Introduction
Cerebral ischemia-reperfusion (I/R) is a common
feature of ischemic stroke, and its pathogenesis is associated with
oxidative stress, apoptosis and the inflammatory response (1). A previous report demonstrated that
pyroptosis and necroptosis are commonly associated with the
inflammatory response (2-5).
Notably, both pyroptosis and necroptosis aggravate damage during
cerebral I/R and are implicated in the regulation of inflammation.
Gasdermin D (GSDMD), a molecule involved in the execution of
pyroptosis, is activated by the nucleotide-binding oligomerization
domain, leucine-rich repeat and pyrin domain containing 3 (NLRP3)
inflammasome. GSDMD is then cleaved, producing the GSDMD N-terminal
(GSDMD-N) fragment, which forms polymers and channels on the cell
membrane. Necroptosis activation begins with the recruitment of
receptor-interacting protein kinase (RIP)1 and RIP3 (2,3).
Subsequently, RIP3 phosphorylates mixed lineage kinase domain-like
protein (MLKL), and the resulting phosphorylated (p)-MLKL protein
oligomerizes on the cell membrane and forms channels (2,3).
Consequently, both pyroptosis and necroptosis lead to the emission
of pro-inflammatory factors or cell rupture through these channels,
which causes an inflammatory response (4,5) and
cerebral I/R injury (6-8).
Pyroptosis and necroptosis are reported to induce cerebral I/R, and
the inhibition of either one reduces cerebral I/R injury. This
indicates that the suppression of pyroptosis and necroptosis has
the potential to act as an important target for cerebral I/R injury
intervention (6-8).
In Traditional Chinese Medicine (TCM), it is
believed that the combination of the herbal TCMs Astragalus
membranaceus and Panax pseudoginseng nourish Qi and
activate blood circulation, and the combination is used to treat
cardiovascular and cerebrovascular diseases. This combination has
been demonstrated as clinically effective in the treatment of
stroke (9). The main components of
A. membranaceus and P. pseudoginseng are
astragaloside IV (AST IV) and P. notoginseng saponins (PNS),
respectively. These components strongly suppress inflammation in
cerebrally ischemic rats and alleviate cerebral I/R injury
(9-12).
However, whether AST IV and PNS effectively suppress pyroptosis and
necroptosis remains to be elucidated. In the current study, AST IV
and PNS were used to treat rats with middle cerebral artery
occlusion (MCAO) by neurological evaluation,
2,3,5-triphenyltetrazolium chloride (TTC) and hematoxylin and eosin
(H&E) staining and western blot analysis to investigate the
neuroprotective effects of these drugs and to elucidate their
underlying mechanisms.
Materials and methods
Animals
A total of 80 healthy male specific-pathogen-free
(SPF) Sprague-Dawley rats (age, 6-7 weeks; weight, 230-250 g) were
provided by Hunan Slake Jingda Experimental Animal Co., Ltd.
(production license no. 2017-0004). Animals were housed under SPF
conditions at the Experimental Animal Center of Hunan University of
Chinese Medicine on a 12-h light/dark cycle, at an ambient
temperature of 25±1˚C and 60% humidity. After 1 week of adaptive
feeding (12.5 g food/12 h), rats were made to fast with free access
to water for 12 h prior to the experiments.
Chemicals
AST IV (molecular formula,
C41H68O14; molecular weight, 784;
purity, ≥98.05%; cat. no. MUST-14102910), Notoginsenoside
R1 (cat. no. MUST-A0760), Ginsenoside Rg1
(cat. no. MUST-A0237), Ginsenoside Re (cat. no. MUST-A0244),
Ginsenoside Rb1 (cat. no. MUST-A0234), Ginsenoside Rd
(cat. no. MUST-A0245) andPNS (cat. no. DST200706-054) were
purchased from Chengdu Must Biotechnology Co., Ltd. TTC (cat. no.
BCBP3272V) was purchased from Sigma-Aldrich (Merck KGaA). Western
blotting buffer (cat. no. P0013) were purchased from Shanghai
Biyuntian Biotechnology Co., Ltd. Protease inhibitor cocktail
(without EDTA; 100X DMSO; cat. no B14002) and phosphate inhibitor
cocktail (100X; cat. no. B15002) were purchased from Bimake.com, and rabbit polyclonal anti-IL-1β (cat. no.
ab9787) and anti-IL-18 (cat. no. ab191860) were purchased from
Abcam. Rabbit polyclonal anti-caspase 1 (cat. no. NBP1-45433) and
anti-NLRP3 (cat. no. NBP2-12446) were purchased from Novus
Biologicals. Rabbit monoclonal anti-β-actin (cat. no. A2228) was
purchased from Sigma-Aldrich (Merck KGaA), and goat anti-rabbit
(cat. no. AP132P) and goat anti-mouse (cat. no. AP124P) secondary
antibodies were purchased from Merck KGaA. Embolism thread (cat.
no. 2636A2; head diameter, 0.36±0.02 mm) was purchased from Beijing
Xilong Technology Co., Ltd.
High-performance liquid chromatography
(HPLC)
For quantitative determination, standard stock
solutions of five pure saponins, including Notoginsenoside
R1, Ginsenoside Rg1, Ginsenoside Re,
Ginsenoside Rb1 and Ginsenoside Rd, were individually
prepared in methanol. The solutions were then mixed and diluted to
different concentrations for the standard curves. The air-dried PNS
was powdered in a mill and then immersed in 600 ml distilled water
at 50˚C for 30 min in a water bath. Following the addition of 4,000
ml absolute ethanol, the PNS was ultrasonically extracted twice for
1 h each. The temperature of the ultrasonic bath was maintained
(25±2˚C) with running water. Following filtration, the combined
ethanol extracts were concentrated to dryness under reduced
pressure in a rotary evaporator at 55˚C. The residue was
reconstructed in 100 ml distilled water. HPLC analysis was
performed on a Waters Alliance e2695 HPLC system (Waters
Corporation). The chromatographic separation was performed on a
reversed-phase Zorbax C8 column at a temperature of 35˚C. The
mobile phase consisted of acetonitrile (solvent A) and water
(solvent B), eluted with a gradient program as follows: 0-5 min,
15-30% A; 5-15 min, 30-32% A; 15-35 min, 32-32% A; 35-45 min,
32-38% A and 45-60 min, 38-50% A. The flow rate was maintained at
0.8 ml/min. The detection wavelength was set at 203 nm. The five
primary saponins were completely separated within 45 min without
obvious interference. The retention times of Notoginsenoside
R1, Ginsenoside Rg1, Ginsenoside Re,
Ginsenoside Rb1 and Ginsenoside Rd were 16.32, 23.45,
24.53, 36.57 and 40.13 min, respectively. The molecular structure,
compound identification, molecular formula and weight of the five
primary saponins were obtained from PubChem (pubchem.ncbi.nlm.nih.gov/).
Experimental groups and
procedures
Rats were randomly divided into five groups: i) Sham
group, ii) model group, iii) AST IV group, iv) PNS group and v)
combination group. A total of 120 rats were used, 8 of which died
due to unplanned hemorrhage during surgery; 7 died due to
intracranial hemorrhage and brain injury after surgery, and the
remaining rats survived until being euthanized. Each group
comprised 21 rats (8 for brain H&E staining, 8 for TTC staining
and 5 for western blot analysis). The dosage and method of
administration for AST IV (28 mg/kg) and PNS (80 mg/kg) alone or in
combination were based on previous studies (10,11,13).
