Introduction
Vascular calcification (VC), a prevalent
complication of atherosclerosis, aging, chronic kidney disease and
diabetes, is a major contributor to the high morbidity and
mortality observed in cardiovascular diseases (1). Based on the location of the lesion, VC
is classified into either intimal and medial calcification
(2). Vascular medial calcification
is not a passive calcium salt deposition process, but rather an
active osteogenic process regulated by vascular smooth muscle cells
(VSMCs) in the medial layer (3).
Accumulating evidence demonstrates that VC is associated with cell
apoptosis, calcified matrix exosome release and osteogenic
phenotype transformation of VSMCs, the apoptosis of which
influences VC initiation and osteogenic transformation in VSMCs, is
a key event of VC (4).
Specific protein 1 (Sp1) is a transcriptional
activator extensively involved in life-sustaining activities, such
as cell cycle, proliferation, differentiation, chromatin remodeling
and DNA damage (5-9).
Sp1 C-terminal-specific zinc fingers can bind to promoters of
target genes rich in GC boxes and participate in transcriptional
regulation of target genes (10). A
recent study revealed that Sp1 accelerated the process of VC by
promoting VSMC phenotypic transformation or accelerating apoptosis
(11). Sp1 is widely involved in
the basic expression of several genes, including genes associated
with early embryonic development, and regulates cell proliferation
and differentiation (12). The
knockout of Sp1 during embryonic growth and development is
invariably fatal in the Sp1-/- C57BL/6 mouse model
(12), underlining its crucial role
in sustaining basic life activities. Therefore, it is particularly
important to regulate the pro-calcifying effect of Sp1 without
affecting its expression level. Hence, the present study focused on
the post-translational modifications (PTMs) of Sp1, with the aim of
helping to identify potential therapeutic targets.
PTM is a process of chemical modification in
proteins, which alters their structure and function (13). Excluding phosphorylation, lysine
acetylation is the most widely known PTM and it not only enhances
transcription by attenuating interactions between histones and
chromatin, but also promotes the transcriptional activity of
non-histone transcription factors (14). A previous study revealed that the
acetylation of Sp1 occurs in its DNA-binding domain and upregulates
the expression of downstream genes (15). Deacetylation, as opposed to
acetylation, is associated with transcriptional repression
(16).
The aim of the present study was to explore whether
deacetylated Sp1 regulates calcification by inhibiting phenotypic
switching and apoptosis in VSMCs and, if so, to further determine
the potential molecular mechanisms.
Materials and methods
Cell culture and calcification
model
Primary rat VSMCs were extracted from thoracic
aortic arteries of male Wistar rats (8 weeks; weight, 160±10 g)
using the tissue explant adherent method, as previously described
(17). The rats were obtained from
Charles River Laboratories, Inc. and kept in a climate-controlled
room (temperature, 25±1˚C; relative humidity, 50-60%) with free
access to food and water and a 12-h light/dark cycle. All animal
experimental protocols were approved by the Ethics Committee of
Qilu Hospital of Shandong University. In short, the rats were
euthanized by an overdose of pentobarbital (>150 mg/kg; i.p.)
until loss of limb reflexes.
Cells at passages 3-8 were used for experiments.
VSMCs were incubated in high-glucose DMEM/Nutrient Mixture F-12
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 1% penicillin and 1% streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a humidified
incubator with 5% CO2. The culture medium was replaced
once per day in this way until harvesting after 3 days.
To induce calcification, confluent VSMCs were
treated with 10 mmol/l β-glycerophosphate (β-GP; cat. no. G9422;
Merck KGaA) for 2-14 days at 37˚C (11). The culture medium containing
β-glycerophosphate was replaced every 3 days. Cells without any
treatment were used as the normal control (NC).
Western blotting
Western blotting was performed as previously
described (11). Briefly, VSMCs
were treated with 10 mmol/l β-GP for 3 days at 37˚C and dissolved
in RIPA buffer (Beyotime Institute of Biotechnology) after washing
in cold PBS. The supernatant was centrifuged at 14,000 x g at 4˚C
for 10 min to obtain total protein. Protein concentration was
measured using a BCA protein assay kit (Beyotime Institute of
Biotechnology). The proteins (30 µg/lane) were separated by 10-12%
SDS-PAGE and transferred to polyvinylidene fluoride membranes
(0.22/0.45 µm; EMD Millipore) which were then blocked at room
temperature (RT) for 1 h in PBS-Tween-20 (PBS-T) solution
containing 5% skimmed milk. Primary antibodies against Sp1
(1:1,000; cat. no. NBP2-20460; Novus Biologicals, LLC), BMP2
(1:1,000; cat. no. ab214821; Abcam), α-smooth muscle actin (α-SMA)
(1:1,000; cat. no. ab7817; Abcam), calponin 1 (1:1,000; cat. no.
17819; Cell Signaling Technology, Inc.), Bcl-2 (1:1,000; cat. no.
ab196495; Abcam), runt-related transcription factor 2 (Runx2)
(1:1,000; cat. no. 12556; Cell Signaling Technology, Inc.), β-actin
(1:1,000; cat. no. 3700; Cell Signaling Technology, Inc.), Bax
(1:1,000; cat. no. 2772; Cell Signaling Technology, Inc.) were
incubated with membranes overnight at 4 ˚C. On day 2, the membranes
were extensively washed with Tris-buffered saline with 0.1%
Tween-20 and incubated with a horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin g (IgG) secondary antibody
(1:5,000; cat. no. SA00001-9; ProteinTech Group, Inc.) or goat
anti-mouse IgG secondary antibody (1:5,000; cat. no. SA00001-8;
ProteinTech Group, Inc.) for 1.5 h at RT. Protein signals were
visualized using an Amersham Imager 600 electrochemiluminescence
instrument (Cytiva) and semi-quantified using ImageJ Software
(version 1.48; National Institutes of Health).
Immunoprecipitation (IP)
VSMCs were treated with 10 mmol/l β-GP for 3 days at
37˚C and dissolved in RIPA buffer (Beyotime Institute of
Biotechnology) after washing in cold PBS. The supernatant was
centrifuged at 14,000 x g at 4˚C for 10 min to obtain whole-cell
extracts. 400 µl whole-cell extracts (2 x106 cells) were
preincubated with 25 µl magnetic beads (cat. no. HY-K020;
MedChemExpress) on a rotator for 2 h at 4˚C to clear non-specific
bead binding. Following magnetic separation, the extracts were
incubated with anti-Sp1 antibody (1:100; cat. no. NBP2-20460; Novus
Biologicals, LLC) on a rotator overnight at 4˚C. The
protein-antibody mixture was then re-incubated with 40 µl magnetic
beads and cleaned with PBS with 0.5% Triton X-100 (PBST) on a
rotator for 4 h at 4˚C. After washing four times in PBS-T, the
magnetic beads were separated magnetically. The proteins that
remained and had bound to the magnetic beads were released by
boiling in 1X SDS-PAGE loading buffer. The isolated proteins were
then analyzed. Acetyl-lysine antibody (1:1,000; cat. no. sc-81623;
Santa Cruz Biotechnology, Inc.) was used to detect the levels of
acetyl-Sp1 using western blotting as aforementioned.
Plasmid transfection
A previous study showed that the acetylation site of
Sp1 is at Lys703 in human epidermoid carcinoma cells (A431) and
that mutating lysine 703 (K703) to alanine (A) leads to
deacetylation of Sp1(18). In
addition, following the alignment of the genomic sequence between
humans and rats through the GenBank database on the NCBI website
(http://www.ncbi.nlm.nih.gov/), as
previously described (19), it was
found that the acetylation site of Sp1 is at Lys704 (K704) in rats.
