Introduction
A strong immune system is a key component to
efficiently fight both internal problems, such as the proliferation
of abnormal tumor cells, as well as external insults, such as
bacterial or viral infections (1,2). Thus,
in order to be ready to fight such external offenses more
efficiently, an adequately fitted immune system is absolutely
needed. Ocoxin Oral Solution (OOS) and Viusid (VS) are nutritional
supplements that include several plant and natural products with an
ample spectrum of biological activities (3,4). Some
of these products have antioxidant, vitaminic, or antiproliferative
properties. Moreover, several of the constituents of these
nutritional supplements have been reported to stimulate the immune
system (3,4).
VS composition includes several antiviral agents,
antioxidants and anti-free radicals potentially able to boost the
defenses of the immune system (4).
Its formulation comprises licorice extract, several vitamins such
as ascorbic acid or B group vitamins or amino acids (5). This combination has demonstrated to
improve clinical outcomes in several scenarios, such as patients
with chronic hepatitis C that did not respond to standard antiviral
treatments or interferon therapy (4,6),
cirrhotic patients who have failed to achieve sustained virological
response with standard of care treatments (5) or in combination with diet and
exercise, to improve fatty liver disease (7). However, the mechanism of action of VS
has not been fully elucidated, and research in this direction could
potentiate its application to different pathologies.
OOS formulation includes plant extracts from green
tea, cinnamon or licorice as well as ascorbic acid, B vitamins or
several amino acids. Some of these products have been shown to
exert an antitumoral activity in several experimental models
(3). As a result, OOS exhibits
antitumoral activity in breast, lung or colon cancer, among others
[reviewed in (3), as well as
(8-14)].
The molecular mechanism of action of OOS has been described to
include both a decrease in cell proliferation, as well as an
increase in cell death (3,15). The overall effect of OOS is
therefore a decrease in tumoral cell numbers. Molecularly, OOS has
been reported to provoke cell cycle blockade mainly acting by
increasing p27 levels, as well as by modulating the retinoblastoma
pathway (15).
Current antitumoral agents, mostly those with
chemotherapeutic properties, are known to affect the immune system,
impairing it and, therefore, favoring infections (16,17).
The actual scenario of the COVID-19 pandemic worsens such
situations as immunocompromised patients appear particularly
susceptible to the physiopathological consequences of SARS-CoV-2
infection (18,19). Therefore, supplements that may fight
the infectivity of viruses should report benefits to patients under
chemotherapeutic regimens or on those tumor types directly
associated to viral infections, such as papillomavirus or hepatitis
virus-promoted tumors (20,21).
In the present study, it was hypothesized that OOS
and VS could exhibit antiviral properties. Such possibility was
evaluated in vitro on experimental preclinical models. Using
flow cytometry and western blot analysis it was demonstrated that
OOS, and to a lesser degree, VS, could reduce the infection of
epithelial cells with retrovirus or lentivirus.
Materials and methods
Reagents and antibodies
Cell culture media, fetal bovine serum, antibiotics
and trypsin were purchased from Life Technologies. Polybrene was
obtained from Sigma-Aldrich (Merck KGaA), the Immobilon P membranes
from EMD Millipore and the JetPEI™ reagent from
Polyplus-transfection SA. OOS and VS were provided by Catalysis
S.L. Detailed description of the components of OOS has been
recently reported (3) and includes
plant extracts (Glycyrrhiza glabra, Camellia sinensis
and Cinnamomum verum J. Presl. extract), vitamins (ascorbic
acid, pyridoxine, cyanocobalamin, folic acid and calcium
pantothenate), amino acids (glycine, arginine and cysteine) and
sugars (sucralose and glucosamine). As for the VS formulation, it
is composed of malic acid, glycyrrhizic acid, glucosamine,
arginine, calcium pantethonate, ascorbic acid, folic acid,
cyanocobalamine, zinc sulfate and pyrodoxal (6). Other generic chemicals were purchased
from Sigma-Aldrich, Merck KGaA. The anti-hemagglutinin (HA)
monoclonal antibody (12CA5; cat. no. 11583816001) was from Roche
Diagnostics and the anti-calnexin antibody (cat. no. ADI-SPA-860)
from Enzo Life Sciences, Inc. The goat anti-mouse horseradish
perosidase-conjugated secondary antibody (cat. no. 170-6516) was
purchased from Bio-Rad Laboratories.
Cell culture, transfection, viral
preparation and infection
293T, H460, OVCAR-8 and HeLa cells were grown in
Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine
serum and antibiotics. Cells were cultured at 37˚C in a humidified
atmosphere of 5% CO2/95% air. 293T, HeLa and OVCAR-8
cells were usually passaged when confluent, at a 1:10 ratio and
H460 at a 1:12 ratio. 293T, H460 and HeLa cells were obtained from
the American Type Culture Collection (ATCC) and OVCAR-8 from Dr
Faustino Mollinedo (Center for Biomedical Research, Madrid, Spain)
who obtained them from the ATCC.