Drugs were administered three times (10 ml/kg per time) by gavage
in all groups. Optimal administration times were 36 and 12 h before
model establishment and 12 h after model establishment, and the
sham and model groups received equal volumes of physiological
saline. Surgery was performed after anesthetizing rats with an
intraperitoneal (i.p.) injection of 10% chloral hydrate (300 mg/kg;
cat. no. 8MQ20-CC; Tixiai Huacheng Industrial Development Co.,
Ltd.). Rats exhibited no sign of peritonitis post-injection. A
total of 105 rats remained alive 24 h after surgery. Following
this, the rats were euthanized by i.p. injection of sodium
pentobarbital (140 mg/kg body weight). Death was monitored based on
cardiac activity and respiration. All efforts were taken to
minimize animal suffering and reduce the number of animals
used.
MCAO model establishment
The MCAO model was established as previously
described (13). Following
anesthetization, the right common carotid artery (CCA), external
carotid artery (ECA) and internal carotid artery (ICA) were
isolated through a neck incision. Surgical thread was used to
ligate the distal portion of the ECA, and ECA and its branches were
disconnected near the ligation site using electrocoagulation. A
slipknot was made to ligate the distal part of the ICA, and an
arterial clamp was used to clamp the distal ICA and CCA. A small
orifice was made in the ECA stump, and an embolism thread was
introduced into the ECA and directed into the ICA through the
bifurcation of the CCA. The artery clip that had been used to clamp
the distal ICA was removed, and the embolism thread was inserted
into the intracranial portion of the ICA at a distance of 18±2 mm
from the distal portion of the CCA bifurcation. When a slight
resistance to insertion of the thread into the anterior cerebral
artery was felt, insertion was stopped. Finally, the ICA was
clamped to prevent bleeding and movement, and the neck skin was
sutured. Following 2 h of blood flow blocking and a subsequent 24 h
of reperfusion, follow-up experiments were conducted. In the sham
group, the CCA, ECA and ICA were isolated without insertion of an
embolism thread.
Neurological deficit score (NDS)
test
Following 22 h reperfusion, the NDS of each rat in
each group was calculated, as previously described (13). Briefly, the characterization
procedure was as follows: 0, asymptomatic or no neurological
deficit; 1, when lifting the tail, the rats' left forelimb could
not be extended; 2, when walking, rats are circling to the
contralateral side; 3, when walking, rats are tilting to the
contralateral side; and 4, no spontaneous motor activity or loss of
consciousness.
TTC staining
Following 24 h of reperfusion, 8 rats/group were
anesthetized and subsequently euthanized. The whole brain was
rapidly removed from each rat, harvested and placed in a -20˚C
freezer for 20 min. From the beginning of the optic chiasm, the
coronal plane was sectioned at intervals of 2 mm, which yielded
exactly five slices. The slices were incubated in 2% TTC phosphate
buffer and immersed in a constant-temperature water bath in the
dark at 37˚C for 15 min, with stirring performed every 5 min. After
staining, brain slices were fixed with 4% paraformaldehyde at 4˚C
for 24 h. Non-ischemic regions appeared red, whereas infarcted
regions were pale. ImagePro Plus software version 6.0 (Media
Cybernetics, Inc.) was used to calculate the non-ischemic
red-stained TTC areas of the cerebral hemispheres on the normal and
ischemic sides of each slice of brain. The corrected ischemic area
value was obtained by subtracting the two areas in accordance with
a previously described method (14)
to eliminate the effect of cerebral edema on the infarction volume.
The area of each ischemic cerebral hemisphere was multiplied by its
thickness (2 mm) and this value was added to find the entire
approximate cerebral infarction volume. Finally, this approximate
value was divided by the normal hemisphere volume to calculate the
infarction volume rate. As previously described (14), the following formula was used to
calculate cerebral infarction volume rate: (Total contralateral
hemisphere area-ipsilateral hemisphere area)/(total contralateral
hemisphere area).
H&E staining
Following 24 h of reperfusion, 40 rats were
anesthetized and euthanized. Whole brains were fixed in 4%
paraformaldehyde overnight at room temperature and sliced into 2-mm
coronal sections on the optic cross-plane. The brains were
subsequently sliced to 3 µm thickness, dewaxed with xylene and
hydrated using a series of graded concentrations of ethanol (100, 5
min; 95, 1 min; 80, 5 min; 75% ethanol, 5 min; distilled water, 2
min). Following washing three times with distilled water for 5 min
each, sections were stained with hematoxylin for 10 min, washed
with running water for 10 min and counterstained with eosin for 5
min at room temperature. All sections were fixed on
polylysine-treated glass slides for H&E staining. The stained
sections were observed under a light microscope (magnification,
x400), and the cell damage ratio was calculated; five
non-overlapping visual fields were randomly selected for
observation of damaged cells in the ischemic cortex, and the total
count of quantified cells was ~300. Cell vacuolar degeneration,
eosinophilic degeneration, nuclear contraction and nuclear
dissolution were the main characteristics of damaged cells. The
damaged cells and total cells in each high-magnification field were
counted and the cell damage rate was calculated as follows, to
obtain a percentage: (number of damaged cells/total number of
cells) x100.
Western blot analysis
Western blotting was conducted to detect
NLRP3-induced activation of pyroptosis and necroptosis. The
cerebral ischemic penumbras of 5 rats from each group were weighed
and homogenized, and 100 mg of cerebral cortex from the ischemic
penumbra was added to 10X pyrolysis liquid with a phosphatase
inhibitor and protease inhibitor cocktail. The lysate was
centrifuged at 12,000 x g for 10 min at 4˚C, the pellet was
discarded and the protein concentration in the supernatant was
determined using a BCA assay. A total of 40 µg protein/lane were
separated by 8-15% SDS-PAGE and transferred to PVDF membranes (cat.
no. ISEQ00010; Merck KGaA). The membranes were blocked in 5%
non-fat milk in TBS-Tween-20 buffer (100 mM NaCl; 10 mM Tris-HCl;
pH, 7.4; 0.1% Tween-20) for 2 h at room temperature and
subsequently incubated overnight at 4˚C with anti-
apoptosis-associated speck-like protein containing a caspase
activation and recruitment domain (ASC; 1:500; cat. no. NBP1-45453;
Novus Biologicals), anti-NLRP3 (1:1,000), anti-IL-1β (1:1,000),
anti-IL-18 (1:1,000), anti-caspase-1 (1:500), anti-RIP1 (1:1,000;
cat. no. ab106393; Abcam), anti-RIP3 (1:1,000; cat. no. ab56164;
Abcam), anti-MLKL (1:1,000), anti-p-MLKL (1:1,000), anti-GSDMD
(1:1,000), anti-GSDMD-N (1:1,000) and anti-β-actin (1:5,000).
Following a wash with PBS, the membranes were incubated with
horseradish peroxidase-labeled secondary antibody (1:10,000) for 1
h at room temperature. Protein bands were detected using an
enhanced chemiluminescence detection kit (cat. no. 631701; Pierce;
Thermo Fisher Scientific, Inc.), and the integrated optical density
(IOD) of the target protein was determined using Quantity One 1-D
analysis software (Bio-Rad Laboratories, Inc.; version 4.6.8).
