Introduction
Postmenopausal women can experience symptoms that
negatively affect their quality of life. These symptoms may develop
several years prior to menopause (1) and comprise mainly vasomotor symptoms
(VMS), such as hot flushes and night sweats, palpitations,
headache, shoulder stiffness, insomnia, depression, nervousness,
anxiety, fatigue, irritability and dizziness (2). Balance disturbances may be one of the
most common morbidities experienced by postmenopausal women and are
defined as Meniere's disease (MD) (3). These are characterized by vertigo,
fluctuating hearing loss and tinnitus, and may be attributable to
the inability of the cochlea to regulate endolymph absorption,
production and/or circulation (4,5).
The prevalence of MD is 34-190 per 100,000
individuals. The age of onset ranges from the third to the seventh
decades of life, with a female predominance (6). It has been recognized that the
specific reduction in estrogen levels in perimenopausal women may
cause microcirculatory disruption in the inner ear, promoting the
development of MD or contributing to the deterioration of the
symptoms (7). The peak range of the
development of MD is estimated at the age of 40-60 years in women
(8).
In conclusion, postmenopausal women with MD exhibit
lower estrogen levels, increased pure-tone threshold, elevated I-V
interpeak latency and higher incidence of unilateral caloric
weakness. Changes in estrogen levels may contribute to the
deteriorating symptoms of MD (low-frequency hearing loss, episodic
symptoms of vertigo, aural fullness and tinnitus) in postmenopausal
women to a certain extent.
Hormone therapy (HT) is considered as the most
effective therapy for VMS and is currently the first choice of
treatment (9). A continuous HT
combining 2 mg drospirenone and 1 mg 17β-estradiol (E2), denoted as
DRSP/E2, may be used to treat VMS in postmenopausal women. Unlike
other synthetic progestins, DRSP exhibits a similar physiological
profile to that of natural progesterone. DRSP is derived from
spirolactone and inhibits the effects of aldosterone and androgen
(10). Moreover, its
antimineralocorticoid activity reduces salt and water retention and
exerts diuretic and anti-edema effects (11,12).
Notably, DRSP/E2 provides symptomatic relief in women with VMS and
improves genitourinary atrophy, while it protects against
endometrial hyperplasia and reduces the risk of osteoporosis
(13).
Taking into consideration the existing evidence
regarding the pathophysiology of vertigo, the present study aimed
to investigate the effects of DRSP/E2 HT combined with
rehabilitation therapy (study group) and compare them with those of
rehabilitation therapy alone (control group) for postmenopausal
women with MD.
Materials and methods
Patients
The current prospective study was performed at the
Gynecology Clinic of the Department of General Surgery and Medical
Surgical Specialties and at the Otorhinolaryngology Clinic of the
Department of Medical Surgical Sciences and Advanced Technologies,
University of Catania, Catania, Italy. The study conformed to the
ethical guidelines of the 2013 Helsinki Declaration. Written
informed consent was obtained from each participant upon
enrollment. The present study was not advertised and no
remuneration was offered.
A total of 74 postmenopausal women (age range 49-56
years) with MD and severe distress were recruited at the
Otorhinolaryngology Clinic and invited to participate in the study.
The time period of subject recruitment was from to April 2017 to
October 2019. All women had their last spontaneous menstrual
bleeding >6 months earlier. The plasma follicle-stimulating
hormone levels were >40 mIU/ml (pre-menopausal normal range,
3,4-20 mUI/ml) and the serum E2 levels were <30 pg/ml
(pre-menopausal normal range, 40-400 pg/ml). Each participant
underwent general medical and gynecological examination.
Women with a history of thromboembolic and liver
disease who had used steroid treatment in the previous 3 months or
had received phytoestrogens within 1 month prior to the initiation
of the study, were excluded. Furthermore, women with a body mass
index of 35 kg/m2 or higher, with endometrial thickness
≥4 mm measured by transvaginal ultrasound prior to the study
initiation, and/or presenting with abnormal uterine bleeding,
hormone-dependent malignancies, receiving antihypertensive drugs,
or suffering from diabetes or other chronic medical conditions,
were also excluded from the study. The otolaryngological exclusion
criteria included previous vestibular diseases, low compliance due
to physical disabilities, or treatment with vestibular-suppressor
drugs.
