Introduction
Pancreatic cancer (PC) is one of the deadliest
cancers, ranking among the top five causes of cancer-associated
mortality (1,2). Genetics, smoking, diabetes and obesity
have been associated with an increased risk of PC (3). In 2017, the new PC cases in China
accounted for 18.66% of all new PC cases worldwide, and the
incidence of PC is still rising (4,5). Owing
to the invasiveness of PC cells in the early stages, it is
difficult to completely cure PC through surgery (6). Despite the progress made regarding
existing treatments for PC, including surgery, immunotherapy and
chemotherapy, the 5-year survival rate of patients is only 5%
worldwide (7,8). Therefore, it is critical to
investigate the underlying mechanisms of PC and discover novel
targets for PC therapy.
Long non-coding RNAs (lncRNAs) serve major roles in
the complicated regulatory network of the tumour development
process, including PC (9). For
instance, decreased KCNK15-AS1 expression has been indicated to
suppress cell migration and invasion in PC (10). BX111887 downregulation has been
demonstrated to inhibits PC cell migration and invasion and tumour
growth in vivo (11).
Emerging evidence has revealed that the reduction of LINC00657
suppresses the development of human cancers, such as colon
(12), oesophageal squamous cell
(13) and pancreatic ductal
(14) cancer. However, the function
of LINC00657 and its underlying mechanism in PC development
requires further elucidation.
MicroRNAs (miRNAs/miRs) are short non-coding RNAs
that can alter gene expression in several biological processes
(15). Previous studies have
indicated that altered expression of miRNAs, such as miR-9-5p
(16), miR-138-5p (17) and miR-142-5p (18), is associated with PC progression.
miR-520h has been reported to be associated with PC growth. For
instance, Wang et al (19)
demonstrated that miR-520h upregulation inhibited the migration and
invasion of PC cells. It has been reported that the regulatory
function of miRNAs in PC development is generally regulated by
lncRNAs. Gao et al (20)
revealed that decreased expression of lncRNA ZEB2-AS1 inhibited PC
cell growth and invasion by regulating miR-204. The effect of
miR-133a overexpression on the inhibition of PC cell viability has
been indicated to be regulated by lncRNA X-inactive specific
transcript (21). Knockdown of
LINC00657 has been demonstrated to inhibit the viability, migration
and invasion of pancreatic ductal adenocarcinoma (PDAC) cells via
targeting miR-433(14). However,
the interaction between miR-520h and LINC00657 in PC progression
has not been revealed.
In the present study, the expression levels of
LINC00657, miR-520h and cyclin-dependent kinases regulatory subunit
1 (CKS1B) were examined in PC tissues and cell lines. Moreover, the
effects of LINC00657 knockdown on the viability, migration and
invasion of PC cell lines were investigated. The association
between LINC00657 and the miR-520h/CKS1B axis was further
investigated in PC cell lines. The results of the current study may
provide a potential novel target for PC therapy.
Materials and methods
Tissue samples
Sixty patients with PC (31 male and 29 female, mean
age, 62±11 years old) were recruited between January 2017 and
October 2018 in the Affiliated Hospital of Beihua University
(Jilin, China). All patients had not received local or systemic
treatment before surgery and PC was staged (IA/IB/IIA and
IIB/III/IV) in accordance with the 8th edition of the American
Joint Committee on Cancer tumour-node-metastasis (TNM)
classification (22). PC tissue
samples and corresponding adjacent normal tissues (5 cm away from
tumors) from different TNM stages were acquired by surgical
resection. Each specimen was diagnosed by histology. All patients
provided informed consent, and the Ethics Committee of the
Affiliated Hospital of Beihua University approved the present study
(Jilin, China; approval no. 20-18).
Cell culture
The normal human pancreatic duct epithelial cell
line (HPDE6) is a cell line of male origin that contains the E6/E7
gene of human papillomavirus type 16 and is positive for nestin
[karyotype: 45~47, XY, der (21) t
(17; 21) (q21.3; p13) and 46, XY, t (3; 18) (p21.1; q11.2), der
(21) t (17; 21) (q21.3; p13)].
HPDE6 and PC cell lines (SW1990, PACA-2 and BXPC-3) were purchased
from American Tissue Culture Collection. SW1990 cells were cultured
in Leibovitz's L-15 Medium (MilliporeSigma) containing 15% FBS
(Gibco; Thermo Fisher Scientific, Inc.). HPDE6 and PACA-2 cells
were cultured in DMEM containing 10% FBS (both from Gibco; Thermo
Fisher Scientific, Inc.). BXPC-3 cells were cultured in RPMI-1640
medium containing 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.). All cells were cultured at 37˚C in an incubator
(MCO-15AC; Sanyo Electric Co., Ltd.) with 5% CO2.
Cell transfection
Short hairpin (sh)RNA negative control (NC) (sh-NC),
sh-LINC00657-1 and sh-LINC00657-2 were purchased from GeneCopoeia
Inc. miR mimics NC (miR-NC, 5'-UUCUCCGAACGUGUCACGUTT-3'), miR-520h
mimics (5'-ACAAAGUGCUUCCCUUUAGAGU-3'), inhibitor NC
(5'-UUCUCCGAACGUGUCACGUTT-3') and miR-520h inhibitor
(5'-CCCUCUACAGGGAAGCGCUU-3'), as well as pcDNA-NC and pcDNA-CKS1B
were obtained from Shanghai GenePharma Co., Ltd. The aforementioned
mimics, inhibitors, plasmids and NC (all, 50 nM) were then
transfected into PACA-2 and/or SW1990 cells (6x105
cells/well) using Lipofectamine 3000® (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37˚C. Two days after
transfection, the cells were harvested to perform the following
experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from PC tissues and PC cell
lines (SW1990, PACA-2, and BXPC-3) using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). According to the
manufacturer's instructions, cDNA was synthesized using the
First-Strand cDNA Synthesis kit (APeXBIO Technology LLC) and
RT-qPCR was performed with SYBR Green FAST Mastermix (Qiagen GmbH).
The expression of LINC00657 and CKS1B was normalized to that of
GAPDH. The expression of miR-520h was normalized to that of U6. The
primer sequences used for RT-qPCR are listed in Table I. The PCR conditions were as
follows: 95˚C for 10 min, followed by 40 cycles of 95˚C for 15 sec,
60˚C for 1 min and 72˚C for 1 min. The 2-ΔΔCq method was
used to evaluate relative gene expression (23).
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| LINC00657 |
TGATAGGATACATCTTGGACATGGA |
AACCTAATGAACAAGTCCTGACATACA |
| miR-520h |
TCGCGACAAAGTGCTTCCCT |
GTGCAGGGTCCGAGGT |
| CKS1B |
TATTCGGACAAATACGACGACG |
CGCCAAGATTCCTCCATTCAGA |
| GAPDH |
GCGAGATCGCACTCATCATCT |
TCAGTGGTGGACCTGACC |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
Target prediction
The miRNA targets of LINC00657 were predicted using
LncBase Predicted v.2 software (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index-predicted)
and StarBase software v.2 (http://starbase.sysu.edu.cn/). A total of 302 and 272
miRNA targets was predicted, respectively. Among these miRNA
targets, miR-520h was selected because of its important role in PC
(19) and unknown regulatory
relationship with LINC00657. In addition, the mRNA targets of
miR-520h were predicted using miRDB database (http://mirdb.org/), and 916 targets were predicted. As
CKS1B has been associated with the pathogenesis of numerous human
cancers such as retinoblastoma (24), CRC (25) and GC (26), CKS1B was selected for subsequent
experiments.
