Introduction
Ischemia/reperfusion (I/R) brain injury is one of
the leading causes of mortality in cardiovascular and
cerebrovascular diseases, with high morbidity and mortality rates
worldwide (1). Cerebral ischemic
events impact brain physiology (2).
An acute ischemic stroke may occur when an artery supplying blood
to the brain becomes occluded, leading to excitotoxicity and cell
death, ultimately leading to brain tissue death and focal
neurological deficits (3).
Prothrombin is cleaved to form thrombin, which acts
as a serine protease and belongs to the chymotrypsin family
(4). Thrombin activates blood
coagulation factors and the anticoagulant protein C (5). Previous studies have demonstrated that
the expression and activity of thrombin increases in tissues and
cells following cerebral ischemic injury, and brain cell toxicity
is also induced by thrombin (6,7). Bushi
et al (7) reported increased
thrombin activity in the brain following acute ischemic stroke and
demonstrated a close association between thrombin levels and
progression of brain damage. Furthermore, thrombin expression is
commonly upregulated around the lesion site in intracerebral
hemorrhage (8).
Autophagy is a cellular degradation process in which
unnecessary or damaged cytoplasmic contents are removed to maintain
cellular homeostasis (9). Moderate
autophagy has been reported to promote cell survival rate, while
excessive autophagy may induce cell cytotoxicity, leading to cell
death (10); for example, I/R
injury enhanced autophagy (11).
Thrombin levels are higher in the ischemic stroke model compared
with the control group (12).
However, the regulatory association between thrombin and autophagy
remains unclear.
Sprouty-related EVH1 domain (SPRED) proteins bind to
Ras and Raf-1 and inhibit cytokines, growth factors and the
ERK-MAPK pathway (13). A recent
study reported that SPRED2 is an essential regulator of cardiac
autophagy, whereby its deficiency suppresses autophagy (14). However, the role of SPRED2 in
autophagy and the association between SPRED2 and thrombin is yet to
be investigated.
‘Proper’ autophagy is considered to protect against
I/R injury, while excessive autophagy contributes to the injury
(15,16). For astrocytes, activation of
autophagy protects, as well as causes injury (17-19).
I/R injury has been reported to activate autophagy in astrocytes,
causing astrocyte cell death (17).
However, a few studies have demonstrated that the autophagic flux
in astrocytes protects brain I/R injury (18,19).
Generally, thrombin induces autophagy in astrocytes (20,21).
Given that thrombin may contribute to brain I/R injury, the
association between thrombin-mediated autophagy in astrocytes and
hypoxia/reoxygenation (H/R) conditions requires investigation.
The present study aimed to investigate the roles and
molecular mechanisms of thrombin, SPRED2 and autophagy in H/R
induced astrocytes and to provide a novel target therapy against
cerebral I/R injury.
Materials and methods
Cell culture and H/R
Rat primary astrocytes were purchased from the
American Type Culture Collection and maintained in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Cytiva), 100 IU/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA), at 37˚C with 5%
CO2 in a humidified atmosphere.
Initially, cells were cultured in DMEM supplemented
with 0.5% FBS for 12 h and placed in a hypoxia chamber with 5%
CO2/95% N2 and incubated at 37˚C for 24 h.
Subsequently, astrocytes were cultured in DMEM supplemented with
10% FBS at 37˚C with 5% CO2 for 4 h for
reoxygenation.
Cell treatment and transfection
Dabigatran (1 nM; Selleck Chemicals) or thrombin (5
U/ml; Sigma-Aldrich; Merck KGaA) with/without dabigatran were used
to treat the induced cells, or the induced cells were treated with
thrombin (5 U/ml) with/without 3-MA (Sigma-Aldrich; Merck
KGaA).
The H/R induced cells were treated with thrombin (5
U/ml) and transfected with small interfering (si)-SPRED2 or
si-negative control (NC; 5 nM; all synthesized and purchased from
Shanghai GenePharma Co., Ltd.) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. The Lipofectamine®
2000-si-SPRED2 complex was formed by mixing for 20 min at room
temperature. Then, Lipofectamine® 2000-si-SPRED2 complex
was added to the cells and the cells were cultured in serum-free
Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C for 4 h,
followed by incubation in serum-containing medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) at 37˚C for 72 h. After 48 h, the
transfection efficiency was evaluated by reverse
transcription-quantitative (RT-qPCR). The sequences were as
follows: si-SPRED2, 5'-AUAGCAUUCCAAUACAACCAG-3' and si-NC,
5'-AAUUGACCCAACAAUCACAU-3'.
MTT assay
The MTT assay was performed to assess cell
viability. Briefly, astrocytes in different groups were seeded into
96-well plates at a density of 3x103 cells/well and were
incubated for 48 h at 37˚C, with 5% CO2. Subsequently,
cells were incubated with 10 µl MTT of 5 mg/ml (Roche Diagnostics)
for 4 h at 37˚C. Following the MTT incubation, the purple formazan
crystals were dissolved using 180 µl DMSO and viability was
subsequently analyzed at a wavelength of 450 nm, using a
spectrophotometer (Omega Bio-Tek).
