Introduction
The small intestinal mucosa functions as a
protective barrier against various environmental stimuli and is
considered to be the most sensitive and vulnerable site of
ischemia/reperfusion (I/R) injury (1). Intestinal I/R injury typically occurs
after acute mesenteric ischemia, small bowel volvulus, major
surgical procedures, hemorrhagic shock and sepsis (2,3). The
development of intestinal I/R is associated with intestinal mucosa
injury, serious impairment of the local microvasculature, increased
vascular and mucosal permeability and multiple organ failure, all
of which contribute to increasing the risk of mortality (4,5). To
date, although significant improvements have been achieved in
medical and surgical techniques, including hyperbaric oxygen
treatment, ulinastatin and sodium pyruvate intravenous
resuscitation combined with abdominal resuscitation, intestinal I/R
injury remains to be a serious clinical challenge (6-8).
A growing body of evidence has shown that the
oxidative stress response, inflammation and cell apoptosis are
involved in the progression of intestinal I/R, which can lead to
the disruption of the intestinal barrier and distant organ injury
(9,10). Previous studies have proposed that
I/R-induced production of reactive oxygen species (ROS) and
apoptosis can cause damage to DNA and other cellular biomolecules,
such as proteins and saccharides (11-13).
In addition, activation of inflammation induced by ROS, which
releases inflammatory cytokines and oxygen-derived free radicals,
can aggravate intestinal injury further (14,15).
It has been reported that tofacitinib (Tofa), an oral
small-molecule Janus kinase (JAK) inhibitor, is a potentially
effective inductive therapeutic option for patients with
moderate-to-severe ulcerative colitis (16). Additionally, Tofa was found to
inhibit T-cell homing and activation during chronic intestinal
inflammation in an experimental mouse colitis model (17), whereas another study revealed that
Tofa could rescue human intestinal epithelial cells and colonoids
from interferon-γ-induced barrier dysfunction (18). Tofa has also been shown to suppress
the activities of all JAKs, particularly JAK1 and JAK3 in mammalian
inflammatory bowel disease (19,20).
JAK1 and JAK3 can activate STATs by phosphorylation (21). An increasing number of studies have
suggested that the JAK/STAT signaling is involved in the
pathogenesis of tissue and organ I/R injury, including intestinal
I/R injury (22-24).
Therefore, the present study aimed to investigate the role of Tofa
and its possible regulatory effects on the JAK/STAT signaling
pathway in intestinal I/R injury.
In the present study, an oxygen-glucose
deprivation/reoxygenation (OGD/R)-induced normal rat small
intestinal epithelial cell model was established to simulate the
physiological environment of intestinal I/R injury. The effects of
Tofa on oxidative stress, inflammation and apoptosis caused by
OGD/R-induced intestinal I/R injury were investigated and the
regulatory mechanisms associated with JAK/STAT3 signaling pathway
were explored.
Materials and methods
Cell culture and treatment
The normal rat small intestinal epithelial cell
line, IEC-6, was obtained from the American Type Culture
Collection. Cells were routinely cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) in a 5% CO2 incubator at 37˚C.
Tofa was purchased from Selleckchem. Tofa was dissolved in 100%
dimethyl sulfoxide (DMSO; Sigma-Aldrich, Merck KGaA) to a stock
concentration of 10 mM and stored at -20˚C until use as the
vehicle. For 50, 100 and 200 nM Tofa concentrations, the stock was
diluted using DMEM. After reaching 80% confluence, cells were
pretreated with 0.5 µM colivelin (Shanghai Aladdin Biochemical
Technology Co., Ltd.) for 6 h at 37˚C and were then incubated in
the presence of different doses of Tofa (50, 100 or 200 nM) for an
additional 24 h 37˚C. Colivelin, an agonist of the JAK/STAT3
pathway, was used to investigate whether the effects of Tofa on
intestinal I/R injury were mediated by this signaling pathway
(25).
Construction of the OGD/R model
Briefly, IEC-6 cells were first grown under normal
conditions until they reached 80% confluence. Subsequently, the
DMEM was replaced with D-Hanks buffer (Sigma-Aldrich; Merck KGaA)
and cells were incubated in a modular incubator chamber filled with
a 95% N2 and 5% CO2 gas mixture for 4 h at
37˚C. The medium was then changed back to DMEM supplemented with
10% FBS and cells were reoxygenated for 4 h. Control cells were not
deprived of oxygen and glucose and were maintained in complete DMEM
in a fully oxygenated environment.
Cell viability assay and lactate
dehydrogenase (LDH) detection
Cell viability was assessed using a Cell Counting
Kit-8 (CCK-8) reagent (Shanghai YiSheng Biotechnology Co., Ltd.)
according to manufacturer's protocol. Briefly, 3x103
IEC-6 cells were plated into 96-well culture plates. At the
indicated time, 10 µl CCK-8 solution was added into each well and
cells were incubated at 37˚C for 2 h. The absorbance values in each
well were recorded at a wavelength of 450 nm using a microplate
reader (Bio-Rad Laboratories, Inc.).
Furthermore, cell death was evaluated by measuring
the activity of LDH in the supernatant using a LDH assay kit (cat.
no. A020-2-2; Nanjing Jiancheng Bioengineering Institute) according
to the manufacturer's instructions.
Detection of intracellular ROS
The production of intracellular ROS in IEC-6 cells
was detected utilizing a ROS assay kit (cat. no. S0033; Beyotime
Institute of Biotechnology) with cell-permeable
2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA), according to
the manufacturer's protocol. Briefly, following OGD/R induction,
cells were stained with DCFH-DA (10 µmol/l) and DAPI (1 µg/l) for
15 min in the dark at 37˚C. The fluorescence intensity of ROS was
measured under a confocal microscope (magnification, x200; Olympus
Corporation) using the excitation and emission wavelengths of 488
and 530 nm, respectively. ROS activity was quantified using ImageJ
software (version 1.45; National Institutes of Health).
Measurement of oxidative
stress-related factors
Following cell treatment, the content of
malondialdehyde (MDA; cat. no. A003-4-1) and the activity of
superoxide dismutase (SOD; cat. no. A001-3-2) in the culture media
were detected using commercially available kits according to the
protocols provided by the supplier (Nanjing Jiancheng
Bioengineering Institute).
Determination of the secretion levels
of inflammatory factors
After the end of the reoxygenation stage, the
culture supernatants were collected. The concentration of
inflammatory cytokines TNF-α (cat. no. F16960), IL-6 (cat. no.
F15870) and IL-1β (cat. no. F15810), was determined using ELISA
according to the manufacturer's protocol (Shanghai Xitang
Biotechnology Co., Ltd.). Briefly, the treated cells were harvested
by centrifugation at 4˚C, 12,000 x g for 10 min. The supernatant
was then collected and plated into ELISA microplates to measure the
absorbance of each well at a wavelength of 450 nm using an
automatic microplate reader (Syngene).
