Introduction
Glaucoma is a leading cause of irreversible
blindness worldwide, and its prevalence increases with age. In
2013, the global prevalence of glaucoma for people aged 40-80 years
was 3.54%, and the number of people in this age range with glaucoma
worldwide was estimated to be 64.3 million, increasing to 76.0
million in 2020 and 111.8 million in 2040(1). Intraocular pressure (IOP) is a notable
modifiable risk factor that depends on the balance of aqueous humor
production and outflow, and it is thought that prolonged IOP
elevation leads to optic nerve damage. However, the existing
treatments to control IOP, including anti-glaucoma medication,
laser therapy and surgery, cannot meet clinical needs. In 2010, 2.1
million people went blind and 4.2 million people were visually
impaired due to glaucoma around the world (2).
Secreted protein acidic and rich in cysteine
(SPARC), also known as osteonectin or BM-40, is a promising
matricellular protein that is involved in glaucoma and functions
primarily to promote extracellular matrix (ECM) deposition
(3). In human eyes, it is
distributed throughout the trabecular meshwork (TM), with
pronounced expression in the juxtacanalicular tissue (JCT)
(3). Research indicates that, in
porcine TM cells, SPARC is one of the most highly upregulated genes
following mechanical stretching (4), while IOP drops by 15-20% in SPARC-null
mice (5). In addition, elevated
SPARC expression has been found in the iris of patients with
primary open angle glaucoma (POAG) (6). Together, these indicate that SPARC
could be involved in POAG, potentially by compromising IOP
regulation. SPARC is a potential therapeutic target, and the
mechanism by which it regulates IOP remains to be clarified.
Physiologically, the TM mediates major aqueous humor
outflow and maintains the IOP. Previous research indicates that the
remodeling of the TM cellular cytoskeleton and ECM metabolism has
significant effects on the regulation of IOP within TM tissues
(7,8). The present study hypothesized that
SPARC could regulate the expression of cytoskeleton-associated
proteins and cytoskeletal rearrangement in TM cells, and in turn
affect cell-ECM interactions, just as it does in some other tissues
(9,10). To test this hypothesis, the
regulation of SPARC was observed with regard to F-actin, zonula
occludens-1 (ZO-1) and phagocytosis in human TM cells in
vitro in the present study.
Materials and methods
Cell culture and identification
Primary human TM cells (cat. no. 634K-05a; Cell
Applications, Inc.) were stored in liquid nitrogen (-196˚C).
Following resuscitation, the cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) (cat. no. 11966025; Gibco; Thermo
Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS;
cat. no. SH30084.03, HyClone; Cytiva) at 37˚C in a 5%
CO2 cell incubator and TM cells from passages 3-4 were
used for subsequent experiments.
Glucocorticoid induction of myocilin (MYOC) is a
method to identify TM cells (11).
TM cells were seeded at a density of 1x105 cells per
well in six-well culture plates and allowed to attach at 37˚C
overnight. The medium was changed the next day for fresh medium
containing 100 nM dexamethasone (cat. no. D1756-25MG;
MilliporeSigma) or vehicle control (1% ethanol), and the cells were
incubated at 37˚C for another 5 days (11,12).
Subsequently, the cells were harvested and total RNA was extracted.
The expression level of the MYOC gene was determined in each sample
using reverse transcription-quantitative PCR (RT-qPCR). GAPDH was
used as the internal control.
Construction of SPARC suppressor
gene-containing lentiviral vectors
Viral vectors were constructed using 293T cells
(cat. no. CRL-11268; American Type Culture Collection). The 293T
cells were cultured at 37˚C in Opti-MEM (cat. no. 31985; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS.
TM cells were infected with different titers
[multiplicity of infection (MOI) 0, 20, 50 and 100] of empty
control lentiviruses to assess infection efficiency. The infected
cells were observed and counted under light (cat. no. CX43; Olympus
Corporation; magnification, x200) and fluorescence microscopy (cat.
no. IX51; Olympus Corporation; magnification, x200).
The whole sequence of SPARC was acquired from The
National Center for Biotechnology Information database (accession
no. NM_003118.3, ncbi.nlm.nih.gov/genome/gdv/browser/gene/?id=6678).
The oligonucleotides were designed by Virus Lab of Jiao Tong
University (Shanghai, China). Three pairs of oligonucleotides were
designed and synthesized according to the sequence of SPARC before
annealing. The annealed products were cloned into the empty vector
PDS134_pL_shRNA_mKate2 (Shanghai Nuobai Biotechnology Co., Ltd.).
Thus, three interfering vectors were obtained, including
pL_shRNA_mKate2-SPARC-543, pL_shRNA_mKate2-SPARC-582 and
pL_shRNA_mKate2-SPARC-347 (Table
I). The sequences of these vectors were confirmed by Single
Molecule Real-Time sequencing (PacBio Sequel; Pacific Biosciences
of California).
 | Table IConstruction of SPARC suppressor
gene-containing lentiviral vectors. |
Table I
Construction of SPARC suppressor
gene-containing lentiviral vectors.
| shRNA | Sequences
(5'-3') |
|---|
|
pL_shRNA_mKate2-SPARC-543 | |
|
Forward |
CACCGGATGAGGACAACAACCTTCTCGAAAGAAGGTTGTTGTCCTCATCC |
|
Reverse |
AAAAGGATGAGGACAACAACCTTCTTTCGAGAAGGTTGTTGTCCTCATCC |
|
pL_shRNA_mKate2-SPARC-582 | |
|
Forward |
CACCGCGGGTGAAGAAGATCCATGACGAATCATGGATCTTCTTCACCCGC |
|
Reverse |
AAAAGCGGGTGAAGAAGATCCATGATTCGTCATGGATCTTCTTCACCCGC |
|
pL_shRNA_mKate2-SPARC-347 | |
|
Forward |
CACCGCCAGGTGGAAGTAGGAGAATTCGAAAATTCTCCTACTTCCACCTGG |
|
Reverse |
AAAACCAGGTGGAAGTAGGAGAATTTTCGAATTCTCCTACTTCCACCTGGC |
Subsequently, the 293T cells were co-transfected
with the three types of interfering lentiviral vectors separately
and the ratio of the lentiviral vector plasmid and packaging
plasmid and envelope plasmid was 3:2:1. Lentiviruses were produced
using a second-generation packaging system (13). Briefly, 6x106 293T cells
were plated in a 15-cm dish and transfected with 9 µg Packaging Mix
(cat. no. K4975-00; Invitrogen; Thermo Fisher Scientific, Inc.), 3
µg transfer plasmid and 36 µl Lipofectamine 2000 (cat. no.
11668019; Invitrogen; Thermo Fisher Scientific, Inc.), which were
combined in 5 ml Opti-MEM. The transfection mix was removed once
cells were cultured for 6 h at 37˚C and fresh Opti-MEM supplemented
with 10% FBS was added. Virus-containing medium was collected at 48
h post-transfection. Subsequently, the collected medium was
filtered through a cellulose acetate membrane (pore size, 0.45 mm).