RT-qPCR
RT-qPCR analysis was performed to detect the
expression levels of SPRED2 and thrombin. Briefly, total RNA was
extracted from cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed
into cDNA using the PrimeScript RT reagent kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). RT conditions were as follows:
30˚C for 10 min, 42˚C for 50 min and 95˚C for 5 min. PCR reactions
were performed in an ABI 7500 Fast RealTime PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). qPCR was subsequently
performed using the Fast Start Universal SYBR-Green Master (ROX,
Takara Biotechnology Co., Ltd.) under the following thermocycling
conditions: 95˚C for 30 sec, 95˚C for 5 sec and 60˚C for 35 sec for
40 cycles, then 72˚C for 10 min. The following primer sequences
were used for qPCR: SPRED2 forward, 5'-TGTGAGCACCGGAAGATTTATACC-3'
and reverse, 5'-CGCGGCGGCTTTGTGCTT-3'; thrombin forward,
5'-ATGGCTGCAATCCGAAAGAAG-3' and reverse,
5'-ACAGTAGGGACGTAGACCTCC-3'; and GAPDH forward,
5'-ACCACAGTCCATGCCATCAC-3' and reverse, 5'-TCCACCACCCTGTTGCTGTA-3'.
Relative expression levels were calculated using the
2-ΔΔCq method and normalized to the internal reference
gene GAPDH (22).
Western blotting
Western blot analysis was performed to detect the
protein expression levels of SPRED2, microtubule-associated protein
light chain 3 (LC3)-II, LC3-I and Beclin 1. Briefly, treated cell
lines were harvested and washed with PBS. Total protein was
extracted from cells using RIPA buffer (Vazyme Biotech Co., Ltd.).
Protein was then quantified using the BCA™ Protein Assay kit (Merck
KGaA). Samples (30 µg) were then separated by 10% SDS-PAGE (Thermo
Fisher Scientific, Inc.). The separated proteins were subsequently
transferred onto PVDF membranes (Amersham; Cytiva) and blocked with
5% non-fat milk at room temperature for 2 h. The membranes were
incubated with primary antibodies against thrombin (cat. no.
ab92621; 1:1,000), SPRED2 (cat. no. ab153700; 1:500), Beclin 1
(cat. no. ab210498; 1:1,000), LC3-II/I (cat. no. ab62721; 1:2,000)
and GAPDH (cat. no. ab181602; 1:10,000) overnight at 4˚C (all
purchased from Abcam). Following the primary incubation, membranes
were incubated with a horseradish peroxidase-conjugated secondary
antibody (cat. no. ab205718; 1:2,000; Abcam) at 37˚C for 45 min.
Protein bands were visualized by Pierce ECL Western Blotting
Substrate (Pierce; Thermo Fisher Scientific, Inc.) using the
Bio-Image Analysis System (Bio-Rad Laboratories, Inc.). GAPDH
served as the internal control.
ELISA
ELISA kits were used to measure the protein
expression levels of interleukin (IL)-1β, IL-6 and tumor necrosis
factor (TNF)-α in the cell supernatant. The following ELISA kits
were used: Mouse IL-1β ELISA kit (cat. no. ab197742), Mouse IL-6
ELISA kit (cat. no. ab100712) and Mouse TNF-α ELISA kit (cat. no.
ab208348), according to the manufacturer's instructions (all
purchased from Abcam).
Measurement of superoxide dismutase
(SOD), malondialdehyde (MDA) and reactive oxygen species (ROS)
generation
Following H/R treatment, SOD activity was detected
using the xanthine oxidase method and MDA content was quantified
via the thiobarbituric acid assay (TBA) using MDA (cat. no.
A003-1-2) and SOD (cat. no. A001-3-2) kits (Nanjing Jiancheng
Bioengineering Institute). ROS generation during reoxygenation was
detected using CM-H2DCFDA (cat. no. C6827; Invitrogen; Thermo
Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp.). All experiments were repeated in triplicate.
Data are presented as the mean ± standard deviation. Unpaired
Student's t-test was used to compare differences between two
groups, while one-way ANOVA followed by Tukey's post hoc test were
used to compare differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Thrombin aggravates inflammatory
factor secretion and oxidative stress in H/R induced
astrocytes
The effect of thrombin on astrocytes induced by H/R
treatment was assessed by detecting inflammatory factor secretion,
oxidative stress and cell viability. The results demonstrated that
SOD activity was inhibited following H/R treatment, whereas MDA
content and ROS levels increased (Fig.
1A). In addition, the cell supernatant levels of IL-1β, IL-6
and TNF-α increased following H/R treatment (Fig. 1B), whereas cell viability was
suppressed, according to the MTT assay (Fig. 1C). In addition, the effect of H/R on
astrocytes for SOD activity, MDA content, ROS level, the cell
supernatant levels of IL-1β, IL-6 and TNF-α, and cell viability was
promoted by thrombin, the effects of which were reversed following
treatment with dabigatran (Fig.
1A-C). Taken together, these results suggest that thrombin can
aggravate inflammatory factor secretion and oxidative stress in H/R
induced astrocytes, and inhibit cell viability.
