Introduction
Subarachnoid hemorrhage (SAH) is a severe type of
stroke with high mortality worldwide of ~50% in a 2019 study
(1), and numerous survivors of SAH
suffer from long-term disabling sequelae, including sensorimotor,
neuropsychiatric and cognitive deficits (2). Several of these impairments can be
recapitulated in animal models of SAH, providing opportunities for
the elucidation of fundamental pathogenic mechanisms and the
identification of potential treatments (3). Depression is a particularly
deleterious complication as it impedes rehabilitation and
functional recovery following SAH (4). Neuronal apoptosis, oxidative stress
and inflammation are seminal pathogenic processes leading to early
brain injury and the associated functional deficits after SAH
(2,3,5).
Multiple cognitive domains are strongly dependent on the prefrontal
cortex (PFC), including response inhibition and the encoding of
contextual information (6), and
inhibition of neuronal apoptosis in the PFC can improve the
cognitive performance of SAH model mice (7). A previous study also reported that
anxiety behavior was associated with neuronal apoptosis (8). Thus, we hypothesized that cognitive
impairment and anxiety behaviors after SAH are associated with
neuronal apoptosis in the ventromedial (vm)PFC.
Hydrogen gas (H2) administration via
inhalation or dissolution in drinking water has been shown to
protect neurons in rodent models of traumatic brain injury
(9), intracerebral hemorrhage
(10) and ischemia-reperfusion
injury (11), possibly by
suppressing endoplasmic reticulum stress (12), autophagy (13), neuronal apoptosis (14) and/or) inflammatory cell infiltration
into cerebral cortex (15). Xie
et al (16) reported that
H2 provided a neuroprotective effect by alleviating
mitochondrial dysfunction via suppressing the nuclear factor
erythroid 2-related factor 2-mediated NLR family pyrin domain
containing 3 pathway. In addition, hemeoxygenase-1 (HO-1) is
involved in H2-mediated neuroprotective effects via the
MAPK/HO-1/proliferator-activated receptor γ coactivator 1-α
signaling pathway (17). While the
precise molecular mechanisms for these effects remain unknown,
recent evidence has suggested modulation of p38 MAPK signaling
after SAH exposure may be involved (18).
The MAPK serine/threonine kinases consist of three
distinct subtypes, p38 MAPK, JNKs and ERKs 1 and 2, of which p38
MAPK and JNKs are known regulators of apoptosis (19). Activation of p38 MAPK results in the
production of cleaved caspase-3, the principal effector of
apoptosis, the upregulation of the pro-apoptotic modulator Bax and
the downregulation of anti-apoptotic Bcl-2(20). Inhibition of p38 MAPK-related
proapoptotic pathways can ameliorate early brain injury after
experimental SAH (21), and
inhibition of p38 MAPK signaling appears to contribute to the
neuroprotective effects of H2 (22). Thus, the aim of the current study
was to investigate the potential neuroprotective effects of
H2 inhalation on neuronal apoptosis, anxiety-like
behaviors and cognitive impairments in SAH model rats and to assess
the role of the p38 MAPK pathway.
Materials and methods
Animals
Adult male Sprague-Dawley rats (n=108; age, 6-8
weeks; weight, 200-250 g) were purchased from Liaoning Changsheng
Biotechnology and raised under controlled temperature (25±1˚C),
humidity (50±10%) and a 12/12-h light/dark cycle with free access
to food and water. All study procedures and animal care protocols
were approved by the Animal Review Board of Cangzhou Central
Hospital (Cangzhou, China) and conformed to National Institutes of
Health guidelines (23).
SAH model and H2
administration
A total of 108 rats were divided into a sham group
(n=36), a SAH group (n=36) and a SAH + H2 group (n=36).
A SAH model was established via the endovascular perforation method
as described previously (23).
Compared with using two injections of arterial blood solvate into
the cisterna magna, a rat model of endovascular perforation was
performed in this current study based on the good consistency with
the pathophysiological process of SAH (23). Briefly, rats were anesthetized with
sevoflurane (7-8% for induction, 3-4% for maintenance), intubated
and ventilated at a tidal volume of 4-5 ml/1,000 g body weight
(fraction of inspired oxygen=40%) using a ventilator (ALC-V;
Shanghai Alcott Biological Technology, Inc.) with continuous body
temperature control at 36.0±0.5˚C using a heating pad. The oxygen
concentration was set to 40% during surgical preparation and SAH,
as 40% of oxygen could lead to 99-100% oxygen saturation during
mechanical ventilation (24).
Following exposure of the surgical field via lateral
neck incision, the blood flow of the common carotid artery (CCA)
was blocked using a vascular clamp. A polytetrafluoroethylene tube
was then guided up the right internal carotid artery (ICA) through
the external carotid artery stump until resistance was detected. A
2-mm protrusion of a tungsten wire inside the tube was used to
puncture the intracranial segment of the ICA. After retracting the
wire, the CCA was reopened to allow reperfusion via the ICA, and
then the external carotid artery was ligatured using a silk thread.
At last, the wounds were sutured layer-by-layer. The sham group was
subjected to the same procedure but without ICA puncture. In the
SAH + H2 group, rats inhaled 2.9% H2
(25) (Gilmore Liquid Air Company)
mixed with 20% oxygen (flow rate 1 l/min, oxygen saturation 96-99%)
for 2 h under anesthesia (2-3% sevoflurane) immediately after SAH
was successfully established. Due to oxidative stress induced by
high concentration of oxygen (compared with 40% oxygen during
surgery), 20% oxygen was used to avoid producing an effect against
neuroprotection of H2. The H2 concentration
was monitored periodically using a handheld hydrogen detector
(H2 scan).
Western blotting (n=6) and immunofluorescence assays
(n=6) were performed at 24 h after SAH, and an MRI study was held
at 7 (n=6) and 30 days (n=6) after SAH surgery. In addition, 30 day
post-surgery, Nissl staining (n=6) and behavioral tests (n=6) were
conducted. After anesthesia with 8% sevoflurane, cerebral tissue
for western blotting (perfusion with 0.9% ice-saline via
ventriculus sinister), immunofluorescence and Nissl staining assays
(perfusion with 0.9% ice-saline and 10% neutral-buffered formalin
via ventriculus sinister) was extracted. Moreover, once MRI and
behavioral tests were finished, the rats were euthanized via
cervical dislocation under 8% sevoflurane anesthesia. The duration
of this experiment was 30 days as indicated in Fig. 1. If rats were still alive after 30
days, euthanasia was performed as aforementioned.
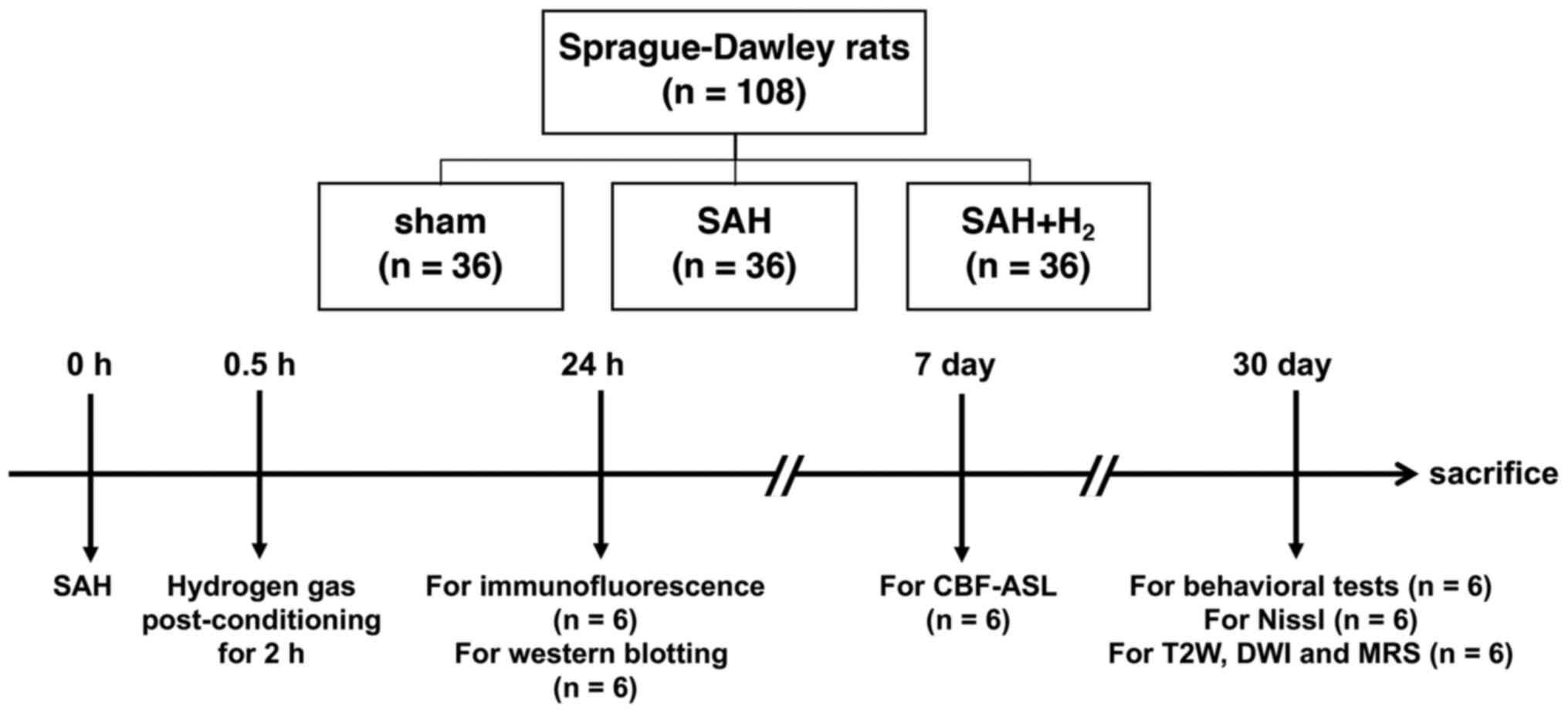 | Figure 1Schematic diagram of the experimental
procedures. Rats were subjected to SAH using a model of
endovascular perforation and inhaled 2.9% H2 mixed with
40% oxygen and balanced nitrogen (flow rate 1 l/min) for 2 h
post-SAH. MRI, including CBF-ASL, T2W, DWI and 1H MR spectra,
pathology and protein expression analyses, and behavior tests
including the open field, novel object recognition, were evaluated.