β-actin was used as the internal reference and the ratio of the
target protein IOD to the β-actin IOD was calculated.
Statistical analysis
The data were analyzed using SPSS software version
22.0 (IBM Corp.). All continuous data are expressed as the mean ±
standard deviation. Differences among the experimental groups were
evaluated using one-way ANOVA followed by Tukey's post hoc test to
compare the differences between groups. For NDS data, values are
presented as the median (interquartile range), and groups were
analyzed using Kruskal-Wallis test followed by Dunn's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
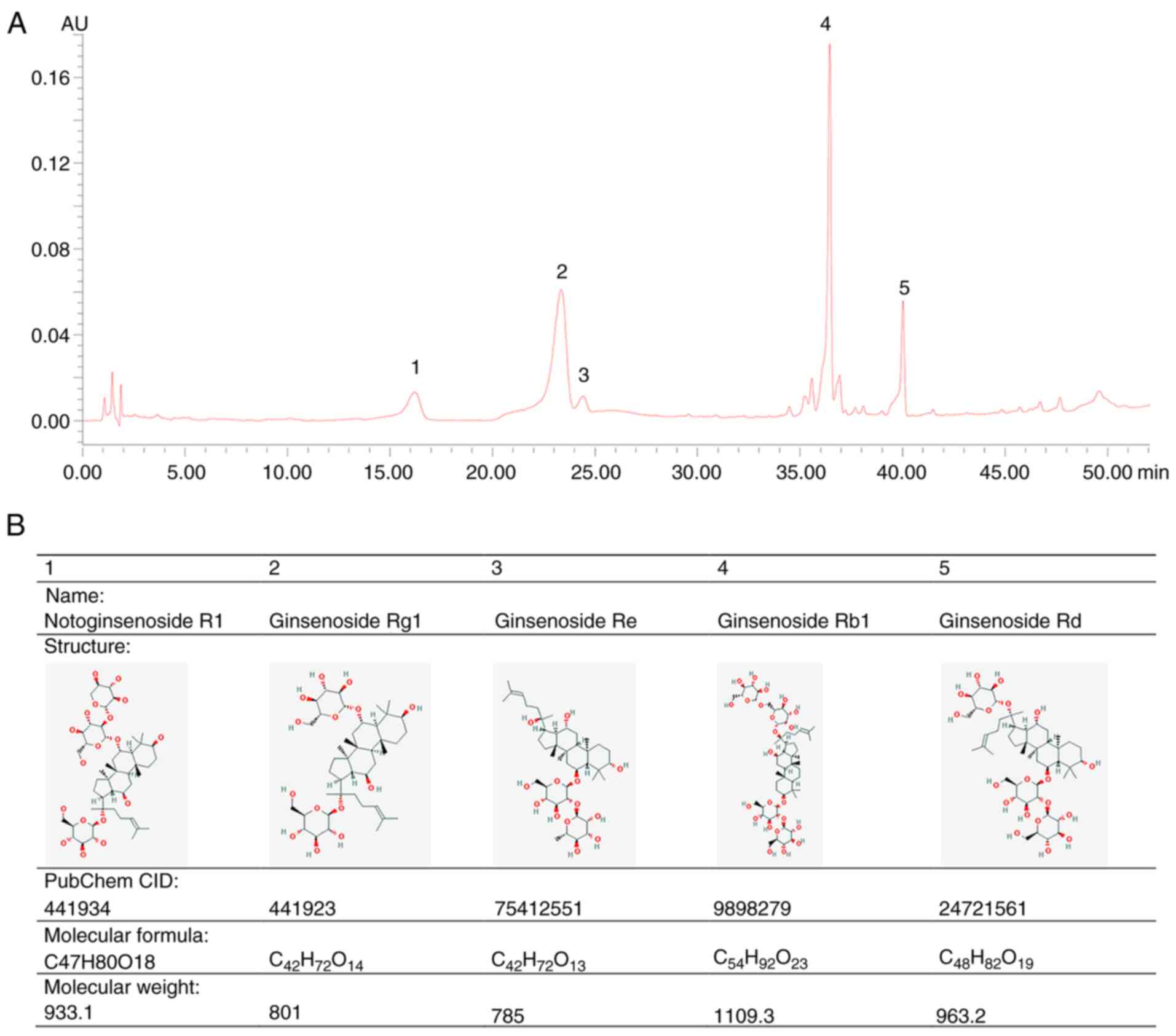
PNS component identification
HPLC was used to identify PNS compounds by measuring
the absorbance at 203 nm. The main saponins of PNS were completely
separated without interference. The major effective constituents
included: i) 6.96% (v/v) notoginsenoside R1; ii) 34.29%
(v/v) ginsenoside Rg1; iii) 2.65% (v/v) ginsenoside Re;
iv) 41.34% (v/v) ginsenoside Rb1; and v) 10.19% (v/v)
ginsenoside Rd. The chemical fingerprint and molecular structures
of PNS are displayed in Fig. 1.
AST IV and PNS reduce cerebral
infarction volume and NDS in rats with cerebral I/R
TTC staining of brain tissue revealed no infarct
areas nor clear neurological deficits in sham group rats (Fig. 2A). However, rats in the MCAO model
group presented large areas of pale infarction as well as
neurological deficits, with larger cerebral infarction volumes and
higher NDSs compared with those of rats in the sham group (Fig. 2A-C). Further results indicated that
treatment with AST IV and PNS alone or in combination significantly
decreased both the volume of cerebral infarction (Fig. 2A and B) and the relative NDS (Fig. 2C) compared with the model group.
Moreover, the volume of cerebral infarction and the NDSs were
decreased significantly in the combination group compared with the
AST IV and PNS groups alone (Fig.
2A-C).