Bioinformatics analysis
The Cancer Genome Atlas (TCGA; https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
was used to examine the expression levels of LINC00657 and CKS1B in
PC patients (n=179) and normal controls (n=171).
Dual-luciferase reporter (DLR)
assay
The 3'-untranslated region of LINC00657
incorporating the predicted wild type (Wt) or mutant (Mut) binding
sites of miR-520h were cloned into the pGL3 vector (Promega
Corporation) to construct LINC00657 Wt and LINC00657 Mut plasmids.
The CKS1B Wt and CKS1B Mut reporter plasmids were constructed in a
similar way. Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to co-transfect a Wt or Mut luciferase
reporter and miR-520h mimics or miR-NC into SW1990 and PACA-2
cells. Then, the DLR assay kit (Promega Corporation) was used for
detecting the relative luciferase activity 48 h after transfection.
The activity of firefly luciferase was normalized to that of
Renilla luciferase.
MTT assay
After transfection, PACA-2 and/or SW1990 cells were
plated in 96-well plates (1x103 cells/well) and cultured
for 0, 24, 48, 72 and 96 h. Subsequently, 15 µl MTT solution (5
mg/ml) was added to each well. After 4 h, 200 µl DMSO was added.
The optical density value was measured at 570 nm using a
spectrophotometer (Thermo Fisher Scientific, Inc.).
Cell doubling time assay
After reaching 80% confluence, the transfected
SW1990 and PACA-2 cells were trypsinized with 0.25% trypsin at 37˚C
for 10 min to create a single-cell suspension. The suspension was
seeded in a six-well plate at 2x103 cells/well. The
cells were cultured for 7 days and then digested and counted.
Doubling time was calculated as 7x [lg2/(lgN0-lgN7)] x24 (N0, cell
number at day 0; N7, cell number at day 7).
Wound healing assay
PACA-2 and/or SW1990 cells were seeded into a
six-well plate and cultured in medium (Leibovitz's L-15 Medium for
SW1990 and DMEM for PACA-2) supplemented with 10% FBS until the
cells were grown to 100% confluence. A 10 µl pipette tip was used
to create a scratch wound in the cell monolayer. The medium was
aspirated to remove the detached cells, and serum-free DMEM and/or
Leibovitz's L-15 Medium were added to the 6-well plate. At 0 and 24
h, cell migration was observed using an Olympus inverted light
microscope (magnification, x400; Olympus Corporation) and
photographed using a Cyber-shot camera (Sony Corporation).
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.) was used to
measure the wound distance at 0 and 24 h. The wound heading rate
was calculated as follows: (0 h width -24 h width)/0 h width
x100%.
Transwell assay
Transwell inserts (8.0 µm, BD Biosciences) were
placed on 24-well plates to create top and bottom chambers. The
transfected PACA-2 and/or SW1990 cells in serum-free DMEM and/or
Leibovitz's L-15 Medium were cultured in the top compartment
precoated at 37˚C for 30 min with Matrigel (BD Biosciences). The
bottom compartment was filled with DMEM and/or Leibovitz's L-15
Medium containing 20% FBS. After 24 h, the cells on the lower
compartment were fixed in 75% methanol at 4˚C for 10 min and
stained with 0.3% crystal violet at 37˚C for 30 min. Finally, an
inverted optical microscope (magnification, x400; Olympus
Corporation) was used to capture the photos of migrated cells.
Western blot analysis
RIPA lysis solution (Beyotime Institute of
Biotechnology) was used to obtain total protein from SW1990 and
PACA-2 cells. The protein concentration was detected by the BCA
Protein Assay kit (Abcam). Protein samples (20 µg/lane) were
separated via 10% SDS-PAGE and then transferred onto PVDF membranes
(MilliporeSigma). The membranes were blocked with 5% skimmed milk
at 25˚C for 1 h and incubated overnight at 4˚C with anti-CKS1B
(1:1,000; cat. no. ab72639; Abcam) and anti-β-tubulin (1:1,000;
cat. no. ab7291; Abcam) antibodies. Subsequently, the membranes
were incubated with an horse-radish peroxidase (HRP)-conjugated
goat anti-rabbit secondary antibody (1:5,000, cat. no. ab205718;
Abcam) at 37˚C for 2 h. The signals were detected using ECL reagent
(Thermo Fisher Scientific, Inc.), and the immunoblots were analysed
using Image Lab™ Software v.1.46 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were conducted in triplicate in at
least 3 independent experiments. Data analysis was performed using
GraphPad Prism version 6 (GraphPad Software, Inc.), and the data
are presented as the mean ± standard deviation. The differences
between two groups or among multiple groups were analysed by
Student's t-test (paired, Figs. 1B,
3D and 5D; unpaired, Figs. 1C, 2C-E and 4C-E) or one-way ANOVA followed by Tukey's
post-hoc test, respectively. Pearson's correlation analysis was
carried out to assess correlations between the expression levels of
different molecules. The differences of clinicopathological
characteristics between patients with PC with low and high
expression of LINC00657 was analysed via χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
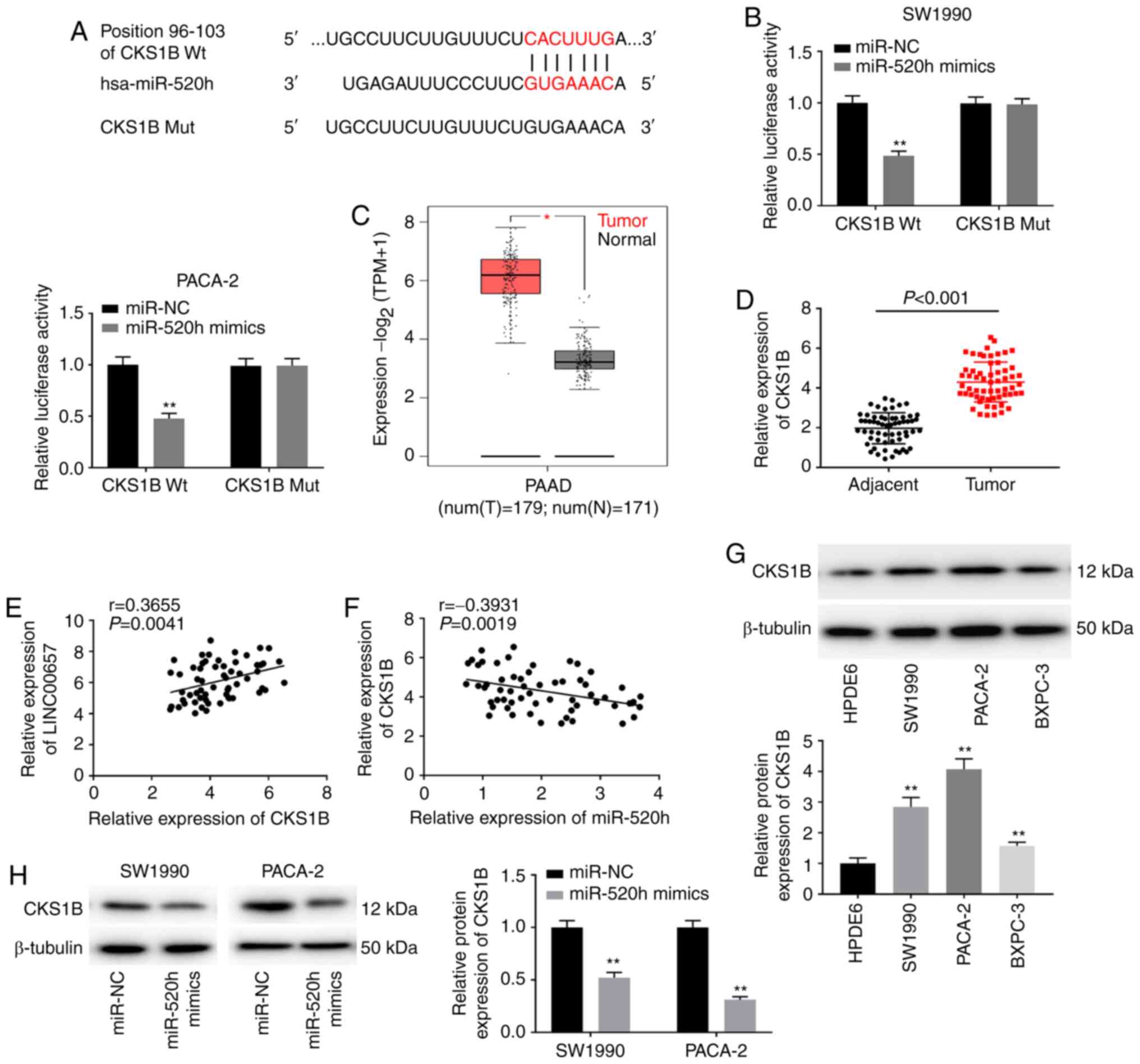 | Figure 5CKS1B is a target of miR-520h. (A)
The putative binding sites of miR-520h and CKS1B were predicted
using TargetScan. (B) Dual-luciferase reporter assay confirmed the
association between miR-520h and CKS1B in SW1990 and PACA-2 cells.