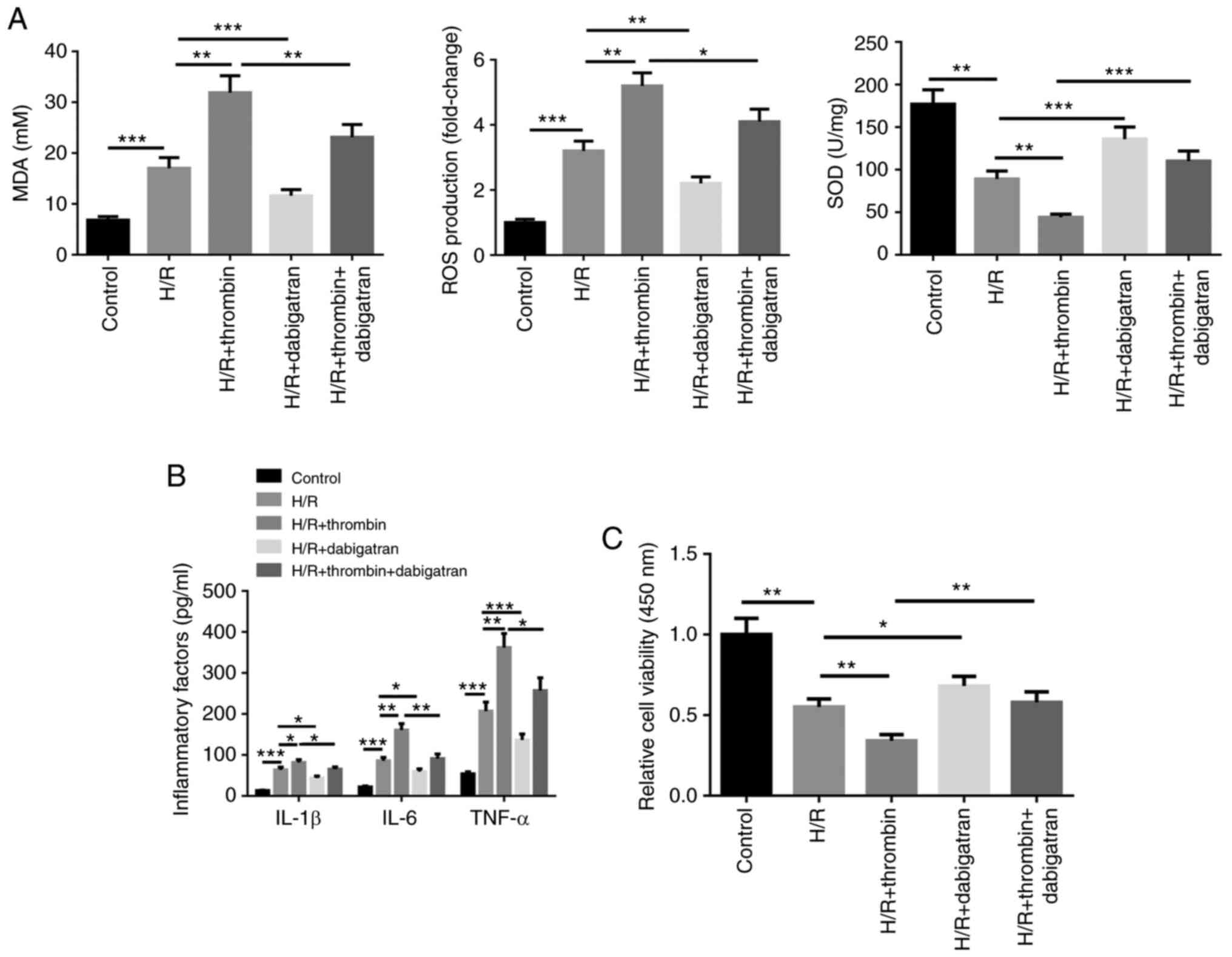 | Figure 1Thrombin aggravates inflammatory
factor secretion and oxidative stress in H/R induced astrocytes.
(A) The xanthine oxidase method was used to detect SOD activity,
the thiobarbituric acid assay was performed to quantify MDA content
and CM-H2DCFDA was used to detect ROS generation in H/R induced
cells. H/R induced cells were treated with thrombin, dabigatran,
and thrombin and dabigatran, respectively. (B) The cell supernatant
levels of IL-1β, IL-6 and TNF-α were detected via ELISA. (C) The
MTT assay was performed to assess cell viability.
*P<0.05; **P<0.01;
***P<0.001. H/R, hypoxia/reoxygenation; SOD,
superoxide dismutase; MDA, malondialdehyde; ROS, reactive oxygen
species; IL, interleukin; TNF, tumor necrosis factor. |
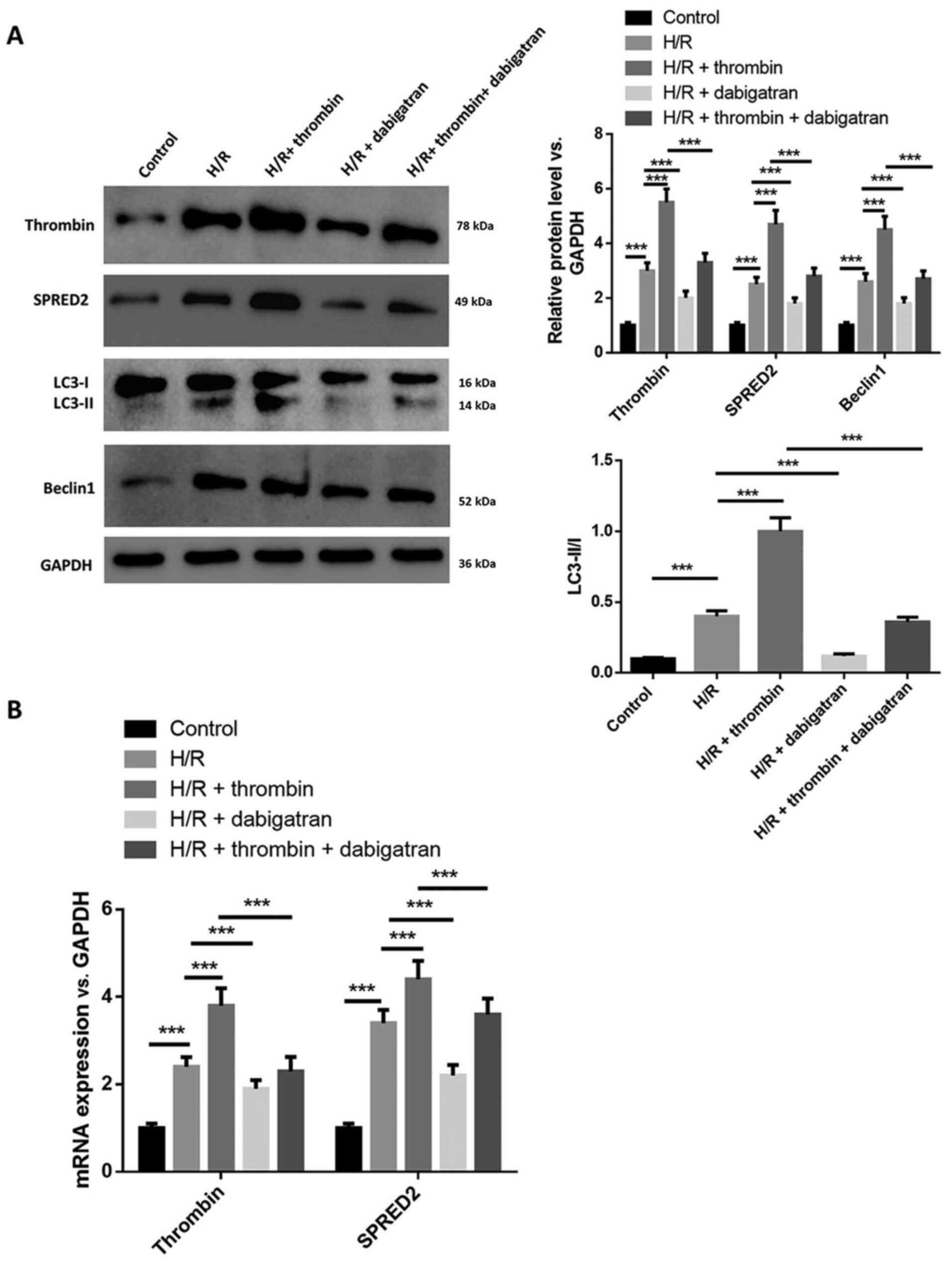
Thrombin aggravates autophagy in H/R
induced astrocytes
The effect of thrombin on autophagy in H/R induced
astrocytes was investigated. Western blot analysis demonstrated
that the protein expression levels of thrombin, SPRED2 and Beclin
1, and the ratio of LC3-II/I increased following H/R induction,
which was promoted by thrombin but attenuated by dabigatran
(Fig. 2A). In addition, the
regulation of thrombin on the expression levels of SPRED2, Beclin 1
and the ratio of LC3-II/I was reversed by dabigatran. RT-qPCR
analysis demonstrated that the mRNA expression levels of thrombin
and SPRED2 increased following treatment with thrombin and
decreased following treatment with dabigatran, and dabigatran