SAH, subarachnoid hemorrhage; CBF-ASL, cerebral blood flow arterial
spin labeling; T2W, T2-weighted; DWI, diffusion weighted
imaging. |
Assessment of mortality and SAH
grade
The mortality rate of rats was observed within 24 h
after SAH, and the death was verified by the lack of cardiac
electric activity. The SAH grade was determined using a previously
published grade scale (26). Scores
ranging from 0-18, including spontaneous movement of four limbs
(0-3), spontaneous activity (0-3), forelimbs outstretching (0-3),
body proprioception (0-3), vibrissa touch (0-3) and climbing
capacity (0-3), represented the severity of SAH. SAH model rats
were divided into three categories according to the severity of
bleeding: Mild (SAH grade from 0-7), moderate (SAH grade from 8-12)
and severe (SAH grade from 13-18) (27). Once rats were unable to eat food or
drink water, euthanasia was performed. Sham-operated rats had a
score of 0. The grading, health and behavior of rats were examined
by a blinded observer every 1 h within 24 h, and every day from 24
h to 30 days after SAH. The rats with SAH grades ≥8 were included
in the following study. The rats with mild SAH were euthanized via
cervical dislocation.
MRI study
At 7 and 30 days post-surgery, randomly chosen rats
from each group were anesthetized with sodium pentobarbital (65
mg/kg) (28) and the gross brain
structure evaluated using a 3.0 T MRI scanner equipped with a
special coil (DISCOVERY MR750; Cytiva). As described in a previous
study (29), a predetermined
central voxel was used for registration of diffusion-weighted
images. Conventional coronal T2-weighted (T2W) images were obtained
with the following parameters: Repetition time (TR)/echo time (TE),
3,500/85 msec; number of excitations (NEX), 2; phase, 256;
frequency, 320/sec; slices, 21; slice thickness, 1.5 mm; field of
view, 80 mm; and acquisition time, 2 min. Then, 7 days post-SAH,
cerebral blood flow (CBF) was assessed using CBF-arterial spin
labeled (ASL), as the vasospasm was most severe at 7 day after SAH.
An axial pseudocontinuous 3D ASL sequence was also performed using
the following parameters: TE, 11.1 msec; TR, 4,326 msec; field of
view, 240x240 mm; and section thickness, 3 mm, spiral readout. Maps
of CBF were generated using Functool software v. 4.5.3 (Cytiva).
1H-magnetic resonance spectra were also obtained via
multivoxel pattern analysis with the following parameters: TR/TE,
1,500/35 msec; NEX, 1; phase, 18; frequency, 16/sec; and
acquisition time, 7 min 20 sec.
The ventromedial PFC (vmPFC) in T2W and spectral
images were further analyzed for infarcts by calculating the ratio
of average signal intensity (SI) relative to the temporalis [T2W
standardized SI (SSI)]. In addition, the ratio of N-acetylaspartate
(NAA) to creatinine (Cr) peak area was calculated to assess
neuronal metabolism and integrity using the AW 4.5 Workstation
(Cytiva). The positions of NAA and Cr (as an internal spectral
reference) on the nuclear spectrum were 2.02 and 3.05 part per
million, respectively.
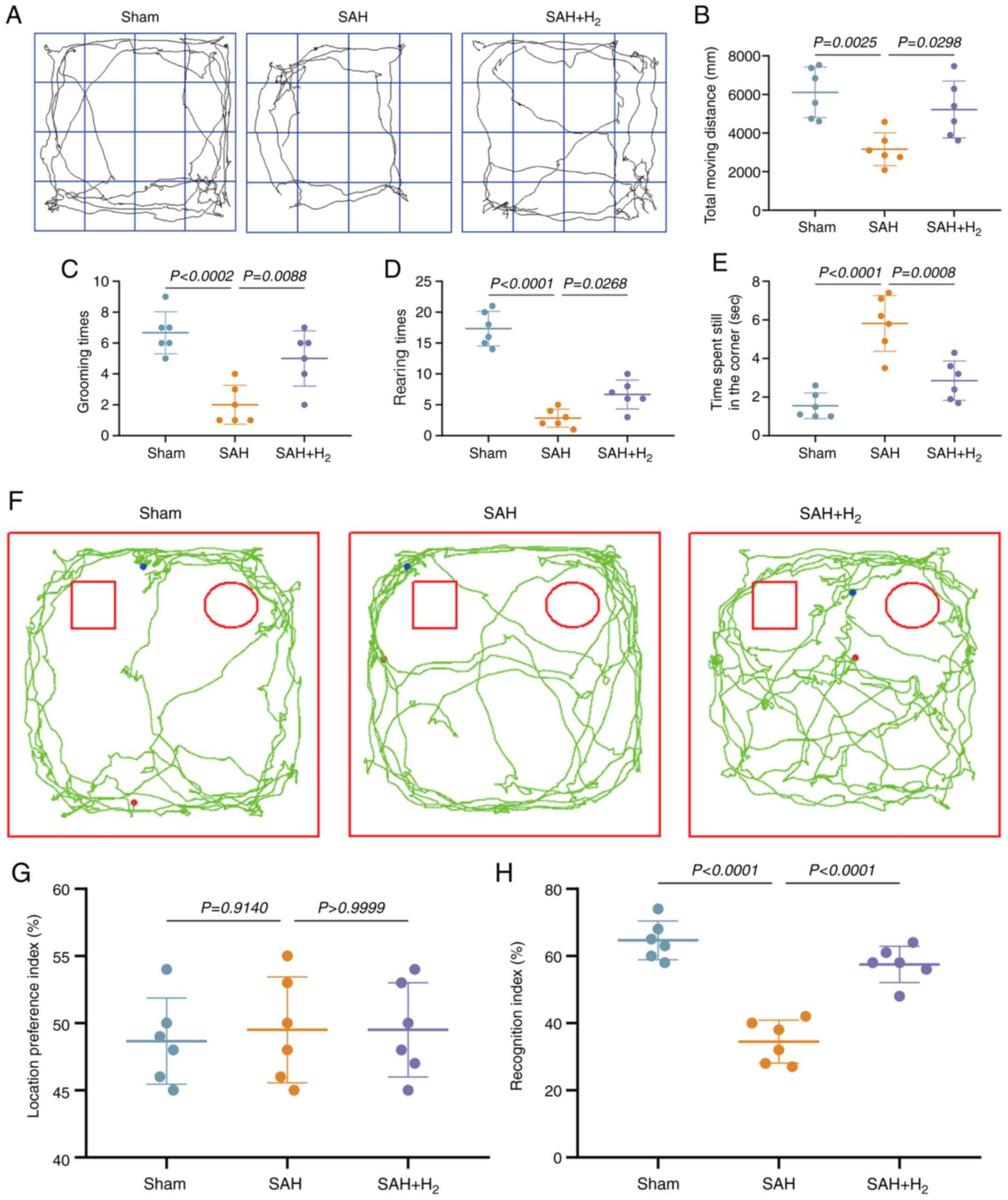
Open field test (OFT)
Considering the motor deficits between rats,
long-term anxiety was assessed using an OFT, which was performed at
30 days after SAH (30). The
experimental apparatus was a large open field box (60x60x40 cm)
divided into 16 equal squares. The test was conducted 30 day
post-SAH. An individual rat was placed in the center of the box,
and the distance traveled, grooming and rearing times, and time
spent in the corners were recorded over a 90-sec test period and
analyzed with a computerized tracking system (XR-XZ301; Shanghai
Xinruan Software Co., Ltd.).
