Introduction
Uterine cervical cancer is the fourth most common
cancer and the leading cause of death from cancer among women
worldwide (1). Although cervical
cancer incidence and mortality rates have decreased in recent years
because of screening programs and early detection of preinvasive
cervical lesions, cervical cancer remains the second most common
female cancer and the third leading cause of cancer-associated
death among women. The mortality rate of cervical cancer is
exceptionally high in developing countries (1). The standard of care for patients with
recurrent cervical cancer is platinum-based chemotherapy; however,
chemotherapy is mostly palliative, and new targeted therapeutic
agents are urgently needed (2).
Phosphatase and tensin homolog deleted in chromosome
10 (PTEN) is an important tumor suppressor that is frequently
mutated or downregulated in various human cancer types (3-9).
PTEN mutation or downregulation can significantly enhance
carcinogenesis and worsen treatment outcomes (3-9).
In a recent study, in contrast to previous reports, high PTEN
expression was associated with a worse prognosis in patients with
wild-type p53 breast cancer (10).
PTEN and p53 interact closely, with PTEN regulating
p53 function and p53 enhancing PTEN transcription (11). In PTEN-/-mice, the loss of PTEN
dramatically decreased p53 protein levels (12). Nevertheless, acute loss of PTEN
increased the levels and enhanced the function of p53 in prostate
cancer cells (13). In a xenograft
model of prostate cancer, long-term PTEN inhibition with the
water-soluble vanadium-based complex VO-OHpic significantly
decreased the tumor burden and prolonged the survival of
tumor-bearing mice (14).
Furthermore, PTEN downregulation in renal epithelial cells resulted
in dedifferentiation and growth arrest (15). These anti-proliferative effects of
PTEN silencing were p53-dependent (15).
However, the role of PTEN in cervical cancer remains
unclear. In the present study, the effects of PTEN silencing on the
proliferation, apoptosis and cell cycle of cervical cancer cells
were investigated. The mechanisms underlying the effects of PTEN
downregulation on the proliferation of cervical cancer cells were
also explored.
Materials and methods
Cell lines and cultures
The human cervical cancer cell lines HeLa and CaSki
(Korean Cell Line Bank) were used. Both cell lines were cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) containing 10%
(v/v) fetal bovine serum (FBS, Welgene, Inc.), 1% (v/v) penicillin
and streptomycin. Cells were maintained in a humidified incubator
with 5% CO2 at 37˚C.
Chemicals and reagents
To minimize the possibility of cross-reactivity,
cells were transfected with two different small interfering
(si)RNAs targeting PTEN: siRNA1, CCA GUC AGA GGC GCU AUG UdTdT; and
siRNA2, CAA GAU GUU UGA AAC UAU (16,17). A
p53-targeting siRNA (CUA CUU CCU GAA AAC GdTdT) was also used
(18). A commercial scrambled siRNA
was used as a negative control (Bioneer Corporation). Lipofectamine
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) was used as
the transfection reagent. Opti-MEM (minimal essential medium;
Gibco; Thermo Fisher Scientific, Inc.) was used to prepare the
siRNA-lipid complexes. For cell cycle analyses, a FITC BrdU Flow
kit (BD PharMingen; BD Biosciences) was used. An APO-BrdU TUNEL
Assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
assess apoptosis. An Annexin V-FITC kit (BD PharMingen; BD
Biosciences) was used to visualize apoptotic cells under a
fluorescence microscope. An EZ-cyTox kit (DoGenBio) was used to
evaluate cell viability. The following primary antibodies were
used: PTEN (1:1,000; cat. no. SC-6817-R; Santa Cruz Biotechnology,
Inc.), cyclin D1 (1:1,000; cat. no. SC-718; Santa Cruz
Biotechnology, Inc.), p53 (1:1,000; cat. no. SC-126; Santa Cruz
Biotechnology, Inc.), actin (1:5,000; cat. no. SC-1615; Santa Cruz
Biotechnology, Inc.), p27 (1:1,000; cat. no. 3686; Cell Signaling
Technologies, Inc.), p21 (1:1,000; cat. no. 2947; Cell Signaling
Technologies, Inc.), cyclin dependent kinase (cdk)-6 (1:1,000; cat.