Western blot analysis
Following treatment, IEC-6 cells were harvested and
the cellular lysates were extracted with RIPA lysis buffer
(Beyotime Institute of Biotechnology). The protein concentration in
each group was measured using a BCA Protein Assay Kit (Beyotime
Institute of Biotechnology). The proteins (40 µg/lane) were
separated by 10% SDS-PAGE and were then transferred onto
nitrocellulose membranes (Merck KGaA). Following incubation with 5%
non-fat milk for 1 h at room temperature, the membranes were probed
with the following primary antibodies overnight at 4˚C: Anti-Bcl-2
(1:1,000; cat. no. sc-7382; Santa Cruz Biotechnology, Inc.),
anti-cleaved caspase-3 (1:1,000; cat. no. 9664T; Cell Signaling
Technology, Inc.), anti-caspase-3 (1:1,000; cat. no. 9662S; Cell
Signaling Technology, Inc.), anti-cleaved caspase-9 (1:1,000; cat.
no. 20750S; Cell Signaling Technology, Inc.), anti-caspase-9
(1:1,000; cat. no. 9508T; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-STAT3 (1:1,000; cat. no. 9145S; Cell
Signaling Technology, Inc.), anti-STAT3 (1:1,000; cat. no. 4904T;
Cell Signaling Technology, Inc.), anti-GAPDH (1:1,000; cat. no.
5174T; Cell Signaling Technology, Inc.), anti-p-JAK1 (1:1,000; cat.
no. ab138005; Abcam), anti-JAK1 (1:1,000; cat. no. ab47435; Abcam),
anti-p-JAK3 (1:1,000; cat. no. ab278789; Abcam) and anti-JAK3
(1:1,000; cat. no. ab45141; Abcam). Following primary incubation,
the membranes were incubated with the corresponding secondary
antibody conjugated with horseradish peroxidase (1:3,000; cat. no.
7074S; Cell Signaling Technology, Inc.) for 1 h at room
temperature. The protein bands were visualized with using enhanced
chemiluminescence substrate (Pierce; Thermo Fisher. Scientific,
Inc.) in a chemiluminescence imaging equipment (Ultra-Lum, Inc.).
Band intensity was semi-quantified via the ImageJ software (version
1.45; National Institutes of Health). GAPDH served as an internal
control for normalization.
Statistical analysis
All experiments were repeated three times and the
measurement data are expressed as the mean ± SD. Data management
and analysis were performed using the GraphPad Prism software
(version 6.0; GraphPad Software, Inc.). Comparisons between two
groups were conducted by an unpaired Student's t-test. One-way
analysis of variance followed by Tukey's post hoc test was applied
to compare differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
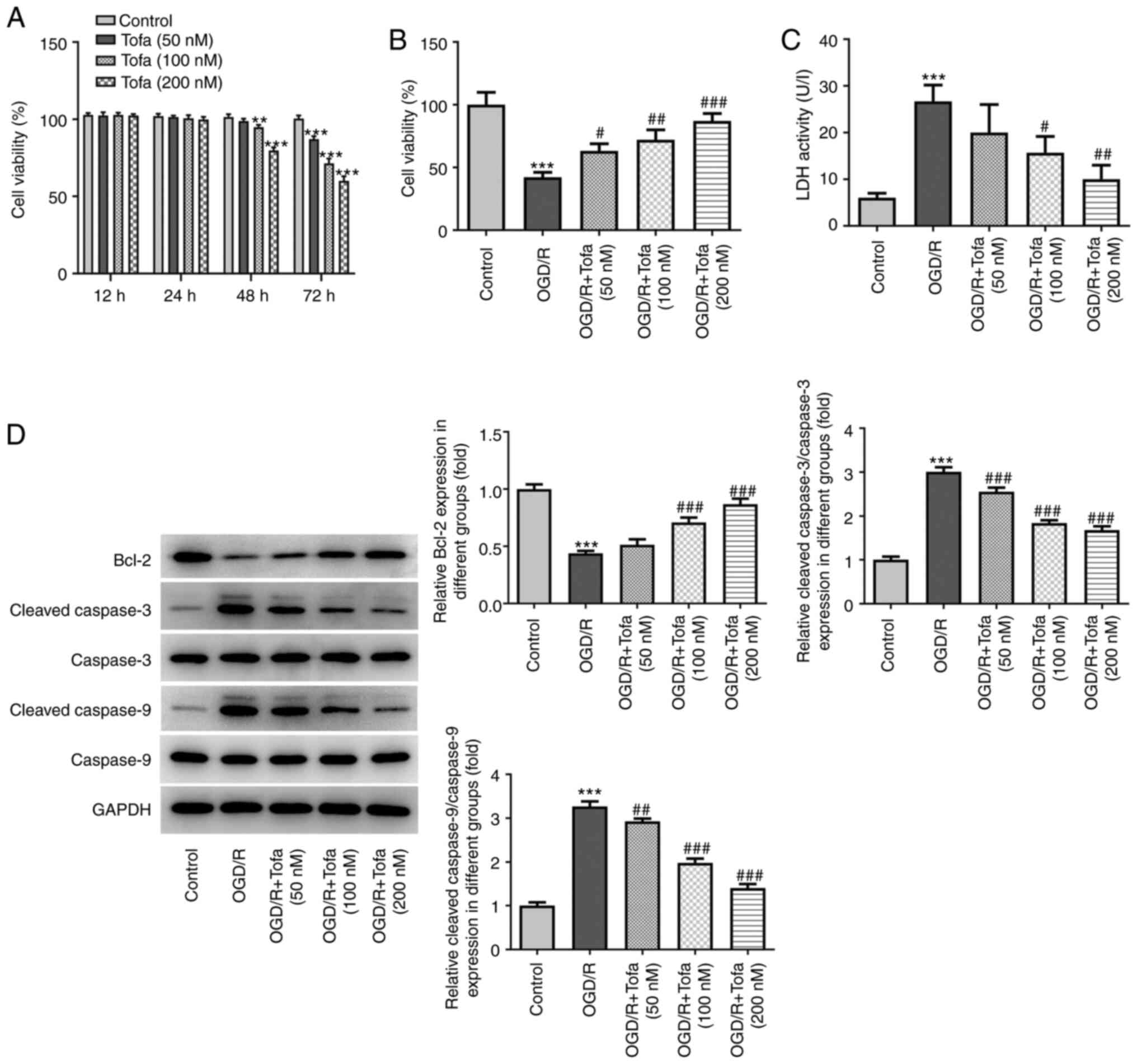
Tofa treatment enhances the viability
and attenuates apoptosis of OGD/R-induced IEC-6 cells
Firstly, IEC-6 cell viability was evaluated using a
CCK-8 kit after treatment with Tofa (50, 100 and 200 nM) for 24, 48
and 72 h. As shown in Fig. 1A, none
of the doses of Tofa exerted significant effects on cell viability
after Tofa treatment for 12 and 24 h. However, 100 and 200 nM Tofa
intervention for 48 h, in addition to 50, 100 and 200 nM Tofa
treatment for 72 h, significantly decreased cell viability compared
with that in the Control group. Therefore, treatment of IEC-6 cells
with 50, 100 and 200 nM for 24 h was chosen to be the regimen used
for subsequent analyzes. Following Tofa preconditioning in IEC-6
cells exposed to OGD/R, cell viability was assessed using CCK-8
assay. As shown in Fig. 1B, OGD/R
challenge significantly attenuated cell viability compared with
that in the untreated control group. By contrast, Tofa treatment
dose-dependently elevated IEC-6 cell viability compared with that
in the OGD/R group. Additionally, compared with that in the
OGD/R-induced group, cell treatment with 100 and 200 nM Tofa
markedly reduced the activity of LDH in the culture supernatant
(Fig. 1C). According to Fig. 1D, the expression levels of Bcl-2
were significantly downregulated, whilst those of
cleaved-caspase-3/9 were significantly upregulated, in the OGD/R
group compared with those in the control group. However, these
effects aforementioned were markedly reversed following Tofa
pretreatment in a dose-dependent manner (Fig. 1D). These findings suggest that Tofa
treatment can enhance cell viability whilst inhibiting apoptosis in
OGD/R-induced IEC-6 cells.