For the generation of viral particles,
2.5x106 293T cells were transfected using the JetPEI™
reagent following the manufacturer's instructions. To produce
lentiviruses, the transfection mix containing the pLKO-GFP plasmid
(8 µg), as well as the packaging vectors pMDLg/RRE, pRSV-Rev and
pMD2.G plasmids (4 µg each) and the JetPEI™ reagent (50 µl), were
incubated for 30 min at room temperature prior to addition to the
cells, which were maintained for 12 h at 37˚C, as previously
described (22). The
lentivirus-containing supernatants were filtered 48 h after
transfection and used to infect HeLa, H460 or OVCAR-8 cells by
incubation at 37˚C in the presence of 6 µg/ml polybrene and the
corresponding nutritional supplement (OOS or VS, at dilutions that
ranged from 1:2,000 to 1:100). After 24 h of incubation, media
containing viral supernatants were replaced by fresh culture media
in which none of the supplements was added, and 48 h later, samples
were used for subsequent experiments.
Similarly, to generate retroviruses, either 5 µg
pLZR-IRES-GFP or the pLZR-HA-ERK5 were co-transfected with the
retroviral accessory plasmids pMDG-VSV (2,5 µg) and
pNGUL-MLV-gag-pol (3 µg) as previously described (23), and viral supernatants were collected
and used as in the lentiviral transduction.
All the plasmids used for transfection were obtained
from Addgene, Inc., collaborators (Dr A. Bernard, National Center
of Biotechnology, Madrid, Spain) or prepared in the laboratory
(pLZR-HA-ERK5).
Flow cytometry and microscopy
To determine the percentage of infected
(GFP+) populations, cells were detached by trypsin
treatment, washed twice in PBS and acquired using an Accuri C6 Flow
Cytometer (BD Biosciences). A total of 20,000 events were collected
for each sample and analyzed using the C6 (version 1.0.264.21)
software (BD Biosciences). For each experimental condition and
time-point, three independent wells were analyzed. Before the
cytometric analysis, cells were observed under a fluorescence
microscope, and photomicrographs were acquired using the EVOS Floid
Cell Imaging Station (Life Technologies).
Preparation of cell lysates and
western blot analysis
Cells were washed with PBS and lysed, as described
previously (23). Briefly, cells
were washed with cold PBS and lysed in ice-cold lysis buffer (140
mM NaCl; 50 mM EDTA; 10% glycerol; 1% Nonidet P-40; 20 mM Tris-HCl,
pH 7.0; 1 mM PMSF; 1 mM Na3O4V; 1 µM
pepstatin; 1 µg/ml aprotinin; 1 µg/ml leupeptin; 25 mM
β-glycerolphosphate; 10 mM NaF; 1 mM sodium orthovanadate),
centrifuged at 4˚C at 15,000 x g for 10 min, and the supernatants
were transferred to new tubes. Then, 50 µg total protein per lane
was resolved by SDS-PAGE on 6% gels, transferred to PVDF membranes,
blocked for at least 1 h in blocking buffer (140 mM NaCl, 10 mM
Tris-HCl, pH 7.5, 0.05% Tween-20, 1% BSA) at room temperature, and
then incubated overnight at 4˚C with the primary antibodies diluted
1:10,000 (anti-HA) or 1:30,000 (anti-calnexin). After extensive
washing, the membranes were incubated for 30 min at room
temperature with horseradish peroxidase-conjugated goat anti-mouse
antibody diluted 1:10,000. Bands were visualized using a
luminol-based detection system with p-iodophenol enhancement
(Clarity Max™ Western ECL Substrate; Bio-Rad Laboratories, Inc.).
Protein content was determined using a BCA assay, as previously
described (23). Densitometrical
analysis of the gels was performed with Image Lab Touch Software,
version 2.4.0.03 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Each condition was analyzed in triplicate and data
are presented as the mean ± SD of at least three independent
experiments. One-way ANOVA was used to compare more than two
groups. Tukey's post hoc test or Games-Howell's post hoc test were
used in case of variance homogeneity or heterogeneity,
respectively. Data distributions were checked for normality using
the Shapiro-Wilk test, and homogeneity of variances was verified by
the Levene test. P<0.05 was considered to indicate a
statistically significant difference.
Results
With the aim of testing the potential antiviral
protection conferred by OOS or VS, an experimental system in which
HeLa cells were infected with two types of RNA virus (lentivirus
and retrovirus) was used (Fig. 1A).
Both viral genomes included a coding sequence for GFP. GFP
fluorescence was used to follow up the infection burden by
microscopy (Fig. 1B) or flow
cytometry (Fig. 1C). Infections
were carried out as described in the Materials and methods section,
using viral supernatant generated in 293T cells and based in the
retroviral vector pLZR-IRES-GFP or the lentiviral vector pLKO-GFP
(22,23). Microscopic analyses verified that
infection with the retroviral particles induced GFP expression in a
substantial number of cells (Fig.
1B). Moreover, flow cytometry showed that both vectors gave
infection efficiencies as high as 90% (Fig. 1C).