Instruments
The modified Kupperman index (KI) was used to
evaluate menopausal symptoms by 13 items (14), including somatic (hot flushes/night
sweats, paresthesia, dizziness, arthralgia/myalgia, headache,
palpitations and formication), psychological (insomnia/sleep
disturbance, depression, irritability and fatigue) and urogenital
(urinary infection and sexual complaints) symptoms. The scoring
system used was as follows: 0=none, 1=mild, 2=moderate and
3=severe. The following score range system was used to rate the
degree of severity as follows: 0-6 (none), 7-15 (mild), 16-30
(moderate) and >30 (severe). The range was from 0 to 63
points.
The participants underwent Semont's maneuver
(15) to assess the characteristics
of positional vertigo (16). During
this assessment, the women were sat upright in the middle of a
stretcher. The head was rotated by the therapist towards the
healthy ear; this rotation was maintained throughout the maneuver.
Subsequently, the therapist lowered the body of the patient
sideways onto the stretcher so that she was allowed to lie on the
side of the affected ear, with her nose pointing upwards. This
position was maintained for 3 min and the therapist instructed the
woman to adopt an upright position without straightening her head,
which was subsequently lowered to her opposite side with the nose
pointing downwards. Finally, after maintaining this position for 3
min, the woman was slowly brought back to an upright position and
her head was rotated back to the normal position.
Stabilometry was used to evaluate and measure the
balance of each patient through a platform connected to a computer
by three transducers. The platform was sensitive to the pressure
applied on it due to the movements of each patient (17). The computer recorded the changes of
the position at the center of pressure (CoP). The movements on the
CoPs of the platform were presented on the monitor as an ellipsoid
graphic. The recorded movements indicated that the patients may
have moved on the medial-lateral (x-axis, right-left), or
anterior-posterior (y-axis, back-forth) direction (18). Each subject performed the test
sequentially with her eyes open, closed and the retroflex, and then
open again. The displacements on the platform and the surface of
the ellipse were calculated (mm2).
Finally, the Dizziness Handicap Inventory (DHI)
questionnaire was used for the self-assessment of severe discomfort
due to vertigo (19). This
questionnaire is based on 25 items investigating physical,
functional and emotional aspects of dizziness and unsteadiness. The
scores ranged between 0, indicating no discomfort, and 100,
indicating significant self-perceived discomfort. Each instrument
was used at baseline (T0) and at the 3-month (T1) and 6-month (T2)
follow-up visits.
Statistical analysis
The demographic non categorical variables,
stabilometric and DHI intergroup data were compared by one-way
analysis of variance with Bonferroni as a post hoc test. The
χ2 was used to compare the categorical demographic data.
Intragroup analysis was performed to compare baseline stabilometry
and DHI values at follow-up by paired Student's t-test. All values
are shown as mean ± SD. P<0.05 was considered to indicate
statistically significant differences. Statistical analysis was
carried out using software package for Windows 95 (Grantz SA;
Primer of Biostatistics).
Results
Clinicopathological parameters
A total of 9 women refused to participate in the
study; consequently, 65 women were finally included in the
analysis, of whom 31 (47.7%) consented to receiving HT and
rehabilitation. These patients comprised the study group (group A).
A total of 34 women (52.3%) refused to receive HT and comprised the
control group (group B), which selected rehabilitation alone. A
total of 6 (17.6%) of the 34 study subjects did not come to the
appointment T1, whereas 28 (82.4%) completed the study.
Table I indicates
the baseline demographic characteristics of the two groups. The
vertiginous crises suffered by each woman lasted for 20 sec, and
had paroxysmal onset and rotatory character, mainly during the
morning and night. At baseline, the women were affected by
menopausal symptoms, including severe dizziness, associated with
mild hot flushes, night sweats, sleep disturbances and fatigue
[group A (32±7.3) vs. group B (30±6.1; P=0.2)].
 | Table IDemographic characteristics at
baseline of women on 2 mg drospirenone and 1 mg 17β-estradiol
hormone therapy and rehabilitation (Study group) or on
rehabilitation (Control group) for the Maniere's disease. |
Table I
Demographic characteristics at
baseline of women on 2 mg drospirenone and 1 mg 17β-estradiol
hormone therapy and rehabilitation (Study group) or on
rehabilitation (Control group) for the Maniere's disease.