**P<0.01 vs. miR-NC. (C) The expression of CKS1B was
evaluated using The Cancer Genome Atlas in PAAD tumours.
*P<0.05 vs. normal tissues. (D) CKS1B expression was
detected via reverse transcription-quantitative PCR in PC tissues
and adjacent normal tissues. P<0.001. (E) The correlation
between CKS1B and LINC00657 was evaluated using Pearson's
correlation analysis. r=0.3655, P=0.0041. (F) The correlation
between CKS1B and miR-520h was evaluated using Pearson's
correlation analysis. r=-0.3931, P=0.0019. (G) The expression of
CKS1B protein was detected via western blotting in PC cell lines
and HPDE6 cells. **P<0.01 vs. HPDE6. (H) The
expression of CKS1B protein was detected via western blotting in
SW1990 and PACA-2 cells transfected with miR-520h mimics or miR-NC.
**P<0.01 vs. miR-NC. NC, negative control; miR,
microRNA; CKS1B, cyclin-dependent kinases regulatory subunit 1;
PAAD, pancreatic adenocarcinoma; PC, pancreatic cancer; TNM,
tumour-node-metastasis; num, number; T, tumour; N, normal; Wt,
wild-type; Mut, mutant. |
Results
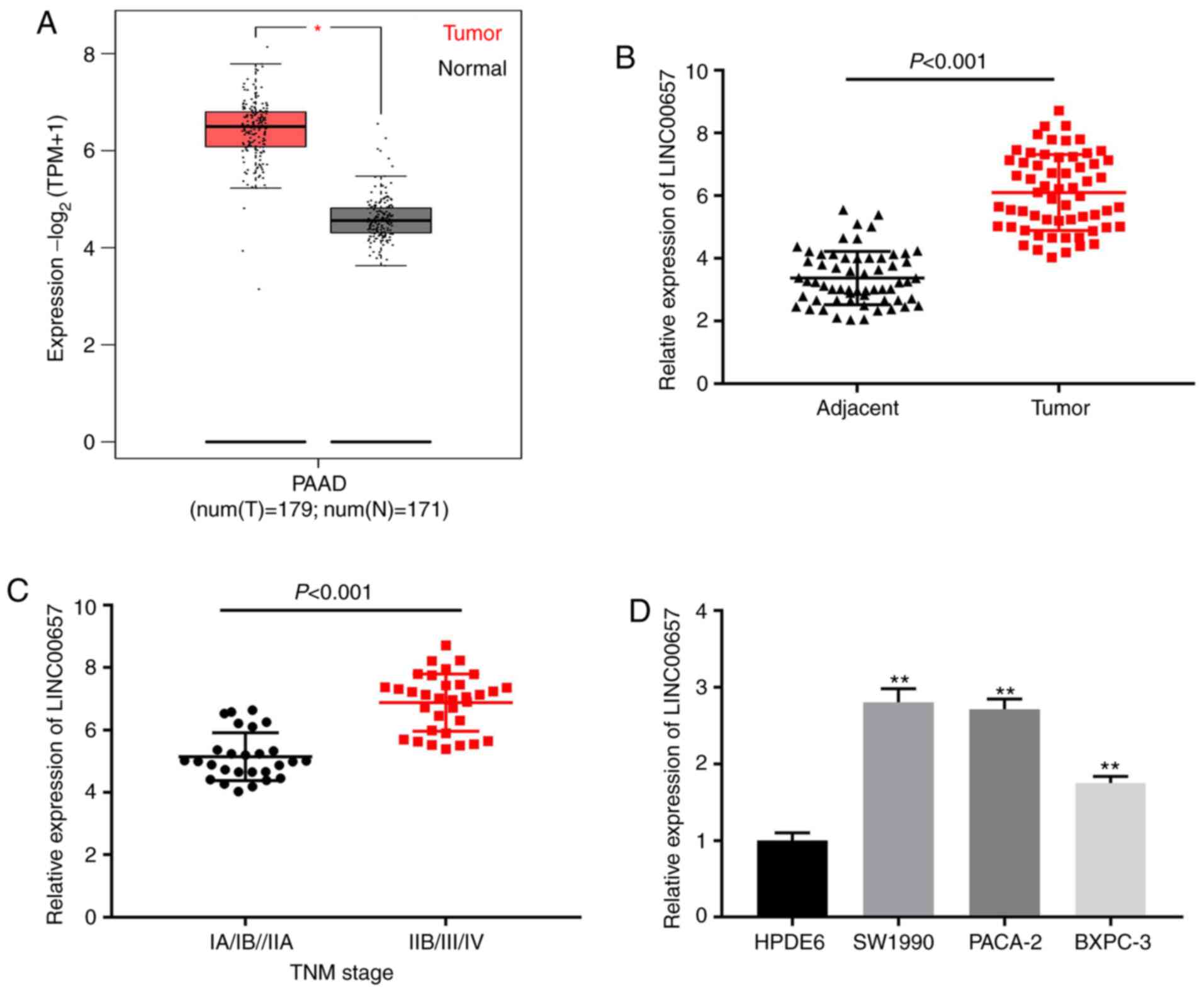
LINC00657 expression is increased in
PC tissues and cell lines
Firstly, The Cancer Genome Atlas (TCGA) was used to
examine LINC00657 expression in PC, and it was revealed that
LINC00657 expression was markedly upregulated in pancreatic
adenocarcinoma (PAAD) compared with normal tissues (P<0.05;
Fig. 1A). RT-qPCR data indicated
that LINC00657 expression in PC tissues was enhanced compared with
that in adjacent normal tissues (P<0.001; Fig. 1B). LINC00657 expression in TNM stage
IIB/III/IV was higher than that of stage IA/IB/IIA (P<0.001;
Fig. 1C). In addition, LINC00657
expression was examined in PC cell lines and HPDE6 cells. The
results indicated that LINC00657 expression was notably increased
in PC cell lines, especially in SW1990 and PACA-2 cells, compared
with HPDE6 cells (all P<0.01; Fig.
1D). Thus, these two cell lines were selected for subsequent
experiments. As presented in Table
II, there were significant differences among patients with PC
with high and low expression of LINC00657 in the diameter
(P=0.017), metastasis (P=0.002) and TNM stage parameters (P=0.010).