Next, Sp1 overexpression plasmid (pCMV; Sp1-WT), Sp1 point mutant
(K704A) plasmid (pCMV; Sp1-K704A) and control plasmid (pCMV;
control plasmid) were synthesized by Shanghai Genechem Co. Ltd.
SMCs (1x105 cells/ml) were seeded in 6-well plates. In
total, 6 µg plasmid was transfected into VSMCs with 6 µl
Micropoly-transfecter Cell Reagent (Micropoly) for 24 h at 37˚C and
used for subsequent experiments. For in vitro analysis, 48 h
after cell transfection, the cells were treated with 10 mmol/l β-GP
for 2-14 days.
Immunofluorescence staining
VSMCs (1x105 cells/ml) were seeded in
24-well climbing slice culture plates treated with 10 mmol/l β-GP
for 3 days at 37˚C following plasmid transfection. They were then
fixed in 4% paraformaldehyde for 1 h at RT. Following washing with
PBS, cells were permeabilized using 0.5% Triton X-100 for 10 min at
RT. Next, cells were washed with PBS and blocked with 5% bovine
serum albumin (cat. no. A8850-5; Beijing Solarbio Science &
Technology Co., Ltd.) for 30 min at RT. The cells were then
double-stained with primary antibodies against BMP2 (1:100; cat.
no. ab214821; Abcam) and α-SMA (1:100; cat. no. 48938; Cell
Signaling Technology, Inc.) in PBS overnight at 4˚C. On day 2,
following extensive washing with PBS, cells were incubated with
Alexa Fluor 488-conjugated goat anti-mouse (1:200; cat. no.
ZF-0511; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
or Alexa Fluor 594-conjugated goat anti-rabbit (1:200; cat. no.
ZF-0516; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
for 1 h at RT. Following staining with DAPI for 10 min at 37˚C,
cells were viewed by fluorescence microscopy (magnification, x200;
Nikon Eclipse NI-E; Nikon Corporation) and analyzed using ImageJ
Software (version 1.48; National Institutes of Health).
Calcium staining
Calcium staining was performed as previously
described (17). Briefly,
calcification induction was performed by 10 mmol/l β-GP for 12 days
at 37˚C following plasmid transfection, VSMCs were washed with PBS
and fixed in 70% ethanol for 1 h at RT. Following rinsing with PBS,
VSMCs were exposed to 1 mg/ml Alizarin red S solution (pH 4.2) in
the dark for another 1 h at RT. Images were captured using an
inverted motorized microscope (magnification, x40; Nikon Ti-E;
Nikon Corporation).
Calcium deposition detection, ALP
activity and caspase-3 activity assay
VSMCs were treated with 10 mmol/l β-GP for 6 days at
37˚C following plasmid transfection and dissolved in RIPA buffer
(Beyotime Institute of Biotechnology) after washing in cold PBS.
The supernatant was centrifuged at 14,000 x g at 4˚C for 10 min to
obtain whole-cell extracts. The calcium content was determined
using the Calcium Assay kit at RT (cat. no. C004-2; Nanjing
Jiancheng Bio-engineering Institute Co., Ltd.), and ALP and
caspase-3 activities were detected using an ALP assay kit at RT
(cat. no. P0321; Beyotime Institute of Biotechnology) and caspase-3
activity assay kit at 37˚C (cat. no. C1116; Beyotime Institute of
Biotechnology), respectively. The testing of calcium content, ALP
and caspase-3 activities were performed according to the
manufacturers' instructions. The results were then normalized to
protein concentrations measured using an enhanced BCA protein assay
kit (cat. no. P0010; Beyotime Institute of Biotechnology).
TUNEL assay
Apoptosis in calcified VSMCs was measured using an
In Situ Cell Death Detection kit at RT, TMR red (cat. no.
12156792910; Roche Diagnostics), following the manufacturer's
instructions. In brief, VSMCs (1x105 cells/ml) were
incubated in a 24-well plate and treated with 10 mmol/l β-GP for 3
days at 37˚C following plasmid transfection. The steps to fix and
permeabilize cells were the same as those for immunofluorescence.
Calcified VSMCs were then stained using TUNEL dyes for a minimum of
60 min at 37˚C. Following DAPI staining for 10 min at 37˚C,
TUNEL-positive VSMCs were manually counted via fluorescence
microscopy (magnification, x200; Nikon Eclipse NI-E; Nikon
Corporation).
Annexin V/propidium iodide
double-staining
VSMCs (1x105 cells/ml) were incubated in
a 6-well plate and treated with 10 mmol/l β-GP for 3 days at 37˚C
following plasmid transfection. Following the manufacturer's
instructions, Annexin V/propidium iodide double-staining was
performed using an Annexin V-FITC Apoptosis Detection kit at RT
(cat. no. 556547; BD Pharmingen), and apoptotic cells were detected
using BD FACSCalibur (BD Biosciences) and analyzed using FlowJo
software (version 7.6; FlowJo LLC).
Chromatin immunoprecipitation (ChIP)
assay
VSMCs (2x107 cells/ml) were treated with
10 mmol/l β-GP for 3 days at 37˚C following plasmid transfection.
ChIP assay was performed using SimpleChIP® Plus
Enzymatic ChIP kit at RT (cat. no. 9005; Cell Signaling Technology,
Inc.), according to the manufacturer's instructions. Anti-sp1
antibody (1:50; cat. no. NBP2-20460; Novus Biologicals, LLC) was
used to bind chromatin-bound proteins for 24 h at 4˚C. The primers
used to amplify the fragments containing the BMP2 promoter were as
follows: BMP2 forward, 5'-TTACACTCAGCCGGGACGC-3' and reverse,
5'-GAACACCTCCCCCTCGGA-3'. The PCR products were analyzed on 2%
agarose gel and then visualized using an electrochemiluminescence
instrument (Bio-Rad Laboratories, Inc.). ChIP signal was normalized
to total input. A positive control (Anti-Histone H3; 1:50; cat. no.
9005; Cell Signaling Technology, Inc.) and a negative control
(normal IgG; 1:50; cat. no. 9005; Cell Signaling Technology, Inc.)
were employed for each immunoprecipitation.
Statistical analysis
Each experiment was performed >3 times
independently. Data are presented as the mean ± SEM. GraphPad Prism
8.0 (GraphPad Software, Inc.) was used for statistical analysis.
Unpaired Student's t-test was used for comparisons between two
groups, and one-way ANOVA, followed by Tukey's post hoc test for
comparisons among multiple groups. P<0.05 was considered to
indicate a significantly significant different.
Results
Expression levels of acetylated Sp1
are increased in β-GP-treated VSMCs
As previously described, VSMCs were treated with
β-GP for 72 h at 37˚C to induce calcification (17). Western blotting was performed to
assess the calcification of VSMCs, and it was found that the
expression levels of the osteogenic markers BMP2 and Runx2 were
upregulated and those of α-SMA, a marker of the contractile
phenotype of VSMCs, was reduced (Fig.
1A; P<0.05). Next, the expression levels of acetylated Sp1
in calcified VSMCs was investigated. The IP results showed that,
compared with the control group, the expression levels of
acetylated Sp1 were increased in a time-dependent manner in the
calcification group (Fig. 1B and
C; P<0.05).