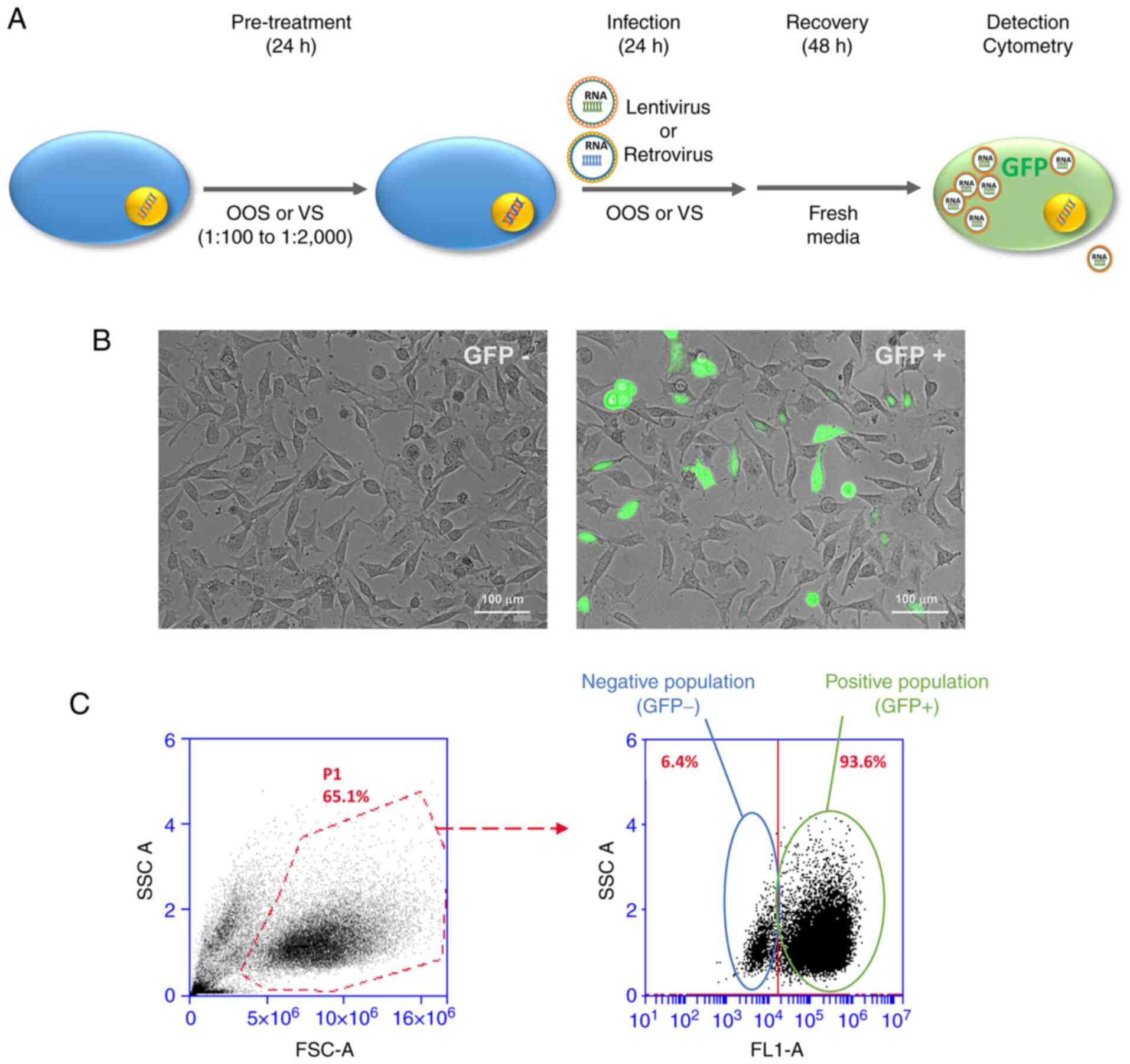 | Figure 1Representation of the experimental
design. (A) Schematic representation of the experimental design.
HeLa cells were pre-treated for 24 h with the indicated compounds,
before their transduction with retro or lentiviruses, which was
carried out for another 72 h. After transduction, the incidence of
transduced cells was determined by flow cytometry or western
blotting. (B) Viral infection could be followed by fluorescence
microscopy, since the viral particles express GFP, visible in those
conditions. Scale bar, 100 µm. (C) Follow up of infection by flow
cytometry. FSC-A and SSC-A indicate the population of living cells
(P1 gate). Of those cells included in P1, infected
(GFP+) cells are identified, and their frequency
evaluated. OOS, Ocoxin oral solution; VS, Viusid; GFP, green
fluorescence protein; SSC, side scatter; FSC, forward scatter. |
To determine the capacity of OOS or VS to protect
from viral infection, HeLa cells were incubated for 24 h with
different dilutions of these products (1:100-1:2,000, schematized
in Fig. 1A), then exposed to the
lentiviruses in culture media containing the indicated amounts of
the corresponding products. Infections in the absence and presence
of the products proceeded for 24 h and analyzed 72 h
post-transduction. Two control groups were included: Uninfected
cells (control) and infected cells without pre-treatment (untreated
positive control to which the OOS or VS-treated samples were
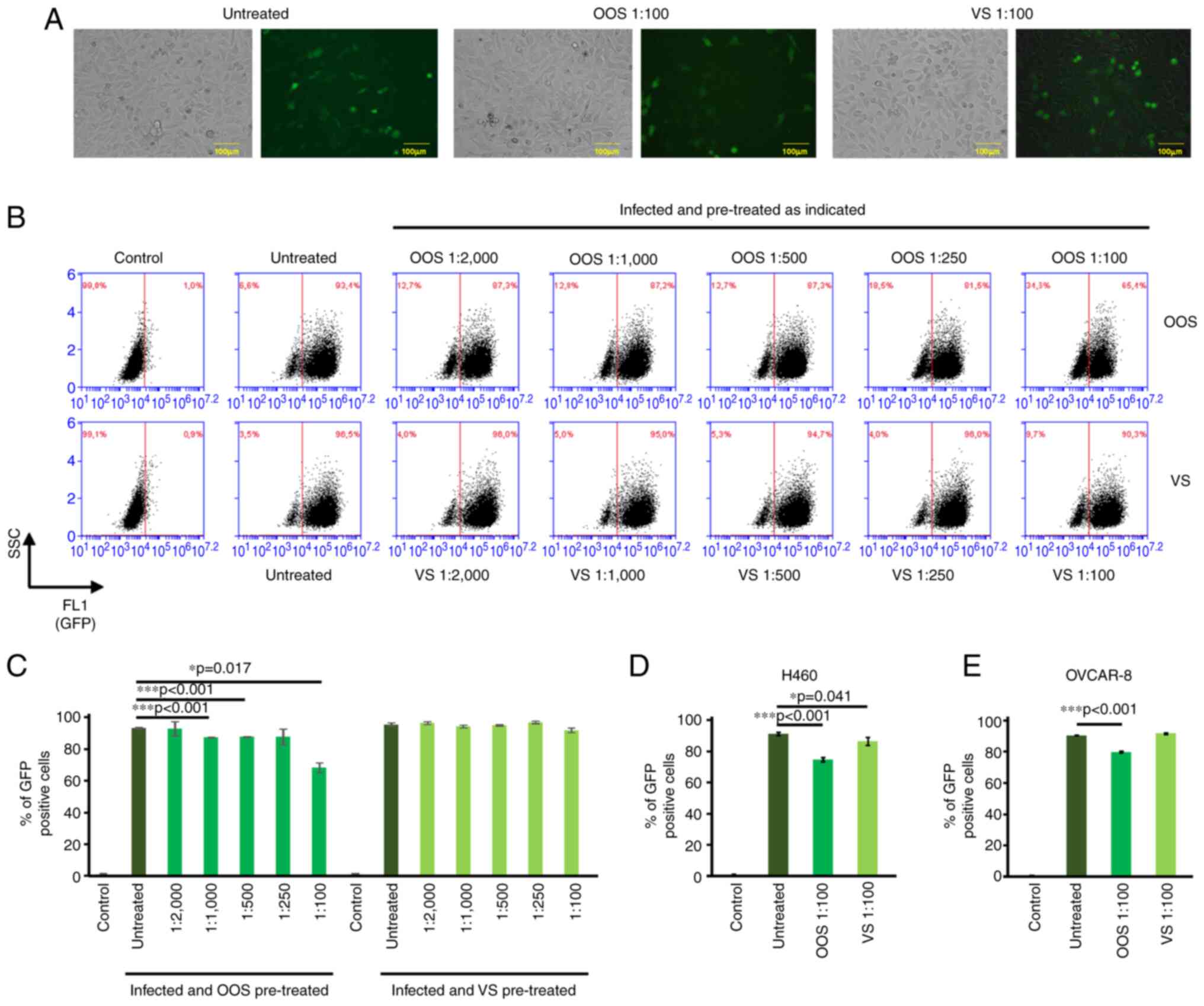
compared). Fig. 2A shows bright
