Introduction
Preeclampsia is a pregnancy-specific clinical
syndrome with an incidence rate of 3-6%, which is responsible for
10-15% of maternal deaths (1,2). The
classical definition of preeclampsia is a novel-onset hypertension
combined with novel-onset proteinuria after gestational week 20, or
a novel-onset preeclampsia-associated signs without proteinuria
(3). Preeclampsia can cause
maternal pulmonary edema, abnormal expression of liver enzymes,
eclampsia and central nervous system complications, but can also
cause fetal growth and development-related complications (4). The clinical characteristics of
preeclampsia demonstrate notable heterogeneity, suggesting that it
might be difficult to explain the pathogenesis of preeclampsia
based on a single etiological factor (5). At present, preeclampsia is generally
considered to involve multiple factors, pathways and mechanisms
(6). Its etiology may be closely
associated with insufficient invasion of trophoblasts, increased
oxidative stress, abnormal immune regulation mechanisms, vascular
endothelial damage and genetic mechanisms (7). However, there is a consensus that
placental dysfunction serves a crucial role in the development of
preeclampsia (8).
The placenta is the foundation of the
maternal-infant relationship, and maternal-fetal nutrient transport
is the basis of fetal growth and development (9). Lipid transport in the placenta is not
only the primary source of fetal energy supply, but also the key
material that promotes the development of the fetal nervous system
(10,11). Lipid metabolism disorder can cause
inflammatory reactions and accumulation of oxidative stress
products, affecting trophoblast invasion and placental development
and causing vascular endothelial damage and inhibition, leading to
a series of pathological changes (12). Compared with normal pregnant women,
the serum lipid levels and lipoprotein metabolism of patients with
preeclampsia is abnormal (13).
High blood lipid levels in patients with preeclampsia lead to
atherosclerotic changes during pregnancy, which can persist for
several years after delivery, increasing the risk of cardiovascular
disease (14). This may be due to
the increase in blood vessel injury components, such as serum
C-reactive protein, homocysteine and dimethylarginine and a
decrease in vascular protective components, such as vascular
endothelial growth factor and angiopoietins 2 in patients with
preeclampsia (15). Lipid
metabolism disorders might therefore lead to the production of a
large number of lipid peroxides, resulting in vasospasm and
endothelial cell damage (16).
Placental lipid transport is an important energy
source for the fetus. Maternal triglycerides (TGs) need to be
decomposed into fatty acids by the adipose TG lipase (ATGL) of the
placenta before entering the fetus (17). Lipoprotein lipase (LPL) is a member
of the ATGL family that is widely present in adipocytes,
macrophages, myocardium and skeletal muscle (18). LPL is involved in the hydrolysis of
TGs in chylomicrons and very low-density lipoprotein granules, and
their products can be used for oxidative energy supply and lipid
metabolism (18). The disorder of
placental lipid transport can lead to lipid accumulation and
increased lipid peroxidation with oxidative activity, resulting in
inflammation, inward flow of extravascular lipids, and a further
increase in vascular permeability, resulting in endothelial damage
(19). Resistin can upregulate the
expression of LPL in macrophages via the PPARγ-dependent PI3K/Akt
signaling pathway, thus accelerating the transfer of extracellular
oxidized low-density lipoprotein (oxLDL) to macrophages (20). The increase of LPL expression not
only contributes to the hydrolysis of intracellular TG, but also
promotes the accumulation of lipids in macrophages (20).
Comparative gene identification-58 (CGI-58) is a
glycoprotein with a molecular weight of 28 kb, which is highly
expressed in fat, liver, testis, muscle and other tissues, and can
be used as an agonist in the hydrolysis of ATGL and
lysophosphatidic acid acyltransferase (21,22).
Due to co-activation, LPL can increase its autocatalytic activity
by 20 times by binding with co-activating protein CGI-58(13). Under the influence of maternal
obesity, dyslipidemia and elevated insulin levels, CGI-58 is
involved in the regulation of the TG hydrolysis pathway in fetal
placenta. CGI-58 knockout macrophages are similar to foam cells,
which contain large amounts of triglycerides and cholesteryl esters
(23). Triglycerides and
cholesteryl esters are easy to deposit under vascular endothelial
cells and cause oxidative stress due to defective PPARγ signaling
(24). Under the stimulation of
oxLDL, the deposition of lipid droplets in macrophages is
increased, which aggravates the formation of atherosclerosis
(25). Lipid metabolism disorder
can cause hyperlipidemia and accumulation of lipolysis products,
which might cause endothelial cell damage, such as vasoconstriction
and hypercoagulability, which are related to the pathogenesis of a
preeclampsia (26). The oxidative
stress caused by lipid metabolism disorder in the placenta is
enhanced, and the invasive ability as well as apoptosis in the
trophoblasts is decreased, which affect the lipid transport
function of the placenta (27).
CGI-58 and LPL are therefore involved in the process of placental
TG hydrolysis; however, whether both are related to preeclampsia
requires further investigation.
In the present study, the expression of CGI-58 and
LPL in the placenta and the blood lipid levels were determined. The
association between CGI-58 and LPL in the placenta with
preeclampsia and blood lipid levels were further examined. The
results from the present study may improve the current
understanding of the pathogenesis of preeclampsia and highlight
novel treatment strategies for patients.
Materials and methods
Patients
A total of 37 pregnant women with preeclampsia and
40 normal pregnant women in the Department of Obstetrics and
Gynecology of the North China University of Science and Technology
Affiliated Hospital (Tangshan, China) were recruited between
September 2018 and July 2019. The present study was approved by the
Ethics Committee of the North China University of Science and
Technology Affiliated Hospital (approval no. 20180718A) and
performed in accordance with the Declaration of Helsinki. All
participants provided written informed consent for the use of their
placenta, blood and clinical information.
The diagnostic criteria of preeclampsia were as
follows: After 20 weeks of gestation, systolic blood pressure was
≥140 mmHg and/or diastolic blood pressure was ≥90 mmHg, with
proteinuria ≥0.3 g/24 h, or random urine protein (+); or, if there
was no proteinuria, any of the following: i) thrombocytopenia
(platelet <100x109 /l); ii) liver function damage
(serum transaminase level >2 times that of normal values; iii)
impairment of renal function (serum creatinine levels >1.1 mg/dl
or 2x the normal value); iv) pulmonary edema; or v) novel central
nervous system abnormalities or visual disorders (3). The inclusion criteria for the
preeclampsia group were as follows: Diagnosis of preeclampsia,
gestational weeks between 37 and 41 and singleton birth by cesarean
section. Were excluded from the preeclampsia group pregnant women
with the following diseases: Chronic hypertension, diabetes
mellitus, gestational diabetes mellitus, thyroid dysfunction,
nephritis, cholestasis syndrome and autoimmune diseases, severe
infectious diseases or premature rupture of membranes during
pregnancy. The inclusion criteria for the control group were as
follows: Healthy pregnant women who gave birth by cesarean section
due to social factors, scarred uterus or cephalopelvic
disproportion, no history of hypertension, singleton delivery and
gestational weeks between 37 and 41. The exclusion criteria for the
control group was pregnant woman who did not provide consent.
