormalities | 0.838 | 1 | 0.360 |
| Sinusitis | 2.143 | 1 | 0.143 |
| Mega cisterna
magna | 2.392 | 1 | 0.122 |
| Doubling septum
pellucidum | 0.517 | 1 | 0.472 |
| Cortical
atrophy | 0.828 | 1 | 0.363 |
| Hemosiderotic
spots | 1.607 | 1 | 0.205 |
| Calcification of
the falx cerebri | 2.392 | 1 | 0.122 |
| Lacuna | 2.813 | 1 | 0.094 |
| Venous
malformation | 0.895 | 1 | 0.344 |
Sinusitis was one of the most common abnormalities
in our sample, but it did not predominate statistically
significantly in either sex, although the abnormality was present
in 15 men and 12 women (P>0.05) (Tables I and II).
Calcification of the falx cerebri, mega cisterna
magna and hemosiderotic spots were present in 2 of the men,
respectively. However, this aspect is not characteristic of the
male group as no significant association was found between these
variables (P>0.05) (Tables I and
II).
Lacuna and venous malformation were present in 3 and
1 of the women, respectively; however, no significant correlation
was found between sex and the two anomalies (P>0.05) (Tables I and II).
Doubling septum pellucidum and cortical atrophy were
present in both men and women; however, there were no significant
differences between them (P>0.05) (Tables I and II).
Relationship between age and brain
abnormalities
Enlarged Virchow spaces, sinusitis and white
substance abnormalities were the most common abnormalities of the
brain by age category albeit these abnormalities were not a
characteristic of any age category from a statistical point of view
(P>0.05) (Tables III and
IV).
 | Table IIIContingency table concerning the
association between age and brain abnormalities in the patients
with schizophrenia (N=45). |
Table III
Contingency table concerning the
association between age and brain abnormalities in the patients
with schizophrenia (N=45).
| | Age range |
|---|
| Variables | 13-28 years | 29-61 years | Total |
|---|
| Enlarged Virchow
spaces | | | |
|
Absent | 1 | 0 | 1 |
|
Present | 24 | 20 | 44 |
|
Total | 25 | 20 | 45 |
| White substance
abnormalities | | | |
|
Absent | 19 | 10 | 29 |
|
Present | 6 | 10 | 16 |
|
Total | 25 | 20 | 45 |
| Sinusitis | | | |
|
Absent | 11 | 7 | 18 |
|
Present | 14 | 13 | 27 |
|
Total | 25 | 20 | 45 |
| Mega cisterna
magna | | | |
|
Absent | 25 | 18 | 43 |
|
Present | 0 | 2 | 2 |
|
Total | 25 | 20 | 45 |
| Doubling septum
pellucidum | | | |
|
Absent | 23 | 19 | 42 |
|
Present | 2 | 1 | 3 |
|
Total | 25 | 20 | 45 |
| Cortical
atrophy | | | |
|
Absent | 25 | 16 | 41 |
|
Present | 0 | 4 | 4 |
|
Total | 25 | 20 | 45 |
| Hemosiderotic
spots | | | |
|
Absent | 24 | 16 | 40 |
|
Present | 1 | 4 | 5 |
|
Total | 25 | 20 | 45 |
| Lacuna | | | |
|
Absent | 23 | 19 | 42 |
|
Present | 2 | 1 | 3 |
|
Total | 25 | 20 | 45 |
| Venous
malformation | | | |
|
Absent | 24 | 20 | 44 |
|
Present | 1 | 0 | 1 |
|
Total | 25 | 20 | 45 |
| Calcification of
the falx cerebri | | | |
|
Absent | 25 | 18 | 43 |
|
Present | 0 | 2 | 2 |
|
Total | 25 | 20 | 45 |
 | Table IVThe χ² tests concerning the
association between age and brain abnormalities. |
Table IV
The χ² tests concerning the
association between age and brain abnormalities.