The viral stock was collected and concentrated via
ultracentrifugation (82,700 x g for 2 h at 4˚C). Viral titers were
measured using flow cytometry (On-Chip Sort; On-chip
Biotechnologies Co., Ltd.).
TM cells were infected with the three types of
packaged lentiviruses at an MOI of 50. After 24 h of incubation,
the infection efficiency was determined by fluorescence microscopy.
The relative expression of SPARC mRNA in TM cells after viral
infection were determined using RT-qPCR, as aforementioned.
mRNA and protein expression levels of
SPARC, F-actin and ZO-1 following downregulation of SPARC
TM cells at a confluence of ~80% were selected for
this test. The culture medium was removed, and the cells were
rinsed with PBS (25X; cat. no. ab64026; Abcam) twice before
exposure to serum-free DMEM. The cells were then divided into three
groups: i) The blank control group (HTMC group); ii) the empty
vector negative control (NC) group (HTMC-NC group), in which target
cells were infected with the NC lentivirus
(PDS134_pL_shRNA_mKate2); and iii) the lentivirus group (HTMC-SPARC
group), in which target cells were infected with
pL_shRNA_mKate2-SPARC-543 lentivirus, which demonstrated the
greatest efficiency. After 48 h of culture at 37˚C, the mRNA levels
of SPARC, F-actin and ZO-1 were determined using RT-qPCR. The
protein levels of SPARC, F-actin and ZO-1 were determined using
western blotting. GAPDH was used as the internal control.
Escherichia coli phagocytosis
experiment in TM cells
TM cells at a confluence of ~50% were selected for
this test. The culture medium was removed, and the cells were
rinsed with PBS twice before exposure to DMEM containing 10% FBS.
The cells were then divided into 3 groups: HTMC group, the HTMC-NC
group and the HTMC-SPARC group. Subsequently, 0.1 ml inactivated
Escherichia coli suspension (1.6x109 CFU/ml) was added
to the cells. After culture for another 24 or 48 h, the culture
medium was removed and the cells were rinsed with PBS twice before
extraction of RNA. RT-qPCR was used to determine the expression of
bacterial 16S RNA in the cells to evaluate phagocytosis, as
aforementioned.
RT-qPCR
Human TM cells were harvested and total RNA was
extracted. The expression levels of MYOC, GAPDH, SPARC, ZO-1,
F-actin, and 16S were detected. Total RNA was extracted using
TRIzol® (cat. no. 15596018; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. A
SuperScript First-Strand Synthesis system (cat. no. 11904-018;
Invitrogen; Thermo Fisher Scientific, Inc.) was used for RT. Each
reaction contained 0.5 µl random primers (0.2 µg/µl) and 1.0 µl
SuperScript III reverse transcriptase (200 U/µl). The specific
primers used are listed in Table
II. qPCR was performed using the SYBR® Premix Ex Taq
(cat. no. RR420L; Takara Bio, Inc.). The thermocycling conditions
were as follows: Initial denaturation at 95˚C for 2 min; denaturing
at 95˚C for 10 sec; annealing at 60˚C for 30 sec and polymerization
at 70˚C for 45 sec. A total of 40 PCR cycles was performed. PCR was
performed using a CFX96 Touch Real-Time PCR Detection system. Gene
expression was determined as the ratio of relative optical density
of target gene to GAPDH. The 2-ΔΔCq method was utilized
to measure PCR results (14).
 | Table IIPrimers used in reverse
transcription-quantitative PCR. |
Table II
Primers used in reverse
transcription-quantitative PCR.
| Primer | Sequence,
5'→3' |
|---|
| MYOC | |
|
Forward |
TACCGAGACAGTGAAGGCTG |
|
Reverse |
TGTAGCTGCTGACGGTGTAC |
| GAPDH | |
|
Forward |
CGTATTGGGCGCCTGGTCACC |
|
Reverse |
GGGATGATGTTCTGGAGAGCCC |
| SPARC | |
|
Forward |
AGGAAACCGAAGAGGAGG |
|
Reverse |
GCAAAGAAGTGGCAGGAA |
| ZO-1 | |
|
Forward |
TATTCACGCAGTTACGAGCAAG |
|
Reverse |
AAGGTATCAGCGGAGGGACA |
| F-actin | |
|
Forward |
GTCACCAACTGGGACGACA |
|
Reverse |
CACAGCCTGGATAGCAACG |
| 16S | |
|
Forward |
CCGCATAATGTCGCAAGACC |
|
Reverse |
TCAGACCAGCTAGGGATCGT |
Western blot analysis
Protein expression levels of SPARC, F-actin and ZO-1
in TM cells were detected using western blot analysis. The cells
were lysed in lysis buffer (cat. no. P0013, Beyotime Institute of
Biotechnology) at 4˚C with phosphatase and protease inhibitors
(cat. no. P1045; Beyotime Institute of Biotechnology). The lysis
mixture was centrifuged at 4˚C for 10 min at 10,000 x g, and the
supernatant containing cellular proteins was utilized for
subsequent experiments. The protein concentration was measured
using a BCA kit. Proteins were separated using SDS-PAGE (10% gel;
40 µg/lane; 120 V). The separated proteins were then transferred to
polyvinylidene fluoride membranes (100 V for 120 min; cat. no.
FFP24; Beyotime Institute of Biotechnology). The membranes were
blocked with 5% non-fat milk at room temperature for 1 h and
incubated with anti-SPARC (400 µl; cat. no. ab51399; Abcam;
1:1,000), anti-ZO-1 (50 µg; cat. no. ab59720, Abcam; 1:250), or
anti-F-actin (cat. no. ab205, Abcam; 1:1,000) primary antibodies at
4˚C overnight. Membranes were washed with Tris-buffered saline
containing 0.1% Tween-20 and incubated with horseradish
peroxidase-conjugated secondary antibodies (goat anti-chicken IgY,
anti-rabbit IgG and anti-mouse IgM; all 1:2,000; cat. nos. ab6877,
ab6721 and ab47827; all Abcam) at room temperature for 1 h.
Membranes were incubated in enhanced chemiluminescence solution
(cat. no. P0018A; Beyotime Institute of Biotechnology). Images were
captured on film (cat. no. FF057, Beyotime Institute of
Biotechnology) in a dark room. Experiments were repeated three
times. Blot images were quantified in greyscale using ImageJ
(version 1.5.2; National Institutes of Health).
Flow cytometry to titrate lentiviral
vectors
The cells were seeded at a density of
5x104 cells/well in a 6-well plate. At 24 h
post-seeding, the number of cells was counted in two wells using a
hemacytometer. The medium in other wells was replaced with 0.5 ml
fresh Opti-MEM supplemented with 10% FBS containing 8 mg/ml
polybrene. Next, the cells were transduced by adding 0.5, 5.0 and
50.0 ml aliquots vector stock/well and placed back in the incubator
at 37˚C. After 20 h, the medium was replaced with 2 ml fresh medium
and incubated at 37˚C for another 2 days, Then, the medium was
removed and cells were washed with 1 ml PBS. After that, 0.5
ml/well trypsin-EDTA was added and the cells were incubated at 37˚C
for 2 min. Next, 1 ml medium was added to each well and contents
were mixed. The cell suspension was transferred into a 5-ml
round-bottomed tube and centrifuged at 500 x g for 5 min at 20˚C.