no. 3136; Cell Signaling Technologies, Inc.), cyclin E (1:1,000;
cat. no. 4129; Cell Signaling Technologies, Inc.), cyclin A
(1:1,000; cat. no. 4656; Cell Signaling Technologies, Inc.), cyclin
B1 (1:1,000; cat. no. 4138; Cell Signaling Technologies, Inc.),
PARP (1:1,000; cat. no. 9542; Cell Signaling Technologies, Inc.),
caspase-3 (1:1,000; cat. no. 9665; Cell Signaling Technologies,
Inc.), AKT (1:2,000; cat. no. 4691; Cell Signaling Technologies,
Inc.), pAKT (Thr308) (1:1,000; cat. no. 13038; Cell Signaling
Technologies, Inc.), ERK1/2 (1:1,000; cat. no. 9122; Cell Signaling
Technologies, Inc.), pERK1/2 (1:1,000; cat. no. 4370, Cell
Signaling Technologies, Inc.), Bax (1:1,000; cat. no. 5023; Cell
Signaling Technologies, Inc.), and MDM2 (1:1,000; cat. no. ab16895;
Abcam). Goat anti-mouse IgG-HRP (1:5,000; cat. no. sc-2005) and
mouse anti-rabbit IgG-HRP (1:5,000; cat. no. sc-2357) secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
PTEN downregulation by siRNA
To synchronize the cell cycle, HeLa and CaSki cells
were cultured in 100-mm culture dishes (1x106
cells/dish) under serum-starvation and antibiotic-free conditions.
After 48 h, the cell culture medium was refreshed and the cells
were maintained under serum-starvation and antibiotic-free
conditions. Cells were transfected with siRNAs targeting PTEN and a
negative control using the Lipofectamine RNAiMAX transfection
reagent according to the manufacturer's instructions. Briefly,
siRNA-lipid complexes were prepared in 500 µl Opti-MEM media by
mixing 20 µl transfection reagent and 20 µM siRNAs (PTEN and
negative control) for 5 min at room temperature. The mixtures were
added to the cells in 10 ml culture medium. After 6 h of
transfection, the medium was replaced with RPMI-1640 containing 10%
(v/v) FBS, 1% (v/v) penicillin and streptomycin. Cells were
incubated in complete growth medium for 48 h.
Western blot analysis
Transfected cells were harvested and lysed in RIPA
buffer supplemented with protease inhibitors. Protein concentration
was determined using BCA protein assay (Thermo Fisher Scientific,
Inc.). After incubation for 30 min on ice, cell lysates containing
10-30 µg protein per well were resolved by 10-12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked with 5% (w/v) nonfat dried skimmed milk in
Tris-buffered saline containing 0.1% Tween-20 (pH 7.6) overnight at
4˚C with agitation. Subsequently, membranes were incubated with
primary antibodies overnight at 4˚C with agitation. After three
washes with 1X Tris-buffered saline containing 0.1% Tween-20, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:5,000) for 1 h at room temperature. Signals
were developed with a chemiluminescence reagent (Advansta);
chemiluminescence was detected using an Amersham Imager 600 (GE
Healthcare Biosciences AB).
Flow cytometry
Bromodeoxyuridine (BrdU) incorporation analysis was
conducted to evaluate cell-cycle progression. Briefly,
serum-starved siRNA-transfected cells (5x105 cells in
100-mm culture dish) were cultured in complete growth media for 48
h and then labeled with 10 µM BrdU at 37˚C for 60 min. After
labeling with BrdU, cells were harvested and immunostained with
FITC-conjugated anti-BrdU according to the manufacturer's
instructions. BrdU incorporation was evaluated by flow cytometry
(BD-FACSCalibur; BD Biosciences).
Apoptosis was assessed by measuring the amount of
5-bromo-2-deoxyuridine 5-triphosphate (BrdUTP) incorporated into
the DNA using flow cytometry. Transfected cells were harvested,
fixed with paraformaldehyde [1% (w/v) in PBS], and labeled with 8.0
µl BrdUTP (APO-BrdU TUNEL Assay kit) and 31.25 µl H2O
according to the manufacturer's instructions. Stained cells were
analyzed on a BD-FACSCalibur, (BD Biosciences).
Visualization of apoptotic cells by
fluorescence microscopy
To confirm the role of PTEN silencing in apoptosis,
Annexin V-FITC staining was performed, followed by fluorescence
microscopy (magnification, x200). Briefly, transfected HeLa cells
were washed twice with PBS and once with Annexin V binding buffer.
Subsequently, cells were stained with Annexin V-FITC (1:10 in
Annexin V binding buffer) for 15 min at room temperature. Cells
were washed and observed under a confocal microscope (LSM 700; Carl
Zeiss AG). ImageJ software was used for quantification.
MTT assay
The viability of HeLa and CaSki cells was analyzed
following transfection with PTEN and control siRNAs. Briefly,
siRNA-transfected cells were incubated with water-soluble
tetrazolium salts (1/10 of the volume of the culture medium;
EZ-cyTox, DoGenBio) at 37˚C for 2 h. Optical absorbance at 450 nm
was measured using a microplate reader. The optical absorbance of
the culture medium alone was measured as blank.