| Variables | Value | df | P-value |
|---|
| Enlarged Virchow
spaces | 0.978 | 1 | 0.323 |
| White substance
abnormalities | 0.841 | 1 | 0.175 |
| Sinusitis | 1.201 | 1 | 0.273 |
| Mega cisterna
magna | 2.188 | 1 | 0.139 |
| Doubling septum
pellucidum | 0.311 | 1 | 0.577 |
| Cortical
atrophy | 4.590 | 1 | 0.032 |
| Hemosiderotic
spots | 5.881 | 1 | 0.015 |
| Calcification of
the falx cerebri | 2.392 | 1 | 0.122 |
| Lacuna | 0.313 | 1 | 0.564 |
| Venous
malformation | 0.978 | 1 | 0.322 |
Mega cisterna magna (4 participants) and
calcification of the falx cerebri, (2 participants) and lacuna were
present in the second age category (13-28 years) more frequently
than in the first age category (29-61 years) albeit there were no
statistically significant differences between the two age
categories (P>0.05) (Tables
III and IV).
Cortical atrophy and hemosiderotic spots were
present in the second age category (29-61 years) (8 patients) more
frequently than in the first category (13-28 years) (1 patient) and
there are statistically significant differences between the two age
categories (P<0.05) (Tables
III and IV). The statistical
significance of cortical atrophy and hemosiderotic spots by age
category is shown in Table V
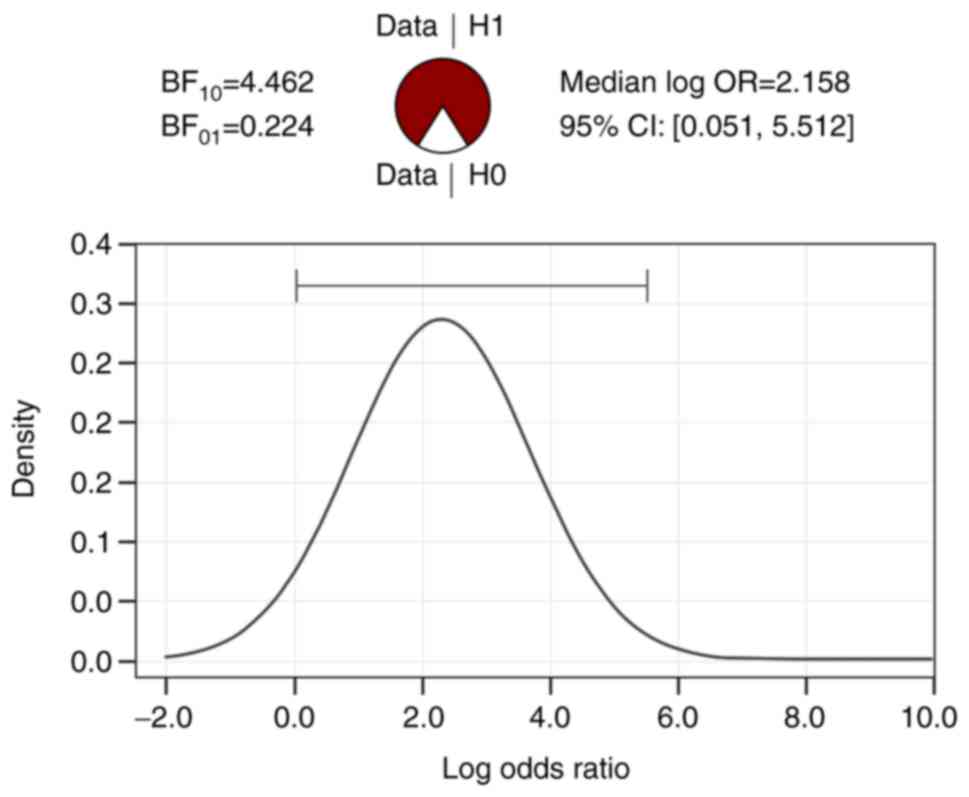
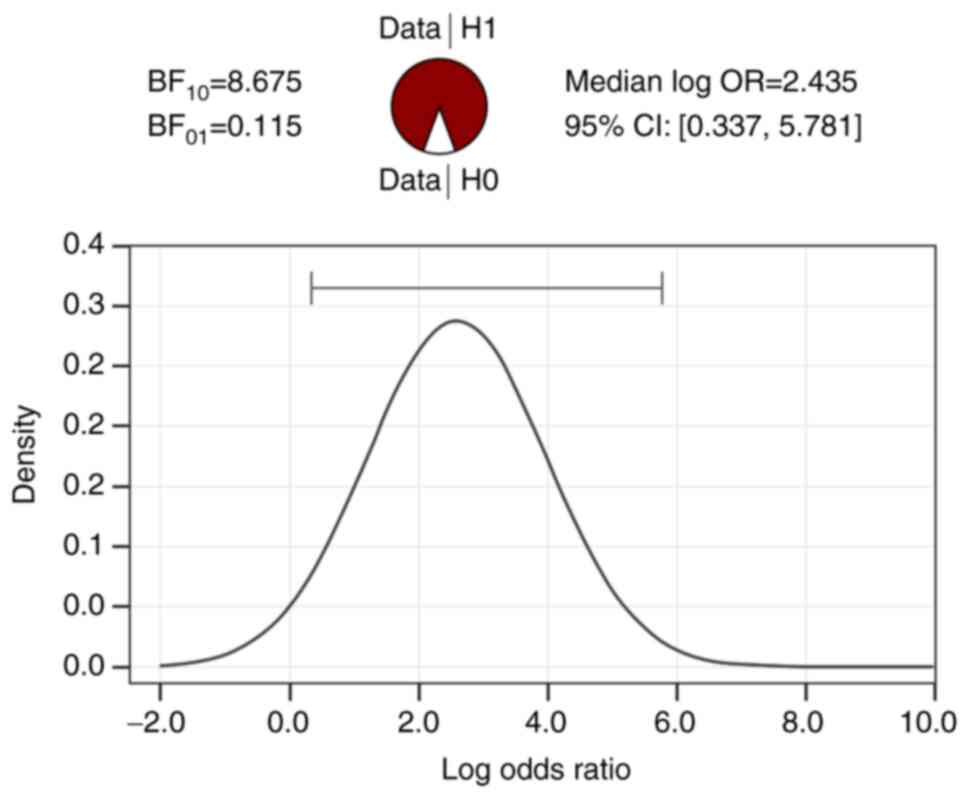
(BF10 independent multinomial cortical atrophy=4.462,
BF10 independent multinomial hemosiderotic spots=8.675).
In addition, in Figs. 1 and