These data suggested that LINC00657 may be a lncRNA with on
oncogenic role in PC development.
 | Table IIDifferences of clinicopathological
characteristics between patients with pancreatic cancer with low
and high expression of LINC00657. |
Table II
Differences of clinicopathological
characteristics between patients with pancreatic cancer with low
and high expression of LINC00657.
| | LINC00657
expression | |
|---|
|
Characteristics | Total number | Low | High | P-value |
|---|
| Sex | | | | 0.796 |
|
Male | 31 | 16 | 15 | |
|
Female | 29 | 14 | 15 | |
| Age, years | | | | 0.602 |
|
<60 | 26 | 12 | 14 | |
|
≥60 | 34 | 18 | 16 | |
|
Differentiation | | | | 0.284 |
|
Well/moderate | 22 | 9 | 13 | |
|
Poor | 38 | 21 | 17 | |
| Diameter, cm | | | | 0.017a |
|
<2 | 23 | 16 | 7 | |
|
≥2 | 37 | 14 | 23 | |
| Metastasis | | | | 0.002a |
|
No | 24 | 18 | 6 | |
|
Yes | 36 | 12 | 24 | |
| TNM stage | | | | 0.010a |
|
IA/IB+IIA | 27 | 21 | 6 | |
|
IIB+III+IV | 33 | 9 | 24 | |
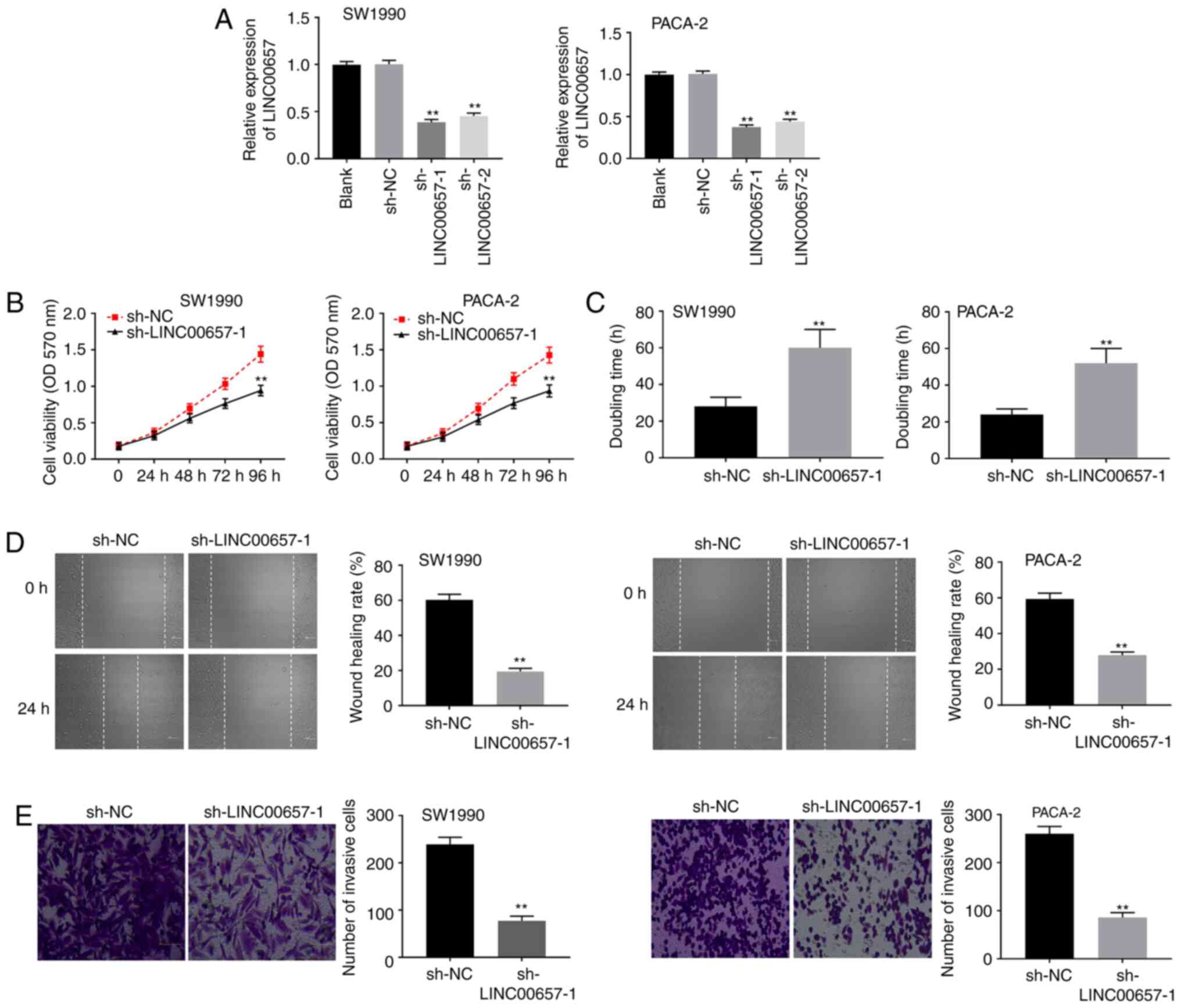
Knockdown of LINC00657 inhibits the
viability, migration and invasion of PC cells
To explore the function of LINC00657 in PC,
sh-LINC00657-1 or sh-LINC00657-2 was initially transfected into
SW1990 and PACA-2 cells. LINC00657 expression was significantly
reduced both in the sh-LINC00657-1 and sh-LINC00657-2 group
compared with the sh-NC group (both P<0.01; Fig. 2A). sh-LINC00657-1 was utilized in
subsequent experiments because of its greater silencing efficiency.
MTT assay demonstrated that the cell viability of the
sh-LINC00657-1 group was markedly inhibited compared with that of
the sh-NC group in SW1990 and PACA-2 cells (both P<0.01;
Fig. 2B). As illustrated in
Fig. 2C, a longer cell doubling
time was determined in the sh-LINC00657-1 group than that in the
sh-NC group (P<0.01). Moreover, LINC00657 knockdown inhibited
the migration and invasion of SW1990 and PACA-2 cells (all
P<0.01; Fig. 2D and E). The aforementioned results indicated
that knockdown of LINC00657 could inhibit the viability, migration
and invasion of PC cells.