The medium was removed and cells were resuspend in 2 ml Hank's
balanced salt solution and centrifuged at 500 x g for 5 min at
20˚C. Subsequently, Hank's balanced salt solution was removed and
the cells were resuspend in 300 ml Hank's balanced salt solution.
Then, the cells were analyzed using a flow cytometer and titers
were calculated (15).
Statistical analysis
All the experiments were repeated in triplicate. The
statistical analysis was performed using SPSS 19.0 software (IBM
Corp.). Data are presented as the mean ± standard deviation.
Differences between two groups were measured using Student's t-test
(paired). Differences among multiple groups were measured using
Student Newman Keuls test or two-way ANOVA followed by Tukey's post
hoc. P<0.05 was considered to indicate a statistically
significant difference.
Results
MYOC expression after dexamethasone
exposure
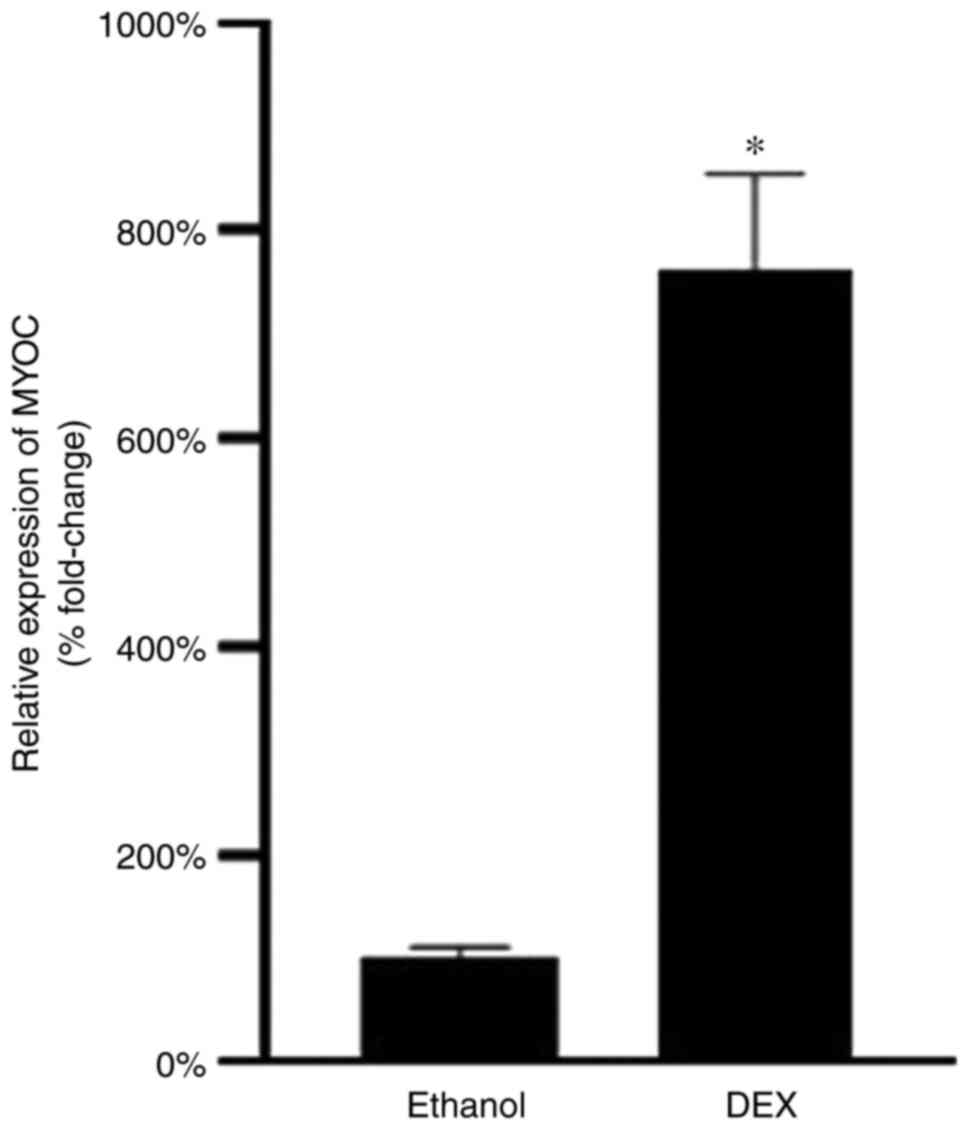
After 5 days of exposure to 100 nM dexamethasone,
the TM cells in the dexamethasone group exhibited a mean 7.62-fold
increase in MYOC gene expression compared with those in vehicle
control group (Fig. 1). This
indicated that the MYOC could be induced by glucocorticoids in this
commercial human TM cell strain and that this cell strain met the
needs of subsequent experiments.