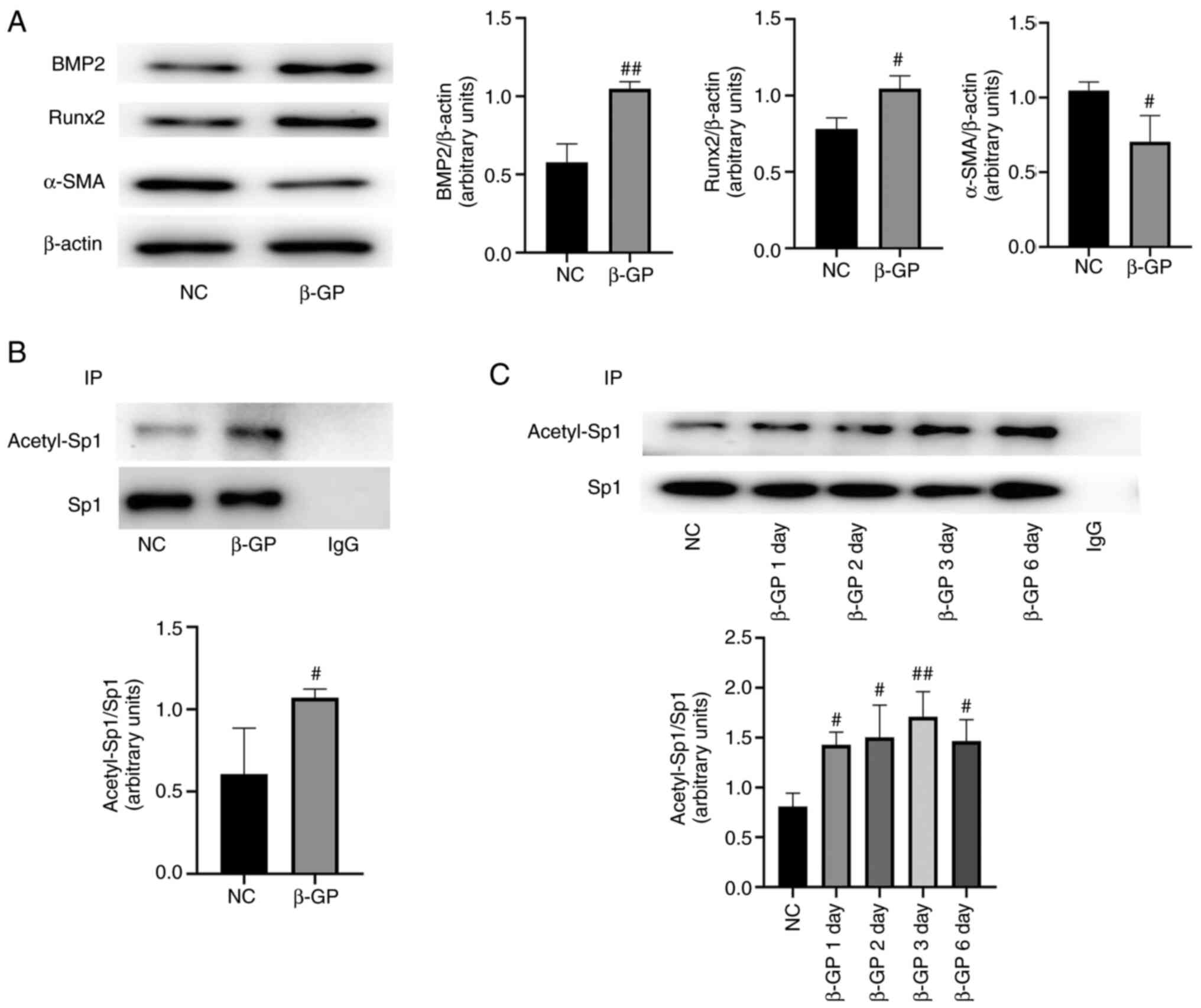 | Figure 1Acetylated Sp1 expression levels are
higher in calcified VSMCs. (A) BMP2, Runx2, α-SMA and calponin 1
expression levels were measured by western blotting and normalized
to β-actin. (B and C) Acetylated Sp1 expression was measured by IP
and normalized to Sp1. Data are presented as the mean ± SEM. n=3.
#P<0.05; ##P<0.01 vs. NC group. NC
group, normal cultured VSMCs; β-GP group,
β-glycerophosphate-induced VSMCs; IP, immunoprecipitation; Sp1,
specific protein 1; VSMCs, vascular smooth muscle cells; BMP2, bone
morphogenetic protein 2; Runx2, runt-related transcription factor
2; α-SMA, α-smooth muscle actin; β-GP, β-glycerophosphate. |
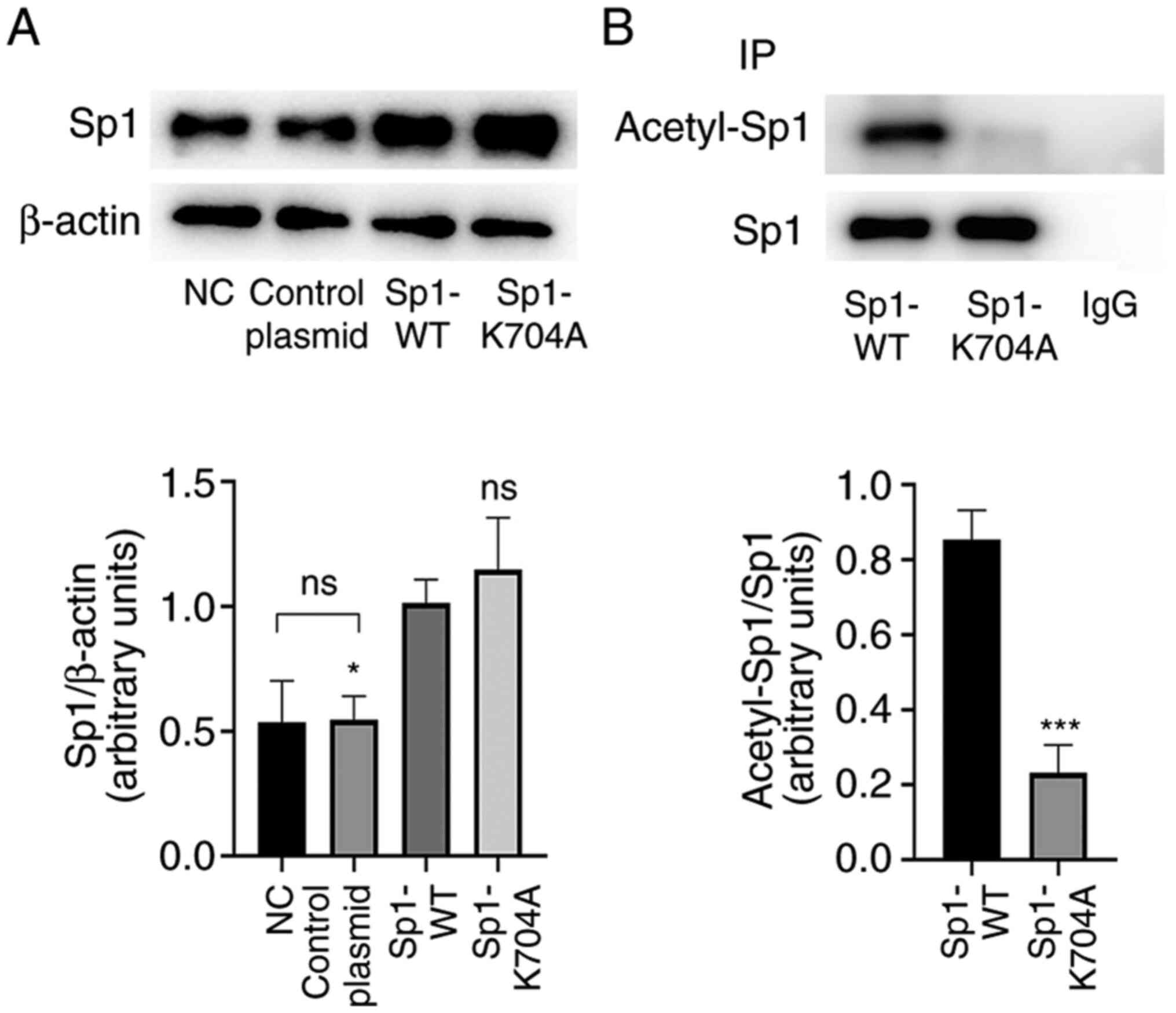
Sp1 acetylation site is at K704 in rat
VSMCs
Next, Sp1 K704A mutant plasmid was constructed to
mimic the deacetylation status of Sp1, and then Sp1-WT plasmid and
Sp1-K704A mutant plasmid were transfected into VSMCs. At 48 h from
infection, the acetylation levels of Sp1 were examined. As shown by
western blotting (Fig. 2A;
P<0.05), protein levels of Sp1 remained unchanged following
negative control plasmid transfection, but were higher following
Sp1 WT and K704A mutant plasmid transfection, as compared with the
NC. The IP results revealed that acetylated Sp1 levels were
markedly reduced by Sp1 K704A plasmid, as comparison with plasmid
Sp1 WT plasmid (Fig. 2B;
P<0.05).