field, as well as fluorescence analysis, of control HeLa cells
(left panels), or infected and pre-treated with OOS (middle panels)
or VS (right panels) both at a 1:100 dilution in culture media.
Treatment with OOS slightly decreased the number of cells
GFP+ cells, which was verified by flow cytometry. Under
these conditions, the control cells did not express GFP and were
used to establish the GFP+ population (Fig. 2B; control). In untreated HeLa cells
a GFP+ population could be clearly identified and
included >90% of the cells (95.5±1.1%; Fig. 2B, infected and untreated).
Pre-treatment with OOS decreased the infection by the lentivirus
(Fig. 2B and C). Moreover, the observed antiviral effect
was dose-dependent, reaching its maximal inhibition at the dilution
of 1:100 (68.2±3.1%; Fig. 2B and
C). VS induced a much milder
effect, which was only detectable at the 1:100 dilution (91.9±1.4%;
Fig. 2B and C). These experiments were also carried out
using retroviral instead of lentiviral infections, with similar
results (data not shown). To determine whether this protective
effect on viral infection was unique to HeLa cells or could be more
general, H460 (Fig. 2D) or OVCAR-8
(Fig. 2E) cells were pre-treated
with OOS or VS (both diluted 1:100) for 24 h prior to viral
infection. In both cases, OOS significantly reduced viral
infection, from 89.2±1.08 to 73.6±1.36% in H460 cells (Fig. 2D and S1A) and from 91.4±0.26 to 80.47±0.47% in
OVCAR-8 cells (Fig. 2E and S1B). VS had a much milder effect, which
was only detectable in H460 cells (89.2±1.08 to 84.6±2.46%).