| Variable | Study group
(n=31) | Control group
(n=34) | P-value |
|---|
| Age range, years | 49-56 | 50-56 | 0.9 |
| Mean age, years (mean
± SD) | 52.6±3.1 | 53.1±3.3 | 0.5 |
| BMI, kg/m2
(mean ± SD) | 26.3±4.1 | 25.5±5.1 | 0.4 |
| Age at menopause,
years (mean ± SD) | 50.5±6.1 | 51.3±2.8 | 0.4 |
| Years from menopause
(mean ± SD) | 5.0±3.4 | 6.0±4.1 | 0.2 |
| Parity, n (%) | | | |
|
One | 5 (16.1) | 6 (17.6) | 0.8 |
|
Two or more
children | 26 (83.9) | 28 (82.4) | 0.8 |
| Kupperman index (mean
± SD) | 32.0±7.3 | 30.0±6.1 | 0.2 |
| Cigarette smoking,
n (%) | | | |
|
Non-smoker | 28 (90.3) | 29 (85.3) | 0.9 |
|
Current
smoker | 3 (9.7) | 5 (14.7) | 0.8 |
| Systolic blood
pressure, mmHg (mean ± SD) | 132.5±11.6 | 134.2±15.4 | 0.6 |
| Diastolic blood
pressure, mmHg (mean ± SD) | 83.8±8.3 | 82.7±7.5 | 0.5 |
| Heart rate, bpm
(mean ± SD) | 65.8±10.3 | 67.4±12.2 | 0.5 |
Following Semont's maneuver, the subjects in group A
exhibited an improvement at T1 (26/31, 83.8%; P<0.001) and at T2
(31/31, 100%; P<0.001) compared with T0. In group B, 7/28 (25%)
patients exhibited an improvement at T1 (P<0.03) with regard to
Semont's maneuver alone, whereas 10 (35.7%) women exhibited an
improvement at T2 (P<0.01) compared with T0. A total of 11
(39.3%) subjects reported no benefit. The women of group A
exhibited an improvement compared with those of group B at both
follow-up visits when intergroup comparison was performed
(P<0.001).
Stabilometry and DHI measurements
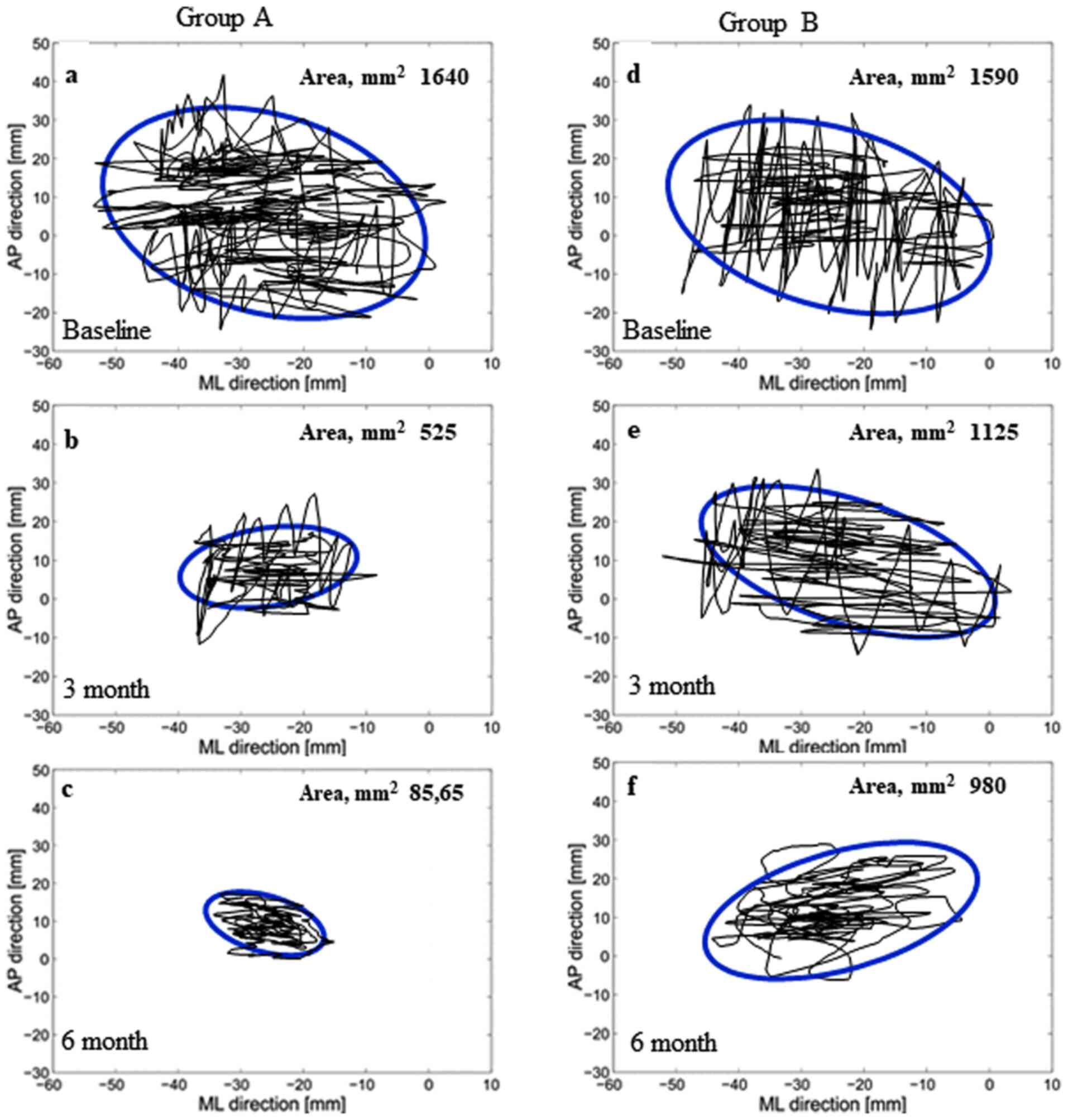