Sp1 deacetylation ameliorates calcium
deposition and phenotype switching in calcified VSMCs
Following transfection with Sp1-WT and Sp1-K704A
plasmids, calcification was induced in VSMCs by β-GP for 3-12 days
at 37˚C. As shown in Fig. 3A-C,
Alizarin red S staining, ALP activity assay and calcium content
assay (P<0.05) indicated that compared with the Sp1
overexpression group, deacetylated Sp1 clearly inhibited calcium
deposition in calcified VSMCs. Moreover, using western blot
analysis (Fig. 4A; P<0.05) and
immunofluorescence staining (Fig.
4B; P<0.05), it was observed that the decreased levels of
VSMC contractile markers α-SMA and calponin 1 were rescued, while
those of osteogenic markers Runx2 and BMP2 were suppressed.
Simultaneously, it was observed that calcium deposition and
phenotype switching were enhanced by Sp1 overexpression in the Sp1
overexpression group, as compared with the induced calcification
group. The β-GP groups and NC were used as a reference to confirm
the state of calcification and Sp1 overexpression in VSMCs,
respectively.
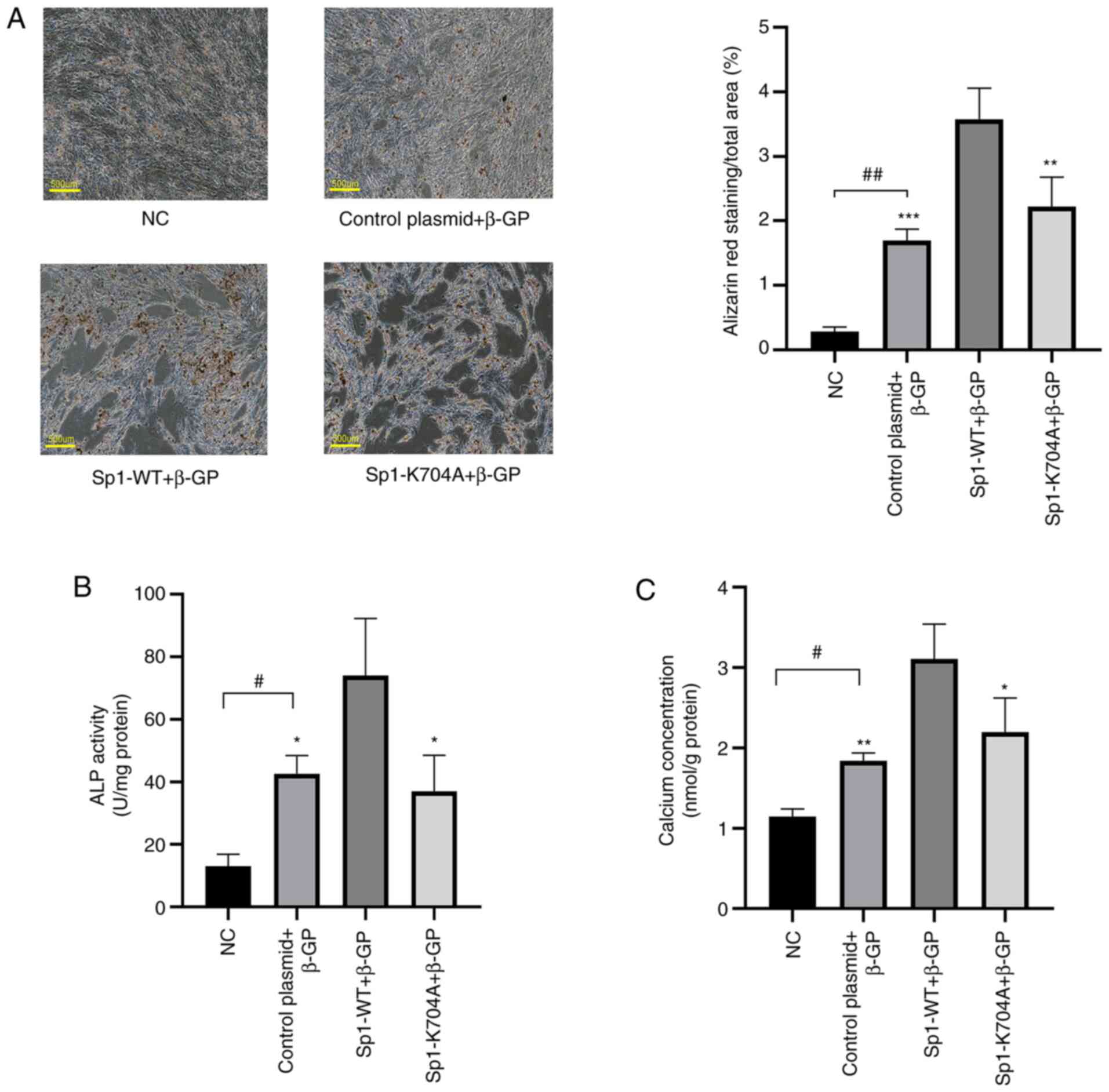 | Figure 3Deacetylated Sp1 ameliorates ALP
activity and calcium deposition in calcified VSMCs. (A) Alizarin
red S staining of VSMCs with β-GP induction for 12 days. Scale bar,
500 µm. (B) ALP activity and (C) calcium deposition in calcified
VSMCs with β-GP induction for 6 days were measured and normalized
to protein content for quantitative analysis. Data are presented as
mean ± SEM. n=3. #P<0.05; ##P<0.01 vs.