Statistical analysis
Data were expressed as mean ± standard deviation.
All experiments were performed more than three times. Independent-t
tests and Mann-Whitney U tests were performed using SPSS 17.0
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
PTEN downregulation enhances the
expression of apoptosis-associated proteins
In order to assess the effects of PTEN
downregulation on the expression levels of proteins involved in
cellular fate, western blotting was performed to measure the levels
of proteins associated with cellular apoptosis and senescence.
Notably, PTEN silencing in HeLa cells increased the levels of p53
and p53-associated proteins (Fig.
1A). Although PTEN silencing in CaSki cells did not alter the
levels of p53, levels of p21 and p27 were increased in PTEN
siRNA-transfected cells.
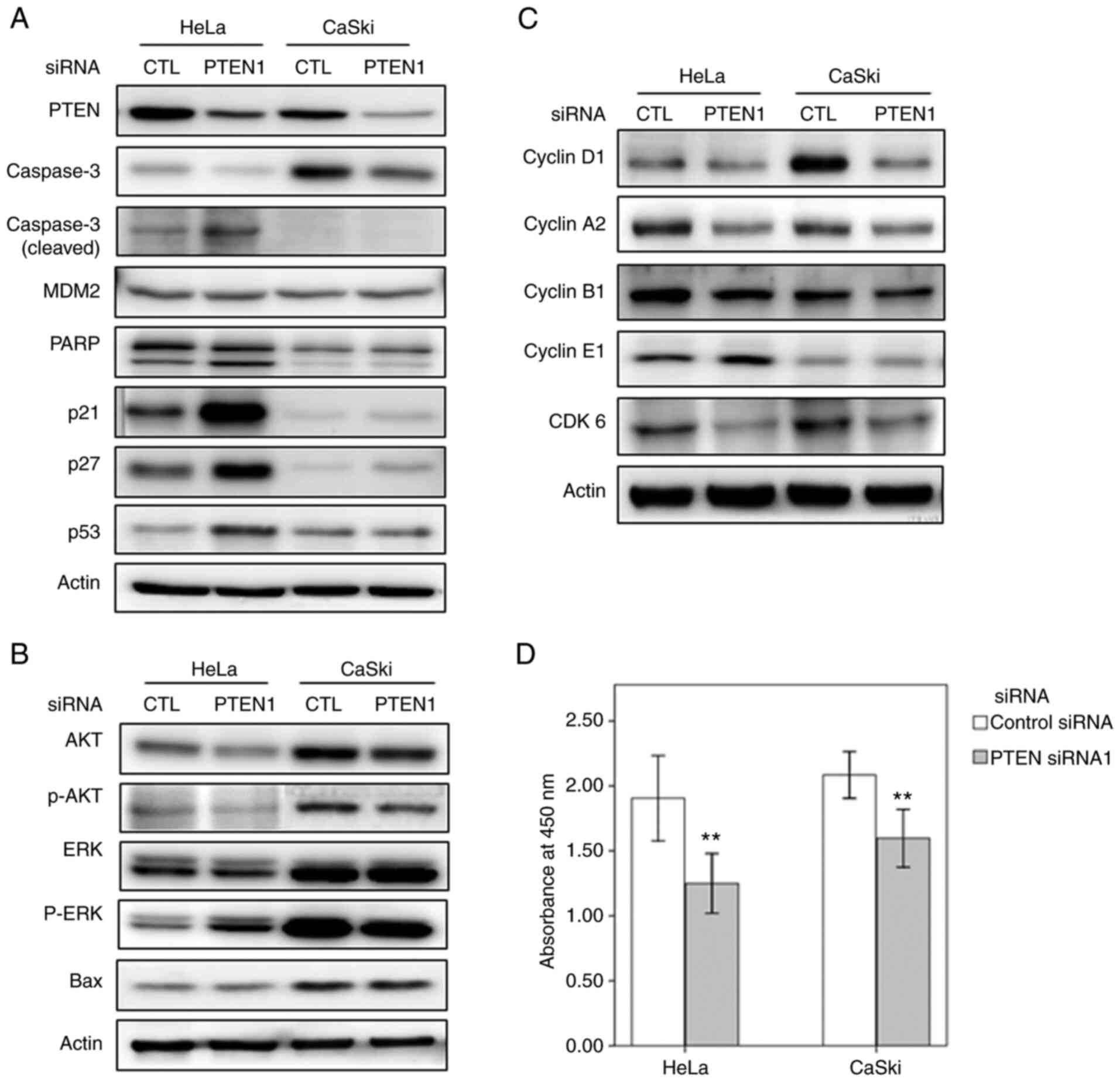 | Figure 1PTEN silencing regulates the
expression levels of proteins involved in apoptosis and the cell
cycle. (A-C) Western blots showing the levels of proteins involved
in apoptosis (caspase-3, MDM2, PARP, p21, p27, p53 and Bax), cell
cycle (cyclin D1, cyclin A2, cyclin B1, cyclin E1 and CDK6), and
AKT and ERK signaling pathways in cervical cancer cells transfected
with PTEN siRNA1. (D) Cell viability of HeLa and CaSki cells
transfected with PTEN-targeting and control siRNAs (error bars
represent 95% confidence intervals). **P<0.01 vs.