reversed the effects of thrombin on the levels of thrombin and
SPRED2 (Fig. 2B). Collectively,
these results suggest that thrombin aggravates autophagy in H/R
induced astrocytes.
SPRED2 knockdown suppresses the effect
of thrombin on inflammatory factor secretion and oxidative stress
in H/R induced astrocytes
The molecular mechanism of SPRED2 for thrombin on
inflammatory factor secretion, oxidative stress and cell viability
in H/R induced astrocytes was investigated. As presented in
Fig. 3A, SPRED2 knockdown
significantly decreased SPRED2 protein expression. MDA content and
ROS levels increased following H/R treatment, whereas SOD activity
was suppressed (Fig. 3B). In
addition, the levels of IL-1β, IL-6 and TNF-α in cell supernatant
increased following H/R treatment (Fig.
3C), whereas cell viability was inhibited (Fig. 3D). The effect of H/R induction on
astrocytes was aggravated following addition of thrombin. However,
the expression levels of the inflammatory factors (IL-1β, IL-6 and
TNF-α) and oxidative stress markers (MDA and ROS) in cells treated
with thrombin were inhibited following SPRED2 knockdown, while the
level of SOD was elevated (Fig. 3B
and C). Furthermore, the expression
levels of the inflammatory factors and oxidative stress markers in
cells treated with thrombin were suppressed by autophagy inhibitor
3-MA. Taken together, these results suggest that SPRED2 knockdown
suppresses the effect of thrombin on inflammatory factor secretion
and oxidative stress in H/R induced astrocytes.