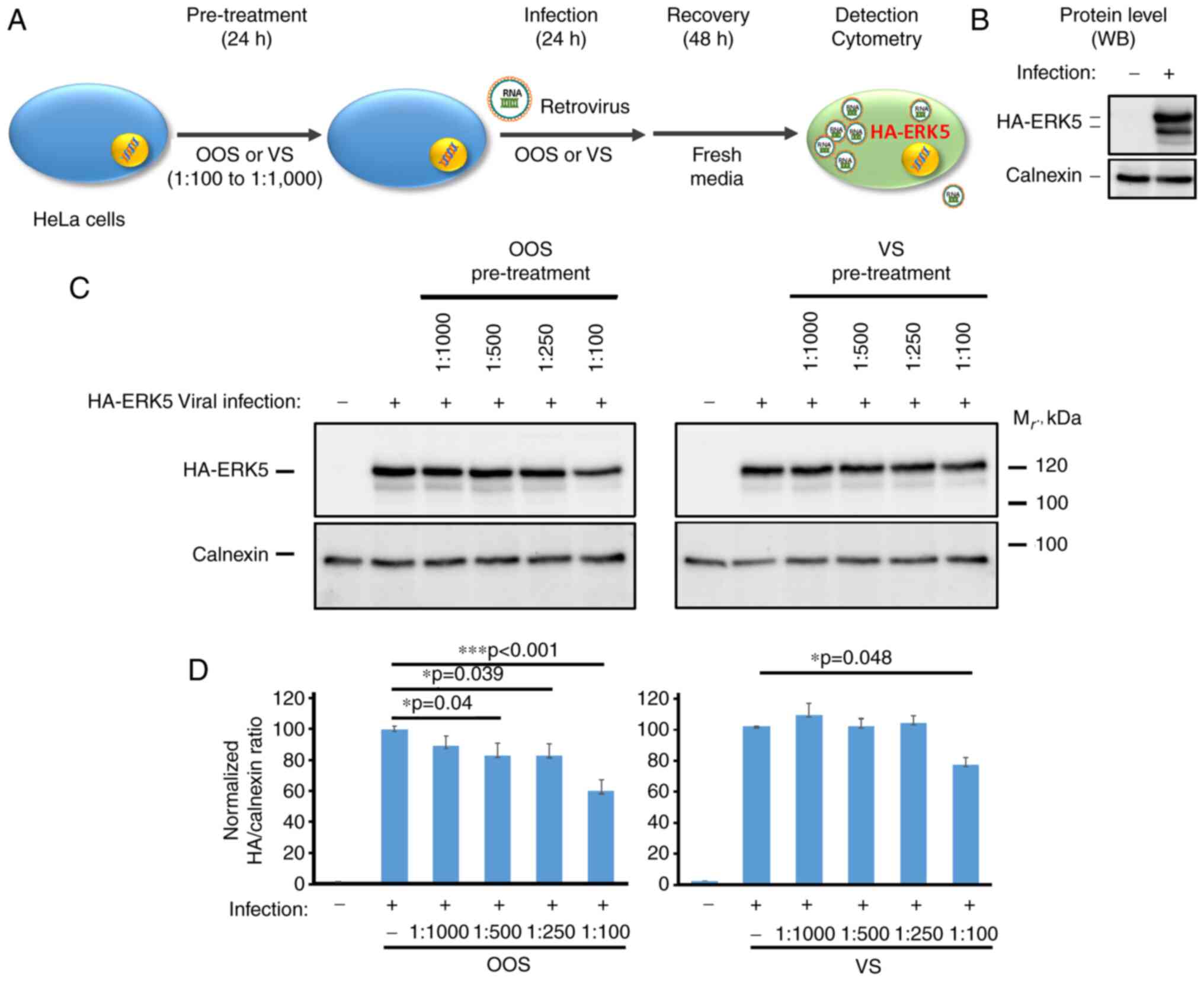
In the case of HeLa cells transduced with the
pLZR-IRES-GFP retroviral vector, an alternative approach using
western blot analysis was used to detect viral infection (Fig. 3A and B). To that end, since the vector includes
an internal ribosomal entry site, the sequence coding for an
HA-tagged version of the human mammalian mitogen-activated protein
kinase ERK5 was inserted. HeLa cells were plated and pre-treated
with OOS or VS as aforementioned, then infected with viral
particles containing pLZR-IRES-GFP-HA-ERK5. Protein lysates were
prepared three days later and quantitated, and equal amounts of
protein resolved by SDS-PAGE. Separated proteins in gels were
transferred to PVDF membranes which were probed with the anti-HA
antibody. As shown in Fig. 3B and
C, the anti-HA antibody failed to
recognize any band in the uninfected HeLa cells. In contrast, in
cells transduced with the retrovirus, the anti-HA antibody strongly
reacted with a 120 kDa protein, which corresponded to HA-tagged
ERK5. In this context, pre-treatment with both OOS and VS prevented
the infection with pLZR-HA-ERK5 in a dose-dependent manner,
reaching an inhibition rate of up to 40±1.4 and 24.8±4.9%,
respectively. In this experimental setting, OOS also demonstrated a
much stronger effect than that caused by VS (Fig. 3C and D). Reprobing of the blots with an antibody
to calnexin was used to verify equal amounts of protein loading as
well as to use that protein as an internal standard to normalize
expression amounts (Fig. 3D).
Discussion
In the present study, the potential antiviral
activities of the nutritional supplements OOS and VS were
investigated, demonstrating that OOS pre-treatment was able to
reduce both retroviral and lentiviral infection. OOS has
demonstrated its antitumoral activity in several clinical trials
including hepatocellular carcinoma (24), melanoma (25), head and neck (26), prostate (27), cervical and endometrial cancer
(28) among others (29,30),
and can improve the quality of life of patients (3). On the other hand, VS is a nutritional
supplement that has properties as immunomodulator and
hepatoprotector (4,5,7,31,32)
which have also been tested in a number of trials (31-33).