Placental tissue collection
Within 30 min of delivery, two placental tissue
sections of ~1 cm3 were randomly taken from the maternal
surface of the placenta under strict aseptic conditions. Embolic
and calcified sites were avoided. One sample was stored at -80˚C
for western blotting and RT-qPCR analysis. The other sample was
fixed using 10% formalin for 24 h at room temperature, embedded in
paraffin and sectioned with thickness of ~3 mm for
immunohistochemical analysis.
Biochemical analyses
Fasting venous blood (fasting for ≥8 h) was
collected 1 week before delivery, and the blood lipid levels were
measured. Based on cholesterol oxidase method, total cholesterol
(TC) was determined with the total cholesterol test kit (cat. no.
OSR6116; Beckman Coulter) according to the manufacturers'
instructions. TG level was evaluated by GPO-PAP method (cat. no.
GS111Z), high density lipoprotein cholesterol (HDL-C) was evaluated
by selective inhibition method (cat. no. GS131Z), low density
lipoprotein cholesterol (LDL-C) was evaluated by surfactant removal
method (cat. no. GS141Z), lipoprotein small a [Lp(a)] was evaluated
by immunological turbidimetry assay (cat. no. GS151Z),
apolipoprotein A (ApoA) was evaluated by immunological turbidimetry
assay (cat. no. GS161Z), and apolipoprotein B (ApoB) was evaluated
by immunological turbidimetry assay (cat. no. GS171Z). All these
kits were purchased from Beijing Jiuqiang Biotechnology Co., Ltd.
Routine blood tests were performed by the Laboratory Department of
North China University of Science and Technology Affiliated
Hospital using AU5800 Chemistry Analyzer (Beckman Coulter). The
Department of Obstetrics and Gynecology collected the clinical
examination data, including age, gestational week, number of
pregnancies, number of births, uterine height, abdominal
circumference, neonatal weight, BMI before pregnancy and
gestational weight gain.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from placental tissue using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and 5 µg RNA was reverse transcribed into cDNA using an
All-in-one™ First-Strand cDNA Synthesis kit (Guangzhou Fansi
Biotechnology Co., Ltd) according to the manufacturers' protocol.
Subsequently, qPCR was performed using a SYBR Green Realtime PCR
Mix kit (Mei5bio; Beijing Jumei Biotechnology Co., Ltd.). Gene
accession numbers were 51099 for CGI-58 (www.ncbi.nlm.nih.gov/gene/51099) and 4023 for LPL
(www.ncbi.nlm.nih.gov/gene/4023). The sequences of the
primers used were as follows: CGI-58, forward
5'-ATCAAGGGTTAATCATCTCA-3', reverse 5'-CTGGAATTGGTCTGTCTT-3'; LPL,
forward 5'-CATAGCCTATAATTGGTTAG-3, reverse
5'-GTGTAGATGAGTCTGATT-3'; and β-actin, forward
5'-ATATGAGATGCGTTGTTA-3' and reverse 5'-AAGTATTAAGGCGAAGAT-3'.
β-actin was used as the internal reference. The thermocycling
conditions were as follows: Initial denaturation at 95˚C for 10
min; followed by 40 cycles of 95˚C for 15 sec, 60˚C for 20 sec,
72˚C for 30 sec and 60˚C for 1 min. The relative expression levels
of CGI-58 and LPL were normalized to the internal reference control
β-actin and quantified using the 2-ΔΔCq method (ΔCq=Cq
value of CGI-58 or LPL-Cq value of β-actin; ΔΔCq=Cq value of
preeclampsia-Cq value of control) (28).
Immunohistochemistry analysis
Paraffin-embedded placental tissue samples with
thickness of ~3 mm were successively dewaxed and rehydrated using
xylene, ethanol (100, 95, 85 and 75%) and tap water. Sections were
immersed in 0.01 mol/l sodium citrate buffer above 100˚C for
antigen retrieval, and peroxide activity was quenched using 3%
H2O2 at room temperature for 15 min.
Subsequently, sections were incubated separately with either rabbit
anti-human CGI-58 antibody (1:100; cat. no. DF12065; Affinity
Biosciences) or rabbit anti-human LPL antibody (1:100; cat. no.
A9228; ABclonal) at 4˚C for 12 h. Then, sections were incubated
with goat anti-rabbit IgG horseradish peroxidase-conjugated
secondary antibody (1:100; cat. no. ab97051; Abcam.) for 30 min at
37˚C. Samples were washed with PBS and stained using a DAB Staining
kit (Sigma-Aldrich; Merck KGaA) according to the manufacturers'
protocol. The samples were counterstained with hematoxylin, rinsed,
dehydrated (70, 80, 95 and 100% ethanol, analytical reagent grade)
and sealed with neutral resin sequentially. The immunohistochemical
staining was imaged using a Micro Publisher 5.0 microscope (Roper
Industries; magnification, x400) and staining was analyzed using
Image-Pro-Plus 6.0 software (Media Cybernetics, Inc.).
The CGI-58 and LPL staining were classified as
positive or negative, depending on the presence or absence of
brownish yellow granules in the cell membrane and cytoplasm,
respectively. A total of 10 randomly selected fields of view were
selected to count the total number of cells and the number of
positive cells. The positive cell rate (%) was calculated using the
following formula: (Number of positive cells/total number of cells)
x100. If the positive cell rate was >10%, the sample was
considered as positive (+), otherwise, it was considered as
negative.
Western blotting
Total proteins from placental tissue were extracted
using RIPA protein lysate (Beyotime Institute of Biotechnology) on
ice and quantified using a BCA Protein assay kit (Beyotime
Institute of Biotechnology) according to the manufacturers'
protocols. Proteins (15 µg) were denatured and separated by 12%
SDS-PAGE and transferred onto a PVDF membrane at 100 V for 2 h.
Once nonspecific binding was blocked using 5% skimmed milk at 4˚C
overnight, membranes were incubated with the rabbit anti-human
primary antibodies against CGI-58 (1:1,000; cat. no. DF12065;
Affinity Biosciences), LPL (1:1,000; cat. no. A9228; ABclonal) and
β-actin (1:3,000; cat. no. ab8226; Abcam) overnight at 4˚C.
Membranes were then incubated with goat anti-rabbit IgG secondary
antibody (1:3,000; cat. no. ab97051; Abcam) at room temperature for
2 h. Eventually, antibody binding was detected using enhanced
chemiluminescence substrate (National Diagnostic) and visualized
using autoradiography. Relative expression levels of CGI-58 and LPL
were normalized to endogenous control β-actin using Image Lab v.5.0
software (Bio-Rad Laboratories, Inc.).