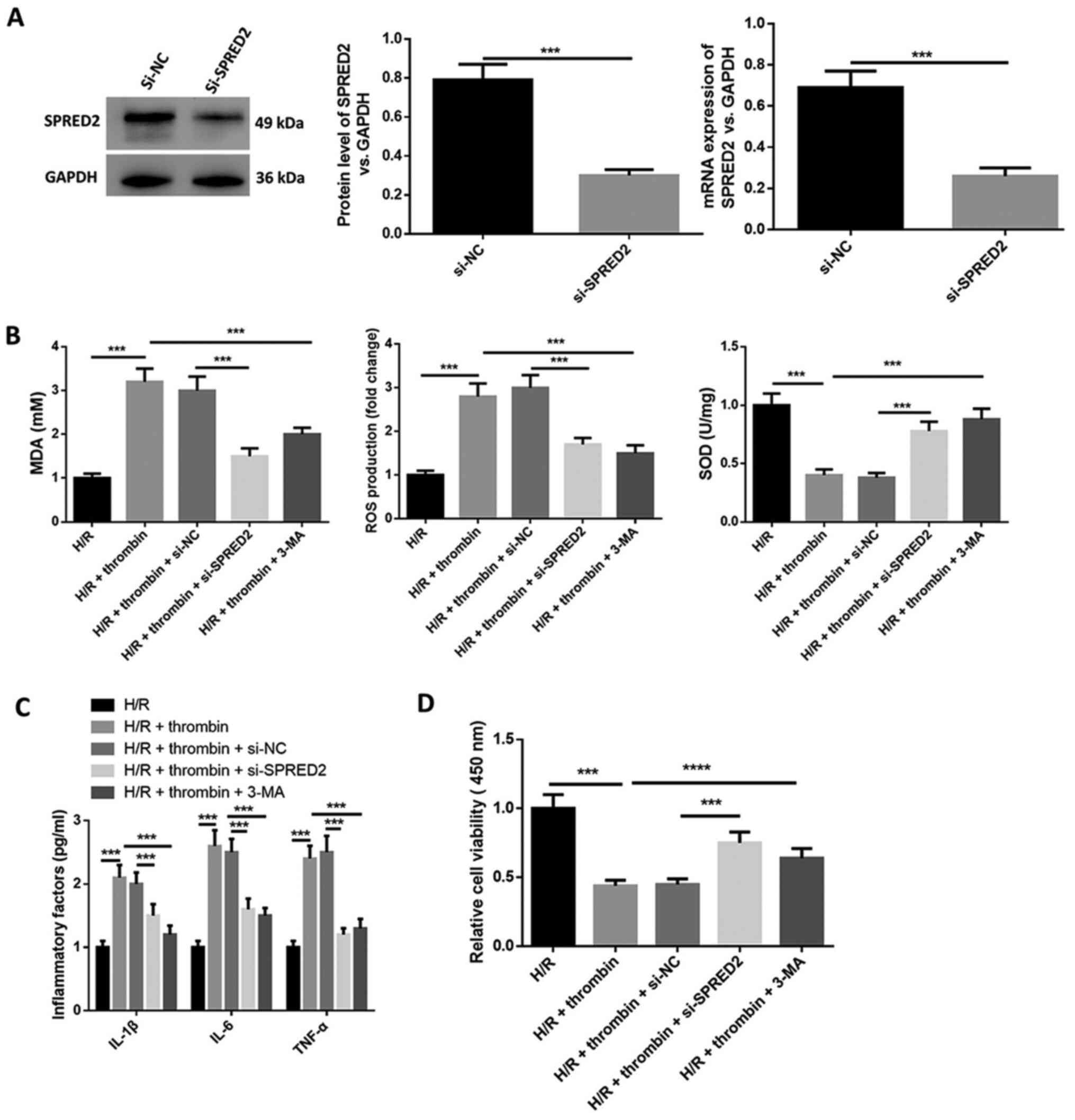 | Figure 3SPRED2 knockdown suppresses the effect
of thrombin on inflammatory factor secretion and oxidative stress
in H/R induced astrocytes. (A) Western blot and RT-qPCR analysis
were performed to determine transfection efficiency of si-SPRED2.
(B) The xanthine oxidase method was used to detect SOD activity,
the thiobarbituric acid assay was performed to quantify MDA content
and CM-H2DCFDA was used to detect ROS generation in H/R induced
cells. H/R induced cells were treated with thrombin, and thrombin
and 3-MA, and transfected with si-SPRED2 or si-NC. (C) The cell
supernatant levels of IL-1β, IL-6 and TNF-α were detected via
ELISA. (D) The MTT assay was performed to assess cell viability.