control siRNA. siRNA, small interfering RNA; CTL, control siRNA;
PTEN1, PTEN siRNA1. |
p53 is regulated by the extracellular signal
regulated kinase (ERK) and is essential for the activation of
downstream ERK pathway components. Activation of caspase-3 and poly
(ADP-ribose) polymerase (PARP) by ERK induces apoptosis (19,20).
PARP is a substrate for caspase-3, and PARP cleavage promotes
apoptosis by inhibiting DNA repair (21). In the study, PTEN silencing in HeLa
cells increased the levels of phosphorylated (p)ERK, p53, caspase-3
and cleaved PARP; Bax, levels remained unchanged (Fig. 1B). It was also investigated whether
PTEN downregulation affects cell proliferation and viability.
Indeed, PTEN silencing inhibited the proliferation of HeLa and
CaSki cells, implying that PTEN downregulation induces apoptosis in
cervical cancer cells (Fig.
1D).
PTEN downregulation represses the
expression of cell cycle-associated proteins
To achieve cellular senescence, an increased amount
of Cdk inhibitor p21, a target of p53, can inactivate both cyclin E
and cyclin D1-associated kinase (22,23).
In order to examine the role of PTEN in cell-cycle
regulation in cervical cancer cells, the levels of
cell-cycle-associated proteins were analyzed after PTEN silencing.
The levels of Cdk6, cyclin D1 and cyclin A2 were decreased after
PTEN silencing (Fig. 1C). However,
PTEN downregulation did not alter the levels of cyclin E1 and
cyclin B1 in HeLa or CaSki cells (Fig.
1C).
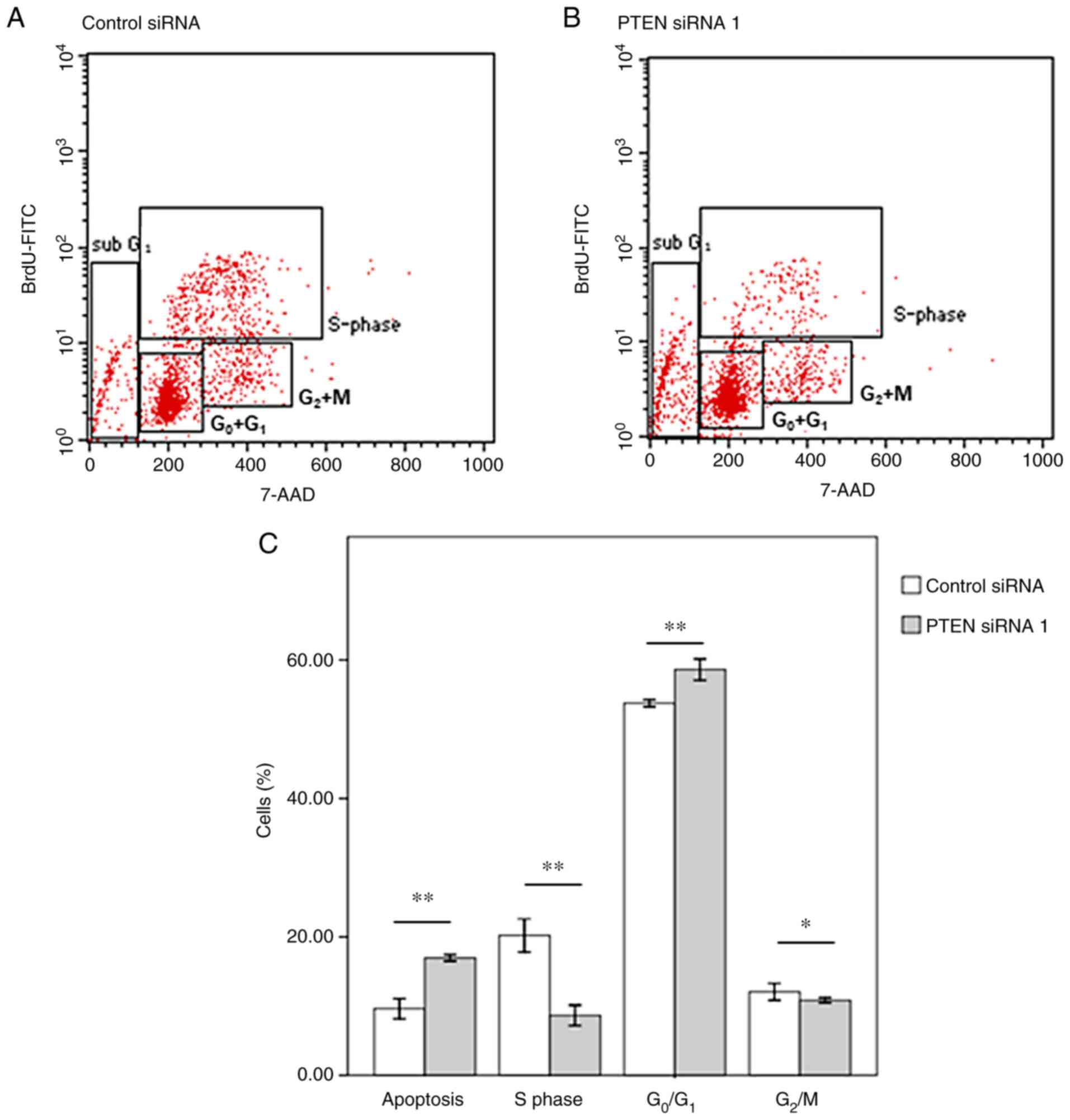
Furthermore, flow cytometry analyses revealed that
the proportion of cells in the G0/G1 and
sub-G1 phases was increased after PTEN silencing. On the
other hand, the proportion of cells in the S-phase was decreased
when PTEN was downregulated (Figs.
2 and S1). These findings
imply that PTEN downregulation promotes overexpression of p53 and
p21, and causes apoptosis and G1 cell-cycle arrest in