2, it can be seen that the
differences between the two age categories in terms of the
anomalies discussed are strongly supported by the data collected,
BF10>BF01.
 | Table VBayesian contingency table tests and
the association between age, cortical atrophy, and hemosiderotic
spots. |
Table V
Bayesian contingency table tests and
the association between age, cortical atrophy, and hemosiderotic
spots.
| Variable | Value |
|---|
| BF10
independent multinomial cortical atrophy | 4.462 |
| N | 45 |
| BF10
independent multinomial hemosiderotic spots | 8.675 |
Relationship between sex and brain
abnormalities in children
The study included 25 pediatric patients (12 boys
and 13 girls). The age interval was 13-18 years (mean age of 15.08
years; SD of 1.68). Of the 25 patients, 24 had enlarged Virchow
spaces, 14 had sinusitis, 6 had white substance abnormalities, 1
had hemosiderotic spots, 2 had lacuna and doubling septum
pellucidum, respectively, and 1 of the participants had venous
malformation.
Although, 13 of the girls in the sample and 11 of
the boys had enlarged Virchow spaces, this brain abnormality no
longer predominated in one of the sexes from a statistical point of
view (P>0.05) (Tables VI and
VII).
 | Table VIContingency table for the association
between sex and brain abnormalities in children (N=25). |
Table VI
Contingency table for the association
between sex and brain abnormalities in children (N=25).