The changes in the stabilometric area of the
ellipses of both groups were assessed (Fig. 1). The intragroup stabilometric
evaluation indicated improved normalization in group A (T1 and T2
vs. T0, P<0.001) compared with group B (T1 vs. T0, P<0.01; T2
vs. T0, P<0.002). The intergroup statistical comparison
indicated an improvement in group A compared with group B at each
follow-up assessment (P<0.001; Table II).
 | Table IIStabilometry of female patients
treated with 2 mg drospirenone and 1 mg 17β-estradiol hormone
therapy combined with rehabilitation (group A), or rehabilitation
alone (group B), for Meniere's disease. |
Table II
Stabilometry of female patients
treated with 2 mg drospirenone and 1 mg 17β-estradiol hormone
therapy combined with rehabilitation (group A), or rehabilitation
alone (group B), for Meniere's disease.
| Stabilometric
values | Baseline (T0) | 3 months (T1) | 6 months (T2) |
P-valuea
(T1 vs. T0) |
P-valuea
(T2 vs. T0) |
|---|
| Group A | n=31;
1,640±655 | n=31; 525±125 | n=31; 85.65±25 | 0.001 | 0.001 |
| Group B | n=34;
1,590±590 | n=28;
1,125±880 | n=28; 980±660 | 0.016 | 0.002 |
|
P-valueb | 0.747 | 0.001 | 0.001 | | |
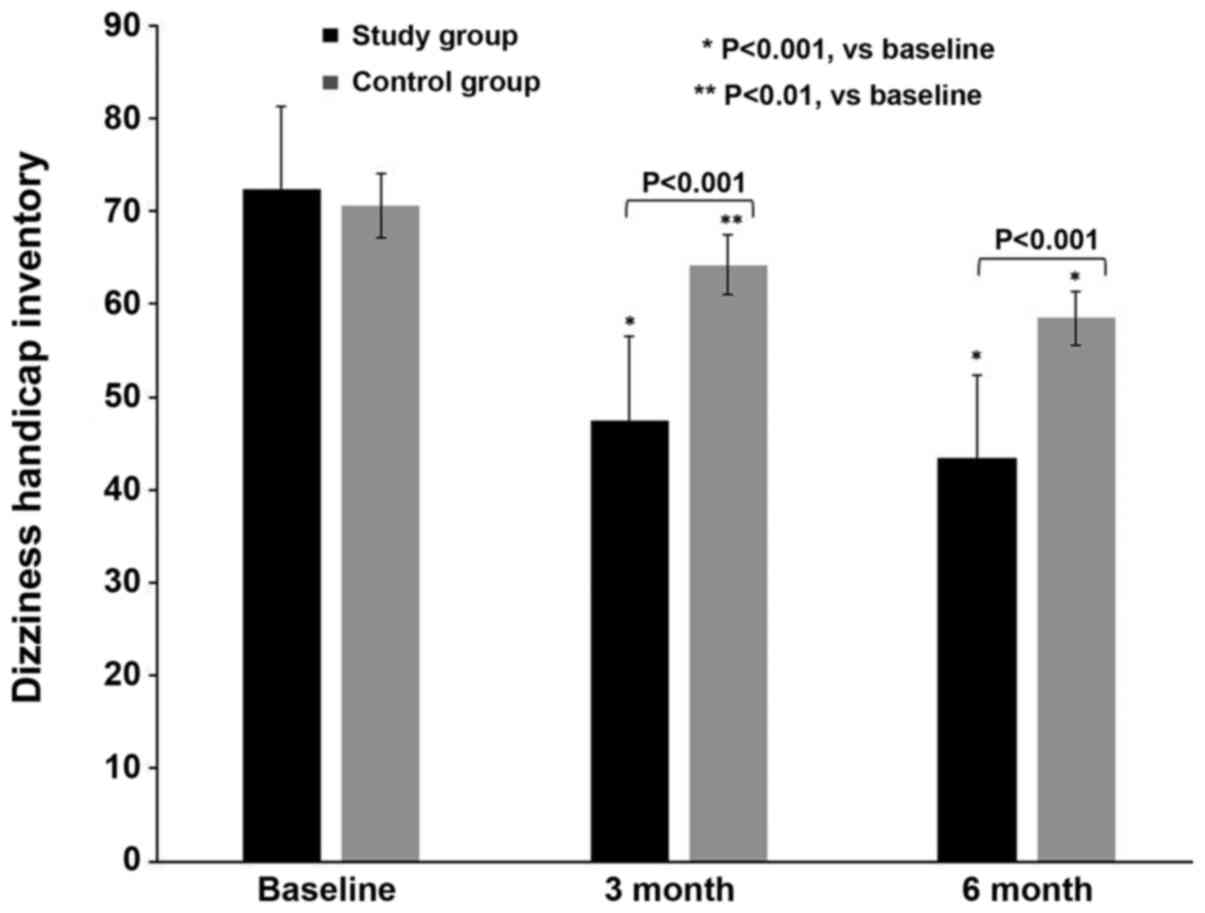
The DHI scores of both groups were assessed during
the study (Fig. 2). Table III summarizes the DHI intra- and
intergroup statistical evaluation. At T0, both groups exhibited
severe self-perceived discomfort, with similar DHI scores of
72.3±3.7 (group A) and 70.6±3.9 (group B; P=0.07). At T1, both