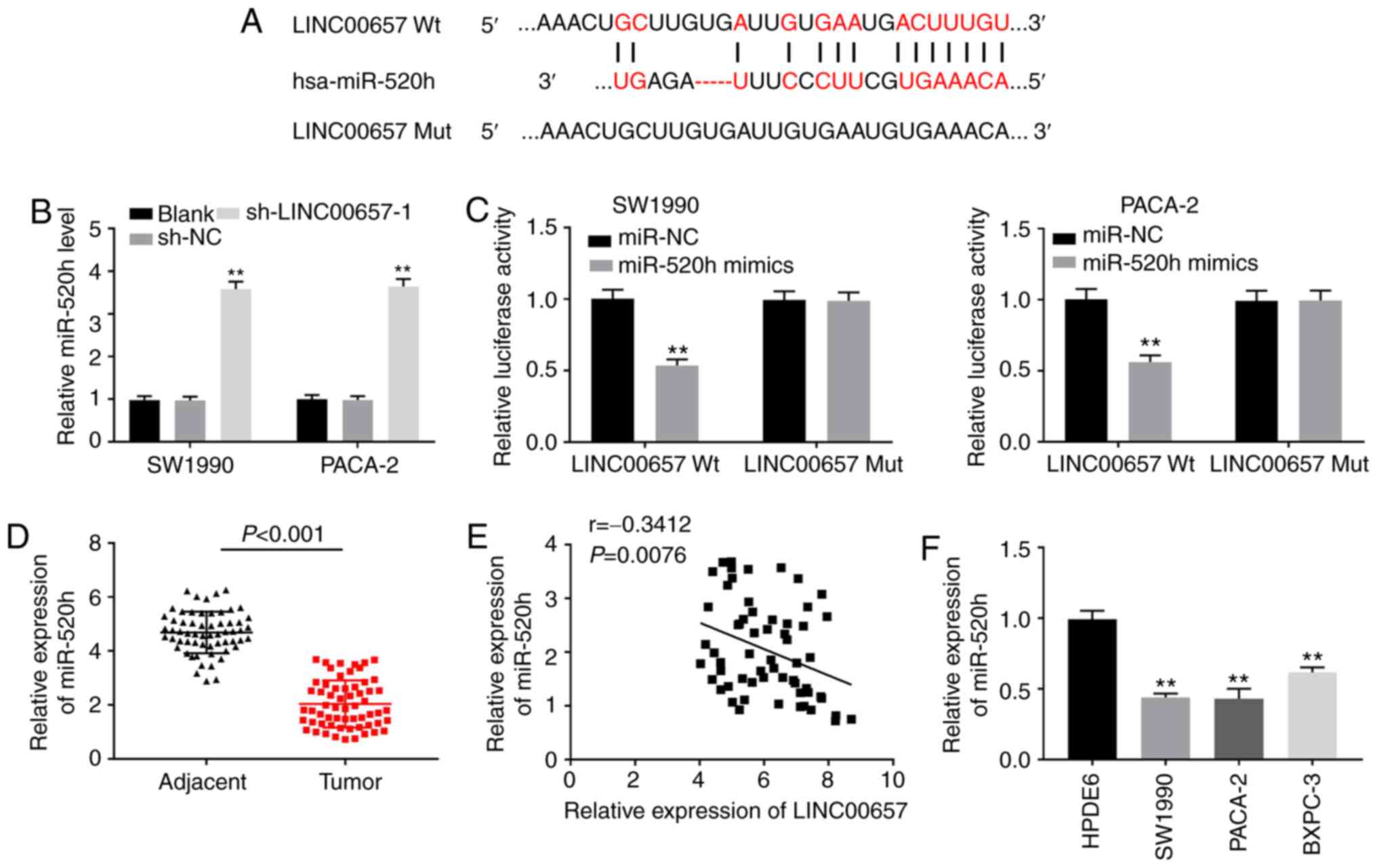
miR-520h is a target of LINC00657
To reveal the mechanism of LINC00657 function in PC,
LncBase Predicted v.2 and StarBase software v.2 softwares were used
to predict its target genes. A binding site between LINC00657 and
miR-520h was observed (Fig. 3A).
Silencing of LINC00657 increased the expression level of miR-520h
in SW1990 and PACA-2 cells (both P<0.01; Fig. 3B). In addition, the DLR assay
demonstrated that miR-520h mimics reduced the luciferase activity
of LINC00657 Wt in SW1990 and PACA-2 cells (both P<0.01;
Fig. 3C), further validating the
association between LINC00657 and miR-520h. miR-520h expression in
PC tissues lower than that in adjacent normal tissues (P<0.001;
Fig. 3D). As illustrated in
Fig. 3E, there was a negative
correlation between the expression levels of LINC00657 and miR-520h
in PC tissues (r=-0.3412; P=0.0076). Furthermore, miR-520h
expression was also studied at the cellular level. miR-520h
expression in PC cell lines was decreased compared with that in
HPDE6 cells (all P<0.01; Fig.
3F). These results indicated that miR-520h may be a target of
and negatively modulated by LINC00657, as well as a suppressor of
PC.
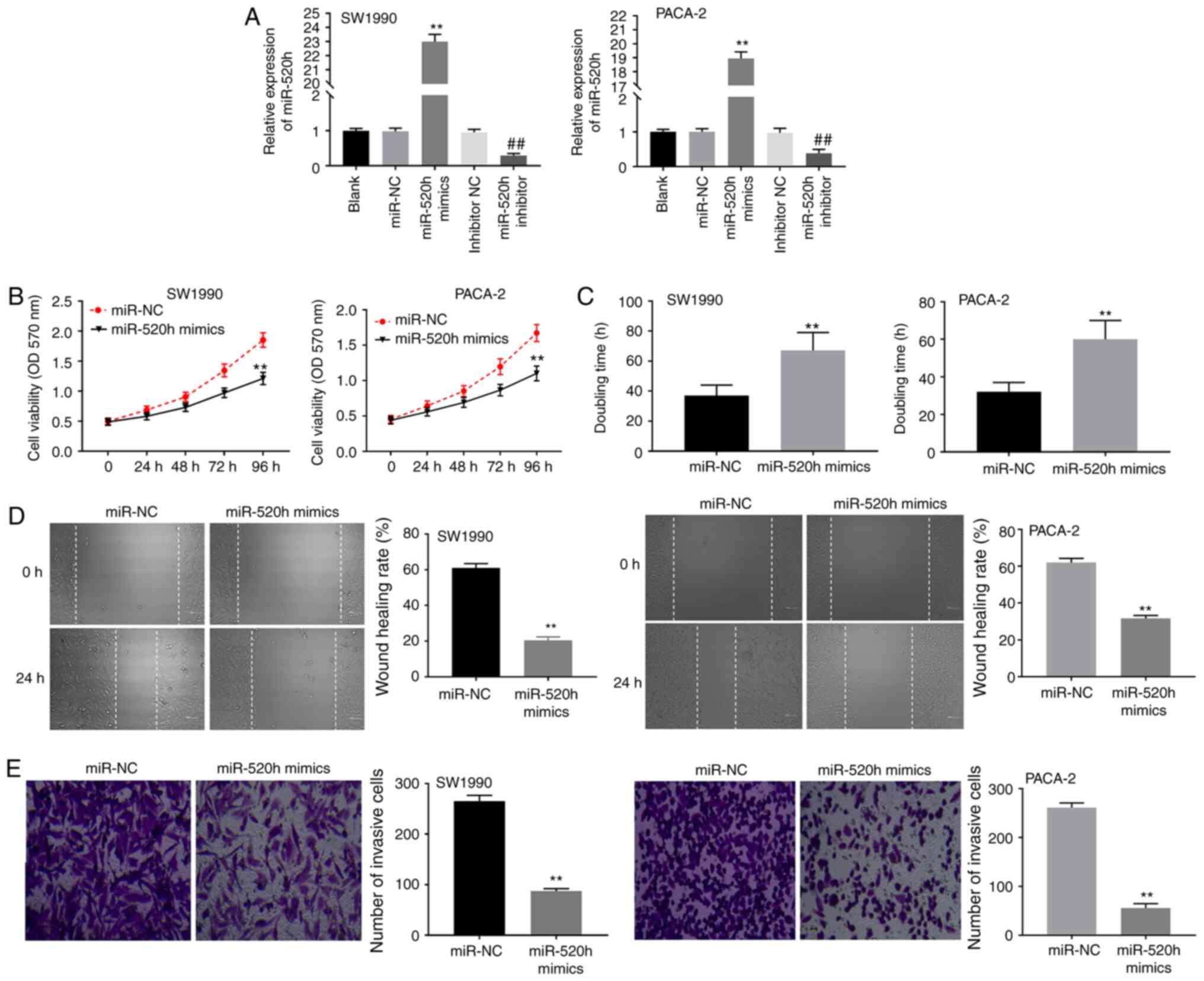
miR-520h overexpression inhibits the
viability, migration and invasion of PC cells
To further explore the potential role of miR-520h in
PC, miR-520h mimics or inhibitor was primarily transfected into
SW1990 and PACA-2 cells to determine the transfection efficiency.