| | Sex |
|---|
| Variables | M | F | Total |
| Enlarged Virchow
spaces | | | |
|
Absent | 1 | 0 | 1 |
|
Present | 11 | 13 | 24 |
|
Total | 12 | 13 | 25 |
| White substance
abnormalities | | | |
|
Absent | 9 | 10 | 19 |
|
Present | 3 | 3 | 6 |
|
Total | 12 | 13 | 25 |
| Sinusitis | | | |
|
Absent | 5 | 6 | 11 |
|
Present | 7 | 7 | 14 |
|
Total | 12 | 13 | 25 |
| Mega cisterna
magna | | | |
|
Absent | 12 | 13 | 25 |
|
Present | 0 | 0 | 0 |
|
Total | 12 | 13 | 25 |
| Doubling septum
pellucidum | | | |
|
Absent | 10 | 13 | 23 |
|
Present | 2 | 0 | 2 |
|
Total | 12 | 13 | 25 |
| Cortical
atrophy | | | |
|
Absent | 12 | 13 | 25 |
|
Present | 0 | 0 | 0 |
|
Total | 12 | 13 | 25 |
| Hemosiderotic
spots | | | |
|
Absent | 12 | 12 | 24 |
|
Present | 0 | 1 | 1 |
|
Total | 12 | 13 | 25 |
| Calcification of
the falx cerebri | | | |
|
Absent | 12 | 13 | 25 |
|
Present | 0 | 0 | 0 |
|
Total | 12 | 13 | 25 |
| Venous
malformation | | | |
|
Absent | 12 | 12 | 24 |
|
Present | 0 | 1 | 1 |
|
Total | 12 | 13 | 25 |
| Lacuna | | | |
|
Absent | 12 | 11 | 23 |
|
Present | 0 | 2 | 2 |
|
Total | 12 | 13 | 25 |
 | Table VIIThe χ² test for the association
between sex and brain abnormalities in children. |
Table VII
The χ² test for the association
between sex and brain abnormalities in children.
| Variable | Value | df | P-value |
|---|
| Enlarged Virchow
spaces | 1.128 | 1 | 0.288 |
| Sinusitis | 0.051 | 1 | 0.821 |
Sinusitis was one of the most common abnormalities
in patients in our sample, but did not predominate statistically
significantly in either sex (P>0.05) (Tables VI and VII).
Discussion
The results obtained in patients of the present
study partially overlap with those reported in the literature, in
the sense that the enlargement of the Virchow spaces was observed
in a high percentage of cases, as was the case for white matter
demyelinating lesions, and cortical atrophy. Akhtar et al
(13) conducted a comparative
study, with a group of 33 schizophrenic patients and a control
group of 33 healthy individuals with a mean age of 30 years, in
order to determine whether there is any structural changes in brain
matter. Enlarged Virchow spaces were identified in 10 (30%) of all
those with schizophrenia and zero of the control subjects. In
addition, in that study there were no significant differences by
sex, compared to the control group or between individuals in the
same group. The presence of enlarged Virchow spaces in adults was
associated with different situations including vascular ectasia,
CSF pulsations, abnormal arterial wall permeability, dementia, and
hypertension (42,43). Wuerfel et al suggested a role
of Virchow spaced in inflammatory processes of the brain (44). The significance of enlarged Virchow
spaces in children and adolescents is not clear. A study on
children with autism spectrum disorder (ASD) showed an increased
incidence of enlarged Virchow spaces in ASD children compared with
control group; this anomaly was correlated with the expression of
symptoms and with a low adaptative functioning (42).
Sinusitis was also reported in 10 of the cases of
schizophrenia and 22 of the individuals in the control group, while
in our case sinusitis had a frequency of 60% of the total number of
patients. Davis et al (45)
suggested that the MRI shows in patients with schizophrenia a
slight, but substantial white matter decrease. Of the 45 patients
in the present study, 16 patients had white matter abnormalities,
which means a percentage of 35.5%.
In a large number of cases, the presence of a sinus
reaction has been identified, both in pediatric and adult patients.
Findings have shown there is an association between mental
disorders and sinusitis (46,47).
The link between schizophrenia and sinusitis could be investigated
in order to demonstrate whether patients with schizophrenia have a
predisposition to developing sinus disorders or whether they
develop a chronic inflammatory process. Mega cisterna magna,
calcification at falx cerebri level, doubling of the septum
pellucidum and gap-type lesions were more frequent in the age
category 29-61 years, with no statistically significant differences
between the two age categories. Cortical atrophy and hemosiderotic
spots were more frequently described in patients aged 29-61 years,
with statistically significant differences between the two age
categories. Regarding the hemosiderotic spots, lacuna, the venous
malformation and the calcification of the falx cerebri found on MRI
in the current study, few reports in the literature discuss their
manifestation and it is reasonable to conclude that they are not
frequent abnormalities of the brain in patients with
schizophrenia.