OOS and VS are formulations that include several
compounds with anti-oxidant, anti-inflammatory and antiviral
properties (3). These properties
make those products attractive to help in fighting different
diseases. Given the fact that some of the constituent components of
OOS and VS have been reported to act as antiviral compounds, the
aim of the present study was to further confirm whether OOS or VS
could have antiviral properties. Such study is important, since
patients who receive OOS or VS suffer from diseases in which the
immune system may be compromised, and therefore are more
susceptible to infections. Such is the case of oncological
disorders (16,17). In pathologies such as leukemia,
glioblastoma, hepatocellular carcinoma, or breast, lung, prostate,
colon or pancreatic cancer, OOS has been shown to reduce the
proliferation of a number of cell lines and xenografted tumors in
mice (8-14).
Moreover, several clinical studies have indicated that this product
is not toxic and may favor quality of life of patients, supporting
its use as a nutritional supplement (24,27,29,30).
In case that OOS or VS would exhibit antiviral capabilities, they
should be considered for its use in the treatment and prevention of
other cancer types that are known to be caused or associated to
viral infections, such as non-Hodgkin's lymphoma, or gastric,
hepatic or cervical cancer, among others (20,21,34-36).
In the present study, OOS and VS exerted antiviral
effects, as demonstrated by their ability to reduce viral infection
caused by two types of RNA viruses. This effect was evidenced by a
reduction in GFP fluorescence in several cell lines infected with
retroviral or lentiviral vectors, as well as a decreased production
of retrovirus-derived HA-ERK5. The date indicated that, while the
antiviral effect of OOS was larger than that of VS, such effect was
far from being high. Although the effect of these compounds could
have been potentiated by increasing its concentration, this option
was discarded because this higher concentration may affect not only
viral infection, but also cell proliferation as happens in several
cellular models (8-11),
making the effects more difficult to interpret. Nevertheless, while
the antiviral benefit may be small it may be useful to decrease
viral infective capacity and therefore increase the body's defense
mechanism against infections. Given the special susceptibility of
some patients to viral infections, the fact that OOS or VS may have
antiviral properties, in addition to their immunostimulatory
effects, appears an attractive property that should further
evaluated.
Finally, the actual threat that the COVID-19
pandemic represents for oncological patients also needs to be
considered (18,19). Given the discrete but reproducible
antiviral effect of VS and especially OOS, and the fact that those
products have demonstrated to be safe and well-tolerated in several
clinical contexts (3-7,37),
they may be considered in the fight against SARS-CoV-2 infections.
Furthermore, several recent reports indicate that some of the
constituents of OOS and VS may be effective at counteracting
COVID-19 both reducing the infectivity or severity of the disease
(8-11,38-40).
Besides the use of food supplements with antioxidant and
anti-inflammatory properties seems to be beneficial in that context
(41,42). In fact, clinical trials in that
direction, evaluating the antiviral protective actions of VS are
currently ongoing (https://www.clinicaltrials.gov/ct2/show/NCT04407182),
while others have recently been published (43,44).
However, the exact mechanism through which OOS or VS cause their
antiviral effect is unknown, although these products could affect
viral entry or replication. The present study suggested that
exploration of the antiviral actions of OOS is worthwhile, and may
help understand whether this supplement could help prevent
infections in oncological patients whose immune system is
immunocompromised.
Supplementary Material
Antiviral effect of OOS and VS on H460
and OVCAR-8 cells. Effect of OOS and VS pre-treatment on H460 (A)
or OVCAR-8 cells (B) measured by flow cytometry. GFP positive
populations were detected in living untreated cells, and compared
with those that had been pre-treated with a 1:100 dilution of OOS
or VS. Images of a representative experiment that was repeated at
least 4 times. OOS, Ocoxin oral solution; VS, Viusid; GFP, green
fluorescence protein.
Acknowledgements
The authors would like to acknowledge the Pandiella
group (Cancer Research Center of Salamanca, Spain) for their
helpful discussions and continuous support, and specially Ms. L.
Gandullo, for her help with the statistical analysis.
Funding
Funding: AP is a staff employee of the Spanish Research Council
(CSIC) and EDR is supported by a postdoctoral contract from the
same institution. ES is an employee of Catalysis S.L. The work on
ocoxin oral solution in preclinical models carried out by AP and
EDR has received partial support from Catalysis S.L.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EDR carried out the experiments. Both EDR and AP
designed the research, analyzed the experiments, wrote the paper
and prepared the figures. ES assisted in data analysis and
manuscript preparation. All authors read and approved the final
manuscript. AP and EDR confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
ES is an employee of Catalysis S.L. The work on OOS
in preclinical models carried out by AP and EDR has received
partial support from Catalysis S.L.
References
|
1
|
McComb S, Thiriot A, Akache B, Krishnan L
and Stark F: Introduction to the immune system. Methods Mol Biol.
2024:1–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parkin J and Cohen B: An overview of the
immune system. Lancet. 357:1777–1789. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pandiella-Alonso A, Diaz-Rodriguez E and
Sanz E: Antitumoral properties of the nutritional supplement ocoxin
oral solution: A comprehensive review. Nutrients.
12(2661)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gomez EV, Perez YM, Sanchez HV, Forment
GR, Soler EA, Bertot LC, Garcia AY, del Rosario Abreu Vazquez M and
Fabian LG: Antioxidant and immunomodulatory effects of Viusid in
patients with chronic hepatitis C. World J Gastroenterol.