groups exhibited a gradual improvement, which was more prominent in
group A (47.5±3.7) compared with group B (64.2±3.3; P<0.001). At
T2, the DHI scores had improved in group A (43.4±3.4) compared with
those in group B (58.5±3.1; P<0.001).
 | Table IIIDHI of female patients with Meniere's
disease. |
Table III
DHI of female patients with Meniere's
disease.
| DHI | Baseline (T0) | 3 months (T1) | 6 months (T2) |
P-valuea
(T1 vs. T0) |
P-valuea
(T2 vs. T0) |
P-valuea
(T2 vs. T1) |
|---|
| Group A | 72.3±3.7 | 47.5±3.7 | 43.4±3.4 | 0.001 | 0.001 | 0.001 |
| Group B | 70.6±3.9 | 64.2±3.3 | 58.5±3.1 | 0.010 | 0.001 | 0.004 |
|
P-valueb | 0.077 | 0.001 | 0.001 | | | |
Discussion
To the best of our knowledge, the present study was
the first to evaluate the therapeutic effects of DRSP/E2 HT on
postmenopausal women affected by severe MD associated with mild
VMS. Initially, women on DRSP/E2 exhibited an improvement of VMS.
Moreover, they exhibited a more notable improvement of the
vertiginous crisis and of DHI compared with women on rehabilitation
alone. The improvement of DHI observed in group B was less
pronounced compared with that in group A, which may be attributed
to the effects of Semont's maneuver. Of note, stabilometric
evaluation indicated an optimal normalization with a more evident
reduction of the areas of the ellipses in women on DRSP/E2 than in
those who refused to receive HT. These data indicated that the
women acquired more balance skills following treatment
initiation.