NC group; *P<0.05; **P<0.01;
***P<0.001 vs. Sp1-WT group. NC, normal control;
control plasmid + β-GP, β-glycerophosphate-induced VSMCs
transfected with negative control plasmid; Sp1-WT + β-GP,
β-glycerophosphate-induced VSMCs transfected with Sp1-WT plasmid;
Sp1-K704A + β-GP, β-glycerophosphate-induced VSMCs transfected with
Sp1-K704A plasmid; NC, normal control; ALP, alkaline phosphatase;
VSMCs, vascular smooth muscle cells; β-GP, β-glycerophosphate; WT,
wild-type. |
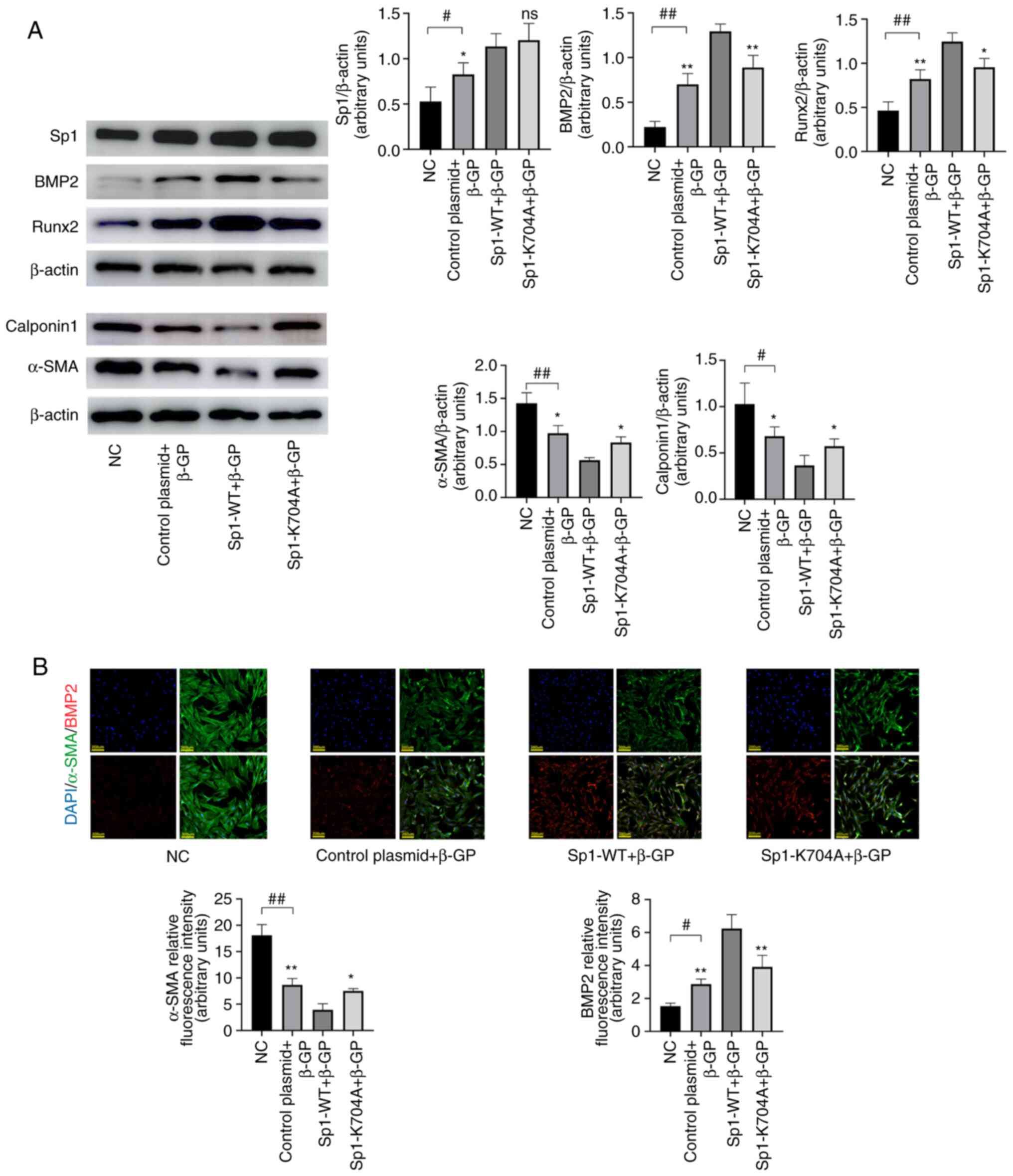 | Figure 4Deacetylated Sp1 ameliorates
phenotypic switching in calcified VSMCs. Calcified VSMCs were
induced by β-GP for 3 days after transfection. (A) Sp1, BMP2,
Runx2, α-SMA and calponin 1 protein expression was measured by
western blotting and normalized to β-actin. (B) Immunofluorescence
staining of BMP2 (red) and α-SMA (green), Scale bar=200 µm. Data
are presented as the mean ± SEM. n=3. #P<0.05;
##P<0.01 vs. NC group; *P<0.05;
**P<0.01 vs. Sp1-WT group; ns, P>0.05. NC, normal
control; control plasmid + β-GP, β-glycerophosphate-induced VSMCs
transfected with negative control plasmid; Sp1-WT + β-GP,
β-glycerophosphate-induced VSMCs transfected with Sp1-WT plasmid;
Sp1-K704A + β-GP, β-glycerophosphate-induced VSMCs transfected with
Sp1-K704A plasmid; NC, normal control; Sp1, specific protein 1;
VSMCs, vascular smooth muscle cells; β-GP, β-glycerophosphate;
BMP2, bone morphogenetic protein 2; Runx2, runt-related
transcription factor 2; α-SMA, α-smooth muscle actin; WT,
wild-type. |
Deacetylated Sp1 reduces apoptosis in
calcified VSMCs
To investigate the role of deacetylated Sp1 on
apoptosis, Annexin V-PI flow cytometry (Fig. 5C; P<0.05) and TUNEL analysis
(Fig. 5D; P<0.05) were
performed. The number of apoptotic VSMCs was markedly reduced by
deacetylated Sp1 (Sp1-K704A). In addition, the expression levels of
the apoptosis-related protein Bax and the anti-apoptotic protein
Bcl-2 were determined via western blotting. It was revealed that,
compared with the Sp1 overexpression (Sp1-WT) group, the Bcl-2/Bax
ratio was increased in the Sp1-K704A group (Fig. 5A; P<0.05). Caspase-3 activity
assay showed that deacetylated Sp1 significantly reduced the
activity of caspase3 to inhibit VSMCs apoptosis (Fig. 5B; P<0.05).