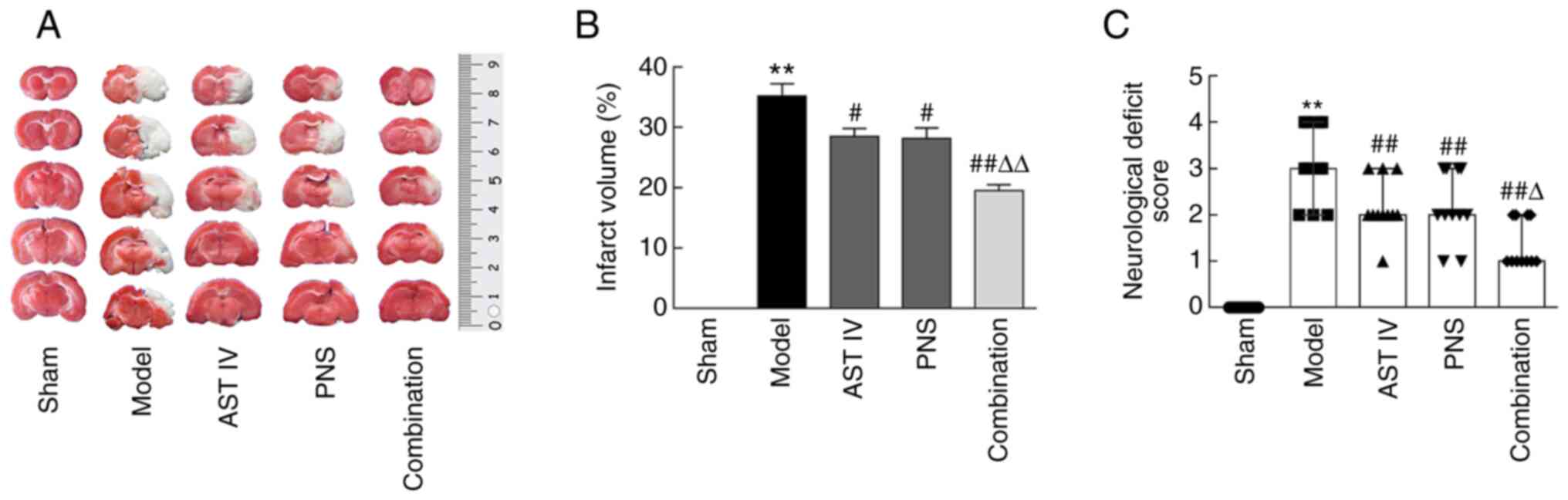 | Figure 2AST IV and PNS decrease cerebral
infarction volume and the NDSs in rats with cerebral
ischemia-reperfusion. (A) TTC-stained brain slices; pale areas
indicate infarction (n=8). (B) Cerebral infarct volumes were
calculated using TTC staining of brain sections (n=8 rats/group).
(C) Functional deficits were examined via NDS (n=11 rats/group).
The groups are as follows: i) Sham, rats with sham operation; ii)
model, rats with MCAO; iii) AST IV, rats with MCAO + 28 mg/kg AST
IV; iv) PNS, rats with MCAO + 80 mg/kg PNS; and v) combination,
rats with MCAO + 28 mg/kg AST IV + 80 mg/kg PNS.
**P<0.01 vs. Sham; #P<0.05,
##P<0.01 vs. Model group; ΔP<0.05,
ΔΔP<0.01 vs. AST IV or PNS. AST IV, astragaloside IV;
MCAO, middle cerebral artery occlusion; TTC,
2,3,5-triphenyltetrazolium chloride; NDS, neurological deficit
score; PNS, Panax notoginseng saponins. |
Combination of AST IV and PNS reduces
cerebral cortical-cell injury in rats
H&E-stained brain tissue slices were observed
under a light microscope. The results demonstrated that the
cellular structure of the cerebral cortex in the sham group was
complete. That is, nucleoli were clearly visible with regular
structures and there was no interstitial inflammatory-cell
infiltration; instead, there was a tight cell arrangement and
occasional injury (Fig. 3A).
However, following MCAO treatment, the pericellular space in the
cerebral cortex was widened; the nuclei were irregular, and
capillary atrophy, clear interstitial edema and a disordered cell
arrangement were observed. An example of the damaged cells along
with their site of vacuolation are displayed in Fig. 3B. Moreover, nuclei were
hyperchromatic and condensed or displayed acidophilic degeneration.
The results further demonstrated that the ratio of damaged cells in
the cerebral cortex was significantly higher in the model group
compared with the sham group (Fig.
3F). Compared with the model group, cells in the AST IV, PNS
and combination groups were mildly swollen, and the widened
pericellular space had narrowed (Fig.
3C-E, respectively). In addition, acidophilic degeneration of
nuclei was reduced, and cell damage was attenuated compared with
the model group (Fig. 3C-F).
Furthermore, cell damage was significantly decreased in the
combination group compared with the AST IV and PNS groups (Fig. 3F).
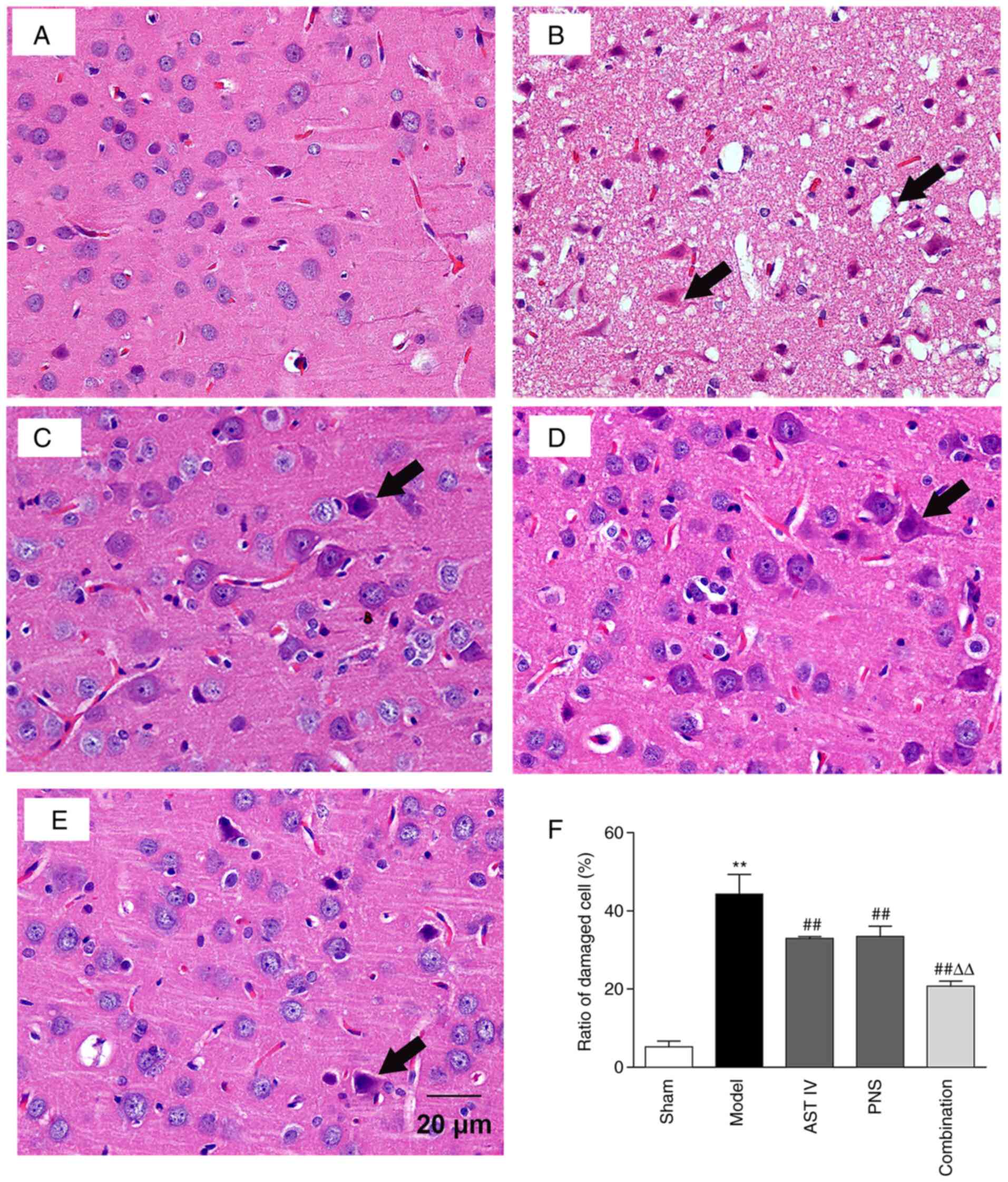 | Figure 3Combination treatment with AST IV and
PNS alleviates cerebral cortical cell injury in rats. H&E
staining analysis of pathological changes in the cerebral cortices
of rats in the. Black arrows indicate damaged cells. (A) sham, (B)
model, (C) AST IV, (D) PNS and (E) combination groups (n=8
rats/group). (F) Damaged cell ratio. The groups are as follows: i)
Sham, rats with sham operation; ii) model, rats with MCAO; iii) AST
IV, rats with MCAO + 28 mg/kg AST IV; iv) PNS, rats with MCAO + 80
mg/kg PNS; and v) combination, rats with MCAO + 28 mg/kg AST IV +
80 mg/kg PNS. **P<0.01 vs. Sham;
##P<0.01 vs. Model; ΔΔP<0.01 vs. AST IV
or PNS. AST IV, astragaloside IV; MCAO, middle cerebral artery
occlusion; PNS, Panax notoginseng saponins. |
Combination of AST IV and PNS inhibits
pyroptosis in rats with cerebral I/R
MCAO induced NLRP3 inflammasome activation in the
ischemic area, as evidenced by the increased expression of NLRP3
inflammasome components in the model group compared with the sham
group. These included NLRP3, ASC, caspase-1, IL-1β and IL-18
(Fig. 4A-F). The results of the
present study also revealed that MCAO significantly increased the
expression of GSDMD and GSDMD-N in the cerebral ischemic region
compared with the sham group, indicating that MCAO induced
pyroptosis in rats (Fig. 4G and
H). Furthermore, the MCAO-induced
expression of these proteins was inhibited by treatment with AST
IV, PNS or a combination of both, compared with the model group
(Fig. 4A-H). The combination of
both drugs was more effective than either AST IV or PNS alone in
inhibiting such expression (Fig.