HeLa cells (4,13,15,24).
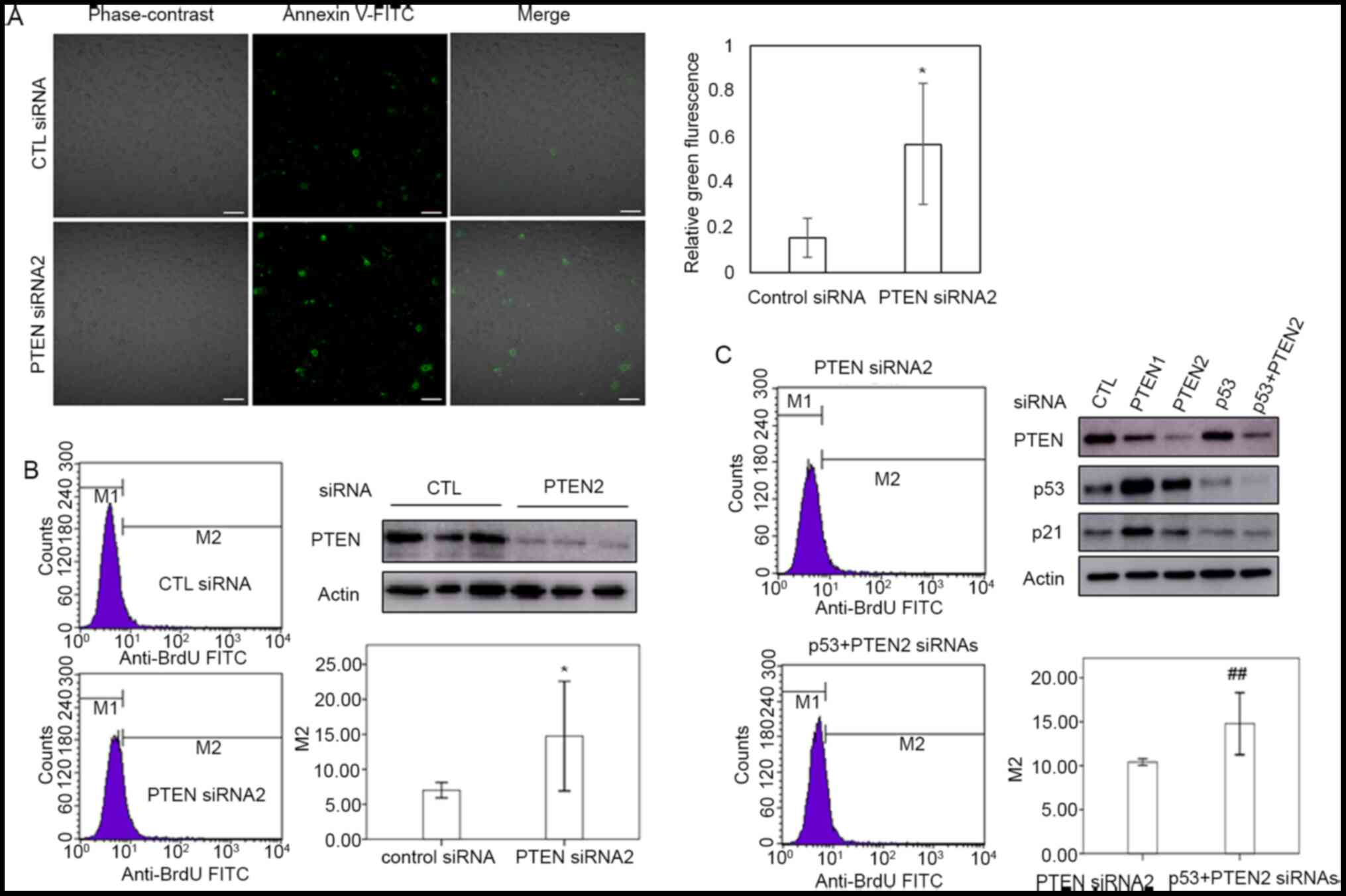
PTEN downregulation induces apoptosis
independently of p53
In order to confirm the role of PTEN in the
apoptosis of cervical cancer cells, TUNEL assay and flow cytometry
were performed using PTEN siRNA-transfected HeLa cells. As shown in
Fig. 3A and B, PTEN silencing increased the proportion
of HeLa cells undergoing apoptosis. To investigate the role of p53
in the apoptosis of cells with PTEN downregulation, HeLa cells were
co-transfected with siRNAs targeting PTEN and p53. Cells
co-transfected with PTEN-targeting and scrambled siRNAs were used
as a control. PTEN promoted apoptosis in cervical cancer cells
regardless of the levels of p53 (Fig.
3B and C). These results imply
that p53 is not essential for the induction of apoptosis in HeLa
cells in response to PTEN downregulation.
Discussion
Regulation of DNA damage during the cell cycle is
essential for cell survival. Cells with unrepaired DNA damage
undergo apoptosis or cell-cycle arrest due to the activation of
DNA-damage-response pathways (25).
PTEN and p53 are crucial for DNA damage repair and
the maintenance of genomic integrity (26). Although PTEN and p53 are often
downregulated in cervical cancer, somatic mutations in PTEN are
rare, ranging from 0 to 2% (7,27); p53
mutations have been reported in 13.3% of cervical adenocarcinomas
and 5.9% of cervical squamous cell carcinomas (28). Although downregulation of PTEN and
p53 has been reported in cervical cancer (8,29), no
somatic mutations in these genes were detected in HeLa and CaSki
cells (30,31), allowing cancer cells to survive
despite having unresolved DNA damage. In the present study, PTEN
downregulation promoted apoptosis and cell-cycle arrest in cervical
cancer cells (Figs. 1 and 2), implying that loss of PTEN impairs the
ability of cells to repair DNA damage. Unrepaired DNA damage in