16:2638–2647. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vilar Gomez E, Sanchez Rodriguez Y, Torres
Gonzalez A, Calzadilla Bertot L, Arus Soler E, Martinez Perez Y,
Yasells Garcia A and Abreu Vazquez Mdel R: Viusid, a nutritional
supplement, increases survival and reduces disease progression in
HCV-related decompensated cirrhosis: A randomised and controlled
trial. BMJ Open. 1(e000140)2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vilar Gomez E, Gra Oramas B, Soler E,
Llanio Navarro R and Ruenes Domech C: Viusid, a nutritional
supplement, in combination with interferon alpha-2b and ribavirin
in patients with chronic hepatitis C. Liver Int. 27:247–259.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vilar Gomez E, Rodriguez De Miranda A, Gra
Oramas B, Arus Soler E, Llanio Navarro R, Calzadilla Bertot L,
Yasells Garcia A and Del Rosario Abreu Vazquez M: Clinical trial: A
nutritional supplement Viusid, in combination with diet and
exercise, in patients with nonalcoholic fatty liver disease.
Aliment Pharmacol Ther. 30:999–1009. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Diaz-Rodriguez E, El-Mallah AM, Sanz E and
Pandiella A: Antitumoral effect of Ocoxin in hepatocellular
carcinoma. Oncol Lett. 14:1950–1958. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Diaz-Rodriguez E, Hernandez-Garcia S, Sanz
E and Pandiella A: Antitumoral effect of Ocoxin on acute myeloid
leukemia. Oncotarget. 7:6231–6242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Diaz-Rodriguez E, Sanz E and Pandiella A:
Antitumoral effect of Ocoxin, a natural compound-containing
nutritional supplement, in small cell lung cancer. Int J Oncol.
53:113–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hernandez-Garcia S, Gonzalez V, Sanz E and
Pandiella A: Effect of oncoxin oral solution in HER2-overexpressing
breast cancer. Nutr Cancer. 67:1159–1169. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hernandez-SanMiguel E, Gargini R, Cejalvo
T, Segura-Collar B, Núñez-Hervada P, Hortigüela R,
Sepúlveda-Sánchez JM, Hernández-Laín A, Pérez-Núñez A, Sanz E and
Sánchez-Gómez P: Ocoxin modulates cancer stem cells and M2
macrophage polarization in glioblastoma. Oxid Med Cell Longev.
2019(9719730)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hernandez-Unzueta I, Benedicto A, Olaso E,
Sanz E, Viera C, Arteta B and Márquez J: Ocoxin oral
solution® as a complement to irinotecan chemotherapy in
the metastatic progression of colorectal cancer to the liver. Oncol
Lett. 13:4002–4012. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hernandez-Unzueta I, Benedicto A, Romayor
I, Herrero A, Sanz E, Arteta B, Olaso E and Márquez J: Ocoxin oral
solution exerts an antitumoral effect in pancreatic cancer and
reduces the stromal-mediated chemoresistance. Pancreas. 48:555–567.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Perez-Pena J, Diaz-Rodriguez E, Sanz E and
Pandiella A: Central role of cell cycle regulation in the
antitumoral action of ocoxin. Nutrients. 11(1068)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rusu RA, Sirbu D, Curseu D, Năsui B, Sava
M, Vesa ŞC, Bojan A, Lisencu C and Popa M: Chemotherapy-related
infectious complications in patients with Hematologic malignancies.
J Res Med Sci. 23(68)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vento S and Cainelli F: Infections in
patients with cancer undergoing chemotherapy: Aetiology,
prevention, and treatment. Lancet Oncol. 4:595–604. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z,
Zhang Z, You H, Wu M, Zheng Q, et al: Patients with cancer appear
more vulnerable to SARS-CoV-2: A multicenter study during the
COVID-19 outbreak. Cancer Discov. 10:783–791. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fuentes-Antras J, Manzano A, Marquina G,
Paz M, Aguado C, Granja M, Benítez J, Ortega J, Priego A, González
C, et al: A snapshot of COVID-19 infection in patients with solid
tumors. Int J Cancer: Dec 3, 2020 (Epub ahead of print). doi:
10.1002/ijc.33420.
|
|
20
|
Bouvard V, Baan R, Straif K, Grosse Y,
Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L, et al: A review of human carcinogens-Part B: Biological
agents. Lancet Oncol. 10:321–322. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fernandez AF and Esteller M: Viral
epigenomes in human tumorigenesis. Oncogene. 29:1405–1420.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Diaz-Rodriguez E and Pandiella A:
Modulation of cereblon levels by anti-myeloma agents. Leuk
Lymphoma. 57:167–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Diaz-Rodriguez E, Álvarez-Fernández S,
Chen X, Paiva B, López-Pérez R, García-Hernández JL, San Miguel JF
and Pandiella A: Deficient spindle assembly checkpoint in multiple
myeloma. PLoS One. 6(e27583)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Al-Mahtab M, Akbar SM, Khan MS and Rahman
S: Increased survival of patients with end-stage hepatocellular
carcinoma due to intake of ONCOXIN(R), a dietary supplement. Indian
J Cancer. 52:443–446. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gray Lovio OR, Abreu Daniel A, García
Yánez LA, Rodríguez MO, González CV, Lence Anta JJ and Sanz E:
Efficacy and safety of oncoxin-viusid, a nutritional supplement, in
twenty patients with stage IIB-III of cutaneous melanoma: An
open-label proof of concept study. J Cancer Sci Ther. 11:263–268.