Statistical analysis
SPSS version 17.0 (SPSS, Inc.) was used for
statistical analysis. Data are presented as the means ± standard
deviation and compared using a Student's t-test. A χ2
test was used to analyze the classified count data. Pearson's
linear correlation analysis was used to analyze the relationship
between two variables. A multivariate logistic regression model was
used for multivariate analysis. α=0.05 was used as the test level
(bilateral) and P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of clinical data
As presented in Table
I, there were no significant differences in age, gestational
week, pregnancy time, production time and neonatal weight between
the preeclampsia and control groups (all P>0.05). However, the
uterine height, abdominal circumference, BMI before pregnancy and
gestational weight gain in the preeclampsia group were
significantly higher compared with the control group
(P<0.05).
 | Table IClinical data analysis of the
preeclampsia and control groups. |
Table I
Clinical data analysis of the
preeclampsia and control groups.
| Variable | Preeclampsia
(n=37) | Control (n=40) | P-value |
|---|
| Age, years | 29.85±4.78 | 28.69±3.60 | 0.21 |
| Gestational week,
weeks | 37.8±0.63 | 39.1±1.25 | 0.08 |
| Number of
pregnancies, n | 1.85±0.87 | 1.90±1.30 | 0.81 |
| Number of births,
n | 1.41±0.54 | 1.36±0.49 | 0.61 |
| Uterine height,
cm | 34.52±3.82 | 32.88±2.30 | 0.02a |
| Abdominal
circumference, cm | 106.61±9.58 | 97.95±15.05 | 0.00a |
| Neonatal weight,
g |
3,105.20±583.33 |
3,295.33±385.57 | 0.08 |
| BMI before
pregnancy | 24.46±5.06 | 22.27±3.15 | 0.02a |