4A-H).
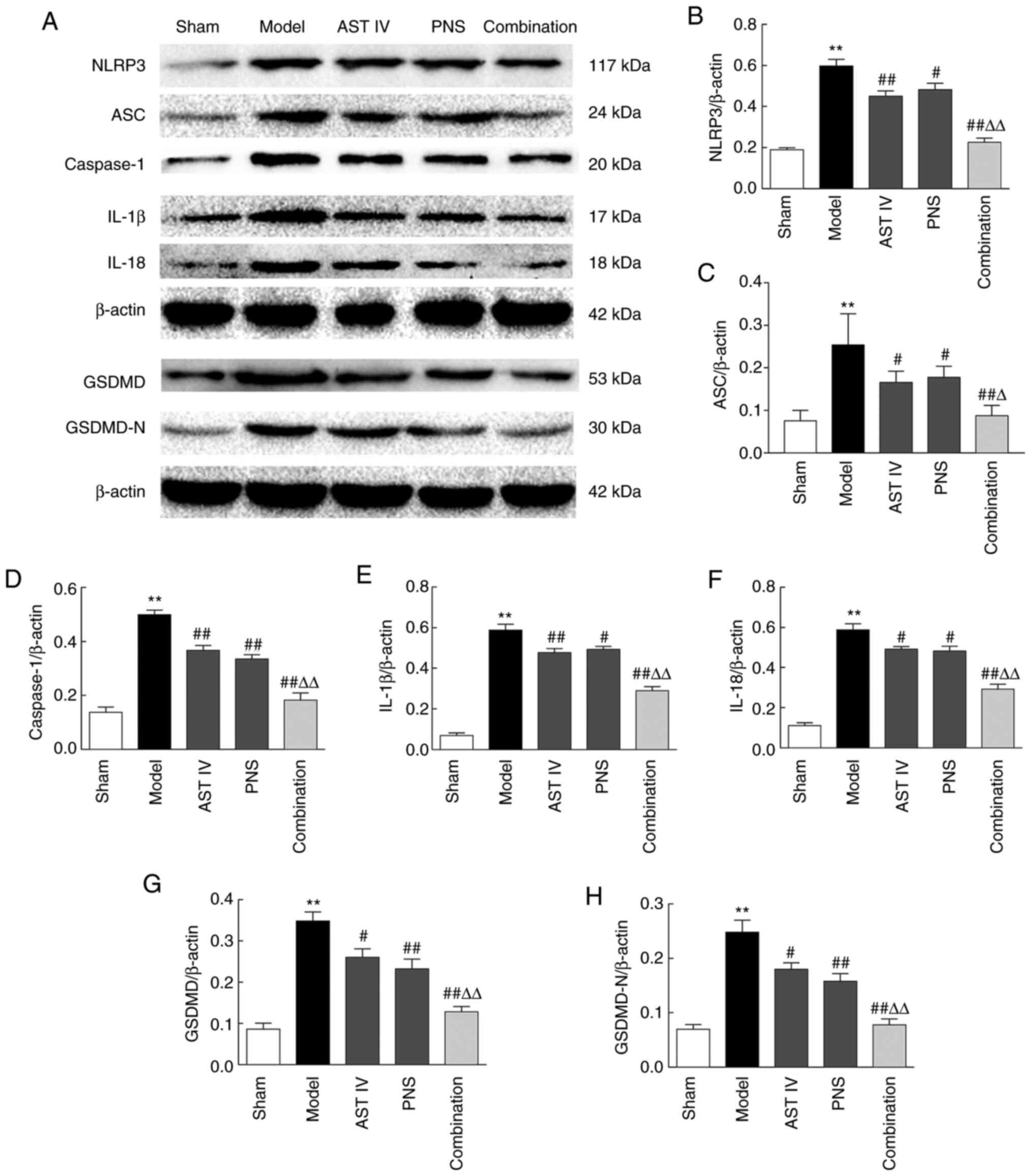 | Figure 4Combination treatment with AST IV and
PNS suppresses pyroptosis in rats with cerebral
ischemia-reperfusion. (A) Western blot analysis of
pyroptosis-associated proteins (n=5). β-actin was used as the
internal reference. Quantification of (B) NLRP3, (C) ASC, (D)
caspase-1, (E) IL-1β, (F) IL-18, (G) GSDMD and (H) GSDMD-N protein
expression levels (n=5). The groups are as follows: i) Sham, rats
with sham operation; ii) model, rats with MCAO; iii) AST IV, rats
with MCAO + 28 mg/kg AST IV; iv) PNS, rats with MCAO + 80 mg/kg
PNS; and v) combination, rats with MCAO + 28 mg/kg AST IV + 80
mg/kg PNS. **P<0.01 vs. Sham; #P<0.05,
##P<0.01 vs. Model; ΔP<0.05,
ΔΔP<0.01 vs. AST IV or PNS. ASC, apoptosis-associated
speck-like protein containing a caspase activation and recruitment
domain; AST IV, astragaloside IV; GSDMD, gasdermin D; MCAO, middle
cerebral artery occlusion; N, N-terminal; NLRP3, nucleotide-binding
oligomerization domain, leucine-rich repeat and pyrin domain
containing 3; PNS, Panax notoginseng saponins. |
Combination of AST IV and PNS reduces
the expression of necroptosis-related proteins
RIP1, RIP3 and MLKL are necroptosis-related proteins
(8). The results of the present
study revealed that the expression of RIP1 and RIP3 and ratio of
MLKL and p-MLKL significantly increased in the cerebral cortices of
rats in the model group compared with the sham group (Fig. 5A-E). Furthermore, treatment with AST
IV and PNS alone or in combination significantly suppressed
MCAO-induced increases in RIP1, RIP3, MLKL and p-MLKL levels in the
rats compared with the model group (Fig. 5A-E). Moreover, the expression of
these proteins in the cerebral cortices of rats treated with the
drug combination was significantly lower compared with the protein
expression levels in rats treated with either AST IV or PNS alone
(Fig. 5A-E).