Tofa treatment alleviates oxidative
stress and inflammation in OGD/R-treated IEC-6 cells
To investigate the effects of Tofa on oxidative
stress in OGD/R-treated IEC-6 cells, the production of
intracellular ROS was measured. The results demonstrated that
exposure to OGD/R significantly increased the intracellular
production of ROS compared with that in the control group, whereas
Tofa intervention dose-dependently and significantly decreased ROS
levels in OGD/R-treated IEC-6 cells (Fig. 2A and B). Consistently, the content of MDA was
also significantly enhanced after OGD/R challenge, accompanied by
significantly reduced SOD activity, compared with those in the
control group (Fig. 2C and D). However, these effects were
dose-dependently reversed following treatment with Tofa.
Furthermore, Tofa dose-dependently reversed the significant
inflammatory responses in IEC-6 cells induced by OGD/R, as
indicated by the significantly decreased concentrations of TNF-α,
IL-6 and IL-1β (Fig. 2E-G). These
findings suggest that Tofa exert inhibitory effects on
OGD/R-induced oxidative stress and inflammation in IEC-6 cells.
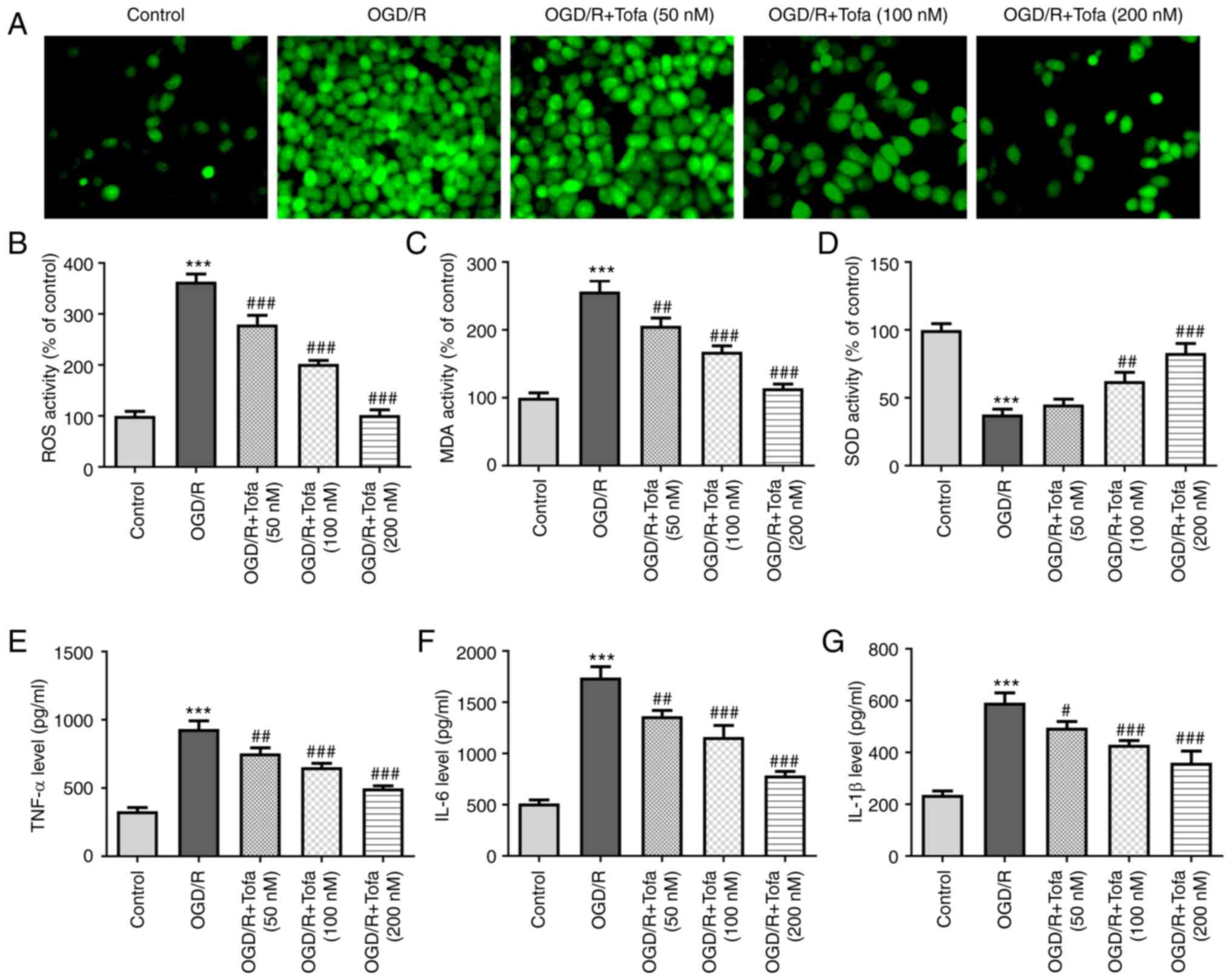 | Figure 2Tofa treatment attenuates oxidative
stress and inflammation in IEC-6 cells that were exposed to OGD/R.