| Gestational weight
gain, kg | 18.48±5.96 | 14.01±4.74 | 0.00a |
A seen in Table
II, there was no significant difference in TC and Lp(a) levels
between the preeclampsia and control groups (P>0.05). The TG,
LDL-C and ApoB levels in the preeclampsia group were significantly
increased compared with those in the control group (all P<0.05).
HDL-C and ApoA levels in the preeclampsia group were significantly
decreased compared with the control group (P<0.05). As an index
to predict atherosclerosis, the atherosclerotic index (AI) can
directly reflect the degree of lipid metabolism disorder, and was
calculated as follows: AI=(TC-HDL-C)/HDL-C. The AI value of the
preeclampsia group was significantly higher than that of the
control group (P<0.05).
 | Table IIComparison of blood lipid levels
between preeclampsia and control groups. |
Table II
Comparison of blood lipid levels
between preeclampsia and control groups.
| Blood lipids | Preeclampsia
(n=37) | Control (n=40) | P-value |
|---|
| TC, mmol/l | 6.19±1.23 | 5.93±1.09 | 0.30 |
| TG, mmol/l | 3.57±1.09 | 2.83±1.08 | 0.00a |
| HDL-C, mmol/l | 1.76±0.50 | 2.02±0.38 | 0.01a |
| LDL-C, mmol/l | 3.57±0.78 | 3.21±0.78 | 0.03a |
| Lp(a), mg/l | 205.17±92.05 | 135.74±83.48 | 0.07 |
| ApoA, g/l | 2.01±0.34 | 2.18±0.26 | 0.01a |
| ApoB, g/l | 1.28±0.27 | 1.14±0.29 | 0.02a |
| AI | 3.88±0.97 | 2.95±1.14 | 0.00a |
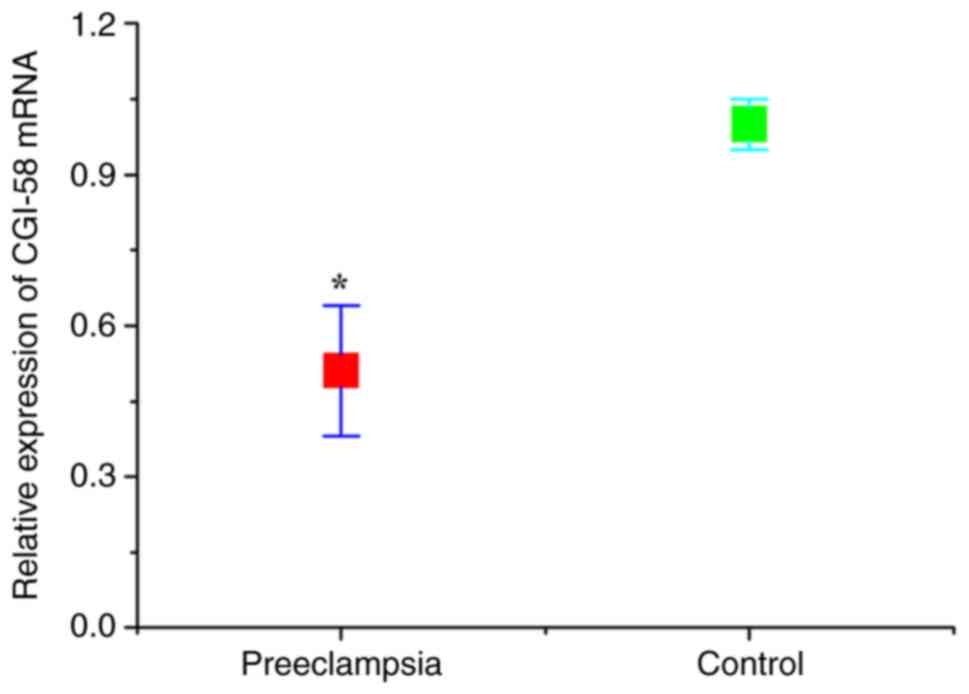
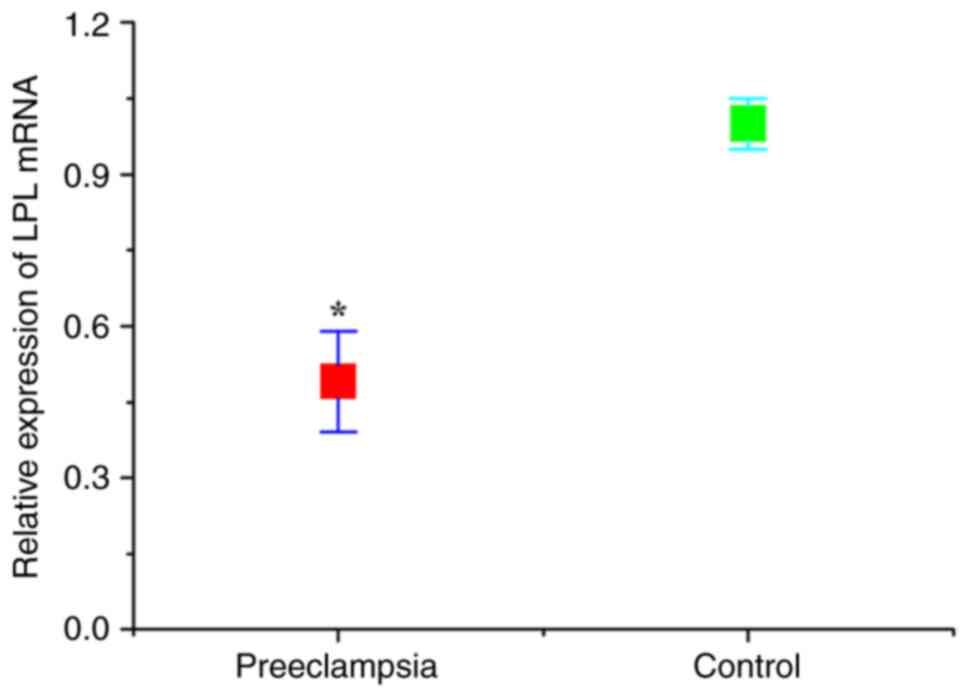
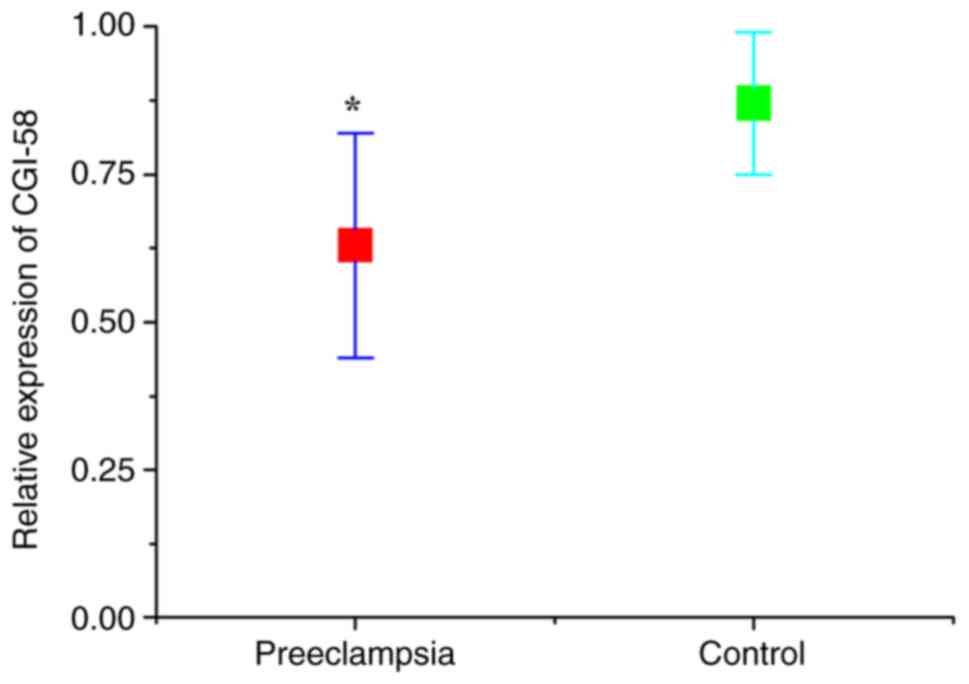
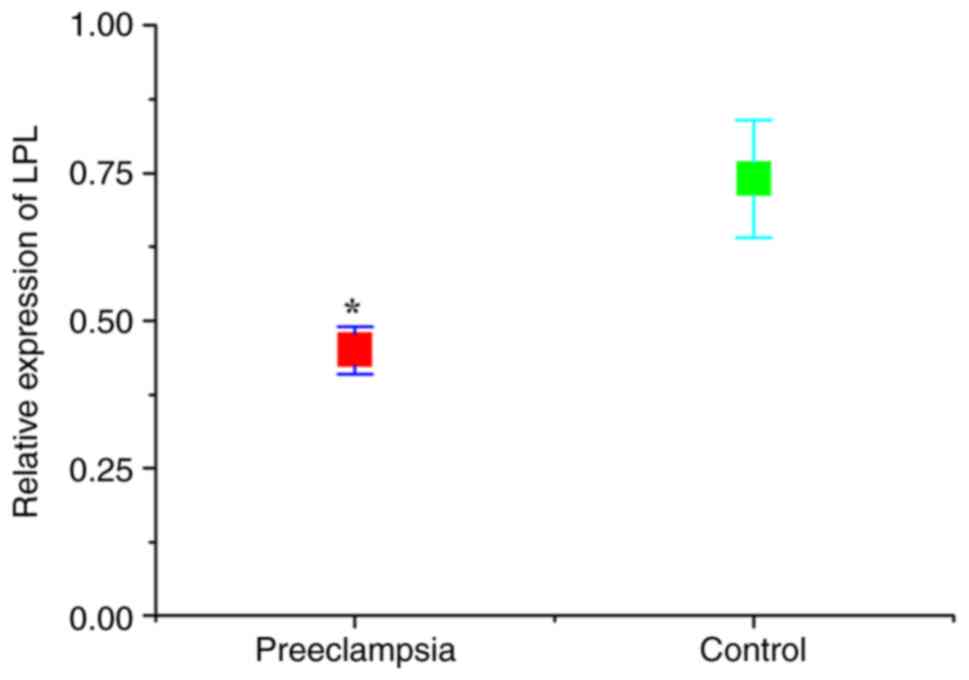
CGI-58 and LPL levels