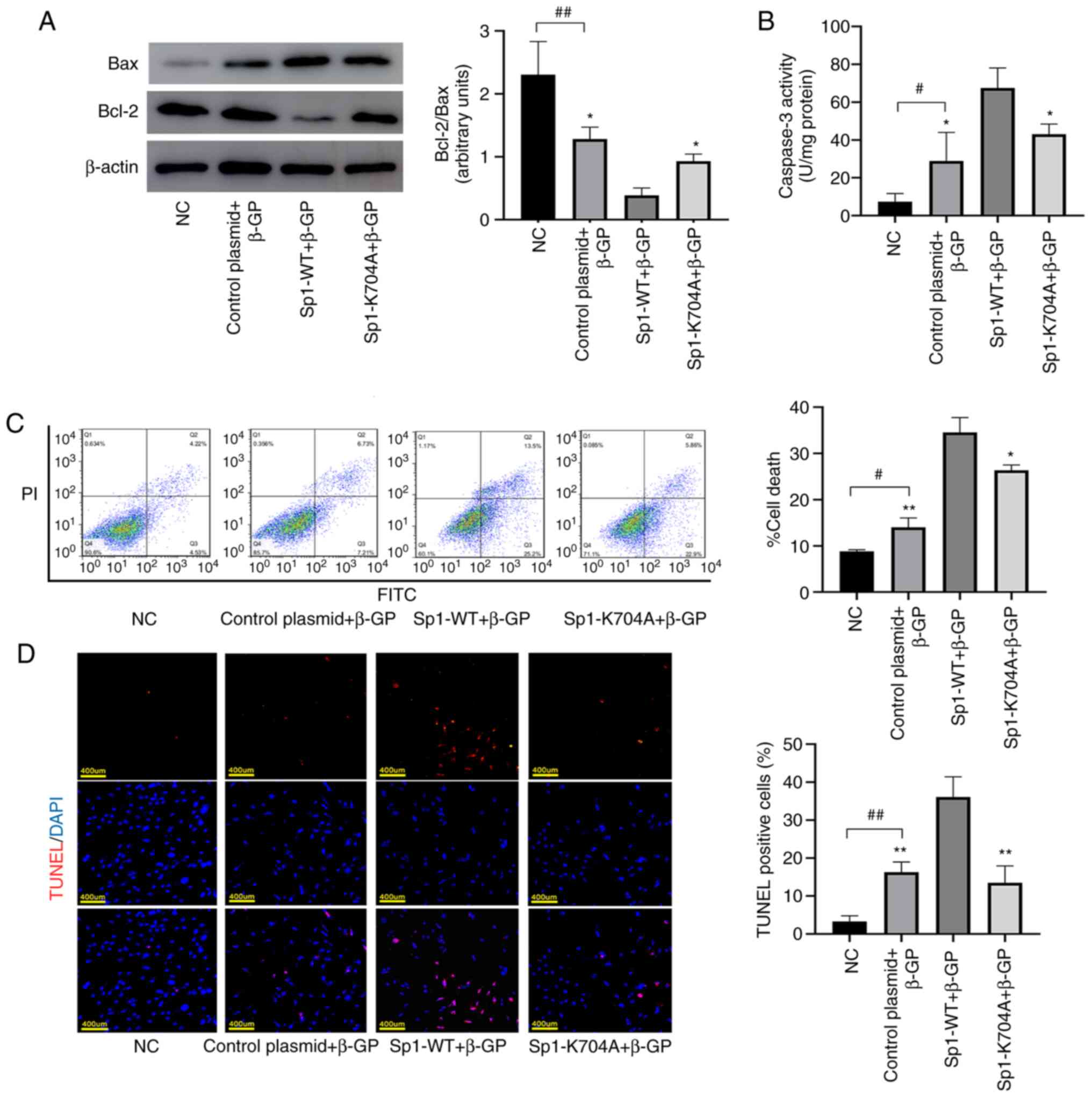 | Figure 5Deacetylated Sp1 reduces apoptosis in
calcified VSMCs. Calcified VSMCs were induced by β-GP for 3 days
after transfection. (A) Expression of apoptosis related-proteins
Bcl-2 and Bax was measured by western blotting and the levels of
Bcl-2/Bax were detected by densitometric analysis. (B) Caspase-3
activity was assessed using a caspase-3 activity assay kit.
Apoptotic rate was determined by (C) TUNEL staining (Scale bar=400
µm) and (D) Annexin V/propidium iodide double staining. Data are
presented as the mean ± SEM. n=3. #P<0.05;
##P<0.01 vs. NC group; *P<0.05;
**P<0.01 vs. Sp1-WT group. NC, normal control;
control plasmid + β-GP, β-glycerophosphate-induced VSMCs
transfected with negative control plasmid; Sp1-WT + β-GP,
β-glycerophosphate-induced VSMCs transfected with Sp1-WT plasmid;
Sp1-K704A + β-GP, β-glycerophosphate-induced VSMCs transfected with
Sp1-K704A plasmid; NC, normal control; Sp1, specific protein 1;
VSMCs, vascular smooth muscle cells; β-GP, β-glycerophosphate;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; terminal
deoxynucleotidyl transferase dUTP nick end labeling; WT,
wild-type. |
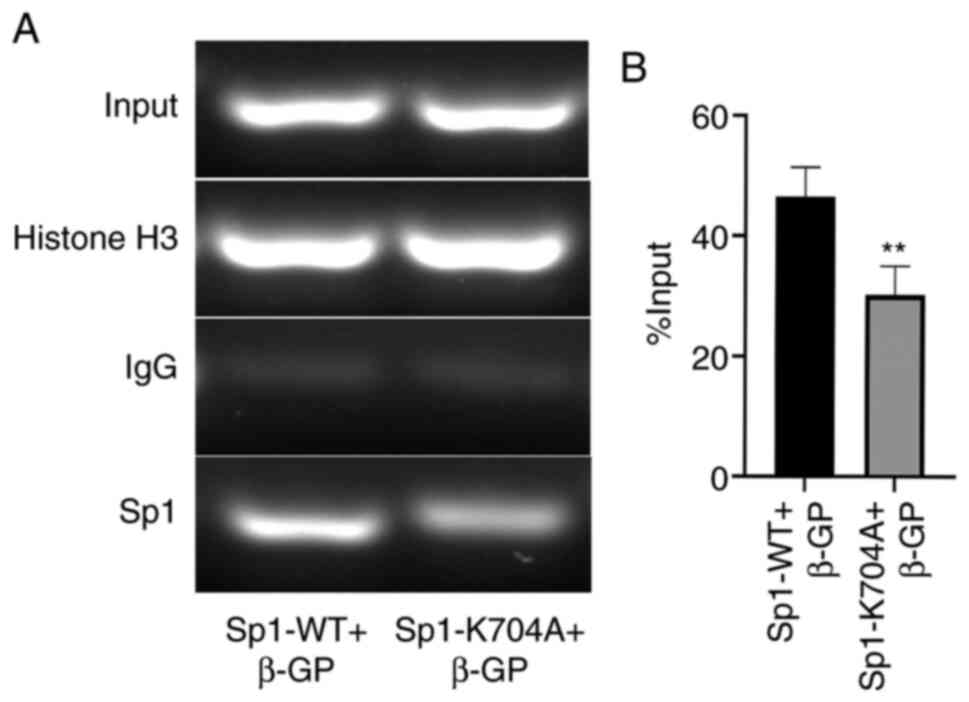
Deacetylated Sp1 inhibits VSMC
phenotype switching and apoptosis by decreasing Sp1 binding to BMP2
promoter
Based on the aforementioned findings, the underlying
mechanism mediating the protective role of deacetylated Sp1 in VSMC
calcification was explored. Considerable evidence has demonstrated
that BMP2 is not only involved in VSMC phenotype switching, but
also in VSMC apoptosis (20,21).
Furthermore, in our previous study, it was revealed that Sp1
binding to BMP2 promoter was elevated in β-GP-induced calcified
VSMCs (11). To determine whether
deacetylated Sp1 regulated VC by inhibiting Sp1 binding activity to
the BMP2 promoter, a ChIP assay was performed in β-GP-induced
calcified VSMCs following plasmid transfection. As shown in
Fig. 6A and B, it was observed that Sp1 binding to the
BMP2 promoter was downregulated in the Sp1-K704A group compared
with that in the Sp1 overexpression (Sp1-WT) group.