Novel object recognition (NOR)
The NOR test (30)
was conducted in an empty white box (60x60x40 cm). During the
adaptation phase, individual rats were permitted to freely explore
the box for 5 min/day for 2 consecutive days. In the
familiarization phase, individual rats were placed in the same box
with two identical objects (tasteless, not smooth) placed in the
left and right corners (5 cm from the walls) and allowed to freely
investigate both until they had explored each for a total of 30 sec
(defined as contact by the front paws or nose). The testing phase
was conducted after 24 h. Each rat was placed in the same box with
one of the two familiar objects replaced by a novel object. At this
stage, continuous monitoring was conducted for 10 min and
preference for the novel object relative to the familiar object was
calculated as an index of recognition memory according to the
equation: Recognition index (RI)=Novel object exploration
time/(Novel object exploration time + Familiar object exploration
time).
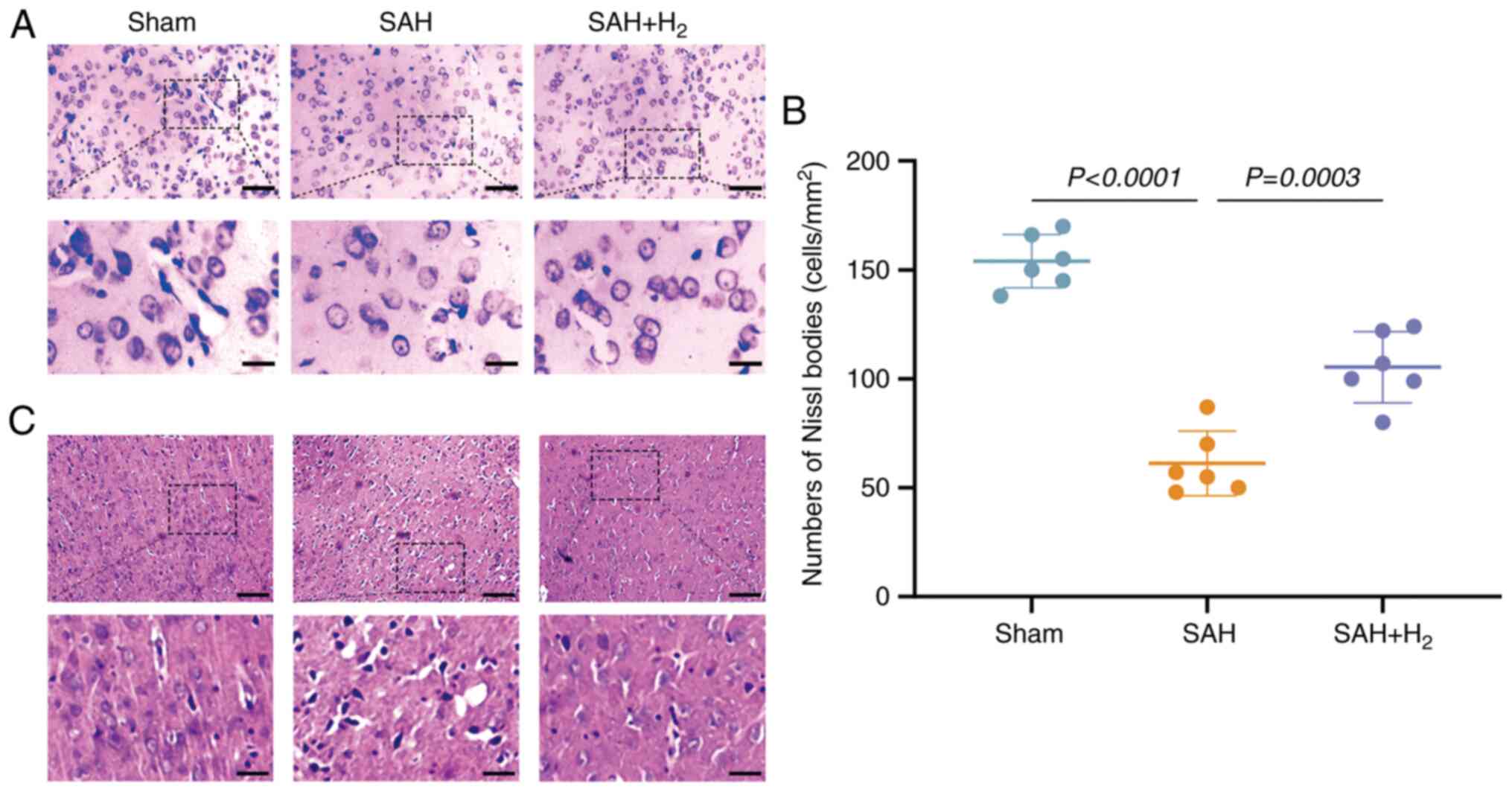
H&E and Nissl staining
At 30 days post-SAH, pathological changes in the
vmPFC were evaluated via H&E and Nissl staining of coronal
brain slices. Briefly, brains were fixed with 10% neutral-buffered
formalin for 48 h at room temperature, embedded in paraffin, slices
at 4 µm, deparaffinized for 10 min and were stained with
hematoxylin for 5 min and eosin for 10 sec at 25˚C. For Nissl, the
slices were stained as described (C0117; Beyotime Institute of
Biotechnology) for 10 min at room temperature. As Nissl bodies are
indicative of protein synthesis, reduced numbers are a sign of
nerve cell injury (31). Image-Pro
Plus 6.0 (Media Cybernetics, Inc.) was used to count and analyze
the number of Nissl-stained cells in six randomly selected fields
per animal at magnification of x200 under a light microscope (BX51;
Olympus Corporation).
Immunofluorescence
At 24 h post-surgery, the vmPFC was fixed with 10%
neutral-buffered formalin at room temperature for 48 h, embedding
in paraffin, sectioned at 4-µm thickness, dewaxed in xylene,
hydrated in gradient ethanol at room temperature and heated in
sodium citrate for 20 min at 100˚C. Slices were then incubated
overnight at 4˚C with polyclonal rabbit anti-rat p38 MAPK antibody
(1:100; cat. no. AF5887; Beyotime Institute of Biotechnology) and
polyclonal mouse anti-rat-neuronal nuclei (NeuN; 1:200; cat. no.
ab104224; Abcam). Slices were washed three times with PBS and
incubated with Cy3-conjugated goat anti-rabbit IgG (1:500; cat. no.
A0516; Beyotime Institute of Biotechnology) and FITC-conjugated
goat anti-mouse IgG (1:500; cat. no. A0568; Beyotime Institute of
Biotechnology) for 1 h at 25˚C. Finally, cell nuclei were
counterstained with 5 µg/ml DAPI (cat. no. P0131; Beyotime
Institute of Biotechnology) for 3 min at 25˚C. A fluorescence
microscope (MF43; Guangzhou Micro-shot Technology Co., Ltd.) was
used to observe the sections.
TUNEL staining was used to identify apoptotic
neurons. Slices prepared as described above were dewaxed, washed
three times in PBS and incubated in 20 µg/ml protease K (cat. no.
st533; Beyotime Institute of Biotechnology) at 37˚C for 35 min.
Then, the slices were incubated with polyclonal mouse anti-rat NeuN
(1:200; cat. no. ab104224; Abcam) overnight at 4˚C, following by
washing with PBS and incubation with TDT enzyme containing
fluorescent labeling solution (cat. no. C1088; Beyotime Institute
of Biotechnology) in the dark for 60 min at 37˚C. TUNEL-stained
slices were then treated with 5 µg/ml Antifade Mounting Medium with
DAPI (cat. no. P0131; Beyotime Institute of Biotechnology) for 3
min at 25˚C. Apoptotic neurons were identified by overlapping
TUNEL, NeuN and DAPI staining. A fluorescence microscope (MF43;
Guangzhou Micro-shot Technology Co., Ltd.) was used to count
stained (apoptotic) cells in six fields (magnification, x200) of
six sections randomly selected from each rat. The average density
of apoptotic cells was determined using Image-pro plus 6.0 (Media
Cybernetics, Inc.).
Western blotting
At 24 h post-surgery, total protein from the vmPFC
was extracted in lysis buffer (cat. no. P0013; Beyotime Institute
of Biotechnology), and the concentration measured using a BCA
Protein Assay kit (cat. no. P0012S; Beyotime Institute of
Biotechnology). Equal amounts of protein per gel lane (30 µg) were
separated using 10% polyacrylamide gels and transferred to PVDF
membranes. Membranes were blocked in QuickBlock™
Blocking Buffer (Beyotime Institute of Biotechnology) at 25˚C for
10 min, and probed overnight at 4˚C with rabbit anti-rat Bax
antibody (1:500; cat. no. AB016; Beyotime Institute of
Biotechnology), rabbit anti-Bcl-2 antibody (1:500; cat. no.
K003505P; Beijing Solarbio Science & Technology Co., Ltd.),
anti-caspase-3 antibody (1:1,000; cat. no. ab13847; Abcam), rabbit
anti-rat phosphorylated (p)-p38 MAPK antibody (1:1,000; cat. no.
AF5887; Beyotime Institute of Biotechnology), rabbit anti-p38 MAPK
antibody (1:1,000; cat. no. AF7668; Beyotime Institute of
Biotechnology), rabbit anti-p-JNK1/2 (1:1,000; cat. no. AF1762;
Beyotime Institute of Biotechnology), rabbit anti-JNK1/2 (1:1,000;
cat. no. AF1048; Beyotime Institute of Biotechnology), rabbit
anti-p-AKT (1:500; cat. no. AA329; Beyotime Institute of
Biotechnology), rabbit anti-AKT (1:500; cat. no. AA326; Beyotime
Institute of Biotechnology) and anti-GAPDH (1:1,000; cat. no.