The treatment usually recommended for women with MD
exacerbations includes restriction of dietary salt intake and the
use of diuretics (20). In the
present study, an HT containing DRSP was selected to regulate the
anti-aldosterone and antimineralocorticoid activities of
progestogen, which, in turn, decreased salt and water retention
(21).
The effects of sex on medical care and behavioral
therapies have not been examined following treatment of women with
MD (22). MD is most prevalent
between the ages of 40 and 60 years, with a peak onset at 40-50
years (23,24). It also exhibits a slightly higher
prevalence in women than in men (23,24). A
correlation has been observed between VMS and vertigo exacerbation
(3). However, that study did not
record differences in vertigo prevalence between users and
non-users of HRT (3). This could
depend on the composition and regimen of the HT used to treat
symptomatic postmenopausal women. DRSP derived from
17a-spironolactone is a progestin with an important
antimineralocorticoid activity, which is able to reduce salt and
water retention and to exert diuretic and anti-edema effects
(11).
Moreover, it has been shown that the low estrogen
levels noted in postmenopausal women may cause microcirculatory
disorders in the inner ear, leading to the development of MD
(7). Consequently, the present
study indicated that the benefits obtained by postmenopausal women
with MD using DRSP/E2 treatment may be due to the type, dosage,
combination and regimen of progestogen and E2. Furthermore, the use
of an oral hormonal contraceptive containing DRPS and
ethinylestradiol was effective in improving the exacerbation of MD
in fertile women affected by premenstrual syndrome (25).
Estrogen receptors are expressed in various tissues
and their activation is implicated in several biological functions.
In addition, progesterone receptors are expressed at variable
levels, and their effects display different intensities or,
occasionally, are unknown, in different tissues (26). Although estradiol increases neuronal
excitability, progesterone metabolites enhance the inhibitory
effects of gamma-amino butyric acid (GABA) (27). In women, electroencephalography and
auditory processes may change during the menstrual cycle and
postmenopausally (28,29). Of note, estrogen fluctuations during
the menstrual cycle may affect neuronal plasticity and the
metabolic levels of neurotransmitters and, consequently, the
neuronal conduction time into the audiological system. This was
observed by studying the auditory brainstem response via
auditory-evoked potentials (30).
It has been shown that psychological stress and
plasma levels of antidiuretic hormones may exacerbate MD symptoms
(31). The cochlea and central
auditory system have receptors for both α- and β-estrogen sex
hormones, and the female cyclical hormonal changes may influence
the inner ear anatomy and function. Moreover, complex genomic and
non-genomic mechanisms may affect MD symptoms by regulating the
auditory system pathways, GABA, serotonin, dopamine and glutamate
levels, as well as the levels of other sex hormones, including
testosterone (32).
In addition, olfactory sensitivity in postmenopausal
women was found to be enhanced during treatment with DRSP/E2 HT:
The antimineralocorticoid activity of DRSP may decrease nasal
edema, promoting efficient interaction between odorous substances
and receptors (33), further
emphasizing the role of sex steroids on non-genital targets.
Moreover, the protective activities against osteoporosis and
cardiovascular diseases in postmenopausal women are widely known
(34). Finally, the risk factors of
vertigo include vitamin D deficiency, decreased ionized Ca levels
and osteoporosis (35,36). Moreover, low estrogen levels
predispose to bone loss following menopause and HT is considered
the primary preventative method for the development of osteoporosis
(37). The risk of fractures can be
reduced in postmenopausal women affected by MD. HT, such as
DRSP/E2, can reduce both MD exacerbation and the risk of
osteoporosis, thereby reducing the risk of fractures. In
conclusion, DRSP, which is the main component of HT, can reduce
fluid overload, which serves as the main trigger for MD, and may
prove effective for improving the symptoms of this disease.
Despite its significant findings, the present study
comes with certain limitations. The therapeutic regimen included
DRSP/E2 and was not compared with HTs containing different
progestins other than DRSP. Moreover, the present study did not
compare women with naturally occurring or surgically induced
menopause using estrogen replacement therapy alone, in order to
verify whether estrogen was sufficient to improve vertigo, as
previously reported (38).