***P<0.001. SPRED2, Sprouty-related EVH1 domain-2;
H/R, hypoxia/reoxygenation; si, small interfering; SOD, superoxide
dismutase; MDA, malondialdehyde; ROS, reactive oxygen species; IL,
interleukin; TNF, tumor necrosis factor; NC, negative control. |
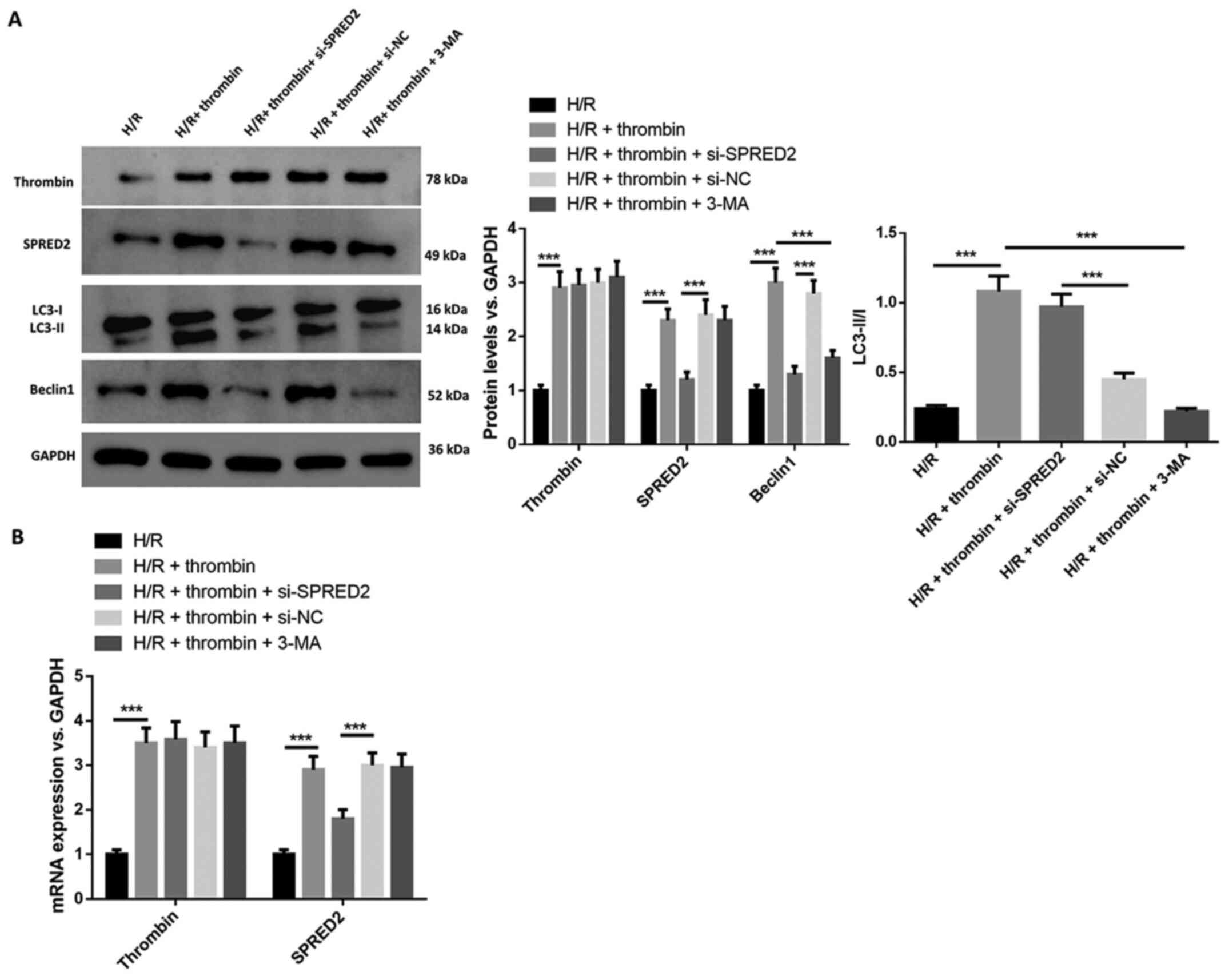
SPRED2 knockdown suppresses the
regulation of thrombin on autophagy in H/R induced astrocytes
The regulation of SPRED2 on autophagy in H/R induced
astrocytes was investigated. The results demonstrated that thrombin
increased SPRED2 expression, the ratio of LC3-II/I and Beclin 1
expression (Fig. 4A), the effects
of which were reversed following transfection with si-SPRED2. The
effect of thrombin on cells induced by H/R was suppressed following
treatment with 3-MA. RT-qPCR analysis demonstrated that SPRED2
expression decreased following transfection with si-SPRED2, and
3-MA reversed the effect of thrombin on cells with H/R induction
(Fig. 4B). Collectively, these
results suggest that thrombin regulation on autophagy in H/R
induced astrocytes can be inhibited by silencing SPRED2.
Discussion
Irreversible damages, such as oxidative stress,
death associated with inflammation, neuronal injury and
excitotoxicity, are the results of I/R brain injury (23). Oxidative stress plays an important
role in the development of the ischemic cascade. Thus, the
intervention of oxidative damage may prevent diseases induced by
oxidative stress (24).
Furthermore, inflammation plays an important role in the
pathophysiology of cerebral ischemia, as well as ischemic
cerebrovascular diseases (25). The
roles and molecular mechanisms of thrombin and SPRED2 in the I/R
induced rat astrocytes model were investigated in the present
stud.
Increasing evidence suggest that thrombin is a
mediator for cerebrovascular inflammation and hypoxia, with obvious
value in hypoxia, inflammation and oxidative stress (26,27). A
previous study reported that thrombin promotes macrophage
differentiation into an M1-like phenotype, closely associated with
the expression of classical pro-inflammatory markers (28). Another study demonstrated that
thrombin promotes inflammatory gene expression and enhances
sustained signaling in astrocytes (29). The results of the present study
demonstrated that thrombin aggravates inflammatory factor secretion
and oxidative stress induced by H/R in astrocytes, which is
consistent with the aforementioned reports (28,29).
Autophagy usually occurs in response to cellular stresses,
including oxidative stress, infection and nutrient starvation
(15,30). However, the molecular mechanism of
autophagy remains unknown. Hu et al (21) reported that thrombin increases the
ratio of LC3-II/LC3-I and the level of cathepsin D; thus, thrombin
activates autophagy and plays an important role in ICH-induced
autophagy. Another report demonstrated that the level of
perihematomal neuron autophagy was correlated with
thrombin-antithrombin plasma levels in patients with intracerebral
hemorrhage (31). The present study
demonstrated that thrombin aggravated autophagy in H/R induced
astrocytes.