K106389P; Beijing Solarbio Science & Technology Co., Ltd.),
which was used as the loading control. Blotted membranes were then
incubated in horseradish peroxidase-labeled goat anti-rabbit
secondary antibodies (1:1,000; cat. no. A0208; Beyotime Institute
of Biotechnology) at room temperature for 1 h. After three washes
in TBS-Tween-20 (0.05%; cat. no. ST825; Beyotime Institute of
Biotechnology), slices were incubated with ECL reagent (cat. no.
P0018FM; Beyotime Institute of Biotechnology) for 5 min at room
temperature, and protein bands detected using a western blot
detection system (Gel Doc XRS; Bio-Rad Laboratories, Inc.) and
quantified by Image Lab software 6.0.1 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All statistical analyses were conducted using SPSS
20.0 (IBM Corp). Group data are presented as the mean ± SD. The
log-rank test of Kaplan-Meier analysis followed by a Bonferroni's
test for correction (K=3) was performed to assess the percentage
survival of SAH model rats. In the Kaplan-Meier analysis, if a
P-value was less than the Bonferroni-corrected threshold
(P<0.016), then the comparison can be said to be statistically
significant. A Mann-Whitney U test was used to assess the
difference of SAH grade between groups. The Levene test was
conducted to check the assumption of homogeneity of variance.
Statistical differences among groups were then evaluated via
one-way ANOVA followed by Bonferroni's post hoc tests for pair-wise
comparisons. P<0.05 (two-tailed) was considered to indicate a
statistically significant difference for all tests.
Results
H2 post-conditioning
mitigates the neuroimaging manifestations of SAH-induced neural
damage
In total, 21 rats died before the scheduled
euthanasia time. None of them were sham-operated animal (0 of 36
rats). The mortality in the SAH group was 26.2% (16 of 61 rats;
sham vs. SAH, P<0.016; Fig. 2A),
which was consistent with previous study (32). However, the mortality dropped to
10.2% (5 of 49 rats) in the SAH + H2 group compared with
the SAH group (P>0.016; Fig.
2A). The death of rat all occurred with 5 h after SAH, and the
cause of death was cerebral hernia induced by bleeding volume. In
total, 17 rats with mild SAH were excluded from this study. The
rats with moderate and severe SAH were included for the following
studies. There was no difference in the average SAH grades between
the SAH and SAH + H2 groups. (P>0.05; Fig. 2B).
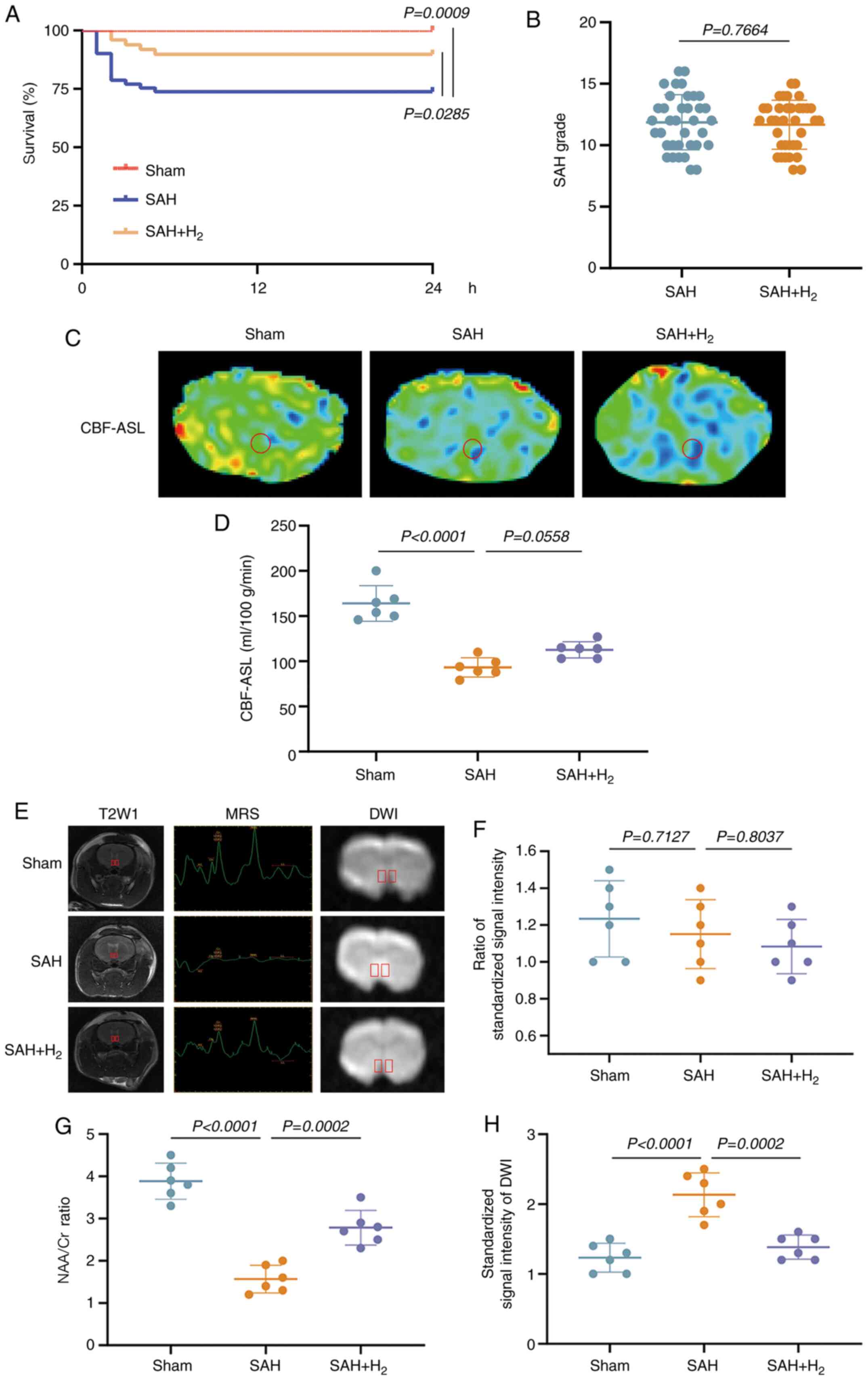 | Figure 2H2 post-conditioning
mitigates the neuroimaging manifestations of SAH-induced neuronal
damages. (A) Survival analysis during 24 h after SAH (n=36 in sham
group, n=61 in SAH group, n=49 in SAH + H2 group). (B)
SAH grade of the SAH group (n=36) and SAH + H2 group
(n=36). (C) CBF-ASL coronal views at 7 day post-SAH. (D) CBF-ASL
value in the region of the vmPFC caused by the indicated stimuli.
(E) Representative T2W MRI, 1H MR spectra and DWI of the vmPFC in
the coronal view at 30 day post-SAH (n=6). The (F) ratio of
standardized signal intensity, (G) the ratio of NAA/Cr and (H) the
signal intensity of DWI in the region of the vmPFC caused by the
indicated stimuli. SAH, subarachnoid hemorrhage; CBF-ASL, cerebral
blood flow arterial spin labeling; NAA, N-acetylaspartate; Cr,
creatinine; T2W, T2-weighted; DWI, diffusion weighted imaging;
vmPFC, ventromedial prefrontal cortex. |
At 7 days post-SAH, the absolute CBF-ASL map
(Fig. 2C) demonstrated that the
vmPFC perfusion in SAH and SAH + H2 groups was
significantly reduced (vs. sham, P<0.05; Fig. 2D). vmPFC perfusion between both the
SAH and SAH + H2 groups did not show significant
difference, indicating that H2 inhalation did not
improve vasospasm.
At 30 days post-surgery, T2W MRI revealed no
significant differences in SSI ratios among sham, SAH and SAH +
H2 groups (P>0.05; Fig.
2E and F). However,
1H MRS at 30 days post-surgery revealed a significant
decrease in the vmPFC NAA/Cr metabolite ratio among SAH group rats
compared with sham group rats, which was partially reversed by
H2 inhalation (P<0.05; Fig. 2E and G). The result indicated the signal
intensity of diffusion weighted imaging in the vmPFC was increased
in the SAH and SAH + H2 groups, but compared with SAH
group, the signal intensity was decreased in SAH + H2
group (P<0.05; Fig. 2E and
H).
H2 inhalation alleviates
anxiety-like behavior following SAH
The OFT is a well-established method to measure
anxiety-like behavior in rodents (33). At 30 days post-surgery, SAH group
rats showed reduced total travel distance, spent more time in the
corners of the open field and less time in the center region, with
fewer grooming times and fewer rearings compared with sham group
rats (P<0.05; Fig. 3A-E), which
are all behavioral signs of anxiety. By contrast, all of these
indices of anxiety were partially reversed by H2
inhalation (SAH + H2 vs. SAH; P<0.05; Fig. 3).