Construction of SPARC suppressor
gene-containing lentiviral vectors
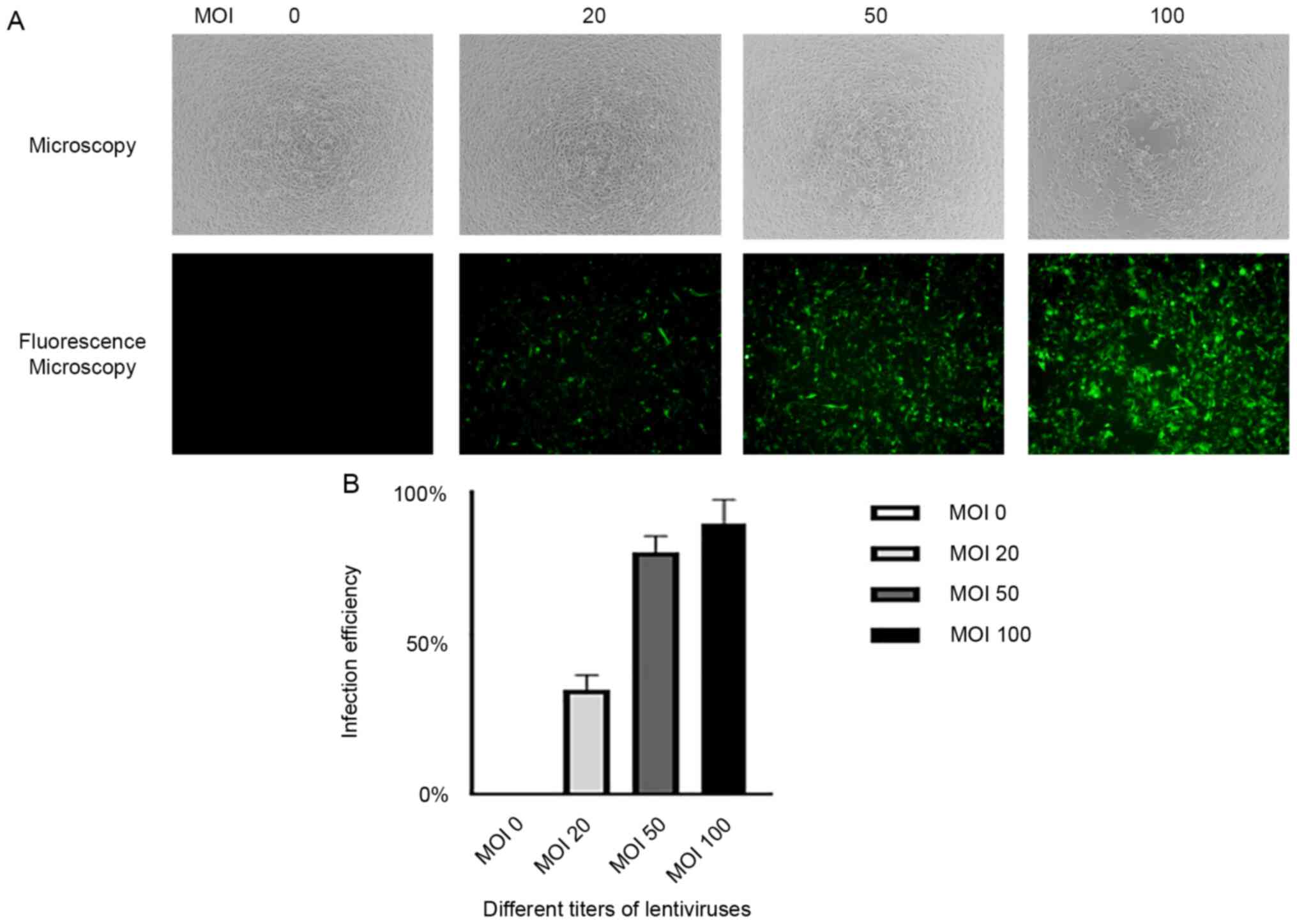
TM cells were infected with different titers (MOI 0,
20, 50 and 100) of empty control lentiviruses to assess infection
efficiency. It was revealed that 80.2 and 89.7% of TM cells were
infected with empty control lentiviruses at MOIs of 50 and 100,
respectively, which was satisfactory infection efficiency for
subsequent experiments, as revealed by non-fluorescence microscopy
and fluorescence microscopy (Fig.
2A and B). To avoid cell
physiological dysfunction or death, MOI of 50 was selected for
subsequent experiments.
Three pairs of oligonucleotides were designed
according to the sequence of SPARC. The annealed products were
cloned into the empty vector PDS134_pL_shRNA_mKate2. The 293T cells
were co-transfected with the three types of interfering lentiviral
vectors separately and lentiviruses were produced using a
second-generation packaging system. The fluorescence ratio was
determined to calculate viral titers, which were revealed to be
similar among the three types of lentiviruses (Table III).
 | Table IIIFluorescence ratio of the three viral
titers. |
Table III
Fluorescence ratio of the three viral
titers.
| Virus
pL_shRNA_mKate2-SPARC-543 | µl 1 | Fluorescence ratio,
% 100 | Viral titers, TU/ml
2.0x108 |
|---|
|
pL_shRNA_mKate2-SPARC-582 | 1 | 90 |
1.8x108 |
|
pL_shRNA_mKate2-SPARC-347 | 1 | 80 |
1.6x108 |
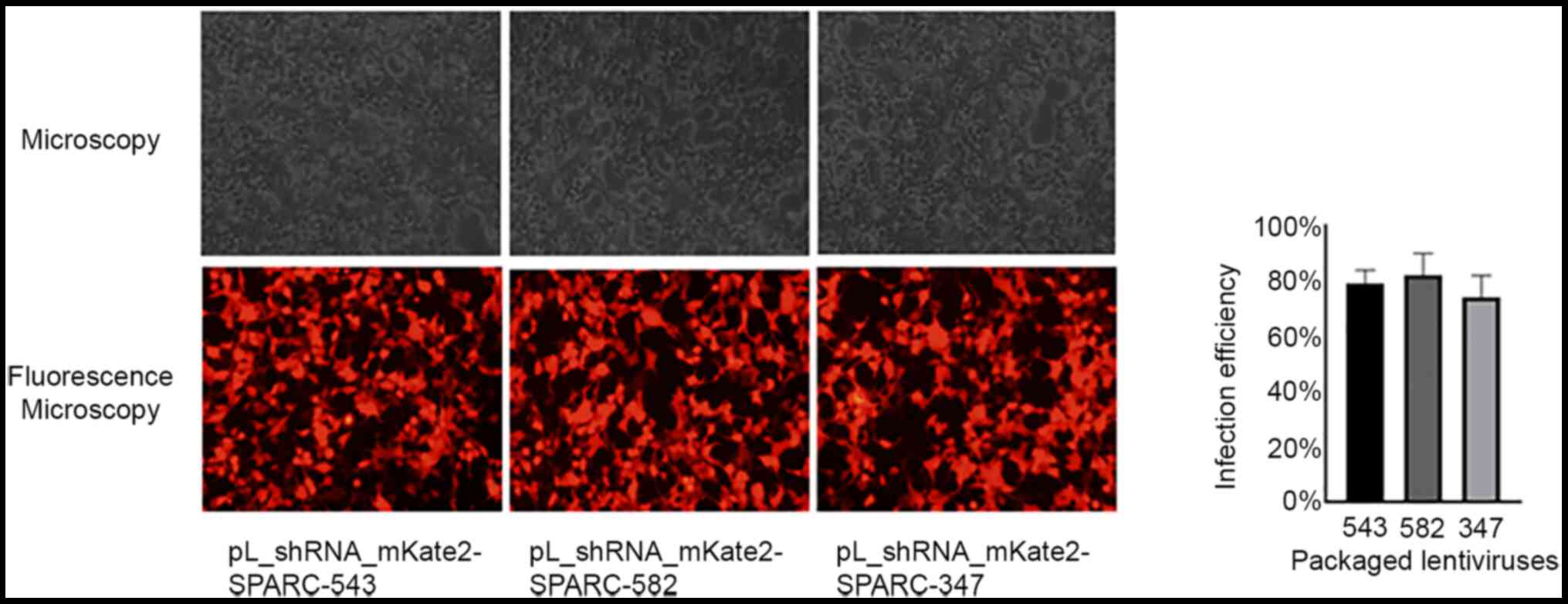
Subsequently, TM cells were infected with the three
types of packaged lentiviruses at an MOI of 50. After 24 h of
incubation, the mean infection efficiency was 79.2, 82.1 and 74.0%
in pL_shRNA_mKate2-SPARC-543, pL_shRNA_mKate2-SPARC-582 and
pL_shRNA_mKate2-SPARC-347, respectively, which were showed that all
three lentiviruses achieved satisfactory results, as revealed by
fluorescence microscopy (Fig.
3).