(A) Intracellular ROS production was measured using
2',7'-dichlorodihydrofluorescein diacetate as a fluorescence probe,
(B) which was quantified. Magnification, x200. (C) MDA content and
(D) SOD activity were assessed in the cell culture supernatant by
corresponding MDA and SOD assay kits. Secretion levels of
inflammatory factors, namely (E) TNF-α, (F) IL-6 and (G) IL-1β,
were measured using ELISA. ***P<0.001 vs. Control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. OGD/R. Tofa, tofacitinib; OGD/R,
oxygen-glucose deprivation/reoxygenation; ROS, reactive oxygen
species; MDA, malondialdehyde; SOD, superoxide dismutase. |
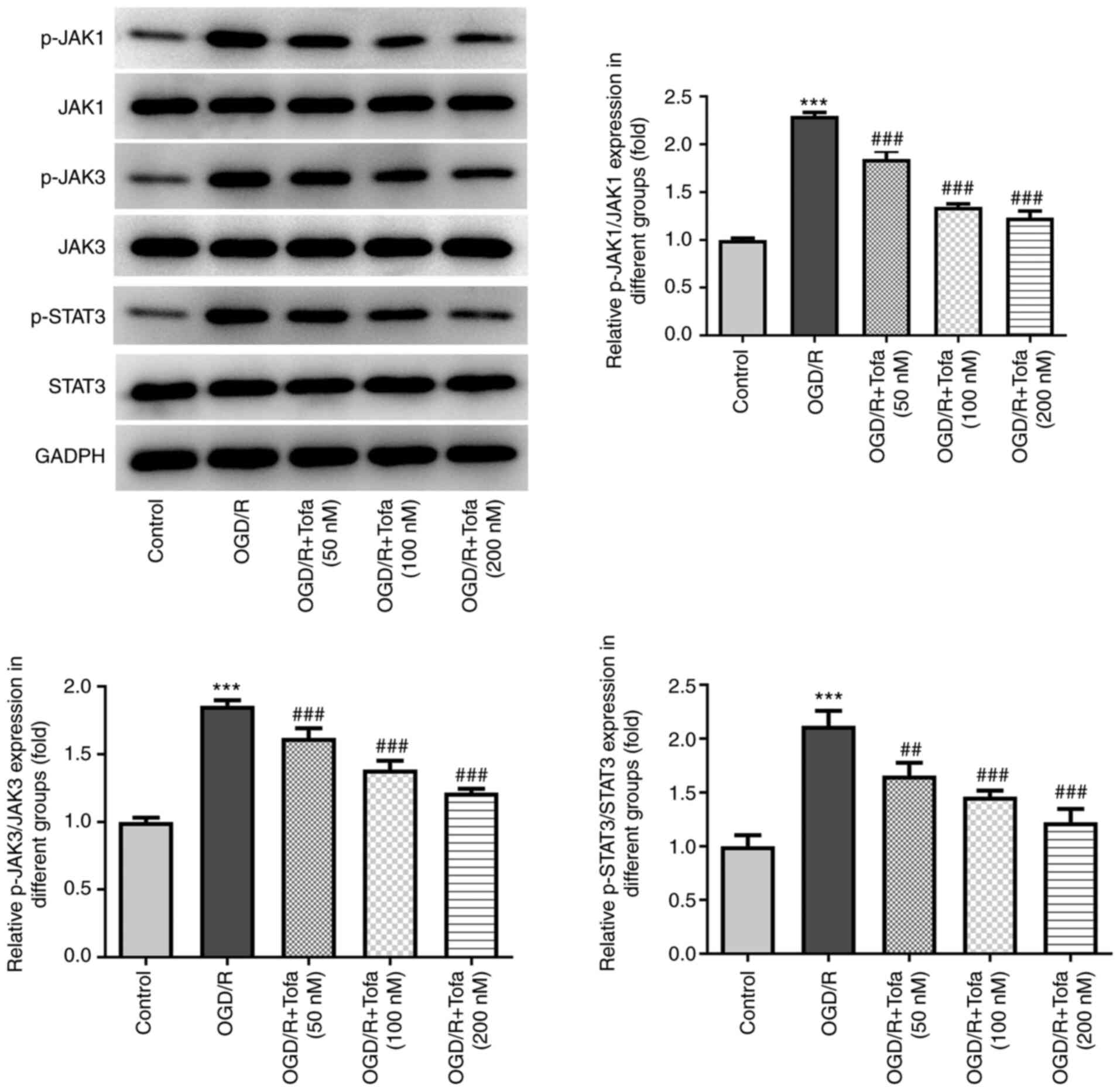
Tofa preconditioning inhibits
JAK/STAT3 signaling in OGD/R-stimulated IEC-6 cells
To investigate the potential mechanism of Tofa in
OGD/R-induced IEC-6 cells, the expression levels of the JAK/STAT3
signaling pathway-related proteins were detected by the means of
western blot analysis. OGD/R challenge significantly upregulated
the levels of phosphorylated (p)-JAK1, p-JAK3 and p-STAT3 compared
with those in the control group (Fig.
3). By contrast, Tofa treatment dose-dependently and
significantly downregulated the phosphorylation of JAK1, JAK3 and
STAT3 compared with those in the OGD/R group (Fig. 3). These findings suggest that Tofa
can inhibit the JAK/STAT3 signaling pathway in OGD/R-induced IEC-6
cells.
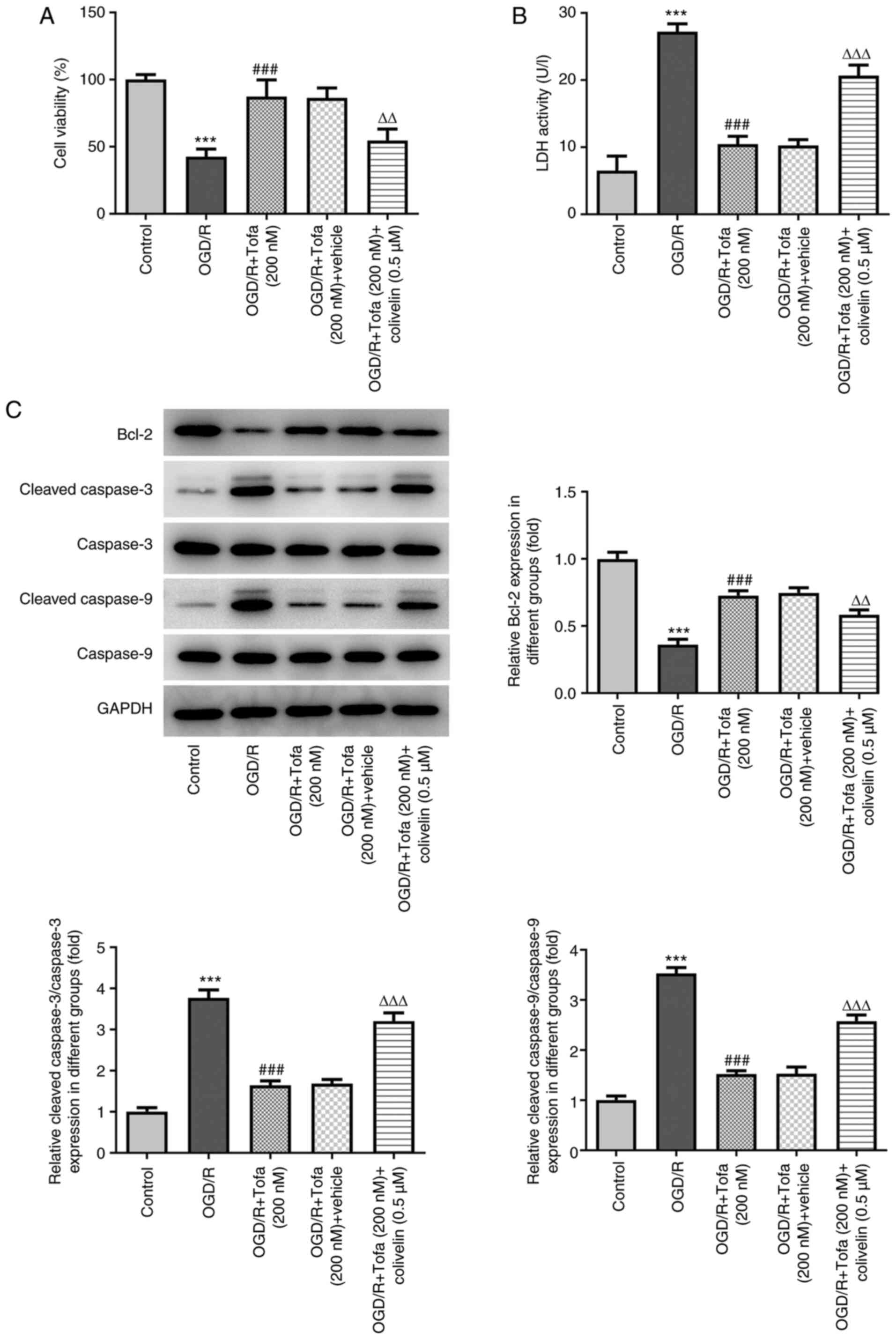
Activation of JAK/STAT3 signaling
rescues the inhibitory effects of Tofa on OGD/R-induced IEC-6 cell
injury
To verify the importance of the JAK/STAT3 pathway
for the protective effects of Tofa on OGD/R-induced IEC-6 cell
injury, cells were treated with colivelin, an agonist of the
JAK/STAT3 pathway (25). As shown
in Fig. 4A, colivelin significantly
abrogated the therapeutic effects of Tofa on cell viability after
exposure to OGD/R. Additionally, significantly enhanced LDH
activity was observed in the OGD/R + Tofa + colivelin group
compared with that in the OGD/R + Tofa + vehicle group (Fig. 4B). Colivelin also significantly
reversed the effects of Tofa on the expression of Bcl-2, cleaved
caspase-3 and cleaved caspase-9 in OGD/R-induced IEC-6 cells
(Fig. 4C).