Discussion
The present study demonstrated that deacetylated Sp1
can significantly reverse the calcification of VSMCs. The levels of
acetylated Sp1 were clearly increased by β-GP-induced calcification
and altered depending the β-GP stimulation time. Following plasmid
transfection conducted to interfere with the levels of acetylated
Sp1, osteogenic transformation, calcium deposition and apoptosis of
calcified VSMCs were improved. Deacetylated Sp1 was found to exert
an anti-VC effect by downregulating the binding of Sp1 to the
promoter of the target gene BMP2.
VC is recognized as a common vascular complication
during chronic kidney disease (CKD), aging and diabetes mellitus.
The selection of different VC models is based on the underlying
disease. For example, glycation end-products (AGEs) produced by
diabetics are important calcification promoters, thus
diabetes-associated VC is studied using a calcification model
established by AGEs (22).
Anti-aging genes, such as klotho and sirt1 are downregulated during
calcification, so the VC model of aging is established by
inhibiting the associated anti-aging genes (23). The high-phosphorus-induced VC model
is established based on the perivascular high phosphorus
environment in patients with CKD (24). Since VC is more widespread in
patients with CKD and hyperphosphatemia-related VC is currently a
hot research topic, a β-GP-induced VC model was selected to study
CKD-related VC (25). A previous
study reported that blocking the phenotypic transformation of VSMC
is essential for the prevention and treatment of VC (26). Therefore, phenotypic transformation
of VSMCs can be used as a reliable index for assessing VC.
Sp1, a member of the Sp family (Sp1-Sp8), was found
to be the key regulator in the proliferation and invasion of tumor
cells (9,27). Recently, the role of Sp1 in the
occurrence and development of cardiovascular diseases was also
investigated. Studies have suggested that Sp1 is involved in the
regulation of myocardial cell apoptosis, myocardial fibrosis,
inflammation, oxidative stress and vascular endothelial cell injury
(28,29). In our previous study, it was
demonstrated that Sp1 also regulates calcification and apoptosis in
β-GP-induced calcified VSMCs, in which its pro-calcific role was
performed by regulating BMP2 transcriptional activation (11). As the main promoter of medial
calcification, BMP2 induces the expression of MSX2 and Runx2, which
are key factors of VSMC phenotypic transformation (20). Sp1 is extensively involved in the
basic expression of multiple genes, and the deletion of Sp1 is
invariably fatal during fetal development (12). Therefore, it is important to
downregulate the pro-calcified effect of Sp1 without affecting its
expression level. Protein PTMs can increase the functional
diversity of Sp1, therefore Sp1 PTMs were investigated in order to
identify novel therapeutic targets in VC. The majority of the
research conducted on Sp1 PTMs is on phosphorylation, but the level
of phosphorylated Sp1 in calcified VSMCs exhibited non-significant
changes compared with the NC group in our unpublished data (data
not shown).
Histone acetylation serves an important role in gene
regulation. A growing number of studies have suggested that,
besides histones, non-histone transcription factors can also be
acetylated (14,30). Acetylated Sp1 has been reported to
upregulate the binding activity to target genes during different
pathological processes (31-33).
However, to the best of our knowledge, no research has explored the
association between acetylated Sp1 and VC. The present study
revealed that the acetylated Sp1 expression is significantly
elevated in calcified VSMCs. This result demonstrated that
acetylated Sp1 promotes the development of VC. Therefore, it was
suggested that inhibiting the acetylation of Sp1 may reduce VC.
Hepp et al (34) reported
that mutating K703 to A not only results in the deacetylation of
Sp1, but also downregulates its transcriptional activity. Following
genetic comparison, Sp1 K704A mutant plasmid was synthesized based
on Sp1 overexpression to achieve the deacetylated state of Sp1
(deacetylated Sp1). In the present study, deacetylated Sp1 was
found to suppress the expression of osteogenic markers and reduce
calcium deposition in VC, in line with our hypothesis. It was also
observed that Sp1 overexpression promoted VC, which, to the best of
our knowledge, has not been explored in other studies.
Apoptosis, a type of programmed cell death, has been
found to be associated with the initiation of VC (35,36).
Our previous study confirmed that, in addition to up-regulating
BMP2 transcriptional activity, Sp1 also participated in VC-related
apoptosis compared with the NC group (11). Of note, the apoptosis-promoting role
of BMP2 in VSMCs was also reported in a previous study, which
demonstrated that BMP2 promoted VSMC apoptosis via the
Wnt/β-catenin pathway (21).
Therefore, it was hypothesized that deacetylated Sp1 may ameliorate
VSMC apoptosis by repressing BMP2 transcription. In the present
study, VSMC apoptosis was indeed shown to be ameliorated by
inhibiting the acetylation of Sp1. This was then confirmed by ChIP
assay, which demonstrated that deacetylation of Sp1 decreased its
binding to BMP2 promoter, in line with our hypothesis.
There were certain limitations to the present study.
First, an acetyl-Sp1 overexpression plasmid could not be
constructed, so the elevated level of acetylated Sp1 in calcified
VSMCs was used to illustrate that acetylated Sp1 is responsible for
the development of VC. Second, whether deacetylated Sp1 affects
other vital activities regulated by Sp1, in addition to VC, was not
explored. Further research is therefore required to address these
issues.
In conclusion, the present study provided strong
evidence supporting that acetylated Sp1 promotes β-GP-induced VSMCs
calcification. The deacetylation of Sp1 by Sp1-K704A plasmid
prevents the calcification of VSMCs by inhibiting BMP2
transcription. These meaningful findings may provide new options
for the treatment and prevention of VC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81873516, 81873522 and
81900444), the National Key Research and Development Program of
Shandong Province (grant no. 2017YFC1308303), the Shandong
Provincial Natural Science Foundation of China (grant no.
ZR2019PH030) and the Clinical Research Center of Shandong
University (grant no. 2020SDUCRCA009).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XPJ designed the study. ZHZ, XYZ, PZ, JX, HZ LW and
YZ performed experiments. CWW and SBY performed the statistical
analysis. ZHZ prepared the manuscript and performed the literature
search. XPJ and XYZ have seen and can confirm the authenticity of
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All applicable international, national and/or
institutional guidelines for the care and use of animals were
followed. All procedures performed in studies involving animals
were approved by the Ethics Committee of Qilu Hospital of Shandong
University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lanzer P, Boehm M, Sorribas V, Thiriet M,
Janzen J, Zeller T, St Hilaire C and Shanahan C: Medial vascular
calcification revisited: Review and perspectives. Eur Heart J.
35:1515–1525. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Demer LL and Tintut Y: Vascular
calcification: Pathobiology of a multifaceted disease. Circulation.