2019.
|
|
26
|
Rivas IC, Silva JA, Alfonso G, Candanedo
H, Cuervo Y, Mestre B, Mestre Cabello JR, Lence J, Lugoyo M and
Sanz E: Oncoxin-viusid with radiotherapy and chemotherapy in
patients with head and neck cancer: Results from a phase II,
randomised, double-blind study. J Cancer Sci Ther. 10:317–327.
2018.
|
|
27
|
Fundora Ramos MI, Maden LB, Casanova FO,
Cruz FH, Reyes CS, Gato AH, Lyncon IB, González EV, Morales KP,
Lence JJ and Sanz E: Oncoxin-Viusid® may improve quality
of life and survival in patients with hormone-refractory prostate
cancer undergoing onco-specific treatments. Mol Clin Oncol.
14(5)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ruiz Lorente R, Hernández Durán D, García
Viamontes J, Lence Anta J, Ortiz Reyes R and Sanz E: Efficacy of
Oncoxin-viusid on the reduction of adverse reactions to
chemotherapy and radiotherapy in patients diagnosed with cervical
cancer and endometrial adenocarcinoma. J Cancer Ther. 11:276–295.
2020.
|
|
29
|
Uddin D, Islam MA, Mahmood I, Ghosh AK,
Khatun RA and Kundu S: Findings of the 3-month supportive treatment
with ocoxin solution beside the standard modalities of patients
with different neoplastic diseases. TAJ. 22:172–175. 2009.
|
|
30
|
Kaidarova DR, Kopp MV, Pokrovsky VS,
Dzhugashvili M, Akimzhanova ZM, Abdrakhmanov RZ, Babich EN, Bilan
EV, Byakhov AV, Gurov SN, et al: Multicomponent nutritional
supplement Oncoxin and its influence on quality of life and therapy
toxicity in patients receiving adjuvant chemotherapy. Oncol Lett.
18:5644–5652. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ashraf S, Alam J, Sarkar JA, Khondaker FA,
Farhana Y and Khan NA: An open-label randomized clinical study to
compare the effects of a nutritional supplement versus vitamin E on
fibroscan score in nonalcoholic steatohepatitis (NASH) patients.
Univ J Public Health. 6:56–62. 2018.
|
|
32
|
Pascal R and Russu E: ‘Viusud’ as a
immnunomodultaor drug for the treatment of immunological
disturbances in psoriatic arthritis. Arta Medica. 4:38–42.
2006.
|
|
33
|
Valencia MH, Pacheco AC, Quijano TH, Girón
AV and López CV: Clinical response to glycyrrhizinic acid in
genital infection due to human papillomavirus and low-grade
squamous intraepithelial lesion. Clin Pract. 1(e93)2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Martel C, Georges D, Bray F, Ferlay J
and Clifford GM: Global burden of cancer attributable to infections
in 2018: A worldwide incidence analysis. Lancet Glob Health.
8:e180–e190. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Plummer M, de Martel C, Vignat J, Ferlay
J, Bray F and Franceschi S: Global burden of cancers attributable
to infections in 2012: A synthetic analysis. Lancet Glob Health.
4:e609–e616. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schelhaas M: Viruses and cancer: Molecular
relations and perspectives. Biol Chem. 398:815–816. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dominguez Gomez J, Simon RD, Abreu Daniel
A and Zelenkova H: Effectiveness of glycyrrhizinic Acid (glizigen)
and an immunostimulant (viusid) to treat anogenital warts. ISRN
Dermatol. 2012(863692)2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bae M and Kim H: Mini-review on the roles
of vitamin C, vitamin D, and selenium in the immune system against
COVID-19. Molecules. 25(5346)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Menegazzi M, Campagnari R, Bertoldi M,
Crupi R, Di Paola R and Cuzzocrea S: Protective effect of
epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled
immune activation: Could such a scenario be helpful to counteract
COVID-19? Int J Mol Sci. 21(5171)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mhatre S, Srivastava T, Naik S and
Patravale V: Antiviral activity of green tea and black tea
polyphenols in prophylaxis and treatment of COVID-19: A review.
Phytomedicine. 85(153286)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mrityunjaya M, Pavithra V, Neelam R,
Janhavi P, Halami PM and Ravindra PV: Immune-boosting, antioxidant
and anti-inflammatory food supplements targeting pathogenesis of
COVID-19. Front Immunol. 11(570122)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lammi C and Arnoldi A: Food-derived
antioxidants and COVID-19. J Food Biochem.
45(e13557)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Benites W, Heras MV, Mero ML and Marquez
D: Effectiveness of VIUSID® and ASBRIP® in
hospitalized patients infected by SARS-CoV-2 and mild-to-moderate
respiratory illness. An observational prospective study. Clin
Infect Dis. (5)2021.
|
|
44
|
Petrov P, Mihaylov A, Shopove M, Boncheva
M and Marquez D: Efficacy and safety of viusid and asbrip in
hospitalized patients with mild and moderate COVID-19: A randomized
controlled trial. Adv Infectious Dis. 11:171–184. 2021.
|