Subsequently, the contents of ROS and MDA were
significantly enhanced after colivelin treatment, which was also
accompanied by the significantly decreased activity of the
antioxidant enzyme SOD, compared with those in the OGD/R + Tofa +
vehicle group (Fig. 5A-D). In
addition, colivelin partially but significantly counteracted the
inhibitory effects of Tofa on the secretion levels of TNF-α, IL-6
and IL-1β (Fig. 5E-G).
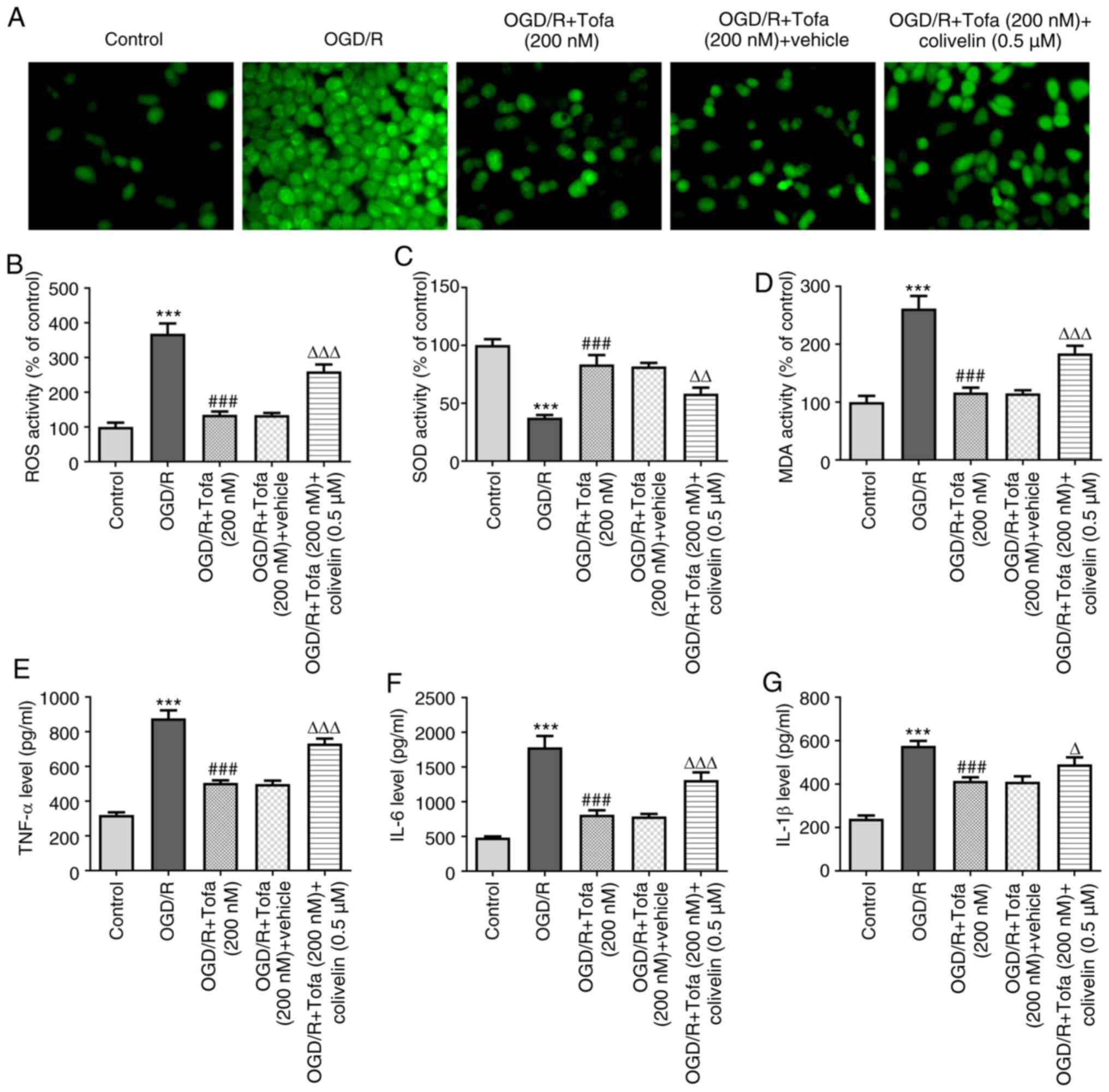 | Figure 5Activation of the JAK/STAT3 signaling
reverses the inhibitory effects of Tofa on oxidative stress and
inflammation in OGD/R-induced IEC-6 cells. (A) The levels of ROS
were measured using 2',7'-dichlorodihydrofluorescein diacetate as a
fluorescence probe, (B) which were quantified in OGD/R-induced
IEC-6 cell apoptosis in the presence or absence of Tofa (200 nM)
and colivelin (0.5 µM). Magnification, x200. The activity of (C)
SOD and (D) the content of MDA in the cell culture supernatant were
measured using corresponding MDA and SOD assay kits. ELISA was used
to measure the concentration of (E) TNF-α, (F) IL-6 and (G) IL-1β.