Therefore, future studies should aim to address these issues.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CMG, LM and SC contributed to protocol and project
development, follow-up/examination/treatment of the patients, data
collection or management, data analysis and manuscript
writing/editing. AMCR, GP and GC contributed to conception and
design, follow-up/examination/treatment of the patients, data
collection or management and manuscript writing/editing. SC and LM
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The authors declare that the study protocol
conformed to the ethical guidelines of the 2013 Helsinki
Declaration. The approval of the institutional review board of the
department research committees was obtained (Ethics
Committee-Catania 1, Azienda Ospedaliero-Universitaria Policlinico
‘G. Rodolico-San Marco’ Catania, Italy; reference number:
0033012/TMP/10-2015). Written informed consent was obtained
directly from all women.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hale GE, Zhao X, Hughes CL, Burger HG,
Robertson DM and Fraser IS: Endocrine features of menstrual cycles
in middle and late reproductive age and the menopausal transition
classified according to the staging of reproductive Aging workshop
(STRAW) staging system. J Clin Endocrinol Metab. 92:3060–3067.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
National Institutes of Health. National
Institute of Health State-of-the-Science Conference statement:
Management of menopause-related symptoms. Ann Intern Med.
142:1003–1013. 2005.PubMed/NCBI
|
|
3
|
Ekblad S, Bergendabl A, Enler P, Ledin T,
Möllen C and Hammar M: Disturbances in postural balance are common
in postmenopausal women with vasomotor symptoms. Climacteric.
3:192–198. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Muncie HL, Sirmans SM and James E:
Dizziness: Approach to evaluation and management. Am Fam Physician.
95:154–162. 2017.PubMed/NCBI
|
|
5
|
Bruderer SG, Bodmer D, Stohler NA, Jick SS
and Meier CR: Population-based study on the epidemiology of
Ménière's disease. Audiol Neurootol. 22:74–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lopez-Escamez JA, Carey J, Chung WH,
Goebel JA, Magnusson M, Mandalà M, Newman-Toker DE, Strupp M,
Suzuki M, Trabalzini F, et al: Diagnostic criteria for Menière's
disease. J Vestib Res. 25:1–7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jian H, Yu G, Chen G, Lin N and Wang H:
Correlation between auditory-vestibular functions and estrogen
levels in postmenopausal patients with Meniere's disease. J Clin
Lab Anal. 33(e22626)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shojaku H, Watanabe Y, Fujisaka M, Tsubota
M, Kobayashi K, Yasumura S and Mizukoshi K: Epidemiologic
characteristics of definite Meniere's disease in Japan. A long-term
survey of Toyama and Niigata prefectures. ORL J Otorhinolaryngol
Relat Spec. 67:305–309. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Baber RJ, Panay N and Fenton A: IMS
Writing Group. 2016 IMS recommendations on women's midlife health
and menopause hormone therapy. Climacteric. 19:109–150.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Foidart JM: Added benefits of drospirenone
for compliance. Climacteric 8 Suppl. 3:S28–S34. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sitruk-Ware R: Pharmacology of different
progestogens: The special case of drospirenone. Climacteric. 8
(Suppl 3):S4–S12. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Palacios S, Foidart JM and Genazzani AR:
Advances in hormone replacement therapy with drospirenone, a unique
progestogen with aldosterone receptor antagonism. Maturitas.
55:297–307. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Archer DF: Drospirenone and estradiol: A
new option for the postmenopausal woman. Climacteric. 10 (Suppl
1):S3–S10. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schneider HP, Heinemann LA, Rosemeier HP,
Potthoff P and Behre HM: The menopause rating scale (MRS):
Comparison with Kupperman index and quality-of-life scale SF-36.
Climacteric. 3:50–58. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Semont A, Freyss G and Vitte E: Curing the
BPPV with a liberatory maneuver. Adv Otorhinolaryngol. 42:290–293.
1988.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang X, Qian X, Lu L, Chen J, Liu J, Lin
C and Gao X: Effects of Semont maneuver on benign paroxysmal
positional vertigo: A meta-analysis. Acta Otolaryngol. 137:63–70.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kapteyn TS, Bles W, Njiokiktjien CJ, Kodde
L, Massen CH and Mol JM: Standardization in platform stabilometry
being a part of posturography. Agressologie. 24:321–326.