The occurrence of cortical atrophy and the abnormal
cavum septum pellucidum are the most common changes on MRI in
patients with schizophrenia, according to the literature. Given
that 25 of the 45 patients in the current study were children, it
is not surprising that only 7 (15.5%) of them documented cortical
atrophy or the presence of double septum pellucidum.
Childhood-onset schizophrenia (COS) exhibits brain anatomic
abnormalities that are close to those observed in adult
populations, suggesting that there is a general continuity between
these exceptional childhood cases and adult schizophrenia
populations.
The research included patients with schizophrenia
who were already taking antipsychotic treatment. Concerning
utilization of antipsychotic medications by a large number of
participants in neuroimaging trials, the effect of antipsychotic
medications as a cause of MRI changes was not previously explored
(48). Lieberman et al
concluded that prescribing haloperidol to patients caused a marked
reduction in the grey matter after 12 and 52 weeks, but no
reduction was observed in patients who were prescribed olanzapine.
Patients were investigated using MRI brain imaging (49). The brain seems to be more damaged
after taking typical antipsychotics than atypical ones. This is not
the only study reported in medical literature that observed the
impact of antipsychotics upon the brain structure. However, data
from studies of wider groups of participants shows a progressive
association between antipsychotic usage and brain volumetric
decrease. Cahn et al reported MRI modifications in 34
schizophrenic patients undergoing treatment with typical and
atypical antipsychotics, modification such as increased volume of
lateral ventricles and decreased brain volume, especially in grey
matter. Patients were prospectively studied for 16 weeks and
compared to a control group that did not receive any antipsychotic
treatment (50). In a study of 18
antipsychotic-naive schizophrenia patients and 18 stable controls,
Jayakumar et al (51) found
gray-matter volumetric decreases and higher cerebrospinal fluid
volumes, as did Davatzikos et al (52).
Since prior imaging was not available for
comparison, the causal relationship between schizophrenia and
observed brain changes could not be determined. The relatively
small number of investigated cases did not allow the establishment
of imaging abnormalities with the role of biomarker, thus an
extension of the number of investigated cases being necessary, as
well as the comparative evaluation with a similar group of healthy
subjects. To create a link between schizophrenia and brain
structural abnormalities, larger longitudinal studies with
functional imaging are needed.
In summary, MRI can be a helpful method in
identifying brain changes in patients with schizophrenia and can
also contribute to establishing a long-term prognosis for them.
Additionally, MRI may be used to make a differential diagnosis
between organic psychoses and those that do not have a neurological
substrate (53,54).
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported partially by a grant
from the Romanian National Authority for Scientific Research and
Innovation CCCDI-UEFISCDI project number Cofund-ERANET NEURON
SYNSCHIZ 6/2018.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FPI conceived and designed the study, wrote the
manuscript, contributed in all stages of the article. MCM analyzed
and collecting data regarding MRI findings. MB performed
statistical analysis and was involved in revising the manuscript
critically for important intellectual content. EA and FL
contributed to the data collection and interpretation. FR
contributed to reviewing the data and editing of the manuscript. RC
confirmed the authenticity of all the imaging investigations and
interpreted the results. AMC finalized the analysis and gave the
final approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
‘Prof. Dr. Alexandru Obregia’ Clinical Hospital of Psychiatry,
Bucharest, Romania (approval no. 10/21.03.2018). Written informed
consent was obtained from each patient/legal guardian of each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rofman ES: Kaplan and Sadock's synopsis of
psychiatry. J Clin Psychiatry. 11(303)2015.
|
|
2
|
Murray RM, Bhavsar V, Tripoli G and Howes
O: 30 years on: How the neurodevelopmental hypothesis of
schizophrenia morphed into the developmental risk factor model of
psychosis. Schizophr Bull. 43:1190–1196. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Howes OD and Murray RM: Schizophrenia: An
integrated sociodevelopmental-cognitive model. Lancet.