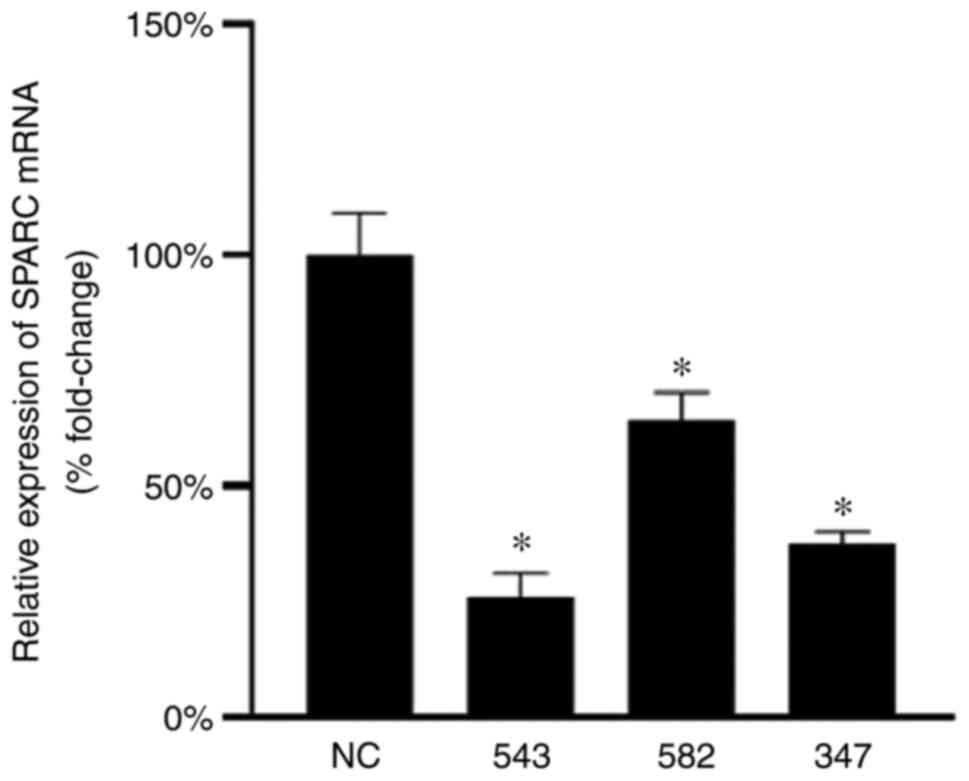
The relative expression levels of SPARC mRNA in TM
cells after viral infection were determined using RT-qPCR. The mean
interference efficiency of pL_shRNA_mKate2-SPARC-543,
pL_shRNA_mKate2-SPARC-582 and pL_shRNA_mKate2-SPARC-347 was 74.2,
36.0 and 62.5%, respectively. The pL_shRNA_mKate2-SPARC-543 had a
significantly decreased expression level of SPARC compared with the
NC; it was also the most efficient in interfering with SPARC mRNA
expression in human TM cells and, therefore, it was used for
subsequent experiments (Fig.
4).
Effects of SPARC downregulation on
F-actin and ZO-1 expression
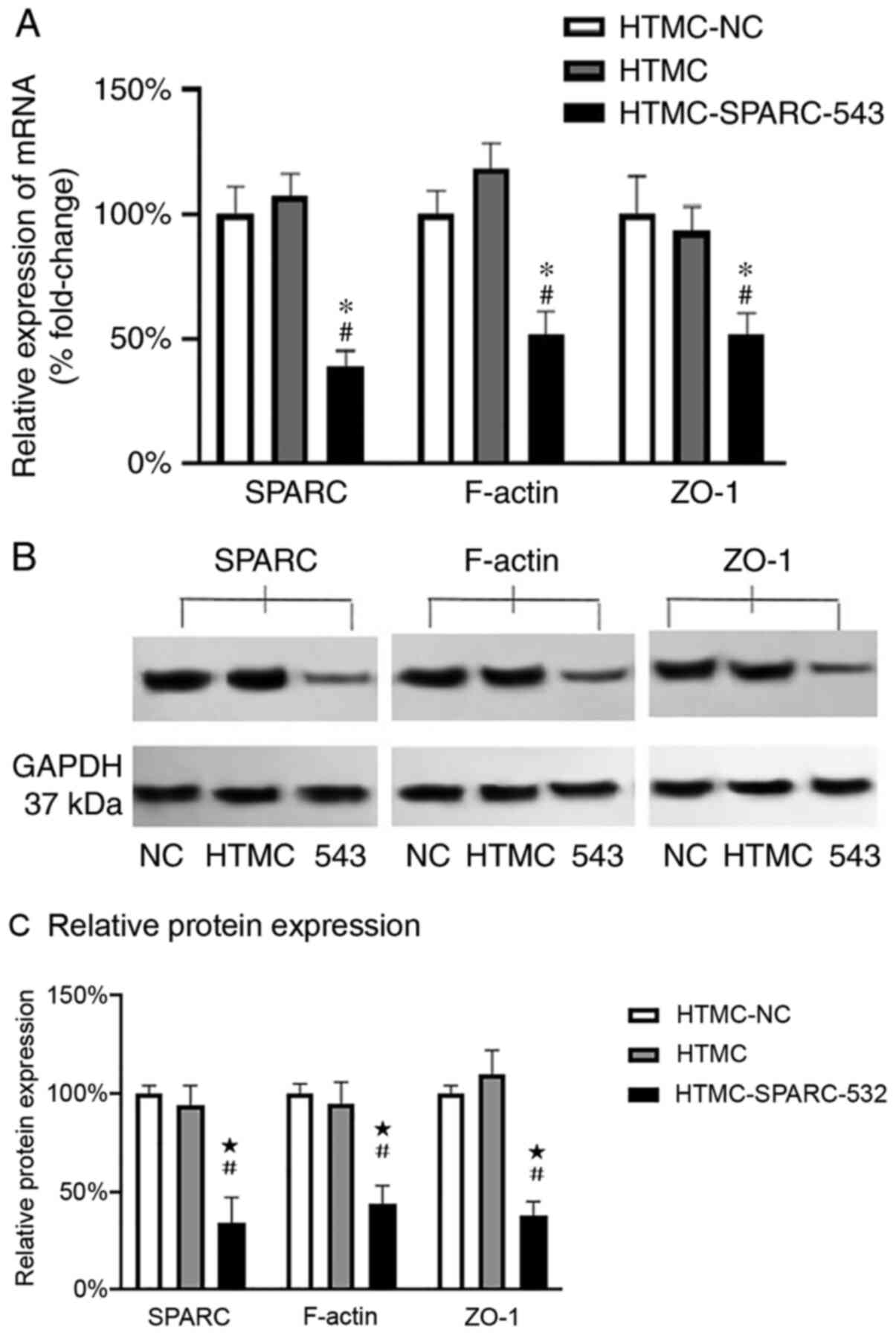
The mRNA expression levels of SPARC, F-actin and
ZO-1 were analyzed after 48 h of culture in the empty vector
control (HTMC-NC) group, which was used to represent 100%
expression. The relative mRNA expression levels of SPARC, F-actin
and ZO-1 were 107, 118 and 93% in the blank control (HTMC) group
and 39, 52 and 52% in the lentivirus (HTMC-SPARC-543) group,
respectively. RT-qPCR revealed that the mRNA expression levels of
SPARC, F-actin and ZO-1 were significantly lower in the
HTMC-SPARC-543 group compared with the levels in the HTMC and
HTMC-NC groups (Fig. 5A).