The expression levels of CGI-58 and LPL in placental
tissues were detected using RT-qPCR. As presented in Figs. 1 and 2, the relative expression of both CGI-58
and LPL mRNA in placental tissues of the preeclampsia group was
significantly lower than that of control (normal pregnant) group
(both P<0.05). The expression levels of CGI-58 and LPL in
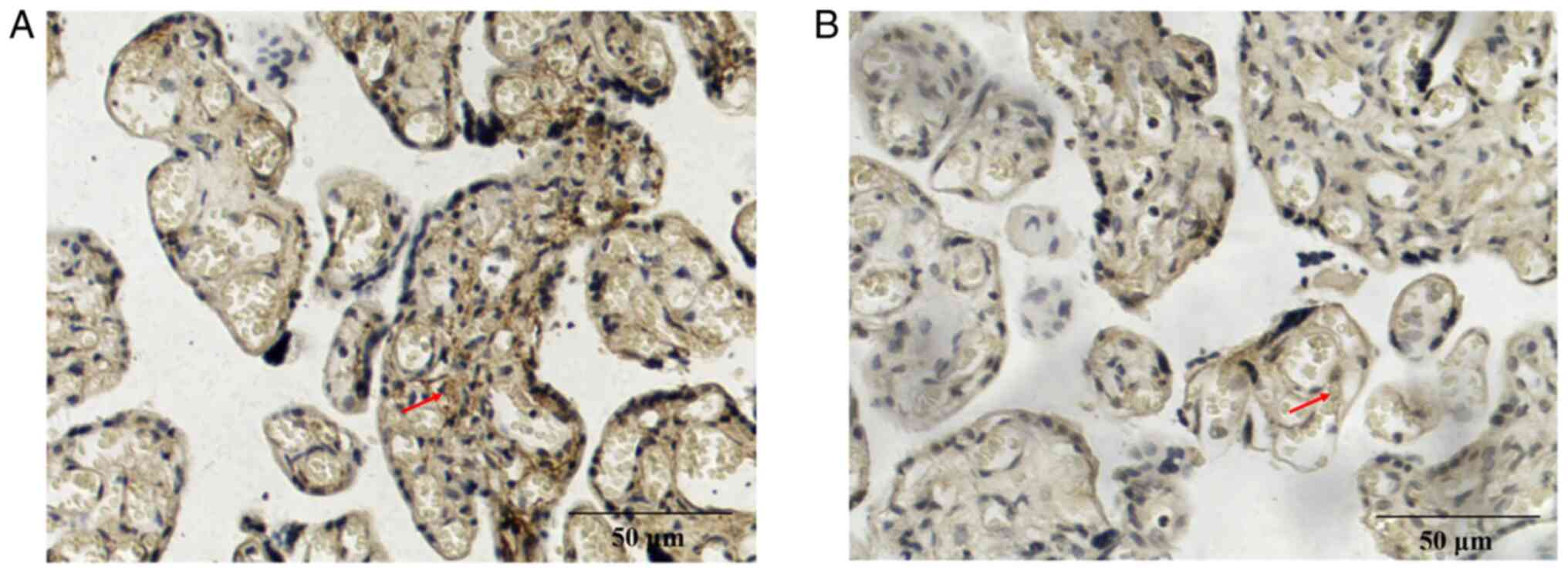
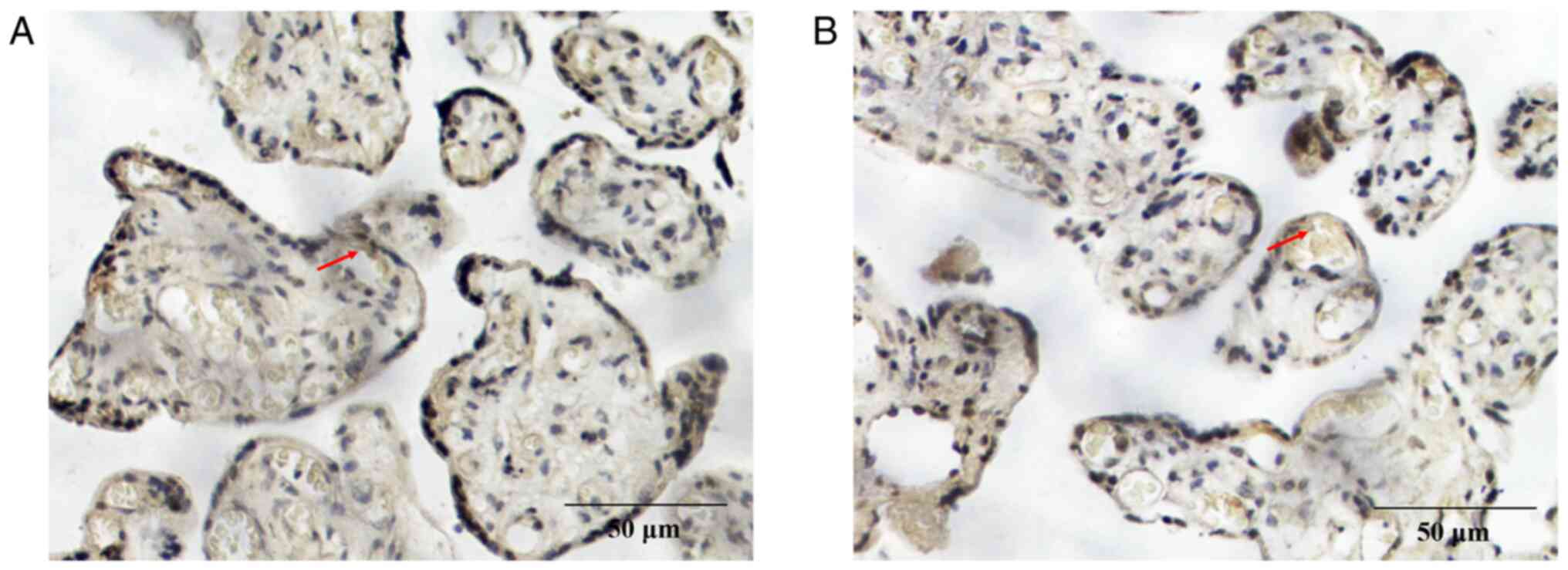
placental tissue were further assessed using immunohistochemistry.
The positive expression of CGI-58 (Fig. 3) and LPL (Fig. 4) was characterized by brown and
yellow granules in the cell membrane and cytoplasm, and their
expression was weakly positive in the preeclampsia group (Figs. 3A and 4A) and positive in the control group
(Figs. 3B and 4B). The positive rates of CGI-58 and LPL
were 40.54 and 29.73%, respectively, in the preeclampsia group,
which were both significantly lower compared with the control group
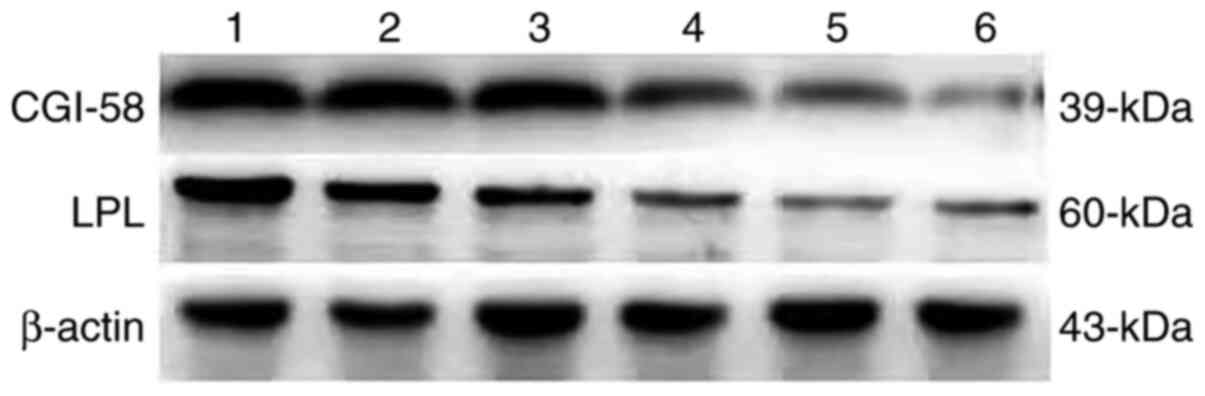
(72.50 and 67.50%, respectively; P<0.05; Table III). Semi quantitative western
blot method was also used to test the expression of CGI-58 and LPL
(Fig. 5). The expression of both
CGI-58 and LPL in the placental tissue of the preeclampsia group
was significantly lower than in the control group (P<0.05;
Figs. 6 and 7). The results from RT-qPCR,
immunohistochemistry and western blotting were therefore
consistent.
 | Table IIIImmunohistochemical examination for
CGI-58 and LPL in placenta of preeclampsia and control groups. |
Table III
Immunohistochemical examination for
CGI-58 and LPL in placenta of preeclampsia and control groups.