The results indicated that the expression of miR-520h was markedly
increased after transfection with miR-520h mimics, and was
decreased after transfection with miR-520h inhibitor in SW1990 and
PACA-2 cells compared with their respective controls (all
P<0.01; Fig. 4A), suggesting
that miR-520h mimics and miR-520h inhibitor was successfully
transfected. miR-520h overexpression reduced the viability of
SW1990 and PACA-2 cells (both P<0.01; Fig. 4B). The results of the cell doubling
time experiment demonstrated that the miR-520h mimics group
exhibited a longer doubling time compared with that of the miR-NC
group (both P<0.01; Fig. 4C).
Furthermore, miR-520h overexpression notably suppressed migration
and invasion in SW1990 and PACA-2 cells (all P<0.01; Fig. 4D and E). These results indicated that miR-520h
overexpression could suppress the viability, invasion and migration
of PC cells.
CKS1B is a target of miR-520h
The target genes of miR-520h were predicted using
miRDB database, and CKS1B was indicated to have binding sites for
miR-520h (Fig. 5A). The DLR assay
revealed that miR-520h mimics notably inhibited the luciferase
activity of CKS1B Wt in SW1990 and PACA-2 cells (both P<0.01;
Fig. 5B). TCGA analysis indicated
that CKS1B expression was enhanced in PAAD compared with normal
tissues (P<0.05; Fig. 5C).
Similarly, CKS1B expression in PC tissues was higher than that in
adjacent normal tissues (P<0.001; Fig. 5D). CKS1B expression was positively
associated with that of LINC00657 (r=0.3655; P=0.0041; Fig. 5E) and negatively associated with
miR-520h expression in PC tissues (r=-0.3931; P=0.0019; Fig. 5F). Subsequently, CKS1B expression
was examined in vitro. The results revealed that CKS1B
expression in PC cell lines was notably increased compared with
that in HPDE6 cells (all P<0.01; Fig. 5G). Overexpression of miR-520h
significantly decreased CKS1B expression in SW1990 and PACA-2 cells
(both P<0.01; Fig. 5H). These
findings indicated that miR-520h directly targeted CKS1B and
inversely modulated CKS1B expression, as well as it may be an
oncogene in PC.
LINC00657 knockdown inhibits PC
tumorigenesis by regulating miR-520h and CKS1B expression
Finally, the effect of the LINC00657/miR-520h/CKS1B
axis on PC progression was explored. As presented in Fig. 6A, western blotting demonstrated that
the CKS1B protein level was significantly elevated in PACA-2 cells
transfected with pcDNA-CKS1B compared with pcDNA-NC (P<0.01),
which indicated that pcDNA-CKS1B was successfully transfected.
LINC00657 knockdown inhibited the viability of PACA-2 cells, while
this inhibitory effect was partially reversed by miR-520h
inhibition and CKS1B overexpression (both P<0.01; Fig. 6B). Moreover, miR-520h inhibition and
CKS1B overexpression alleviated LINC00657 knockdown-induced
inhibition of migration and invasion in PACA-2 cells (all
P<0.01; Fig. 6C and D). The aforementioned data indicated that
knockdown of LINC00657 inhibited the development of PC by
regulating miR-520h and CKS1B expression.
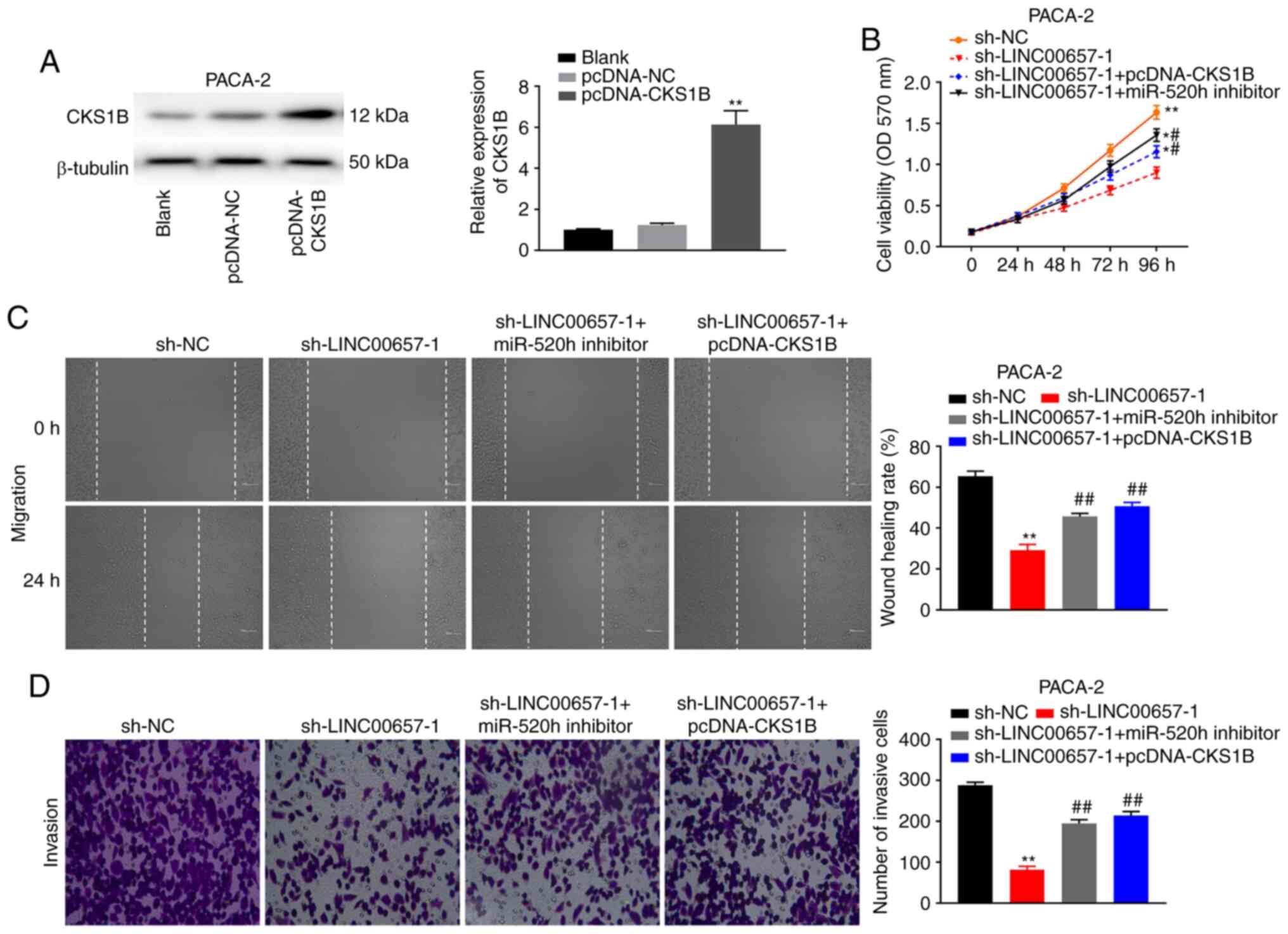 | Figure 6LINC00657 knockdown inhibits
pancreatic cancer tumorigenesis by regulating miR-520h and CKS1B
expression. (A) The expression of CKS1B protein was detected via
western blotting in PACA-2 cells transfected with pcDNA-NC or
pcDNA-CKS1B. **P<0.01 vs. pcDNA-NC. (B) Cell
viability was detected via MTT assay in PACA cells transfected with
sh-NC, sh-LINC00657-1, sh-LINC00657-1 + pcDNA-CKS1B or
sh-LINC00657-1 + miR-520h. *P<0.05,
**P<0.01 vs. sh-NC; #P<0.05 vs.
sh-LINC00657-1. (C) Cell migration was examined via wound healing
assay and (D) cell invasion was detected via Transwell assay in
PACA-2 cells transfected with sh-NC, sh-LINC00657-1, sh-LINC00657-1
+ pcDNA-CKS1B or sh-LINC00657-1 + miR-520h. Magnification, x400.