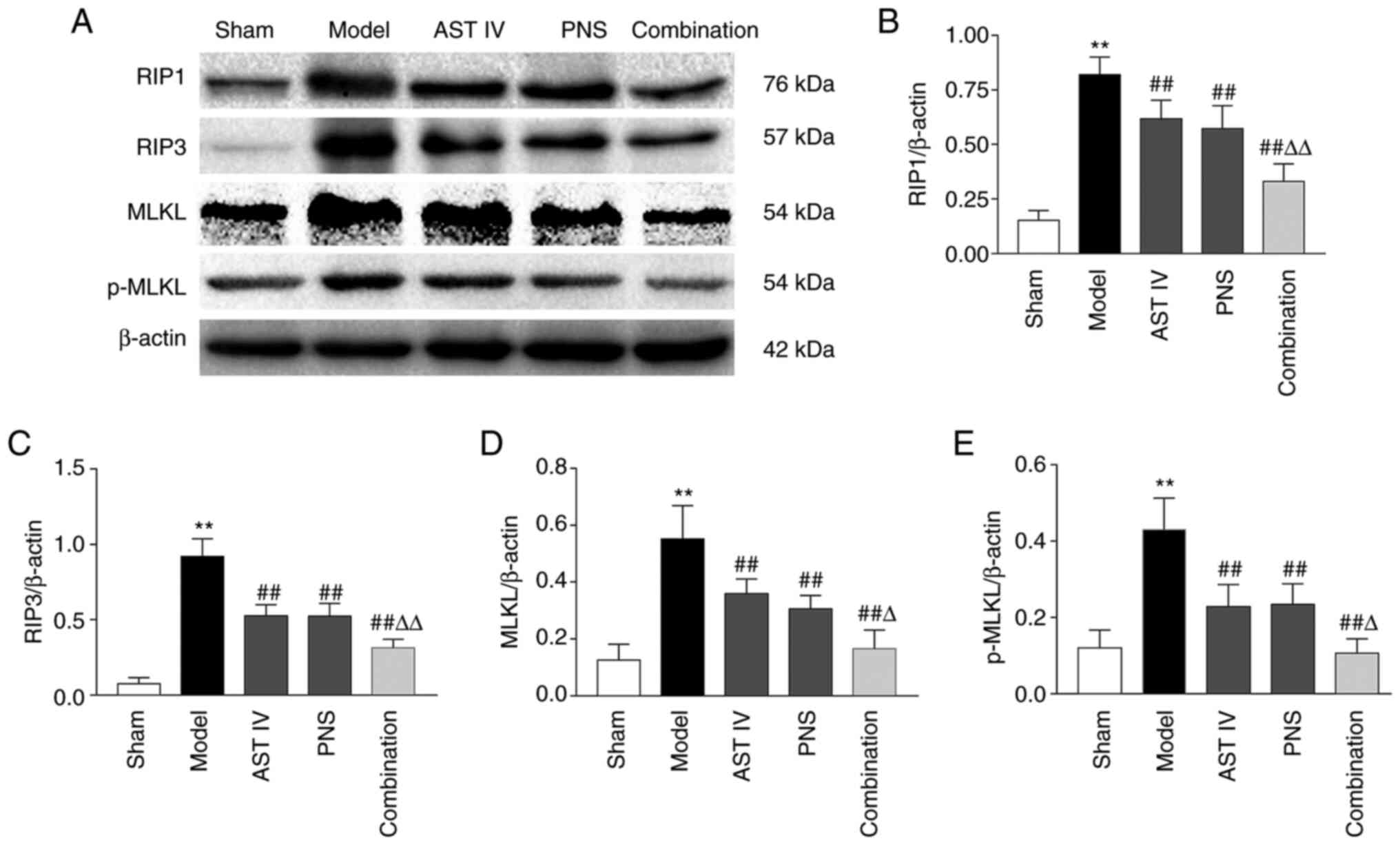 | Figure 5Combination treatment with AST IV and
PNS decreases necroptosis-related protein expression. (A) Western
blot analysis of necroptosis-related proteins (n=5). β-actin was
used as the internal reference. Quantification of (B) RIP1, (C)
RIP3, (D) MLKL and (E) p-MLKL protein expression levels (n=5). The
groups are as follows: i) Sham, rats with sham operation; ii)
model, rats with MCAO; iii) AST IV, rats with MCAO + 28 mg/kg AST
IV; iv) PNS, rats with MCAO + 80 mg/kg PNS; and v) combination,
rats with MCAO + 28 mg/kg AST IV + 80 mg/kg PNS.
**P<0.01 vs. Sham; ##P<0.01 vs. Model;
ΔP<0.05, ΔΔP<0.01 vs. AST IV or PNS.
AST IV, astragaloside IV; MCAO, middle cerebral artery occlusion;
MLKL, mixed-lineage kinase domain-like protein; p, phosphorylated;
PNS, Panax notoginseng saponins; RIP, receptor-interacting
protein kinase. |
Discussion
In the present study, an MCAO rat model similar to
human cerebral I/R was established, and the effects of AST IV and
PNS treatment, alone or in combination, on cerebral I/R injury were
detected by measuring the cerebral infarct volume, NDS and cerebral
pathomorphological indicators. The results revealed that cerebral
infarction volume, NDS and the number of damaged cells were
significantly increased following cerebral I/R, and H&E
staining indicated that cell injury was markedly increased.
Intervention with AST IV, PNS or a combination of both led to a
decrease in all of the aforementioned indicators. In addition, the
effects of the drug combination were stronger than those of AST IV
or PNS alone. Previous studies have demonstrated that both AST IV
and PNS have protective effects against I/R injury to the brain,
and primary components of A. membranaceus and P.
notoginseng have been demonstrated to have synergistic effects
in the treatment of I/R injury (12-17).
The results of the present study were consistent with those of the
preceding studies, demonstrating that AST IV and PNS reduced
cerebral I/R injury and that the combination of both drugs
exhibited the largest effect on cerebral I/R injury in rats.
The expression of GSDMD-N in cells is sufficient to
induce pyroptosis and necroptosis, and the NLRP3 inflammasome
pathway is the classical pathway for activating cleavage of GSDMD
(18). Extracellular stimulation
activates NLRP3, which activates caspase-1; caspase-1 in turn
cleaves the pro-inflammatory factors IL-1β and IL-18, and
simultaneously cleaves GSDMD into GSDMD-N to form the membrane pore
(19). Pro-inflammatory factors are
released through this membrane pore into the extracellular space,
causing inflammation (20).