| | Number of
specimens | |
|---|
| Protein | Groups | Number | Negative
expression | Positive
expression | Positive rate
(%) | P-value |
|---|
| CGI-58 | Preeclampsia | 37 | 22 | 15 | 40.54 | 0.01a |
| | Control | 40 | 11 | 29 | 72.50 | |
| LPL | Preeclampsia | 37 | 26 | 11 | 29.73 | 0.00a |
| | Control | 40 | 13 | 27 | 67.50 | |
Association analysis
As presented in Table
IV, the mean protein expression levels of CGI-58 (0.75) and LPL
(0.59) in placental tissue were selected as the threshold between
high and low expression. A χ2 test revealed that the
expression levels of CGI-58 and LPL were associated with
preeclampsia. The risk of preeclampsia in pregnant women with high
expression levels of CGI-58 and LPL was only 0.19 and 0.22 in
pregnant women with low expression levels of CGI-58 and LPL,
respectively. These results demonstrated that low expression of
CGI-58 and LPL are risk factors for preeclampsia in pregnant
women.
 | Table IVRelationship between preeclampsia and
the expression of CGI-58 and LPL in placenta. |
Table IV
Relationship between preeclampsia and
the expression of CGI-58 and LPL in placenta.
| Factors | n | High level | Low level | χ2 | P-value | OR | 95% CI |
|---|
| CGI-58a | | ≥0.75 | <0.75 | | | | |
| Preeclampsia | 37 | 10 | 27 | 11.13 | 0.00b | 0.20 | 0.15-0.65 |
| Control | 40 | 26 | 14 | | | | |
| LPLa | | ≥0.59 | <0.59 | | | | |
| Preeclampsia | 37 | 13 | 24 | 5.76 | 0.02b | 0.33 | 0.17-0.75 |
| Control | 40 | 25 | 15 | | | | |
To investigate the risk factors of preeclampsia, the
indicators that differed significantly between the preeclampsia and
control groups, including uterine height, abdominal circumference,
BMI before pregnancy, gestational weight gain, TG, HDL-C, LDL-C,
ApoA, ApoB, AI, CGI-58 and LPL levels, were selected as independent
variables, and their corresponding means (33.73, 102.47, 23.41,
16.34, 3.22, 1.88, 3.40, 2.08, 1.21, 3.42, 0.75 and 0.59,
respectively) were used as the threshold between high and low
expression. As presented in Table
V, multivariate logistic regression analysis demonstrated that
gestational weight gain, TG, AI, CGI-58 and LPL levels were
significant different (all P<0.05), and the odds ratios were
7.61, 4.60, 5.98, 1.19 and 1.23, respectively. Their corresponding
95% confidence intervals were 2.22-26.04, 1.36-15.54, 1.87-19.09,
1.02-1.39 and 1.05-2.20, respectively.
 | Table VMultivariate logistic regression
analysis of factors affecting the development of preeclampsia. |
Table V
Multivariate logistic regression
analysis of factors affecting the development of preeclampsia.
| Factors | β | SE | Wald | P-value | OR | 95% CI |
|---|
| Gestational weight
gain, kg | 2.03 | 0.63 | 10.45 | 0.00a | 7.61 | 2.22-26.04 |
| TG, mmol/l | 1.53 | 0.62 | 6.02 | 0.01a | 4.60 | 1.36-15.54 |
| AI | 1.79 | 0.59 | 9.11 | 0.00a | 5.98 | 1.87-19.09 |
| CGI-58 | 0.17 | 0.08 | 4.76 | 0.03a | 1.19 | 1.02-1.39 |
| LPL | 0.21 | 0.09 | 5.62 | 0.04a | 1.23 | 1.05-2.20 |
Pearson's linear correlation analysis was used to
study the association between the expression of CGI-58 and LPL in
the placenta and the blood lipid levels. As presented in Table VI, CGI-58 was positively
correlated with HDL-C (r=0.63; P<0.01), negatively correlated
with TG and ApoB (r=0.84; P<0.01; and r=0.51; P<0.05,
respectively), and not correlated with TC, LDL-C, Lp(a), ApoA and
AI (P>0.05). CGI-58 was positively correlated with HDL-C
(r=0.52; P<0.01) and negatively correlated with TG and AI
(r=0.66; P<0.01; and r=0.47; P<0.05, respectively). In
addition, CGI-58 was positively correlated with LPL (r=0.60;
P<0.05).
 | Table VICorrelation analysis between CGI-58
and LPL expression in placenta and maternal blood lipid levels. |
Table VI
Correlation analysis between CGI-58
and LPL expression in placenta and maternal blood lipid levels.
| | CGI-58 | LPL |
|---|
| Factor | r | P-value | r | P-value |
|---|
| TC | -0.278 | 0.249 | -0.187 | 0.469 |
| TG | -0.840 | 0.000 | -0.659 | 0.000a |
| HDL-C | 0.625 | 0.003 | 0.524 | 0.009a |
| LDL-C | -0.373 | 0.174 | -0.372 | 0.095 |
| Lp(a) | -0.312 | 0.210 | -0.234 | 0.249 |
| ApoA | 0.393 | 0.097 | 0.313 | 0.186 |
| ApoB | -0.514 | 0.026 | -0.428 | 0.074 |
| AI | -0.486 | 0.063 | -0.466 | 0.038 |
Discussion
Preeclampsia is an idiopathic pregnancy disease
which seriously endangers the life of both the mother and the
infant. Although the etiology of preeclampsia has not been fully
elucidated, it is established that abnormal lipid metabolism during
pregnancy serves an important role in its pathogenesis (29,30).
Disorders of lipid metabolism and abnormal transformation of
lipoproteins can cause atherosclerosis, leading to the accumulation
of lipid peroxides, promoting oxidative stress and aggravating
damage to vascular endothelial function (31). Acute atherosclerotic changes are
observed in 20-40% of the placental tissues of patients with
preeclampsia, in which the vascular resistance of the spiral artery
increases and microthrombosis leads to placental villi infarction
and therefore, a decrease in placental perfusion (32). TG in maternal blood is converted
into fatty acids via the placenta, which is an important source of
energy for the fetus. CGI-58 and LPL are involved in the hydrolysis
of TG, and their abnormal expression can result in lipid transport
disorders in the placenta and affect the regulation of placental
metabolism and energy supply (13). In the present study, the expression
of CGI-58 and LPL in the placenta were therefore detected to
examine the relationship between CGI-58 and LPL expression and the
pathogenesis of preeclampsia.
To meet the needs of fetal growth and development
during pregnancy, the maternal blood lipid levels increase
gradually with time (11). Unlike
the changes in blood lipids during normal pregnancy, the higher
blood lipids level in preeclampsia pregnant women lead to
atherosclerotic changes, which can cause oxidative stress due to
high production of lipid peroxides and the subsequent release of
reactive oxygen species, resulting in decreased vasodilation and
increased endothelial damage (33). Wojcik-Baszko et al (12) reported that the levels of TC and TG
in preeclampsia pregnant women are >205 and >133 mg/dl, which
are higher than those in normal pregnant women by 3.6x and 4.15x,
respectively. LDL-C and HDL-C are also increased by 10.4% and
decreased by 7%, respectively. In the present study, the serum
levels of TG, LDL-C, ApoB and AI in the preeclampsia group were
significantly higher than those in the control group, whereas the
serum levels of HDL-C and ApoA in the preeclampsia group were lower
than those in the control group, which was consistent with a
previous study (18).