383:1677–1687. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Weinberger DR and Levitt P:
Neurodevelopmental origins of schizophrenia. In: Schizophrenia 3rd
Edition. Weinberger DR and Harrison PE (eds.) Wiley Blackwell,
Oxford, England, pp393-412, 2010.
|
|
5
|
Andreasen NC, Flashman L, Flaum M, Arndt
S, Swayze V 2nd, O'Leary DS, Ehrhadt JC and Yuh WT: Regional brain
abnormalities in schizophrenia measured with magnetic resonance
imaging. JAMA. 272:1763–1769. 1994.PubMed/NCBI
|
|
6
|
Bilder RM, Wu H, Bogerts B, Degreef G,
Ashtari M, Alvir JM, Snyder PJ and Lieberman JA: Absence of
regional hemispheric volume asymmetries in first-episode
schizophrenia. Am J Psychiatry. 151:1437–1447. 1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bilder RM, Wu H, Bogerts B, Ashtari M,
Robinson D, Woerner M, Lieberman JA and Degreef G: Cerebral volume
asymmetries in schizophrenia and mood disorders: A quantitative
magnetic resonance imaging study. Int J Psychophysiol. 34:197–205.
1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Narr KL, Thompson PM, Sharma T, Moussai J,
Cannestra AF and Toga AW: Mapping morphology of the corpus callosum
in schizophrenia. Cereb Cortex. 10:40–49. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Downhill JE Jr, Buchsbaum MS, Wei T,
Spiegel-Cohen J, Hazlett EA, Haznedar MM, Silverman J and Siever
LJ: Shape and size of the corpus callosum in schizophrenia and
schizotypal personality disorder. Schizophr Res. 42:193–208.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chua SE, Sharma T, Takei N, Murray RM and
Woodruff PW: A magnetic resonance imaging study of corpus callosum
size in familial schizophrenic subjects, their relatives, and
normal controls. Schizophr Res. 41:397–403. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shenton ME, Dickey CC, Frumin M and
McCarley RW: A review of MRI findings in schizophrenia. Schizophr
Res. 49:1–52. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kwon JS, Shenton ME, Hirayasu Y, Salisbury
DF, Fischer IA, Dickey CC, Yurgelun-Todd D, Tohen M, Kikinis R,
Jolesz FA and McCarley RW: MRI study of cavum septi pellucidi in
schizophrenia, affective disorder, and schizotypal personality
disorder. Am J Psychiatry. 155:509–515. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Akhtar W, Naqvi HA, Hussain S, Ali A and
Ahmad N: Magnetic Resonance Imaging Findings in Patients with
Schizophrenia. J Coll Physicians Surg Pak. 20:167–170.
2010.PubMed/NCBI
|
|
14
|
Najjar S and Pearlman DM:
Neuroinflammation and white matter pathology in schizophrenia:
Systematic review. Schizophr Res. 161:102–112. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chew LJ, Fusar-Poli P and Schmitz T:
Oligodendroglial alterations and the role of microglia in white
matter injury: Relevance to schizophrenia. Dev Neurosci.
35:102–129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ohnuma T, Kimura M, Takahashi T, Iwamoto N
and Arai H: A magnetic resonance imaging study in first-episode
disorganized-type patients with schizophrenia. Psychiatry Clin
Neurosci. 51:9–15. 1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
DeLisi LE, Sakuma M, Tew W, Kushner M,
Hoff AL and Grimson R: Schizophrenia as a chronic active brain
process: A study of progressive brain structural change subsequent
to the onset of schizophrenia. Psychiatry Res. 74:129–140.
1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
DeLisi LE, Stritzke P, Riordan H, Holan V,
Boccio A, Kushner M, McClelland J, Van Eyl O and Anand A: The
timing of brain morphological changes in schizophrenia and their
relationship to clinical outcome. Biol Psychiatry. 31:241–254.
1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma
M, Kushner M, Lee G, Shedlack K, Smith AM and Grimson R: A
prospective follow-up study of brain morphology and cognition in
first-episode schizophrenic patients: Preliminary findings. Biol
Psychiatry. 38:349–360. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Coulon N, Godin O, Bulzacka E, Dubertret
C, Mallet J, Fond G, Brunel L, Andrianarisoa M, Anderson G, Chereau
I, et al: Early and very early-onset schizophrenia compared with
adult-onset schizophrenia: French FACE-SZ database. Brain Behav.