cervical cancer cells may promote apoptosis by activating
DNA-damage-response pathways.
The PTEN, PI3K and AKT signaling pathways are
critical regulators of cell proliferation and apoptosis (3,32). The
downregulation of PTEN is associated with the activation of PI3K
and AKT signaling, cell proliferation and a worse prognosis in
various cancer types (3-5).
However, Tan and Chiu (20)
reported that the activation of ERK inhibited the AKT pathway. In
the present study, loss of PTEN activated the ERK pathway;
nevertheless, the expression levels of MDM2 and phospho-AKT were
unchanged regardless of the PTEN status in HeLa cells (Fig. 1A and B). This implies that PTEN regulates p53
levels independently of MDM2 and AKT, consistent with a previous
report (12). In contrast to the
present observations in HeLa cells, PTEN silencing did not affect
AKT activation or MDM expression in CaSki cells. Thus, the
regulation of the PTEN-ERK-p53-PARP axis may differ depending on
cell type.
Although the loss of both PTEN and p53 enhances
tumor aggressiveness, the inactivation of PTEN with intact p53
induces cellular senescence (13).
Similar regulatory mechanisms have been reported for BRCA1/2 and
p53 in breast cancer (33,34). Furthermore, PTEN inhibition in
hepatocarcinoma cells expressing low levels of PTEN has been shown
to drive senescence (35). Although
PTEN downregulation is common in cervical cancer (8,9),
somatic mutations in PTEN are rare (7,27).
Therefore, most cervical cancer have intact PTEN, but expressed at
low levels, similar to reports regarding hepatocarcinoma (35). In the present study, PTEN inhibition
in cervical cancer cells expressing low levels of PTEN promoted
cellular senescence and apoptosis, which was independent of p53
status. Therefore, PTEN downregulation in cervical cancer may
result in p53 upregulation, thereby inducing apoptosis in cervical
cancer cells (Fig. 1).