***P<0.001 vs. Control; ###P<0.001 vs.
OGD/R; ΔP<0.05, ΔΔP<0.01 and
ΔΔΔP<0.001 vs. OGD/R + Tofa + vehicle. JAK, Janus
kinase; Tofa, tofacitinib; OGD/R, oxygen-glucose
deprivation/reoxygenation; ROS, reactive oxygen species; SOD,
superoxide dismutase; MDA, malondialdehyde. |
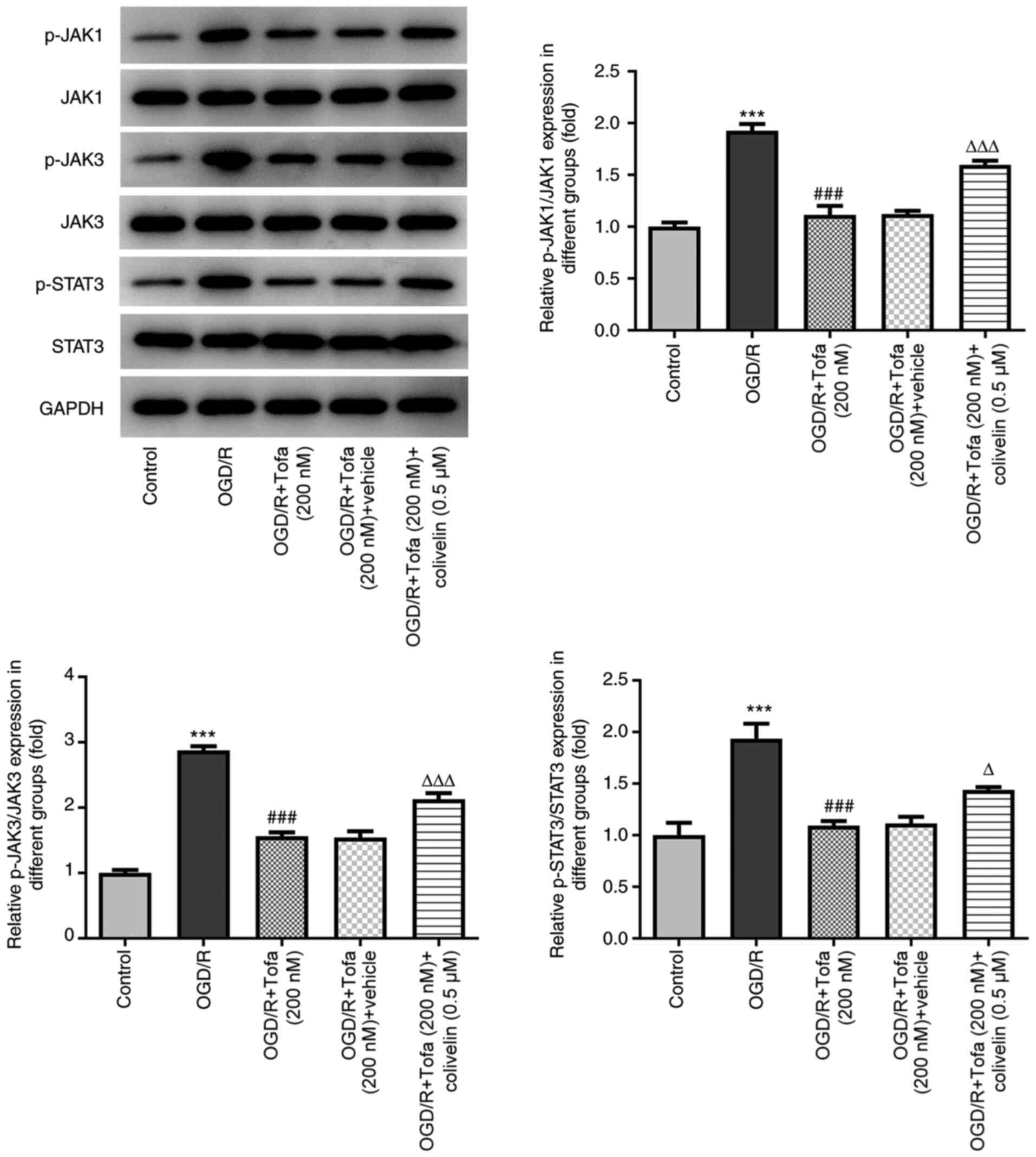
Consistent with the aforementioned observations,
colivelin significantly upregulated the protein phosphorylation of
JAK1, JAK3 and STAT3 (Fig. 6).
Overall, these data suggest that the activation of JAK/STAT3
signaling could negate the effects of Tofa on OGD/R-induced IEC-6
cell injury.
Discussion
Intestinal I/R injury caused by the clamping of the
superior mesenteric artery is a common life-threatening
complication that can be observed in multiple clinical conditions,
such as small intestinal volvulus, acute mesenteric ischemia, shock
and trauma (26). The present study
provided useful findings using an OGD/R IEC-6 cell model to
simulate the physiological environment during intestinal I/R
injury. The results demonstrated that Tofa preconditioning exerted
protective effects on apoptosis, oxidative stress and inflammation
in IEC-6 cells in a dose-dependent manner during OGD/R.
Mechanically, the aforementioned beneficial effects of Tofa were
partially abrogated by the agonist of the JAK/STAT3 pathway.
A growing body of evidence suggests that intestinal
I/R injury is characterized by disruption of the mucosal barrier,
which may result in systemic inflammatory response syndrome and
multiple organ failure (27-29).
Apoptosis is a major mechanism of mucosal epithelial cell death
during intestinal I/R-induced destruction of the intestinal
epithelial barrier (30).
Consistent with the results of the present study, a previous study
demonstrated that OGD/R challenge markedly enhanced the apoptosis
of IEC-6 cells (31). Additionally,
intestinal I/R injury is caused by oxidative damage due to the
imbalance in oxidation and antioxidation in ischemic tissues and
cells, such that ROS cannot be removed efficiently after blood
supply is restored (32). MDA
represents one of the end products of lipid peroxidation and is an
oxidative stress marker, whereas SOD is a crucial antioxidant
enzyme that is part of the defense system against oxidative stress
and can protect intestinal epithelial cells against ROS-induced
cell death (33,34). Tofa has been reported to inhibit the
production of ROS triggered by oxidized low-density lipoprotein in
cultured primary human aortic endothelial cells (35). In the present study, Tofa alleviated
oxidative stress in OGD/R-induced intestinal epithelial cells, as
indicated by the reduced levels of ROS and MDA and enhanced
activity of SOD, which were in accordance with previous studies
(15,36). Emerging evidence has suggested that
the predominant cause of intestinal I/R damage is the excessive
release of inflammatory factors (14,37).
In addition, it has been reported that during intestinal I/R, the
levels of proinflammatory cytokines, including TNF-α, IL-6 and
IL-1β, are notably enhanced, which contribute to the induction of
the systemic inflammatory response and even damage of distant
organs (38). Tofa is a well-known
small-molecule JAK inhibitor that has been approved for the
treatment of rheumatoid arthritis (39). Furthermore, Tofa is recommended for
the treatment of adult ulcerative colitis, which is a type of
inflammatory bowel disease (40).
Tofa can suppress T-cell homing and activation during chronic
intestinal inflammation (17) and
rescue human intestinal epithelial cells and colonoids from
cytokine-induced barrier dysfunction (18). The present study revealed that Tofa
could dose-dependently alleviate OGD/R-induced IEC-6 cell damage,
suggesting the potential use of Tofa for treating intestinal I/R
injury.