**P<0.01 vs. sh-NC; ##P<0.01 vs.
sh-LINC00657-1. NC, negative control; CKS1B, cyclin-dependent
kinases regulatory subunit 1; miR, microRNA; sh, short hairpin; OD,
optical density. |
Discussion
PC is a fatal malignant tumour that is mainly
observed in males and exhibits an aggressive course (27). The existing literature has indicated
that abnormally expressed lncRNAs serve a major role in the
formation and growth of PC (28).
In the present study, LINC00657 expression was indicated to be
increased in PC tissues and cell lines compared with healthy
tissues and control cells, respectively. Silencing of LINC00657
suppressed the viability, migration and invasion of PC cells. In
addition, LINC00657 directly targeted miR-520h, and miR-520h
directly targeted CKS1B. Further studies revealed that knockdown of
LINC00657 inhibited PC tumorigenesis via targeting the
miR-520h/CKS1B axis.
Previous studies have revealed that LINC00657
functioned as a cancer-promoting molecule in certain cancers, and
its expression was enhanced in non-small cell lung cancer (NSCLC)
(29), colorectal cancer (CRC)
(30) and breast cancer (31). Bi et al (14) demonstrated that LINC00657 expression
was increased in PDAC tissues and cell lines. Similarly, the
present study revealed LINC00657 was highly expressed in PC tissues
and cell lines. The increase of LINC00657 was notably associated
with tumour diameter, metastasis and TNM stage among the patients
with PC. The aforementioned results indicated that LINC00657 was
involved in PC development. Previous studies have reported that
LINC00657 could regulate cellular processes in certain cancers. For
instance, silencing LINC00657 has been indicated to inhibit the
viability and migration of NSCLC cells (29). In oesophageal cancer, LINC00657
knockdown has been revealed to limit cell viability, migration and
invasion (32). In the current
study, LINC00657 knockdown inhibited the viability, migration and
invasion of SW1990 and PACA-2 cells. The results of the present
study suggested that silencing LINC00657 could inhibit PC
development.
Numerous studies have indicated that LINC00657
regulates cancer development by interacting with miRNAs. For
example, LINC00657 can competitively bind to miR-165-3p in
oesophageal squamous cell carcinoma (13) and to miR-190a-3p in glioblastoma
(33). In the present study, it was
demonstrated that LINC00657 could target miR-520h. Additionally,
LINC00657 knockdown increased miR-520h expression in PC cells,
suggesting that LINC00657 was negatively associated with miR-520h.
It has been indicated that miR-520h serves a pivotal role in the
development of several cancers, and its expression has been
demonstrated to be reduced in multiple myeloma cells (34) and breast cancer cells (35). In the current study, miR-520h was
also weakly expressed in PC tissues and cell lines, suggesting that
miR-520h may be a tumour suppressor gene in PC development. The
tumour-suppressive function of miR-520h has been recognized in
certain cancers. For example, overexpression of miR-520h inhibited
cell migration and invasion in PC (19). Furthermore, miR-520h overexpression
decreased the viability, migration and invasion of CRC cells
(36). Similar to previous studies,
in the present study miR-520h overexpression also suppressed the
viability, invasion and migration of SW1990 and PACA-2 cells. The
aforementioned results suggested that miR-520h overexpression could
inhibit PC growth. As LINC00657 inversely regulated miR-520h, we
hypothesized that LINC00657 knockdown may inhibit PC development by
targeting miR-520h.
CKS1B is a member of the CKS protein family and is
related to cell proliferation (37). CKS1B has been demonstrated to
participate cancer growth and perform its function by acting as a
target for miR-204 in GC (38) and
miR-197 in NSCLC (39). In the
current study, CKS1B was revealed to be a target of miR-520h and
was reversely modulated by miR-520h in PC. Upregulation of CKS1B
has been widely observed in multiple cancers, such as
retinoblastoma (24), CRC (25) and GC (26). The present study demonstrated that
CKS1B expression was also enhanced in PC tissues and cell lines
compared with healthy tissues and control cells, respectively,
suggesting that CKS1B may be an oncogenic molecule in PC
development. In addition, Fujita et al (39) reported that the inhibitory effect of
CKS1B knockdown on the viability, migration and invasion of NSCLC
cells can be regulated by miR-197. miR-1258 overexpression has been
indicated to suppress cell growth by targeting CKS1B in
hepatocellular carcinoma cells (40). Thus, we hypothesized that miR-520h
overexpression may inhibit the viability, migration and invasion of
PC cells by targeting CKS1B. Further rescue experiments revealed
that miR-520h inhibition and CKS1B overexpression alleviated the
inhibition of LINC00657 knockdown on the viability, migration and
invasion of PACA-2 cells. As LINC00657 interacted with miR-520h and
miR-520h directly targeted CKS1B, it was concluded that LINC00657
knockdown may suppress PC development by regulating the
miR-520h/CKS1B axis.
In conclusion, it was revealed that LINC00657
expression was increased in PC tissues and cell lines compared with
healthy tissues and control cells, respectively. Silencing
LINC00657 suppressed the viability, migration and invasion of PC
cells by regulating the miR-520h/CKS1B axis, indicating that
LINC00657 may constitute a novel target for PC treatment. The
present study may offer a potential target for PC therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL, HW and GC made substantial contributions to the
conception and design of the study. HW and GC performed the
experiments. PL, YT, SS, YM and YX made substantial contributions
to the acquisition, analysis and interpretation of data, as well as
the drafting and revision of the manuscript. All authors confirmed
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All patients signed informed consents and the Ethics
Committee of the Affiliated Hospital of Beihua University approved
the present study (Jilin, China; approval no. 20-18).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oberstein PE and Olive KP: Pancreatic
cancer: Why is it so hard to treat? Therap Adv Gastroenterol.
6:321–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xu XH, Zeng XY, Wang LJ, Liu YN, Liu JM,
Qi JL, Yin P and Zhou MG: The disease burden of pancreatic cancer
in China in 1990 and 2017. Zhonghua Liu Xing Bing Xue Za Zhi.
40:1084–1088. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
GBD 2017 Pancreatic Cancer Collaborators.
The global, regional, and national burden of pancreatic cancer and
its attributable risk factors in 195 countries and territories,
1990-2017: A systematic analysis for the Global Burden of Disease
Study 2017. Lancet Gastroenterol Hepatol. 4:934–947.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li Y, Vandenboom TG II, Wang Z, Kong D,
Ali S, Philip PA and Sarkar FH: MiR-146a suppresses invasion of
pancreatic cancer cells. Cancer Res. 70:1486–1495. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brunner M, Wu Z, Krautz C, Pilarsky C,
Grützmann R and Weber GF: Current clinical strategies of pancreatic
cancer treatment and open molecular questions. Int J Mol Sci.
20(4543)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Duguang L, Jin H, Xiaowei Q, Peng X,
Xiaodong W, Zhennan L, Jianjun Q and Jie Y: The involvement of
lncRNAs in the development and progression of pancreatic cancer.