In conclusion, the present data imply that PTEN
downregulation in cervical cancer cells induces cell-cycle arrest
and apoptosis. Furthermore, decreased cell viability was observed
as a result of PTEN downregulation in cervical cancer cells. Taken
together, the present study findings may present a possible
strategy for cervical cancer treatment via the regulation of PTEN.
More studies will need to follow that will not only detail the
effects of PTEN regulation but also reinforce some of the
limitations of the present study, which include lack of various
forms of analysis and clear mechanistic study. The limitations of
the present study will be addressed in a subsequent study, which
will mainly focus on elucidating the role of p53 in cervical cancer
cell apoptosis by checking gene expression levels of components
that are essential for apoptosis induced by PTEN
downregulation.
Supplementary Material
PTEN silencing regulates cell-cycle
progression in HeLa cells. Side scatter vs. forward scatter plots;
FL3-A (area) vs. FL3-W (width) scatter plots; SSC vs. BrdU-FITC
scatter plots; SSC vs. 7-AAD scatter plots; and histograms of 7-AAD
expression in (A) control and (B) PTEN siRNA-transfected HeLa
cells. siRNA, small interfering RNA; SSC, side angle light scatter
channel.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Gil Medical Center,
Gachon University (grant no. 2012-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JWS and JYY conducted the experiments and analyzed
the results. JWS designed the study and wrote the manuscript. SHK
performed analysis and interpretation of data, and the overall
editing of the manuscript. JYY and SHK confirmed the authenticity
of all the raw data. All the authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Boussios S, Seraj E, Zarkavelis G,
Petrakis D, Kollas A, Kafantari A, Assi A, Tatsi K, Pavlidis N and
Pentheroudakis G: Management of patients with recurrent/advanced
cervical cancer beyond first line platinum regimens: Where do we
stand? A literature review. Crit Rev Oncol Hematol. 108:164–174.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim J, Eltoum IE, Roh M, Wang J and
Abdulkadir SA: Interactions between cells with distinct mutations
in c-MYC and Pten in prostate cancer. PLoS Genet.
5(e1000542)2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yanagawa N, Leduc C, Kohler D, Saieg MA,
John T, Sykes J, Yoshimoto M, Pintilie M, Squire J, Shepherd FA and
Tsao MS: Loss of phosphatase and tensin homolog protein expression
is an independent poor prognostic marker in lung adenocarcinoma. J
Thorac Oncol. 7:1513–1521. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tashiro H, Blazes MS, Wu R, Cho KR, Bose
S, Wang SI, Li J, Parsons R and Ellenson LH: Mutations in PTEN are
frequent in endometrial carcinoma but rare in other common
gynecological malignancies. Cancer Res. 57:3935–3940.
1997.PubMed/NCBI
|
|
8
|
Lu D, Qian J, Yin X, Xiao Q, Wang C and
Zeng Y: Expression of PTEN and survivin in cervical cancer:
Promising biological markers for early diagnosis and prognostic
evaluation. Br J Biomed Sci. 69:143–146. 2012.PubMed/NCBI
|
|
9
|
Lee JS, Choi YD, Lee JH, Nam JH, Choi C,
Lee MC, Park CS, Kim HS and Min KW: Expression of PTEN in the
progression of cervical neoplasia and its relation to tumor
behavior and angiogenesis in invasive squamous cell carcinoma. J
Surg Oncol. 93:233–240. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yndestad S, Austreid E, Knappskog S,
Chrisanthar R, Lilleng PK, Lønning PE and Eikesdal HP: High PTEN
gene expression is a negative prognostic marker in human primary
breast cancers with preserved p53 function. Breast Cancer Res
Treat. 163:177–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakanishi A, Kitagishi Y, Ogura Y and
Matsuda S: The tumor suppressor PTEN interacts with p53 in
hereditary cancer (Review). Int J Oncol. 44:1813–1819.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Blanco-Aparicio C, Renner O, Leal JF and
Carnero A: PTEN, more than the AKT pathway. Carcinogenesis.