The protein expression levels of SPARC, F-actin and
ZO-1 were analyzed and the empty vector control (HTMC-NC) groups
were used to represent 100% expression. The relative protein
expression levels of SPARC, F-actin and ZO-1 were 94, 95 and 110%
in the blank control (HTMC) group and 34, 44 and 38% in the
lentivirus (HTMC-SPARC-543) group, respectively. The western
blotting results also revealed that the protein expression levels
of SPARC, F-actin and ZO-1 were significantly lower in the
HTMC-SPARC-543 group compared with those in the HTMC and HTMC-NC
groups (Fig. 5B and C). Overall, these results demonstrated
that downregulation of SPARC significantly decreased the expression
of F-actin and ZO-1.
Effects of SPARC downregulation on
phagocytosis
Research has indicated that phagocytosis in TM cells
is active, especially in the uveal and corneoscleral meshwork
(11). Shirato et al
(16) demonstrated that
phagocytosis reached half-maximum at 40 h and maximum at 96 h in TM
cells in vitro; therefore 24 and 48 h were selected as
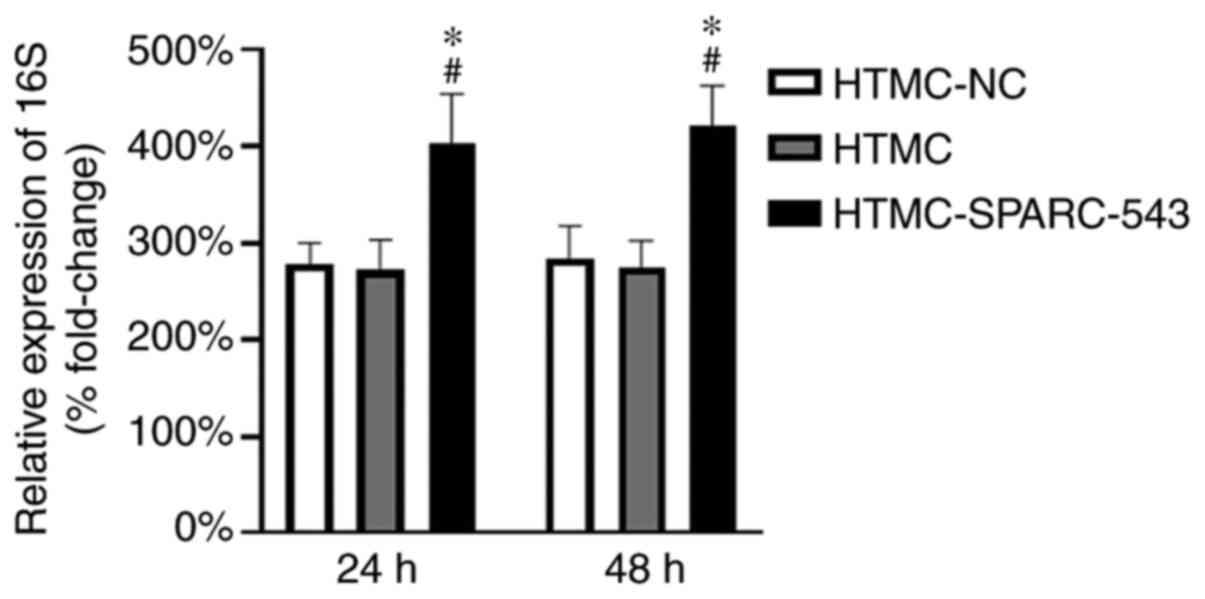
observational time points in the present study. An equal amount of
inactivated Escherichia coli suspension was added to each group.
After 24 or 48 h of culture, the expression levels of bacterial 16S
RNA in TM cells were detected by RT-qPCR. The expression of 16S RNA
in the HTMC-NC group after 5 min of culture was used as a control
to represent 100% expression. Thus, the relative expression levels
in the HTMC-NC, HTMC and SPARC-543 groups were 278, 272 and 403%
after 24 h of culture, and 283, 274 and 421% after 48 h of culture,
respectively. The expression levels of 16S RNA were significantly
increased in the SPARC-543 groups compared with those in the
HTMC-NC and HTMC groups. However, the expression of 16S RNA in
these three groups did not differ between 24 and 48 h of culture
(Fig. 6). These results indicated
that SPARC downregulation significantly promoted the phagocytosis
of TM cells and that phagocytosis of TM cells reached a peak within
24 h.
Discussion
IOP, as determined by the production, circulation
and drainage of aqueous humor, is the main risk factor for
glaucoma. The trabecular outflow pathway via which aqueous humor
passes through the TM into Schlemm's canal, known as the
conventional outflow pathway, serves a key function in maintaining
the IOP. TM cells are the primary cell type that occupy and form
this outflow pathway, in coordination with the inner wall of
Schlemm's canal. Cellular dysfunction of TM cells results in the
generation of extra resistance that causes elevated IOP. To ensure
effective regulation of outflow resistance, TM tissue has two
primary responsibilities, namely, filtration and resistance
generation (11). Accordingly, TM
cells display different morphologies and characteristics to support
these two primary responsibilities (11).
TM cells in the inner TM tissue, the uveal meshwork
and the corneoscleral meshwork have macrophage-like activity and
act as professional endothelial cells. Phagocytosis is an essential
function of TM cells in this role (11). Notably, rapid clearance of debris by
the inner TM occurs prior to it reaching into the deep TM where it
could accumulate and disrupt resistance generation and regulation
(11). Cultured TM cells and TM
cells in vivo are actively phagocytic, and this is an
important part of maintaining a clean outflow filter (17). TM cell phagocytosis changes in
response to environmental changes. A series of studies have
indicated that TM cell phagocytosis can be inhibited by
dexamethasone (18), γ-interferon
(19), epinephrine and cortisone
(20), and that impaired TM cell
phagocytosis may be one of the pathogeneses of some types of
glaucoma. By contrast, promoting phagocytosis shows several
prospects for clinical application. The results of the present
study demonstrated that SPARC downregulation significantly
increased the phagocytosis of TM cells and contributed to aqueous
humor outflow. Although the mechanism remains to be clarified,
SPARC downregulation shows promise for application as an
anti-glaucoma treatment, especially for pseudoexfoliation syndrome
or pigmented glaucoma, in which large amounts of pigment granules
and debris are produced.
TM cells in the outer TM tissue, the JCT region,
have both fibroblastic and smooth muscle-like qualities (11). Previous research has demonstrated
that fibrosis of the JCT is a major cause of impaired aqueous humor
outflow and glaucoma (21). The
mechanical and biological changes associated with JCT fibrosis, in
turn, are caused by structural changes in the cytoskeleton
(22) and ECM (23). These structural changes are
considered upstream signals in the pathological process of glaucoma
(24). The present study indicated
that downregulation of SPARC lowered the expression of F-actin in
TM cells, leading to rearrangement of the actin cytoskeleton.