The present study also investigated the role of the
JAK/STAT3 signaling in the protective effects of Tofa on
OGD/R-induced IEC-6 cell injury. To the best of our knowledge, Tofa
is a well-known small-molecule JAK inhibitor, which inhibits all
JAKs, particularly JAK1 and JAK3 (19,20).
JAK1 and JAK3 activate STATs by phosphorylation (21). It has been previously reported that
JAK/STAT signaling is involved in several important cellular
processes, including cell proliferation, migration, apoptosis and
inflammation (41-43).
A number of studies have suggested that JAK/STAT signaling is
involved in the pathogenesis of tissue and organ I/R injury,
including intestinal I/R injury (22-24).
By inactivating JAK/STAT3 signaling, dexmedetomidine was shown to
inhibit the apoptosis of astrocytes induced by OGD/R (44). Fish oils have also been found to
provide protection against cecal ligation and puncture-induced
septic acute kidney injury by regulating inflammation, oxidative
stress and apoptosis by suppressing JAK/STAT3 signaling (45). The present study suggests that Tofa
can dose-dependently downregulate the phosphorylation and therefore
activation of JAK1, JAK2 and STAT3 in IEC-6 cells under OGD/R
conditions. Importantly, colivelin, which is a JAK activator,
partially counteracted the beneficial effects of Tofa on
OGD/R-induced IEC-6 cell injury.
To conclude, to the best of our knowledge, the
present study was the first to demonstrate that Tofa exerted
anti-apoptotic, antioxidant and anti-inflammatory effects during
intestinal I/R injury in vitro by inactivating the JAK/STAT3
signaling pathway. This suggests that Tofa may be a promising
candidate for the treatment and prevention of intestinal I/R injury
in the clinic. However, the lack of in vivo experiments
using an intestinal I/R injury animal model is a limitation of the
present study. Therefore, further intestinal I/R animal experiments
to explore the potential effects of Tofa on I/R-induced intestinal
damage should be performed in future studies to support the present
conclusions.
Acknowledgements
Not applicable.
Funding
Funding: Not funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and XX searched the literature, designed and
conducted the experiments. JY analyzed and interpreted the data and
wrote the manuscript. XX revised the manuscript. JY and XX confirm
the authenticity of all the raw data. All the authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian
D, Liu D, Zhang F, Ning S, Yao J and Tian X: Ischemia-induced ACSL4
activation contributes to ferroptosis-mediated tissue injury in
intestinal ischemia/reperfusion. Cell Death Differ. 26:2284–2299.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sun Y, Lian M, Lin Y, Xu B, Li Y, Wen J,
Chen D, Xu M, Almoiliqy M and Wang L: Role of p-MKK7 in
myricetin-induced protection against intestinal
ischemia/reperfusion injury. Pharmacol Res. 129:432–442.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Karhausen J, Bernstock JD, Johnson KR,
Sheng H, Ma Q, Shen Y, Yang W, Hallenbeck JM and Paschen W: Ubc9
overexpression and SUMO1 deficiency blunt inflammation after
intestinal ischemia/reperfusion. Lab Invest. 98:799–813.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leone M, Bechis C, Baumstarck K, Ouattara
A, Collange O, Augustin P, Annane D, Arbelot C, Asehnoune K,
Baldési O, et al: Outcome of acute mesenteric ischemia in the
intensive care unit: A retrospective, multicenter study of 780
cases. Intensive Care Med. 41:667–676. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu ZM, Zhang XY, Chen J, Shen JT, Jiang
ZY and Guan XD: Terlipressin protects intestinal epithelial cells
against oxygen-glucose deprivation/re-oxygenation injury via the
phosphatidylinositol 3-kinase pathway. Exp Ther Med. 14:260–266.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yu JS, Yan S, Liu XS, Wu YJ, Fu PF, Wu LH
and Zheng SS: Attenuation of graft ischemia-reperfusion injury by
urinary trypsin inhibitor in mouse intestinal transplantation.
World J Gastroenterol. 11:1605–1609. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Daniel RA, Cardoso VK, Góis E Jr, Parra
RS, Garcia SB, Rocha JJ and Féres O: Effect of hyperbaric oxygen
therapy on the intestinal ischemia reperfusion injury. Acta Cir
Bras. 26:463–469. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miyake H, Koike Y, Seo S, Lee C, Li B,
Ganji N and Pierro A: The effect of pre- and post-remote ischemic
conditioning reduces the injury associated with intestinal
ischemia/reperfusion. Pediatr Surg Int. 36:1437–1442.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Feinman R, Deitch EA, Watkins AC, Abungu
B, Colorado I, Kannan KB, Sheth SU, Caputo FJ, Lu Q, Ramanathan M,
et al: HIF-1 mediates pathogenic inflammatory responses to
intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest
Liver Physiol. 299:G833–G843. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang G, Yao J, Li Z, Zu G, Feng D, Shan W,
Li Y, Hu Y, Zhao Y and Tian X: miR-34a-5p inhibition alleviates
intestinal ischemia/reperfusion-induced reactive oxygen species
accumulation and apoptosis via activation of SIRT1 signaling.
Antioxid Redox Signal. 24:961–973. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rodriguez-Lara SQ, Cardona-Muñoz EG,
Ramirez-Lizardo EJ, Totsuka-Sutto SE, Castillo-Romero A,
García-Cobián TA and García-Benavides L: Alternative interventions
to prevent oxidative damage following ischemia/reperfusion. Oxid
Med Cell Longev. 2016(7190943)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pérez S, Taléns-Visconti R, Rius-Pérez S,
Finamor I and Sastre J: Redox signaling in the gastrointestinal
tract. Free Radic Biol Med. 104:75–103. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ameli M, Hashemi MS, Moghimian M and
Shokoohi M: Protective effect of tadalafil and verapamil on
testicular function and oxidative stress after torsion/detorsion in
adult male rat. Andrologia. 50(e13068)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Groot H and Rauen U:
Ischemia-reperfusion injury: Processes in pathogenetic networks: A
review. Transplant Proc. 39:481–484. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zu G, Guo J, Che N, Zhou T, Zhang X, Wang
G, Ji A and Tian X: Protective effects of ginsenoside Rg1 on
intestinal ischemia/reperfusion injury-induced oxidative stress and
apoptosis via activation of the Wnt/β-catenin pathway. Sci Rep.
6(38480)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sandborn WJ, Ghosh S, Panes J, Vranic I,
Su C, Rousell S and Niezychowski W: Study A3921063 Investigators.
Tofacitinib, an oral janus kinase inhibitor, in active ulcerative
colitis. N Engl J Med. 367:616–624. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lechner K, Gerlach K, Popp V, Offensperger
L, Zundler S, Wiendl M, Becker E, Atreya R, Rath T, Neurath MF and
Weigmann B: The JAK1/3 inhibitor tofacitinib suppresses T cell
homing and activation in chronic intestinal inflammation. J Crohns
Colitis: Aug 18, 2020 (Epub ahead of print).
|
|
18
|
Sayoc-Becerra A, Krishnan M, Fan S,
Jimenez J, Hernandez R, Gibson K, Preciado R, Butt G and McCole DF:
The JAK-inhibitor tofacitinib rescues human intestinal epithelial
cells and colonoids from cytokine-induced barrier dysfunction.