28:1379–1386. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen Z, Trotman LC, Shaffer D, Lin HK,
Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al:
Crucial role of p53-dependent cellular senescence in suppression of
Pten-deficient tumorigenesis. Nature. 436:725–730. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alimonti A, Nardella C, Chen Z, Clohessy
JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G,
et al: A novel type of cellular senescence that can be enhanced in
mouse models and human tumor xenografts to suppress prostate
tumorigenesis. J Clin Invest. 120:681–693. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Samarakoon R, Helo S, Dobberfuhl AD,
Khakoo NS, Falke L, Overstreet JM, Goldschmeding R and Higgins PJ:
Loss of tumour suppressor PTEN expression in renal injury initiates
SMAD3- and p53-dependent fibrotic responses. J Pathol. 236:421–432.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gupta A and Dey CS: PTEN and SHIP2
regulates PI3K/Akt pathway through focal adhesion kinase. Mol Cell
Endocrinol. 309:55–62. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mise-Omata S, Obata Y, Iwase S, Mise N and
Doi TS: Transient strong reduction of PTEN expression by specific
RNAi induces loss of adhesion of the cells. Biochem Biophys Res
Commun. 328:1034–1042. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Scacheri PC, Rozenblatt-Rosen O, Caplen
NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS,
Bhattacharjee A, Meyerson M and Collins FS: Short interfering RNAs
can induce unexpected and divergent changes in the levels of
untargeted proteins in mammalian cells. Proc Natl Acad Sci USA.
101:1892–1897. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma
WY, Dong Z, Pike HM, Brown RE and Reed JC: Calcium-activated
RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and
is abrogated by alpha B-crystallin through inhibition of RAS
activation. Mol Biol Cell. 16:4437–4453. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan BJ and Chiu GN: Role of oxidative
stress, endoplasmic reticulum stress and ERK activation in
triptolide-induced apoptosis. Int J Oncol. 42:1605–1612.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
D'Amours D, Sallmann FR, Dixit VM and
Poirier GG: Gain-of-function of poly(ADP-ribose) polymerase-1 upon
cleavage by apoptotic proteases: Implications for apoptosis. J Cell
Sci. 114:3771–3778. 2001.PubMed/NCBI
|
|
22
|
Stein GH, Drullinger LF, Soulard A and
Dulić V: Differential roles for cyclin-dependent kinase inhibitors
p21 and p16 in the mechanisms of senescence and differentiation in
human fibroblasts. Mol Cell Biol. 19:2109–2117. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Althubiti M, Lezina L, Carrera S,
Jukes-Jones R, Giblett SM, Antonov A, Barlev N, Saldanha GS,
Pritchard CA, Cain K and Macip S: Characterization of novel markers
of senescence and their prognostic potential in cancer. Cell Death
Dis. 5(e1528)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim JS, Lee C, Bonifant CL, Ressom H and
Waldman T: Activation of p53-dependent growth suppression in human
cells by mutations in PTEN or PIK3CA. Mol Cell Biol. 27:662–677.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou BB and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Ming M and He YY: PTEN in DNA damage
repair. Cancer Lett. 319:125–129. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Su TH, Chang JG, Perng LI, Chang CP, Wei
HJ, Wang NM and Tsai CH: Mutation analysis of the putative tumor
suppressor gene PTEN/MMAC1 in cervical cancer. Gynecol Oncol.
76:193–199. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tornesello ML, Buonaguro L and Buonaguro
FM: Mutations of the TP53 gene in adenocarcinoma and squamous cell
carcinoma of the cervix: A systematic review. Gynecol Oncol.
128:442–448. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qi Q, Ling Y, Zhu M, Zhou L, Wan M, Bao Y
and Liu Y: Promoter region methylation and loss of protein
expression of PTEN and significance in cervical cancer. Biomed Rep.
2:653–658. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yaginuma Y and Westphal H: Analysis of the
p53 gene in human uterine carcinoma cell lines. Cancer Res.
51:6506–6509. 1991.PubMed/NCBI
|
|
31
|
Meric-Bernstam F, Akcakanat A, Chen H, Do
KA, Sangai T, Adkins F, Gonzalez-Angulo AM, Rashid A, Crosby K,
Dong M, et al: PIK3CA/PTEN mutations and Akt activation as markers
of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res.
18:1777–1789. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu X, Wagner KU, Larson D, Weaver Z, Li C,
Ried T, Hennighausen L, Wynshaw-Boris A and Deng CX: Conditional
mutation of Brca1 in mammary epithelial cells results in blunted
ductal morphogenesis and tumour formation. Nat Genet. 22:37–43.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Jonkers J, Meuwissen R, van der Gulden H,
Peterse H, van der Valk M and Berns A: Synergistic tumor suppressor
activity of BRCA2 and p53 in a conditional mouse model for breast
cancer. Nat Genet. 29:418–425. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Augello G, Puleio R, Emma MR, Cusimano A,
Loria GR, McCubrey JA, Montalto G and Cervello M: A PTEN inhibitor
displays preclinical activity against hepatocarcinoma cells. Cell
Cycle. 15:573–583. 2016.PubMed/NCBI View Article : Google Scholar
|