Cancer Biol Ther. 18:927–936. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P,
Liu D, Tian L, Yin J, Jiang K and Miao Y: ALKBH5 inhibits
pancreatic cancer motility by decreasing long non-coding RNA
KCNK15-AS1 methylation. Cell Physiol Biochem. 48:838–846.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deng SJ, Chen HY, Ye Z, Deng SC, Zhu S,
Zeng Z, He C, Liu ML, Huang K, Zhong JX, et al: Hypoxia-induced
LncRNA-BX111 promotes metastasis and progression of pancreatic
cancer through regulating ZEB1 transcription. Oncogene.
37:5811–5828. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lei Y, Wang YH, Wang XF and Bai J:
LINC00657 promotes the development of colon cancer by activating
PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 22:6315–6323.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun Y, Wang J, Pan S, Yang T, Sun X, Wang
Y, Shi X, Zhao X, Guo J and Zhang X: LINC00657 played oncogenic
roles in esophageal squamous cell carcinoma by targeting miR-615-3p
and JunB. Biomed Pharmacother. 108:316–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bi S, Wang Y, Feng H and Li Q: Long
noncoding RNA LINC00657 enhances the malignancy of pancreatic
ductal adenocarcinoma by acting as a competing endogenous RNA on
microRNA-433 to increase PAK4 expression. Cell Cycle. 19:801–816.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv Biol Regul. 57:1–9.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang J, Wang B, Ren H and Chen W: MiR-9-5p
inhibits pancreatic cancer cell proliferation, invasion and
glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun.
509:241–248. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tian S, Guo X, Yu C, Sun C and Jiang J:
MiR-138-5p suppresses autophagy in pancreatic cancer by targeting
SIRT1. Oncotarget. 8:11071–11082. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yao R, Xu L, Wei B, Qian Z, Wang J, Hui H
and Sun Y: MiR-142-5p regulates pancreatic cancer cell
proliferation and apoptosis by regulation of RAP1A. Pathol Res
Pract. 215(152416)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang F, Xue X, Wei J, An Y, Yao J, Cai H,
Wu J, Dai C, Qian Z, Xu Z and Miao Y: hsa-miR-520h downregulates
ABCG2 in pancreatic cancer cells to inhibit migration, invasion,
and side populations. Br J Cancer. 103:567–574. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gao H, Gong N, Ma Z, Miao X, Chen J, Cao Y
and Zhang G: LncRNA ZEB2-AS1 promotes pancreatic cancer cell growth
and invasion through regulating the miR-204/HMGB1 axis. Int J Biol
Macromol. 116:545–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wei W, Liu Y, Lu Y, Yang B and Tang L:
LncRNA XIST promotes pancreatic cancer proliferation through
miR-133a/EGFR. J Cell Biochem. 118:3349–3358. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
van Roessel S, Kasumova GG, Verheij J,
Najarian RM, Maggino L, de Pastena M, Malleo G, Marchegiani G,
Salvia R, Ng SC, et al: International validation of the eighth
edition of the American joint committee on cancer (AJCC) TNM
staging system in patients with resected pancreatic cancer. JAMA
Surg. 153(e183617)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zeng Z, Gao ZL, Zhang ZP, Jiang HB, Yang
CQ, Yang J and Xia XB: Downregulation of CKS1B restrains the
proliferation, migration, invasion and angiogenesis of
retinoblastoma cells through the MEK/ERK signaling pathway. Int J
Mol Med. 44:103–114. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hwang JS, Jeong EJ, Choi J, Lee YJ, Jung
E, Kim SK, Min JK, Han TS and Kim JS: MicroRNA-1258 inhibits the
proliferation and migration of human colorectal cancer cells
through suppressing CKS1B expression. Genes (Basel).
10(912)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang J, Liu X, Yu G, Liu L, Wang J, Chen
X, Bian Y, Ji Y, Zhou X, Chen Y, et al: UBE2C is a potential
biomarker of intestinal-type gastric cancer with chromosomal
instability. Front Pharmacol. 9(847)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Goral V: Pancreatic cancer: Pathogenesis
and diagnosis. Asian Pac J Cancer Prev. 16(5619)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang W, Lou W, Ding B, Yang B, Lu H, Kong
Q and Fan W: A novel mRNA-miRNA-lncRNA competing endogenous RNA
triple sub-network associated with prognosis of pancreatic cancer.
Aging (Albany NY). 11:2610–2627. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang R, Niu Z, Pei H and Peng Z: Long
noncoding RNA LINC00657 induced by SP1 contributes to the non-small
cell lung cancer progression through targeting miR-26b-5p/COMMD8
axis. J Cell Physiol. 235:3340–3349. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shaker OG, Ali MA, Ahmed TI, Zaki OM, Ali
DY, Hassan EA, Hemeda NF and AbdelHafez MN: Association between
LINC00657 and miR-106a serum expression levels and susceptibility
to colorectal cancer, adenomatous polyposis, and ulcerative colitis
in Egyptian population. IUBMB Life. 71:1322–1335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu H, Li J, Koirala P, Ding X, Chen B,
Wang Y, Wang Z, Wang C, Zhang X and Mo YY: Long non-coding RNAs as
prognostic markers in human breast cancer. Oncotarget.
7:20584–20596. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang XM, Wang J, Liu ZL, Liu H, Cheng YF
and Wang T: LINC00657/miR-26a-5p/CKS2 ceRNA network promotes the
growth of esophageal cancer cells via the MDM2/p53/Bcl2/Bax
pathway. Biosci Rep. 40(BSR20200525)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chu L, Yu L, Liu J, Song S, Yang H, Han F,
Liu F and Hu Y: Long intergenic non-coding LINC00657 regulates
tumorigenesis of glioblastoma by acting as a molecular sponge of
miR-190a-3p. Aging (Albany NY). 11:1456–1470. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yuan X, Ma R, Yang S, Jiang L, Wang Z, Zhu
Z and Li H: MiR-520g and miR-520h overcome bortezomib resistance in
multiple myeloma via suppressing APE1. Cell Cycle. 18:1660–1669.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Su JL, Chen PB, Chen YH, Chen SC, Chang
YW, Jan YH, Cheng X, Hsiao M and Hung MC: Downregulation of
microRNA miR-520h by E1A contributes to anticancer activity. Cancer
Res. 70:5096–5108. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou T, Wu L, Ma N, Tang F, Zong Z and
Chen S: LncRNA PART1 regulates colorectal cancer via targeting
miR-150-5p/miR-520h/CTNNB1 and activating Wnt/β-catenin pathway.
Int J Biochem Cell Biol. 118(105637)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stella F, Pedrazzini E, Baialardo E, Fantl
DB, Schutz N and Slavutsky I: Quantitative analysis of CKS1B mRNA
expression and copy number gain in patients with plasma cell
disorders. Blood Cells Mol Dis. 53:110–117. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shrestha S, Yang CD, Hong HC, Chou CH, Tai
CS, Chiew MY, Chen WL, Weng SL, Chen CC, Chang YA, et al:
Integrated MicroRNA-mRNA analysis reveals miR-204 inhibits cell
proliferation in gastric cancer by targeting CKS1B, CXCL1 and
GPRC5A. Int J Mol Sci. 19(87)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fujita Y, Yagishita S, Hagiwara K,
Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Tsuta K, Nokihara H,
Tamura T, et al: The clinical relevance of the
miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant
non-small-cell lung cancer. Mol Ther. 23:717–727. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hu M, Wang M, Lu H, Wang X, Fang X, Wang
J, Ma C, Chen X and Xia H: Loss of miR-1258 contributes to
carcinogenesis and progression of liver cancer through targeting
CDC28 protein kinase regulatory subunit 1B. Oncotarget.
7:43419–43431. 2016.PubMed/NCBI View Article : Google Scholar
|