F-actin in the cytoskeleton is one of the key factors that
determines cell mechanics (25).
F-actin morphology is closely associated with functional disorders
of TM cells (26,27). The cytoskeleton plays notable roles
in maintaining cell polarity and initiating intracellular
metabolism and signaling (28,29).
Cytoskeletal rearrangement is also closely associated with
phagocytosis, cell secretion (30,31),
cell junction changes and changes in the composition of the ECM
(32,33). In addition, the distribution and
structure of the cytoskeleton directly affects contraction of the
TM (28). These factors can
directly or indirectly change the outflow resistance of aqueous
humor.
The results of the present study also indicated that
SPARC downregulation led to decreased expression of ZO-1, causing
F-actin to detach from the cell membrane. ZO-1 plays a central role
in cytoskeleton-associated proteins; it is attached to the cell
membrane, with one end linked to occludin, a tight junction protein
in the cell membrane, and the other end linked to F-actin in the
cytoplasm. Together, these proteins together form a functional
complex that is the main permeability barrier among cells. The
barrier regulates intercellular transport of water, ions and
macromolecules, bridges internal and external cell communication,
modulates cell movement and maintains the cell microenvironment
(33). A previous study has
demonstrated that changes in ZO-1 expression or location and/or
structural dysfunction of ZO-1 may disrupt the integrity of tight
junctions, resulting in loss of intercellular junctions and
impaired permeability (34). ZO-1
is involved in cell and tissue remodeling. ZO-1-deficient mice
exhibit delayed growth (35).
Previous research has demonstrated that ZO-1 is also involved in
the regulation of TM function. Increased expression of ZO-1 in TM
cells has been noted in patients with neovascular glaucoma
(36). Increased IOP can lead to
decreased expression of ZO-1 and affect the cytoskeleton of TM
cells and intercellular adhesion (37). Dexamethasone increases the protein
expression level of ZO-1 in cultured TM cells (38). These results indicate that ZO-1 is
closely associated with cytoskeletal rearrangement and cellular
contractile tone, therefore affecting the drainage of the aqueous
humor, and that SPARC may initiate this process.
In human eyes, SPARC is present in numerous tissues,
including the lens (39), corneal
epithelium (39), TM cells
(3), ciliary body smooth muscle
cells (40), aqueous and vitreous
humors (39), and retinal pigment
epithelium (39,41). Immunofluorescence staining revealed
that SPARC is present throughout the TM and that it is among the
most abundantly expressed genes in cultured human TM cells
(4,42). In the TM of human eyes at
postmortem, SPARC and MYOC, another glaucoma gene, increased
significantly with increased levels of IOP (43). SPARC-null mice were demonstrated to
have lower IOP compared with wild-type mice, potentially due to
decreased outflow resistance (5),
and TGF-β2 failed to induce ocular hypertension in such mice
(44). These results indicate that
SPARC is important in the regulation of aqueous humor outflow via
the TM, although the underlying mechanism remains unclear. The
results of the present study suggested that SPARC may work
throughout the whole TM tissue by a variety of mechanisms,
including improving phagocytosis and cytoskeletal
rearrangement.
The current study used only one commercial human TM
cell strain to test MYOC as the cell marker. By contrast, directly
acquiring cells from human TM tissues would increase the validity
of the results. Meanwhile, precise cell identification is
indispensable in primary cells. Furthermore, in vivo tests
are needed to validate the conclusions the present study revealed
in vitro. The mechanism whereby SPARC regulates IOP, the
signaling pathways upstream of SPARC and its regulation of ECM
metabolism represent future research directions in this field. We
consider that SPARC will hold promise as a novel therapeutic target
for glaucoma as more information is revealed on this matricellular
protein.
In conclusion, downregulation of SPARC decreased the
expression levels of the cytoskeleton-associated proteins F-actin
and ZO-1, promoted phagocytosis in human TM cells and may affect
the outflow of aqueous humor through the TM pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YaF, LL, YiF and MT designed the experimental
protocol. MT contributed to the study design. LL performed the
experimental procedures. YiF drafted the paper. YiF and MT
performed the critical revision of the manuscript. All authors have
read and approved the final version of the manuscript. YiF and MT
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
Shanghai General Hospital, Shanghai Jiao Tong University (Shanghai,
China; approval no. 2016KY034).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: A systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bourne RR, Taylor HR, Flaxman SR, Keeffe
J, Leasher J, Naidoo K, Pesudovs K, White RA, Wong TY, Resnikoff S,
et al: Number of people blind or visually impaired by glaucoma
worldwide and in world regions 1990-2010: A meta-analysis. PLoS
One. 11(e0162229)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rhee DJ, Fariss RN, Brekken R, Sage EH and
Russell P: The matricellular protein SPARC is expressed in human
trabecular meshwork. Exp Eye Res. 77:601–607. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vittal V, Rose A, Gregory KE, Kelley MJ
and Acott TS: Changes in gene expression by trabecular meshwork
cells in response to mechanical stretching. Invest Ophthalmol Vis
Sci. 46:2857–2868. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haddadin RI, Oh DJ, Kang MH, Filippopoulos
T, Gupta M, Hart L and Rhee DJ: SPARC-null mice exhibit lower
intraocular pressures. Invest Ophthalmol Vis Sci. 50:3771–3777.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chua J, Seet LF, Jiang Y, Su R, Htoon HM,
Charlton A, Aung T and Wong TT: Increased SPARC expression in
primary angle closure glaucoma iris. Mol Vis. 14:1886–1892.
2008.PubMed/NCBI
|
|
7
|
Stamer WD: The cell and molecular biology
of glaucoma: Mechanisms in the conventional outflow pathway. Invest
Ophthalmol Vis Sci. 53:2470–2472. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tian B, Gabelt BT, Geiger B and Kaufman
PL: The role of the actomyosin system in regulating trabecular
fluid outflow. Exp Eye Res. 88:713–717. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bradshaw AD: The role of secreted protein
acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis:
Does expression of SPARC by macrophages influence outcomes? J Mol
Cell Cardiol. 93:156–161. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nie J and Sage EH: SPARC functions as an
inhibitor of adipogenesis. J Cell Commun Signal. 3:247–254.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stamer WD and Clark AF: The many faces of
the trabecular meshwork cell. Exp Eye Res. 158:112–123.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fan BJ, Wang DY, Tham CC, Lam DS and Pang
CP: Gene expression profiles of human trabecular meshwork cells
induced by triamcinolone and dexamethasone. Invest Ophthalmol Vis
Sci. 49:1886–1897. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zufferey R, Nagy D, Mandel RJ, Naldini L
and Trono D: Multiply attenuated lentiviral vector achieves
efficient gene delivery in vivo. Nat Biotechnol. 15:871–875.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kutner RH, Zhang XY and Reiser J:
Production, concentration and titration of pseudotyped HIV-1-based
lentiviral vectors. Nat Protoc. 4:495–505. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shirato S, Murphy CG, Bloom E,
Franse-Carman L, Maglio MT, Polansky JR and Alvarado JA: Kinetics
of phagocytosis in trabecular meshwork cells. Flow cytometry and
morphometry. Invest Ophthalmol Vis Sci. 30:2499–2511.