H2 alleviates cognitive
impairment following SAH
The NOR test exploits the innate tendency of animals
to preferentially explore a novel object over a familiar object
(34). In the familiarization phase
conducted 30 days post-surgery, the total time spent in proximity
of the two identical objects did not differ, yielding a RI of ~50%
in all groups (P>0.05; Fig. 3F
and G). In the testing phase,
however, SAH group rats spent significantly less time in the
defined zone surrounding the novel object compared with sham group
rats (P<0.05; Fig. 3F and
H), and spent more time in the zone
surrounding the familiar object, indicating a deficit in
recognition memory (lower RI). Inhalation of H2
following SAH significantly increased the RI compared with SAH
group rats, indicating partial preservation of recognition memory
(P<0.05; Fig. 3H).
H2 inhalation reduces
neuronal damage following SAH
Nissl staining of vmPFC tissue from sham group rats
revealed structurally intact neurons with the expected somal
distribution and abundant Nissl bodies, while vmPFC neurons in the
SAH group were disorganized, reduced in number and contained fewer
Nissl bodies (vs. sham; P<0.05; Fig.
4A and B). These signs of
neurodegeneration were partially reversed by H2
inhalation following SAH (P<0.05; Fig. 4A and B). In addition, H&E staining was
performed to assess the effect of H2 administration, and
the data revealed that H2 administration alleviated
histological impairments in the vmPFC induced by SAH exposure
(Fig. 4C).
H2 reduces neuronal
apoptosis following SAH
To investigate the neuroprotective efficacy of
H2 inhalation following SAH, neuronal apoptosis in the
vmPFC was assessed via TUNEL staining (Fig. 5A and C). At 24 h post-surgery, the
TUNEL-positive cell number was greater in the SAH group compared
with the sham group (P<0.05; Fig.
5A and C), and this increase
was partially reversed by H2 inhalation (SAH +
H2 vs. SAH; P<0.05; Fig.
5A and C).
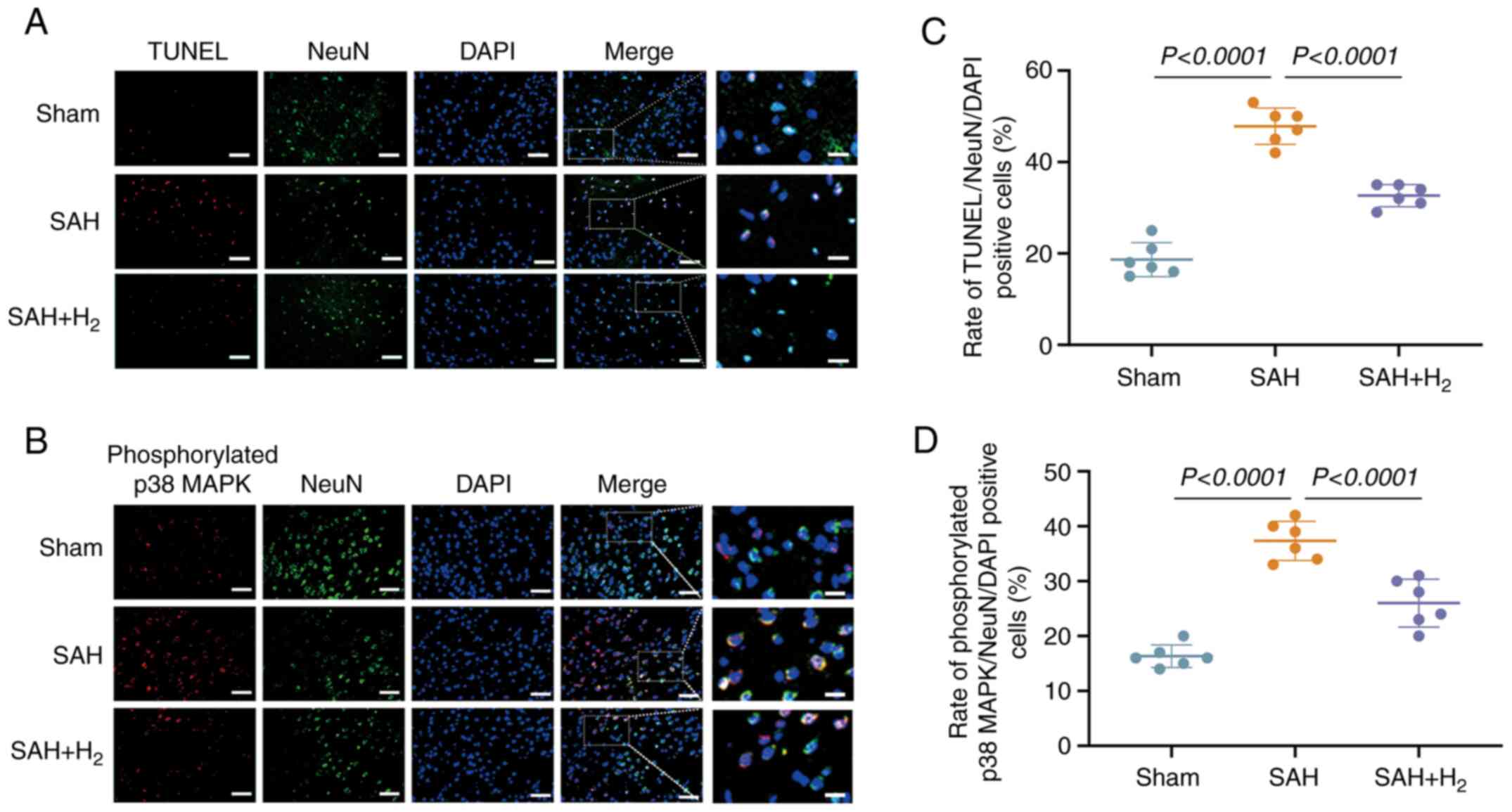 | Figure 5H2 post-conditioning
reduces neuronal apoptosis and alters SAH-induced
phosphorylated-p38 MAPK in neurons. (A) Representative
photomicrographs of NeuN and TUNEL staining (NeuN, green; TUNEL,
red; DAPI, blue), showing apoptotic neurons in the vmPFC on 24 h
after SAH (n=6). Scale bar, 50 or 15 µm. (B) Representative
photomicrographs of NeuN and phosphorylated p38 MAPK staining
(NeuN, green; p38 MAPK, red; DAPI, blue), showing phosphorylated
p38 MAPK-positive neurons in the vmPFC on 24 h after SAH (n=6).
Scale bar, 50 or 15 µm. (C) Rate of apoptotic neurons in the vmPFC,
induced by the indicated stimuli. (D) Rate of phosphorylated p38
MAPK-positive neurons in the vmPFC, induced by the indicated
stimuli. SAH, subarachnoid hemorrhage; vmPFC, ventromedial
prefrontal cortex; NeuN, neuronal nuclei. |
H2 inhalation inhibits the
phosphorylation of p38 MAPK in neuronal cells
Immunostaining revealed the increased expression of
p-p38 MAPK in NeuN-positive cells at 24 h post-SAH compared with
the sham group (P<0.05; Fig. 5B
and D), a response significantly
attenuated by H2 inhalation. Western blotting (Fig. 6A) also demonstrated the increased
expression levels of p-p38 MAPK (P<0.05; Fig. 6B), p-JNK1/2 (P<0.05; Fig. 6C), p-AKT (P<0.05; Fig. 6D) and cleaved caspase-3 (P<0.05;
Fig. 6E) in the SAH group, as well
as an elevated Bax/Bcl-2 ratio (vs. sham; P<0.05; Fig. 6F). Again, each of these changes were
reversed by H2 inhalation following SAH.