10:e01495. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wright IC, Rabe-Hesketh S, Woodruff PW,
David AS, Murray RM and Bullmore ET: Meta-analysis of regional
brain volumes in schizophrenia. Am J Psychiatry. 157:16–25.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Matsumoto H, Simmons A, Williams S, Pipe
R, Murray R and Frangou S: Structural magnetic imaging of the
hippocampus in early onset schizophrenia. Biol Psychiatry.
49:824–831. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Margari F, Presicci A, Petruzzelli MG,
Ventura P, Di Cuonzo F, Palma M and Margari L: Very early onset and
greater vulnerability in schizophrenia: A clinical and neuroimaging
study. Neuropsychiatr Dis Treat. 4:825–830. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Degreef G, Bogerts B, Falkai P, Greve B,
Lantos G, Ashtari M and Lieberman J: Increased prevalence of the
cavum septum pellucidum in magnetic resonance scans and post-mortem
brains of schizophrenic patients. Psychiatry Res. 45:1–13.
1992.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tordesillas-Gutierrez D, Koutsouleris N,
Roiz-Santiañez R, Meisenzahl E, Ayesa-Arriola R, Marco de Lucas E,
Soriano Mas C, Suarez Pinilla P and Crespo-Facorro B: Grey matter
volume differences in non-affective psychosis and the effects of
age of onset on grey matter volumes: A voxelwise study. Schizophr
Res. 164:74–82. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Narr KL, Bilder RM, Toga AW, Woods RP, Rex
DE, Szeszko PR, Robinson D, Sevy S, Bruce HG, Wang YP, DeLuca H and
Thompson PM: Mapping cortical thickness and gray matter
concentration in first episode schizophrenia. Cereb Cortex.
15:708–719. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gogtay N: Cortical brain development in
schizophrenia: Insights from neuroimaging studies in
childhood-onset schizophrenia. Schizophr Bull. 34:30–36.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mathalon DH, Sullivan EV, Lim KO and
Pfefferbaum A: Progressive brain volume changes and the clinical
course of schizophrenia in men: A longitudinal magnetic resonance
imaging study. Arch Gen Psychiatry. 58:148–157. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gur RE, Cowell P, Turetsky BI, Gallacher
F, Cannon T, Bilker W and Gur RC: A follow-up magnetic resonance
imaging study of schizophrenia. Relationship of neuroanatomical
changes to clinical and neurobehavioral measures. Arch Gen
Psychiatry. 55:145–152. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lieberman J, Chakos M, Wu H, Alvir J,
Hoffman E, Robinson D and Bilder R: Longitudinal study of brain
morphology in first episode schizophrenia. Biol Psychiatry.
49:487–499. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ho BC, Andreasen NC, Nopoulos P, Arndt S,
Magnotta V and Flaum M: Progressive structural brain abnormalities
and their relationship to clinical outcome: A longitudinal magnetic
resonance imaging study early in schizophrenia. Arch Gen
Psychiatry. 60:585–594. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rapoport JL, Castellanos FX, Gogate N,
Janson K, Kohler S and Nelson P: Imaging normal and abnormal brain
development: New perspectives for child psychiatry. Aust N Z J
Psychiatry. 35:272–281. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nopoulos P, Torres I, Flaum M, Andreasen
NC, Ehrhardt JC and Yuh WT: Brain morphology in first-episode
schizophrenia. Am J Psychiatry. 152:1721–1723. 1995.PubMed/NCBI View Article : Google Scholar
|
|
34
|
van Haren NEM, Cahn W, Hulshoff Pol HE and
Kahn RS: Schizophrenia as a progressive brain disease. Eur
Psychiatry. 23:245–254. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kasai K, Shenton ME, Salisbury DF,
Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R,
Jolesz FA and McCarley RW: Progressive decrease of left superior
temporal gyrus gray matter volume in patients with first-episode
schizophrenia. Am J Psychiatry. 160:156–164. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kay SR, Fiszbein A and Opler LA: The
Positive and Negative Syndrome Scale (PANSS) for schizophrenia.
Schizophr Bull. 13:261–276. 1987.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Addington D, Addington J and Schissel B: A
depression rating scale for schizophrenics. Schizophr Res.