Therefore, the expression levels of NLRP3, ASC, caspase-1, IL-1β,
IL-18, GSDMD and GSDMD-N are key indicators for verifying NLRP3
inflammasome activation and, consequently, pyroptosis (21). In the present study, the effects of
AST IV, PNS or a combination of both on the expression of cerebral
cortical NLRP3, ASC, caspase-1, IL-1β, IL-18, GSDMD and GSDMD-N
were observed in MCAO rats, and the mechanisms underlying AST IV
and PNS in I/R injury were investigated.
The results of the present study demonstrated that
expression of the aforementioned proteins in the rat cerebral
cortex increased significantly following cerebral I/R, which was
consistent with the results of a previous study in vitro
(21). This indicated that NLRP3
inflammasome-mediated pyroptosis activation occurred in cerebral
I/R. In addition, the data of the present study revealed that
expression of these proteins in the cerebral cortices of model rats
was significantly decreased in the AST IV, PNS and combination
groups, which suggested that AST IV and PNS may inhibit NLRP3
inflammasome-mediated activation of pyroptosis in rats with
cerebral I/R. It has previously been reported that glycosides serve
a neuroprotective role by inhibiting pyroptosis of neurons
following brain I/R injury, and that the underlying mechanism is
closely associated with NLRP3 inflammasome-related pyroptosis
(16). The subsequent results of
the current study demonstrated that the combination of AST IV and
PNS was more effective than either drug alone in inhibiting
pyroptosis. A recent study reported that the inhibition of NLRP3
inflammasome-mediated pyroptosis activation attenuated I/R injury
(17). Collectively, these results
suggested that AST IV and PNS attenuated cerebral I/R injury by
suppressing NLRP3 inflammasome-mediated pyroptosis activation.
The most typical induction of necroptosis is
mediated by RIP3 and its substrate MLKL (22). RIP3-mediated MLKL phosphorylation
leads to p-MLKL oligomerization, and the oligomers translocate to
the plasma membrane to form channels (19), which leads to membrane rupture.
Consequently, a number of damage-associated molecular patterns,
such as IL-1α, IL-1β and IL-18, are released following necrotic
cell rupture, which is a strong trigger for inflammation (22). Therefore, RIP1, RIP3, MLKL and
p-MLKL are key factors for evaluating the activation of necroptosis
(8).
To further explore the regulatory roles of AST IV
and PNS in necroptosis in cerebral I/R, the effects of both on
cortical RIP1, RIP3 expression, and p-MLKL/MLKL ratios in cerebral
I/R were observed. The expression of all four proteins in the
cerebral cortices of rats increased significantly following
cerebral I/R, suggesting that cerebral I/R induced programmed
necroptosis, which was consistent with a recent study (8). These events were significantly
inhibited after treatment with AST IV, PNS or a combination of
both, which indicated that both drugs suppressed necroptosis
induced by cerebral I/R in rats. The results of the present study
further revealed that a combination of AST IV and PNS inhibited
necroptosis in the cerebral cortices of rats more potently compared
with treatment with either drug alone. Although recent studies have
demonstrated that inhibition of necroptosis improved cerebral I/R
injury (8,23), the results of the present study are
the first to indicate that the underlying mechanisms by which AST
IV and PNS reduce I/R injury are associated with necroptotic
inhibition.
In conclusion, AST IV, PNS or a combination of both
exerted protective effects in brain I/R injury. The underlying
mechanisms were associated with inhibition of NLRP3
inflammasome-mediated pyroptosis and RIP1-, RIP3- and MLKL-mediated
necroptosis in cerebral I/R (Fig.
6).
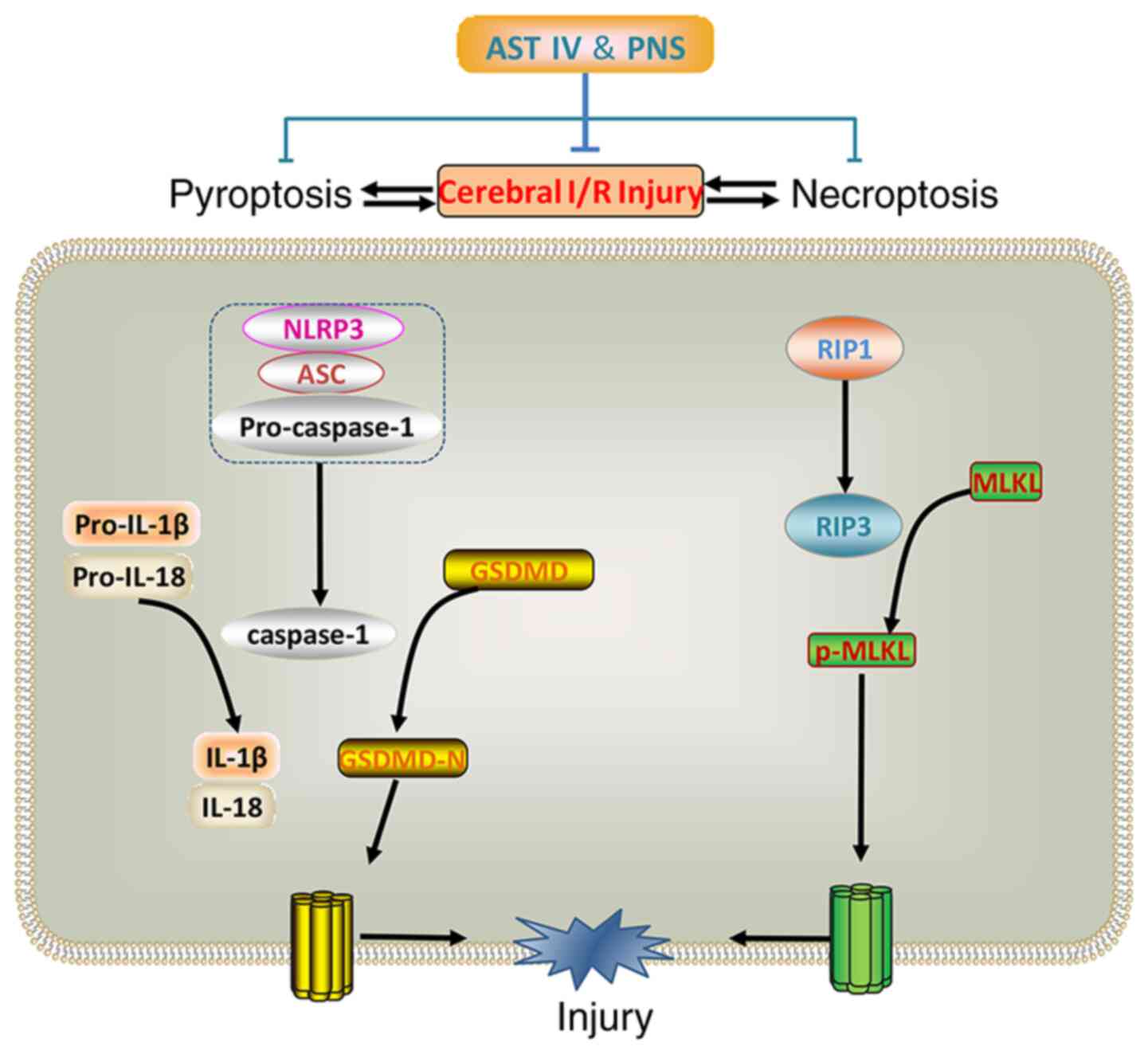 | Figure 6Combination treatment with AST IV and
PNS inhibits pyroptosis and necroptosis to attenuate cerebral I/R
injury. Pyroptosis and necroptosis are activated in cerebral I/R.