SPRED2 belongs to a family of proteins containing a
cysteine-rich domain and is widely expressed in several tissues,
including the brain (32). SPRED2
regulates adipose tissue inflammation and metabolic abnormalities
induced by a high-fat diet in mice (33). Downregulated SPRED2 expression
attenuates epithelial cell injury and inflammation in dextran
sulfate sodium-induced acute colitis in mice (34). Itakura et al (35) reported that inflammatory responses
decrease by inhibiting SPRED2 from protecting mice from
polymicrobial sepsis by activating the ERK/MAPK pathway. The
results of the present study demonstrated that the promotion of
inflammatory factor secretion and oxidative stress in H/R induced
astrocytes by thrombin was suppressed following SPRED2 knockdown.
However, very few studies have investigated the role of SPRED2 in
autophagy (32-35).
In addition, autophagy may be a promising target for promoting
SPRED2-mediated antitumor activity (36). The results of the present study
demonstrated that SPRED2 knockdown suppressed the promotional
effect of thrombin on autophagy in H/R induced astrocytes.
The present study is not without limitations. First,
deeper insights on the regulatory association between SPRED2 and
autophagy in the I/R process were not investigated in the present
study. Secondly, it is unclear whether other signaling pathways are
involved in thrombin aggravated I/R injury. Thus, further studies
are required to confirm the results presented here.
In conclusion, the results of the present study
demonstrated that inflammatory factor secretion, oxidative stress
and autophagy in H/R induced astrocytes were aggravated by
thrombin, which was inhibited by SPRED2 knockdown. To the best of
our knowledge, the present study is the first to demonstrate H/R
injury of astrocytes aggravated by thrombin by activating the
autophagy pathway mediated by SPRED2. This research will help to
identify novel targets against I/R brain injury.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Achievement
Transformation Project of the First Hospital of Jilin University
(grant no. JDYYZH-2102057).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed and performed the experiments, analyzed
data and wrote the manuscript. WL and BL performed experiments and
collected and analyzed data. YX performed data analysis and
reviewed and revised the manuscript. WL and YX confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Disdier C, Chen X, Kim JE, Threlkeld SW
and Stonestreet BS: Anti-cytokine therapy to attenuate
ischemic-reperfusion associated brain injury in the perinatal
period. Br Sci. 8(101)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de la Monte SM, Gallucci GM, Lin A, Tong M
and Stonestreet BS: Critical shifts in cerebral white matter lipid
profiles after ischemic-reperfusion brain injury in fetal sheep as
demonstrated by the positive ion mode MALDI-mass spectrometry. Cell
Medicine: Feb 7, 2020 (Epub ahead of print).
|
|
3
|
Vermani B, Mukherjee S, Kumar G and
Patnaik R: Prolactin attenuates global cerebral ischemic injury in
rat model by conferring neuroprotection. Brain Inj. 34:685–693.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Scalambrino E, Padovan L, Chantarangkul V,
Clerici M, Artoni A, Peyvandi F and Tripodi A: Responsiveness of
the activated partial thromboplastin time and dilute thrombin time
to argatroban: Results of an in vitro study. Int J Lab Hematol.
42:e128–e131. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zavyalova EG, Ustinov NB and Kopylov AM:
Exploring the efficiency of thrombin inhibitors with a quantitative
model of the coagulation cascade. FEBS Lett. 594:995–1004.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bushi D, Chapman J, Wohl A, Stein ES,
Feingold E and Tanne D: Apixaban decreases brain thrombin activity
in a male mouse model of acute ischemic stroke. J Neurosci Res.
96:1406–1411. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bushi D, Stein ES, Golderman V, Feingold
E, Gera O, Chapman J and Tanne D: A linear temporal increase in
thrombin activity and loss of its receptor in mouse brain following
ischemic stroke. Front Neurol. 8(138)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hu S, Wu G, Zheng J, Liu X and Zhang Y:
Astrocytic thrombin-evoked VEGF release is dependent on p44/42
MAPKs and PAR1. Biochem Biophys Res Commun. 509:585–589.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rao JY, Wang Q, Wang YC, Xiang F, Tian XC,
Liu DH and Dong Z: β-caryophyllene alleviates cerebral
ischemia/reperfusion injury in mice by activating autophagy.
Zhongguo Zhong Yao Za Zhi. 45:932–936. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
10
|
Zhang Y, Tian Z, Wan H, Liu W and Ma G:
Deltonin ameliorates cerebral ischemia/reperfusion injury in
correlation with modulation of autophagy and inflammation.
Neuropsychiatr Dis Treat. 16:871–879. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qiu R, Li W and Liu Y: MicroRNA-204
protects H9C2 cells against hypoxia/reoxygenation-induced injury
through regulating SIRT1-mediated autophagy. Biomed Pharmacother.
100:15–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bushi D, Chapman J, Katzav A, Shavit-Stein
E, Molshatzki N, Maggio N and Tanne D: Quantitative detection of
thrombin activity in an ischemic stroke model. J Mol Neurosci.
51:844–850. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Susanto A, Zhao G, Wazin F, Feng Y, Rasko
JEJ, Bailey CG and Lovicu FJ: Spred negatively regulates lens
growth by modulating epithelial cell proliferation and fiber
differentiation. Exp Eye Res. 178:160–175. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ullrich M, Aßmus B, Augustin AM, Häbich H,
Abeßer M, Machado JM, Werner F, Erkens R, Arias-Loza AP, Umbenhauer
S, et al: SPRED2 deficiency elicits cardiac arrhythmias and
premature death via impaired autophagy. J Mol Cell Cardiol.