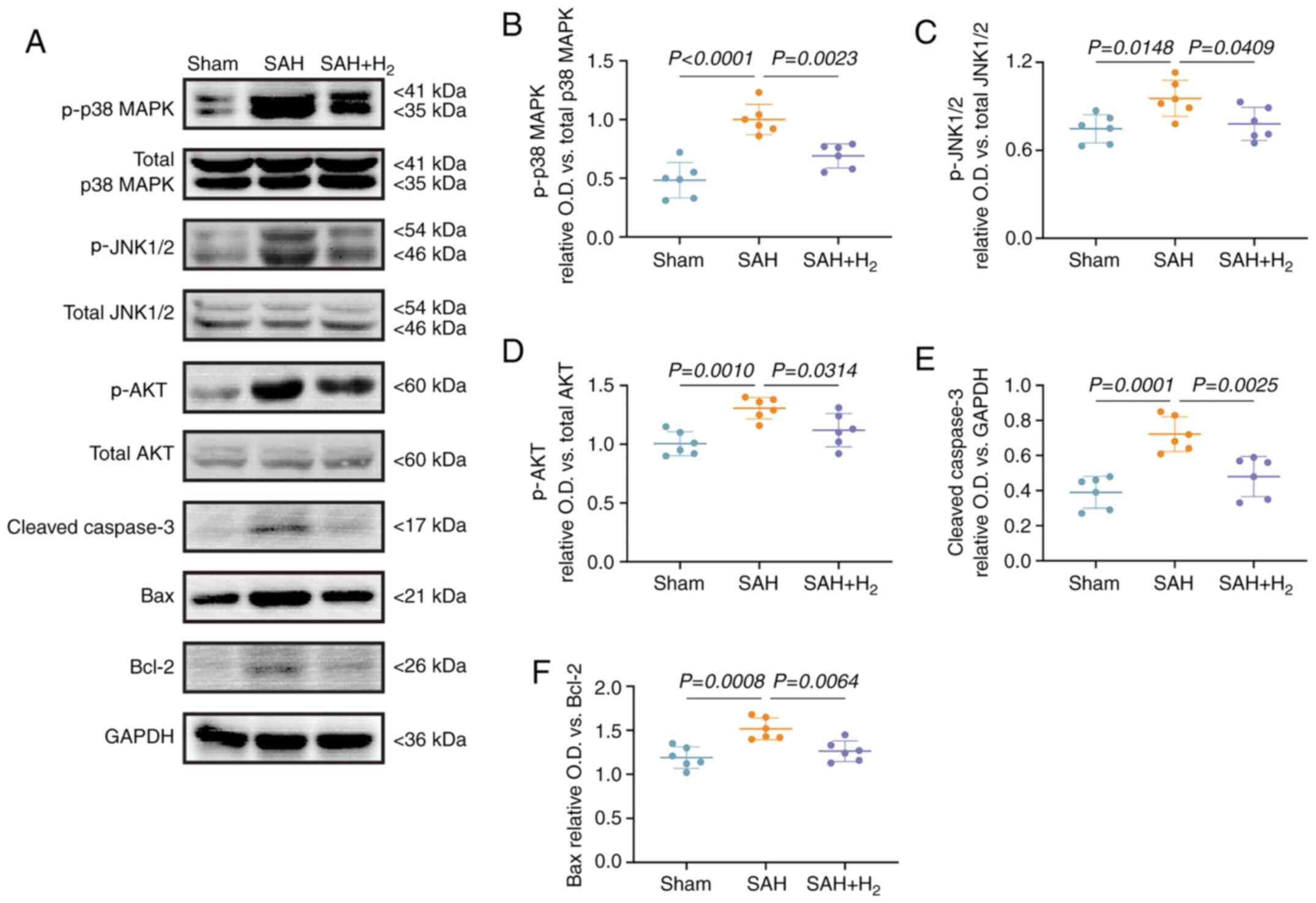 | Figure 6H2 post-conditioning
inhibits the phosphorylation of p38 MAPK in neuronal cells. (A)
Representative results of immunoblotting of p-p38 MAPK, total p38
MAPK, p-JNK1/2, total JNK1/2, p-AKT, total AKT (a second band of
total Akt may be a non-specificity band), cleaved caspase-3, Bax
and Bcl-2 in the vmPFC at 24 h post-SAH (n=6). (B) The ratio of
p-p38 MAPK/total p38 MAPK. (C) The ratio of p-JNK1/2/total JNK1/2.
(D) The ratio of p-AKT/total AKT. (E) The protein relative
expression level of cleaved caspase-3. (F) The ratio of Bax/Bcl-2.
SAH and H2 are as described previously. SAH,
subarachnoid hemorrhage; p-, phosphorylated; O.D., optical
density. |
Discussion
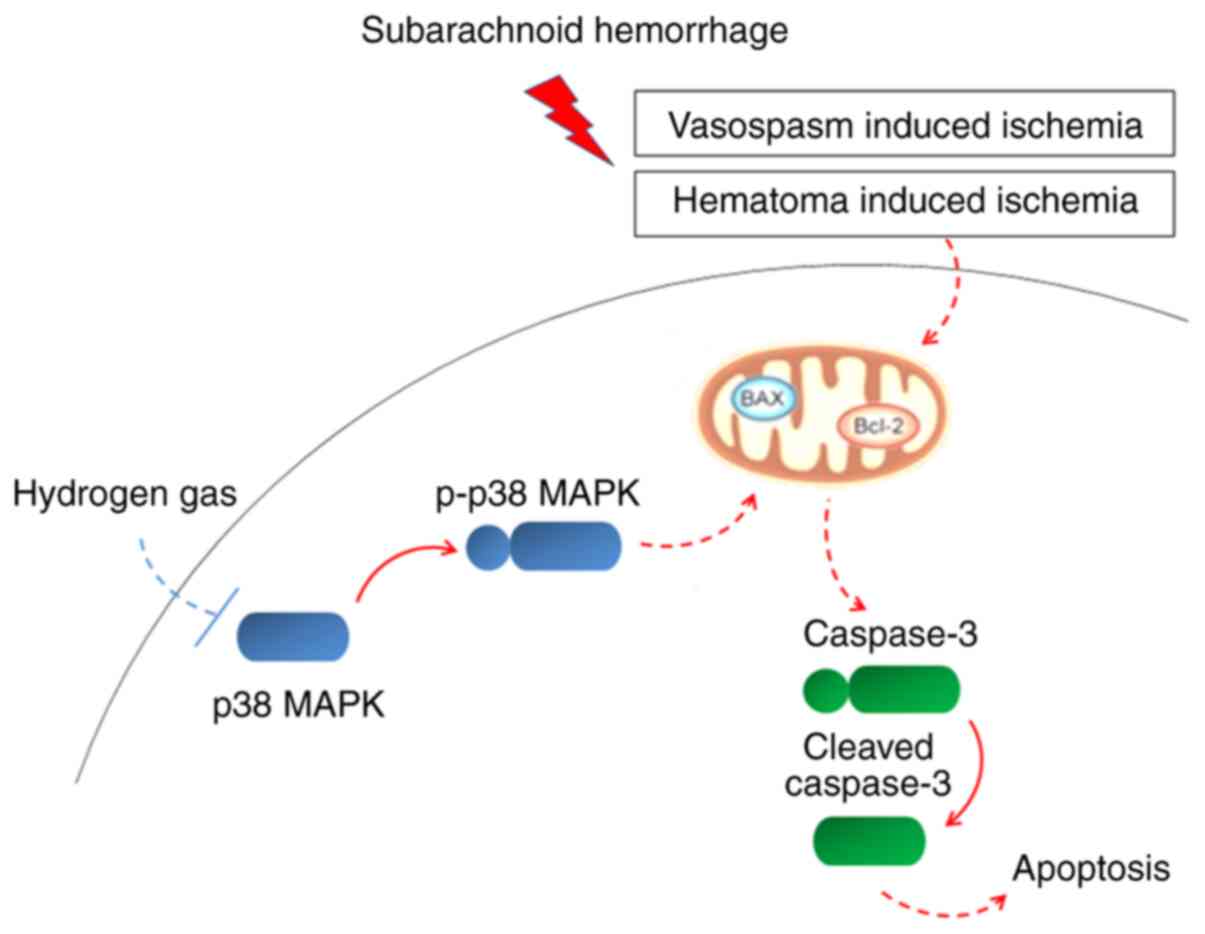
In this current study, it was demonstrated that
relatively brief H2 inhalation following experimental
SAH could mitigate metabolic disruption, long-term recognition
memory impairment, anxiety-like behaviors and neuronal apoptosis in
PFC, possibly by inhibiting p38 MAPK activity (Fig. 7).
Previous studies have reported that a single and
double blood injection into cisterna magna, as well as endovascular
perforation, can be used to establish a rodent model of SAH
(35,36). Compared with injections of arterial
blood solvate into the cisterna magna, a rat model of endovascular
perforation was performed in this current study due to a good
consistency with the pathophysiological process of SAH (37). Neurological damage following SAH may
result in cognitive decline and psychiatric disorders, such as
anxiety and depression, which are major obstacles to rehabilitation
and recovery (4). Previous studies
have shown that rodent models of SAH were impaired in spatial
memory as evidenced by longer escape latencies and swim paths in
the Morris water maze test (38)
and exhibited long-lasting state anxiety (39,40).
Considering motor deficits post-SAH, the present study assessed
cognitive dysfunction and anxiety-like behavior as long-term
behavioral changes at 30 days post-SAH. The current object
recognition and OFT results were consistent with these previous
studies (41,42) and underscore the utility of this
rodent model for investigation of SAH pathogenesis, behavioral
impairments and potential treatment strategies. Furthermore,
neuronal damage in the vmPFC is strongly associated with cognitive
dysfunction and emotional changes following SAH (43,44),
and the present study identified significant neurocellular
abnormalities in this region. While there were no significant
differences in vmPFC SSI between sham and SAH groups, the NAA
signal, a general marker of neuronal integrity and viability, was
decreased after SAH exposure. The NAA pool is considered to act as
a reservoir for glutamate synthesis that allows the brain to
maintain lower glutamate concentrations and thereby reduce the risk
of excitotoxicity (29).
Collectively, these results suggested that long-term cognitive
dysfunction and emotional changes induced by SAH may be associated
with neuronal death in the vmPFC.
Recently, H2 administration was reported
to attenuate neuronal injury in models of ischemia/reperfusion
injury and hemorrhage-associated stoke (45,46).