Being overweight or obese are the most important
risk factors for preeclampsia with attributable risk percentages of
64.9 and 64.4%, respectively (34). However, the relationship between
obesity and the onset of preeclampsia has not been fully
elucidated. Obese pregnant women may develop preeclampsia through a
lipid metabolism disorder (35).
In the present study, the BMI before pregnancy and the gestational
weight gain of preeclampsia patients were significantly higher than
those of normal pregnant women. The weight gain during pregnancy
was an independent risk factor of preeclampsia. With an increase in
gestational weight gain, the risk of preeclampsia increases. The
unbalanced diet and excessive intake of fat and other nutrients
during pregnancy lead to excessive weight gain, which may be
important factors causing lipid metabolism disorder. Weight control
and proper physical exercise during pregnancy can increase insulin
sensitivity, relieve sympathetic nervous tension and reduce serum
TG concentration and blood glucose levels (36). These measures may help obese people
reducing their risk of preeclampsia. The detection of blood lipid
levels can help identifying high-risk pregnant women earlier,
allowing a better tracking and targeted preventative interventions
and treatments, in order to reduce maternal mortality caused by
preeclampsia.
Fatty acids are the key substances for fetal brain
development and adipose tissue formation. Maternal TG is the most
important source of fetal fatty acids, and 20-50% of fetal fatty
acids come from the maternal circulation (7). TG is present in adipocytes in the
form of lipid droplets, which cannot pass through the cell membrane
directly. TG must be hydrolyzed by lipase to be transported in
vivo and deposited in adipose tissue or directly used for
energy. LPL participates in the hydrolysis of TG and promotes the
transport of lipids to embryos. CGI-58 can increase the catalytic
activity of LPL by 20x (13).
Disorders of placental lipid transport can lead to lipid
accumulation and increased lipid peroxide formation, resulting in
endothelial cell injury (37). In
the present study, the mRNA and protein expression of CGI-58 and
LPL in placenta was decreased, and their expression levels were
positively correlated, which may have led to the accumulation of
lipid hydrolysates and oxidative stress reactions via TG metabolism
and lipid transport.
Dyslipidemia and abnormal expression of lipoproteins
may be closely associated with the pathogenesis of preeclampsia.
Disorders of placental lipid transport can lead to lipid
accumulation and the production of a large number of peroxides,
which can cause endothelial cell damage (16). In addition, inflammatory factors
released by the placenta can cause systemic vascular endothelial
dysfunction (12). In pregnant
women with preeclampsia, glomerular endothelial hyperplasia and
alterations to the umbilical vein and placental uterine vascular
endothelial cells are observed (38). The levels of markers of endothelial
cell activation in serum, including adhesion molecules, cytokines,
procoagulant factors and antiangiogenic factors are increased
(16). Oxidative stress at the
placental interface can lead to disordered placental spiral artery
remodeling, resulting in placental dysplasia, insufficient
trophoblast cell infiltration and preeclampsia (31). CGI-58 and LPL are involved in the
first step of triglyceride hydrolysis and serve an important role
in lipid transport in the placenta (13). In the present study, CGI-58
expression was positively correlated with maternal serum HDL-C
levels and negatively correlated with TG and ApoB levels. Placental
LPL expression was positively correlated with HDL-C and negatively
correlated with TG level and AI. These results suggested that the
expression of CGI-58 and LPL in the placenta may be associated with
disorders of maternal lipid metabolism. As placental detection can
only be performed following delivery and since the expression of
CGI-58 and LPL in the placenta is associated with maternal blood
lipid levels, blood lipid levels during pregnancy may be used to
assist the diagnosis of preeclampsia. In addition, maternal serum
CGI-58 and LPL expression levels may also be associated with
preeclampsia or maternal blood lipid levels, and both these
hypotheses will be assessed. In future studies, the expression
levels of maternal CGI-58 and LPL will be assessed to determine the
relationship between lipid metabolism and the expression levels of
CGI-58 and LPL and to further clarify the mechanism by which
upregulated expression of CGI-58 and LPL can increase the risk of
preeclampsia.
In conclusion, compared with normal pregnant women,
TG, LDL-C, ApoB levels and AI in the serum of preeclampsia pregnant
women were significantly increased, whereas HDL-C and ApoA levels
were significantly decreased, suggesting that patients with
preeclampsia exhibited dyslipidemia. CGI-58 and LPL expression in
placental tissue of preeclampsia pregnant women was decreased, and
the expression levels of CGI-58 and LPL were positively correlated.
Therefore, the risk of preeclampsia was increased when the
expression levels of CGI-58 or LPL were decreased. It is
hypothesized that CGI-58 and LPL in the placenta may affect lipid
metabolism in the serum and placenta, and that CGI-58 and LPL may
be considered as important indicators for the diagnosis of
preeclampsia. CGI-58 and LPL could therefore serve an important
role in the pathogenesis of preeclampsia, which may be related to
the accumulation of lipid peroxides in the placenta of preeclampsia
women, leading to increased systemic oxidative stress.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD and YC designed the study. JD, MW and JG
performed the experiments. JD and JL analyzed the data. JD and YC
wrote the manuscript. JD and YC confirmed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the North China University of Science and Technology
Affiliated Hospital (approval no. 20180718A). Informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rana S, Lemoine E, Granger JP and
Karumanchi SA: Preeclampsia: Pathophysiology, challenges, and
perspectives. Circ Res. 124:1094–1112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
De Kat AC, Hirst J, Woodward M, Kennedy S
and Peters SA: Prediction models for preeclampsia: A systematic
review. Pregnancy Hypertens. 16:48–66. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Staff AC: The two-stage placental model of
preeclampsia: An update. J Reprod Immunol. 134-135:1–10.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Perucci LO, Corrêa MD, Dusse LM, Gomes KB
and Sousa LP: Resolution of inflammation pathways in preeclampsia-a
narrative review. Immunol Res. 65:774–789. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sheridan MA, Yang Y, Jain A, Lyons AS,
Yang P, Brahmasani SR, Dai A, Tian Y, Ellersieck MR, Tuteja G, et
al: Early onset preeclampsia in a model for human placental
trophoblast. Proc Natl Acad Sci USA. 116:4336–4345. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang T, Shi XZ and Wu WH: Crosstalk
analysis of dysregulated pathways in preeclampsia. Exp Ther Med.
17:2298–2304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Geldenhuys J, Rossouw TM, Lombaard HA,
Ehlers MM and Kock MM: Disruption in the regulation of immune
responses in the placental subtype of preeclampsia. Front Immunol.
9(1659)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Travaglino A, Raffone A, Saccone G,
Migliorini S, Maruotti GM, Esposito G, Mollo A, Martinelli P, Zullo
F and D'Armiento M: Placental morphology, apoptosis, angiogenesis
and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet
Gynecol Reprod Biol. 234:200–2006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Valencia-Ortega J, Zárate A, Saucedo R,
Hernández-Valencia M, Cruz JG and Puello E: Placental
proinflammatory state and maternal endothelial dysfunction in
preeclampsia. Gynecol Obstet Invest. 84:12–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang H, He B, Yallampalli C and Gao H:
Fetal macrosomia in a hispanic/Latinx predominant cohort and
altered expressions of genes related to placental lipid transport
and metabolism. Int J Obes (Lond). 44:1743–1752. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lewis RM, Wadsack C and Desoye G:
Placental fatty acid transfer. Curr Opin Clin Nutr Metab Care.