1989.PubMed/NCBI
|
|
17
|
Johnson DH, Richardson TM and Epstein DL:
Trabecular meshwork recovery after phagocytic challenge. Curr Eye
Res. 8:1121–1130. 1989.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang X, Ognibene CM, Clark AF and Yorio
T: Dexamethasone inhibition of trabecular meshwork cell
phagocytosis and its modulation by glucocorticoid receptor beta.
Exp Eye Res. 84:275–284. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Park CH and Latina MA: Effects of
gamma-interferon on human trabecular meshwork cell phagocytosis.
Invest Ophthalmol Vis Sci. 34:2228–2236. 1993.PubMed/NCBI
|
|
20
|
Yang X and Li M: Establishment of in vitro
culture of bovine trabecular meshwork cells and their phagocytosis.
Zhonghua Yan Ke Za Zhi. 32:136–139. 1996.PubMed/NCBI(In Chinese).
|
|
21
|
Last JA, Pan T, Ding Y, Reilly CM, Keller
K, Acott TS, Fautsch MP, Murphy CJ and Russell P: Elastic modulus
determination of normal and glaucomatous human trabecular meshwork.
Invest Ophthalmol Vis Sci. 52:2147–2152. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hoare MJ, Grierson I, Brotchie D, Pollock
N, Cracknell K and Clark AF: Cross-linked actin networks (CLANs) in
the trabecular meshwork of the normal and glaucomatous human eye in
situ. Invest Ophthalmol Vis Sci. 50:1255–1263. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lutjen-Drecoll E: Morphological changes in
glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of
the disease. Exp Eye Res. 81:1–4. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Morgan JT, Raghunathan VK, Chang YR,
Murphy CJ and Russell P: The intrinsic stiffness of human
trabecular meshwork cells increases with senescence. Oncotarget.
6:15362–15374. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen QM, Tu VC, Catania J, Burton M,
Toussaint O and Dilley T: Involvement of Rb family proteins, focal
adhesion proteins and protein synthesis in senescent morphogenesis
induced by hydrogen peroxide. J Cell Sci. 113:4087–4097.
2000.PubMed/NCBI
|
|
26
|
Tian B, Geiger B, Epstein DL and Kaufman
PL: Cytoskeletal involvement in the regulation of aqueous humor
outflow. Invest Ophthalmol Vis Sci. 41:619–623. 2000.PubMed/NCBI
|
|
27
|
Clark AF, Brotchie D, Read AT, Hellberg P,
English-Wright S, Pang IH, Ethier CR and Grierson I: Dexamethasone
alters F-actin architecture and promotes cross-linked actin network
formation in human trabecular meshwork tissue. Cell Motil
Cytoskeleton. 60:83–95. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Stumpff F and Wiederholt M: Regulation of
trabecular meshwork contractility. Ophthalmologica. 214:33–53.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kaufman PL: Enhancing trabecular outflow
by disrupting the actin cytoskeleton, increasing uveoscleral
outflow with prostaglandins, and understanding the pathophysiology
of presbyopia interrogating Mother Nature: Asking why, asking how,
recognizing the signs, following the trail. Exp Eye Res. 86:3–17.
2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sanka K, Maddala R, Epstein DL and Rao PV:
Influence of actin cytoskeletal integrity on matrix
metalloproteinase-2 activation in cultured human trabecular
meshwork cells. Invest Ophthalmol Vis Sci. 48:2105–2114.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gilles C, Bassuk JA, Pulyaeva H, Sage EH,
Foidart JM and Thompson EW: SPARC/osteonectin induces matrix
metalloproteinase 2 activation in human breast cancer cell lines.
Cancer Res. 58:5529–5536. 1998.PubMed/NCBI
|
|
32
|
Liu X, Wu Z, Sheibani N, Brandt CR,
Polansky JR and Kaufman PL: Low dose latrunculin-A inhibits
dexamethasone-induced changes in the actin cytoskeleton and alters
extracellular matrix protein expression in cultured human
trabecular meshwork cells. Exp Eye Res. 77:181–188. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamada KM and Geiger B: Molecular
interactions in cell adhesion complexes. Curr Opin Cell Biol.
9:76–85. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shen L: Tight junctions on the move:
Molecular mechanisms for epithelial barrier regulation. Ann N Y
Acad Sci. 1258:9–18. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Katsuno T, Umeda K, Matsui T, Hata M,
Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, et
al: Deficiency of zonula occludens-1causes embryonic lethal
phenotype associated with defected yolk sac angiogenesis and
apoptosis of embryonic cells. Mol Biol Cell. 19:2465–2475.
2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang JG, Zhou CJ, Li XY, Sun PR, Li SP and
Ren BC: Alteration of UCP2 and ZO-1 expression in trabecular
meshwork of neovascular glaucoma patients. J Glaucoma. 24:291–296.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang X, Liu B, Bai Y, Chen M, Li Y, Chen
M, Wei Y, Ge J and Zhuo Y: Elevated pressure downregulates ZO-1
expression and disrupts cytoskeleton and focal adhesion in human
trabecular meshwork cells. Mol Vis. 17:2978–2985. 2011.PubMed/NCBI
|
|
38
|
Zhuo YH, He Y, Leung KW, Hou F, Li YQ,
Chai F and Ge J: . Dexamethasone disrupts intercellular junction
formation and cytoskeleton organization in human trabecular
meshwork cells. Mol Vis. 16:61–71. 2010.PubMed/NCBI
|
|
39
|
Yan Q, Clark JI and Sage EH: Expression
and characterization of SPARC in human lens and in the aqueous and
vitreous humors. Exp Eye Res. 71:81–90. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gilbert RE, Cox AJ, Kelly DJ,
Wilkinson-Berka JL, Sage EH, Jerums G and Cooper ME: Localization
of secreted protein acidic and rich in cysteine (SPARC) expression
in the rat eye. Connect. Tissue Res. 40:295–303. 1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rodriguez IR, Moreira EF, Bok D and
Kantorow M: Osteonectin/SPARC secreted by RPE and localized to the
outer plexiform layer of the monkey retina. Invest Ophthalmol Vis
Sci. 41:2438–2444. 2000.PubMed/NCBI
|
|
42
|
Tomarev SI, Wistow G, Raymond V, Dubois S
and Malyukova I: Gene expression profile of the human trabecular
meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci.
44:2588–2596. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Comes N and Borras T: Individual molecular
response to elevated intraocular pressure in perfused postmortem
human eyes. Physiol Genomics. 38:205–225. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Swaminathan SS, Oh DJ, Kang MH, Shepard
AR, Pang I and Rhee DJ: TGFβ2-mediated ocular hypertension is
attenuated in SPARC-null mice. Invest Ophthalmol Vis Sci.
55:4084–4097. 2014.PubMed/NCBI View Article : Google Scholar
|