Inflamm Bowel Dis. 26:407–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Flanagan ME, Blumenkopf TA, Brissette WH,
Brown MF, Casavant JM, Shang-Poa C, Doty JL, Elliott EA, Fisher MB,
Hines M, et al: Discovery of CP-690,550: A potent and selective
janus kinase (JAK) inhibitor for the treatment of autoimmune
diseases and organ transplant rejection. J Med Chem. 53:8468–8484.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Danese S, Grisham M, Hodge J and Telliez
JB: JAK inhibition using tofacitinib for inflammatory bowel disease
treatment: A hub for multiple inflammatory cytokines. Am J Physiol
Gastrointest Liver Physiol. 310:G155–G162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Clark JD, Flanagan ME and Telliez JB:
Discovery and development of janus kinase (JAK) inhibitors for
inflammatory diseases. J Med Chem. 57:5023–5038. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang H, Liu X, Yang F, Cheng D and Liu W:
Overexpression of HIF-1α protects PC12 cells against OGD/R-evoked
injury by reducing miR-134 expression. Cell Cycle. 19:990–999.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fang H, Zhang FX, Li HF, Yang M, Liao R,
Wang RR, Wang QY, Zheng PC and Zhang JP: PRR34-AS1 overexpression
promotes protection of propofol pretreatment against
ischemia/reperfusion injury in a mouse model after total knee
arthroplasty via blockade of the JAK1-dependent JAK-STAT signaling
pathway. J Cell Physiol. 235:2545–2556. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang XK, Zhou XP, Zhang Q and Zhu F: The
preventive effects of dexmedetomidine against intestinal
ischemia-reperfusion injury in wistar rats. Iran J Basic Med Sci.
18:604–609. 2015.PubMed/NCBI
|
|
25
|
Zhao H, Feng Y, Wei C, Li Y, Ma H, Wang X,
Cui Z, Jin WN and Shi FD: Colivelin rescues ischemic neuron and
axons involving JAK/STAT3 signaling pathway. Neuroscience.
416:198–206. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou J, Huang WQ, Li C, Wu GY, Li YS, Wen
SH, Lei WL and Liu KX: Intestinal ischemia/reperfusion enhances
microglial activation and induces cerebral injury and memory
dysfunction in rats. Crit Care Med. 40:2438–2448. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vollmar B and Menger MD: Intestinal
ischemia/reperfusion: Microcirculatory pathology and functional
consequences. Langenbecks Arch Surg. 396:13–29. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dai H, Wang M, Patel PN, Kalogeris T, Liu
Y, Durante W and Korthuis RJ: Preconditioning with the
BKCa channel activator NS-1619 prevents
ischemia-reperfusion-induced inflammation and mucosal barrier
dysfunction: Roles for ROS and heme oxygenase-1. Am J Physiol Heart
Circ Physiol. 313:H988–H999. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin ZL, Tan SJ, Cheng MH, Zhao CY, Yu WK,
He YL, Li J and Li N: Lipid-rich enteral nutrition controls
intestinal inflammation, improves intestinal motility and mucosal
barrier damage in a rat model of intestinal ischemia/reperfusion
injury. J Surg Res. 213:75–83. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ikeda H, Suzuki Y, Suzuki M, Koike M,
Tamura J, Tong J, Nomura M and fItoh G: Apoptosis is a major mode
of cell death caused by ischaemia and ischaemia/reperfusion injury
to the rat intestinal epithelium. Gut. 42:530–537. 1998.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shen JT, Li YS, Xia ZQ, Wen SH, Yao X,
Yang WJ, Li C and Liu KX: Remifentanil preconditioning protects the
small intestine against ischemia/reperfusion injury via intestinal
δ- and µ-opioid receptors. Surgery. 159:548–559. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Li LX, Yin LH, Gao M, Xu LN, Qi Y and Peng
JY: MiR-23a-5p exacerbates intestinal ischemia-reperfusion injury
by promoting oxidative stress via targeting PPAR alpha. Biochem
Pharmacol. 180(114194)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu MB, Ma B, Zhang TX, Zhao K, Cui SM and
He SC: Propofol improves intestinal ischemia-reperfusion injury in
rats through NF-κB pathway. Eur Rev Med Pharmacol Sci.
24:6463–6469. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang X, Wan M, Cheng Z, Wang Z and Wu Q:
Tofacitinib inhibits ox-LDL-induced adhesion of THP-1 monocytes to
endothelial cells. Artif Cell Nanomed Biotechnol. 47:2775–2782.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang AL, Niu Q, Shi N, Wang J, Jia XF,
Lian HF, Liu Z and Liu CX: Glutamine ameliorates intestinal
ischemia-reperfusion injury in rats by activating the Nrf2/Are
signaling pathway. Int J Clin Exp Pathol. 8:7896–7904.
2015.PubMed/NCBI
|
|
37
|
Wang H, Cai D, Chen Z and Wang Y: GTS-21
promotes α7 nAChR to alleviate intestinal
ischemia-reperfusion-induced apoptosis and inflammation of
enterocytes. Med Sci Monit. 26(e921618)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Meng QT, Chen R, Chen C, Su K, Li W, Tang
LH, Liu HM, Xue R, Sun Q, Leng Y, et al: Transcription factors Nrf2
and NF-κB contribute to inflammation and apoptosis induced by
intestinal ischemia-reperfusion in mice. Int J Mol Med.
40:1731–1740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee EB, Fleischmann R, Hall S, Wilkinson
B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV,
Zang C, et al: Tofacitinib versus methotrexate in rheumatoid
arthritis. N Engl J Med. 370:2377–2386. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Palasik BN and Wang HM: Tofacitinib, the
first oral janus kinase inhibitor approved for adult ulcerative
colitis. J Pharm Pract: Sep 2, 2020 (Online ahead of print).
|
|
41
|
Liu W, Singh SR and Hou SX: JAK-STAT is
restrained by notch to control cell proliferation of the drosophila
intestinal stem cells. J Cell Biochem. 109:992–999. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lu KH, Wu HH, Lin RC, Lin YC, Lu PW, Yang
SF and Yang JS: Curcumin analogue L48H37 suppresses human
osteosarcoma U2OS and MG-63 Cells' migration and invasion in
culture by inhibition of uPA via the JAK/STAT signaling pathway.
Molecules. 26(30)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yue Y, Zhang Q, Wu S, Wang S, Cui C, Yu M
and Sun Z: Identification of key genes involved in JAK/STAT pathway
in colorectal cancer. Mol Immunol. 128:287–297. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Feng P, Zhang A, Su M, Cai H, Wang X and
Zhang Y: Dexmedetomidine inhibits apoptosis of astrocytes induced
by oxygen-glucose deprivation via targeting JAK/STAT3 signal
pathway. Brain Res. 1750(147141)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lin Z, Rn J and Shan X: Fish oils protects
against cecal ligation and puncture-induced septic acute kidney
injury via the regulation of inflammation, oxidative stress and
apoptosis. Int J Mol Med. 44:1771–1780. 2019.PubMed/NCBI View Article : Google Scholar
|