21:78–82. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wojcik-Baszko D, Charkiewicz K and
Laudanski P: Role of dyslipidemia in preeclampsia-A review of
lipidomic analysis of blood, placenta, syncytiotrophoblast
microvesicles and umbilical cord artery from women with
preeclampsia. Prostaglandins Other Lipid Mediat. 139:19–23.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kulminskaya N and Oberer M:
Protein-protein interactions regulate the activity of adipose
triglyceride lipase in intracellular lipolysis. Biochimie.
169:62–68. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Benschop L, Bergen NE,
Schalekamp-Timmermans S, Jaddoe VW, Mulder MT, Steegers EAP and van
Lennep JE: Maternal lipid profile 6 years after a gestational
hypertensive disorder. J Clin Lipidol. 12:428–436. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Onda K, Tong S, Beard S, Binder N, Muto M,
Senadheera SN, Parry L, Dilworth M, Renshall L, Brownfoot F, et al:
Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1
and soluble endoglin secretion, decrease hypertension, and rescue
endothelial dysfunction. Hypertension. 69:457–468. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Townsend R, Khalil A, Premakumar Y,
Allotey J, Snell KIE, Chan C, Chappell LC, Hooper R, Green M, Mol
BW, et al: Prediction of pre-eclampsia: Review of reviews.
Ultrasound Obstet Gynecol. 54:16–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lorentzen B, Drevon CA, Endresen MJ and
Henriksen T: Fatty acid pattern of esterified and free fatty acids
in sera of women with normal and pre-eclamptic pregnancy. Br J
Obstet Gynaecol. 102:530–537. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Olivecrona G: Role of lipoprotein lipase
in lipid metabolism. Curr Opin Lipidol. 27:233–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Colvin BN, Longtine MS, Chen B, Costa ML
and Nelson DM: Oleate attenuates palmitate-induced endoplasmic
reticulum stress and apoptosis in placental trophoblasts.
Reproduction. 153:369–380. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li B, Fang J, He T, Yin S, Yang M, Cui H,
Ma X, Deng J, Ren Z, Hu Y, et al: Resistin up-regulates LPL
expression through the PPARγ-dependent PI3K/AKT signaling pathway
impacting lipid accumulation in RAW264.7 macrophages. Cytokine.
119:168–174. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Oberer M, Boeszoermenyi A, Nagy HM and
Zechner R: Recent insights into the structure and function of
comparative gene identification-58. Curr Opin Lipidol. 22:149–158.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang J, Xu D, Nie J, Han R, Zhai Y and
Shi Y: Comparative gene identification-58 (CGI-58) promotes
autophagy as a putative lysophosphatidylglycerol acyltransferase. J
Biol Chem. 289:33044–33053. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goeritzer M, Schlager S, Radovic B,
Madreiter CT, Rainer S, Thomas G, Lord CC, Sacks J, Brown AL, Vujic
N, et al: Deletion of CGI-58 or adipose triglyceride lipase
differently affects macrophage function and atherosclerosis. J
Lipid Res. 55:2562–2575. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miao H, Ou J, Ma Y, Guo F, Yang Z, Wiggins
M, Liu C, Song W, Han X, Wang M, et al: Macrophage CGI-58
deficiency activates ROS-inflammasome pathway to promote insulin
resistance in mice. Cell Rep. 7:223–235. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang S, Liu G, Xu C, Liu L, Zhang Q, Xu
Q, Jia H and Li X and Li X: Perilipin 1 mediates lipid metabolism
homeostasis and inhibits inflammatory cytokine synthesis in bovine
adipocytes. Front Immunol. 9(467)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Contini C, Jansen M, König B,
Markfeld-Erol F, Kunze M, Zschiedrich S, Massing U, Merfort I,
Prömpeler H, Pecks U, et al: Lipoprotein turnover and possible
remnant accumulation in preeclampsia: Insights from the freiburg
preeclampsia H.E.L.P.-apheresis study. Lipids Health Dis.
17(49)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chiarello DI, Abad C, Rojas D, Toledo F,
Vázquez CM, Mate A, Sobrevia L and Marín R: Oxidative stress:
Normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol
Basis Dis. 1866(165354)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cao W, Wang X, Chen T, Xu W, Feng F, Zhao
S, Wang Z, Hu Y and Xie B: Maternal lipids, BMI and IL17/IL35
imbalance in concurrent gestational diabetes mellitus and
preeclampsia. Exp Ther Med. 16:427–435. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun W, Cui B, Hong F and Xu Y:
Establishment of ApoE-knockout mouse model of preeclampsia and
relevant mechanisms. Exp Ther Med. 12:2634–2638. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aouache R, Biquard L, Vaiman D and
Miralles F: Oxidative stress in preeclampsia and placental
diseases. Int J Mol Sci. 19(1496)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Staff AC, Dechend R and Redman CWG:
Review: Preeclampsia, acute atherosis of the spiral arteries and
future cardiovascular disease: Two new hypotheses. Placenta. 34
(Suppl):S73–S78. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dolnikoff M, Martín-Hidalgo A, Machado UF,
Lima FB and Herrera E: Decreased lipolysis and enhanced glycerol
and glucose utilization by adipose tissue prior to development of
obesity in monosodium glutamate (MSG) treated-rats. Int J Obes
Relat Metab Disord. 25:426–433. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Antwi E, Amoakoh-Coleman M, Vieira DL,
Madhavaram S, Koram KA, Grobbee DE, Agyepong IA and
Klipstein-Grobusch K: Systematic review of prediction models for
gestational hypertension and preeclampsia. PLoS One.
15(e0230955)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hirschmugl B, Desoye G, Catalano P,
Klymiuk I, Scharnagl H, Payr S, Kitzinger E, Schliefsteiner C, Lang
U and Wadsack C: Maternal obesity modulates intracellular lipid
turnover in the human term placenta. Int J Obes (Lond). 41:317–323.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Brantsæter AL, Haugen M, Samuelsen SO,
Torjusen H, Trogstad L, Alexander J, Magnus P and Meltzer HM: A
dietary pattern characterized by high intake of vegetables, fruits,
and vegetable oils is associated with reduced risk of preeclampsia
in nulliparous pregnant norwegian women. J Nutr. 139:1162–1168.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Song T, Lu J, Deng Z, Xu T, Yang Y, Wei H,
Li S, Jiang S and Peng J: Maternal obesity aggravates the
abnormality of porcine placenta by increasing N6-methyladenosine.
Int J Obes (Lond). 42:1812–1820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Moyes AJ, Maldonado-Pérez D, Gray GA and
Denison FC: Enhanced angiogenic capacity of human umbilical vein
endothelial cells from women with preeclampsia. Reprod Sci.
18:374–382. 2011.PubMed/NCBI View Article : Google Scholar
|