117:2938–2948. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Voelkl J, Lang F, Eckardt KU, Amann K,
Kuro-O M, Pasch A, Pieske B and Alesutan I: Signaling pathways
involved in vascular smooth muscle cell calcification during
hyperphosphatemia. Cell Mol Life Sci. 76:2077–2091. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Durham AL, Speer MY, Scatena M, Giachelli
CM and Shanahan CM: Role of smooth muscle cells in vascular
calcification: Implications in atherosclerosis and arterial
stiffness. Cardiovasc Res. 114:590–600. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bajpai R and Nagaraju GP: Specificity
protein 1: Its role in colorectal cancer progression and
metastasis. Crit Rev Oncol Hematol. 113:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Leigh O, Jane G and Constanze B: The role
of the ubiquitously expressed transcription factor Sp1 in
tissue-specific transcriptional regulation and in disease. Yale J
Biol Med. 89:513–525. 2016.PubMed/NCBI
|
|
7
|
Verrecchia F, Rossert J and Mauviel A:
Blocking sp1 transcription factor broadly inhibits extracellular
matrix gene expression in vitro and in vivo: Implications for the
treatment of tissue fibrosis. J Invest Dermatol. 116:755–763.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Beishline K and Azizkhan-Clifford J: Sp1
and the ‘hallmarks of cancer’. FEBS J. 282:224–258. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vizcaino C, Mansilla S and Portugal J: Sp1
transcription factor: A long-standing target in cancer
chemotherapy. Pharmacol Ther. 152:111–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Solomon SS, Majumdar G, Martinez-Hernandez
A and Raghow R: A critical role of Sp1 transcription factor in
regulating gene expression in response to insulin and other
hormones. Life Sci. 83:305–312. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang X, Li R, Qin X, Wang L, Xiao J, Song
Y, Sheng X, Guo M and Ji X: Sp1 plays an important role in vascular
calcification both in vivo and in vitro. J Am Heart Assoc.
7(e007555)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marin M, Karis A, Visser P, Grosveld F and
Philipsen S: Transcription factor Sp1 is essential for early
embryonic development but dispensable for cell growth and
differentiation. Cell. 89:619–628. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qian M, Yan F, Yuan T, Yang B, He Q and
Zhu H: Targeting post-translational modification of transcription
factors as cancer therapy. Drug Discov Today. 25:1502–1512.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang M, Zhang Y and Ren J: Acetylation in
cardiovascular diseases: Molecular mechanisms and clinical
implications. Biochim Biophys Acta Mol Basis Dis.
1866(165836)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Suzuki T, Kimura A, Nagai R and Horikoshi
M: Regulation of interaction of the acetyltransferase region of
p300 and the DNA-binding domain of Sp1 on and through DNA binding.
Genes Cells. 5:29–41. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kwon DH, Ryu J, Kim YK and Kook H: Roles
of histone acetylation modififiers and other epigenetic regulators
in vascular calcifification. Int J Mol Sci. 21(3246)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou P, Zhang X, Guo M, Guo R, Wang L,
Zhang Z, Lin Z, Dong M, Dai H, Ji X and Lu H: Ginsenoside Rb1
ameliorates CKD-associated vascular calcification by inhibiting the
Wnt/β-catenin pathway. J Cell Mol Med. 23:7088–7098.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hung JJ, Wang YT and Chang WC: Sp1
deacetylation induced by phorbol ester recruits p300 to activate
12(S)-lipoxygenase gene transcription. Mol Cell Biol. 26:1770–1785.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu HL, Xin YF and Xun LY: Distribution,
diversity, and activities of sulfur dioxygenases in heterotrophic
bacteria. Appl Environ Microbiol. 80:1799–1806. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang P, Troncone L, Augur ZM, Kim SSJ,
McNeil ME and Yu PB: The role of bone morphogenetic protein
signaling in vascular calcification. Bone.
141(115542)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rong S, Zhao X, Jin X, Zhang Z, Chen L,
Zhu Y and Yuan W: Vascular calcification in chronic kidney disease
is induced by bone morphogenetic protein-2 via a mechanism
involving the Wnt/β-catenin pathway. Cell Physiol Biochem.
34:2049–2060. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Deng G, Zhang L, Wang C, Wang S, Xu J,
Dong J, Kang Q, Zhai X, Zhao Y and Shan Z: AGEs-RAGE axis causes
endothelial-to-mesenchymal transition in early calcific aortic
valve disease via TGF-β1 and BMPR2 signaling. Exp Gerontol.
141(111088)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pescatore LA, Gamarra LF and Liberman M:
Multifaceted mechanisms of vascular calcification in aging.
Arterioscler Thromb Vasc Biol. 39:1307–1316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shanahan CM, Crouthamel MH, Kapustin A and
Giachelli CM: Arterial calcification in chronic kidney disease: Key
roles for calcium and phosphate. Circ Res. 109:697–711.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schlieper G, Schurgers L, Brandenburg V,
Reutelingsperger C and Floege J: Vascular calcification in chronic
kidney disease: An update. Nephrol Dial Transplant. 31:31–39.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tyson J, Bundy K, Roach C, Douglas H,
Ventura V, Segars MF, Schwartz O and Simpson CL: Mechanisms of the
osteogenic switch of smooth muscle cells in vascular calcification:
WNT signaling, BMPs, mechanotransduction, and endMT. Bioengineering
(Basel). 7(88)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vellingiri B, Iyer M, Devi Subramaniam M,
Jayaramayya K, Siama Z, Giridharan B, Narayanasamy A, Abdal Dayem A
and Cho SG: Understanding the role of the transcription factor Sp1
in ovarian cancer: From theory to practice. Int J Mol Sci.
21(1153)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun S, Li T, Jin L, Piao ZH, Liu B, Ryu Y,
Choi SY, Kim GR, Jeong JE, Wi AJ, et al: Dendropanax morbifera
prevents cardiomyocyte hypertrophy by inhibiting the Sp1/GATA4
pathway. Am J Chin Med. 46:1021–1044. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y, Cao R, Yang W and Qi B:
SP1-SYNE1-AS1-miR-525-5p feedback loop regulates Ang-II-induced
cardiac hypertrophy. J Cell Physiol. 234:14319–14329.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mao Q, Liang X, Wu Y and Lu Y: Resveratrol
attenuates cardiomyocyte apoptosis in rats induced by coronary
microembolization through SIRT1-mediated deacetylation of p53. J
Cardiovasc Pharmacol Ther. 24:551–558. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kou XX, Hao T, Meng Z, Zhou YH and Gan YH:
Acetylated Sp1 inhibits PTEN expression through binding to PTEN
core promoter and recruitment of HDAC1 and promotes cancer cell
migration and invasion. Carcinogenesis. 34:58–67. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Swingler TE, Kevorkian L, Culley KL,
Illman SA, Young DA, Parker AE, Lohi J and Clark IM: MMP28 gene
expression is regulated by Sp1 transcription factor acetylation.
Biochem J. 427:391–400. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Torigoe T, Izumi H, Wakasugi T, Niina I,
Igarashi T, Yoshida T, Shibuya I, Chijiiwa K, Matsuo K, Itoh H and
Kohno K: DNA topoisomerase II poison TAS-103 transactivates
GC-box-dependent transcription via acetylation of Sp1. J Biol Chem.
280:1179–1185. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hepp MI, Escobar D, Farkas C, Hermosilla
VE, Álvarez C, Amigo R, Gutiérrez JL, Castro AF and Pincheira R: A
Trichostatin A (TSA)/Sp1-mediated mechanism for the regulation of
SALL2 tumor suppressor in Jurkat T cells. Biochim Biophys Acta Gene
Regul Mech: May 18, 2018 (Epub ahead of print).
|
|
35
|
Duan X, Zhou Y, Teng X, Tang C and Qi Y:
Endoplasmic reticulum stress-mediated apoptosis is activated in
vascular calcification. Biochem Biophys Res Commun. 387:694–699.
2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ciceri P, Falleni M, Tosi D, Martinelli C,
Cannizzo S, Marchetti G, D'Arminio Monforte A, Bulfamante G, Block
GA, Messa P and Cozzolino M: Therapeutic effect of iron citrate in
blocking calcium deposition in high Pi-calcified VSMC: Role of
autophagy and apoptosis. Int J Mol Sci. 20(5925)2019.PubMed/NCBI View Article : Google Scholar
|