In the current study, 2.9% H2 treatment for 2 h after
SAH, chosen based on previous studies (47,48)
and our preliminary experiments, ameliorated cognitive dysfunction
and anxiety-like behavior, as well as increased the number of Nissl
bodies and elevated the NAA/Cr ratio. Interestingly, a previous
study reported that H2 only improves neuronal apoptosis
at 24 h after SAH, but not 48 h. However, the present study found
significant improvements in neuronal apoptosis in the vmPFC, which
was different from previous studies (10,25).
As indicated by a previous study (49), 7 day post-SAH was the most severe
period of cerebral vasospasm after SAH. In the present study, it
was noted that there was no change of cerebral blood flow indicated
by CBF-ASL after H2 administration 7 day post-SAH, which
was consistent with previous study (50). It has been shown that apoptosis is a
form of programmed cell death dependent on activation of caspase-3
and is regulated by a relative balance between pro- and
anti-apoptotic factors, such as Bax and Bcl-2(51). The decrease in the apoptotic rate
following H2 inhalation was associated with reduced
expression of cleaved (active) caspase-3 and Bax, and upregulated
expression of Bcl-2, as measured via western blotting, which was
consistent with a widespread reduction in apoptotic rate throughout
the vmPFC. Thus, the present study revealed that H2
prevented long-term cognitive and emotional dysfunction following
SAH by preventing neuronal apoptosis in the vmPFC, but not
inhibiting cerebral vasospasm.
Activation of the p38 MAPK signal pathway can
trigger apoptosis via a variety of downstream pathways, such as p53
phosphorylation (52), activation
of caspase cascades and inhibition of anti-apoptotic proteins, such
as Bcl-2(53). The phosphorylation
of p38 MAPK was reported to accelerate caspase-3 cleavage and
increase the Bax/Bcl-2 ratio in a model of traumatic brain injury
(54). The present study
demonstrated that H2 could reverse the increase in p-p38
MAPK expression by vmPFC neurons. Moreover, it was found that
H2 downregulated the expression levels of p-JNK1/2 and
p-AKT in the vmPFC. Previous studies also suggested that inhibition
of JNK1/2 and AKT activations may be involved in the anti-apoptotic
ability of H2 (55,56).
Thus, besides JNK1/2 and AKT signaling, inhibition of p38 MAPK
signaling is also a plausible mechanism for H2-dependent
neuroprotection following SAH. The molecular mechanisms for this
effect, including the contributions of antioxidant activity,
require further study. Collectively, the current data indicated
that single administration of H2 could suppress neuronal
apoptosis via inhibition of p38 MAPK signaling.
Limitations of the present study include the single
administration protocol and focus on the vmPFC. Future studies are
required to define additional efficacious administration regimens,
to determine whether later post-insult administration is also
effective, as this has significant implications for clinical
treatment, and to examine if H2 is neuroprotective in
other regions associated with cognition and emotion, such as the
hippocampus and amygdala. In addition, the current study did not
examine the expression levels of other MAPK-related signaling
factors, such as MAPKKK, MKK3/6 and apoptosis signal-regulating
kinase 1, to define a more precise pathway between p38 MAPK
activation and apoptosis. The therapeutic effects of repeated
H2 administration post-SAH should be also
investigated.
In conclusion, H2 administered 2 h after
experimental SAH mitigates cognitive dysfunction, anxiety-like
behavior and neuronal apoptosis in the PFC, possibly by inhibiting
the p38 MAPK signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Plan of Cangzhou City (grant no. 172302117).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Design of the study: JHS. Editing the manuscript:
JHS and TPS. Statistical analysis: JHS. Experiments and data
collection: JHS, HYJ, TPS, ZBL and YPZ. JHS and HYJ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All study procedures and animal care protocols were
approved by the Animal Review Board of Cangzhou Central Hospital
(Cangzhou, China) and conformed to National Institutes of Health
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buunk AM, Spikman JM, Metzemaekers JDM,
van Dijk JMC and Groen RJM: Return to work after subarachnoid
hemorrhage: The influence of cognitive deficits. PLoS One.
14(e0220972)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wong GK, Lam SW, Ngai K, Wong A, Siu D,
Poon WS and Mok V: Cognitive Dysfunction after Aneurysmal
Subarachnoid Hemorrhage Investigators. Cognitive domain deficits in
patients with aneurysmal subarachnoid haemorrhage at 1 year. J
Neurol Neurosurg Psychiatry. 84:1054–1058. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Miller BA, Turan N, Chau M and Pradilla G:
Inflammation, vasospasm, and brain injury after subarachnoid
hemorrhage. Biomed Res Int. 2014(384342)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tang WK, Wang L, Kwok Chu Wong G, Ungvari
GS, Yasuno F, Tsoi KKF and Kim JS: Depression after subarachnoid
hemorrhage: A systematic review. J Stroke. 22:11–28.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mo J, Enkhjargal B, Travis ZD, Zhou K, Wu
P, Zhang G, Zhu Q, Zhang T, Peng J, Xu W, et al: AVE 0991
attenuates oxidative stress and neuronal apoptosis via
Mas/PKA/CREB/UCP-2 pathway after subarachnoid hemorrhage in rats.
Redox Biol. 20:75–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blackman RK, Crowe DA, DeNicola AL,
Sakellaridi S, MacDonald AW III and Chafee MV: Monkey prefrontal
neurons reflect logical operations for cognitive control in a
variant of the AX continuous performance task (AX-CPT). J Neurosci.
36:4067–4079. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang Y, Liu C, Wang J, Li Q, Ping H, Gao
S and Wang P: MiR-299-5p regulates apoptosis through autophagy in
neurons and ameliorates cognitive capacity in APPswe/PS1dE9 mice.
Sci Rep. 6(24566)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zare Z, Tehrani M, Zarbakhsh S,
Farzadmanesh H, Shafia S, Abedinzade M, Ghanaat A and Mohammadi M:
Effects of paraoxon exposure on expression of apoptosis-related
genes, neuronal survival, and astrocyte activation in rat
prefrontal cortex. Neurotox Res. 37:356–365. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang M, Shan H, Chang P, Wang T, Dong W,
Chen X and Tao L: Hydrogen sulfide offers neuroprotection on
traumatic brain injury in parallel with reduced apoptosis and
autophagy in mice. PLoS One. 9(e87241)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choi KS, Kim HJ, Do SH, Hwang SJ and Yi
HJ: Neuroprotective effects of hydrogen inhalation in an
experimental rat intracerebral hemorrhage model. Brain Res Bull.
142:122–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li S, Fujino M, Ichimaru N, Kurokawa R,
Hirano S, Mou L, Takahara S, Takahara T and Li XK: Molecular
hydrogen protects against ischemia-reperfusion injury in a mouse
fatty liver model via regulating HO-1 and Sirt1 expression. Sci
Rep. 8(14019)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao Y, Yang H, Chi J, Xu Q, Zhao L and
Yang W, Liu W and Yang W: Hydrogen gas attenuates myocardial
ischemia reperfusion injury independent of postconditioning in rats
by attenuating endoplasmic reticulum stress-induced autophagy. Cell
Physiol Biochem. 43:1503–1514. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yan M and Yu Y, Mao X, Feng J, Wang Y,
Chen H, Xie K and Yu Y: Hydrogen gas inhalation attenuates
sepsis-induced liver injury in a FUNDC1-dependent manner. Int
Immunopharmacol. 71:61–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yonamine R, Satoh Y, Kodama M, Araki Y and
Kazama T: Coadministration of hydrogen gas as part of the carrier
gas mixture suppresses neuronal apoptosis and subsequent behavioral
deficits caused by neonatal exposure to sevoflurane in mice.
Anesthesiology. 118:105–113. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bai X, Liu S, Yuan L, Xie Y, Li T, Wang L,
Wang X, Zhang T, Qin S, Song G, et al: Hydrogen-rich saline
mediates neuroprotection through the regulation of endoplasmic
reticulum stress and autophagy under hypoxia-ischemia neonatal
brain injury in mice. Brain Res. 1646:410–417. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xie K, Zhang Y, Wang Y, Meng X, Wang Y, Yu
Y and Chen H: Hydrogen attenuates sepsis-associated encephalopathy
by NRF2 mediated NLRP3 pathway inactivation. Inflamm Res.
69:697–710. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang P, Zhao M, Chen Z, Wu G, Fujino M,
Zhang C, Zhou W, Zhao M, Hirano SI, Li XK and Zhao L: Hydrogen gas
attenuates hypoxic-ischemic brain injury via regulation of the
MAPK/HO-1/PGC-1a pathway in neonatal rats. Oxid Med Cell Longev.
2020(6978784)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li D and Ai Y: Hydrogen saline suppresses
neuronal cell apoptosis and inhibits the p38 mitogen-activated
protein kinase-caspase-3 signaling pathway following cerebral
ischemia-reperfusion injury. Mol Med Rep. 16:5321–5325.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee Y, Kim YJ, Kim MH and Kwak JM: MAPK
cascades in guard cell signal transduction. Front Plant Sci.
7(80)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang G, He J, Ye X, Zhu J, Hu X, Shen M,
Ma Y, Mao Z, Song H and Chen F: β-Thujaplicin induces autophagic
cell death, apoptosis, and cell cycle arrest through ROS-mediated
Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma.