129:13–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bu Q, Liu X, Zhu Y, Liu Y and Wang Y:
w007B protects brain against ischemia-reperfusion injury in rats
through inhibiting inflammation, apoptosis and autophagy. Brain
Res. 1558:100–108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang X, Yan H, Yuan Y, Gao J, Shen Z,
Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, et al: Cerebral
ischemia-reperfusion-induced autophagy protects against neuronal
injury by mitochondrial clearance. Autophagy. 9:1321–1333.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pan R, Timmins GS, Liu W and Liu KJ:
Autophagy mediates astrocyte death during zinc-potentiated
ischemia-reperfusion injury. Biol Trace Elem Res. 166:89–95.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu X, Tian F, Wang S, Wang F and Xiong L:
Astrocyte autophagy flux protects neurons against oxygen-glucose
deprivation and ischemic/reperfusion injury. Rejuvenation Res.
21:405–415. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhu C, Zhou Q, Luo C and Chen Y:
Dexmedetomidine protects against oxygen-glucose deprivation-induced
injury through inducing astrocytes autophagy via TSC2/mTOR pathway.
Neuromolecular Med. 22:210–217. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu S, Wu G, Ding X and Zhang Y: Thrombin
preferentially induces autophagy in glia cells in the rat central
nervous system. Neurosci Lett. 630:53–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu S, Xi G, Jin H, He Y, Keep RF and Hua
Y: Thrombin-induced autophagy: A potential role in intracerebral
hemorrhage. Brain Res. 1424:60–66. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Q, Ye T, Long T and Peng X: Ginkgetin
exerts anti-inflammatory effects on cerebral
ischemia/reperfusion-induced injury in a rat model via the
TLR4/NF-κB signaling pathway. Biosci Biotechnol Biochem.
83:675–683. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kar F, Hacioglu C, Senturk H, Donmez DB
and Kanbak G: The role of oxidative stress, renal inflammation, and
apoptosis in post ischemic reperfusion injury of kidney tissue: The
protective effect of dose-dependent boric acid administration. Biol
Trace Elem Res. 195:150–158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang YS, Li YX, Zhao P, Wang HB, Zhou R,
Hao YJ, Wang J, Wang SJ, Du J, Ma L, et al: Anti-inflammation
effects of oxysophoridine on cerebral ischemia-reperfusion injury
in mice. Inflammation. 38:2259–2268. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tripathy D, Sanchez A, Yin X, Luo J,
Martinez J and Grammas P: Thrombin, a mediator of cerebrovascular
inflammation in AD and hypoxia. Front Aging Neurosci.
5(19)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ohshiro K, Bui-Nguyen TM, Divijendra Natha
RS, Schwartz AM, Levine P and Kumar R: Thrombin stimulation of
inflammatory breast cancer cells leads to aggressiveness via the
EGFR-PAR1-Pak1 pathway. Int J Biol Markers. 27:e305–e313.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
López-Zambrano M, Rodriguez-Montesinos J,
Crespo-Avilan GE, Muñoz-Vega M and Preissner KT: Thrombin promotes
macrophage polarization into M1-like phenotype to induce
inflammatory responses. Thromb Haemost. 120:658–670.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dusaban SS, Kunkel MT, Smrcka AV and Brown
JH: Thrombin promotes sustained signaling and inflammatory gene
expression through the CDC25 and Ras-associating domains of
phospholipase Cϵ. J Biol Chem. 290:26776–26783. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang Y, Karsli-Uzunbas G, Poillet-Perez L,
Sawant A, Hu ZS, Zhao Y, Moore D, Hu W and White E and White E:
Autophagy promotes mammalian survival by suppressing oxidative
stress and p53. Genes Dev. 34:688–700. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu C, Yan X, Liao Y, Liao L, Huang S, Zuo
Q, Zhou L, Gao L, Wang Y, Lin J, Li S, Wang K, Ge X, Song H, Yang R
and Lu F: Increased perihematomal neuron autophagy and plasma
thrombin-antithrombin levels in patients with intracerebral
hemorrhage: An observational study. Medicine. 98((39)
17130)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kawazoe T and Taniguchi K: The
Sprouty/Spred family as tumor suppressors: Coming of age. Cancer
Sci. 110:1525–1535. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ohkura T, Yoshimura T, Fujisawa M, Ohara
T, Marutani R, Usami K and Matsukawa A: Spred2 regulates high fat
diet-induced adipose tissue inflammation, and metabolic
abnormalities in mice. Front Immunol. 10(17)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Takahashi S, Yoshimura T, Ohkura T,
Fujisawa M, Fushimi S, Ito T, Itakura J, Hiraoka S, Okada H,
Yamamoto K and Matsukawa A: A novel role of Spred2 in the colonic
epithelial cell homeostasis and inflammation. Sci Rep.
6(37531)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Itakura J, Sato M, Ito T, Mino M, Fushimi
S, Takahashi S, Yoshimura T and Matsukawa A: Spred2-deficiecy
protects mice from polymicrobial septic peritonitis by enhancing
inflammation and bacterial clearance. Sci Rep.
7(12833)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiang K, Liu M, Lin G, Mao B, Cheng W, Liu
H, Gal J, Zhu H, Yuan Z, Deng W, et al: Tumor suppressor Spred2
interaction with LC3 promotes autophagosome maturation and induces
autophagy-dependent cell death. Oncotarget. 7:25652–25667.
2016.PubMed/NCBI View Article : Google Scholar
|