Components of the NLRP3 inflammasome, including NLRP3 and ASC,
cleave pro-caspase-1 and releases caspase-1. Caspase-1 cleaves
pro-IL-1β/18 into inflammatory factors IL-1β and IL-18, and cleaves
GSDMD into GSDMD-N, inducing pyroptosis. Through RIP3, RIP1
phosphorylates MLKL into p-MLKL, which induces necroptosis. Both
pyroptosis and necroptosis lead to cell death and cerebral I/R
injury. The combination of AST IV and PNS suppresses the expression
of these proteins and reduces cerebral I/R injury. ASC,
apoptosis-associated speck-like protein containing a caspase
activation and recruitment domain; AST IV, astragaloside IV; GSDMD,
gasdermin D; I/R, ischemia-reperfusion; MLKL, mixed-lineage kinase
domain-like protein; N, N-terminal; NLRP3, nucleotide-binding
oligomerization domain, leucine-rich repeat and pyrin domain
containing 3; p, phosphorylated; PNS, Panax notoginseng
saponins; RIP, receptor-interacting protein kinase. |
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by The National Natural Science
Foundation of China (grant no. 81503385), The Scientific Research
Fund of the Hunan Provincial Education Department (grant no.
19B436), The Hunan Administration of Traditional Chinese Medicine
Science Foundation (grant no. 2021215), The Scientific Research
Foundation of Hunan University of Chinese Medicine (grant no.
202024) and The Excellent Teaching Team of Postgraduate in Hunan
Province [Teaching Team of Postgraduate in Basic Medicine; grant
no. (2019)370-118].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BT and CQD conceived the study and designed the
experiments. BT performed the experiments. BT and XS analyzed the
data and drafted the manuscript. BT and CQD reviewed and revised
the manuscript. BT and CQD confirm the authenticity of all the raw
data All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Hunan University of Chinese Medicine (Changsha, China;
approval no. 43004700006817).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shan B, Pan H, Najafov A and Yuan J:
Necroptosis in development and diseases. Genes Dev. 32:327–340.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barbosa LA, Fiuza PP, Borges LJ, Rolim FA,
Andrade MB, Luz NF, Quintela-Carvalho G, Lima JB, Almeida RP, Chan
FK, et al: RIPK1-RIPK3-MLKL-Associated Necroptosis Drives
Leishmania infantum Killing in Neutrophils. Front Immunol.
9(1818)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin SY, Hsieh SY, Fan YT, Wei WC, Hsiao
PW, Tsai DH, Wu TS and Yang NS: Necroptosis promotes
autophagy-dependent upregulation of DAMP and results in
immunosurveillance. Autophagy. 14:778–795. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang D, Qian J, Zhang P, Li H, Shen H, Li
X and Chen G: Gasdermin D serves as a key executioner of pyroptosis
in experimental cerebral ischemia and reperfusion model both in
vivo and in vitro. J Neurosci Res. 97:645–660. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
An P, Xie J, Qiu S, Liu Y, Wang J, Xiu X,
Li L and Tang M: Hispidulin exhibits neuroprotective activities
against cerebral ischemia reperfusion injury through suppressing
NLRP3-mediated pyroptosis. Life Sci. 232(116599)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li X, Cheng S, Hu H, Zhang X, Xu J, Wang R
and Zhang P: Progranulin protects against cerebral
ischemia-reperfusion (I/R) injury by inhibiting necroptosis and
oxidative stress. Biochem Biophys Res Commun. 521:569–576.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang XP, Ding H, Yang XQ, Li JX, Tang B,
Liu XD, Tang YH and Deng CQ: Synergism and mechanism of
Astragaloside IV combined with Ginsenoside Rg1 against autophagic
injury of PC12 cells induced by oxygen glucose
deprivation/reoxygenation. Biomed Pharmacother. 89:124–134.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang XP, Ding H, Wang B, Qiu YY, Tang YH,
Zeng R and Deng CQ: Effects of the main active components
combinations of Astragalus and Panax notoginseng on energy
metabolism in brain tissues after cerebral ischemia-reperfusion in
mice. Pharmacogn Mag. 11:732–739. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang XP, Qiu YY, Wang B, Ding H, Tang YH,
Zeng R and Deng CQ: Effects of Astragaloside IV combined with the
active components of Panax notoginseng on oxidative stress injury
and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1
signaling pathway after cerebral ischemia-reperfusion in mice.
Pharmacogn Mag. 10:402–409. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang XP, Ding H, Lu JD, Tang YH, Deng BX
and Deng CQ: Effects of the combination of the main active
components of astragalus and panax notoginseng on inflammation and
apoptosis of nerve cell after cerebral ischemia-reperfusion. Am J
Chin Med. 43:1419–1438. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang X, Yan H, Yuan Y, Gao J, Shen Z,
Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, et al: Cerebral
ischemia-reperfusion-induced autophagy protects against neuronal
injury by mitochondrial clearance. Autophagy. 9:1321–1333.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang HL, Zhou QH, Xu MB, Zhou XL and Zheng
GQ: Astragaloside IV for experimental focal cerebral ischemia:
Preclinical evidence and possible mechanisms. Oxid Med Cell Longev.
2017(8424326)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
She Y, Shao L, Zhang Y, Hao Y, Cai Y,
Cheng Z, Deng C and Liu X: Neuroprotective effect of glycosides in
Buyang Huanwu Decoction on pyroptosis following cerebral
ischemia-reperfusion injury in rats. J Ethnopharmacol.
242(112051)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu D, Dong Z, Xiang F, Liu H, Wang Y,
Wang Q and Rao J: Dendrobium alkaloids promote neural function
after cerebral ischemia-reperfusion injury through inhibiting
pyroptosis induced neuronal death in both in vivo and in vitro
models. Neurochem Res. 45:437–454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kolbrink B, Riebeling T, Kunzendorf U and
Krautwald S: Plasma membrane pores drive inflammatory cell death.
Front Cell Dev Biol. 8(817)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Heilig R, Dick MS, Sborgi L, Meunier E,
Hiller S and Broz P: The Gasdermin-D pore acts as a conduit for
IL-1β secretion in mice. Eur J Immunol. 48:584–592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Diao MY, Zhu Y, Yang J, Xi SS, Wen X, Gu Q
and Hu W: Hypothermia protects neurons against
ischemia/reperfusion-induced pyroptosis via m6A-mediated activation
of PTEN and the PI3K/Akt/GSK-3β signaling pathway. Brain Res Bull.
159:25–31. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pasparakis M and Vandenabeele P:
Necroptosis and its role in inflammation. Nature. 517:311–320.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Naito MG, Xu D, Amin P, Lee J, Wang H, Li
W, Kelliher M, Pasparakis M and Yuan J: Sequential activation of
necroptosis and apoptosis cooperates to mediate vascular and neural
pathology in stroke. Proc Natl Acad Sci USA. 117:4959–4970.
2020.PubMed/NCBI View Article : Google Scholar
|