1983.PubMed/NCBI
|
|
18
|
Prieto TE, Myklebust JB, Hoffmann RG,
Lovett EG and Myklebust BM: Measures of postural steadiness:
Differences between healthy young and elderly adults. IEEE Trans
Biomed Eng. 43:956–966. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jacobson GP and Newman CW: The development
of the Dizziness handicap inventory. Arch Otolaryngol Head Neck
Surg. 116:424–427. 1990.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Foster CA: Optimal management of Ménière's
disease. Ther Clin Risk Manag. 11:301–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Miyashita T, Inamoto R, Fukuda S,
Hoshikawa H, Hitomi H, Kiyomoto H, Nishiyama A and Mori N: Hormonal
changes following a low-salt diet in patients with Ménière's
disease. Auris Nasus Larynx. 44:52–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pullen RL Jr: Navigating the challenges of
Meniere disease. Nursing. 47:38–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Watanabe I: Ménière's disease in males and
females. Acta Otolaryngol. 91:511–514. 1981.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Basura GJ, Adams ME, Monfared A, Schwartz
SR, Antonelli PJ, Burkard R, Bush ML, Bykowski J, Colandrea M,
Derebery J, et al: Clinical practice guideline: Ménière's disease.
Otolaryngol Head Neck Surg. 162 (Suppl 2):S1–S55. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Caruso S, Mauro D, Maiolino L, Grillo C,
Rapisarda AMC and Cianci S: Effects of combined oral contraception
containing drospirenone on premenstrual exacerbation of Meniere's
disease: Preliminary study. Eur J Obstet Gynecol Reprod Biol.
224:102–107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Farage MA, Neill S and MacLean AB:
Physiological changes associated with the menstrual cycle: A
review. Obstet Gynecol Surv. 64:58–72. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Smith MJ, Keel JC, Greenberg BD, Adams LF,
Schmidt PJ, Rubinow DA and Wassermann EM: Menstrual cycle effects
on cortical excitability. Neurology. 53:2069–2072. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Al-Mana D, Ceranic B, Djahanbakhch O and
Luxon LM: Hormones and the auditory system: A review of physiology
and pathophysiology. Neuroscience. 153:881–900. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Caruso S, Cianci A, Grasso D, Agnello C,
Galvani F, Maiolino L and Serra A: Auditory brainstem response in
postmenopausal women treated with hormone replacement therapy: A
pilot study. Menopause. 7:178–183. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Caruso S, Maiolino L, Agnello C, Garozzo
A, Di Mari L and Serra A: Effects of patch or gel estrogen
therapies on auditory brainstem response in surgically
postmenopausal women: A prospective, randomized study. Fertil
Steril. 79:556–561. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aoki M, Asai M, Nishihori T, Mizuta K, Ito
Y and Ando K: The relevance of an elevation in the plasma
vasopressin levels to the pathogenesis of Meniere's attack. J
Neuroendocrinol. 19:901–906. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Canlon B and Frisina RD: Sex hormones and
hearing: A pioneering area of enquiry. Hear Res. 252:1–4.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Caruso S, Serra A, Grillo C, De Leo V,
Maiolino L, Agnello C and Cianci A: Prospective study evaluating
olfactometric and rhinomanometric outcomes in postmenopausal women
on 1 mg 17beta-estradiol and 2 mg drospirenone HT. Menopause.
15:967–972. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nelson HD, Humphrey LL, Nygren P, Teutsch
SM and Allan JD: Postmenopausal hormone replacement therapy.
Scientific review. JAMA. 288:872–881. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim SY, Han SH, Kim YH and Park MH:
Clinical features of recurrence and osteoporotic changes in benign
paroxysmal positional vertigo. Auris Nasus Larynx. 44:156–161.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yu S, Liu F, Cheng Z and Wang Q:
Association between osteoporosis and benign paroxysmal positional
vertigo: A systematic review. BMC Neurol. 14(110)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vignozzi L, Malavolta N, Villa P, Mangili
G, Migliaccio S and Lello S: Consensus statement on the use of HRT
in postmenopausal women in the management of osteoporosis by SIE,
SIOMMMS and SIGO. J Endocrinol Invest. 42:609–618. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu DH, Kuo CH, Wang CT, Chiu CC, Chen TJ,
Hwang DK and Kao CL: Age-related increases in benign paroxysmal
positional vertigo are reversed in women taking estrogen
replacement therapy: A population-based study in Taiwan. Front
Aging Neurosci. 9(404)2017.PubMed/NCBI View Article : Google Scholar
|