Cell Death Dis. 10(255)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen S, Ma Q, Krafft PR, Chen Y, Tang J,
Zhang J and Zhang JH: P2X7 receptor antagonism inhibits p38
mitogen-activated protein kinase activation and ameliorates
neuronal apoptosis after subarachnoid hemorrhage in rats. Crit Care
Med. 41:e466–e474. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li C, Liu Y, Tang P, Liu P, Hou C, Zhang
X, Chen L, Zhang L and Gu C: Hydrogen sulfide prevents
OGD/R-induced apoptosis by suppressing the phosphorylation of p38
and secretion of IL-6 in PC12 cells. Neuroreport. 27:230–234.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sehba FA: The rat endovascular perforation
model of subarachnoid hemorrhage. Acta Neurochir Suppl.
120:321–324. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang CS, Han Q, Song ZW, Jia HY, Shao TP
and Chen YP: Hydrogen gas post-conditioning attenuates early
neuronal pyroptosis in a rat model of subarachnoid hemorrhage
through the mitoKATP signaling pathway. Exp Ther Med.
22(836)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhan Y, Chen C, Suzuki H, Hu Q, Zhi X and
Zhang JH: Hydrogen gas ameliorates oxidative stress in early brain
injury after subarachnoid hemorrhage in rats. Crit Care Med.
40:1291–1296. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sugawara T, Ayer R, Jadhav V and Zhang JH:
A new grading system evaluating bleeding scale in filament
perforation subarachnoid hemorrhage rat model. J Neurosci Methods.
167:327–334. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kooijman E, Nijboer CH, van Velthoven CT,
Mol W, Dijkhuizen RM, Kesecioglu J and Heijnen CJ: Long-term
functional consequences and ongoing cerebral inflammation after
subarachnoid hemorrhage in the rat. PLoS One.
9(e90584)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marota JJ, Crosby G and Uhl GR: Selective
effects of pentobarbital and halothane on c-fos and jun-B gene
expression in rat brain. Anesthesiology. 77:365–371.
1992.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fu L, Zhang DX, Zhang LM, Song YC, Liu FH,
Li Y, Wang XP, Zheng WC, Wang XD, Gui CX, et al: Exogenous carbon
monoxide protects against mitochondrial DNA-induced hippocampal
pyroptosis in a model of hemorrhagic shock and resuscitation. Int J
Mol Med. 45:1176–1186. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Oh HM, Lee JS, Kim SW, Oh YT, Kim WY, Lee
SB, Cho YR, Jeon YJ, Cho JH and Son CG: Uwhangchungsimwon, a
standardized herbal drug, exerts an anti-depressive effect in a
social isolation stress-induced mouse model. Front Pharmacol.
10(1674)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Carriel V, Campos A, Alaminos M, Raimondo
S and Geuna S: Staining methods for normal and regenerative myelin
in the nervous system. Methods Mol Biol. 1560:207–218.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cohen SJ and Stackman RW Jr: Assessing
rodent hippocampal involvement in the novel object recognition
task. A review. Behav Brain Res. 285:105–117. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vuralli D, Wattiez AS, Russo AF and Bolay
H: Behavioral and cognitive animal models in headache research. J
Headache Pain. 20(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kangas BD and Bergman J: Touchscreen
technology in the study of cognition-related behavior. Behav
Pharmacol. 28:623–629. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ostrowski RP, Colohan AR and Zhang JH:
Neuroprotective effect of hyperbaric oxygen in a rat model of
subarachnoid hemorrhage. Acta Neurochir Suppl. 96:188–193.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Güresir E, Schuss P, Borger V and Vatter
H: Rat cisterna magna double-injection model of subarachnoid
hemorrhage-background, advantages/limitations, technical
considerations, modifications, and outcome measures. Acta Neurochir
Suppl. 120:325–329. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Okada T, Enkhjargal B, Travis ZD, Ocak U,
Tang J, Suzuki H and Zhang JH: FGF-2 attenuates neuronal apoptosis
via FGFR3/PI3k/Akt signaling pathway after subarachnoid hemorrhage.
Mol Neurobiol. 56:8203–8219. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kumagai K, Tomiyama A, Takeuchi S, Otani
N, Fujita M, Fujii K, Wada K and Mori K: New endovascular
perforation subarachnoid hemorrhage model for investigating the
mechanisms of delayed brain injury. J Neurosurg: Nov 22, 2019 (Epub
ahead of print). doi: 10.3171/2019.9.JNS191934.
|
|
39
|
Shen H, Chen Z, Wang Y, Gao A, Li H, Cui
Y, Zhang L, Xu X, Wang Z and Chen G: Role of neurexin-1β and
neuroligin-1 in cognitive dysfunction after subarachnoid hemorrhage
in rats. Stroke. 46:2607–2615. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Persson HC, Törnbom M, Winsö O and
Sunnerhagen KS: Symptoms and consequences of subarachnoid
haemorrhage after 7 years. Acta Neurol Scand. 140:429–434.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nanegrungsunk D, Ragozzino ME, Xu HL,
Haselton KJ and Paisansathan C: Subarachnoid hemorrhage in C57BL/6J
mice increases motor stereotypies and compulsive-like behaviors.
Neurol Res. 43:239–251. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Donatti AF, Soriano RN, Leite-Panissi CR,
Branco LG and de Souza AS: Anxiolytic-like effect of hydrogen
sulfide (H2S) in rats exposed and re-exposed to the
elevated plus-maze and open field tests. Neurosci Lett. 642:77–85.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Boyko M, Azab AN, Kuts R, Gruenbaum BF,
Gruenbaum SE, Melamed I, Brotfain E, Shapira Y, Cesnulis E and
Zlotnik A: The neuro-behavioral profile in rats after subarachnoid
hemorrhage. Brain Res. 1491:109–116. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen J, Liang J, Lin X, Zhang Y, Zhang Y,
Lu L and Shi J: Sleep deprivation promotes habitual control over
goal-directed control: Behavioral and neuroimaging evidence. J
Neurosci. 37:11979–11992. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Henricks AM, Berger AL, Lugo JM,
Baxter-Potter LN, Bieniasz KV, Petrie G, Sticht MA, Hill MN and
McLaughlin RJ: Sex- and hormone-dependent alterations in alcohol
withdrawal-induced anxiety and corticolimbic endocannabinoid
signaling. Neuropharmacology. 124:121–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen L, Chao Y, Cheng P, Li N, Zheng H and
Yang Y: UPLC-QTOF/MS-based metabolomics reveals the protective
mechanism of hydrogen on mice with ischemic stroke. Neurochem Res.
44:1950–1963. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen CH, Manaenko A, Zhan Y, Liu WW,
Ostrowki RP, Tang J and Zhang JH: Hydrogen gas reduced acute
hyperglycemia-enhanced hemorrhagic transformation in a focal
ischemia rat model. Neuroscience. 169:402–414. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang L, Applegate RL II, Applegate PM,
Boling W and Zhang JH: Inhalation of high concentration hydrogen
gas improves short-term outcomes in a rat model of asphyxia
induced-cardiac arrest. Med Gas Res. 8:73–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xu H, Changyaleket B, Valyi-Nagy T, Dull
RO, Pelligrino DA, Schwartz DE and Chong ZZ: The role of HMGB1 in
pial arteriole dilating reactivity following subarachnoid
hemorrhage in rats. J Vasc Res. 53:349–357. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kumagai K, Toyooka T, Takeuchi S, Otani N,
Wada K, Tomiyama A and Mori K: Hydrogen gas inhalation improves
delayed brain injury by alleviating early brain injury after
experimental subarachnoid hemorrhage. Sci Rep.
10(12319)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Park SH, Seong MA and Lee HY: p38
MAPK-induced MDM2 degradation confers paclitaxel resistance through
p53-mediated regulation of EGFR in human lung cancer cells.
Oncotarget. 7:8184–8199. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yang JS, Lin RC, Hsieh YH, Wu HH, Li GC,
Lin YC, Yang SF and Lu KH: CLEFMA activates the extrinsic and
intrinsic apoptotic processes through JNK1/2 and p38 pathways in
human osteosarcoma cells. Molecules. 24(3280)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tan Z, Chen L, Ren Y, Jiang X and Gao W:
Neuroprotective effects of FK866 against traumatic brain injury:
Involvement of p38/ERK pathway. Ann Clin Transl Neurol. 7:742–756.
2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Qiu X, Li H, Tang H, Jin Y, Li W, YuSun
PingFeng, Sun X and Xia Z: Hydrogen inhalation ameliorates
lipopolysaccharide-induced acute lung injury in mice. Int
Immunopharmacol. 11:2130–2137. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang Y, Wang L, Hu T, Wang F, Han Z, Yin
Z, Ge X, Xie K and Lei P: Hydrogen improves cell viability partly
through inhibition of autophagy and activation of PI3K/Akt/GSK3β
signal pathway in a microvascular endothelial cell model of
traumatic brain injury. Neurol Res. 42:487–496. 2020.PubMed/NCBI View Article : Google Scholar
|