3:247–251. 1990.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sheehan KH and Sheehan DV: Assessing
treatment effects in clinical trials with the discan metric of the
sheehan disability scale. Int Clin Psychopharmacol. 23:70–83.
2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mioshi E, Dawson K, Mitchell J, Arnold R
and Hodges JR: The Addenbrooke's Cognitive Examination Revised
(ACE-R): A brief cognitive test battery for dementia screening. Int
J Geriatr Psychiatry. 21:1078–1085. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
American Psychiatric Association:
Diagnostic and statistical manual of mental disorders. 4th edition.
American Psychiatric Publishing, Inc., Washington DC, 1994.
|
|
41
|
World Health Organization: The ICD-10
classification of mental and behavioural disorders: Diagnostic
criteria for research. WHO Press, Geneva, 1993.
|
|
42
|
Taber KH, Shaw JB, Loveland KA, Pearson
DA, Lane DM and Hayman LA: Accentuated Virchow-Robin spaces in the
centrum semiovale in children with autistic disorder. J Comput
Assist Tomogr. 28:263–268. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Patankar TF, Mitra D, Varma A, Snowden J,
Neary D and Jackson A: Dilatation of the virchow-robin space is a
sensitive indicator of cerebral microvascular disease: Study in
elderly patients with dementia. AJNR Am J Neuroradiol.
26:1512–1520. 2005.PubMed/NCBI
|
|
44
|
Wuerfel J, Haertle M, Waiczies H, Tysiak
E, Bechmann I, Wernecke KD, Zipp F and Paul F: Perivascular
spaces-MRI marker of inflammatory activity in the brain? Brain.
131:2332–2340. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Davis KL, Stewart DG, Friedman JI,
Buchsbaum M, Harvey PD, Hof PR, Buxbaum J and Haroutunian V: White
matter changes in schizophrenia: evidence for myelin-related
dysfunction. Arch Gen Psychiatry. 60:443–456. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Smith AB and Ross CM: Nasal sinusitis and
mental disorder. A survey of 818 cases. Edinb Med J. 45:343–356.
1938.PubMed/NCBI
|
|
47
|
Ciobanu AM, Rosca T, Vladescu CT, Tihoan
C, Popa MC, Boer MC and Cergan R: Frontal epidural empyema (Pott's
puffy tumor) associated with Mycoplasma and depression. Rom J
Morphol Embryol. 55:1203–1207. 2014.PubMed/NCBI
|
|
48
|
Moncrieff J and Leo J: A systematic review
of the effects of antipsychotic drugs on brain volume. Psychol Med.
40:1409–1422. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lieberman JA, Tollefson GD, Charles C,
Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy
J, et al: Antipsychotic drug effects on brain morphology in
first-episode psychosis. Arch Gen Psychiatry. 62:361–370.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cahn W, Hulshoff Pol HE, Lems EB, van
Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van
Engeland H and Kahn RS: Brain volume changes in first-episode
schizophrenia: A 1-year follow-up study. Arch Gen Psychiatry.
59:1002–1010. 2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jayakumar PN, Venkatasubramanian G,
Gangadhar BN, Janakiramaiah N and Keshavan MS: Optimized
voxel-based morphometry of gray matter volume in first-episode,
antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol
Psychiatry. 29:587–591. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Davatzikos C, Shen D, Gur RC, Wu X, Liu D,
Fan Y, Hughett P, Turetsky BI and Gur RE: Whole-brain morphometric
study of schizophrenia revealing a spatially complex set of focal
abnormalities. Arch Gen Psychiatry. 62:1218–1227. 2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ciobanu AM, Lisievici MG, Coman TC,
Ciubotaru GV, Drăghia A, Drăghia F and Ciucu AA: Giant wing
sphenoid meningioma with principal manifestation depression. Rom J
Morphol Embryol. 50:713–717. 2009.PubMed/NCBI
|
|
54
|
Ciobanu AM, Popa C, Marcu M and Ciobanu
CF: Psychotic depression due to giant condyloma Buschke-Löwenstein
tumors. Rom J Morphol Embryol. 55:189–195. 2014.PubMed/NCBI
|